1. Introduction
According to recent cancer statistics data, cancer
incidence has been increasing steadily in the past 10 years due to
a growing and aging population, while the mortality obviously
decreased as a result of earlier detection and advances in
treatment (1). Therefore,
understanding the mechanisms of tumorigenesis and recurrence is
urgent to cut the cost of ‘cancer survivors’ and improve their life
quality. First, it is important to design treatment strategies that
will minimize the chance of occurrence and relapse in these
patients. As we know, almost all solid tumors are composed of tumor
cells, a variety of different non-tumor cells, defined as tumor
stromal cells including endothelial cells, fibroblasts, and
inflammatory cells, various cytokines, and finally their skeleton
ECM (2,3). All of these cells, cytokines and ECM
are tightly linked and interact with each other dynamically,
constituting the tumor microenvironment. It has been discovered
that the tumor microenvironment has a great effect on tumorigenesis
by transforming epithelial cells and changing their aptitude to
give rise to malignant tumors (2,3).
Moreover, as for tumor progression, the complex microenvironment
can affect tumor cell invasion and metastasis ability through
promoting angiogenesis, recruitment of reactive stromal
fibroblasts, immunocyte infiltration, extra production of
proteolytic enzymes, and modifying the structure of ECM (2,3).
Normally, ECM works as the obstacle for cell
movement except during certain processes such as tissue healing and
remodeling, inflammation, and neoplasia. Tumor growth and
metastasis depend on the cell-cell and cell-matrix interactions and
also modification of the ECM (4).
In order to invade the adjacent normal parenchyma and metastasize
to distant organs, surrounding ECM need to be degraded (5). Numerous evidence supports that the
capacity of remolding tumor stroma is rendered by a group of
molecules, including cysteine proteases, serine proteases, and
matrix metalloproteinases. The interactions between tumor cells and
their microenvironment reveal the key role of matrix
metalloproteinases (MMPs) during the process of tumorigenesis.
Matrix metalloproteinases are zinc-dependent
endopeptidases with a tremendous capacity of degrading ECM
proteins, including 25 members in human. According to structural
domains or corresponding specific substrates, MMPs are classified
into 4 subgroups: collagenases, gelatinases, stromelysins, and
membrane type MMPs (6). Functions
of MMPs are fundamentally associated with matrix remodeling, such
as breakdown of ECM proteins and cleavage of cell surface
receptors, especially associated with cancer cell proliferation,
invasion, angiogenesis and metastasis (7,8).
MMPs play significant roles in cancer invasion, metastasis, and
angiogenesis also through their direct impact on cell behavior such
as promoting growth of metastasized tumor cells, increasing
motility of epithelial cells and inhibiting apoptosis.
Associations among MMP expression, advanced stages
and poor clinical outcome have been observed in various types of
cancer. On the other hand, MMPs may act in an indirect way. During
invasion and metastasis process, degradation of ECM and basement
membrane may further promote the release and activation of
ECM-bound cytokines. Furthermore, ECM fragment, the products of
degradation, also facilitated cell growth, motility and
angiogenesis process (9). The
expression and activity of these extracellular enzymes are
precisely regulated at different levels. Most MMPs are produced
initially as inactive proenzymes, and then processed to active
forms via proteolytic cleavage. Their biological activity is also
controlled by a family of natural tissue inhibitor proteins
specific for MMPs (TIMPs). The imbalance between MMPs and TIMPs is
implicated in various pathological tissue remodeling processes,
especially during cancer progression and metastasis (10,11).
The invasive nature of malignant tumors largely
contributes to high mortality and poor prognosis of malignant solid
tumors, which is closely associated with MMPs. Targeting MMPs could
work as an optional therapeutic approach for cancer therapy. This
review discusses the functions of a special matrix
metalloproteinase, MMP-11, in solid tumors and its potential as a
biomarker in the detection and a therapeutic target in the
treatment of cancer.
2. MMP-11 and malignant solid tumors
Human MMP-11 (stromelysin-3) is a member of the
stromelysin subgroup (6), which
was first identified in the stromal cells of breast carcinoma
(12). Different from other
members of MMPs family, it has some unique characteristics and
functions. First, it is intracytoplasmically processed and secreted
as an active enzyme, whereas most MMPs are secreted as inactive
zymogens (13). Second, MMP-11
does not appear to hydrolyze classical MMP-affected substrates such
as laminin, fibronectin, and elastin. Instead, MMP-11 has been
found to have a strong activity accelerating the degradation of
serine protease inhibitor α1-antitrypsin, insulin-like growth
factor binding protein-1 (IGFBP-1) (14), and process the capability of
inducing the dedifferentiation of adipocytes and desmoplastic
reaction surrounding the cancer stroma through cleavage of collagen
VI (15,16). These special properties suggest
MMP-11, compared with other MMPs members, have a distinct role in
tumor development and progression.
