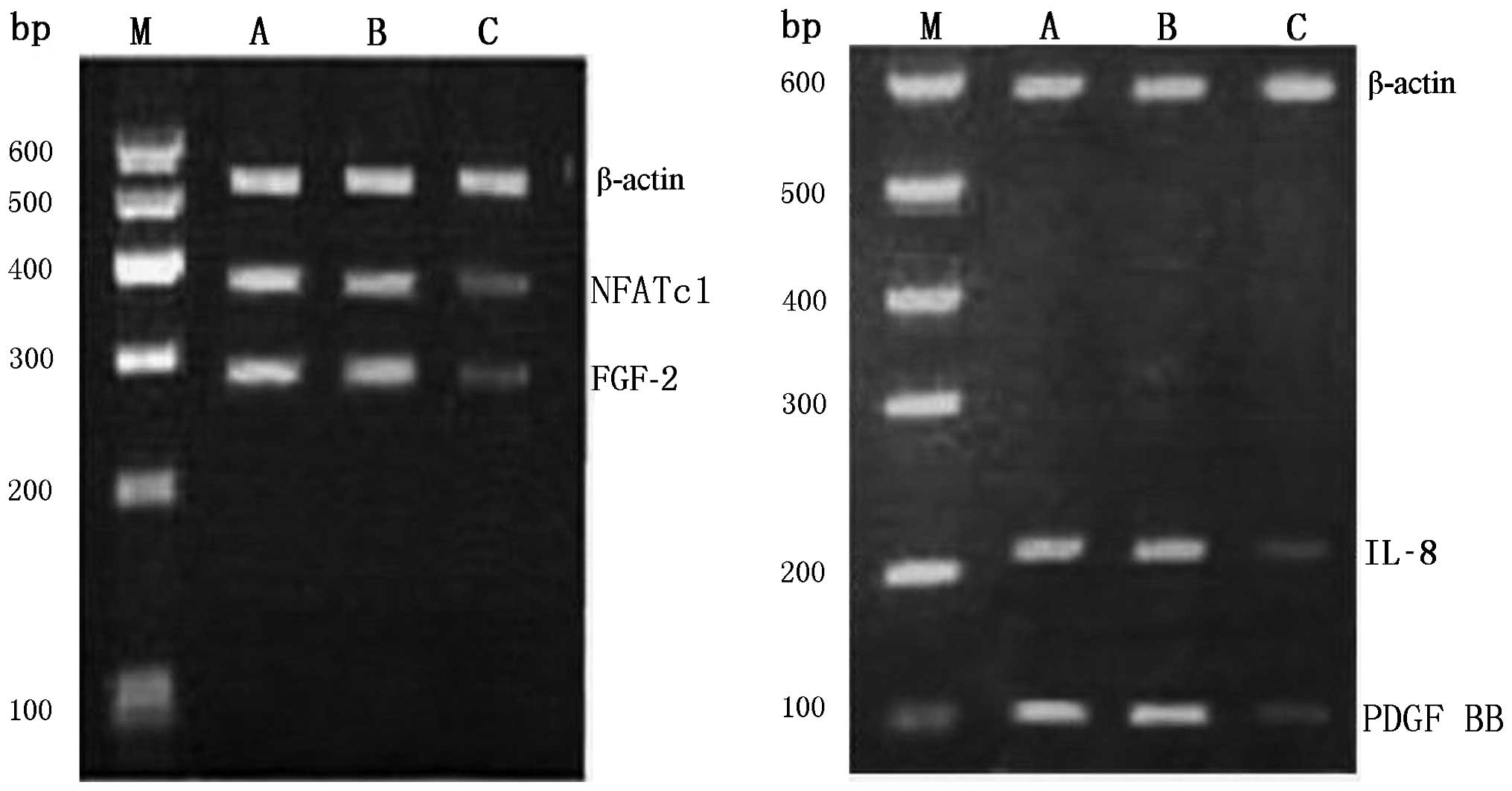
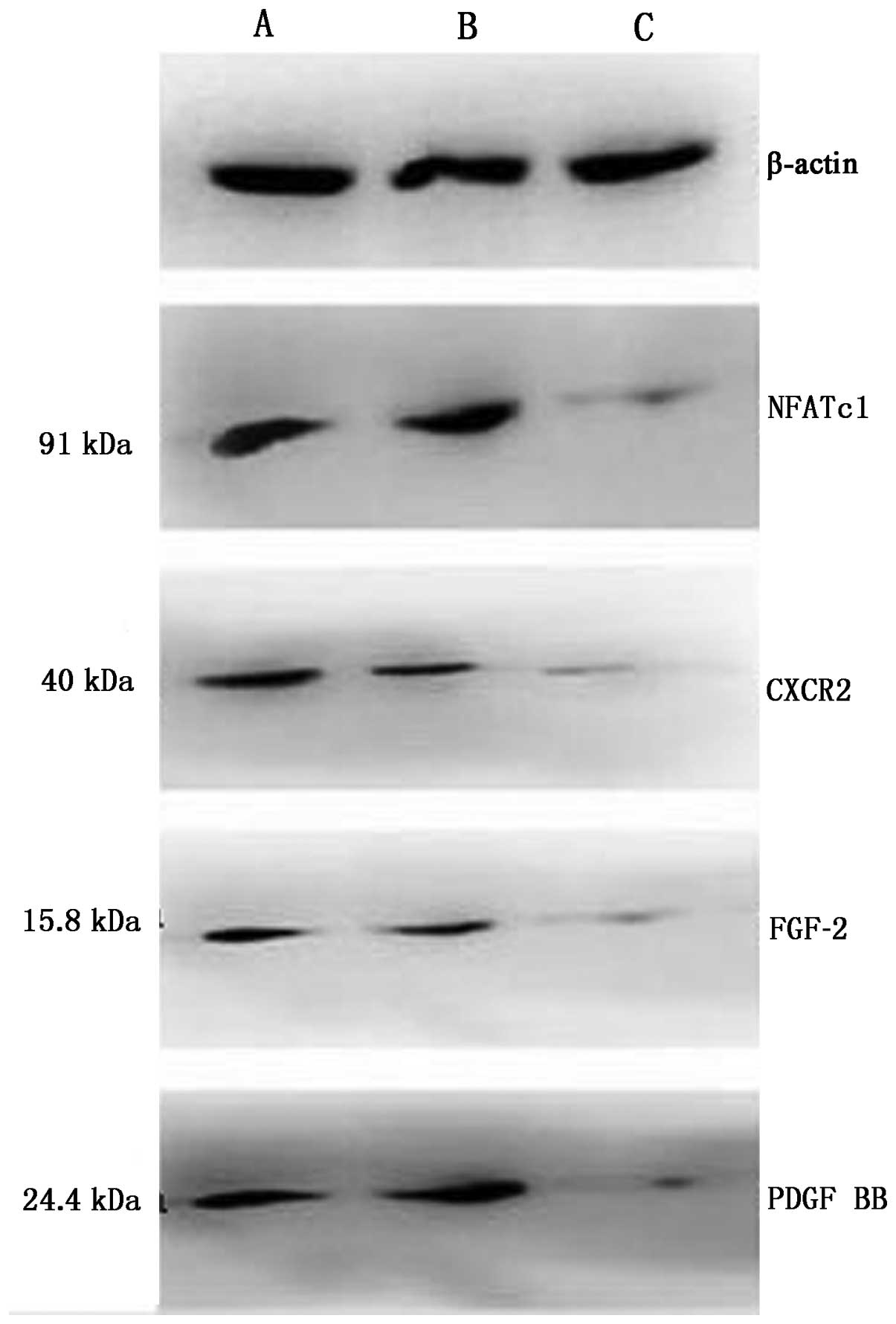
|
1
|
Naito T, Tanaka H, Naoe Y and Taniuchi I:
Transcriptional control of T-cell development. Int Immunol.
23:661–668. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen L, Glover JN, Hogan PG, Rao A and
Harrison SC: Structure of the DNA-binding domains from NFAT, Fos
and Jun bound specifically to DNA. Nature. 392:42–48. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain J, McCaffrey PG, Valge-Archer VE and
Rao A: Nuclear factor of activated T cells contains Fos and Jun.
