Introduction
Renal cell carcinoma (RCC) is the most common solid
neoplasm of adult kidney, accounting for 3% of all adult cancers
(1). Approximately 20–30% of new
RCC patients are diagnosed with metastatic RCC (mRCC), and as many
as 40% of patients with localized RCC may develop mRCC after
radical surgery (2). The prognosis
of mRCC patients is poor and limited treatment options are
available (3). Currently, the
major treatment for mRCC is molecular targeted therapy, which
includes tyrosine kinase inhibitors (TKIs) and mammalian target of
rapamycin (mTOR) inhibitors. As a multi-targeted TKI, sorafenib was
the first targeted agent approved for the treatment of mRCC and
significantly prolonged median progression-free survival in a
randomized phase III trial for advance RCC (4–6).
However, complete or long-term remissions are rarely accomplished
due to intolerance of dose-related side-effects (7). Identifying novel target molecules is
hence necessary to improve the clinical outcome for mRCC
treatment.
Anoikis is a specific type of apoptosis induced by
loss of cell adhesion or inappropriate cell adhesion (8). Anoikis plays a critically important
physiological role in regulating tissue homoeostasis (9). Failure of anoikis execution could
result in adherent cells surviving under suspension conditions or
at ectopic sites. Anoikis-resistance is consequently an essential
prerequisite of progression and metastasis of various human cancer
types (10,11). Anoikis-resistance is closely
related to mRCC (12,13), while the exact mechanisms of
anoikis-resistance in mRCC remain unclear.
Tyrosine receptor kinase B (TrkB) was first
identified as a highly expressed protein-tyrosine kinase in the
brain and subsequently found as the signaling receptor for
brain-derived neurotrophic factor (BDNF), which played a vital role
in the development and repair of the nervous system (14). Activation of TrkB promoted tumor
cell proliferation, survival, angiogenesis, epithelial-mesenchymal
transition (EMT), anoikis-resistance and metastasis through
regulating specific signaling pathways including phosphoinositide
3-kinases (PI3K)/Akt and MEK/ERK (15–17).
Numerous studies have revealed that overexpression of TrkB is
associated with anoikis-resistance and increased metastasis in
various human cancers, such as neuroblastoma (17), hepatic carcinoma (18), lung adenocarcinoma (19), colorectal (20) and pancreatic cancer (21). Nevertheless, the correlation
between TrkB and anoikis-resistance of mRCC is rarely reported.
The present study explored the effects of TrkB on
anoikis-resistance and targeted therapy in mRCC. Our data indicated
that anoikis-resistant ACHN cells were characterized with tolerance
to detachment-induced apoptosis, excessive proliferation and
aggressive invasion, along with upregulation of TrkB expression in
contrast to parental ACHN cells. It was also shown that TrkB
silencing promoted detachment-induced apoptosis, reduced
proliferation and repressed invasion in anoikis-resistant ACHN
cells via inhibiting activities of Akt and ERK. Moreover, TrkB
silencing improved anticancer efficiency of sorafenib in
anoikis-resistant ACHN cells through inactivating PI3K/Akt and
MEK/ERK pathways. Our findings may offer a novel potential
therapeutic strategy for mRCC.
Materials and methods
Pharmaceuticals
Sorafenib (Bayer, Leverkusen, Germany) was dissolved
in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) as
a 10 mM stock solution and stored at −20°C or diluted in cell
culture medium to give the appropriate final concentrations.
Cell culture and establishment of
anoikis-resistant ACHN cell model
Human renal cancer ACHN cell line was obtained from
Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China)
and cultured in minimum essential medium (MEM; Gibco, Grand Island,
NY, USA) containing 10% fetal bovine serum (FBS; Zhejiang Tianhang
Biotechnology Co., Ltd., Hangzhou, China) and 1%
penicillin/streptomycin (Gibco) at 37°C in 5% CO2
incubator. To obtain anoikis-resistant cells, ACHN cells were
continuously cultured in ultra-low attachment 6-well plates
(Corning Life Sciences, Acton, MA, USA) for 10 days (22), then transferred into normal culture
plates and attachment-cultured for 3 days: the re-adherent cells
were anoikis-resistant. The morphology of ACHN cells was observed
with an inverted phase contrast microscope (Olympus, Tokyo,
Japan).
Transfection of small interference RNA
(siRNA)
Three target-specific si-TrkBs and a scramble
control siRNA (si-Ctrl) were synthesized by Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China). The sequences of the siRNAs were as
follows (sense and antisense): si-TrkB-1 (5′-CCGUCACCUUGACUUGU
CU-3′ and 5′-AGACAAGUCAAGGUGACGG-3′); si-TrkB-2
(5′-CCACGAACAGAAGUAAUGA-3′ and 5′-UCAUUACUU CUGUUCGUGG-3′);
si-TrkB-3 (5′-GCGCUUCAGUGGUU CUAUA-3′ and
5′-UAUAGAACCACUGAAGGC-3′); si-Ctrl (5′-UUCUCCGAACGUGUCACGU-3′ and
5′-ACGUGACAC GUUCGGAGAA-3′). Cells were seeded in 6-well plates at
30% confluency. Next day, appropriate amount of si-TrkBs or si-Ctrl
and Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad,
CA, USA) were diluted in 250 μl of opti-MEM reduced-serum medium
(Gibco) respectively, and incubated for 5 min at room temperature.
Diluted siRNAs were added into diluted Lipofectamine 2000 and
incubated for 20 min at room temperature. A total of 500 μl of the
mixture was added each well. The medium was replaced with fresh
culture medium after 6 h.
The
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) assay
Cells (5×103/well) were seeded into
96-well plates and cultured in complete medium for 0, 1, 2, 3, 4, 5
and 6 days, respectively. A total of 20 μl of MTT solution
(Sigma-Aldrich) was added into each well and incubated for 4 h in
the dark, and then 150 μl of DMSO was added into each well for 10
min. Absorbance was measured at 490 nm using a microtiter plate
reader (Thermo Fisher Scientific, Waltham, MA, USA).
Colony formation assay
Isolated cells (5×102/well) were seeded
into 6-well plates and cultured in complete medium for 7 days.
Cells were fixed with methanol and stained with 0.1% crystal violet
(Sigma-Aldrich). Colonies with more than 50 cells were counted with
an inverted phase contrast microscope.