Similar to some members of MMPs, MMP-11 expression
is found upregulated in sera of cancer patients as well as in the
specimens of solid tumor tissues by immunohistochemistry, but
almost absent in normal tissues. Overexpression of MMP-11 has been
identified in various types of human cancers, such as oral cancer
(17,18), desmoid tumors (19), non-small cell lung cancer (20), esophageal adenocarcinoma (21), pancreatic adenocarcinoma (22), aggressive meningioma (23), colon cancer and ovarian carcinoma
(24,25). Apart from in invasive primary
carcinomas, MMP-11 was found expressed and activated in lymph nodes
and distant metastatic lesion as well, but rarely in sarcomas and
other non-epithelial malignancies (26,27).
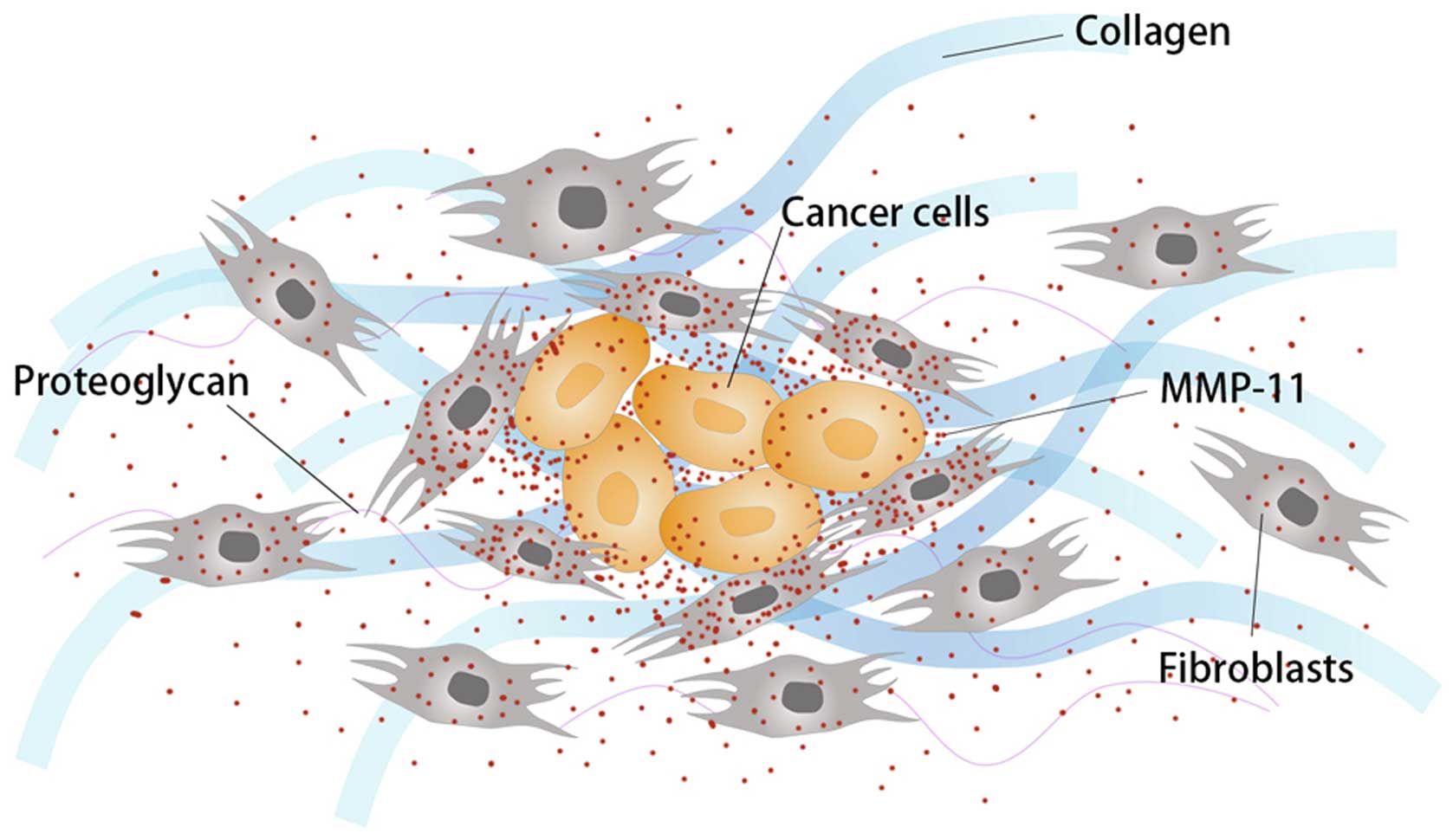
MMP-11 was initially considered expressed in tumor
stroma by fibroblasts surrounding tumor cells instead of by tumor
cells themselves (28). However,
other studies present evidence that both tumor cells and stromal
cells surrounding them express MMP-11 (22,29)
(Fig. 1). Cell-to-cell
interactions between tumor cells and their tumor-associated
fibroblasts were found to facilitate expression and activation of
MMPs in tumor cells, promoting invasion and angiogenesis (30). Some prognostic factors in breast
cancer including p53 expression, ER and HER2 expression, central
tumor fibrosis are found to be correlated with MMP-11 expression in
both tumors and stromal fibroblasts (31). These findings imply that MMP-11 is
probably involved in certain signaling transduction pathways or
unknown biological tumor behavior, indicating a more complex role
in cancer development than its proteolysis activity.
In the following, we comprehensively summarize
existing available findings on the MMP-11 expression pattern in
various human cancers, as well as corresponding functions on the
malignant biological properties of tumor cells.
Breast cancer
One of the main characteristics of breast cancer is
its significantly high capacity of invasion and metastasis
(32). Current evidence reveals
that this property largely derives from interactions between cancer
cells and ECM involved with MMPs and heparanase. MMP-11 was
originally discovered due to its high expression in a c-DNA library
established from a human breast cancer biopsy (12), in which expression of MMP-11 was
found significantly up regulated by 12.45- to 50.45-fold in human
breast cancer tissue samples compared with normal controls using
Real-time RT-qPCR (33).