Nature. 356:801–804. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bengsch B and Wherry EJ: The importance of
cooperation: partnerless NFAT induces T cell exhaustion. Immunity.
42:203–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaw JP, Utz PJ, Durand DB, Toole JJ,
Emmel EA and Crabtree GR: Identification of a putative regulator of
early T cell activation genes. Science. 241:202–205. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bai S and Kerppola TK: Opposing roles of
FoxP1 and Nfat3 in transcriptional control of cardiomyocyte
hypertrophy. Mol Cell Biol. 31:3068–3080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen NM, Singh G, Koenig A, Liou GY, Storz
P, Zhang JS, Regul L, Nagarajan S, Kühnemuth B, Johnsen SA, et al:
NFATc1 links EGFR signaling to induction of Sox9 transcription and
acinar-ductal transdifferentiation in the pancreas.
Gastroenterology. 148:1024–1034.e9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hashizume M, Hayakawa N and Mihara M: IL-6
trans-signalling directly induces RANKL on fibroblast-like synovial
cells and is involved in RANKL induction by TNF-alpha and IL-17.
Rheumatology (Oxford). 47:1635–1640. 2008. View Article : Google Scholar
|
|
10
|
Wang S, Kang X, Cao S, Cheng H, Wang D and
Geng J: Calcineurin/NFATc1 pathway contributes to cell
proliferation in hepatocellular carcinoma. Dig Dis Sci.
57:3184–3188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beals CR, Sheridan CM, Turck CW, Gardner P
and Crabtree GR: Nuclear export of NF-ATc enhanced by glycogen
synthase kinase-3. Science. 275:1930–1934. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Köenig A, Linhart T, Schlengemann K,
Reutlinger K, Wegele J, Adler G, Singh G, Hofmann L, Kunsch S, Büch
T, et al: NFAT-induced histone acetylation relay switch promotes
c-Myc-dependent growth in pancreatic cancer cells.
Gastroenterology. 138:1189–1199.e1-2. 2010. View Article : Google Scholar
|
|
13
|
Neal JW and Clipstone NA: A constitutively
active NFATc1 mutant induces a transformed phenotype in 3T3-L1
fibroblasts. J Biol Chem. 278:17246–17254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang SD, McCrudden CM, Meng C, Lin Y and
Kwok HF: The significance of combining VEGFA, FLT1, and KDR
expressions in colon cancer patient prognosis and predicting
response to bevacizumab. Onco Targets Ther. 8:835–843.
2015.PubMed/NCBI
|
|
15
|
Wu B, Baldwin HS and Zhou B: Nfatc1
directs the endocardial progenitor cells to make heart valve
primordium. Trends Cardiovasc Med. 23:294–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu B, Wang Y, Lui W, Langworthy M,
Tompkins KL, Hatzopoulos AK, Baldwin HS and Zhou B: Nfatc1
coordinates valve endocardial cell lineage development required for
heart valve formation. Circ Res. 109:183–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adinolfi E, Raffaghello L, Giuliani AL,
Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V
and Di Virgilio F: Expression of P2X7 receptor increases in vivo
tumor growth. Cancer Res. 72:2957–2969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shoemaker LD, Fuentes LF, Santiago SM,
Allen BM, Cook DJ, Steinberg GK and Chang SD: Human brain
arteriovenous malformations express lymphatic-associated genes. Ann
Clin Transl Neurol. 1:982–995. 2014. View
Article : Google Scholar
|
|
19
|
Chen HM, Tsai CH and Hung WC: Foretinib
inhibits angiogenesis, lymphangiogenesis and tumor growth of
pancreatic cancer in vivo by decreasing VEGFR-2/3 and TIE-2
signaling. Oncotarget. 6:14940–14952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kähärä J and Lähdesmäki H: Evaluating a
linear k-mer model for protein-DNA interactions using
high-throughput SELEX data. BMC Bioinformatics. 14(Suppl 10):
S22013.
|
|
21
|
Kulkarni RM, Greenberg JM and Akeson AL:
NFATc1 regulates lymphatic endothelial development. Mech Dev.