Flow cytometry
Cell apoptosis was detected by Annexin V-FITC
apoptosis detection kit (KeyGen Biotech., Co., Ltd., Nanjing,
China). Cells (5×105/well) were seeded in ultra-low
attachment 6-well plates for the indicated time, then collected for
incubation with Annexin V-FITC and propidium iodide (PI) for 10 min
in the dark at room temperature, and detected using a FACScan flow
cytometer (BD Biosciences, San Jose, CA, USA).
Transwell assay
Cell invasion assay was performed using the 24-well
Transwell plate with 8-μm pore polycarbonate membrane inserts
(Corning Life Sciences). Chamber inserts were coated with 50 μl
Matrigel (BD Biosciences, Bedford, MA, USA). Cells
(3×104/well) in 200 μl serum-free medium were plated in
the upper chambers, and 500 μl complete medium was added into the
lower chambers. After incubation for 24 h, cells that invaded into
the lower surface of the membrane inserts were fixed in 4%
paraformaldehyde, stained with 0.05% crystal violet and counted in
5 random fields with an inverted phase contrast microscope.
Quantitative real-time polymerase chain
reaction (qRT-PCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen) following the manufacturer's instruction. cDNA
was synthesized from total RNA using First Strand cDNA Synthesis
kit (Toyobo, Co., Ltd., Shanghai, China) following the
manufacturer's protocol. qRT-PCR was performed with the SYBR-Green
qPCR Mix (Toyobo) on the StepOnePlus Real-Time PCR system (Applied
Biosystems, Foster City, CA, USA). The PCR primer pairs synthesized
by Invitrogen and PCR programs were as follows: primer sequences
(forward and reverse) for TrkB was 5′-GGGAACATCTCTCGGT CTATG-3′ and
5′-CAAACTTGGAGTGTCTTGCC-3′ and the following program was
denaturation at 95°C for 60 sec, followed by 40 cycles consisting
of denaturation at 95°C for 30 sec, annealing at 62°C for 20 sec,
and extension at 72°C for 20 sec; primer sequences (forward and
reverse) for internal control β-actin was
5′-GTCCACCGCAAATGCTTCTA-3′ and 5′-TGCTGTCACCTTCACCGTTC-3′ and the
following program was denaturation at 95°C for 60 sec, followed by
40 cycles consisting of denaturation at 95°C for 30 sec, annealing
at 56°C for 20 sec and extension at 72°C for 20 sec. The results
were analyzed using the 2−ΔΔCT method.
Western blot analysis
Total proteins were isolated using
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology, Nanjing, China) and separated by sodium
dodecyl sulfate (SDS)-polyacrylamide (PAGE) gels (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) and transferred onto
nitrocellulose filter membranes (Millipore, Bedford, MA, USA). The
membranes were blocked with 5% non-fat milk in Tris-buffered saline
with Tween-20 (TBST) and incubated with the primary antibodies
overnight at 4°C. Primary antibodies were as follows: polyclonal
rabbit anti-human TrkB (1:500), Mcl-1 (1:1,000), VEGF (1:500), Akt
(1:1,000), p-Akt (1:1,000) and monoclonal rabbit anti-human β-actin
(1:1,000) were purchased from Santa Cruz Biotechnology (Santa Cruz,
CA, USA); polyclonal rabbit anti-human ERK (1:1,000) and p-ERK
(1:1,000) were purchased from Bioworld Technology, Inc. (St. Louis
Park, MN, USA); polyclonal rabbit anti-human MMP-9 (1:1,000) was
purchased from Cell Signaling Technology (Beverly, MA, USA).
β-actin was used as the loading control. The membranes were
incubated with the horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody (1:3,000; Santa Cruz Biotechnology).
Bands were visualized with enhanced chemiluminescence (Beyotime
Institute of Biotechnology). Densitometry was performed using the
ImageJ software (National Institutes of Health, Bethesda, MA,
USA).
Statistical analysis
All experiments were carried out at least in
triplicate. Data were expressed as the mean ± standard deviation
(SD). Statistical analyses were performed using the Statistical
Package for the Social Sciences (SPSS), version 17.0 (SPSS, Inc.,
Chicago, IL, USA). Statistical significances were evaluated using
the Student's t-test or analysis of variance (ANOVA).
*P<0.05 was considered to indicate a statistically
significant result.
Results
Anoikis-resistance inhibits
detachment-induced apoptosis, promoting proliferation and invasion
in ACHN cells
Anoikis-resistant ACHN cell model was established
according to previous reports (23–25).
