Introduction
Molecular factors involved in the progression of
advanced prostate cancer (PC), from an initially well responding
state to a castration-resistant tumor, are multifunctional but can
be attributed to the capability of cell proliferation in a low
androgen environment. Castration-resistant PC is clinically
reflected by rising levels of prostate specific antigen (PSA) and
predominantly driven by changes in androgen receptor (AR)
signaling. Phenotypical alterations in AR functionality enable
tumor cell growth in absence or with near undetectable
concentrations of androgens. Current studies suggest that
initiation and progression of PC are tightly driven by local de
novo synthesis of androgens in tumor tissue and tumor
environment and accessorily accompanied by extragonadal sources, as
the conversion of circulating steroid precursors to testosterone
and dihydrotestosterone (1,2).
Within the group of new generation PC drugs,
abiraterone was shown to provide significant survival advantages
with modest toxicity even in post-chemotherapy settings (3). Abiraterone elicits antitumor activity
by inhibiting cytochrome P450, family 17, subfamily A, polypeptide
1 (CYP17A1) enzyme, which is critical for steroid synthesis from
cholesterol. CYP17A1 studies by conversion of both pregnenolone and
progesterone to 17α-hydroxypregnenolone and 17α-hydroxyprogesterone
(17α-hydroxylase activity) and by subsequent cleavage of
17α-hydroxypregnenolone and 17α-hydroxyprogesterone to
dehydroepiandrosterone (DHEA) and androstenedione (C17,20-lyase
activity) (4). Although less
efficient than CYP17A1 inhibition, abiraterone also blocks some
downstream enzymes of the steroid pathway (5). Additionally to blockading steroid
hormones, the derangement of steroidogenic biosynthesis lowers the
concentration of glucocorticoids and results in exaggerated
production of mineralocorticoids (6). Moreover, clinical and preclinical
studies have shown that abiraterone possesses further activities
which may contribute to tumor biology of advanced PC. Most likely
based on its steroidal structure, abiraterone has been found bound
to the AR and thus competitively antagonizes subsequent AR
signaling cascades, however, not as potent as pure antagonists,
e.g. bicalutamide (7,8). On the contrary, there are lines of
evidence that abiraterone is an inducer of steroidogenic enzymes
including CYP17A1 and may increase expression of steroid synthesis
machinery (9–11). Our present understanding of
molecular properties of abiraterone is still limited. In
particular, combinatorial or sequential combinations of abiraterone
with other drugs as well as the usage of abiraterone in different
settings of PC remain unsolved. Due to multiplicity and complexity
of recently described abiraterone effects, an inhibitory activity
exclusively based on a blockade of androgen synthesis appears
unlikely. With this study we assessed abiraterone effects on
AR-positive and AR-negative PC cells to gain insight into the
molecular mode of action.
Materials and methods
Chemicals and antibodies
Abiraterone acetate was kindly provided by
Janssen-Cilag GmbH (Neuss, Germany) and was used as a 10 mM (LNCaP
cells) and 30 mM (PC-3 cells) stock solution, respectively, with
dimethyl sulfoxide (Carl Roth, Karlsruhe, Germany) as solvent.
Docetaxel was purchased from Sigma-Aldrich (Munich, Germany) and
was used as 10 μM stock solution. Antibodies directed against the
AR and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as well
as peroxidase-coupled secondary antibodies directed against mouse
and rabbit were purchased from Cell Signaling Technology (Danvers,
MA, USA). Antibodies specific for the transforming growth factor β
(TGFβ) receptor type I and II were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA) and antibodies directed
against homolog of Caenorhabditis Sma and Drosophila
Mad 3 (Smad3) and Smad4 were obtained from Zymed Laboratories (San
Francisco, CA, USA).
Cell culture
The PC cell lines LNCaP and PC-3 from Cell Lines
Service (CLS, Heidelberg, Germany) were propagated in RPMI-1640
media supplemented with 10% fetal bovine serum, 1% sodium pyruvate,
and 1% penicillin/streptomycin (all from PAN Biotech, Aidenbach,
Germany) at 5% CO2 atmosphere and 37°C. Cells were
passaged twice per week using a trypsin/ethylenediaminetetraacetic
acid solution (0.1/0.04%) dissolved in phosphate-buffered saline
(PBS) (both from PAN Biotech). For experiments, cells were plated
in poly-L-lysine (Sigma-Aldrich, Deisenhofen, Germany)-coated
24-well (proliferation assay) and 6-well [quantitative reverse
transcription-polymerase chain reaction (RT-PCR), western blot
analysis] cell culture plates, respectively.
Proliferation assay
Cell growth of abiraterone treated cells was
examined by cell counting utilizing a CASY Cell Counter and
Analyzer Model TT (Roche Applied Science, Mannheim, Germany) and
compared to vehicle treated cells. Therefore, 30,000 cells/well
were seeded in 24-well cell culture plates and treated as
indicated. Number of living cells was determined by
trypsin/ethylenediaminetetraacetic acid detachment of adherent
cells and subsequent analysis. The cell suspension (100 μl) was
diluted in 10,000 μl CASYton (Roche Applied Science) and analysis
of 400 μl dilution was performed in triplicates using a capillary
of 150 μm in diameter. Gate settings of 7.20/15.45 μm were used to
ensure the discrimination between living cells and dead cells, as
well as cellular debris.
Annexin V assay
Apoptosis detection of abiraterone-incubated PC-3
cells was performed using a FITC Annexin V Apoptosis Detection kit
I (BD Bioscience, Heidelberg, Germany) as recommended by supplier
instructions. After washing the cells once with PBS,
6×105–1×106 cells were harvested with a cell
scraper and were resuspended in 1 ml PBS. After centrifugation and
discarding the supernatant, the pellet was resuspended in binding
buffer and stained with propidium iodide and FITC Annexin V for 15
min. Data were assessed by a FACSCanto A Cytometer and were
analyzed using BD FACSDiva™ software (both from BD Bioscience).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL apoptosis analysis of abiraterone-incubated
PC-3 cells was performed using the HT TiterTACS assay kit
(Trevigen, Gaithersburg, MD, USA) following supplier
recommendations. After indicated time-points of incubation adherent
cells were detached by 0.1% trypsin/0.04% EDTA. Data were acquired
using an Infinite 200 PRO multimode reader and were analyzed using
the i-control 1.9 software (both from Tecan, Männedorf,
Switzerland). Unlabeled and nuclease treated samples served as
negative and positive control, respectively.
