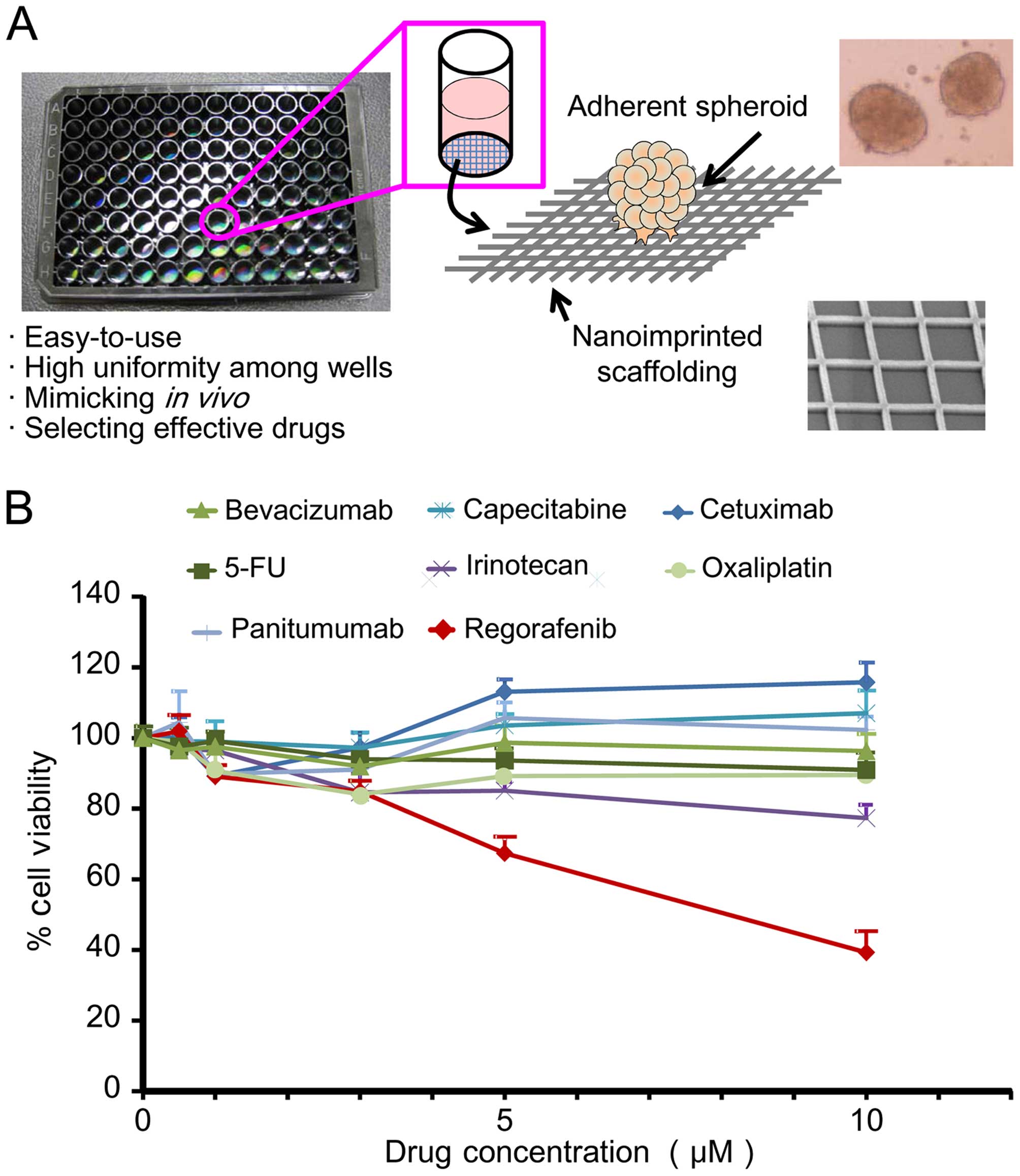
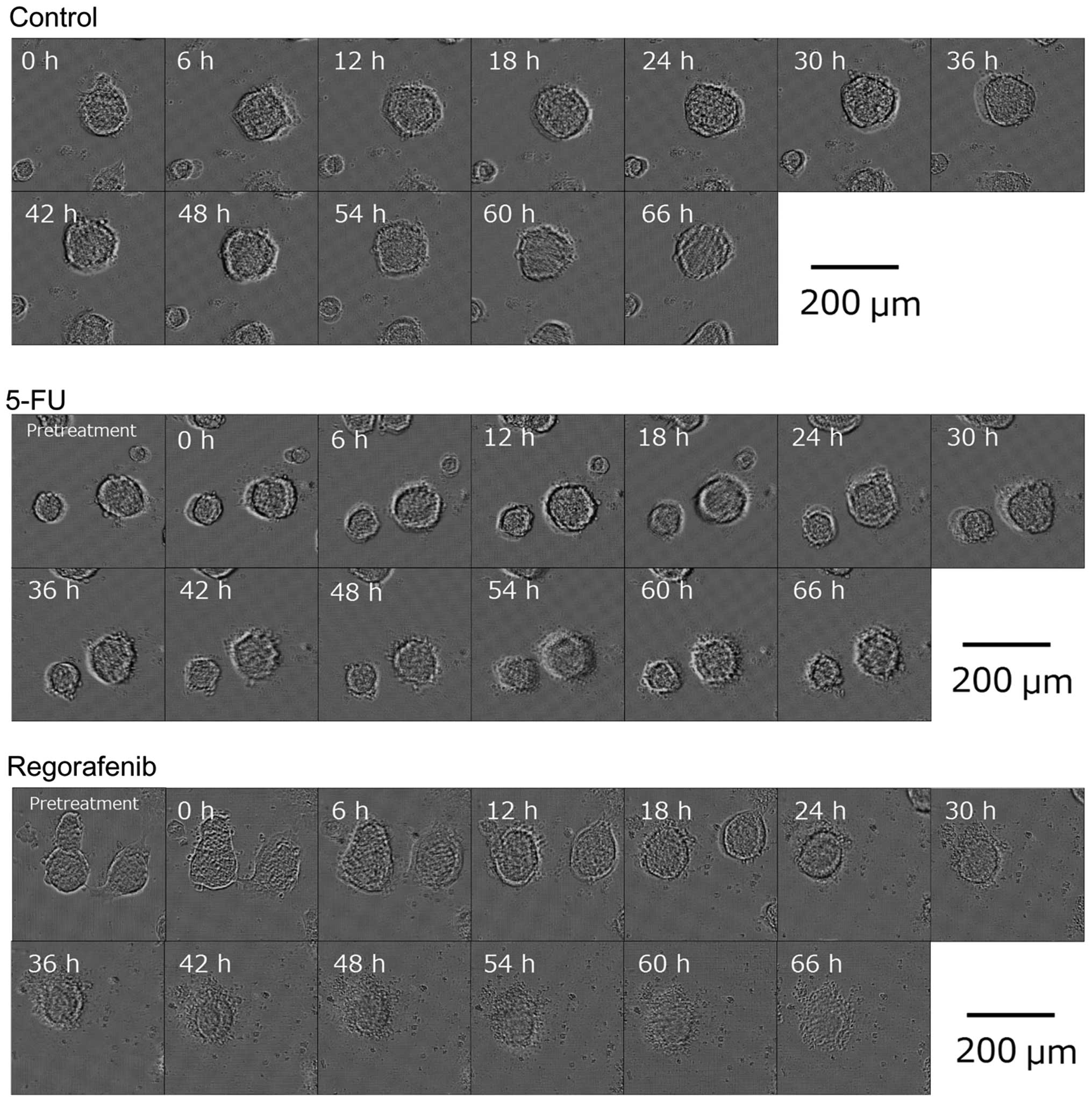
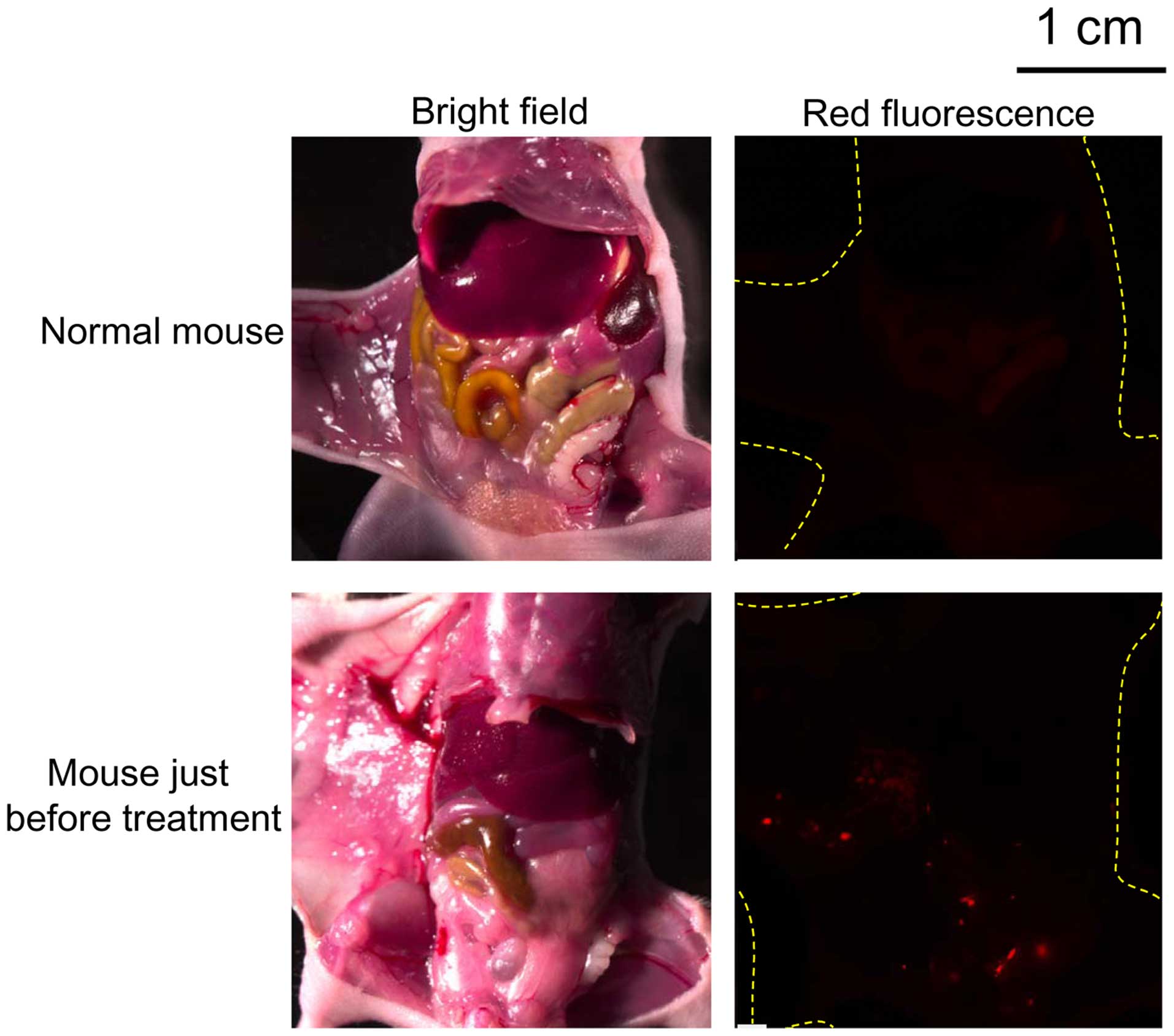
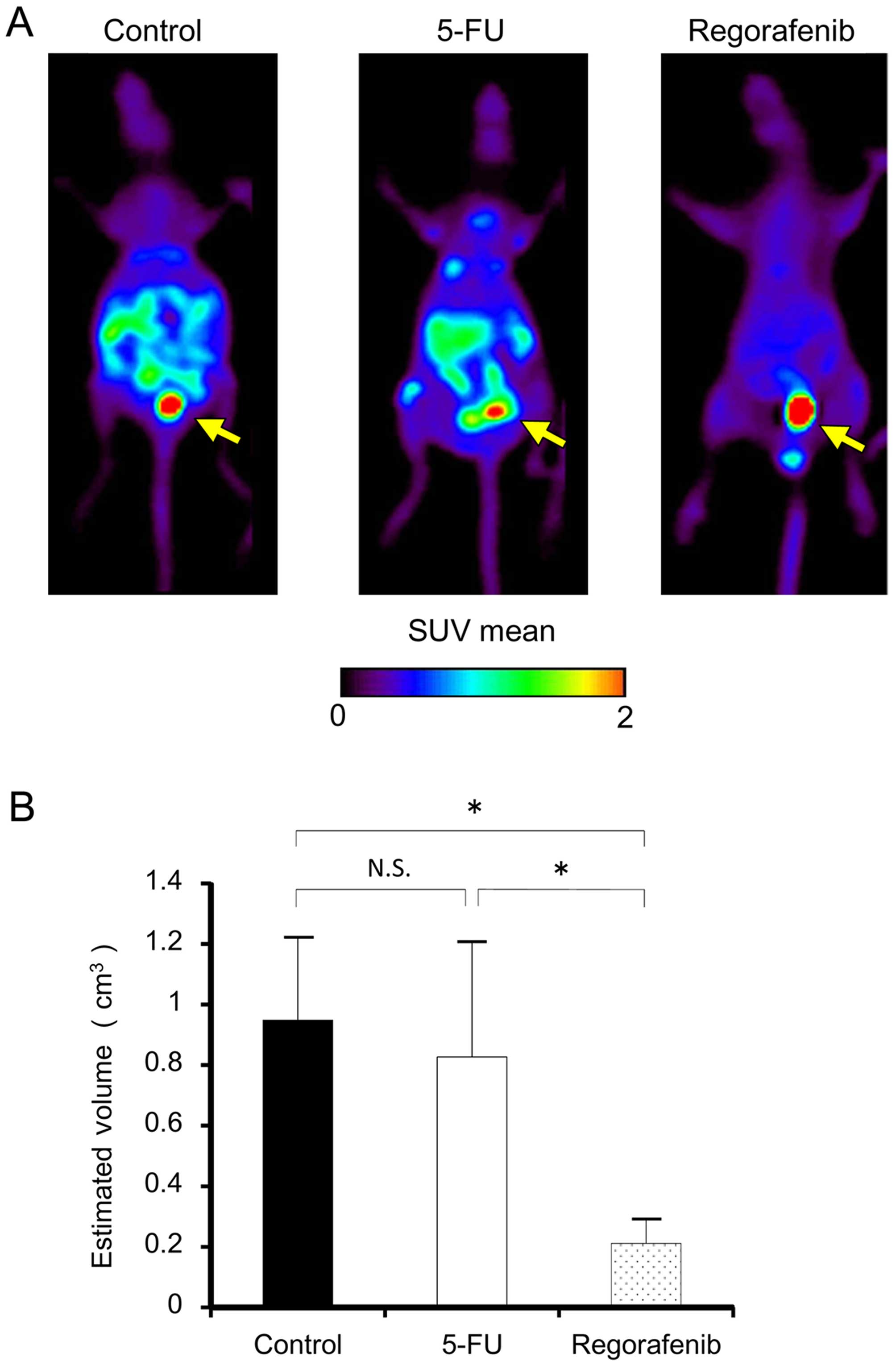
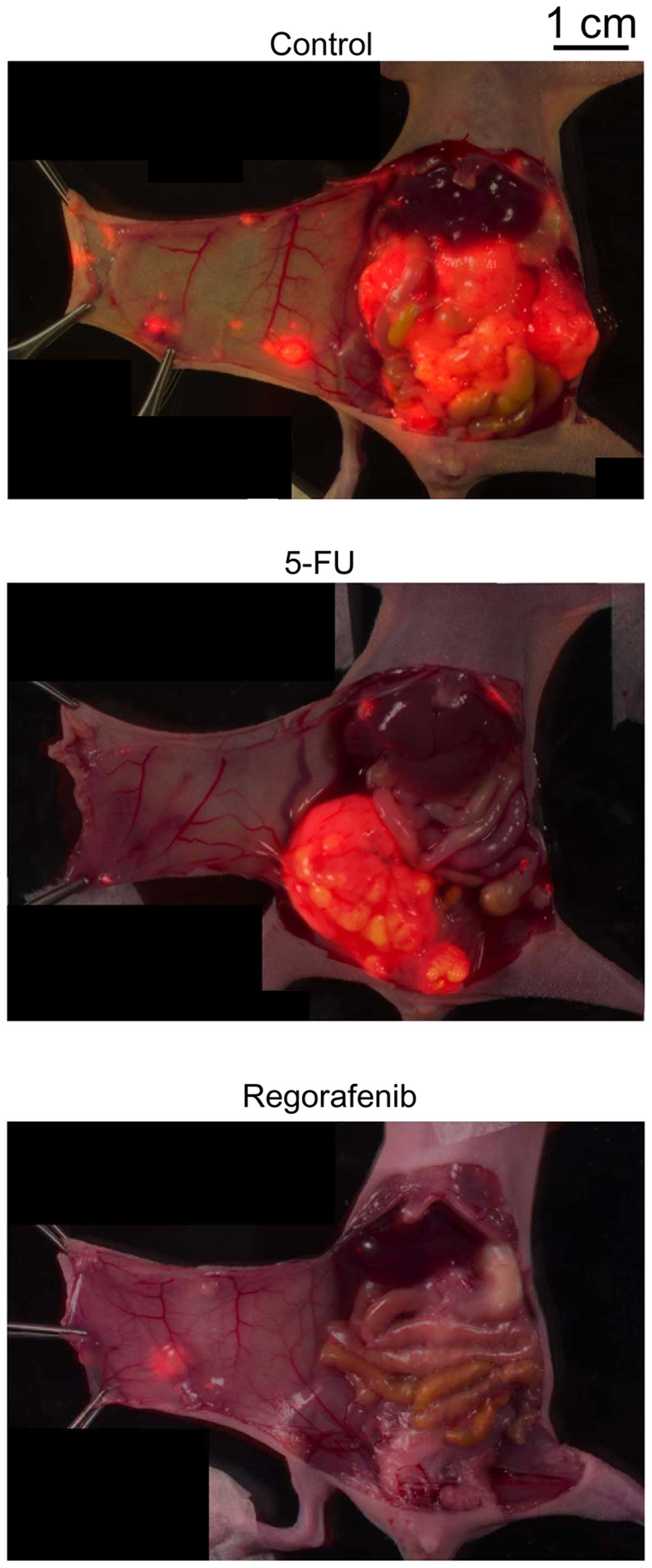
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moertel CG, Fleming TR, Macdonald JS,
Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA,
