Introduction
Colorectal cancer (CRC) ranks the third most common
cancer of all cancer types and is a major public health problem. In
2000, more than 1.2 million people were diagnosed with CRC
(1), and according to the latest
investigation in 2014, CRC is responsible for 8–9% of the
cancer-related deaths in the USA (2). However, the incidence of CRC varies
with geography with the incidence rate in the USA and Europe being
10-fold higher than in African and Asian countries. CRC is quite a
complex disease and tumors with similar histopathological
characteristics will finally develop with variable course in
response to different treatments (3). As with all types of tumors, CRC is
now proved to be a chronic systemic disease: neoplastic cells
develop at tumor sites first and then metastasize to other parts of
the body (4). Fortunately,
advances in molecular biology in the last decades have facilitated
the elucidation of some of the genetic mechanisms involved in the
oncogenesis and development of CRC.
Three major mechanisms that cause aberrant gene
expression results in the carcinogenesis of the colon:
microsatellite instability (MSI), chromosomal instability (CIN),
and the CpG island methylator phenotype (CIMP). These processes
lead to the transition in lesion pathology and progression to
malignancy, which is accompanied by downregulated expression of
tumor suppression genes. Vogelstein et al have inferred that
the accumulation of genetic alterations, in particular APC, TP53,
and KRAS mutation is responsible for CRC development (5). Moreover, increased activity of matrix
metal-loprotease-9 (MMP-9) was also detected in plasma of patients
with colon cancer (6,7). MMP-9 is a matrix metalloproteinase,
which is closely related multi-gene family of zinc-dependent
proteolytic enzymes. It plays a role in normal physiological tissue
remodeling and is capable of degrading all components of the
extracellular matrix. Increasing evidence has proved the important
contribution of MMP-9 to CRC (8,9),
other studies have already revealed the underlying molecular
mechanism involved in MMP-9 inducing cancer cell invasion (10). Taken the above information
together, it is reasonable to consider MMP-9 as a potential
therapeutic target for treatment of CRC.
A great deal of genes associated with metastases of
cancer has been identified in the last decades. One of these genes
is KiSS-1, expression of which is reported to be reduced in
metastatic cancer (11). Moreover,
its ability to suppress the metastatic potential of breast cancer
cells without affecting tumorigenicity was also verified (11,12).
Since MMP-9 has well-established role in tumor cell invasion and
metastases, the interaction between MMP-9 and KiSS-1 has already
drawn attention and the inhibition effect of KiSS-1 on the
expression of MMP-9 has been validated (10). However, to the best of our
knowledge, the mechanism by which KiSS-1 regulates MMP-9 and
suppresses the metastatic phenotype remains partially explored,
especially in CRC, the related studies are scarce.
Thus, in the present study, we hypothesized that
KiSS-1 gene could repress the metastatic potential of
colorectal cancer cells by downregulating the expression of MMP-9.
Based on previous studies (10),
we also investigated the possible molecular mechanism participating
in these processes. Stable regulation of KiSS-1 gene in
HCT-116 cells was achieved by lentivirus infection method. The
effect of KiSS-1 on the cell viability, migration, and
apoptosis was determined. The possible pathway involved in these
processes was also evaluated by western blotting. Our aim was to
assess the role of KiSS-1 and whether it was involved in
oncogenesis and development of CRC to and facilitate the prevention
and treatment of CRC in the clinic.
Materials and methods
Cell cultures and chemicals
Human CRC cell line HCT-116 was purchased from
American Type Culture Collection (ATCC) (Rockville, MD, USA) and
cultured in DMEM/F-12 medium supplemented with 10% (v/v) fetal calf
serum (Gibco Life Technologies, Carlsbad, CA, USA) and 1% (v/v)
anti-biotics mixture in 95% air and 5% CO2 at 37°C. In
addition, cells between passage 3 and 6 were used for further
experiments. PI3K/Akt pathway agonist 740Y-P and PDGF were
purchased from Sigma-Aldrich, St. Louis, MO, USA.
siRNA interference of the KiSS-1
gene
Lentivirus-mediated KiSS-1-specific siRNA
(5′-GCCGAACUACAACUGGA ACTT-3′) and the negative control siRNA (NC)
were obtained from Genechem Biotech (Shanghai, China). The most
effective transfection concentration of lentivirus was determined
by multiplicity of infection (MOI). Based on the results (data not
shown), lentivirus concentration at MOI 100 (10 μl 1×108
TU/ml lentivirus + 90 μl medium) was chosen for further
experiments.
The cell concentration of HCT-116 was adjusted to
1×104/ml and incubated on slides in one well of 24-well
plates for 24 h. Transfection was performed with Lipofectamine 2000
reagent (Invitrogen) according to the manufacturer's protocol and
subsequent selection was conducted using 400-μg/ml puromycin
(Amresco, Solon, OH, USA). HCT-116 cells were grouped into two
treatments: i) NC-siRNA group, HCT-116 cells were transfected with
negative control siRNA. ii) KiSS-1-siRNA group, HCT-116 cells were
transfected with KiSS-1-specific siRNA. Each treatment
consisted of six replicates. All the cells were cultured for 96 h
and cell sampling was conducted every 24 h during the experimental
course from 0 h.
Overexpression of KiSS-1 gene in HCT-116
cells
Lentivirus-mediated KiSS-1 vector and
negative control vector were purchased from Genechem (Shanghai,
China). The most effective transfection concentration of lentivirus
was determined by multiplicity of infection (MOI). Based on the
results (data not shown), lentivirus concentration at MOI 100 (10
μl 1×108 TU/ml lentivirus + 90 μl medium) was chosen for
further experiments. HCT-116 cells were grouped into two treatments
and transfection was conducted as described above: i) control
group, HCT-116 cells transfected with negative control vector. ii)
KiSS-1 group, HCT-116 cells transfected with KiSS-1 vector.
Each treatment consisted of six replicates. Cells were cultured for
96 h and sampling was conducted at 24, 48, 72, and 96 h.
Quantitative real-time PCR (qPCR)
For cell cultures from 24 h in different treatments,
the whole RNA was extracted using TRIzol method according to the
manufacturer's instructions. In addition, NAPDH was selected as the
reference gene. The RNA was reverse transcribed to cDNA using
RT-PCR kit (Fermentas China Co., Ltd., Shenzhen, China), and the
final reaction mixture of volume 20 μl contained 10 μl of SYS BR
Primix Ex Taq 2, 0.5 μl of each primers (KiSS-1, forward:
5′-AGCCGCCAGATCCCCGCA-3′; reverse: 5′-GCCGAA GGAGTTCCAGTTGTAGTT-3′.