Quantitative RT-PCR
Total RNA was extracted using 500 μl peqGold TriFast
reagent (PeqLab, Erlangen, Germany) and 50 μl
1-bromo-3-chloropropane (Sigma-Aldrich) according to manufacturer's
instructions. Prepared RNA was resuspended in diethyl pyrocarbonate
(Carl Roth) treated water and RNA concentration was determined in a
spectral photometer (NanoDrop 2000c; Peqlab). One microgram of
total RNA was applied in RT using MMLV Reverse Transcriptase
(Promega, Mannheim, Germany), Ribolock RNAse Inhibitor (Thermo
Fisher Scientific, Rockford, IL, USA) and an oligo(dT)18
oligonucleotide. Subsequently, mRNA amounts were quantified by
real-time PCR using the following pairs of oligonucleotides: PSA
forward, 5′-CCGGAGAGCTGTGTCACCAT-3′ and reverse,
5′-GTGCAGCACCAATCCACGTC-3′; caspase-3 forward,
5′-GCTCCTAGCGGATGGGTGCT-3′ and reverse, 5′-GATTCCAAGGCGACGCCAAC-3′;
cyclin-dependent kinase inhibitor 1 (p21) forward,
5′-TGGAGACTCTCAGGGT CGAAA-3′ and reverse, 5′-GGCGTTTGGAGTGGTAGAA
ATC-3′, Bcl-2-associated X protein (Bax) forward, 5′-TCC
CCCCGAGAGGTCTTTT-3′ and reverse, 5′-CGGCCCCA GTTGAAGTTG-3′;
survivin forward, 5′-TGCCCCGACGT TGCC-3′ and reverse,
5′-CAGTTCTTGAATGTAGAGATGC GGT-3′, ribosomal protein large P0
(RPLP0) forward, 5′-CAATGGCAGCATCTACAACC-3′ and reverse, 5′-ACT
CTTCCTTGGCTTCAACC-3′. Real-time PCR was carried out with SensiMix
SYBR Hi-Rox (Bioline, Luckenwalde, Germany) in a CFX96 Real-Time
PCR detection system (Bio-Rad Laboratories, München, Germany). For
quantification, target specific signals were standardized to RPLP0
mRNA as reference.
Western blot analysis
Cells were lysed in buffer containing 50 mM Tris (pH
7.5), 150 mM NaCl, 10 mM K 2HPO4, 5 mM EDTA,
10% glycerol, 1% Triton X-100, 0.05% sodium dodecyl sulfate, 1 mM
Na3VO4, 20 mM NaF, 0.1 mM
phenylmethylsulfonyl fluoride, 20 mM 2-phosphoglycerate, and
complete protease inhibitor cocktail (Roche Applied Science), total
amount of protein was determined by using Bradford reagent
(12), and equal amounts of
protein were conducted to gel electrophoresis. After transfer onto
a nitrocellulose membrane (GE Healthcare Europe, Freiburg, Germany)
and blocking (Roti-Block; Carl Roth), protein detection was done by
incubation with target specific primary antibodies followed by
species specific secondary antibodies and visualized by SuperSignal
West Dura Chemiluminescent Substrate (Thermo Fisher Scientific) in
a ChemiDoc XRS System (Bio-Rad Laboratories). Protein signals were
quantified by Image Lab 3.0 software (Bio-Rad Laboratories) and
standardized to GAPDH signals as reference.
Statistical analysis
Results of at least three independent experiments
were statistically analyzed. Analysis was performed using the
Student's t-test with P≤0.05, P≤0.01 and P≤0.001 given as
significant. Data are expressed as mean ± SD.
Results
Cellular growth of AR-positive and
hormone-sensitive LNCaP cells is attenuated in the presence of
abiraterone by diminishing AR expression and activity
To investigate the effects of abiraterone on
cellular growth of PC cells, AR-positive LNCaP cells were
propagated in the presence of various concentrations of
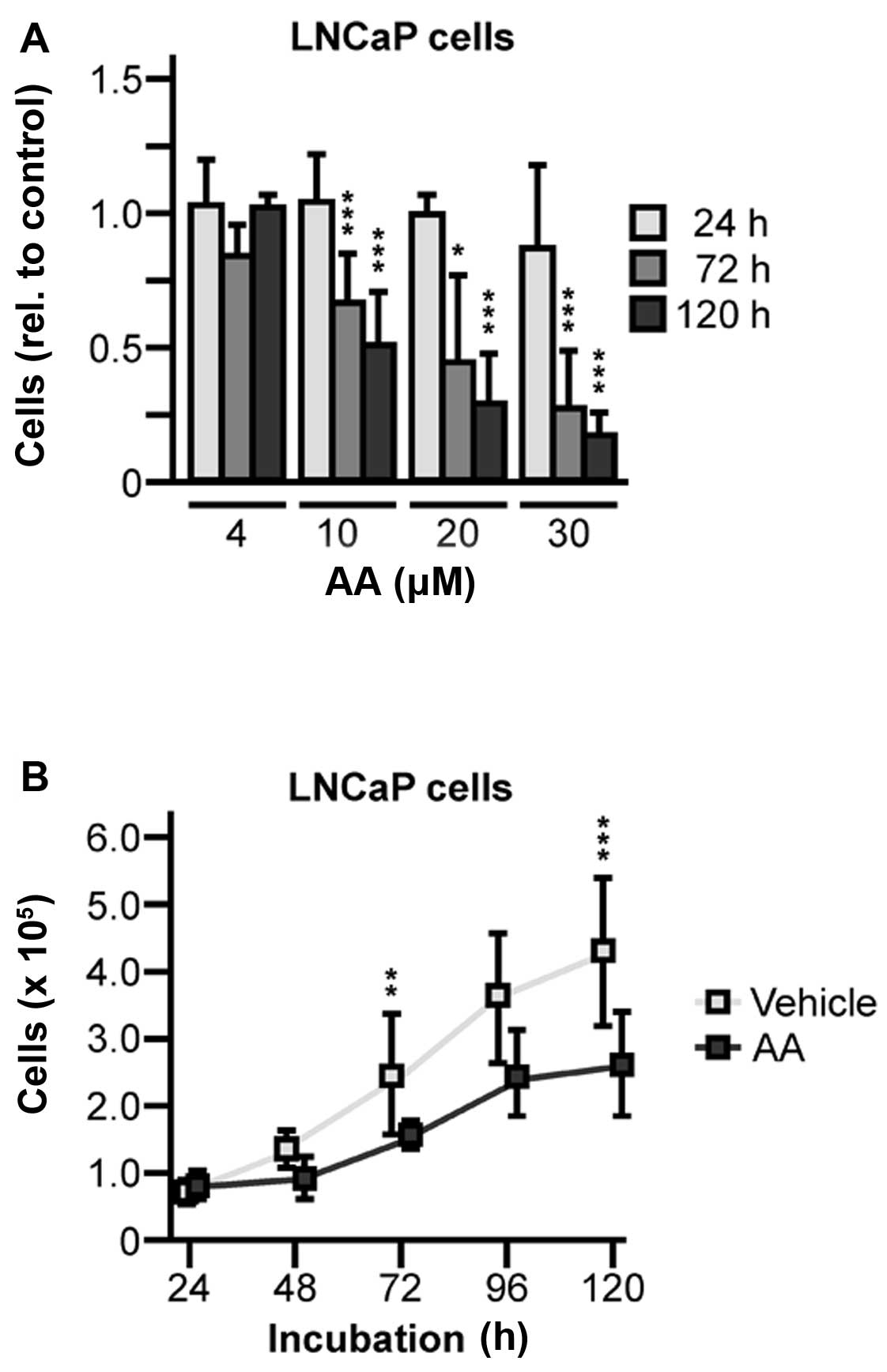
abiraterone, ranging from 4 to 30 μM. We found abiraterone
diminished cellular growth in a concentration-dependent manner
compared to vehicle treated control cells. At intermediate dosage
of 10 μM abiraterone, cell proliferation was inhibited to ~50%
after an exposure of 120 h (Fig.