Briefly, parental ACHN cells were suspension-cultured in ultra-low
attachment 6-well culture plates for 10 days. In suspension
culture, parental ACHN cells gathered into clusters and gradually
formed large cell masses with time (Fig. 1A). On the 10th day, ACHN cells were
collected and attachment-cultured in normal culture plates, and the
re-adherent cells were regarded anoikis-resistant. MTT, colony
formation, flow cytometry and Transwell assays revealed that
anoikis-resistant ACHN cells displayed more rapid proliferation,
less detachment-induced apoptosis and greater capability of
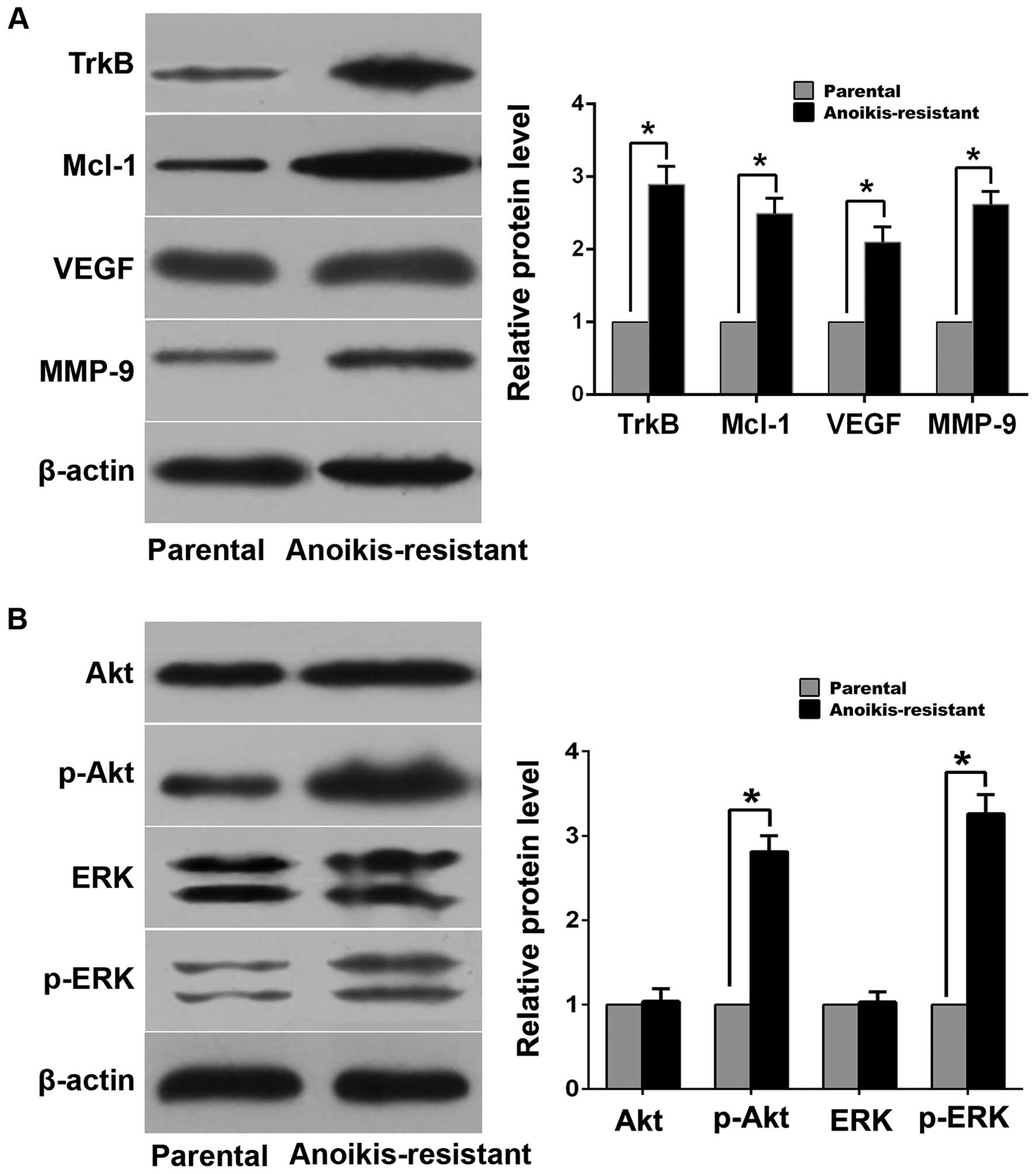
invasion compared with parental cells (Fig. 1B–E). Western blot analysis
demonstrated that expression levels of myeloid cell leukemia-1
(Mcl-1), vascular endothelial growth factor (VEGF), matrix
metalloproteinase-9 (MMP-9) and TrkB in anoikis-resistant ACHN
cells were upregulated compared with parental cells (Fig. 2A). Additionally, expression of
phosphorylated Akt (p-Akt) and phosphorylated ERK (p-ERK) increased
in anoikis-resistant cells while expression of total Akt and ERK
was of no significant difference between the two cell lines
(Fig. 2B).
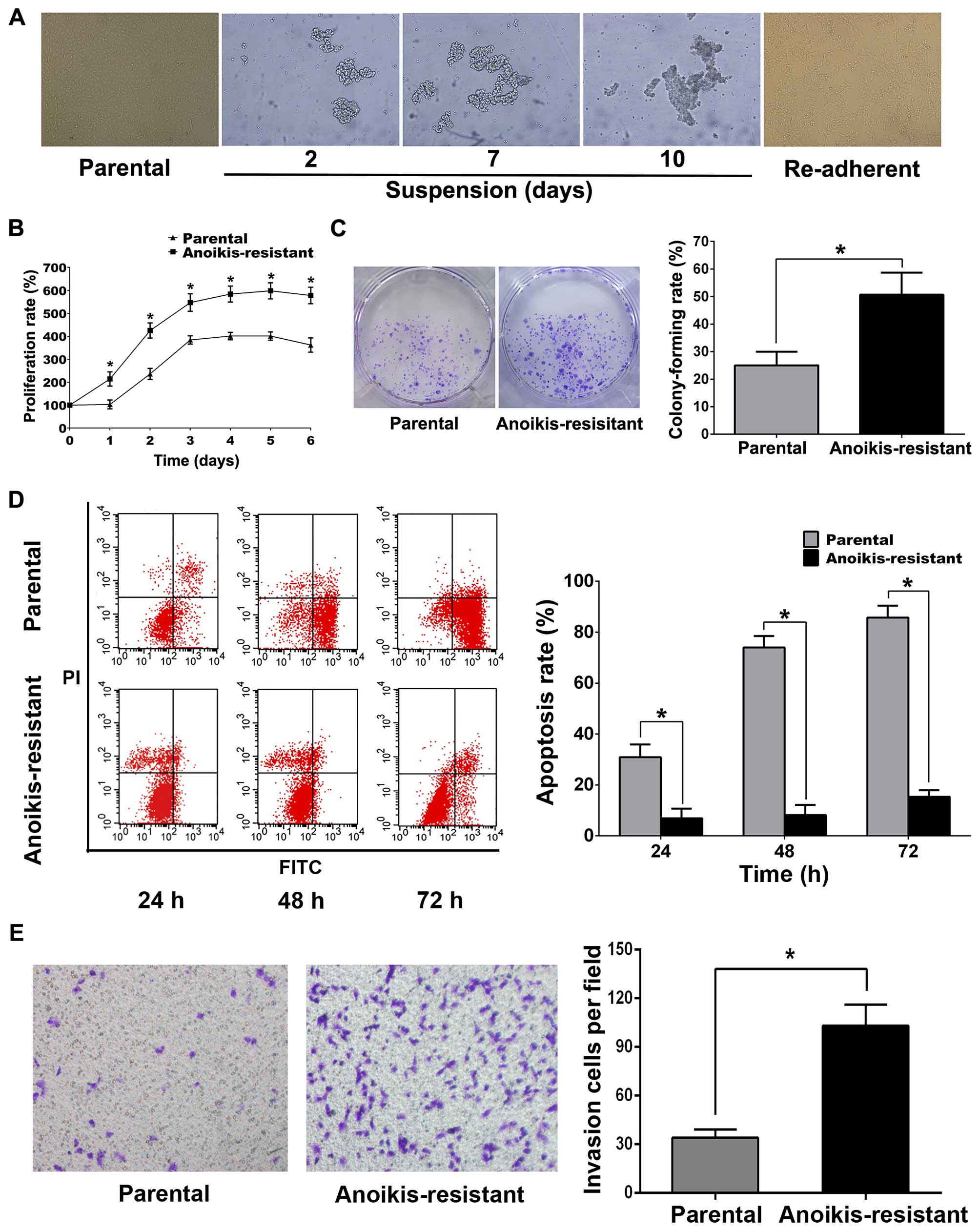 | Figure 1Anoikis-resistance inhibited
detachment-induces apoptosis and promotes proliferation and
invasion in ACHN cells. (A) The morphology of parental,
suspension-cultured and re-adherent ACHN cells (original
magnification, ×200). (B) Parental or anoikis-resistant ACHN cells
(5×103/well) were seeded in 96-well plates respectively;
MTT was used to detect proliferation rates at the indicated
time-points. (C) For colony formation assay, parental or
anoikis-resistant ACHN cells (5×102/well) were seeded in
6-well plates for 7 days. (D) For flow cytometry, parental or
anoikis-resistant ACHN cells (5×105/well) were cultured
in ultra-low attachment 6-well plates, respectively, for the
indicated time. (E) For Transwell assay, parental or
anoikis-resistant ACHN cells (3×104/well) in serum-free
medium were seeded into upper chambers, respectively, and 500 μl
complete medium was added into lower chambers; invasion cells were
counted after 24 h (original magnification, ×200).