Additionally, circulating free MMP-11 and corresponding spontaneous
antibodies were also detected in the sera of breast cancer patients
(34).
Gastric cancer
Approximately 70% of patients presenting with
gastric cancer have a locally advanced and metastatic disease at
the time of diagnosis followed by relatively poor prognosis,
missing the possibility of radical surgical resection (35). Endogenous expression of MMP-11 in
gastric cancer cells has been confirmed by western blotting and IHC
staining (14). Compared with
nonmalignant tissues, MMP-11 expression was significantly higher in
gastric cancer specimens at both transcriptional and protein levels
(36). Consistent with the
expression pattern in tissues, the serum levels of MMP-11 protein
were also higher in patients with advanced gastric adenocarcinoma
than in healthy controls (37).
Colorectal cancer
Colorectal cancer is one of the most common human
malignant neoplasms. Complications from distant metastases rather
than the primary tumor itself often contribute to the death of
patients in most cases. Barrasa et al (29) demonstrated that MMP-11 can be
detected not only in stromal cells, but also in three different
colon adenocarcinoma cell lines from epithelial origin. Of note,
the expression of MMP-11 in one identical individual was finally
confirmed with different patterns among normal colorectal mucosa
tissues, primary colorectal cancerous tissues, and metastatic
sites. The mRNA expression levels of MMP-11 in liver metastatic
lesions were significantly lower compared with matched primary
colon cancer tissues (38).
Nevertheless, a higher expression of MMP-11 was seen in lymph node
metastatic sites by immunohistochemistry (39). We can speculate that MMP-11 may
play different roles in multiple subgroups of metastatic cancer
cells which are genetically different from primary cells.
Pancreatic cancer
The difficulties in early detection, extremely high
mortality and poor prognosis characterize pancreatic cancer.
Surgery, probably the only potential option to cure, is confined
when significant invasion of large peritumoral vessels and distant
metastases exist, which is common for most pancreatic cancer
patients (40). More than 80% of
pancreatic cancer specimens reveal strong signals for MMP-11
located in the epithelial cancer cells as well as in stromal cells
surrounding them, yet MMP-11 has not been detected in mesenchymal
or epithelial cells of normal pancreatic tissues (22).