1A). To verify theses data, growth kinetics of cells treated
with 10 μM abiraterone were done and statistical analysis revealed
that growth suppression was significant (72 h, 1.5-fold, P=0.0082;
120 h, 1.5-fold, P=0.0010) (Fig.
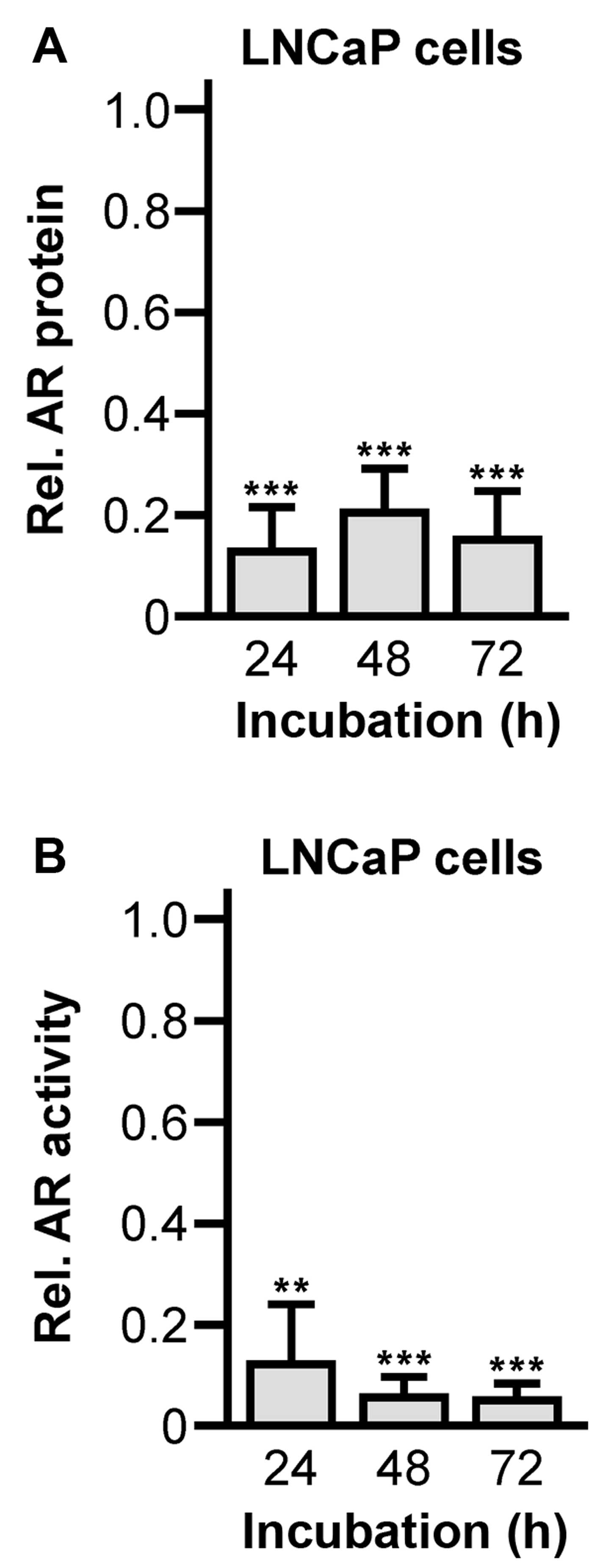
1B). Subsequent western blot analysis of the AR expression
after 72 h lasting abiraterone incubation showed significantly
reduced levels of AR protein (24 h, 0.13±0.09, P<0.0001; 48 h,
0.20±0.10, P<0.0001; 72 h, 0.16±0.10, P=0.0001) (Fig. 2A). The downregulation was
accompanied by strongly attenuated transcriptional activity of the
AR (24 h, 0.12±0.12, P=0.0096; 48 h, 0.06±0.04, P<0.0001; 72 h,
0.05±0.03, P<0.0001) (Fig. 2B),
which was measured by RT-PCR quantification of the AR-dependent PSA
mRNA levels.
