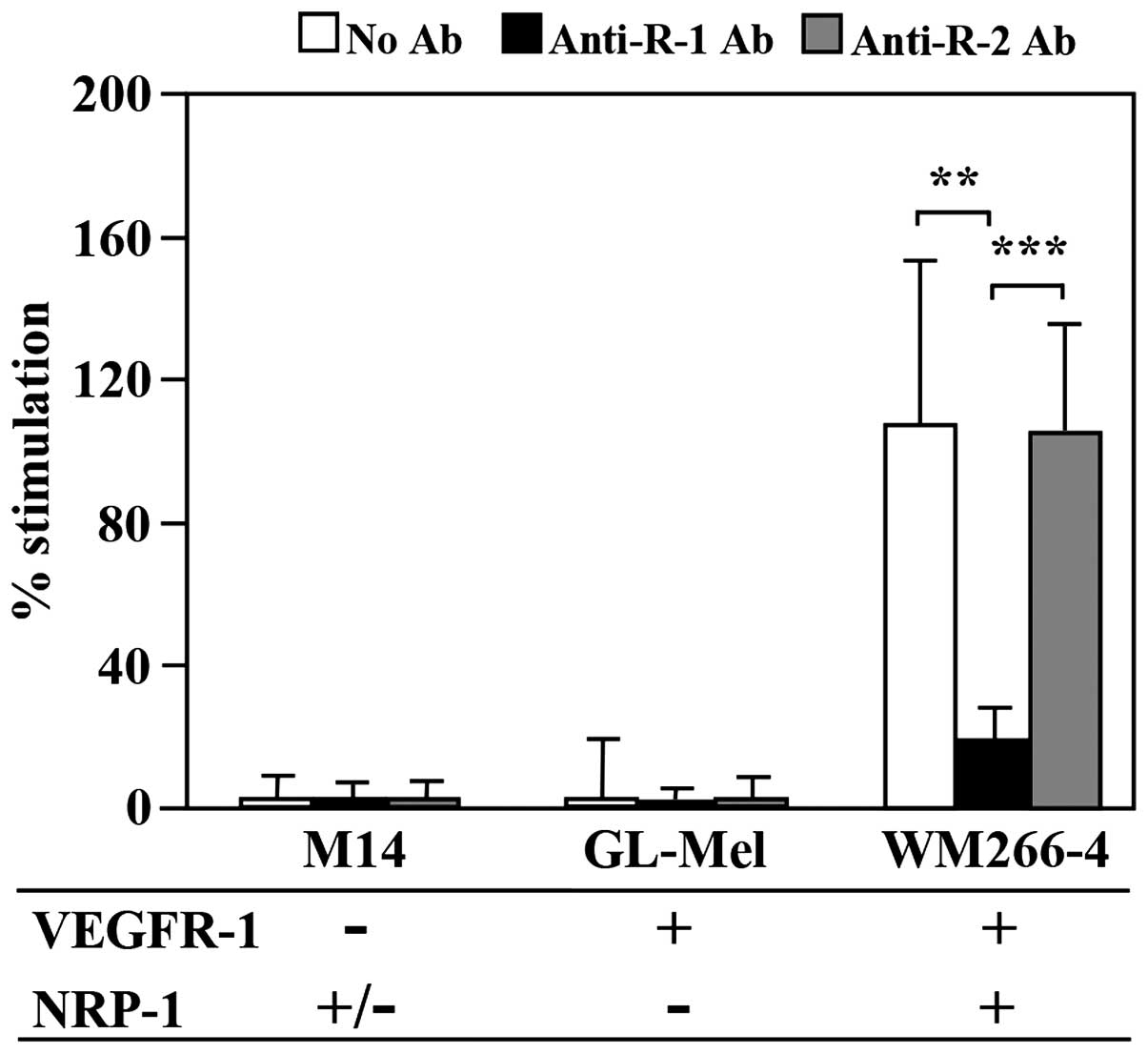
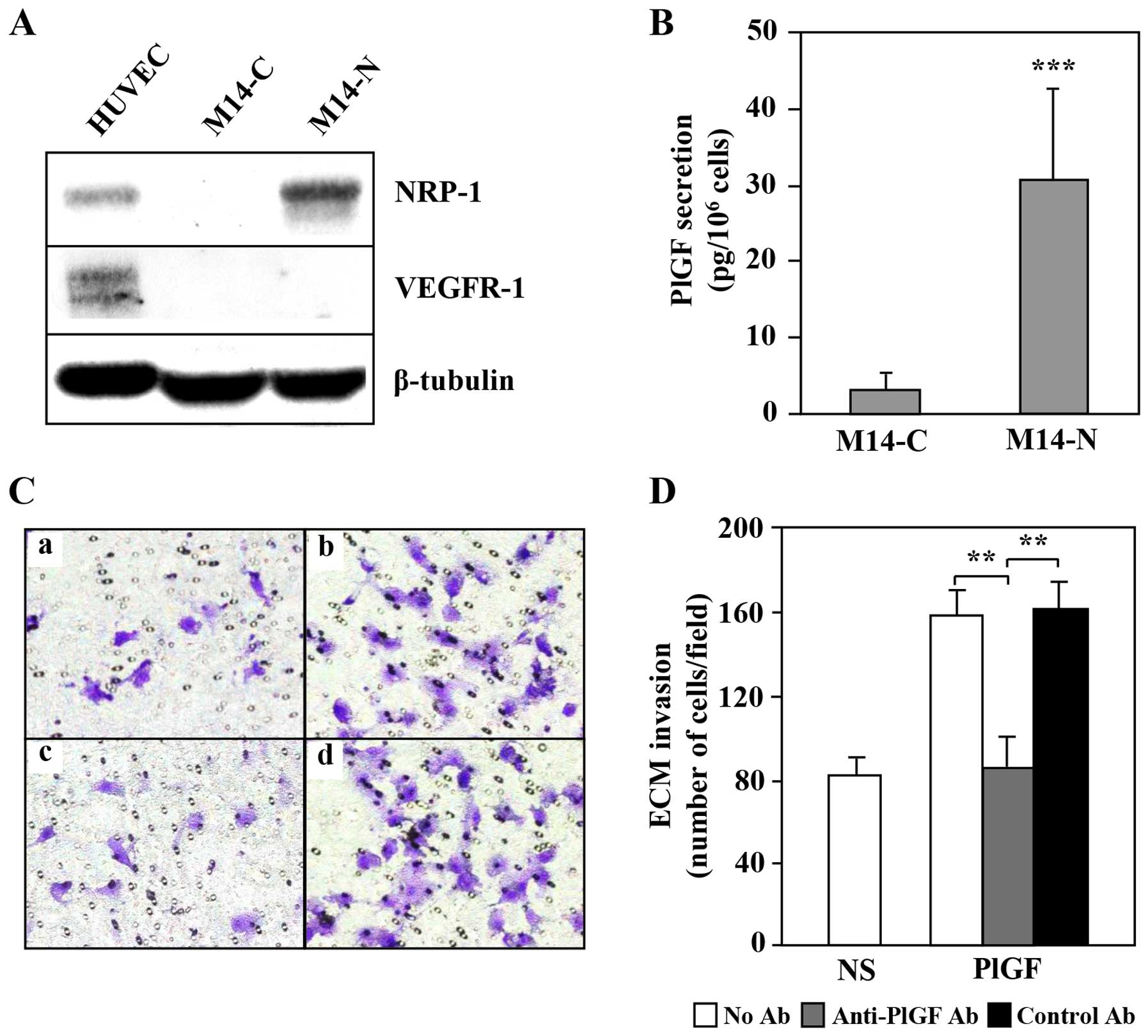
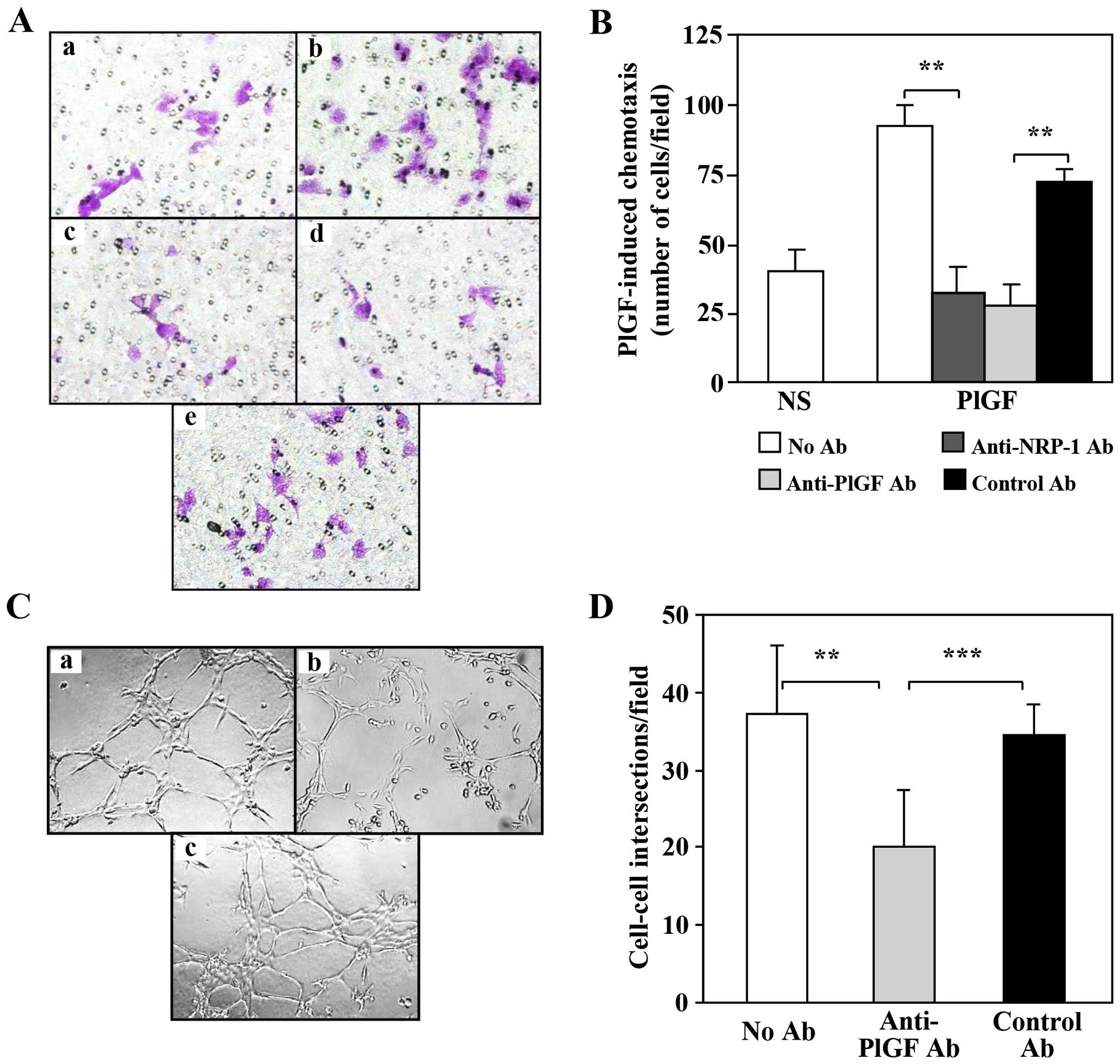
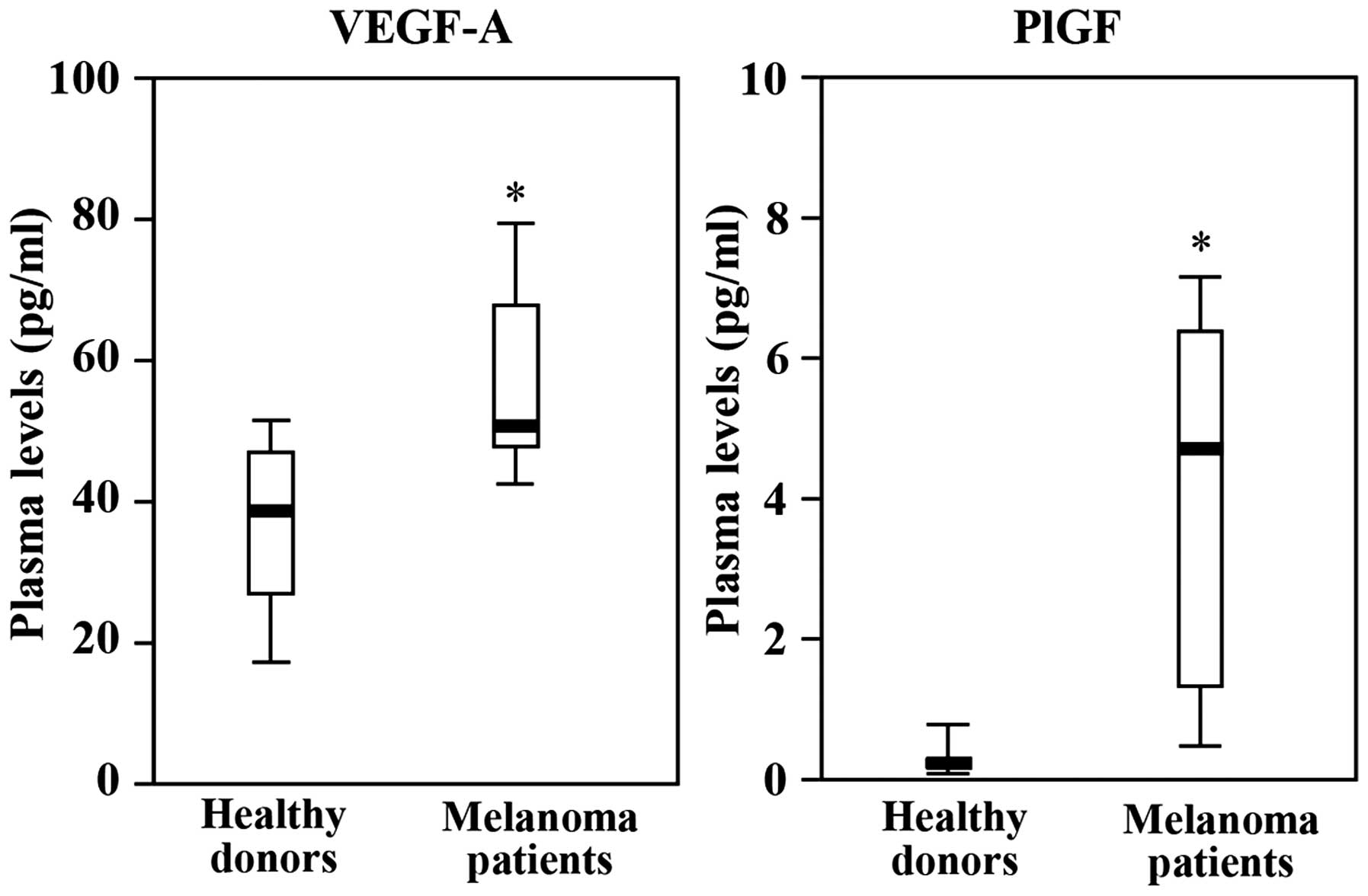
|
1
|
Fischer C, Mazzone M, Jonckx B and
Carmeliet P: FLT1 and its ligands VEGFB and PlGF: drug targets for
anti-angiogenic therapy? Nat Rev Cancer. 8:942–956. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roskoski R Jr: VEGF receptor
protein-tyrosine kinases: structure and regulation. Biochem Biophys
Res Commun. 375:287–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dewerchin M and Carmeliet P: Placental
growth factor in cancer. Expert Opin Ther Targets. 18:1339–1354.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KJ, Cho CS and Kim WU: Role of
placenta growth factor in cancer and inflammation. Exp Mol Med.