GAPDH, forward: 5′-GGG TGGAGCCAAACGGGTC-3′; reverse:
5′-GGAGTTGC TGTTGAAGTCGCA-3′), 1 μl of the cDNA template, and 8 μl
ddH2O. Thermal cycling parameters for the amplification
were as follows: a denaturation step at 94°C for 2 min, followed by
40 cycles at 94°C for 20 sec, 58°C for 30 sec and 72°C for 20 sec.
Relative gene expression was evaluated with Data Assist software
version 3.0 (Applied Biosystems, Foster City, CA, USA). The
relative expression levels of KiSS-1 were determined
according to the expression of 2−ΔΔct.
Western blot assay
Concentrations of protein samples were determined
using the BCA method and western blot assay was performed as
previously described (13); 20 μg
of protein was subject to a 10% sodium dodecylsulfate
polyacrylamide gel electrophoresis (SDS-PAGE). Then targeted
proteins were transferred onto polyvinylidene difluoride (PVDF)
sheets. The membranes were washed with TBST for 5 min and then
transferred into blocking buffer for incubation overnight at 4°C.
After three cycles of 5 min washes with TBST, primary antibodies
(1:2000) against KiSS-1 or GAPDH were incubated with the membranes
for 1 h at room temperature. After additional three washes,
secondary HRP goat anti-rabbit IgG antibodies (1:2000) were added
and incubated with the membranes for 40 min. After final three
washes using TBST, the blots were developed using Beyo ECL Plus
reagent and the results were detected in the Gel Imaging System.
The relative expression levels of different proteins were
calculated with Bio-Rad Quantity One.
Cell proliferation assay
For cell samples from 24, 48, 72, 96 h in both
KiSS-1 interference and KiSS-1 overexpression treatments, cell
viabilities were measured by CCK-8 method: briefly, CCK-8 solution
was added to cells at different time points and cultured at 37°C
for 90 min. OD value at 450 nm was determined using a Microplate
Reader.
Transwell invasion assay
The transwell experiment to evaluate the invasion
ability of HCT-116 cells was performed in cell samples from 96 h in
different treatments: 100 μl of incubation medium (with 1 mM
MgCl2) containing 1×105 cells were seeded
into the upper chamber of BSA coated 8 μM pore size transwell
chambers (Corning Star, Cambridge, MA, USA). Then cells were
incubated at 37°C for 4 h to allow the migration through the porous
membrane. The cells remaining at the upper surface of the chamber
were completely removed. The lower surfaces of the membranes were
stained in a solution containing 1% (w/v) crystal violet in 2%
ethanol for 30 sec and then washed with ddH2O.
Extraction of cell-associated crystal violet was performed by
incubating in 10% acetic acid for 20 min. Results were observed
using an Olympus CX41 microscope and the cell number in different
treatments was determined using Image-Pro Plus 6.0 software
(Nikon).
Fluorescence activated cell sorter
Effect of regulation of KiSS-1 gene on the
apoptosis in HCT-116 cells was also determined using fluorescence
activated cell sorter (FACS): 200 μl Annexin V/7-ADD working
solution (5 μl Annexin V and 10 μl 7-AAD in 0.5 ml 1X Binding
buffer) was added to cell samples from 96 h in different
treatments. After incubation for 15 min at room temperature in the
dark, the apoptotic rate was detected with flow cytometry. Each
treatment was represented by three replicates. Apoptotic rate
(UR+LR-all apoptosis cell percentage) was equal to the sum of the
cell death rate (UR, upper right quadrant-advanced stage apoptosis
cell percentage) and the early apoptosis rate (LR, lower right
quadrant-prophase apoptosis cell percentage).
Detection of inhibition of KiSS-1 gene on
PI3K/Akt/NF-κB- mediated MMP-9 expression
To further determine the molecular mechanism of
KiSS-1 gene reducing the expression of MMP-9, we assessed
the effect of KiSS-1 gene over-expression on PI3K/Akt/NF-κB
signal transduction pathway. HCT-116 cells were classified into 4
groups: i) NC group, HCT-116 cells transfected with negative
control lentivirus vector. ii) KiSS-1 group, HCT-116 cells
transfected with lentivirus-mediated KiSS-1 vector. iii) KiSS-1 +
740Y-P group, KiSS-1 overexpression HCT-116 cells incubated
with PI3K agonist 740Y-P (50 μg/ml) for 90 min. iv) KiSS-1 + PDGF
group, KiSS-1 overexpression HCT-116 cells incubated with
Akt agonist PDGF (100 ng/ml) for 1 h. Each treatment consisted of
six replicates. All the cells were cultured for 96 h and sampled
every 24 h. Cell viabilities of cultures from time points 24, 48,
72, and 96 h were measured using CCK-8 method as described above.
In addition, cell invasion ability and apoptotic rate were
determined using cell samples from 96 h. The expression of PI3K,
Akt, pAkt, NF-κB subunit p65, and MMP-9 was determined with western
blotting as described above.
Statistical analysis
The data are expressed as the mean ± SD. Student
t-test and multiple comparisons with LSD method were conducted by
using GLM model with significant level of p<0.05. All the
statistical analysis were conducted using SPSS version 19.0 (IBM,
Armonk, NY, USA).
