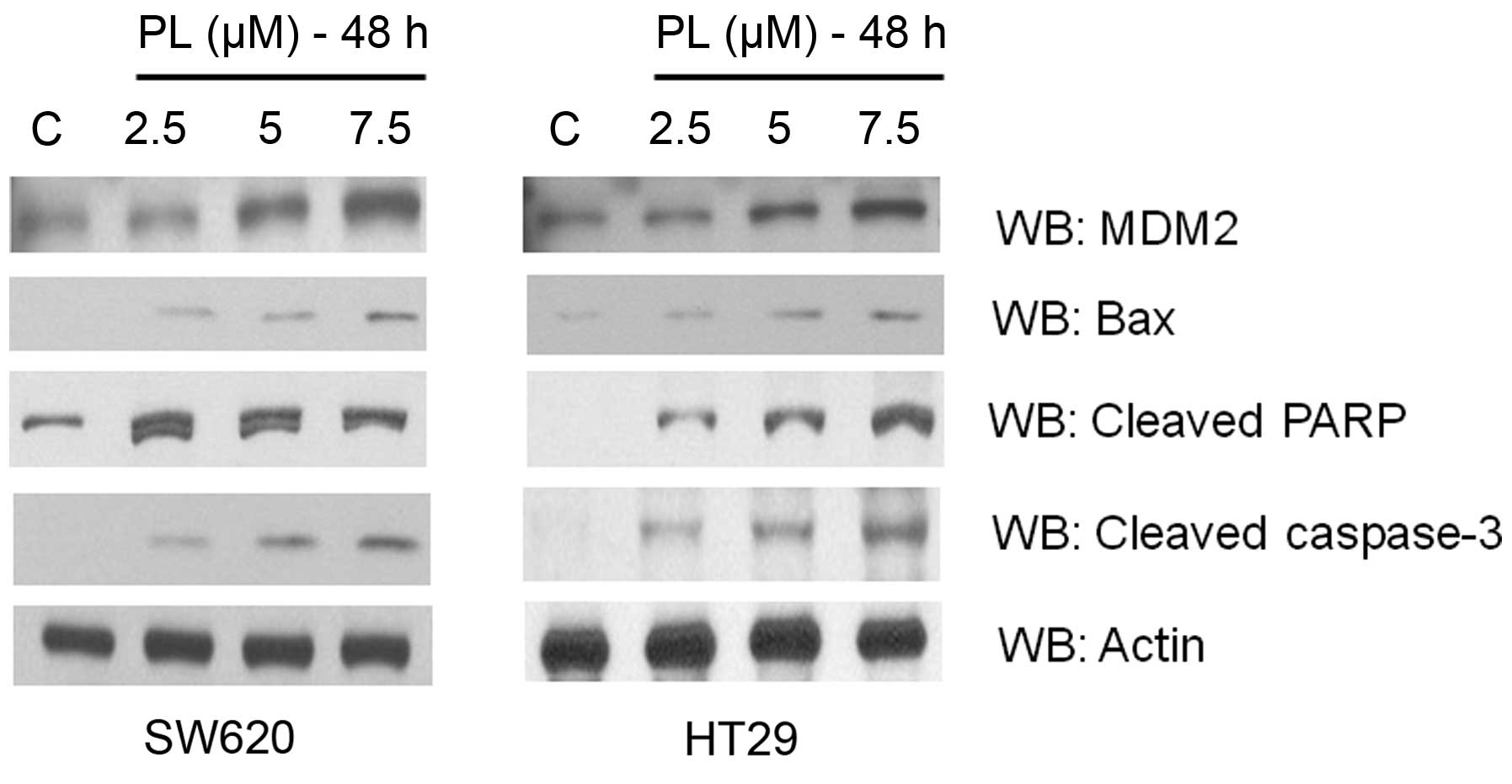
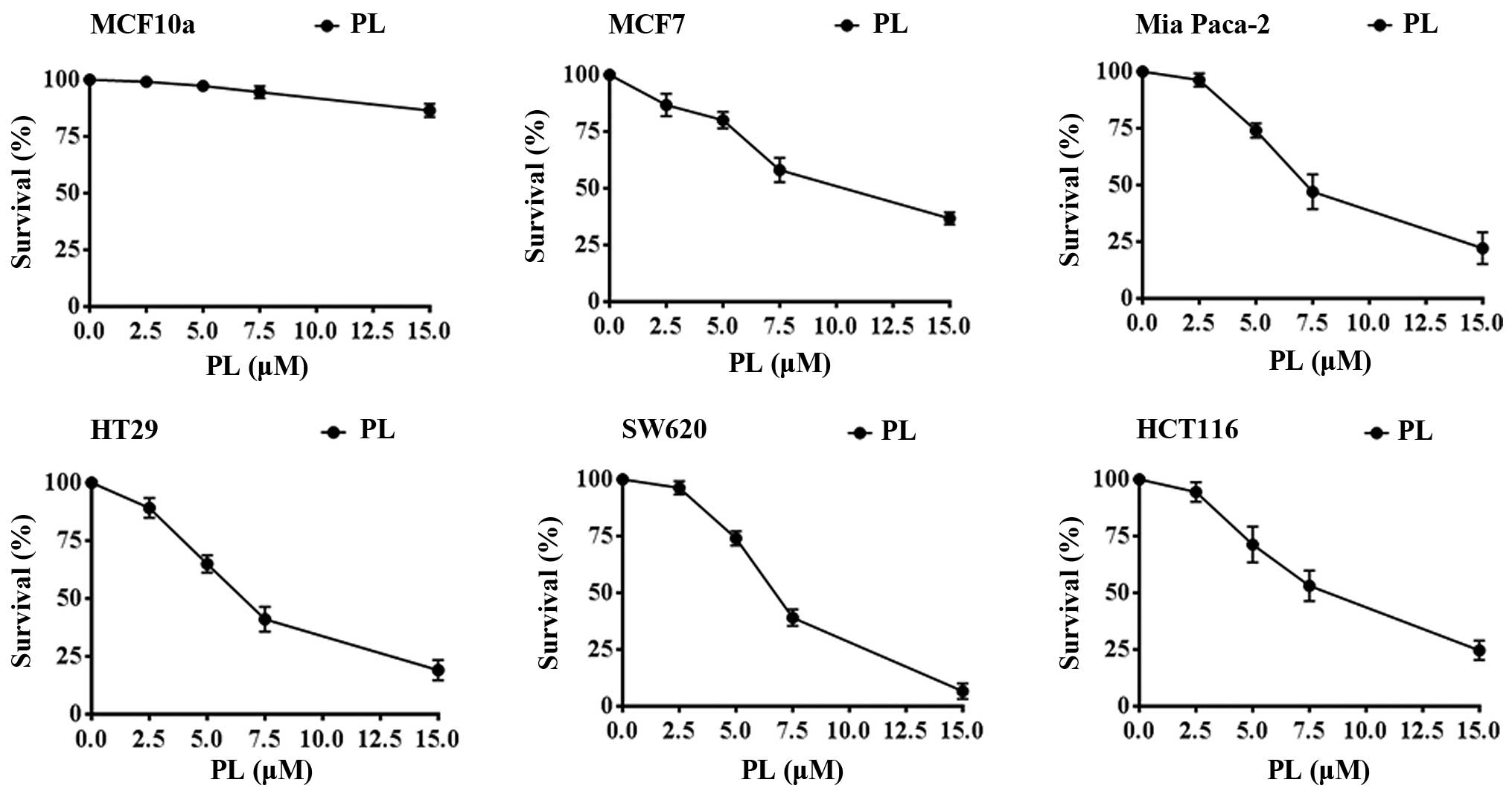
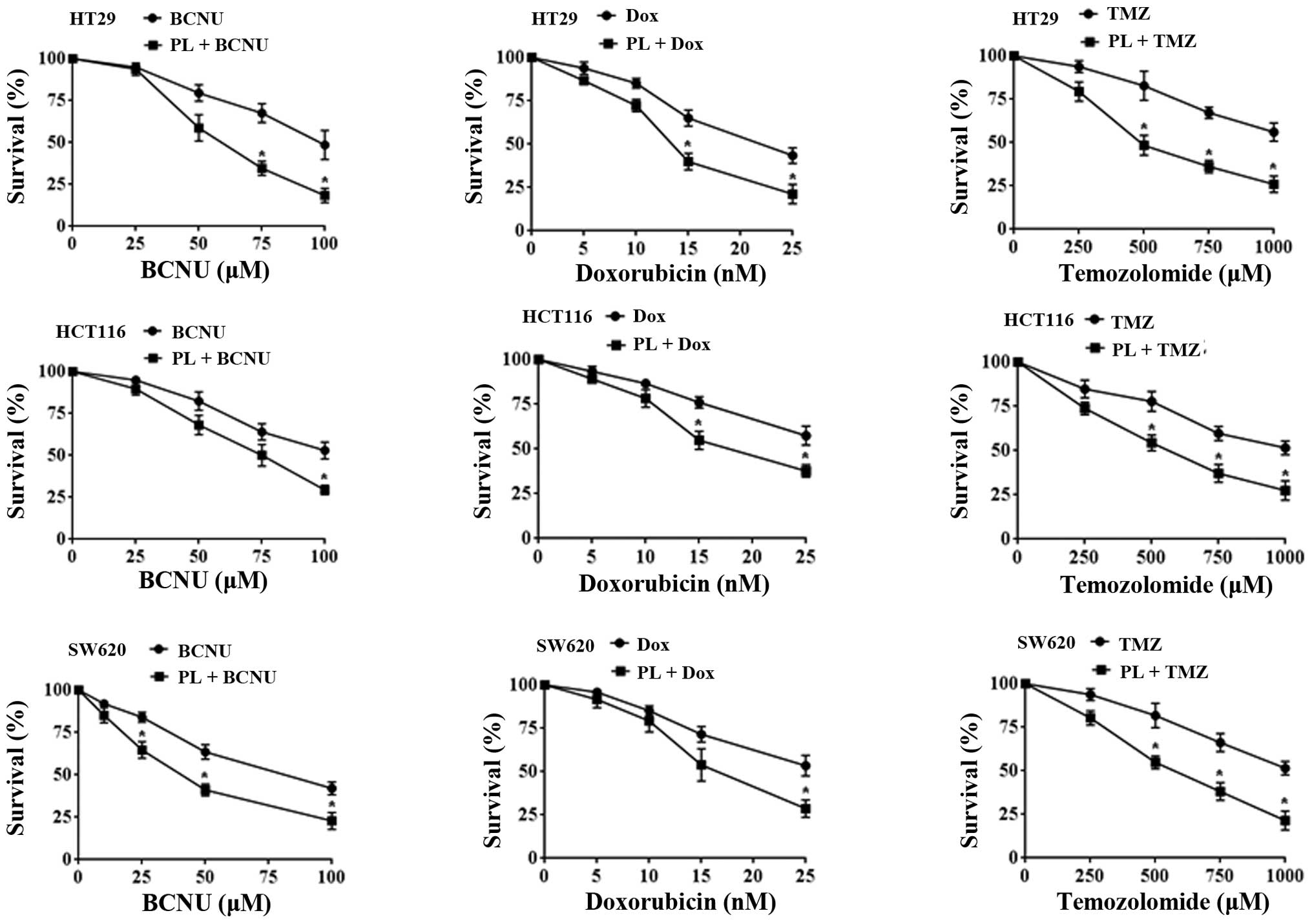
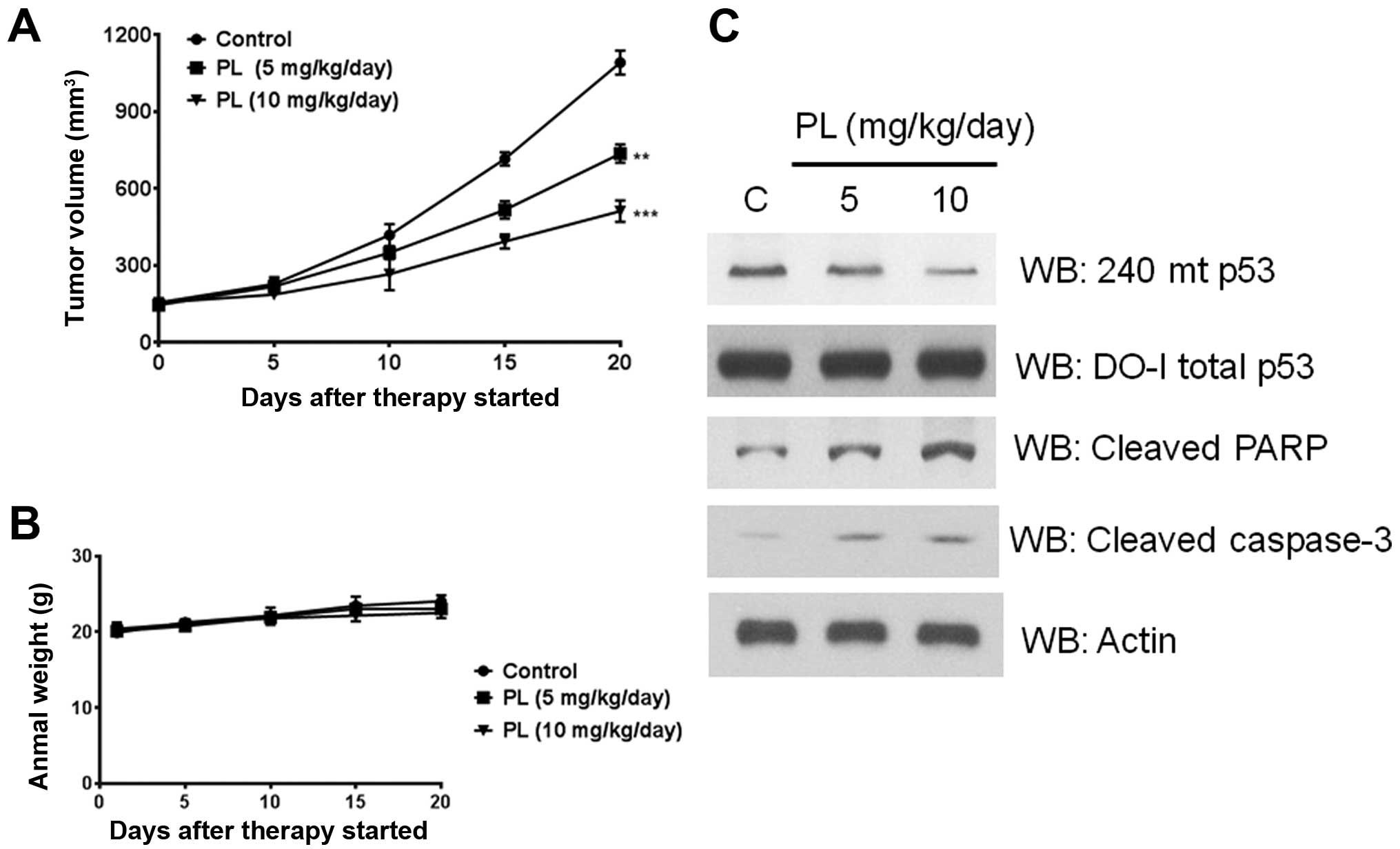
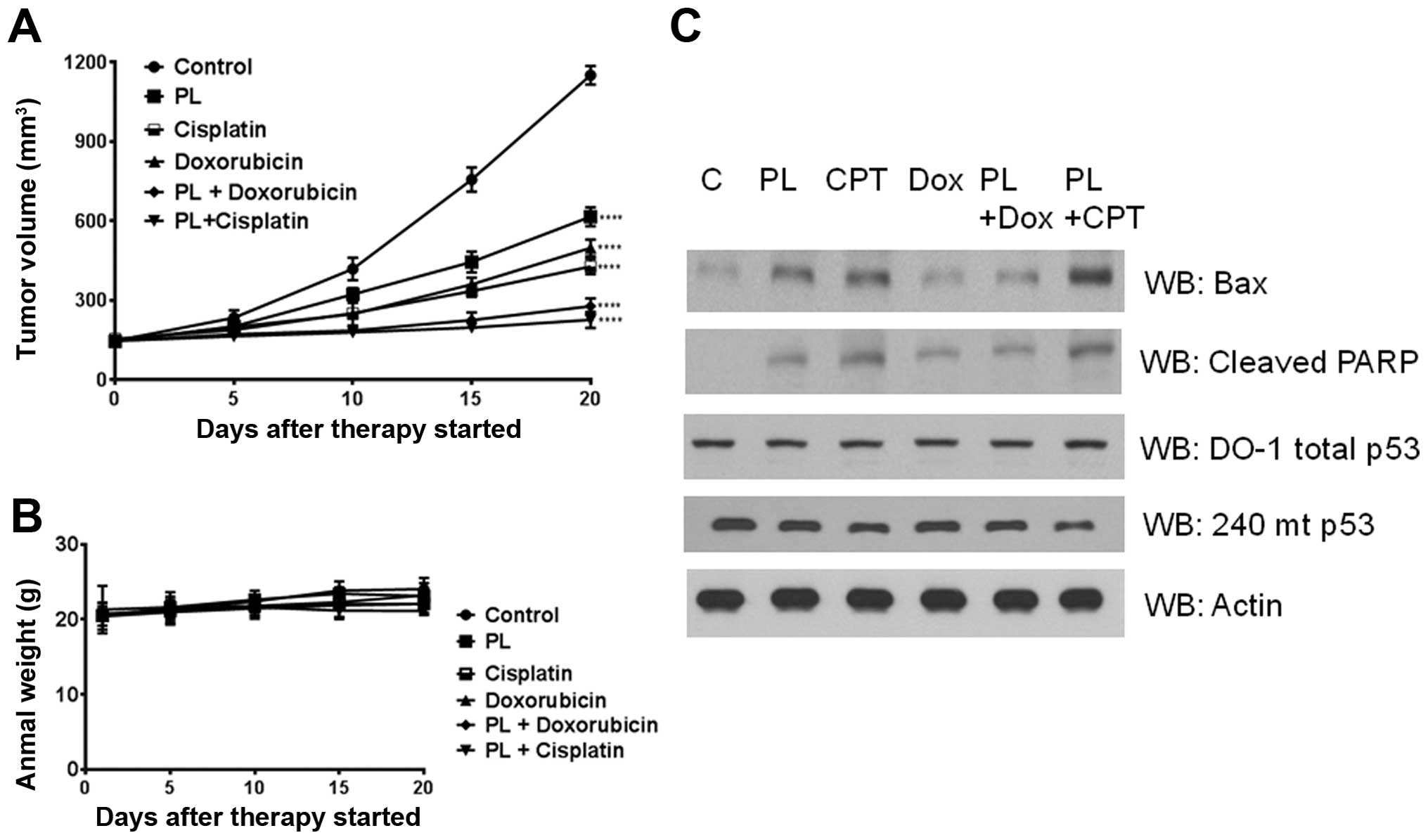
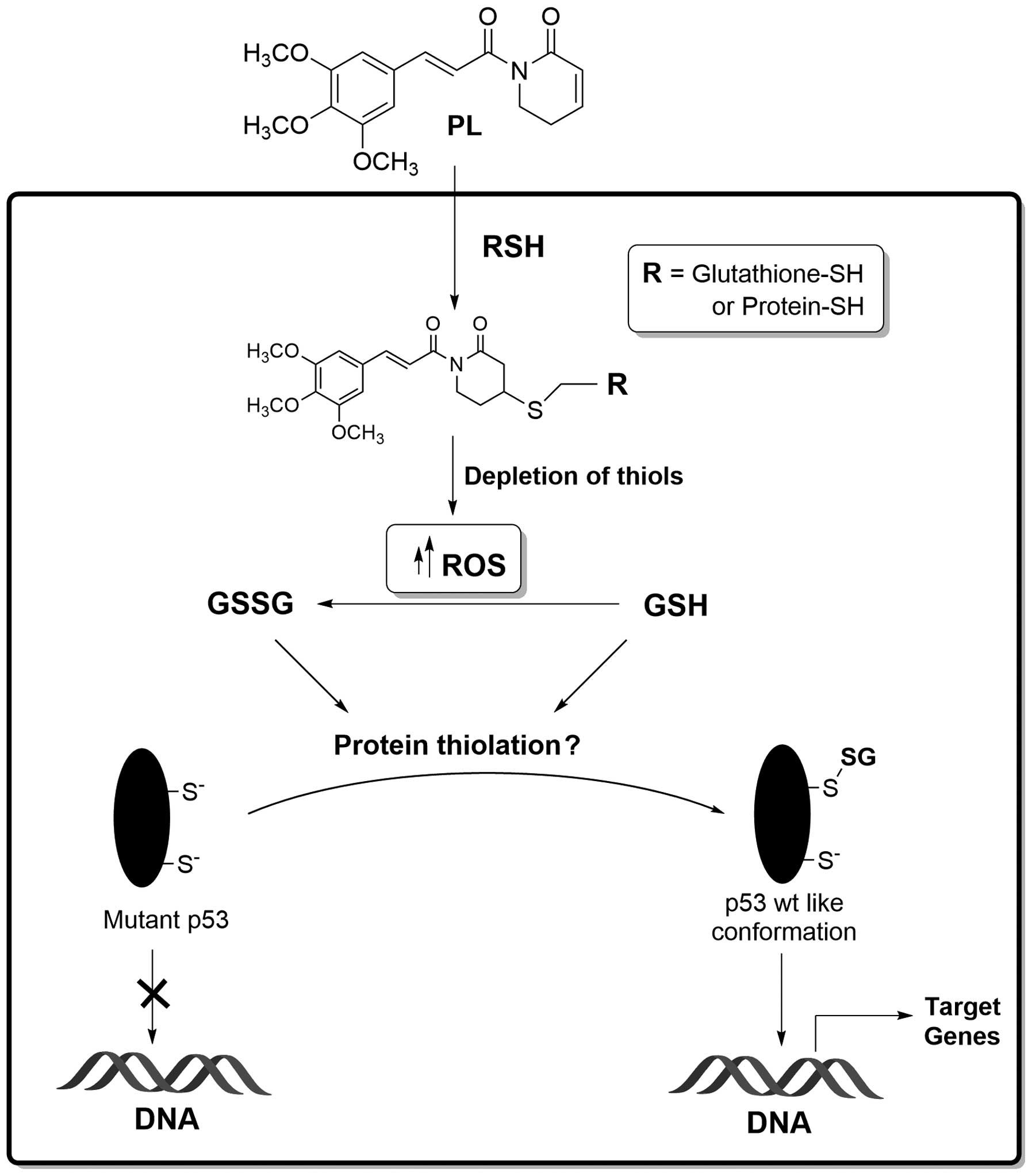
|
1
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and LLeonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar
|
|
3
|
Raj L, Ide T, Gurkar AU, Foley M, Schenone
M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al:
Selective killing of cancer cells by a small molecule targeting the