*P<0.05. |
TrkB silencing promotes apoptosis,
inhibits proliferation and reduces invasion in anoikis-resistant
ACHN cells through inactivating Akt and ERK
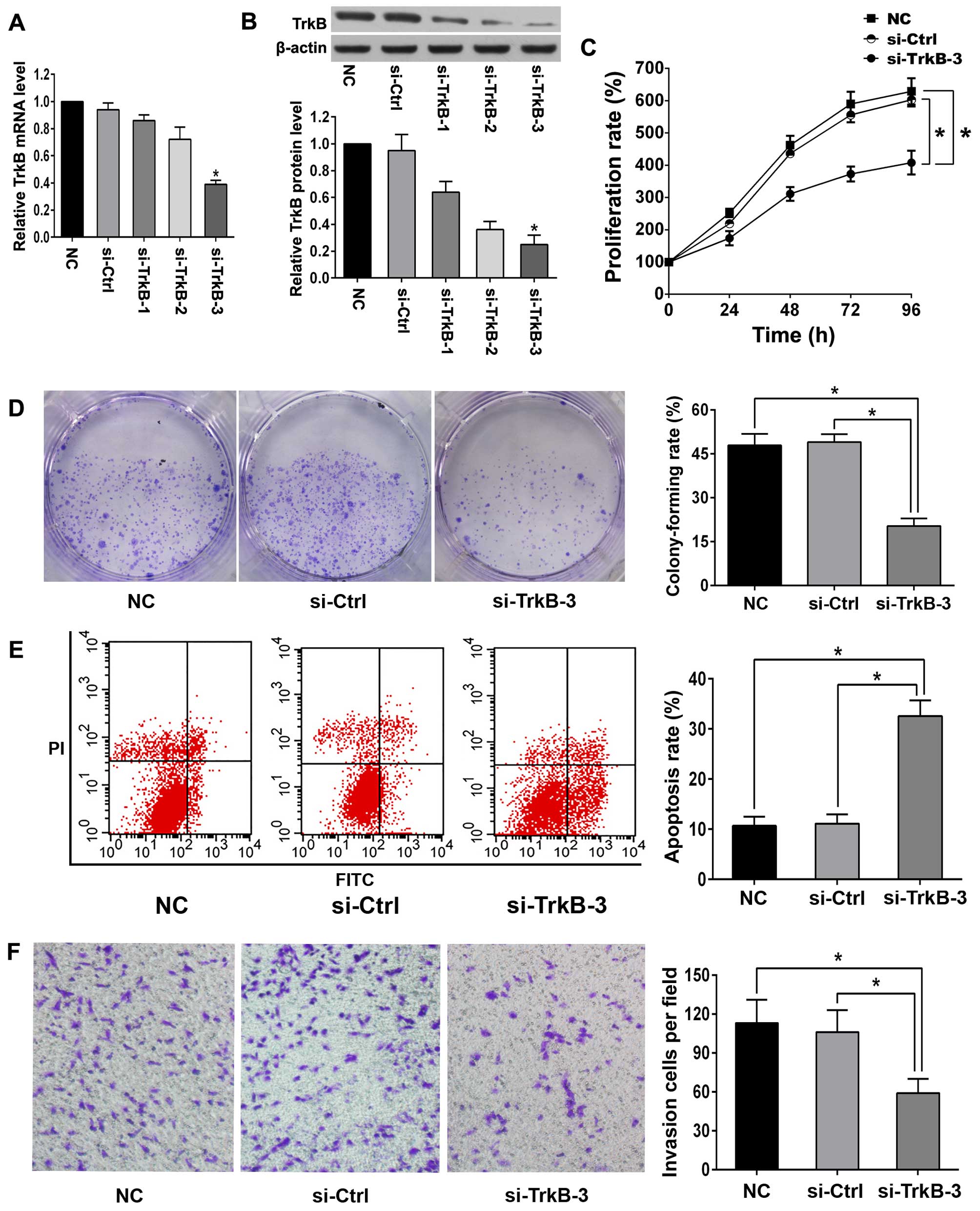
Silencing efficiency of TrkB in anoikis-resistant
ACHN cells was verified by qRT-PCR and western blot analysis
(Fig. 3A and B). TrkB siRNA-3
(si-TrkB-3) showed the highest silencing efficiency and was chosen
for the following experiments. Once TrkB was knocked down,
decreased proliferation rate, enhanced detachment-induced apoptosis
and diminished invasion were observed in anoikis-resistant ACHN
cells (Fig. 3C–F). Western blot
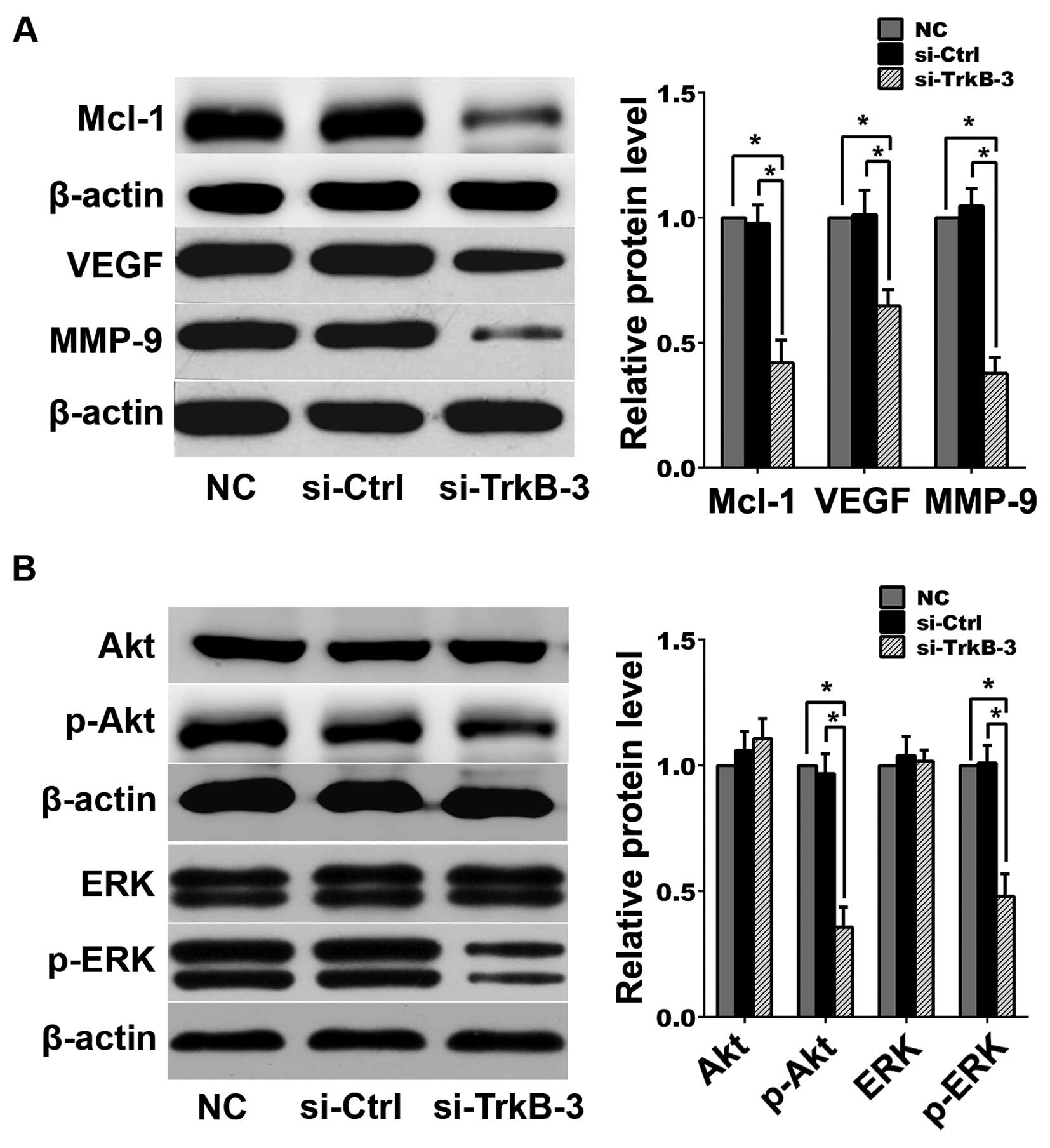