Tormey DC, Glick JH, et al: Levamisole and fluorouracil for
adjuvant therapy of resected colon carcinoma. N Engl J Med.
322:352–358. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Vos tot Nederveen Cappel WH, Meulenbeld
HJ, Kleibeuker JH, Nagengast FM, Menko FH, Griffioen G, Cats A,
Morreau H, Gelderblom H, Vasen HF, et al: Survival after adjuvant
5-FU treatment for stage III colon cancer in hereditary
nonpolyposis colorectal cancer. Int J Cancer. 109:468–471. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vaillant JC, Nordlinger B, Deuffic S,
Arnaud JP, Pelissier E, Favre JP, Jaeck D, Fourtanier G, Grandjean
JP, Marre P, et al: Adjuvant intraperitoneal 5-fluorouracil in
high-risk colon cancer: A multicenter phase III trial. Ann Surg.
231:449–456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshii Y, Waki A, Yoshida K, Kakezuka A,
Kobayashi M, Namiki H, Kuroda Y, Kiyono Y, Yoshii H, Furukawa T, et
al: The use of nanoimprinted scaffolds as 3D culture models to
facilitate spontaneous tumor cell migration and well-regulated
spheroid formation. Biomaterials. 32:6052–6058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshii Y, Furukawa T, Waki A, Okuyama H,
Inoue M, Itoh M, Zhang MR, Wakizaka H, Sogawa C, Kiyono Y, et al:
High-throughput screening with nanoimprinting 3D culture for
efficient drug development by mimicking the tumor environment.