126:350–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Norrmén C, Ivanov KI, Cheng J, Zangger N,
Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, et
al: FOXC2 controls formation and maturation of lymphatic collecting
vessels through cooperation with NFATc1. J Cell Biol. 185:439–457.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JH, Bhang DH, Beede A, Huang TL,
Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S and Kim CF: Lung
stem cell differentiation in mice directed by endothelial cells via
a BMP4-NFATc1-thrombospondin-1 axis. Cell. 156:440–455. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jang GH, Park IS, Yang JH, Bischoff J and
Lee YM: Differential function of genes regulated by VEGF-NFATc1
signaling pathway in migration of pulmonary valve endothelial
cells. FEBS Lett. 584:141–146. 2010. View Article : Google Scholar :
|
|
25
|
Combs MD and Yutzey KE: VEGF and RANKL
regulation of NFATc1 in heart valve development. Circ Res.
105:565–574. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li WH, Qiu Y, Zhang HQ, Tian XX and Fang
WG: P2Y2 Receptor and EGFR cooperate to promote prostate cancer
cell invasion via ERK1/2 pathway. PLoS One. 10:e01331652015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moldobaeva A, Baek A, Eldridge L and
Wagner EM: Differential activity of pro-angiogenic CXC chemokines.
Microvasc Res. 80:18–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joimel U, Gest C, Soria J, Pritchard LL,
Alexandre J, Laurent M, Blot E, Cazin L, Vannier JP, Varin R, et
al: Stimulation of angiogenesis resulting from cooperation between
macrophages and MDA-MB-231 breast cancer cells: Proposed molecular
mechanism and effect of tetrathiomolybdate. BMC Cancer. 10:3752010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarmiento J, Shumate C, Suetomi K,
Ravindran A, Villegas L, Rajarathnam K and Navarro J: Diverging
mechanisms of activation of chemokine receptors revealed by novel
chemokine agonists. PLoS One. 6:e279672011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rabquer BJ, Tsou PS, Hou Y,
Thirunavukkarasu E, Haines GK III, Impens AJ, Phillips K, Kahaleh
B, Seibold JR and Koch AE: Dysregulated expression of MIG/CXCL9,
IP-10/CXCL10 and CXCL16 and their receptors in systemic sclerosis.
Arthritis Res Ther. 13:R182011. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caoa R, Jia H, Fenga N, et al:
Collaborative interplay between FGF-2 and VEGF-C promotes
lymphangiogenesis and metastasis. PNAS. 109:15894–15899. 2012.
View Article : Google Scholar
|
|
32
|
Cao R1, Björndahl MA, Religa P, et al:
PDGF-BB induces intratumoral lymphangiogenesis and promotes
lymphatic metastasis. Cancer Cell. 6:333–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Andrés G1, Leali D, Mitola S, et al: A
pro-inflammatory signature mediates FGF2-induced angiogenesis.
Molecular Medicine. 13:2083–2108. 2009.
|
|
34
|
Chang LK, Garcia-Cardeña G, Farnebo F,
Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J
and Kaipainen A: Dose-dependent response of FGF-2 for
lymhangiongenesis. Proc Natl Acad Sci USA. 101:11658–11663. 2004.
View Article : Google Scholar
|
|
35
|
Schoppmann SF, Alidzanovic L, Schultheis
A, Perkmann T, Brostjan C and Birner P: Thrombocytes correlate with
lymphangiogenesis in human esophageal cancer and mediate growth of
lymphatic endothelial cells in vitro. PLoS One. 8:e669412013.
View Article : Google Scholar :
|
|
36
|
Tung HC, Lee FY, Wang SS, Tsai MH, Lee JY,
Huo TI, Huang HC, Chuang CL, Lin HC and Lee SD: The beneficial
effects of P2X7 antagonism in rats with bile duct ligation-induced
cirrhosis. PLoS One. 10:e01246542015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao Y, Wang Y, Wang Q, Liu Z, Liu Q and
Deng X: Hepatic stellate cells produce vascular endothelial growth
factor via phospho-p44/42 mitogen-activated protein
kinase/cyclooxygenase-2 pathway. Mol Cell Biochem. 359:217–223.
2012. View Article : Google Scholar
|