analysis indicated that following TrkB silencing, expression levels
of Mcl-1, VEGF, MMP-9, p-Akt and p-ERK decreased in
anoikis-resistant ACHN cells (Fig.
4).
TrkB silencing enhanced anticancer
efficiency of sorafenib in anoikis-resistant ACHN cells through
inhibiting activities of Akt and ERK
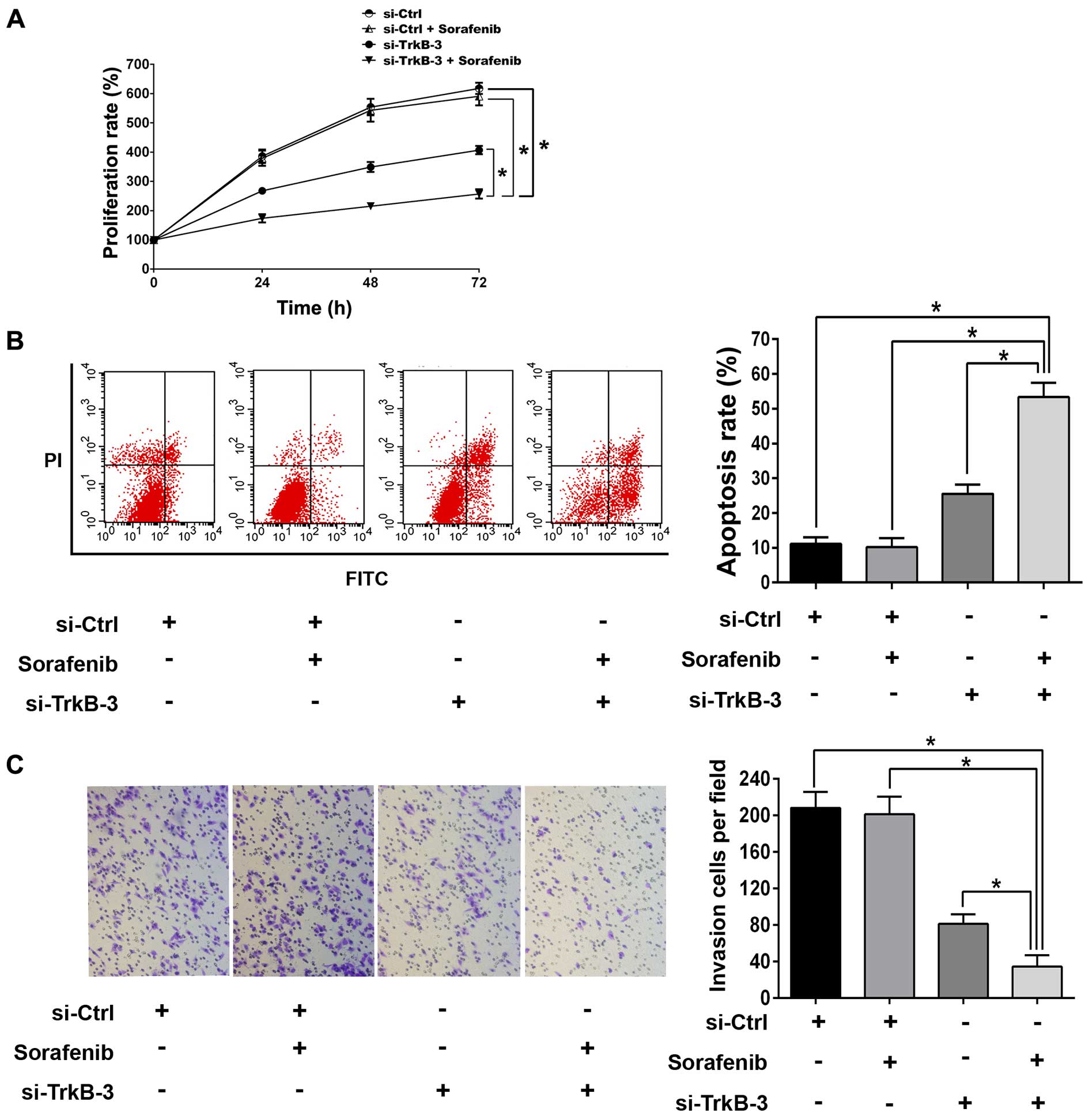
To investigate whether TrkB silencing enhanced
anticancer efficiency of sorafenib in anoikis-resistant ACHN cells,
1.0 μM was chosen as the sub-threshold concentration of sorafenib.
There were no significant difference in proliferation,
detachment-induced apoptosis and invasion between the groups
without or with sorafenib (1.0 μM) treatments (Fig. 5). However, combination of TrkB
