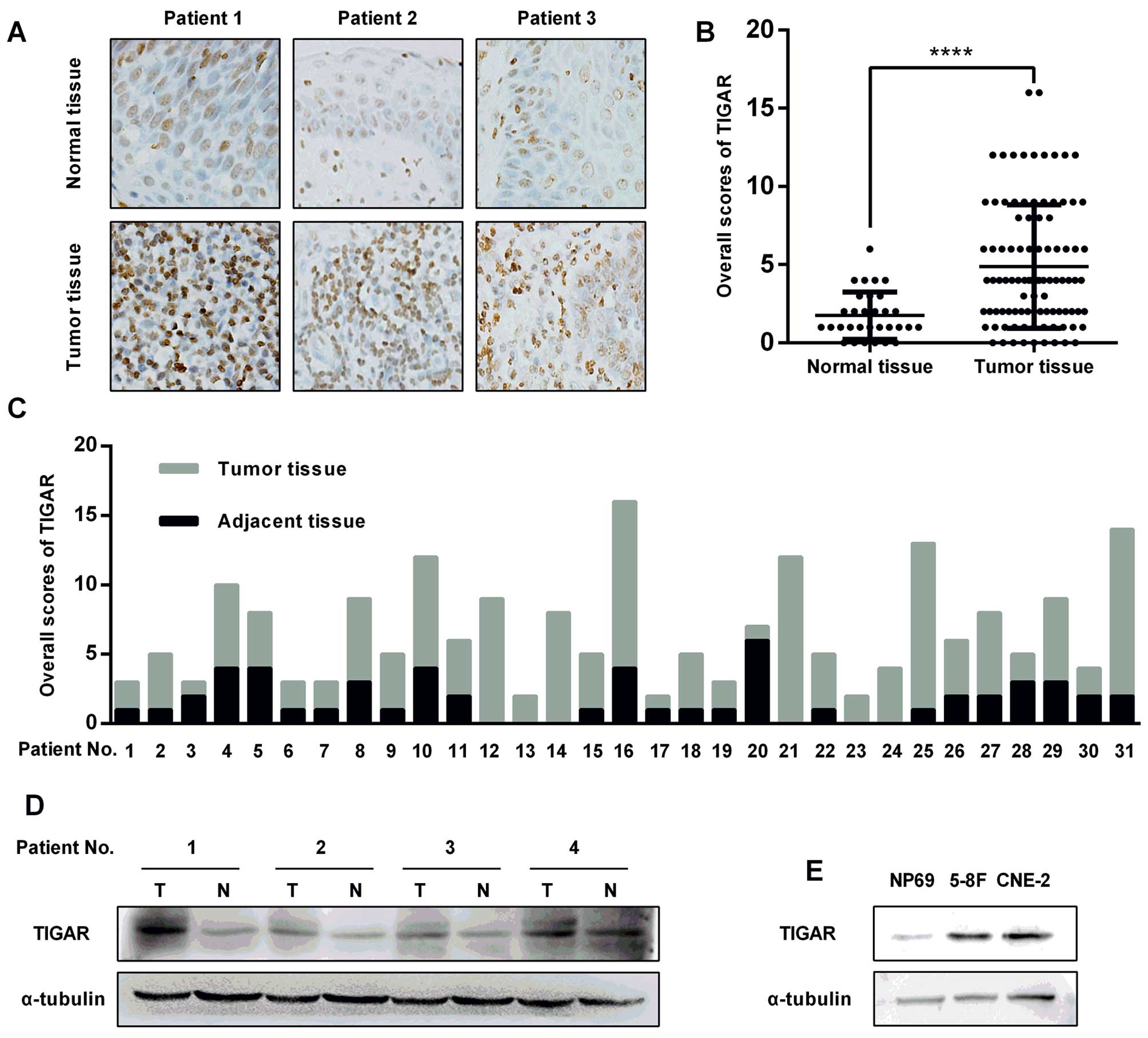
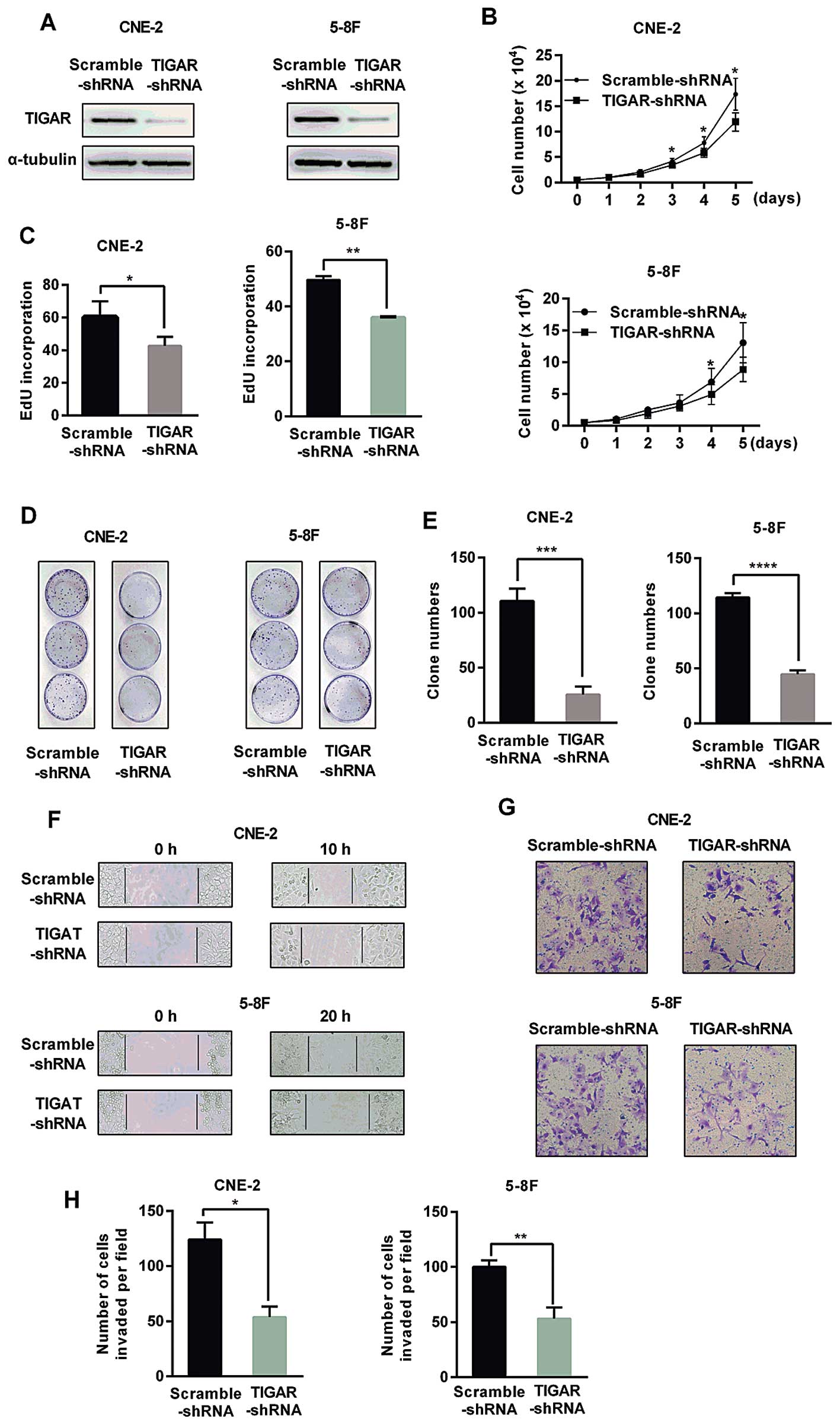
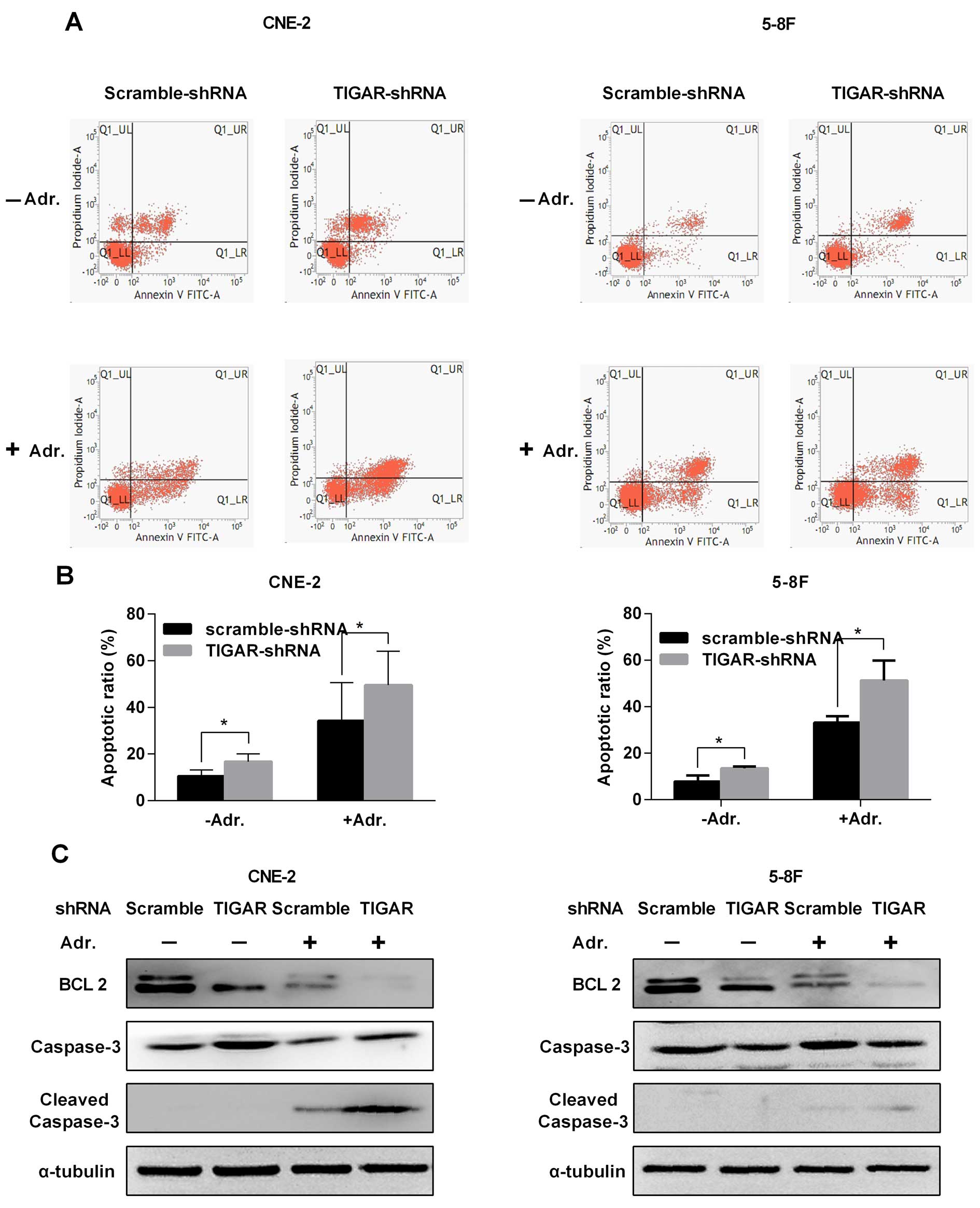
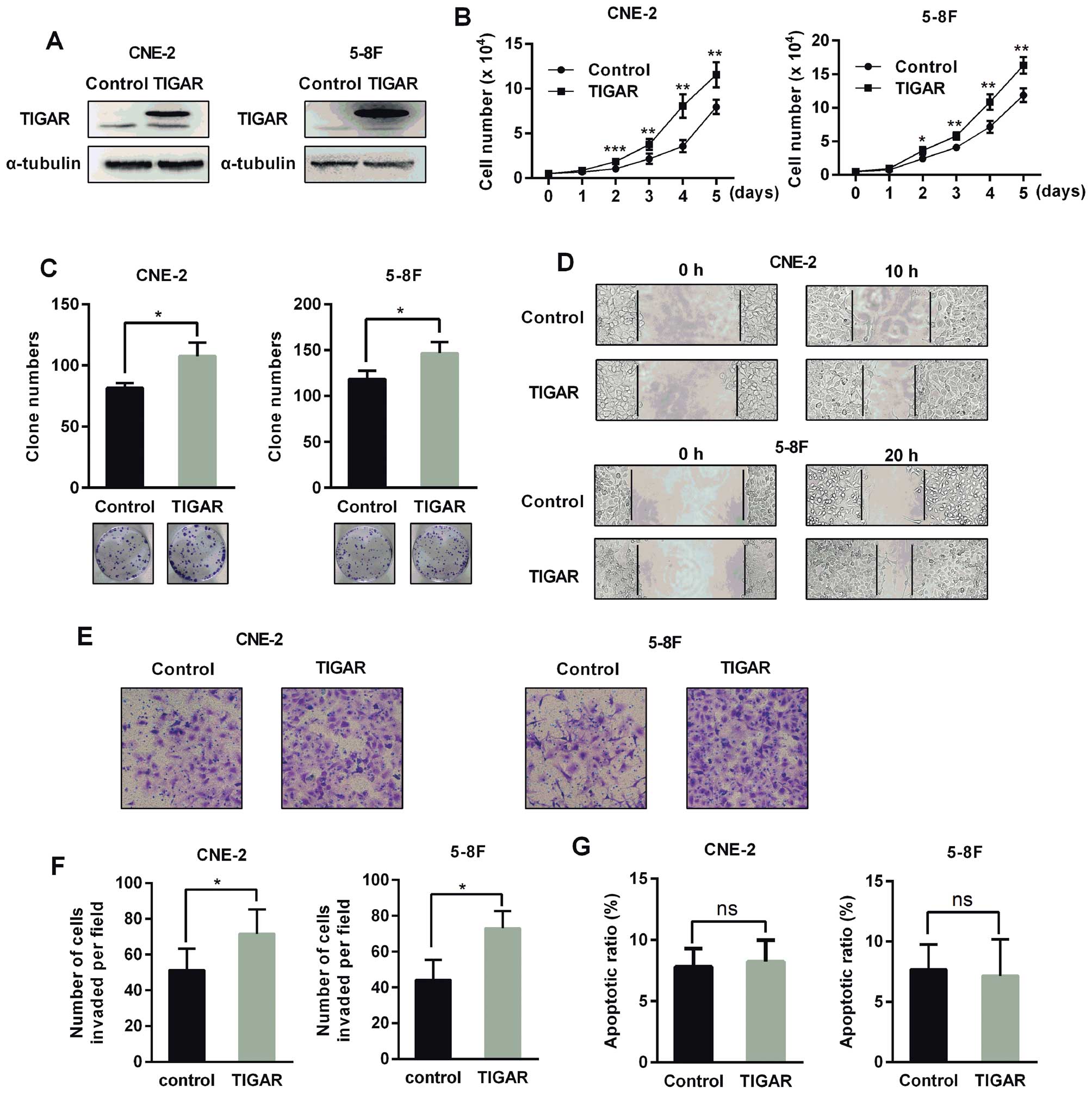
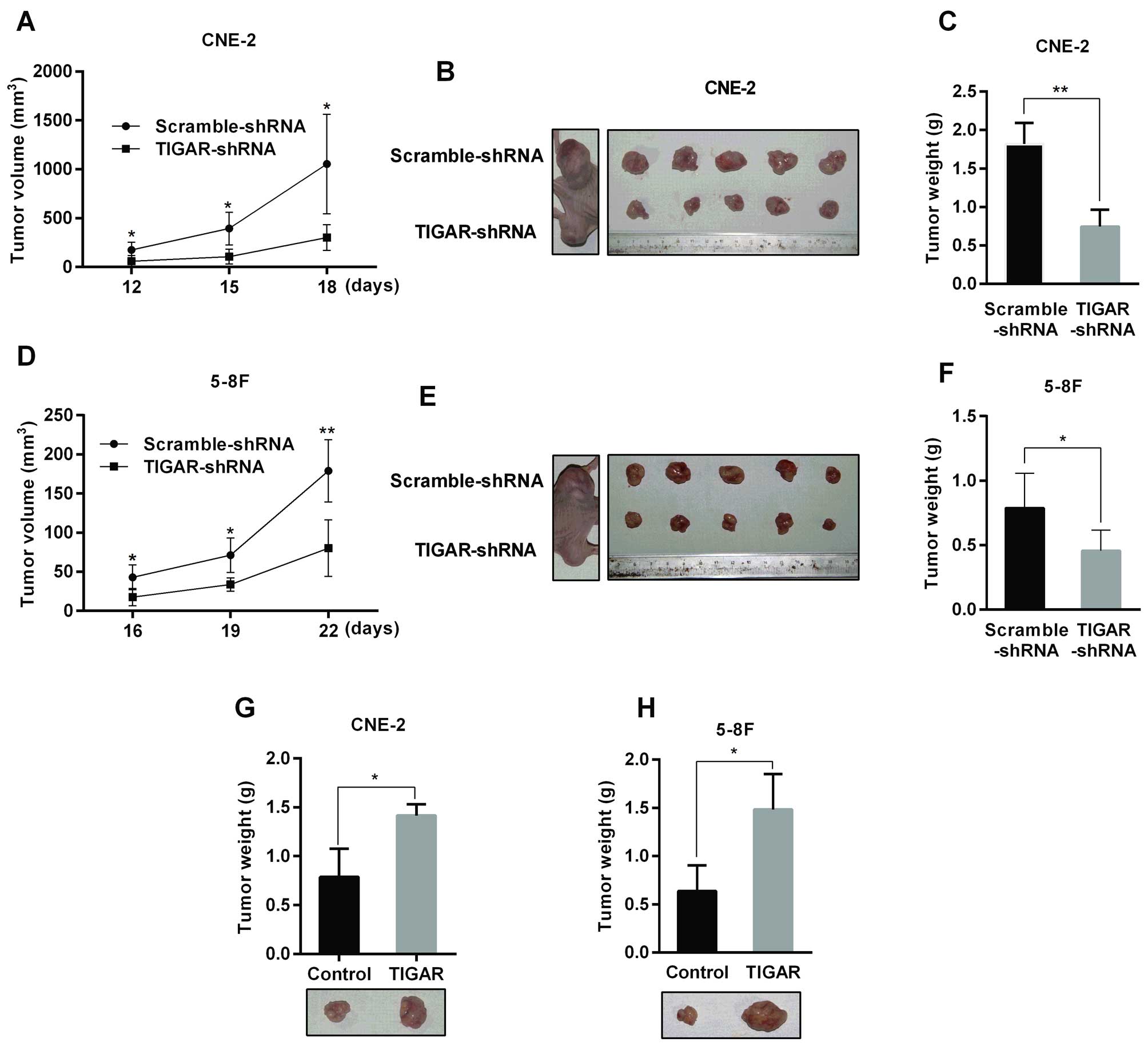
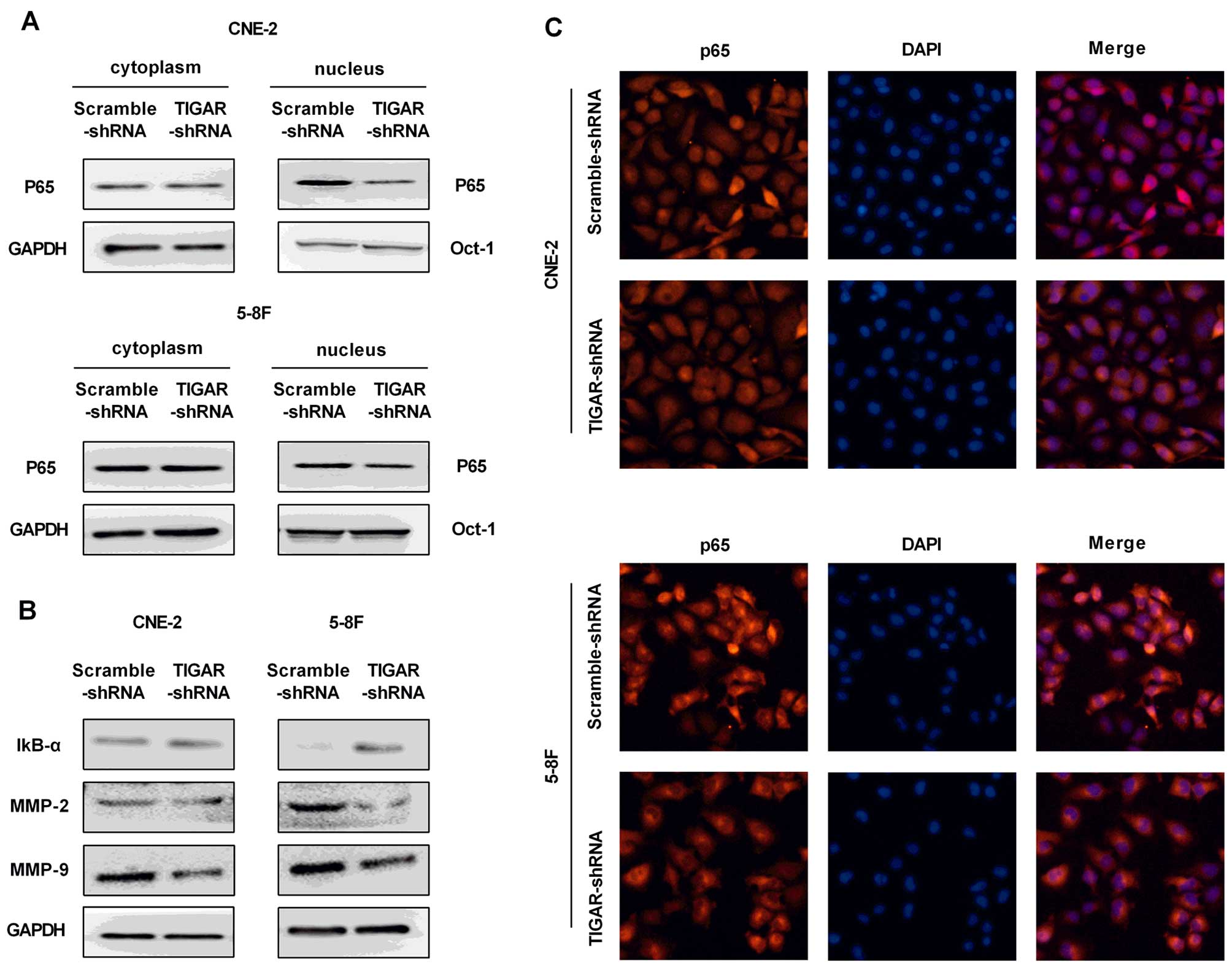
|
1
|
Li XJ, Ong CK, Cao Y, Xiang YQ, Shao JY,
Ooi A, Peng LX, Lu WH, Zhang Z, Petillo D, et al: Serglycin is a
theranostic target in nasopharyngeal carcinoma that promotes