Abiraterone inhibits cellular growth of
hormone-independent PC-3 cells lacking AR
It was commonly hypothesized that abiraterone
inhibits androgen biosynthesis and AR signaling, which is the
primary antitumor activity of the drug. However, participation of
AR protein and AR properties in the molecular mode of action of
abiraterone have not been extensively investigated. Therefore,
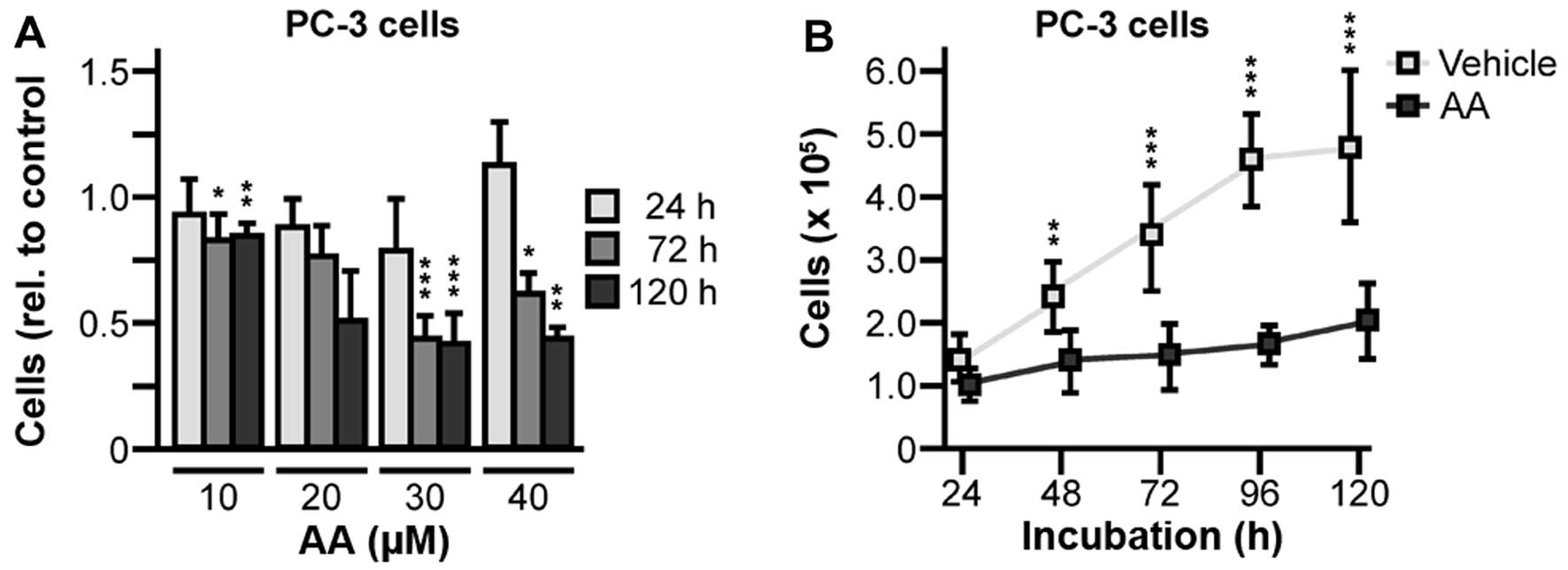
AR-negative PC-3 cells treated with 10 to 40 μM abiraterone were
analyzed and, very notably, presented growth inhibitory effects
(Fig. 3A) comparable to
AR-positive LNCaP cells. Further growth kinetics over 120 h showed
statistically significant reductions of PC-3 proliferation treated
with 30 μM abiraterone (48 h, 1.7-fold, P=0.0026; 72 h, 2.3-fold,
P<0.0001; 96 h, 2.8-fold, P<0.0001; 120 h, 2.4-fold,
P<0.0001) (Fig. 3B).
Abiraterone treatment of PC-3 cells
initiates apoptosis due to modulation of pivotal factors in
proliferation, apoptosis, and cell cycle regulation
To confirm whether the reduction of cell numbers
after CAP treatment is the result of apoptotic cell death, we
further examined the effects of CAP treatment by Annexin V and
TUNEL assay. Previous experiments pointed to the fact that
abiraterone-mediated attenuation of cellular growth can be obtained
in the absence of the AR and AR-dependent signaling cascades.
Therefore, we postulated interference with further regulatory
pathways which may be critical for the abiraterone molecular mode
of action. Primarily, we attempted to clarify whether
abiraterone-dependent reduction of PC cell numbers could be
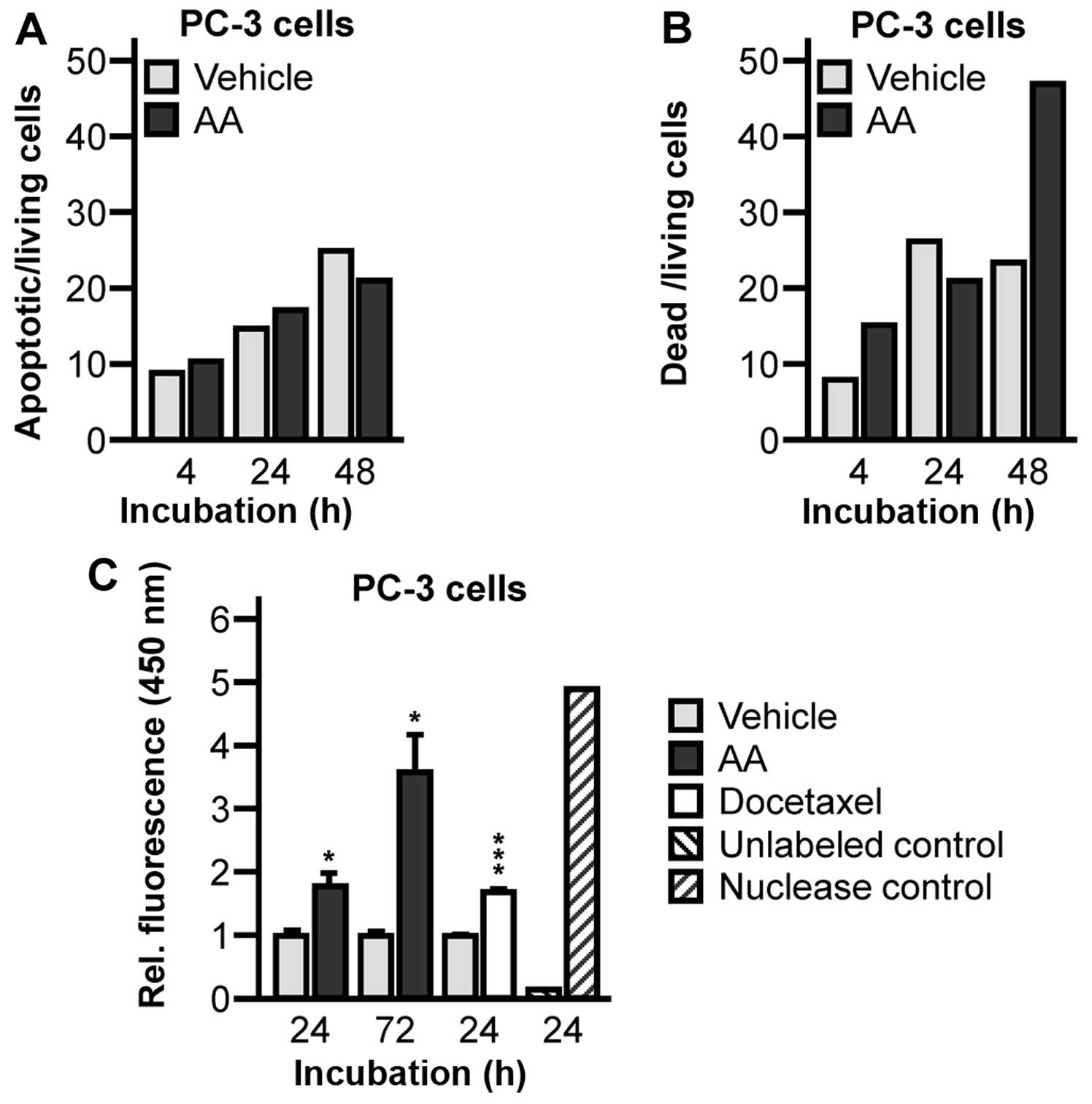
associated with apoptotic cell death. Annexin V analysis in
AR-negative PC-3 cells revealed a 1.09-fold and a 1.13-fold
increase of apoptotic cells after 4 and 24 h following abiraterone
treatment (Fig. 4A) compared to
vehicle treated controls. We detected a 1.86-fold and a 1.93-fold
increase of dead cells after 4 and 48 h following abiraterone
treatment (Fig. 4B). Due to the
ambiguity of the results, we enforced further apoptosis detection
by TUNEL assay. DNA fragment labelling by TUNEL assay showed a
significant increase of DNA fragmentation in abiraterone treated
PC-3 cells after 24 and 72 h (24 h, 1.78±0.40, P=0.0290; 72 h,
3.55±1.04, P=0.0133) (Fig. 4C).