Results
Stable regulation of KiSS-1 gene in
HCT-116 cells
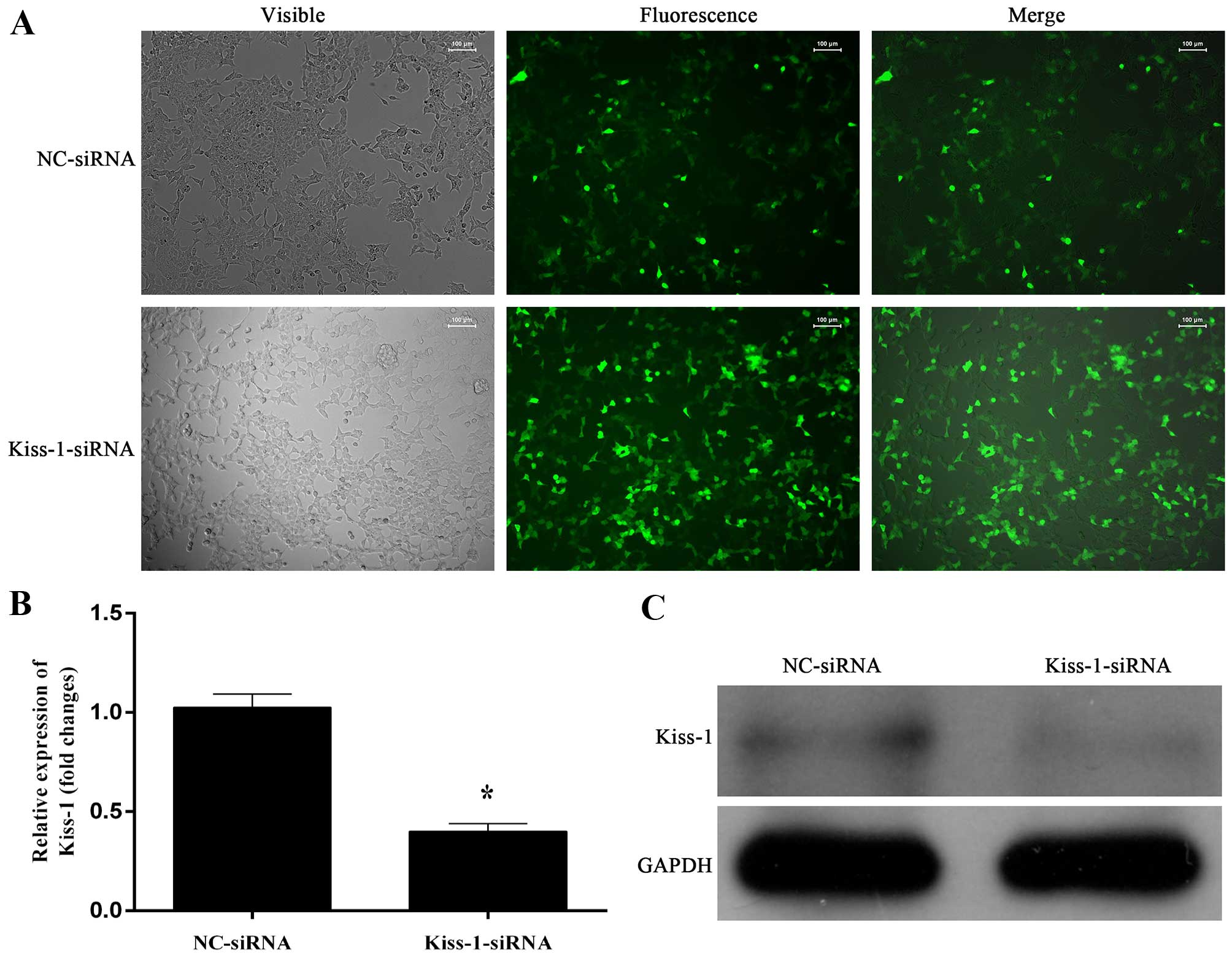
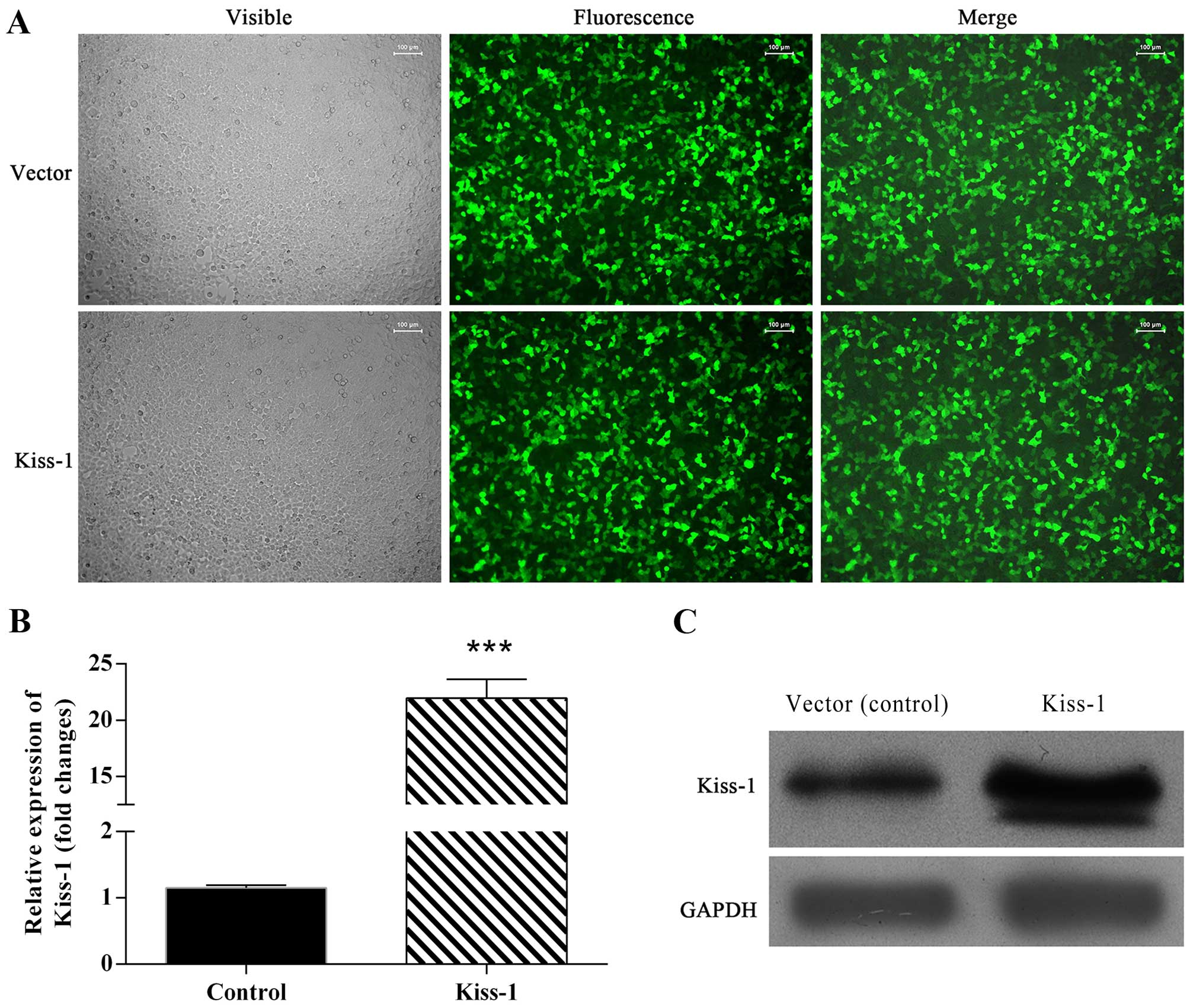
Stable transfection of KiSS-1-specific siRNA
and lentivirus-mediated KiSS-1 vector was detected using
qPCR and western blotting (Figs. 1
and 2). The transfection of
KiSS-1-specific siRNA significantly downregulated the
transcription and synthesis of KiSS-1 (Fig. 1B and C), and the transfection of
KiSS-1 vector significantly upregulated the expression of
KiSS-1 in both mRNA and protein levels (Fig. 2B and C).