stress response to ROS. Nature. 475:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Adams DJ, Dai M, Pellegrino G, Wagner BK,
Stern AM, Shamji AF and Schreiber SL: Synthesis, cellular
evaluation, and mechanism of action of piperlongumine analogs. Proc
Natl Acad Sci USA. 109:15115–15120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han JG, Gupta SC, Prasad S and Aggarwal
BB: Piperlongumine chemosensitizes tumor cells through interaction
with cysteine 179 of IκBα kinase, leading to suppression of
NF-κB-regulated gene products. Mol Cancer Ther. 13:2422–2435. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polyak K, Xia Y, Zweier JL, Kinzler KW and
Vogelstein B: A model for p53-induced apoptosis. Nature.
389:300–305. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petitjean A, Mathe E, Kato S, Ishioka C,
Tavtigian SV, Hainaut P and Olivier M: Impact of mutant p53
functional properties on TP53 mutation patterns and tumor
phenotype: Lessons from recent developments in the IARC TP53
database. Hum Mutat. 28:622–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olivier M, Hollstein M and Hainaut P:
Hollstein M and Hainaut P: TP53 mutations in human cancers:
Origins, consequences, and clinical Use. Cold Spring Harb Perspect
Biol. 2:1–17. 2010. View Article : Google Scholar
|
|
9
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: Consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar
|
|
10
|
Bykov VJN and Wiman KG: Mutant p53
reactivation by small molecules makes its way to the clinic. FEBS
Lett. 588:2622–2627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiman KG: Strategies for therapeutic
targeting of the p53 pathway in cancer. Cell Death Differ.
13:921–926. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saha MN, Qiu L and Chang H: Targeting p53
by small molecules in hematological malignancies. J Hematol Oncol.
6:232013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bykov VJN, Issaeva N, Shilov A, Hultcrantz
M, Pugacheva E, Chumakov P, Bergman J, Wiman KG and Selivanova G:
Restoration of the tumor suppressor function to mutant p53 by a
low-molecular-weight compound. Nat Med. 8:282–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bykov VJN, Issaeva N, Zache N, Shilov A,
Hultcrantz M, Bergman J, Selivanova G and Wiman KG: Reactivation of
mutant p53 and induction of apoptosis in human tumor cells by
maleimide analogs. J Biol Chem. 280:30384–30391. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang X, Zhu Y, Han L, Kim AL, Kopelovich
L, Bickers DR and Athar M: CP-31398 restores mutant p53 tumor
suppressor function and inhibits UVB-induced skin carcinogenesis in
mice. J Clin Invest. 117:3753–3764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zache N, Lambert JMR, Rökaeus N, Shen J,
Hainaut P, Bergman J, Wiman KG and Bykov VJN: Mutant p53 targeting
by the low molecular weight compound STIMA-1. Mol Oncol. 2:70–80.