This was comparable to DNA fragmentation of docetaxel treated
control cells (24 h, 1.67±0.10, P=0.0005) (Fig. 4C).
In order to shed further light on the molecular
mechanisms involved in abiraterone-induced attenuation of cell
growth, we assessed the modulation of factors especially in
apoptosis and cell cycle control. As shown by western blot
analysis, signaling cascades of the cell growth controlling
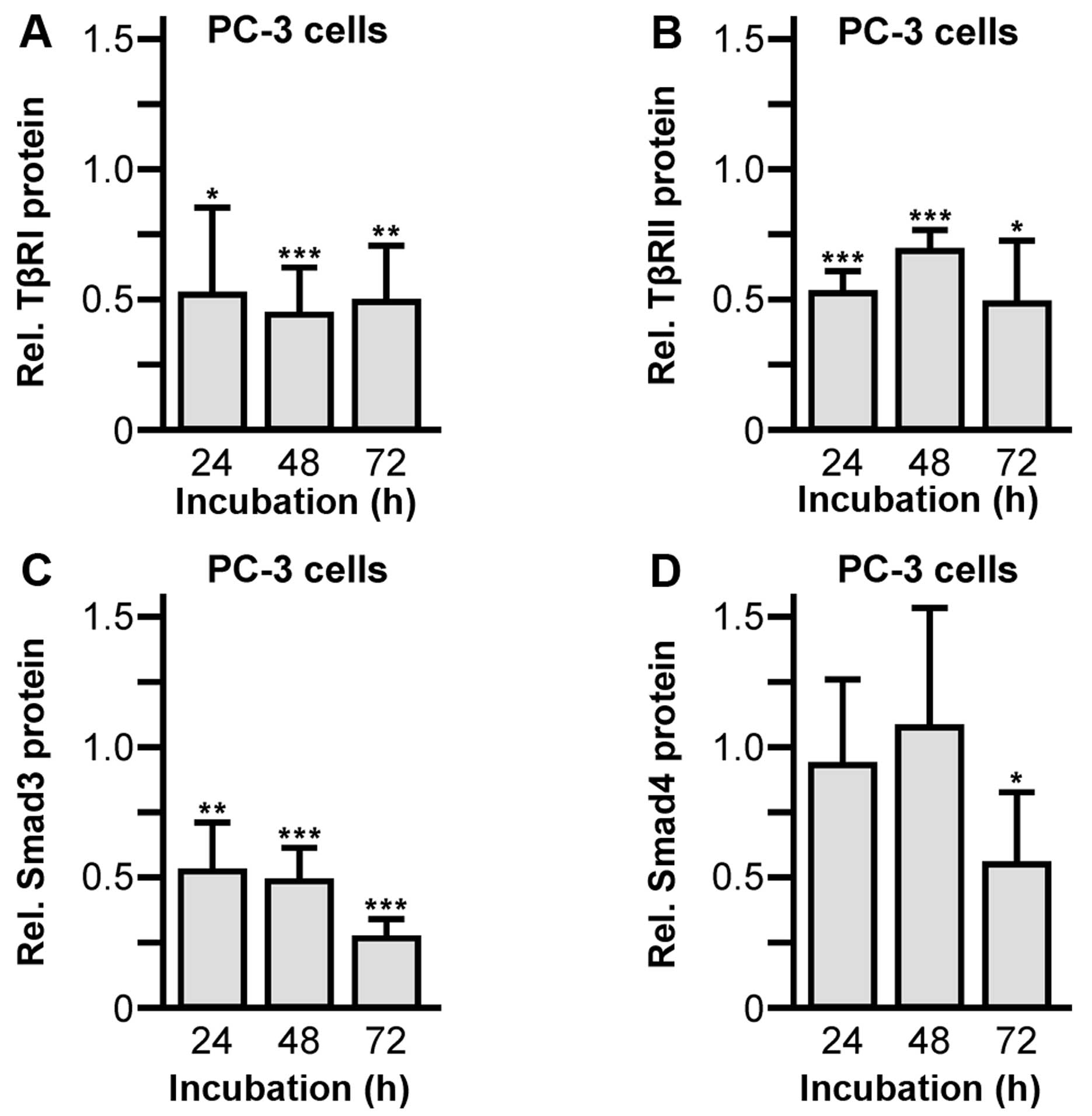
pleiotropic factor TGFβ were affected. Exposure of PC-3 cells to 30
μM abiraterone significantly inhibited protein expression of both
TGFβ receptor isoforms, TGFβ receptor type I (24 h, 0.52±0.43,
P=0.0152; 48 h, 0.44±0.18, P=0.0009; 72 h, 0.49±0.22, P=0.0034)
(Fig. 5A) and type II (24 h,
0.54±0.08, P<0.0001; 48 h, 0.70±0.08, P=0.008; 72 h, 0.50±0.24,
P=0.24) (Fig. 5B), compared to
control treated cells given as 1.0. Furthermore, the TGFβ
intracellular downstream signaling proteins Smad3 (24 h, 0.53±0.18,
P=0.0096; 48 h, 0.49±0.12, P<0.0001; 72 h, 0.27±0.07,
P<0.0001) (Fig. 5C) and Smad4
(72 h, 0.56±0.28, P=0.0071) (Fig.