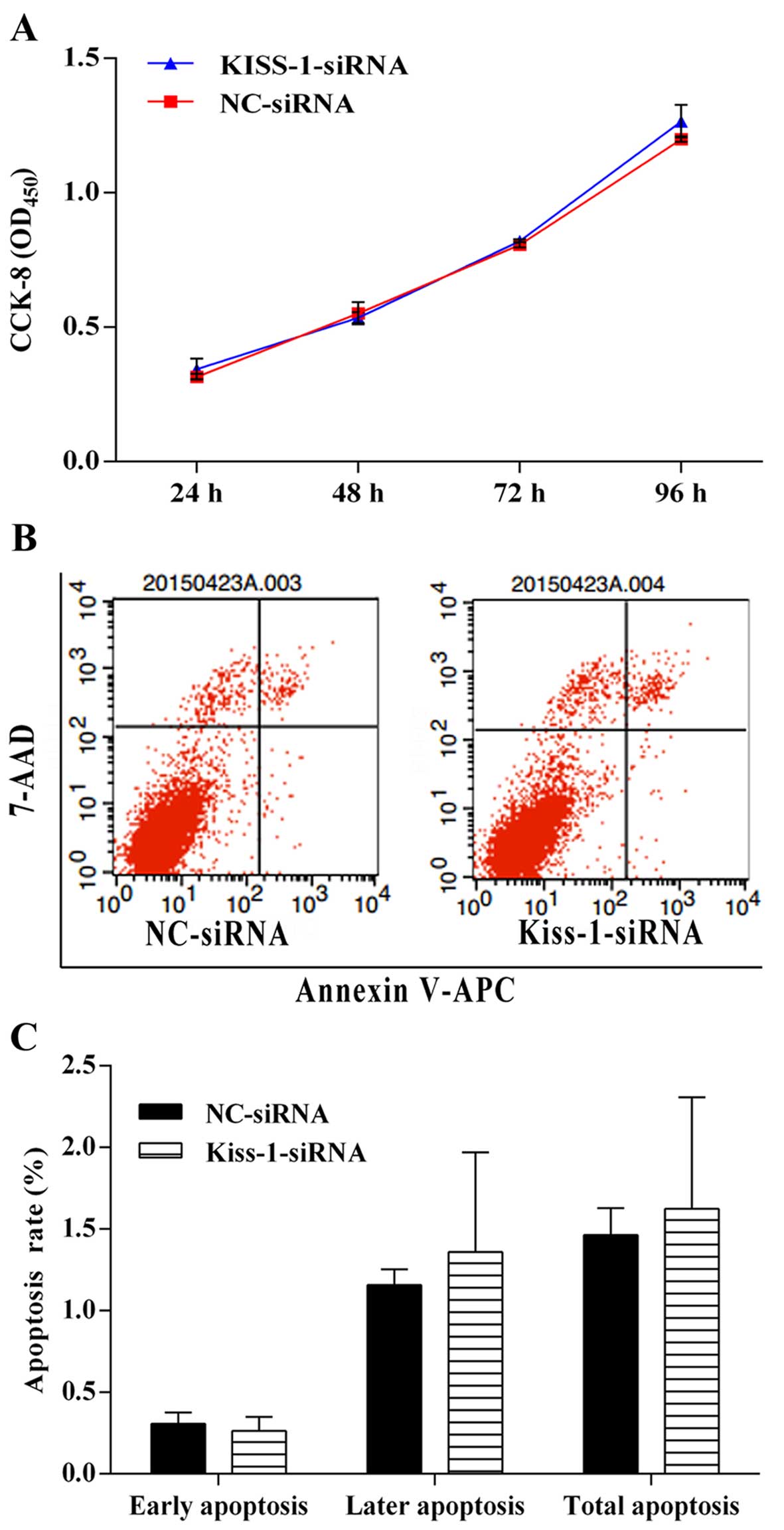
Silencing of KiSS-1 gene by
KiSS-1-specific siRNA has no impact on the cell viability, cell
apoptosis, and invasion ability in HCT-116 cells
Although transfection with KiSS-1-specific
siRNA influenced the transcription and production of KiSS-1,
it seemed that silencing of KiSS-1 gene had no influence on
the on the cell viability, cell invasion ability, or apoptosis
within our experimental period (Figs.
3 and 4), which might indicate
that the expression of KiSS-1 gene in normal HCT-116 cells
was low and inhibition of the transcription of KiSS-1 gene
had little influence on the regular biological activity of CRC
cells.
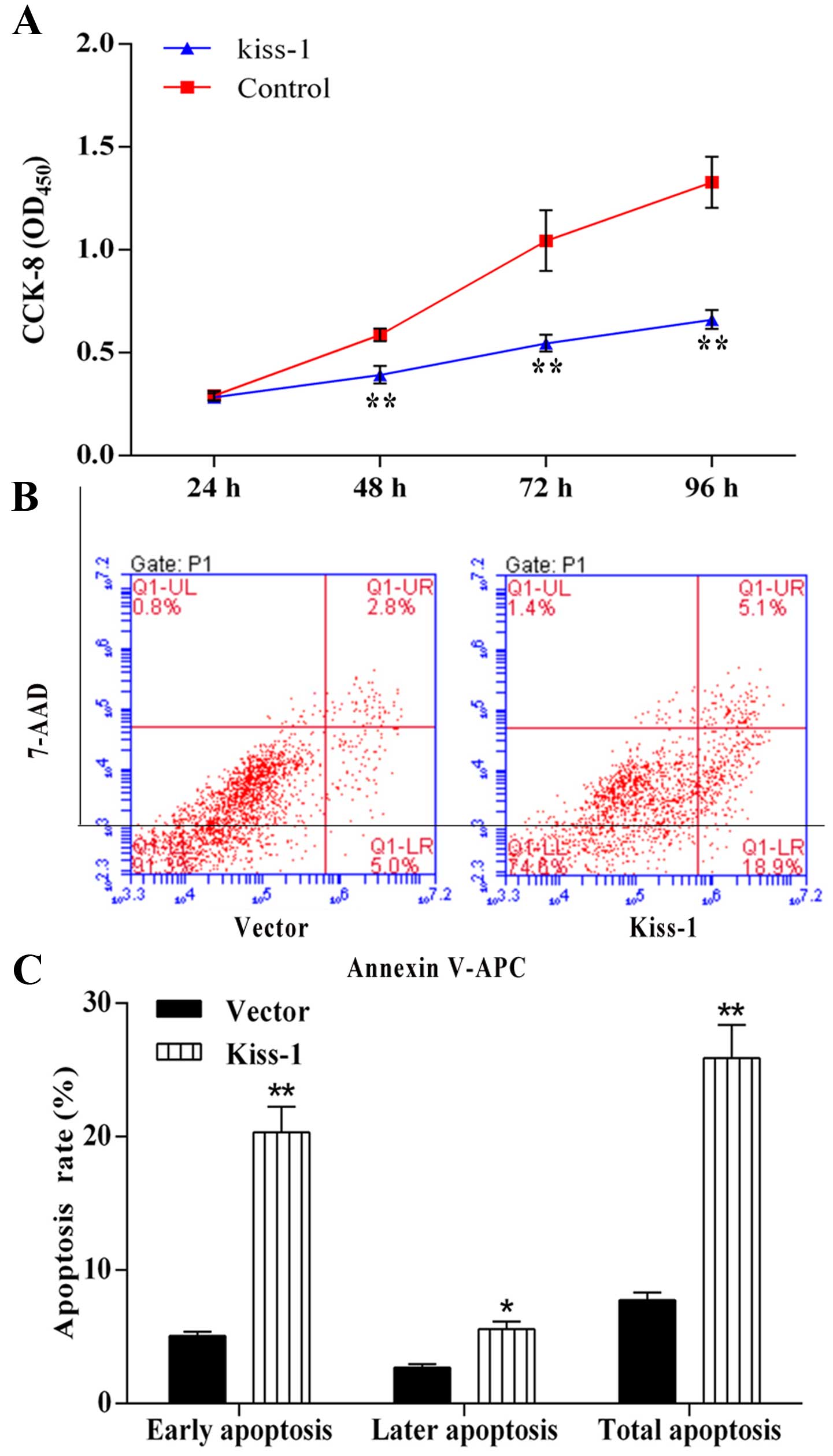
Overexpression of KiSS-1 gene reduces the
cell viability while induces cell apoptosis in HCT-116 cells
Overexpression of KiSS-1 gene led to a
significant decrease in the cell proliferation in HCT-116 cells,
especially at sampling points of 72 and 96 h, showing the potential
of KiSS-1 as an effective anti-tumor target (Fig. 5A). Similar results were also
observed in FACS, overexpression of KiSS-1 significantly
increased the apoptotic rate (24.0%) in KiSS-1 group compared with
NC group (7.1%) (Fig. 5B and C).