metastasis. Cancer Res. 71:3162–3172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao WW, Huang SM, Han F, Wu SX, Lu LX,
Lin CG, Deng XW, Lu TX, Cui NJ and Zhao C: Local control, survival,
and late toxicities of locally advanced nasopharyngeal carcinoma
treated by simultaneous modulated accelerated radiotherapy combined
with cisplatin concurrent chemotherapy: Long-term results of a
phase 2 study. Cancer. 117:1874–1883. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bensaad K, Tsuruta A, Selak MA, Vidal MN,
Nakano K, Bartrons R, Gottlieb E and Vousden KH: TIGAR, a
p53-inducible regulator of glycolysis and apoptosis. Cell.
126:107–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vousden KH and Ryan KM: p53 and
metabolism. Nat Rev Cancer. 9:691–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Won KY, Lim SJ, Kim GY, Kim YW, Han SA,
Song JY and Lee DK: Regulatory role of p53 in cancer metabolism via
SCO2 and TIGAR in human breast cancer. Hum Pathol. 43:221–228.
2012. View Article : Google Scholar
|
|
7
|
Ye L, Zhao X, Lu J, Qian G, Zheng JC and
Ge S: Knockdown of TIGAR by RNA interference induces apoptosis and
autophagy in HepG2 hepatocellular carcinoma cells. Biochem Biophys
Res Commun. 437:300–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheung EC, Athineos D, Lee P, Ridgway RA,
Lambie W, Nixon C, Strathdee D, Blyth K, Sansom OJ and Vousden KH:
TIGAR is required for efficient intestinal regeneration and
tumorigenesis. Dev Cell. 25:463–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wanka C, Steinbach JP and Rieger J:
Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects
glioma cells from starvation-induced cell death by up-regulating
respiration and improving cellular redox homeostasis. J Biol Chem.
287:33436–33446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinha S, Ghildiyal R, Mehta VS and Sen E:
ATM-NFκB axis-driven TIGAR regulates sensitivity of glioma cells to
radio-mimetics in the presence of TNFα. Cell Death Dis. 4:e6152013.
View Article : Google Scholar
|
|
11
|
Pena-Rico MA, Calvo-Vidal MN,
Villalonga-Planells R, Martinez-Soler F, Gimenez-Bonafe P,
Navarro-Sabate A, Tortosa A, Bartrons R and Manzano A: TP53 induced
glycolysis and apoptosis regulator (TIGAR) knockdown results in
radio-sensitization of glioma cells. Radiother Oncol. 101:132–139.