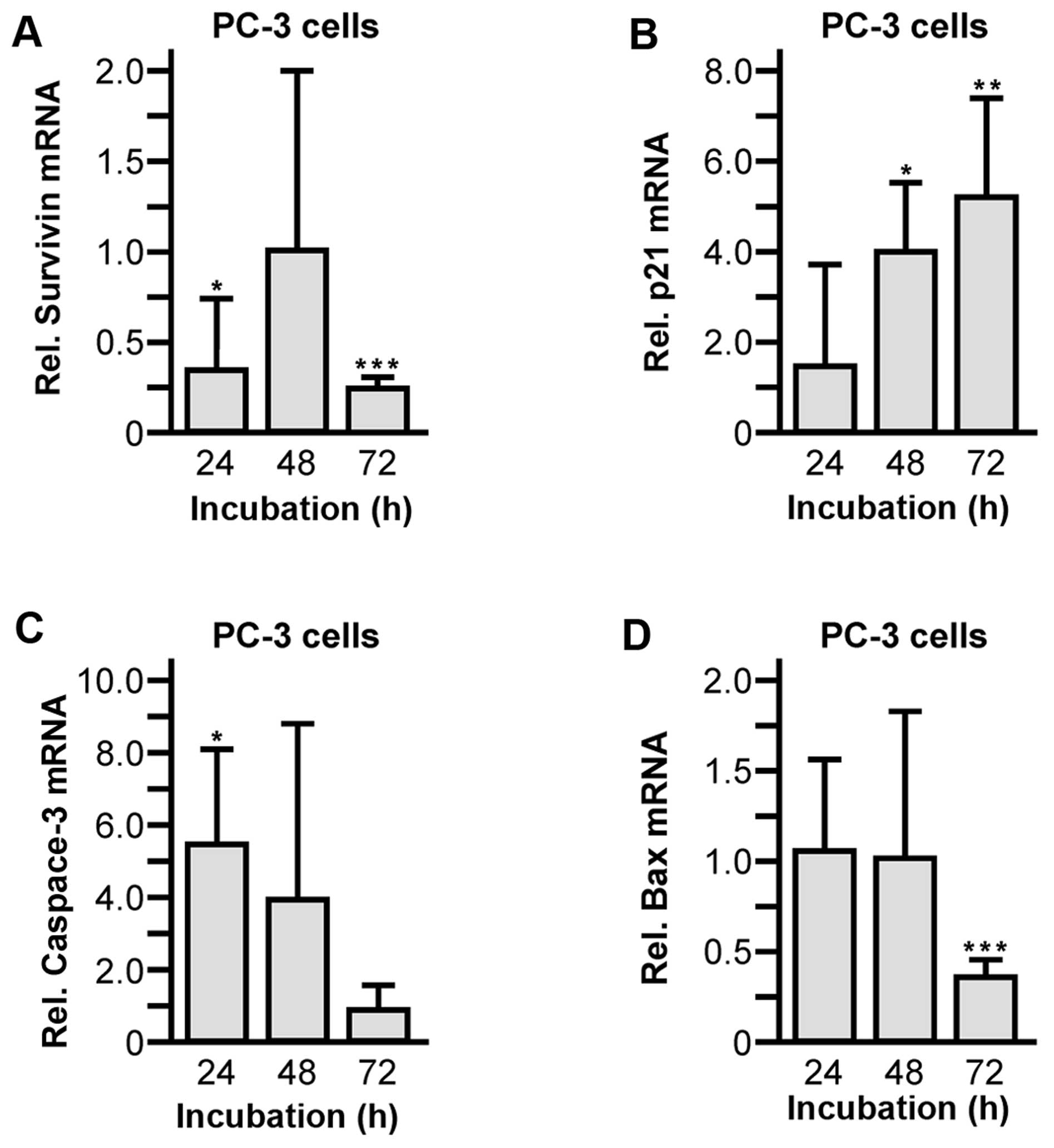
5D) were significantly suppressed. Moreover, alterations of
survivin, cyclin-dependent kinase inhibitor 1 (p21), caspase-3 and
Bcl-2-associated X protein (Bax) mRNA levels were verified by
quantitative RT-PCR and normalized to control cells. During
propagation of PC-3 cells in the presence of 30 μM abiraterone, the
expression of the anti-apoptotic cell survival factor survivin was
suppressed (24 h, 0.33±0.40, P=0.0438; 48 h, 1.01±1.05; 72 h,
0.25±0.08, P<0.0001) (Fig. 6A).
Accordingly, mRNA levels of the pro-apoptotic regulators p21 (24 h,
1.56±2.26; 48 h, 4.00±1.52, P=0.0269; 72 h, 5.19±2.20, P=0.0088)
(Fig. 6B) and caspase-3 (24 h,
5.62±2.63, P=0.0382; 48 h, 4.08±4.84; 72 h, 1.02±0.69) (Fig. 6C) were significantly elevated.
Notably, synthesis of the pro-oncogenic factor Bax was slightly but
significantly reduced within 72 h of abiraterone incubation (24 h,
1.11±0.55; 48 h, 1.00±0.82; 72 h, 0.37±0.09, P<0.0001) (Fig. 6D).
Discussion
Molecular properties of CYP17A1 as a key enzyme in
androgen biosynthesis provided the rationale for development of the
CYP17A1 inhibitor abiraterone. Former studies with regard to the
multiple cytochrome P450 enzyme inhibitor ketoconazole have served
as proof-of-principle: repression of cytochrome P450 enzymes
including CYP17A1 were shown to harbor anticancer activity in PC,
however, ketoconazole administration was of minor benefit and was
accompanied by significant toxicity (13). Recent studies of Brossard et
al utilizing abiraterone derivates with a broad spectrum of
inhibited cytochrome P450 enzymes have confirmed this hypothesis
(14). Therefore, current
understanding of molecular abiraterone efficacy in PC is primarily
based on attenuated androgen biosynthesis/conversion and subsequent
diminished AR signaling by the potent and specific inhibition of
CYP17A1.
The data presented here provide evidence that
abiraterone treatment resulted in significantly reduced
proliferation rates even for PC cells lacking AR signaling. We
previously reported that abiraterone significantly decreased the
viability of AR expressing LNCaP cells most likely by the
antagonistic properties of abiraterone (7,8).
Moreover, growth attenuation was accompanied by a dramatically
diminshed AR protein expression and activity. These observations
are in accordance with a study of Soifer et al, which
considered downregulation of the AR in the presence of 1.0–15.0 μM
abiraterone (15).
Remarkably, AR-negative PC-3 cells exhibited
comparably inhibited growth characteristics in the presence of
abiraterone. The applied drug concentration of 30 μM corresponds to
abiraterone concentrations which were determined in our study (10
μM in LNCaP cell incubation experiments) and in a study of Bruno
et al (10 μM in PC-3 cell incubation experiments) (16). Notably, attenuation of PC-3
proliferation point to additional and AR-independent pathways which
were targeted by abiraterone. Accordingly, further experiments
suggested a broad spectrum of anti-tumor activities of abiraterone
displayed by targeting regulatory factors of TGFβ, apoptosis and
cell cycle pathways.
Besides the numerous AR signaling events, it is well
established that there are several proliferative signal cascades
which are qualified to become active in PC growth, e.g.
phosphati-dylinositide 3-kinase (17), epidermal growth factor (18), and estrogen receptor (19) pathways. The precise role of the
pleiotropic growth factor TGFβ, which is also part of this multiple
and fine-tuned regulatory network, is controversial. On the one
hand, TGFβ has been reported to govern differentiation and
anti-proliferation in non-malignant prostate epithelium as well as
in early stage PC tissue. On the other hand, TGFβ signals are
linked to a tumorigenic phenotype in advanced PC stages (20–23).