44:10–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Autiero M, Waltenberger J, Communi D,
Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M,
Bono F, et al: Role of PlGF in the intra- and intermolecular cross
talk between the VEGF receptors Flt1 and Flk1. Nat Med. 9:936–943.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marcellini M, De Luca N, Riccioni T,
Ciucci A, Orecchia A, Lacal PM, Ruffini F, Pesce M, Cianfarani F,
Zambruno G, et al: Increased melanoma growth and metastasis
spreading in mice overexpressing placenta growth factor. Am J
Pathol. 169:643–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levati L, Ruffini F, Muzi A, Umezawa K,
Graziani G, D'Atri S and Lacal PM: Placenta growth factor induces
melanoma resistance to temozolomide through a mechanism that
involves the activation of the transcription factor NF-κB. Int J
Oncol. 38:241–247. 2011.
|
|
9
|
Schwartz JD, Rowinsky EK, Youssoufian H,
Pytowski B and Wu Y: Vascular endothelial growth factor receptor-1
in human cancer: concise review and rationale for development of
IMC-18F1 (Human antibody targeting vascular endothelial growth
factor receptor-1). Cancer. 116(Suppl): 1027–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adini A, Kornaga T, Firoozbakht F and
Benjamin LE: Placental growth factor is a survival factor for tumor
endothelial cells and macrophages. Cancer Res. 62:2749–2752.
2002.PubMed/NCBI
|
|
11
|
Zhou Y, Bellingard V, Feng K-T, McMaster M
and Fisher SJ: Human cytotrophoblasts promote endothelial survival
and vascular remodeling through secretion of Ang2, PlGF, and
VEGF-C. Dev Biol. 263:114–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu Y, Hooper AT, Zhong Z, Witte L, Bohlen
P, Rafii S and Hicklin DJ: The vascular endothelial growth factor
receptor (VEGFR-1) supports growth and survival of human breast
carcinoma. Int J Cancer. 119:1519–1529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Zhong Z, Huber J, Bassi R, Finnerty
B, Corcoran E, Li H, Navarro E, Balderes P, Jimenez X, et al:
Anti-vascular endothelial growth factor receptor-1 antagonist
antibody as a therapeutic agent for cancer. Clin Cancer Res.
12:6573–6584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fragoso R, Pereira T, Wu Y, Zhu Z,
Cabeçadas J and Dias S: VEGFR-1 (FLT-1) activation modulates acute
lymphoblastic leukemia localization and survival within the bone
marrow, determining the onset of extramedullary disease. Blood.
107:1608–1616. 2006. View Article : Google Scholar
|
|
15
|
Lacal PM, Failla CM, Pagani E, Odorisio T,
Schietroma C, Falcinelli S, Zambruno G and D'Atri S: Human melanoma
cells secrete and respond to placenta growth factor and vascular
endothelial growth factor. J Invest Dermatol. 115:1000–1007. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Graells J, Vinyals A, Figueras A, Llorens
A, Moreno A, Marcoval J, Gonzalez FJ and Fabra A: Overproduction of
VEGF concomitantly expressed with its receptors promotes growth and
survival of melanoma cells through MAPK and PI3K signaling. J
Invest Dermatol. 123:1151–1161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Graeven U, Fiedler W, Karpinski S, Ergün
S, Kilic N, Rodeck U, Schmiegel W and Hossfeld DK:
Melanoma-associated expression of vascular endothelial growth
factor and its receptors FLT-1 and KDR. J Cancer Res Clin Oncol.
125:621–629. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lacal PM, Ruffini F, Pagani E and D'Atri
S: An autocrine loop directed by the vascular endothelial growth
factor promotes invasiveness of human melanoma cells. Int J Oncol.
27:1625–1632. 2005.PubMed/NCBI
|
|
19
|
Grünewald FS, Prota AE, Giese A and
Ballmer-Hofer K: Structure-function analysis of VEGF receptor
activation and the role of coreceptors in angiogenic signaling.