Moreover, most non-viable cells in KiSS-1 group belonged to early
apoptosis stage (18.9%).
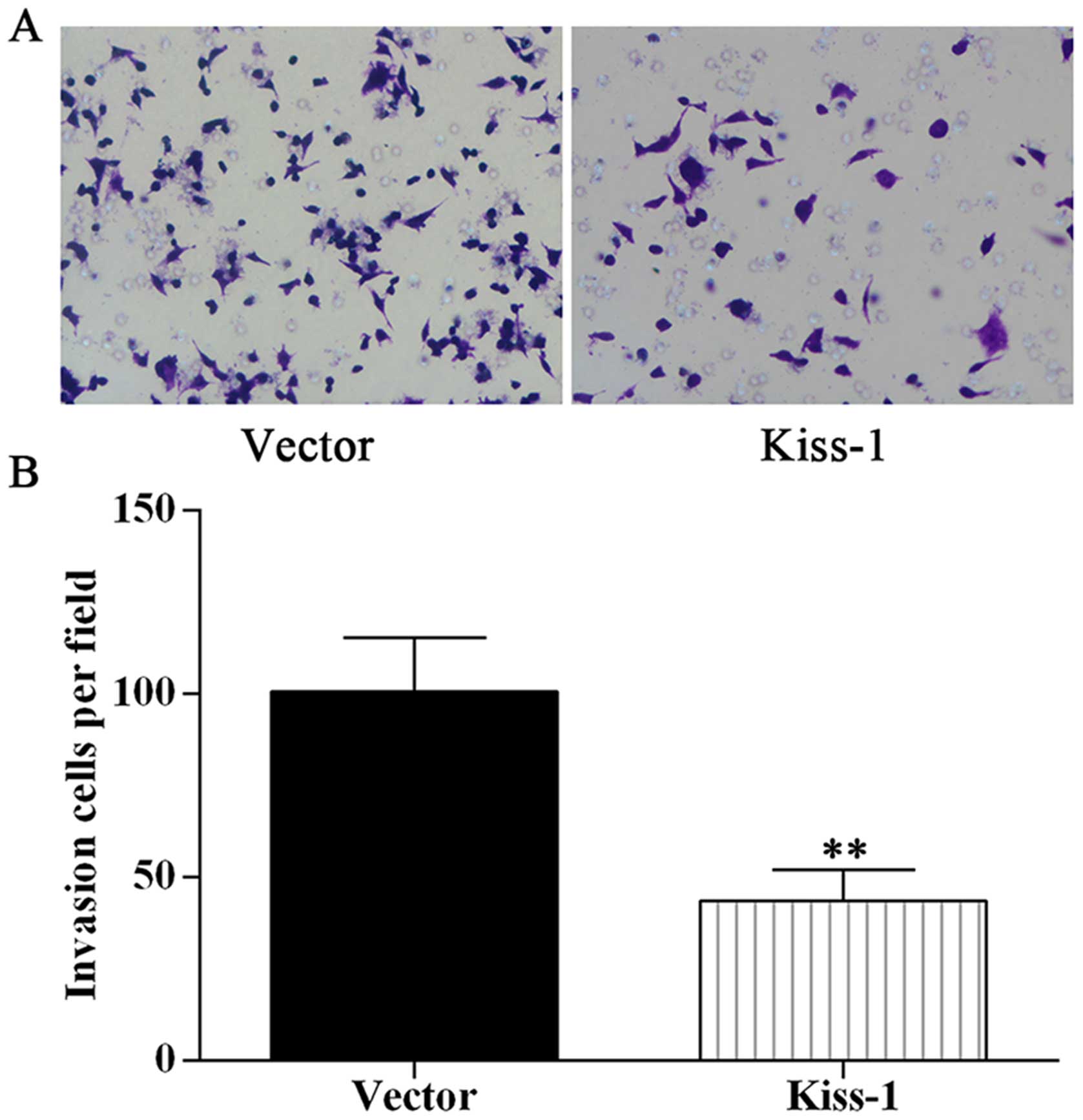
Overexpression of KiSS-1 gene decreases
invasion ability in HCT-116 cells
The cell migration ability was evidently influenced
by overexpression of KiSS-1 gene. To assess the function of
KiSS-1 on cell mobility, we performed migration experiments in a
modified Boyden chamber with HCT-116 cell transfected with
different categories. Overexpression of KiSS-1 strongly
reduced cell migration in KiSS-1 group (Fig. 6), however, given that the silencing
of KiSS-1 had no significant effect on cell migration
(Fig. 4), we inferred that
KiSS-1 might exert its function in metastasis of CRC cells
in an indirect manner.