Even though abiraterone did not affect TGFβ1 and TGFβ2 mRNA levels
(data not shown), analysis of TGFβ pathway proteins revealed a
clear suppression of TGFβ cascades. Abiraterone inhibited both
types of TGFβ receptors, type I and II, as well as the downstream
signaling molecules Smad3 and Smad4; thus, it is reasonable to
hypothesize a strong reduction of TGFβ signaling by abiraterone
treatment. However, since TGFβ signals play a dual role in PC
reflected by its opposing properties of pro- and anti-oncogenic
effects, it is difficult to estimate the TGFβ-dependent impact of
abiraterone. Though, elevated serum levels of TGFβ have been shown
to predict tumor recurrence and metastasis, and the PC cells became
less responsive to TGFβ growth inhibition in later stages of PC
(20,24), therefore, abiraterone efficacy was
probably due to attenuation of TGFβ-dependent oncogenic signal
cascades. However, these results further exemplify the difficulties
of interpretation of TGFβ pathways in PC cell biology and may
suggest further investigations in this field.
Chemotherapy widely exerts its anticancer effect by
triggering apoptotic mechanisms of tumor cells. A primary regulator
of apoptosis is the tumor suppressor p53, which plays a key role in
cell cycle control, genomic stability, and apoptosis. Due to a
frame shift within the p53 gene, PC-3 cells express a truncated and
therefore inactive form of the protein (25,26),
however, the induction of apoptosis pathways in absence of p53 as a
response to drug treatment has been extensively described (27–29).
Notwithstanding, in PC-3 cells lacking functional p53 the
initiation of intrinsic apoptosis pathway factors occurs in the
presence of abiraterone. Increased DNA fragmentation as determined
by TUNEL assay, as well as downregulation of the cell survival
factor survivin and elevated levels of the cell cycle inhibitor p21
and the effector caspase-3 clearly indicated an execution of
apoptotic mechanisms. These data confirm former studies which have
shown that anticancer drugs affect the biosynthesis of these
intrinsic apoptotic factors in PC-3 cells (28,30–32).
Surprisingly, our results could not be definitely verified by
Annexin V staining. Since externalisation of Annexin V to the outer
plasma membrane is a very early effect in apoptosis, it could be
speculated that Annexin V signals have already expired before the
first selected time point. However, it is notable that Annexin V
staining of PC-3 cells failed also previously (33). Curiously, we monitored
significantly reduced levels of pro-apoptotic Bax after 72 h of
abiraterone incubation. This finding is not consistent with earlier
reports in which Bax has been shown being drug-induced in PC-3
cells (28). The intrinsic
apoptosis pathway is controlled by a balance of pro-apoptotic (e.g.
Bax, p21 and caspase-3) and anti-apoptotic (e.g. survivin) factors.
Apoptosis in the absence of p53, however, appears complex.
Therefore, differing cell death cascades compared to the classical
intrinsic apoptosis pathways by interfering with other regulatory
pathways may occur. For instance, there have been reports that
apoptosis induced by the hypoxia response system can provoke a
downregulation of Bax in cancer cells (34,35).
Taken together, our observations suggest that
abiraterone-mediated attenuation of AR signals is not the only
rationale to explain anticancer activity. Besides an expected
inhibition of LNCaP cell growth by targeting the AR, very notably,
abiraterone comparably inhibited proliferation of AR-negative PC-3
cells. Thus, abiraterone's molecular mode of action is proposed to
be mediated through an accessory inhibition of pro-oncogenic TGFβ
signals and through the alteration of apoptotic factors.
Experimental and preclinical data continue to
enlarge our understanding of abiraterone molecular mode of action
and facilitate the opportunity to improve PC treatment regimes as
well as to overcome resistance to abiraterone. Interestingly,
current data showed no, or no detectable expression of CYP17A1
enzyme in LNCaP and PC-3 cell lines (35) and, in this context, abiraterone
efficacy may play a more global role in PC progression control than
originally hypothesized. Consequently, abiraterone is not only a
promising drug for treatment of AR-negative PC stages, even more,
abiraterone may represent a therapeutical alternative for treatment
of other malignancies besides PC.
Acknowledgements
The authors thank Anne Brandenburg and Katja Wittig
for excellent technical assistance. Parts of this study were
supported by a grant of the Gerhard Domagk grant of the University
Medicine Greifswald, Greifswald, Germany. The compound abiraterone
acetate was kindly provided by the Janssen-Cilag GmbH, Neuss,
Germany.
Abbreviations:
|
PC
|
prostate cancer
|
|
TGFβ
|
transforming growth factor β
|
|
AR
|
androgen receptor
|
|
Bax
|
Bcl-2-associated X protein
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick end labeling
|
References
|
1
|
Labrie F, Luu-The V, Lin SX, Simard J,
Labrie C, El-Alfy M, Pelletier G and Bélanger A: Intracrinology:
Role of the family of 17 beta-hydroxysteroid dehydrogenases in
human physiology and disease. J Mol Endocrinol. 25:1–16. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piao YS, Wiesenfeld P, Sprando R and
Arnold JT: TGFβ1 alters androgenic metabolites and hydroxysteroid
dehydrogenase enzyme expression in human prostate reactive stromal
primary cells: Is steroid metabolism altered by prostate reactive
stromal microenvironment? J Steroid Biochem Mol Biol. 138:206–213.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: COU-AA-301 Investigators: Abiraterone and increased survival
in metastatic prostate cancer. N Engl J Med. 364:1995–2005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller WL: Steroidogenic enzymes.
Disorders of the Human Adrenal Cortex. Flück CE and Miller WL:
Karger; Basel: pp. 1–18. 2008, View Article : Google Scholar
|
|
5
|
Yin L and Hu Q: CYP17 inhibitors -
abiraterone, C17,20-lyase inhibitors and multi-targeting agents.
Nat Rev Urol. 11:32–42. 2014. View Article : Google Scholar
|
|
6
|
Pia A, Vignani F, Attard G, Tucci M,
Bironzo P, Scagliotti G, Arlt W, Terzolo M and Berruti A:
Strategies for managing ACTH dependent mineralocorticoid excess
induced by abiraterone. Cancer Treat Rev. 39:966–973. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richards J, Lim AC, Hay CW, Taylor AE,
Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W,
et al: Interactions of abiraterone, eplerenone, and prednisolone
with wild-type and mutant androgen receptor: A rationale for
increasing abiraterone exposure or combining with MDV3100. Cancer
Res. 72:2176–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harshman LC and Taplin ME: Abiraterone
acetate: Targeting persistent androgen dependence in
castration-resistant prostate cancer. Adv Ther. 30:727–747. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stanbrough M, Bubley GJ, Ross K, Golub TR,
Rubin MA, Penning TM, Febbo PG and Balk SP: Increased expression of
genes converting adrenal androgens to testosterone in
androgen-independent prostate cancer. Cancer Res. 66:2815–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai C, Chen S, Ng P, Bubley GJ, Nelson PS,
Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, et al:
Intratumoral de novo steroid synthesis activates androgen receptor
in castration-resistant prostate cancer and is upregulated by
treatment with CYP17A1 inhibitors. Cancer Res. 71:6503–6513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mostaghel EA, Marck BT, Plymate SR,
Vessella RL, Balk S, Matsumoto AM, Nelson PS and Montgomery RB:
Resistance to CYP17A1 inhibition with abiraterone in
castration-resistant prostate cancer: Induction of steroidogenesis
and androgen receptor splice variants. Clin Cancer Res.