Biochim Biophys Acta. 1804:567–580. 2010. View Article : Google Scholar
|
|
20
|
Neagoe PE, Lemieux C and Sirois MG:
Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin
synthesis requires the activation of VEGF receptor-1 and -2
heterodimer. J Biol Chem. 280:9904–9912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allain B, Jarray R, Borriello L, Leforban
B, Dufour S, Liu WQ, Pamonsinlapatham P, Bianco S, Larghero J,
Hadj-Slimane R, et al: Neuropilin-1 regulates a new VEGF-induced
gene, Phactr-1, which controls tubulogenesis and modulates
lamel-lipodial dynamics in human endothelial cells. Cell Signal.
24:214–223. 2012. View Article : Google Scholar
|
|
22
|
Chittenden TW, Claes F, Lanahan AA,
Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar
C, Dedkov E, Tomanek R, et al: Selective regulation of arterial
branching morphogenesis by synectin. Dev Cell. 10:783–795. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Snuderl M, Batista A, Kirkpatrick ND, Ruiz
de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin
JD, Kamoun W, et al: Targeting placental growth factor/neuropilin 1
pathway inhibits growth and spread of medulloblastoma. Cell.
152:1065–1076. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ellis LM: The role of neuropilins in
cancer. Mol Cancer Ther. 5:1099–1107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Holmes K, Roberts OL, Thomas AM and Cross
MJ: Vascular endothelial growth factor receptor-2: structure,
function, intra-cellular signalling and therapeutic inhibition.
Cell Signal. 19:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Beijnum JR, Nowak-Sliwinska P,
Huijbers EJ, Thijssen VL and Griffioen AW: The great escape; the
hallmarks of resistance to antiangiogenic therapy. Pharmacol Rev.
67:441–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lambrechts D, Lenz HJ, de Haas S,
Carmeliet P and Scherer SJ: Markers of response for the
antiangiogenic agent bevacizumab. J Clin Oncol. 31:1219–1230. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ruffini F, D'Atri S and Lacal PM:
Neuropilin-1 expression promotes invasiveness of melanoma cells
through vascular endothelial growth factor receptor-2-dependent and
-independent mechanisms. Int J Oncol. 43:297–306. 2013.PubMed/NCBI
|
|
29
|
Ruffini F, Graziani G, Levati L, Tentori
L, D'Atri S and Lacal PM: Cilengitide downmodulates invasiveness
and vasculogenic mimicry of neuropilin 1 expressing melanoma cells
through the inhibition of αvβ5 integrin. Int J Cancer.
136:E545–E558. 2015. View Article : Google Scholar
|
|
30
|
Orecchia A, Lacal PM, Schietroma C, Morea
V, Zambruno G and Failla CM: Vascular endothelial growth factor
receptor-1 is deposited in the extracellular matrix by endothelial
cells and is a ligand for the alpha 5 beta 1 integrin. J Cell Sci.
116:3479–3489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruffini F, Tentori L, Dorio AS, Arcelli D,
D'Amati G, D'Atri S, Graziani G and Lacal PM: Platelet-derived
growth factor C and calpain-3 are modulators of human melanoma cell
invasiveness. Oncol Rep. 30:2887–2896. 2013.PubMed/NCBI
|
|
32
|
Seftor RE, Hess AR, Seftor EA, Kirschmann
DA, Hardy KM, Margaryan NV and Hendrix MJ: Tumor cell vasculogenic
mimicry: From controversy to therapeutic promise. Am J Pathol.
181:1115–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escudero-Esparza A, Martin TA,
Douglas-Jones A, Mansel RE and Jiang WG: PGF isoforms, PLGF-1 and
PGF-2 and the PGF receptor, neuropilin, in human breast cancer:
prognostic significance. Oncol Rep. 23:537–544. 2010.PubMed/NCBI
|
|
34
|
Banerjee S, Sengupta K, Dhar K, Mehta S,
D'Amore PA, Dhar G and Banerjee SK: Breast cancer cells secreted
platelet-derived growth factor-induced motility of vascular smooth
muscle cells is mediated through neuropilin-1. Mol Carcinog.