Cervical cancer
Similarly, MMP-11 expression was observed in the
tissues of cervical carcinoma, which has also been shown to be
correlated with the development of malignancy. No MMP-11 expression
was detected in normal cervical mucosa tissues, while it can be
detected in approximately 50% of dysplasia, 70% of carcinoma in
situ, and 100% of invasive carcinomas (41). This finding reflects that MMP-11
participates in a cancer-specific development, which needs more
in-depth exploration.
3. Dual functions in tumorigenesis and
cancer progression
While the catalytic activity of MMPs is responsible
for the degrading of ECM components, which is naturally essential
for promoting tumor invasion and metastasis, multiple studies have
demonstrated other roles played by MMPs that are independent of
proteolysis. These findings indicate more complex and even
paradoxical functions of MMPs in tumorigenesis and cancer
progression, possibly through interacting with different signaling
pathways. The roles of MMP-11 in promoting cancer cell
tumorigenesis, proliferation, and invasion have been demonstrated
in many studies, which are shared by other members of MMPs family.
However, it is notable that a study using MMP11-deficient
transgenic mice showed unexpected dual functions of MMP-11.
Overexpression of MMP-11 was found to increase the capacity of
tumorigenesis in early stage, while on the other hand the
metastatic ability of tumor cells are inhibited in advanced stage
(42). The discrepancy whether
MMP-11 during tumor development is a tumor enhancer or repressor
needs clarification (Table I).
 | Table IDual roles of MMP-11 in cancer
development. |
Table I
Dual roles of MMP-11 in cancer
development.
| Critical events
during cancer development | Effects | Results | Potential
mechanisms / hypothesis | Types of study | Types of
cancer | Methodology | Refs. |
|---|
| Tumorigenicity | Positive | Less amount of
colonies were formed without MMP-11 expression. | — | In
vitro | Gastric cancer
(BGC823 cell line) | RNAi by
transfecting cancer cells with siRNA; soft agar colony formation
assay | (14) |
| Positive | Lower incidence,
smaller numbers and sizes of primary cancer were observed in
MMP11−/−transgenic mice. | Cancer cells are
possibly more aggressive in the condition with no MMP-11. | In vivo | Mammary cancer | Transgenic mice
(ras+/+; MMP11+/+ and ras+/+;
MMP11−/−) | (42) |
| Positive | Tumor-free period
was longer without MMP-11 expression. Tumorigenicity depends on
MMP-11's proteolytic activity and some certain ECM factors. | MMP-11 remodels ECM
via its proteolytic activity with some microenvironmental factors
involved in this process. | In vivo and
in vitro | Breast cancer (MCF7
cell line) | MMP-11 gene
mutation; Matrigel assay | (43) |
| Homing of cancer
cells | Positive | Implantation of
cancer cells failed in MMP11−/−fibroblasts. Less tumors
developed in growth factor-depleted condition. | MMP-11, in a
paracrine way, could release or activate ECM-associated growth
factors. | In vivo | Breast cancer (MCF7
cell line) | Transgenic mice
(DMBA-induced tumorigenesis in MMP11−/−and
MMP11+/+ mice); coinjection of cancer cells and
MMP11−/−fibroblasts | (44) |
| Positive | Adipocyte membrane
alteration was observed in MMP11- deficient mice, resulting in fat
infiltration and cancer cell death. | Inhibition of
adipogenesis induced by MMP-11 may be mediated through indirect
downregulation of PPARγ expression. MMP-11 probably participates in
cancer cell-adipocyte crosstalk, adipocyte dedifferentiation and
desmoplasia. | In vivo | Breast cancer (C26
cell line) | Transgenic mice
(MMP11-deficient mice); cancer cell/adipocyte interaction
assay | (15) |
| Cell
proliferation | Undetermined | Proliferation
indexes were not significantly different between
MMP11−/− and MMP11+/+ mice. | — | In vivo | Colon cancer (C26
cell line) | Transgenic mice
(MMP11−/−and MMP11+/+ mice); subcutaneous
injection of cancer cells | (47) |
| Positive | Smaller size of
colonies were formed without MMP-11 expression. | MMP-11 promotes
progression of gastric cancer mainly via regulating proliferation
rather than modulating cell cycle or apoptosis. | In
vitro | Gastric cancer
(BGC823 cell line) | RNAi by