Biomaterials. 51:278–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawada M, Inoue H, Masuda T and Ikeda D:
Insulin-like growth factor I secreted from prostate stromal cells
mediates tumor-stromal cell interactions of prostate cancer. Cancer
Res. 66:4419–4425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73-4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar
|
|
9
|
Schmieder R, Hoffmann J, Becker M,
Bhargava A, Müller T, Kahmann N, Ellinghaus P, Adams R, Rosenthal
A, Thierauch KH, et al: Regorafenib (BAY 73-4506): Antitumor and
antimetastatic activities in preclinical models of colorectal
cancer. Int J Cancer. 135:1487–1496. 2014. View Article : Google Scholar :
|
|
10
|
Kehoe SM, Ma C, Rosales N, Rao T, Dupont J
and Spriggs DR: Effect of combination inhibition of vascular
endothelial growth factor (VEGF) and epidermal growth factor
receptor (EGF-R) on ovarian cancer cell lines. J Clin Oncol.
24(Suppl 13112)2006.
|
|
11
|
Kunnumakkara AB, Diagaradjane P, Anand P,
Harikumar KB, Deorukhkar A, Gelovani J, Guha S, Krishnan S and
Aggarwal BB: Curcumin sensitizes human colorectal cancer to
capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and
CXCR4 expression in an orthotopic mouse model. Int J Cancer.
125:2187–2197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto Y, Fukuda K, Fuchimoto Y,
Matsuzaki Y, Saikawa Y, Kitagawa Y, Morikawa Y and Kuroda T:
Cetuximab promotes anticancer drug toxicity in rhabdomyosarcomas
with EGFR amplification in vitro. Oncol Rep. 30:1081–1086.
2013.PubMed/NCBI
|
|
13
|
Jiang P, Mukthavaram R, Chao Y, Bharati
IS, Fogal V, Pastorino S, Cong X, Nomura N, Gallagher M, Abbasi T,
et al: Novel anti-glioblastoma agents and therapeutic combinations
identified from a collection of FDA approved drugs. J Transl Med.
12:132014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dahan L, Sadok A, Formento JL, Seitz JF
and Kovacic H: Modulation of cellular redox state underlies
antagonism between oxaliplatin and cetuximab in human colorectal
cancer cell lines. Br J Pharmacol. 158:610–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freeman DJ, Bush T, Ogbagabriel S,
Belmontes B, Juan T, Plewa C, Van G, Johnson C and Radinsky R:
Activity of panitumumab alone or with chemotherapy in non-small
cell lung carcinoma cell lines expressing mutant epidermal growth
factor receptor. Mol Cancer Ther. 8:1536–1546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cordel S, Heymann MF, Boisteau O, Oliver
L, Le Pendu J, Grégoire M and Meflah K: 5-Fluorouracil-resistant
colonic tumors are highly responsive to sodium
butyrate/interleukin-2 bitherapy in rats. Int J Cancer. 73:924–928.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Looy T, Gebreyohannes YK, Wozniak A,
Cornillie J, Wellens J, Li H, Vanleeuw U, Floris G, Debiec-Rychter
M, Sciot R, et al: Characterization and assessment of the
sensitivity and resistance of a newly established human
gastrointestinal stromal tumour xenograft model to treatment with
tyrosine kinase inhibitors. Clin Sarcoma Res. 4:102014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gold GL, Hall TC, Shnider BJ, Selawry O,
Colsky J, Owens AH Jr, Dederick MM, Holland JF, Brindley CO and
Jones R: A clinical study of 5-fluorouracil. Cancer Res.