silencing and sorafenib (1.0 μM) remarkably inhibited
proliferation, reduced invasion and promoted detachment-induced
apoptosis as compared with single treatment with TrkB knocked down
(Fig. 5). In line with this,
sorafenib (1.0 μM) alone did not change the expressions of Mcl-1,
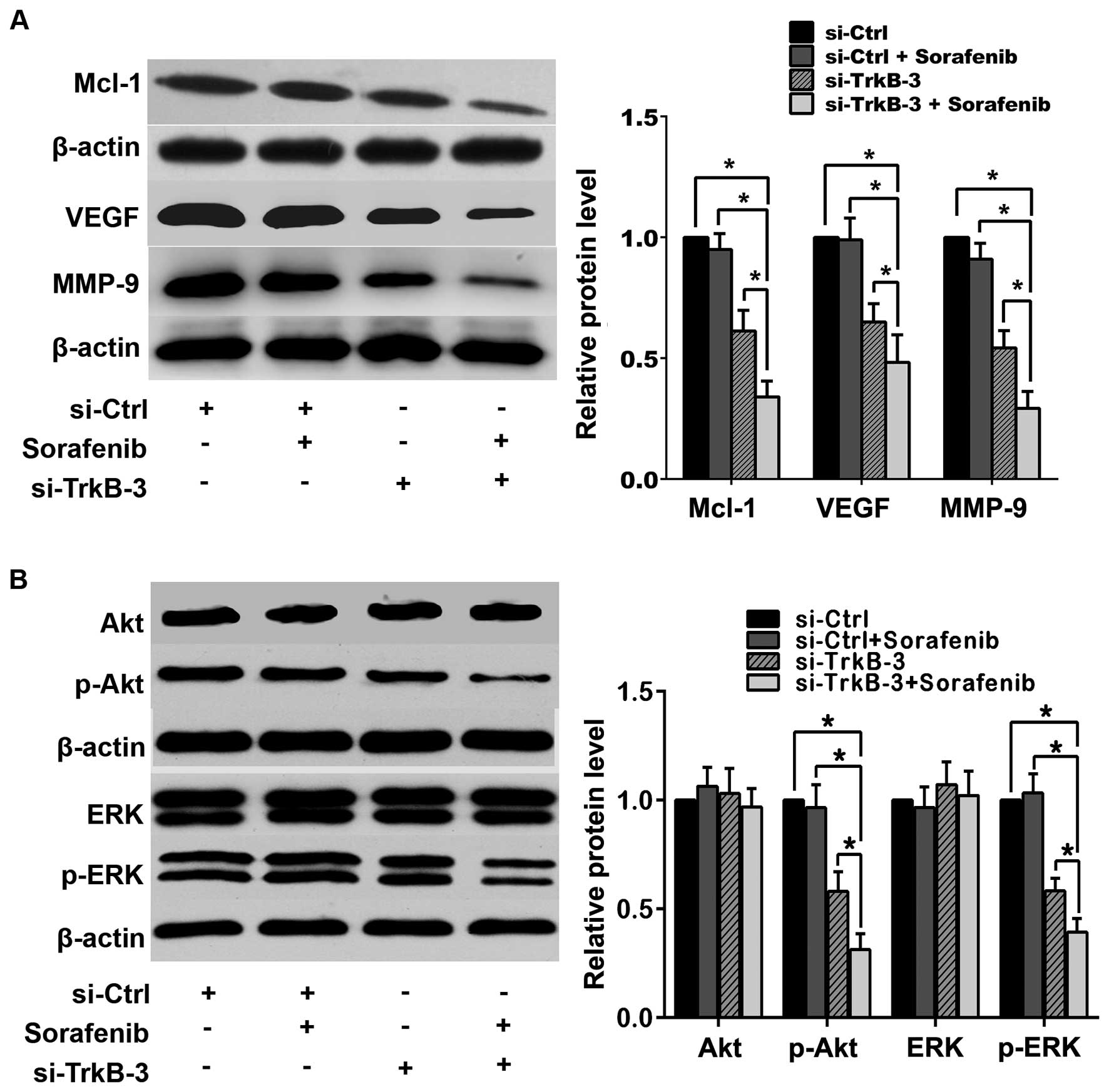
VEGF, MMP-9 or the activity of Akt and ERK (Fig. 6), while combination of TrkB
silencing and sorafenib (1.0 μM) reduced the expression of Mcl-1,
VEGF and MMP-9 significantly, as well as declined the expression of
p-Akt and p-ERK, and such effects were more effective than
treatment with TrkB silencing alone (Fig. 6).