transfecting cancer cells with siRNA; soft agar colony formation
assay | (14) |
| Migration and
invasion | Positive | Silencing MMP-11
inhibited enhancement of migration and invasion induced by Gli1
overexpression. | Gli1 enhances
migration and invasion via upregulation of MMP-11. Overexpression
of MMP-11 probably releases extracellular IGF-1 from IGFBP1, which
could activate Erk1/2 and Akt through IGF-1 receptor, thus
activating Rho family of small GTPases. | In
vitro | Breast cancer
(MDA-MB-231 cell line) | RNA silencing by
lentivirus transduction; Transwell migration and invasion
assays | (46) |
| Angiogenesis | Undetermined | Number of
microvessels were not significantly different between
MMP11+/+ and MMP11−/−mice. | — | In vivo | Colon cancer (C26
cell line) | Transgenic mice
(MMP11−/− and MMP11+/+ mice); subcutaneous
injection of cancer cells | (47) |
| Positive | Higher
microvascular density (MVD) was observed in tissues with higher
expression of MMP-11. | MMP-11's roles in
angiogenesis may rely on releasing of some pro-angiogenic
factors. | In vivo | Prostatic
adenocarcinoma | Immunohistochemical
staining of human prostatic adenocarcinoma tissues | (52) |
| Metastasis | Negative | A lower number and
volume of primary invasive tumors but a higher amount of metastases
were observed without MMP-11 expression. | Cancer cells in the
ECM without MMP-11 may acquire their own anti-apoptotic function,
becoming more aggressive. MMP-11 possibly acts on processes
associated with angiogenesis and spreading of cancer cells. | In vivo | Mammary cancer | Transgenic mice
(ras+/+; MMP11+/+ and ras+/+;
MMP11−/−) | (42) |
|
Apoptosis/Necrosis | Negative | Decreased cancer
cell death through apoptosis and necrosis were observed in
MMP11−/−mice. | Inhibition of
apoptosis may be due to proteolysis of IGFBPs by MMP-11. PMN
infiltration and inflammatory factors (IL-8, G-CSF) could have some
relationships with cancer cell apoptosis. | In vivo | Colon cancer (C26
cell line) | Transgenic mice
(MMP11−/− and MMP11+/+ mice); subcutaneous
injection of cancer cells | (47) |
| Negative | A decrease in the
percentage of cell death was observed with active MMP-11
expression, which was reversed by batimastat, a broad spectrum MMP
inhibitor. | Inhibition of cell
death induced by MMP-11 is not mediated through traditional pro-
and anti-apoptotic proteins such as Bcl-2 and Bax. MMP-11 may
activate p42/p44 MAPK and AKT signaling pathway indirectly by
freeing IGF-1 from IGFBPs. | In
vitro | MCF7 cells | 3-D Culture;
Transfection with vector expressing inactive form of MMP-11 | (46) |
As a tumor enhancer
The tumor enhancer role of MMP-11 was considered to
be natural due to its exclusive expression in most tumor tissues.
Associations between MMP-11 overexpression and advanced
clinicopathological staging as well as poor prognostic outcomes
were also revealed.
Current evidence supports the positive effect of
MMP-11 on tumorigenesis at a relatively early stage of cancer
development. Downregulation of MMP-11 expression via RNA
interference elevated tumorigenicity of cancer cells, leading to
inhibition of tumor growth and colony formation in gastric cancer
cell lines (14). In vivo,
tumor-free period appeared to be longer in nude mice injected with
mixed MMP11-knockdown cancer cells and complete matrigel simulating
micro environment when compared with the control group (43). However, MMP-11 seemed to lose its
ability to promote tumorigenicity when MMP11-expressing cancer
cells were injected with matrigel devoid of some necessary
low-molecular-weight proteins instead of complete matrigel,
indicating MMP-11 may affect tumorigenesis by remodeling ECM
directly and freeing some necessary extracellular growth factors
existing in microenvironment indirectly (43). Results from transgenic mouse models
were consistent with in vitro trials. Compared with
wild-type (MMP11+/+) mice, MMP-11-deficient
(MMP11−/−) transgenic mice presented with lower tumor
incidence rate, decreased numbers, and smaller sizes of primary
cancer. Furthermore, longer tumor-free survival and longer delay
between the first hit of oncogene ras activation and the
primary tumor appearance were observed in MMP-11-deficient mice
(42).