19:935–939. 1959.PubMed/NCBI
|
|
19
|
Miyake M, Anai S, Fujimoto K, Ohnishi S,
Kuwada M, Nakai Y, Inoue T, Tomioka A, Tanaka N and Hirao Y:
5-fluorouracil enhances the antitumor effect of sorafenib and
sunitinib in a xenograft model of human renal cell carcinoma. Oncol
Lett. 3:1195–1202. 2012.PubMed/NCBI
|
|
20
|
Abou-Elkacem L, Arns S, Brix G, Gremse F,
Zopf D, Kiessling F and Lederle W: Regorafenib inhibits growth,
angiogenesis, and metastasis in a highly aggressive, orthotopic
colon cancer model. Mol Cancer Ther. 12:1322–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wieder HA, Geinitz H, Rosenberg R, Lordick
F, Becker K, Stahl A, Rummeny E, Siewert JR, Schwaiger M and
Stollfuss J: PET imaging with
[18F]3′-deoxy-3′-fluorothymidine for prediction of
response to neoadjuvant treatment in patients with rectal cancer.
Eur J Nucl Med Mol Imaging. 34:878–883. 2007. View Article : Google Scholar
|
|
22
|
Hong YS, Kim HO, Kim KP, Lee JL, Kim HJ,
Lee SJ, Lee SJ, Oh SJ, Kim JS, Ryu JS, et al:
3′-Deoxy-3′-18F-fluorothymidine PET for the early
prediction of response to leucovorin, 5-fluo-rouracil, and
oxaliplatin therapy in patients with metastatic colorectal cancer.
J Nucl Med. 54:1209–1216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wodarski CEJ, Weber K, Henze M, Haberkorn
U and Eisenhut M: Synthesis of
3′-deoxy-3′-[18F]fluoro-thymidine with
2,3′-anhydro-5′-O-(4,4′-dimethoxytrityl)-thymidine. J Labelled Comp
Radiopharm. 43:1211–1218. 2000. View Article : Google Scholar
|
|
24
|
Tsuji AB, Kato K, Sugyo A, Okada M, Sudo
H, Yoshida C, Wakizaka H, Zhang MR and Saga T: Comparison of
2-amino-[3-11C]isobutyric acid and
2-deoxy-2-[18F]fluoro-D-glucose in nude mice with
xenografted tumors and acute inflammation. Nucl Med Commun.
33:1058–1064. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuji AB, Morita M, Li XK, Sogawa C, Sudo
H, Sugyo A, Fujino M, Sugioka A, Koizumi M and Saga T:
18F-FDG PET for semiquantitative evaluation of acute
allograft rejection and immunosuppressive therapy efficacy in rat
models of liver transplantation. J Nucl Med. 50:827–830. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugarbaker PH: Colorectal cancer:
Prevention and management of metastatic disease. BioMed Res Int.
2014:7828902014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomassen I, van Gestel YR, Lemmens VE and
de Hingh IH: Incidence, prognosis, and treatment options for
patients with synchronous peritoneal carcinomatosis and liver
metastases from colorectal origin. Dis Colon Rectum. 56:1373–1380.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brodowicz T, Liegl-Atzwager B, Tresch E,
Taieb S, Kramar A, Gruenwald V, Vanseymortier M, Clisant S, Blay
JY, Le Cesne A, et al: Study protocol of REGOSARC trial: activity
and safety of regorafenib in advanced soft tissue sarcoma: a
multinational, randomized, placebo-controlled, phase II trial. BMC
Cancer. 15:1272015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar
|
|
30
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: CONCUR Investigators: Regorafenib
plus best supportive care versus placebo plus best supportive care
in Asian patients with previously treated metastatic colorectal
cancer (CONCUR): A randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jitawatanarat P and Wee W: Update on
antiangiogenic therapy in colorectal cancer: Aflibercept and
regorafenib. J Gastrointest Oncol. 4:231–238. 2013.PubMed/NCBI
|
|
32
|
Boyer J, McLean EG, Aroori S, Wilson P,
McCulla A, Carey PD, Longley DB and Johnston PG: Characterization
of p53 wild-type and null isogenic colorectal cancer cell lines
resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin
Cancer Res. 10:2158–2167. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fan LC, Teng HW, Shiau CW, Lin H, Hung MH,
Chen YL, Huang JW, Tai WT, Yu HC and Chen KF: SHP-1 is a target of
regorafenib in colorectal cancer. Oncotarget. 5:6243–6251. 2014.
View Article : Google Scholar : PubMed/NCBI
|