2011. View Article : Google Scholar
|
|
12
|
Wang L, Wei D, Huang S, Peng Z, Le X, Wu
TT, et al: Transcription factor Sp1 expression is a significant
predictor of survival in human gastric cancer. Clin Cancer Res.
9:6371–6380. 2003.PubMed/NCBI
|
|
13
|
Somasundaram K and El-Deiry WS: Inhibition
of p53-mediated transactivation and cell cycle arrest by E1A
through its p300/CBP-interacting region. Oncogene. 14:1047–1057.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kimata M, Matoba S, Iwai-Kanai E, Nakamura
H, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Mita Y, Okigaki M, et
al: p53 and TIGAR regulate cardiac myocyte energy homeostasis under
hypoxic stress. Am J Physiol Heart Circ Physiol. 299:H1908–H1916.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lui VW, Lau CP, Cheung CS, Ho K, Ng MH,
Cheng SH, Hong B, Tsao SW, Tsang CM, Lei KI, et al: An RNA-directed
nucleoside anti-metabolite, 1-
(3-C-ethynyl-beta-d-ribo-pentofuranosyl) cytosine (ECyd), elicits
antitumor effect via TP53-induced Glycolysis and Apoptosis
Regulator (TIGAR) downregulation. Biochem Pharmacol. 79:1772–1780.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lui VW, Wong EY, Ho K, Ng PK, Lau CP, Tsui
SK, Tsang CM, Tsao SW, Cheng SH, Ng MH, et al: Inhibition of c-Met
down-regulates TIGAR expression and reduces NADPH production
leading to cell death. Oncogene. 30:1127–1134. 2011. View Article : Google Scholar
|
|
17
|
Yin L, Kosugi M and Kufe D: Inhibition of
the MUC1-C oncoprotein induces multiple myeloma cell death by
down-regulating TIGAR expression and depleting NADPH. Blood.
119:810–816. 2012. View Article : Google Scholar :
|
|
18
|
Bensaad K, Cheung EC and Vousden KH:
Modulation of intra-cellular ROS levels by TIGAR controls
autophagy. EMBO J. 28:3015–3026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar :
|
|
20
|
Jacobs MD and Harrison SC: Structure of an
IkappaBalpha/NF-kappaB complex. Cell. 95:749–758. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perkins ND and Gilmore TD: Good cop, bad
cop: The different faces of NF-kappaB. Cell Death Differ.
13:759–772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li F, Zhang J, Arfuso F, Chinnathambi A,
Zayed ME, Alharbi SA, Kumar AP, Ahn KS and Sethi G: NF-κB in cancer
therapy. Arch Toxicol. 89:711–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren Q, Sato H, Murono S, Furukawa M and
Yoshizaki T: Epstein-Barr virus (EBV) latent membrane protein 1
induces interleukin-8 through the nuclear factor-kappa B signaling
pathway in EBV-infected nasopharyngeal carcinoma cell line.
Laryngoscope. 114:855–859. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lo AK, Lo KW, Tsao SW, Wong HL, Hui JW, To
KF, Hayward DS, Chui YL, Lau YL, Takada K, et al: Epstein-Barr
virus infection alters cellular signal cascades in human
nasopharyngeal epithelial cells. Neoplasia. 8:173–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheung AK, Ko JM, Lung HL, Chan KW,
Stanbridge EJ, Zabarovsky E, Tokino T, Kashima L, Suzuki T, Kwong
DL, et al: Cysteine-rich intestinal protein 2 (CRIP2) acts as a
repressor of NF-kappaB-mediated proangiogenic cytokine
transcription to suppress tumorigenesis and angiogenesis. Proc Natl
Acad Sci USA. 108:8390–8395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun W, Guo MM, Han P, Lin JZ, Liang FY,
Tan GM, Li HB, Zeng M and Huang XM: Id-1 and the p65 subunit of
NF-κB promote migration of nasopharyngeal carcinoma cells and are
correlated with poor prognosis. Carcinogenesis. 33:810–817. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kan R, Shuen WH, Lung HL, Cheung AK, Dai
W, Kwong DL, Ng WT, Lee AW, Yau CC, Ngan RK, et al: NF-κB p65
subunit is modulated by latent transforming growth factor-β binding
protein 2 (LTBP2) in nasopharyngeal carcinoma HONE1 and HK1 cells.
PLoS One. 10:e01272392015. View Article : Google Scholar
|
|
29
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|