2008. View Article : Google Scholar
|
|
17
|
Demma M, Maxwell E, Ramos R, Liang L, Li
C, Hesk D, Rossman R, Mallams A, Doll R, Liu M, et al: SCH529074, a
small molecule activator of mutant p53, which binds p53 DNA binding
domain (DBD), restores growth-suppressive function to mutant p53
and interrupts HDM2-mediated ubiquitination of wild-type p53. J
Biol Chem. 285:10198–10212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu X, Vazquez A, Levine AJ and Carpizo DR:
Allele-specific p53 mutant reactivation. Cancer Cell. 21:614–625.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bykov VJN, Lambert JMR, Hainaut P and
Wiman KG: Mutant p53 rescue and modulation of p53 redox state. Cell
Cycle. 8:2509–2517. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Velu CS, Niture SK, Doneanu CE,
Pattabiraman N and Srivenugopal KS: Human p53 is inhibited by
glutathionylation of cysteines present in the proximal DNA-binding
domain during oxidative stress. Biochemistry. 46:7765–7780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yusuf MA, Chuang T, Bhat GJ and
Srivenugopal KS: Cys-141 glutathionylation of human p53: Studies
using specific polyclonal antibodies in cancer samples and cell
lines. Free Radic Biol Med. 49:908–917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Asch H and Kulesz-Martin MF:
Functional quantification of DNA-binding proteins p53 and estrogen
receptor in cells and tumor tissues by DNA affinity immunoblotting.
Cancer Res. 61:5402–5406. 2001.PubMed/NCBI
|
|
23
|
Gallogly MM and Mieyal JJ: Mechanisms of
reversible protein glutathionylation in redox signaling and
oxidative stress. Curr Opin Pharmacol. 7:381–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghezzi P, Bonetto V and Fratelli M:
Thiol-disulfide balance: From the concept of oxidative stress to
that of redox regulation. Antioxid Redox Signal. 7:964–972. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klatt P and Lamas S: Regulation of protein
function by S-glutathiolation in response to oxidative and
nitrosative stress. Eur J Biochem. 267:4928–4944. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Sawaf O, Clarner T, Fragoulis A, Kan
YW, Pufe T, Streetz K and Wruck CJ: Nrf2 in health and disease:
Current and future clinical implications. Clin Sci (Lond).
129:989–999. 2015. View Article : Google Scholar
|
|
27
|
Brandt R and Keston AS: Synthesis of
diacetyldichlorofluorescin: A stable reagent for fluorometric
analysis. Anal Biochem. 11:6–9. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z and Sun Y: Targeting p53 for novel
anticancer therapy. Transl Oncol. 3:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rainwater R, Parks D, Anderson ME,
Tegtmeyer P and Mann K: Role of cysteine residues in regulation of
p53 function. Mol Cell Biol. 15:3892–3903. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Y and Prives C: Are interactions with
p63 and p73 involved in mutant p53 gain of oncogenic function?
Oncogene. 26:2220–2225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonsing BA, Corver WE, Gorsira MCB, van
Vliet M, Oud PS, Cornelisse CJ and Fleuren GJ: Specificity of seven
monoclonal antibodies against p53 evaluated with Western blotting,
immunohistochemistry, confocal laser scanning microscopy, and flow
cytometry. Cytometry. 28:11–24. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang PL, Sait F and Winter G: The
‘wildtype’ conformation of p53: Epitope mapping using hybrid
proteins. Oncogene. 20:2318–2324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gannon JV, Greaves R, Iggo R and Lane DP:
Activating mutations in p53 produce a common conformational effect.
A monoclonal antibody specific for the mutant form. EMBO J.
9:1595–1602. 1990.PubMed/NCBI
|
|
34
|
Niture SK, Velu CS, Bailey NI and
Srivenugopal KS: S-thiolation mimicry: Quantitative and kinetic
analysis of redox status of protein cysteines by
glutathione-affinity chromatography. Arch Biochem Biophys.
444:174–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Punganuru SR, Madala HR, Venugopal SN,
Samala R, Mikelis C and Srivenugopal KS: Design and synthesis of a
C7-aryl piperlongumine derivative with potent antimicrotubule and
mutant p53-reactivating properties. Eur J Med Chem. 107:233–244.
2016. View Article : Google Scholar
|
|
36
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|