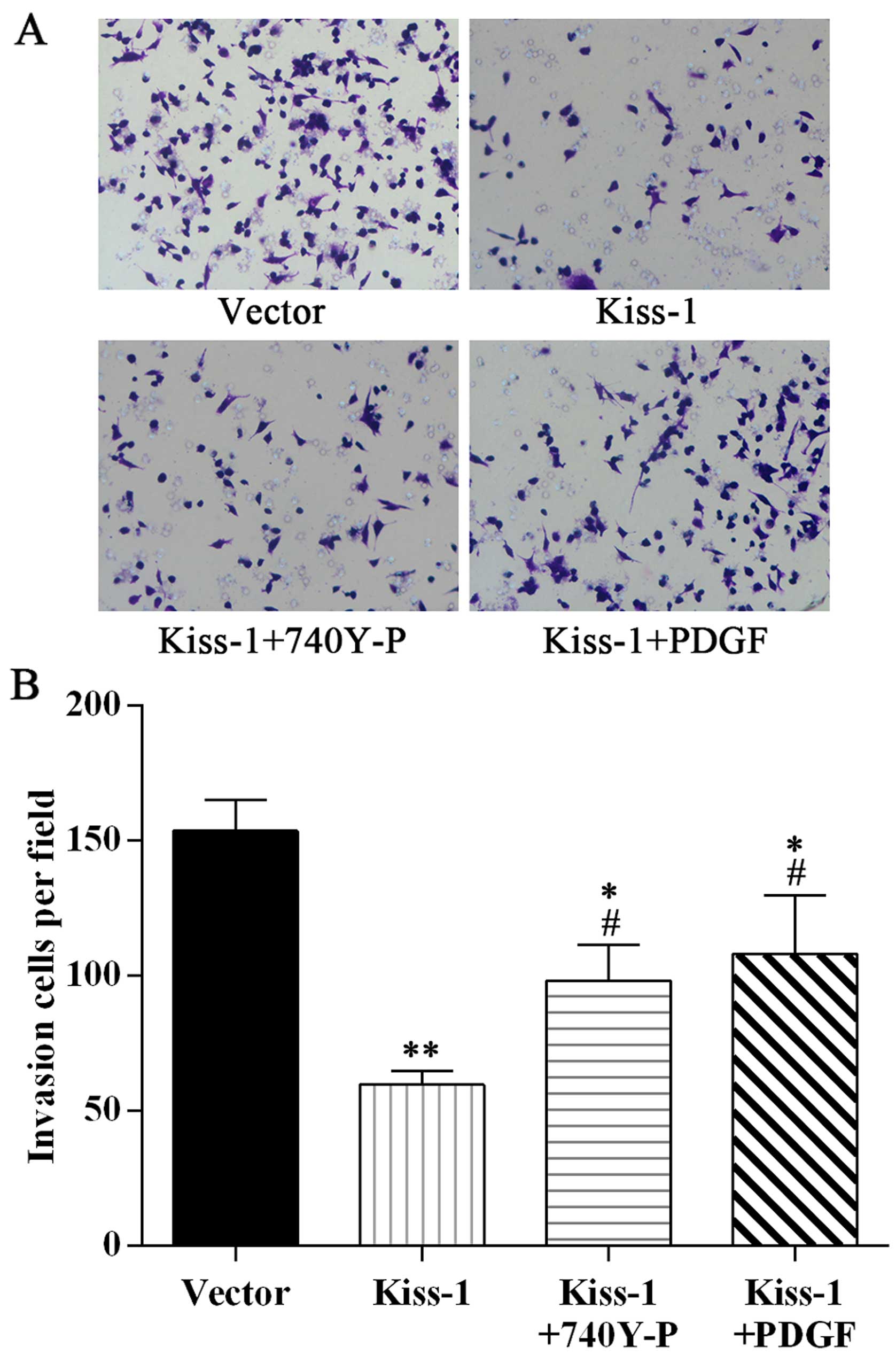
Employment of PI3K/Akt agonists attenuate
the effect of KiSS-1 on the cell viability, cell apoptosis, and
invasion ability in HCT-116 cells
To further explore the mechanism of KiSS-1
influencing the expression of MMP-9 and then reducing the
metastasis of CRC cells, we set up another two groups,
KiSS-1 overexpression HCT-116 cells incubated with PI3K
agonist 740Y-P and Akt agonist PDGF, respectively. Treatments with
the agonists significantly reversed the damage to HCT-116 cells.
The cell viability in KiSS-1 + 740Y-P group and KiSS-1 + PDGF group
was much higher than that in KiSS-1 group (Fig. 7A). The apoptotic rate and migration
ability were also improved by incubation with the agonists
(Figs. 7B and C, and 8). Although the biological condition of
cells in these two groups was still poorer than NC group, the
results indicated the evident involvement of PI3K/Akt pathway in
KiSS-1 reducing proliferation and migration ability of
HCT-116 cells.
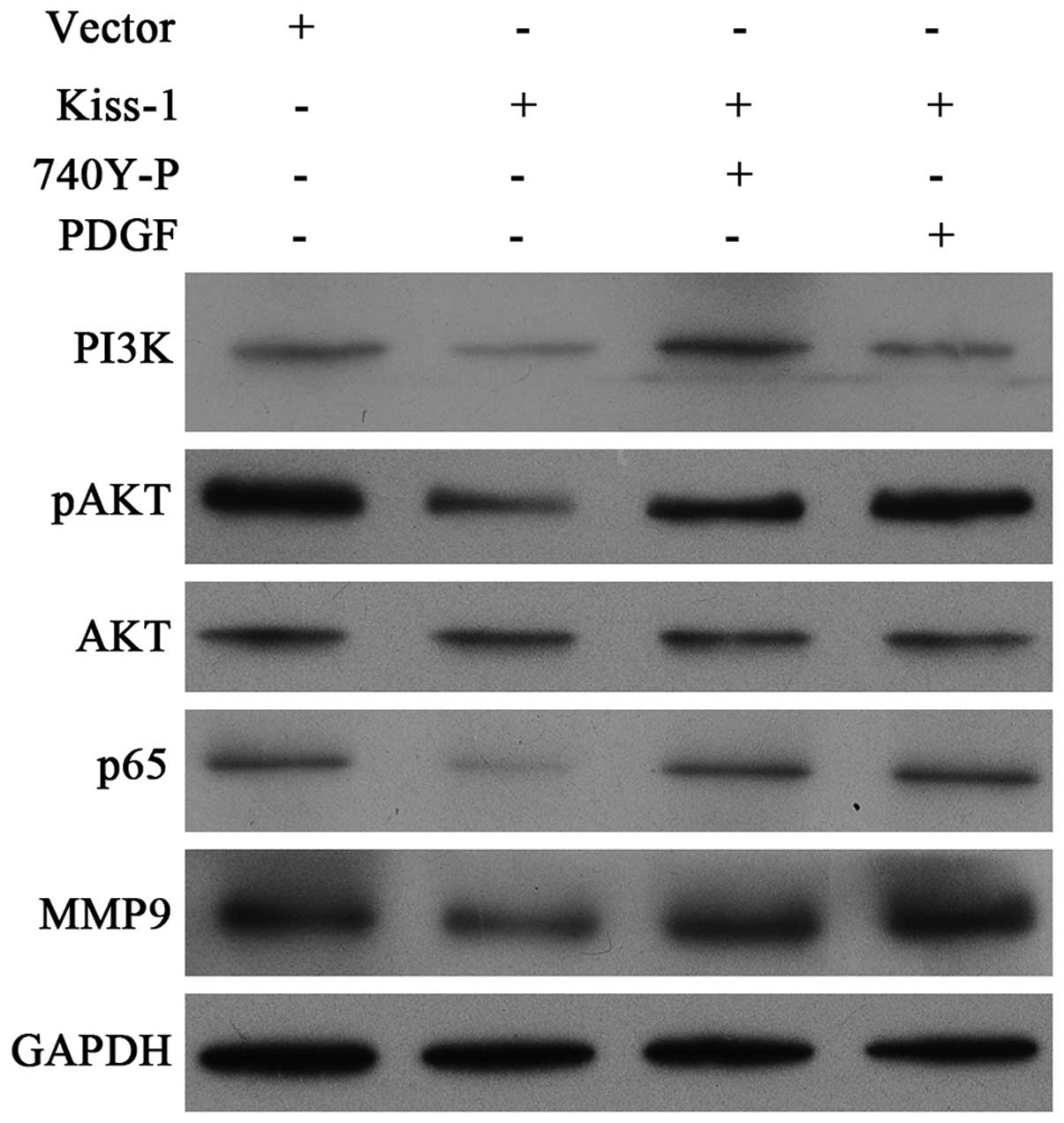
KiSS-1 downregulates the expression of
MMP-9 by blocking PI3K/Akt/NF-κB pathway
We investigated the effect of KiSS-1
overexpression on the downstream activation by detecting the
synthesis of MMP-9, PI3K, p65 and phosphorylation of Akt.