Discussion
Establishment of anoikis-resistant cell model in
vitro is usually on the basis of suspension culture that
prevents cells from attachment (23–25).
Therefore, we chose ultra-low attachment plates for the continuous
suspension culture of the parental ACHN cells for 10 days, and the
re-adherent cells were regarded as anoikis-resistant ACHN cells.
Our data revealed that anoikis-resistant ACHN cells were
characterized with more aggressive malignant biological behavior,
including more rapid proliferation, less detachment-induced
apoptosis and more capable of invasion in contrast to parental
cells.
The molecular mechanisms involved in
anoikis-resistance have been extensively investigated. PI3Ks are a
family of lipid kinases that phosphorylate the 3′-OH group on
phosphatidylinositols in the plasma membrane, resulting in
recruitment of Akt to cell membrane for activation, which regulates
tumor growth and survival (26).
Likewise, MEK/ERK is the most typical mitogen activated protein
kinase (MAPK) pathway, which controls cellular proliferation,
invasion, differentiation and apoptosis, and aberrancy in the
pathway contributes to malignant behavior (27). The activation or overexpression of
PI3K/Akt and MEK/ERK pathways are supposed to promote
anoikis-resistance in certain malignancies (28,29).
It was reported that activated Akt and ERK were associated with
metastasis in RCC (20,21). Our data confirmed that Akt and ERK
activation are responsible for anoikis-resistance of ACHN cells and
metastatic potential of RCC.
Previous studies have reported that anoikis is
closely related to Bcl-2 family-mediated apoptosis pathway and
Mcl-1 is an anti-apoptotic member (8). The combination of PI3K and MEK
inhibition causes concomitant downregulation of Mcl-1 (26). Upregulation of Mcl-1 rendered
anoikis-resistance in cancers, while downregulation of Mcl-1
increased sensitivity of cancer cells to anoikis (30). Similarly to those findings,
expression of Mcl-1 as well as metastasis-related genes VEGF and
MMP-9 were upregulated in anoikis-resistant ACHN cells in the
present study.
TrkB is closely correlated with tumor progression,
anoikis-resistance, metastasis and response to chemotherapy
(17). Binding of BDNF to TrkB
leads to auto-phosphorylation of tyrosines in the intracellular
domain (31,32). Overexpression of TrkB activates
protein kinase B to block anoikis through PI3K signaling (16,33).
It was reported that Trk/PI3K/Akt pathway played an important role
in anoikis-resistance and invasion of cancer cells (17). Activated TrkB induced EMT and
enhanced tumor migration and invasion through MAPK-dependent
Twist-Snail axis (34). The above
evidence indicated that TrkB may serve as a potential therapeutic
target for cancers. The present study illustrated that expression
of TrkB was upregulated in anoikis-resistant ACHN cells, which
implied that TrkB might act as an oncogene. We revealed that TrkB
silencing increased detachment-induce apoptosis, inhibited
proliferation and invasion in anoikis-resistant ACHN cells,
together with downregulated expression of Mcl-1, VEGF, MMP-9 and
reduced activity of Akt and ERK.
Sorafenib is an orally administered small molecule
TKI and has improved progression-free survival for advanced RCC
patients (35). However, it
presented only partial response rates partly due to dose-related
adverse events and clinical toxicities (7,36,37).
In this study, TrkB silencing enhanced anti-proliferative,
pro-apoptotic and anti-invasive effects of sorafenib at a
sub-threshold concentration in anoikis-resistant ACHN cells through
inhibiting the activity of Akt and ERK. Based on our data,
combination of TrkB silencing and sorafenib may be an alternative
to clinical management of mRCC by enhancing the therapeutic effect
of sorafenib and reducing dosage-dependent adverse events.
In summary, the present study revealed that TrkB was
upregulated in anoikis-resistant ACHN cells, and silencing of TrkB
reversed anoikis-resistance and inhibited invasion in ACHN cells;
whereas, silencing of TrkB improved anti-cancer efficiency of
sorafenib in anoikis-resistant ACHN cells through inactivating
PI3K/Akt and MEK/ERK pathways. Our finding might offer a novel
potential therapeutic strategy for mRCC and deserve further
investigations.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 30973008 and
81272847 to Y.X.) and the Program for New Century Excellent Talents
in University of Ministry of Education of China (no. NCET-13-0239
to Y.X.).
References
|
1
|
Ljungberg B, Campbell SC, Choi HY, Jacqmin
D, Lee JE, Weikert S and Kiemeney LA: The epidemiology of renal
cell carcinoma. Eur Urol. 60:615–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim DY, Karam JA and Wood CG: Role of
metastasectomy for metastatic renal cell carcinoma in the era of
targeted therapy. World J Urol. 32:631–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ridge CA, Pua BB and Madoff DC:
Epidemiology and staging of renal cell carcinoma. Semin Intervent
Radiol. 31:3–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ratain MJ, Eisen T, Stadler WM, Flaherty
KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, et
al: Phase II placebo-controlled randomized discontinuation trial of
sorafenib in patients with metastatic renal cell carcinoma. J Clin
Oncol. 24:2505–2512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zustovich F, Lombardi G, Pastorelli D,
Farina P, Bianco MD, De Zorzi L, Palma MD, Nicoletto O and Zagonel
V: Clinical experience and critical evaluation of the role of
sorafenib in renal cell carcinoma. Open Access J Urol. 3:69–82.
2011.PubMed/NCBI
|
|
6
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al; TARGET Study Group. Sorafenib in advanced clear-cell
renal-cell carcinoma. N Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bianchi L, Rossi L, Tomao F, Papa A,
Zoratto F and Tomao S: Thyroid dysfunction and tyrosine kinase
inhibitors in renal cell carcinoma. Endocr Relat Cancer.
20:R233–R245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiarugi P and Giannoni E: Anoikis: A
necessary death program for anchorage-dependent cells. Biochem
Pharmacol. 76:1352–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong X and Rescorla FJ: Cell surface
adhesion molecules and adhesion-initiated signaling: Understanding
of anoikis resistance mechanisms and therapeutic opportunities.