45:871–880. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dhar K, Dhar G, Majumder M, Haque I, Mehta
S, Van Veldhuizen PJ, Banerjee SK and Banerjee S: Tumor
cell-derived PDGF-B potentiates mouse mesenchymal stem
cells-pericytes transition and recruitment through an interaction
with NRP-1. Mol Cancer. 9:2092010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pellet-Many C, Frankel P, Evans IM, Herzog
B, Jünemann-Ramírez M and Zachary IC: Neuropilin-1 mediates PDGF
stimulation of vascular smooth muscle cell migration and signalling
via p130Cas. Biochem J. 435:609–618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frank A, David V, Aurelie TR, Florent G,
William H and Philippe B: Regulation of MMPs during melanoma
progression: from genetic to epigenetic. Anticancer Agents Med
Chem. 12:773–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tamiya M, Tamiya A, Yamadori T, Nakao K,
Asami K, Yasue T, Otsuka T, Shiroyama T, Morishita N, Suzuki H, et
al: Phase2 study of bevacizumab with carboplatin-paclitaxel for
non-small cell lung cancer with malignant pleural effusion. Med
Oncol. 30:6762013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jayasinghe C, Simiantonaki N and
Kirkpatrick CJ: Cell type- and tumor zone-specific expression of
pVEGFR-1 and its ligands influence colon cancer metastasis. BMC
Cancer. 15:1042015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bagley RG, Ren Y, Weber W, Yao M,
Kurtzberg L, Pinckney J, Bangari D, Nguyen C, Brondyk W, Kaplan J,
et al: Placental growth factor upregulation is a host response to
antiangiogenic therapy. Clin Cancer Res. 17:976–988. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fischer C, Jonckx B, Mazzone M, Zacchigna
S, Loges S, Pattarini L, Chorianopoulos E, Liesenborghs L, Koch M,
De Mol M, et al: Anti-PlGF inhibits growth of
VEGF(R)-inhibitor-resistant tumors without affecting healthy
vessels. Cell. 131:463–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang WC, Dennis MS, Stawicki S, Chanthery
Y, Pan Q, Chen Y, Eigenbrot C, Yin J, Koch AW, Wu X, et al:
Function blocking antibodies to neuropilin-1 generated from a
designed human synthetic antibody phage library. J Mol Biol.
366:815–829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pan Q, Chanthery Y, Liang WC, Stawicki S,
Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, et al:
Blocking neuropilin-1 function has an additive effect with
anti-VEGF to inhibit tumor growth. Cancer Cell. 11:53–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xin Y, Li J, Wu J, Kinard R, Weekes CD,
Patnaik A, Lorusso P, Brachmann R, Tong RK, Yan Y, et al:
Pharmacokinetic and pharmacodynamic analysis of circulating
biomarkers of anti-NRP1, a novel antiangiogenesis agent, in two
phase I trials in patients with advanced solid tumors. Clin Cancer
Res. 18:6040–6048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jia H, Cheng L, Tickner M, Bagherzadeh A,
Selwood D and Zachary I: Neuropilin-1 antagonism in human carcinoma
cells inhibits migration and enhances chemosensitivity. Br J
Cancer. 102:541–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zeng F, Luo F, Lv S, Zhang H, Cao C, Chen
X, Wang S, Li Z, Wang X, Dou X, et al: A monoclonal antibody
targeting neuropilin-1 inhibits adhesion of MCF7 breast cancer
cells to fibronectin by suppressing the FAK/p130cas signaling
pathway. Anticancer Drugs. 25:663–672. 2014.PubMed/NCBI
|
|
47
|
Graziani G and Lacal PM: Neuropilin-1 as
therapeutic target for malignant melanoma. Front Oncol. 5:1252015.
View Article : Google Scholar : PubMed/NCBI
|