17:5913–5925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Small EJ, Baron AD, Fippin L and Apodaca
D: Ketoconazole retains activity in advanced prostate cancer
patients with progression despite flutamide withdrawal. J Urol.
157:1204–1207. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brossard D, Zhang Y, Haider SM, Sgobba M,
Khalid M, Legay R, Duterque-Coquillaud M, Galera P, Rault S,
Dallemagne P, et al: N-substituted piperazinopyridylsteroid
derivatives as abiraterone analogues inhibit growth and induce
pro-apoptosis in human hormone-independent prostate cancer cell
lines. Chem Biol Drug Des. 82:620–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soifer HS, Souleimanian N, Wu S,
Voskresenskiy AM, Collak FK, Cinar B and Stein CA: Direct
regulation of androgen receptor activity by potent CYP17 inhibitors
in prostate cancer cells. J Biol Chem. 287:3777–3787. 2012.
View Article : Google Scholar :
|
|
16
|
Bruno RD, Gover TD, Burger AM, Brodie AM
and Njar VC: 17alpha-Hydroxylase/17,20 lyase inhibitor VN/124-1
inhibits growth of androgen-independent prostate cancer cells via
induction of the endoplasmic reticulum stress response. Mol Cancer
Ther. 7:2828–2836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar :
|
|
18
|
Traish AM and Morgentaler A: Epidermal
growth factor receptor expression escapes androgen regulation in
prostate cancer: A potential molecular switch for tumour growth. Br
J Cancer. 101:1949–1956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura Y, McNamara K and Sasano H:
Estrogen receptor expression and its relevant signaling pathway in
prostate cancer: A target of therapy. Curr Mol Pharmacol.
5:392–400. 2012. View Article : Google Scholar
|
|
20
|
Steiner MS and Barrack ER: Transforming
growth factor-beta 1 overproduction in prostate cancer: Effects on
growth in vivo and in vitro. Mol Endocrinol. 6:15–25.
1992.PubMed/NCBI
|
|
21
|
Danielpour D: Functions and regulation of
transforming growth factor-beta (TGF-beta) in the prostate. Eur J
Cancer. 41:846–857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu B and Kyprianou N: Transforming growth
factor beta and prostate cancer. Cancer Treat Res. 126:157–173.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stope MB, Rönnau C, Schubert T, Staar D,
Bradl J, Ziegler P, Streitbörger A, Kroeger N, Zimmermann U,
Walther R, et al: Transforming growth factor β in prostate cancer:
Cellular effects and basic molecular mechanisms. Urologe A.
52:378–383. 2013.(In German). View Article : Google Scholar
|
|
24
|
Shariat SF, Kattan MW, Traxel E, Andrews
B, Zhu K, Wheeler TM and Slawin KM: Association of pre- and
postoperative plasma levels of transforming growth factor beta(1)
and interleukin 6 and its soluble receptor with prostate cancer
progression. Clin Cancer Res. 10:1992–1999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carroll AG, Voeller HJ, Sugars L and
Gelmann EP: p53 oncogene mutations in three human prostate cancer
cell lines. Prostate. 23:123–134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Bokhoven A, Varella-Garcia M, Korch C,
Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS
and Bokhoven van A: Molecular characterization of human prostate
carcinoma cell lines. Prostate. 57:205–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coxon JP, Oades GM, Kirby RS and Colston
KW: Zoledronic acid induces apoptosis and inhibits adhesion to
mineralized matrix in prostate cancer cells via inhibition of
protein prenylation. BJU Int. 94:164–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang F, Yang Z, Yu D, Wang J, Li R and
Ding G: Sepia ink oligopeptide induces apoptosis in prostate cancer
cell lines via caspase-3 activation and elevation of Bax/Bcl-2
ratio. Mar Drugs. 10:2153–2165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raja Singh P, Arunkumar R, Sivakamasundari
V, Sharmila G, Elumalai P, Suganthapriya E, Brindha Mercy A,
Senthilkumar K and Arunakaran J: Anti-proliferative and apoptosis
inducing effect of nimbolide by altering molecules involved in
apoptosis and IGF signalling via PI3K/Akt in prostate cancer (PC-3)
cell line. Cell Biochem Funct. 32:217–228. 2014. View Article : Google Scholar
|
|
30
|
Muenchen HJ, Poncza PJ and Pienta KJ:
Different docetaxel-induced apoptotic pathways are present in
prostate cancer cell lines LNCaP and PC-3. Urology. 57:366–370.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fares M, Abou-Seri SM, Abdel-Aziz HA,
Abbas SE, Youssef MM and Eladwy RA: Synthesis and antitumor
activity of pyrido [2,3-d]pyrimidine and pyrido[2,3-d]
[1,2,4]triazolo[4,3-a] pyrimidine derivatives that induce apoptosis
through G1 cell-cycle arrest. Eur J Med Chem. 83:155–166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu JL, Liu SP, Lee CC, Hsu LC, Ho YF,
Huang HS and Guh JH: A unique amidoanthraquinone derivative
displays antiproliferative activity against human
hormone-refractory metastatic prostate cancers through activation
of LKB1-AMPK-mTOR signaling pathway. Naunyn Schmiedebergs Arch
Pharmacol. 387:979–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weiss M, Gümbel D, Hanschmann EM,
Mandelkow R, Gelbrich N, Zimmermann U, Walther R, Ekkernkamp A,
Sckell A, Kramer A, et al: Cold atmospheric plasma treatment
induces anti-proliferative effects in prostate cancer cells by
redox and apoptotic signaling pathways. PLoS One. 10:e01303502015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erler JT, Cawthorne CJ, Williams KJ,
Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C,
Stratford IJ and Dive C: Hypoxia-mediated down-regulation of Bid
and Bax in tumors occurs via hypoxia-inducible factor 1-dependent
and -independent mechanisms and contributes to drug resistance. Mol
Cell Biol. 24:2875–2889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H, Sun L, Xiao X, Xie R, Liu C, Wang
Y, Wei Y, Zhang H and Liu L: Krüppel-like factor 8 contributes to
hypoxia-induced multidrug resistance in gastric cancer cells.
Cancer Sci. 105:1109–1115. 2014. View Article : Google Scholar : PubMed/NCBI
|