Furthermore, to activate these pathways, we also treated
KiSS-1 overexpression HCT-116 cells with PI3K/Akt agonists
740Y-P and PDGF. The results of western blotting clearly showed
that the transfection with lentivirus-mediated KiSS-1 vector
significantly reduced the synthesis of MMP-9, PI3K, pAkt, and p65
(Fig. 9). By co-incubation with
different agonists, KiSS-1 overexpression-induced inhibition
could be attenuated (Fig. 9).
Taken the above information together, our results demonstrated that
the decrease in cell invasion in CRC cells might result from the
inhibition of MMP-9 due to KiSS-1 blocking the
PI3K/Akt/NF-κB signal transduction pathway.
Discussion
As proposed by Fearon and Vogelstein (14), CRC is a type of malignant neoplasm
resulted from acquired and/or innate molecular alterations in
colonic mucosa. Consumption of red meat and alcohol as well as
obesity is associated with a higher risk of CRC. Moreover,
inflammatory bowel patients also have greater risk for CRC
development than health people. The genetic mutations and
expression patterns of molecular markers involved in the
oncogenesis of CRC and the in CRC are variable (15–17),
which has led scientists to explore further the molecular level to
meet the need for an accurate diagnosis, prognosis, and efficient
therapeutic approach for CRC.
Cancer invasion and metastasis depend critically on
the degradation of the extracellular matrix surrounding the tumor
tissues. The process is believed to be activated by the action of
proteolytic enzymes, including several types of MMPs (18). In the case of CRC, most studies
have reported the increased expression of MMP-9 in patients with
gastrointestinal cancers (8).
Thus, regulation of MMP-9 has become a novel therapeutic target for
prevention and treatment of CRC (8,9).
Previous study of Yan et al (19) showed that KiSS-1 could
repress MMP-9 in the human sarcoma cell line HT-1080, however, few
studies have paid attention to the role of KiSS-1 in
regulating MMP-9 in CRC, neither the related mechanisms. In the
present study, we demonstrated that KiSS-1 gene could reduce
the metastatic potential of colorectal cancer cells by
downregulating the expression of MMP-9 and the interaction between
KiSS-1 and MMP-9 was connected through the PI3K/Akt/NF-κB
signal transduction pathway.
Our data elucidated that overexpression of
KiSS-1 gene significantly suppressed the invasiveness and
proliferation of in human CRC cell line HCT-116 while cell
apoptosis was enhanced. Potential of migration and invasion is
critical for tumor progression and metastasis. Lee and colleague
have indicated that KiSS-1 inhibits invasiveness and
metastasis of cancer cells (11).
They found that loss of expression of KiSS-1 correlated with
metastatic potential in human melanoma cells. This finding implies
the therapeutic effect of KiSS-1 to reduce the migration
ability of tumor cells. Our data derived from CRC model was
different from that of the study by Lee et al (11): it was found that the silencing of
KiSS-1 by specific siRNA had no influence on the cell
viability, invasion, and apoptosis, although the transcription and
synthesis of KiSS-1 in our experiment were significantly
downregulated by RNAi technique. However, overexpression of
KiSS-1 led to a significant decrease in cell invasion and
proliferation. Thus, the regulation of KiSS-1 on the
metastatic phenotype of different tumors is more complicated than
expected.
Exposure to overexpression of KiSS-1 may
induce the alteration of cellular signaling pathway mediators
involved in the invasion of tumor cells, such as PI3K, MAPK, JNK,
Akt, and NF-κB (10,20,21).
However, previous studies showed that PI3K/Akt was one of the
signal pathways to induce MMP-9 secretion and expression of
KiSS-1 could reduce the NF-κB binding to the MMP-9 promoter
(19,22). To elucidate the possible
interactions between these processes, we investigated the
overexpression of KiSS-1 on the synthesis of PI3K, Akt,
pAkt, and NF-κB subunit p65. Our data showed that upregulation of
KiSS-1 was associated with the inhibition of MMP-9, PI3K,
pAkt, and p65. The PI3K/Akt and NF-κB signal transduction pathways
are involved in the resistance of numerous solid tumors against a
variety of anticancer drugs (23–25).
By using array analysis, Agarwal et al (26) found that most genes of PI3K/Akt/IκB
pathway were implicated in tumor angiogenesis and metastasis and
positively regulating NF-κB and β-catenin in CRC cells.
In our experiment, we used agonists of PI3K and Akt
to further detect the influence of KiSS-1 on this pathway.
Both agonists activated the phosphorylation of Akt which was
inhibited by the overexpression of KiSS-1, and then
increased the expression of downstream p65 and MMP-9. However, Akt
agonist PDGF had no effect on the expression of PI3K. Considering
the regulating function of PI3K on Akt, this result confirmed that
regulation of KiSS-1 on this pathway came into play in a
PI3K to Akt pattern. Taken all the results together, we come to a
conclusion that overexpression of KiSS-1 suppressed the
invasiveness of CRC cells, mainly through downregulation of MMP-9
expression mediated by PI3K/Akt/NF-κB signal transduction pathway.