Cell Signal. 24:393–401. 2012. View Article : Google Scholar
|
|
10
|
Simpson CD, Anyiwe K and Schimmer AD:
Anoikis resistance and tumor metastasis. Cancer Lett. 272:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jenning S, Pham T, Ireland SK, Ruoslahti E
and Biliran H: Bit1 in anoikis resistance and tumor metastasis.
Cancer Lett. 333:147–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bouillez A, Gnemmi V, Gaudelot K, Hémon B,
Ringot B, Pottier N, Glowacki F, Butruille C, Cauffiez C, Hamdane
M, et al: MUC1-C nuclear localization drives invasiveness of renal
cancer cells through a sheddase/gamma secretase dependent pathway.
Oncotarget. 5:754–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakamoto S, Schwarze S and Kyprianou N:
Anoikis disruption of focal adhesion-Akt signaling impairs renal
cell carcinoma. Eur Urol. 59:734–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stoilov P, Castren E and Stamm S: Analysis
of the human TrkB gene genomic organization reveals novel TrkB
isoforms, unusual gene length, and splicing mechanism. Biochem
Biophys Res Commun. 290:1054–1065. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Glass DJ, Nye SH, Hantzopoulos P, Macchi
MJ, Squinto SP, Goldfarb M and Yancopoulos GD: TrkB mediates
BDNF/NT-3-dependent survival and proliferation in fibroblasts
lacking the low affinity NGF receptor. Cell. 66:405–413. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Douma S, Van Laar T, Zevenhoven J,
Meuwissen R, Van Garderen E and Peeper DS: Suppression of anoikis
and induction of metastasis by the neurotrophic receptor TrkB.
Nature. 430:1034–1039. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiele CJ, Li Z and McKee AE: On Trk - the
TrkB signal transduction pathway is an increasingly important
target in cancer biology. Clin Cancer Res. 15:5962–5967. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST
and Poon RT: Brain-derived neurotrophic factor promotes
tumorigenesis via induction of neovascularization: implication in
hepatocellular carcinoma. Clin Cancer Res. 17:3123–3133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sinkevicius KW, Kriegel C, Bellaria KJ,
Lee J, Lau AN, Leeman KT, Zhou P, Beede AM, Fillmore CM, Caswell D,
et al: Neurotrophin receptor TrkB promotes lung adenocarcinoma
metastasis. Proc Natl Acad Sci USA. 111:10299–10304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fujikawa H, Tanaka K, Toiyama Y, Saigusa
S, Inoue Y, Uchida K and Kusunoki M: High TrkB expression levels
are associated with poor prognosis and EMT induction in colorectal
cancer cells. J Gastroenterol. 47:775–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sclabas GM, Fujioka S, Schmidt C, Li Z,
Frederick WA, Yang W, Yokoi K, Evans DB, Abbruzzese JL, et al:
Overexpression of tropomysin-related kinase B in metastatic human
pancreatic cancer cells. Clin Cancer Res. 11:440–449.
2005.PubMed/NCBI
|
|
22
|
Mawji IA, Simpson CD, Hurren R, Gronda M,
Williams MA, Filmus J, Jonkman J, Da Costa RS, Wilson BC, Thomas
MP, et al: Critical role for Fas-associated death domain-like
interleukin-1-converting enzyme-like inhibitory protein in anoikis
resistance and distant tumor formation. J Natl Cancer Inst.
99:811–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Geiger TR and Peeper DS: The neurotrophic
receptor TrkB in anoikis resistance and metastasis: A perspective.
Cancer Res. 65:7033–7036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liotta LA and Kohn E: Anoikis: Cancer and
the homeless cell. Nature. 430:973–974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar :
|
|
27
|
Montagut C and Settleman J: Targeting the
RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 283:125–134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frisch SM and Screaton RA: Anoikis
mechanisms. Curr Opin Cell Biol. 13:555–562. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shih YW, Shieh JM, Wu PF, Lee YC, Chen YZ
and Chiang TA: Alpha-tomatine inactivates PI3K/Akt and ERK
signaling pathways in human lung adenocarcinoma A549 cells: effect
on metastasis. Food Chem Toxicol. 47:1985–1995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wongpankam E, Chunhacha P, Pongrakhananon
V, Sritularak B and Chanvorachote P: Artonin E mediates MCL1
down-regulation and sensitizes lung cancer cells to anoikis.
Anticancer Res. 32:5343–5351. 2012.PubMed/NCBI
|
|
31
|
Klein R, Nanduri V, Jing SA, Lamballe F,
Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF and
Barbacid M: The trkB tyrosine protein kinase is a receptor for
brain-derived neurotrophic factor and neurotrophin-3. Cell.
66:395–403. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pearse RN, Swendeman SL, Li Y, Rafii D and
Hempstead BL: A neurotrophin axis in myeloma: TrkB and BDNF promote
tumor-cell survival. Blood. 105:4429–4436. 2005. View Article : Google Scholar
|
|
33
|
Yu X, Liu L, Cai B, He Y and Wan X:
Suppression of anoikis by the neurotrophic receptor TrkB in human
ovarian cancer. Cancer Sci. 99:543–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smit MA and Peeper DS: Zeb1 is required
for TrkB-induced epithelial-mesenchymal transition, anoikis
resistance and metastasis. Oncogene. 30:3735–3744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ramnath N and Adjei A: Inhibitors of Raf
kinase and MEK signaling. Update Cancer Ther. 2:111–118. 2007.
View Article : Google Scholar
|
|
36
|
Kim MJ, Kim DE, Jeong IG, Choi J, Jang S,
Lee JH, Ro S, Hwang JJ and Kim CS: HDAC inhibitors synergize
antiproliferative effect of sorafenib in renal cell carcinoma
cells. Anticancer Res. 32:3161–3168. 2012.PubMed/NCBI
|
|
37
|
Srikanthan A, Ethier JL, Ocana A, Seruga
B, Krzyzanowska MK and Amir E: Cardiovascular toxicity of
multi-tyrosine kinase inhibitors in advanced solid tumors: A
population-based observational study. PLoS One. 10:e01227352015.
View Article : Google Scholar : PubMed/NCBI
|