This pathway also exits in other cell models: Yan et al
demonstrated KiSS-1 could repress MMP-9 via reducing NF-κB
binding to the MMP-9 promoter and Cheng et al showed that
radiation enhanced the expression of MMP-9 via PI3K/Akt/NF-κB
(10,19).
Collectively, our data revealed that overexpression
of KiSS-1 could suppress the invasiveness of CRC cells, and
the gene exerted its function by reducing the expression of MMP-9
by block of the PI3K/Akt/NF-κB pathway. The therapeutic effect of
KiSS-1 depends not only on the elimination of local disease,
but also on inhibiting systemic dissemination of CRC cells. The
clarification of signal transduction mediators involved in this
process might facilitate the development of specific inhibitors to
modulate CRC metastatic signaling in the clinic.
Acknowledgements
The present study was supported by the National Key
Clinical Specialties Construction Projects and the Key Project of
Fujian Science and Technology Department (no. 2014y0021). The
funders had no role in the study design, data collection and
analysis, or preparation of the manuscript.
References
|
1
|
Meijer G: GLOBOCAN 1: Cancer incidence and
mortality worldwide. J Clin Pathol. 53:1642000. View Article : Google Scholar
|
|
2
|
Wang H, Dwyer-Lindgren L, Lofgren KT,
Rajaratnam JK, Marcus JR, Levin-Rector A, Levitz CE, Lopez AD and
Murray CJ: Age-specific and sex-specific mortality in 187
countries, 1970–2010: A systematic analysis for the Global Burden
of Disease Study 2010. Lancet. 380:2071–2094. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blanco-Calvo M, Concha Á, Figueroa A,
Garrido F and Valladares-Ayerbes M: Colorectal cancer
classification and cell heterogeneity: A systems oncology approach.
Int J Mol Sci. 16:13610–13632. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Langley RR and Fidler IJ: The seed and
soil hypothesis revisited - the role of tumor-stroma interactions
in metastasis to different organs. Int J Cancer. 128:2527–2535.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogelstein B, Fearon ER, Hamilton SR, Kern
SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM and Bos
JL: Genetic alterations during colorectal-tumor development. N Engl
J Med. 319:525–532. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zucker S, Lysik RM, Zarrabi MH and Moll U:
M(r) 92,000 type IV collagenase is increased in plasma of patients
with colon cancer and breast cancer. Cancer Res. 53:140–146.
1993.PubMed/NCBI
|
|
7
|
Onisto M, Garbisa S, Caenazzo C, Freda MP,
Di F, Nitti D and Stetler-Stevenson WG: Reverse
transcription-polymerase chain reaction phenotyping of
metalloproteinases and inhibitors involved in tumor matrix
invasion. Diagn Mol Pathol. 2:74–80. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tutton MG, George ML, Eccles SA, Burton S,
Swift RI and Abulafi AM: Use of plasma MMP-2 and MMP-9 levels as a
surrogate for tumour expression in colorectal cancer patients. Int
J Cancer. 107:541–550. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liabakk NB, Talbot I, Smith RA, Wilkinson
K and Balkwill F: Matrix metalloprotease 2 (MMP-2) and matrix
metalloprotease 9 (MMP-9) type IV collagenases in colorectal
cancer. Cancer Res. 56:190–196. 1996.PubMed/NCBI
|
|
10
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JH, Miele ME, Hicks DJ, Phillips KK,
Trent JM, Weissman BE and Welch DR: KiSS-1, a novel human malignant
melanoma metastasis-suppressor gene. J Natl Cancer Inst.
88:1731–1737. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee J-H and Welch DR: Suppression of
metastasis in human breast carcinoma MDA-MB-435 cells after
transfection with the metastasis suppressor gene, KiSS-1. Cancer
Res. 57:2384–2387. 1997.PubMed/NCBI
|
|
13
|
Hamid T, Gu Y, Ortines RV, Bhattacharya C,
Wang G, Xuan YT and Prabhu SD: Divergent tumor necrosis factor
receptor-related remodeling responses in heart failure: Role of
nuclear factor-kappaB and inflammatory activation. Circulation.
119:1386–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinto E, Baker K, Tsuda H, Mochizuki H,
Ueno H, Matsubara O, Foulkes WD and Jass JR: Tumor buds show
reduced expression of laminin-5 gamma 2 chain in DNA mismatch
repair deficient colorectal cancer. Dis Colon Rectum. 49:1193–1202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
García-Solano J, Conesa-Zamora P,
Trujillo-Santos J, Torres-Moreno D, Mäkinen MJ and Pérez-Guillermo
M: Immunohistochemical expression profile of β-catenin, E-cadherin,
P-cadherin, laminin-5γ2 chain, and SMAD4 in colorectal serrated
adenocarcinoma. Hum Pathol. 43:1094–1102. 2012. View Article : Google Scholar
|
|
18
|
Matrisian LM: Metalloproteinases and their
inhibitors in matrix remodeling. Trends Genet. 6:121–125. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan C, Wang H and Boyd DD: KiSS-1
represses 92-kDa type IV collagenase expression by down-regulating
NF-kappa B binding to the promoter as a consequence of Ikappa
Balpha-induced block of p65/p50 nuclear translocation. J Biol Chem.
276:1164–1172. 2001. View Article : Google Scholar
|
|
20
|
Criswell T, Leskov K, Miyamoto S, Luo G
and Boothman DA: Transcription factors activated in mammalian cells
after clinically relevant doses of ionizing radiation. Oncogene.
22:5813–5827. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dent P, Yacoub A, Contessa J, Caron R,
Amorino G, Valerie K, Hagan MP, Grant S and Schmidt-Ullrich R:
Stress and radiation-induced activation of multiple intracellular
signaling pathways. Radiat Res. 159:283–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ruhul Amin AR, Senga T, Oo ML, Thant AA
and Hamaguchi M: Secretion of matrix metalloproteinase-9 by the
proinflammatory cytokine, IL-1beta: a role for the dual signalling
pathways, Akt and Erk. Genes Cells. 8:515–523. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arlt A and Schäfer H: NFkappaB-dependent
chemoresistance in solid tumors. Int J Clin Pharmacol Ther.
40:336–347. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Agarwal A, Das K, Lerner N, Sathe S, Cicek
M, Casey G and Sizemore N: The AKT/I kappa B kinase pathway
promotes angiogenic/metastatic gene expression in colorectal cancer
by activating nuclear factor-kappa B and beta-catenin. Oncogene.
24:1021–1031. 2005. View Article : Google Scholar
|