In early stages of tumorigenesis, cancer cells are
supposed to be compatible with other components in the tissue and
sustained by the microenvironment. Tumor-associated fibroblasts
that express MMP-11 were shown to promote homing of malignant
epithelial cells. MMP11-deficient fibroblasts lost the ability to
promote implantation of cancer cells in mice (44). Furthermore, MMP-11 was also found
as a potent negative regulator of adipogenesis, suppressing
adipocyte infiltration and cancer cell death by interfering with
cancer cell-adipocyte crosstalk during tumor invasion (15).
The roles of MMP-11 in migration and invasion of
cancer cells was first explained by Kwon et al (45), who found that Gli1, an established
oncogene, enhanced migration and invasion via upregulation of
MMP-11. Anti-apoptosis is also a basic function of MMP-11 as a
tumor enhancer. MMP-11 was observed to increase survival of cancer
cells in three-dimensional matrigel culture, which was reversed by
batimastat, a broad spectrum MMP inhibitor. This MMP-11-mediated
cell survival was found accompanied by activation of p42/p44 MAPK
and AKT after analyzing apoptosis-associated proteins (46). In MMP11−/− mice,
decreased cancer cell death contributes to increased tumorigenesis
rate, probably with the assistance of polymorphonuclear (PMN)
infiltration and inflammatory factors (47).
The role of MMP-11 in cell proliferation remains
undetermined. The MMP-11 knockdown cells by using RNAi technology
exhibited significantly decreased growth ability with no remarkable
change in the distribution of different phases in cell cycle
(14). However, proliferation
indexes were not significantly different between
MMP11−/− and MMP11+/+ mice (47). More in vitro and in
vivo studies are needed to validate the effect of MMP-11 on
cell proliferation.
As a tumor repressor
MMPs may also be involved in tumor repression under
certain conditions. In vivo mouse experiments have shown
that MMP inhibitors might promote metastasis (48). Results of the first clinical trials
with broad-spectrum MMPs inhibitors in cancer therapy were
disappointing; no significant therapeutic benefit (49), but rather poorer patient survival
was established (50,51). We consider that possibly the MMP
members actually function using an as yet unknown mechanism.
Unlike several MMPs that have been shown to favor
angiogenesis, MMP-11 in angiogenesis remains undetermined. Though
MMP-11 is found unable to enhance angiogenesis in an animal-based
tumorigenesis model (47),
immunochemical staining of human prostatic adenocarcinoma tissues
revealed that microvascular density (MVD) was significantly
associated with the MMP-11 expression levels (52). Although longer tumor-free survival,
fewer and smaller primary tumors were observed in MMP11-deficient
mice (42), a systemic search for
hidden lung metastases revealed a significant higher number of
metastases in the absence of MMP-11, indicating an increased
possibility for hematogenous dissemination (53). Similar to previous reports, the
number of lung metastases developed in MMP-11-deficient mice was
2–3-fold higher than in wild-type mice using micro-CT and
histological analysis, while primary mammary tumors were
comparatively fewer and smaller (54).
Crosstalk with other tumor-related
molecules
As mentioned above, there still remains controversy
regarding the relationships between MMP-11 expression and malignant
biological properties in various malignant tumors. How MMP-11 acts
in the process of cancer development still remain unclear. It is
assumed that MMP-11 plays an extremely complex role and are
involved in various signal pathways, such as MAP kinase, Wnt, and
PI3-kinase, and/or MMPs inducers, for example CD147 (31,55),
displaying variant biological effects. On the contrary, MMP
expression was found to be modulated at different levels by a
series of growth factors, hormones and cytokines (56,57).
Selvey et al (58) observed
that MMP-11 expression was markedly amplified after treatments with
IL-1β, IL-2, TGF-β1, fibronectin and collagen V. This
overexpression of MMP-11 was inhibited by progesterone, a potent
inhibitor of TGFβ1 (59).
Retinoids were found acting predominantly at a post-transcriptional
level to inhibit MMP-11 expression in human pancreatic carcinoma
cell lines (22). Interactions
among various MMPs also constitute a complex network. Membranous
MMP-14 could hydrolyze and inactivate MMP-11, thus restricting its
functions spatially (60). It is
also found in renal cell carcinoma that microRNA-145 could suppress
cell proliferation, migration and invasion by directly
downregulating the expression of MMP-11 (61). In conclusion, the regulatory
pathways of MMP-11 are complicated and need to be further
explored.
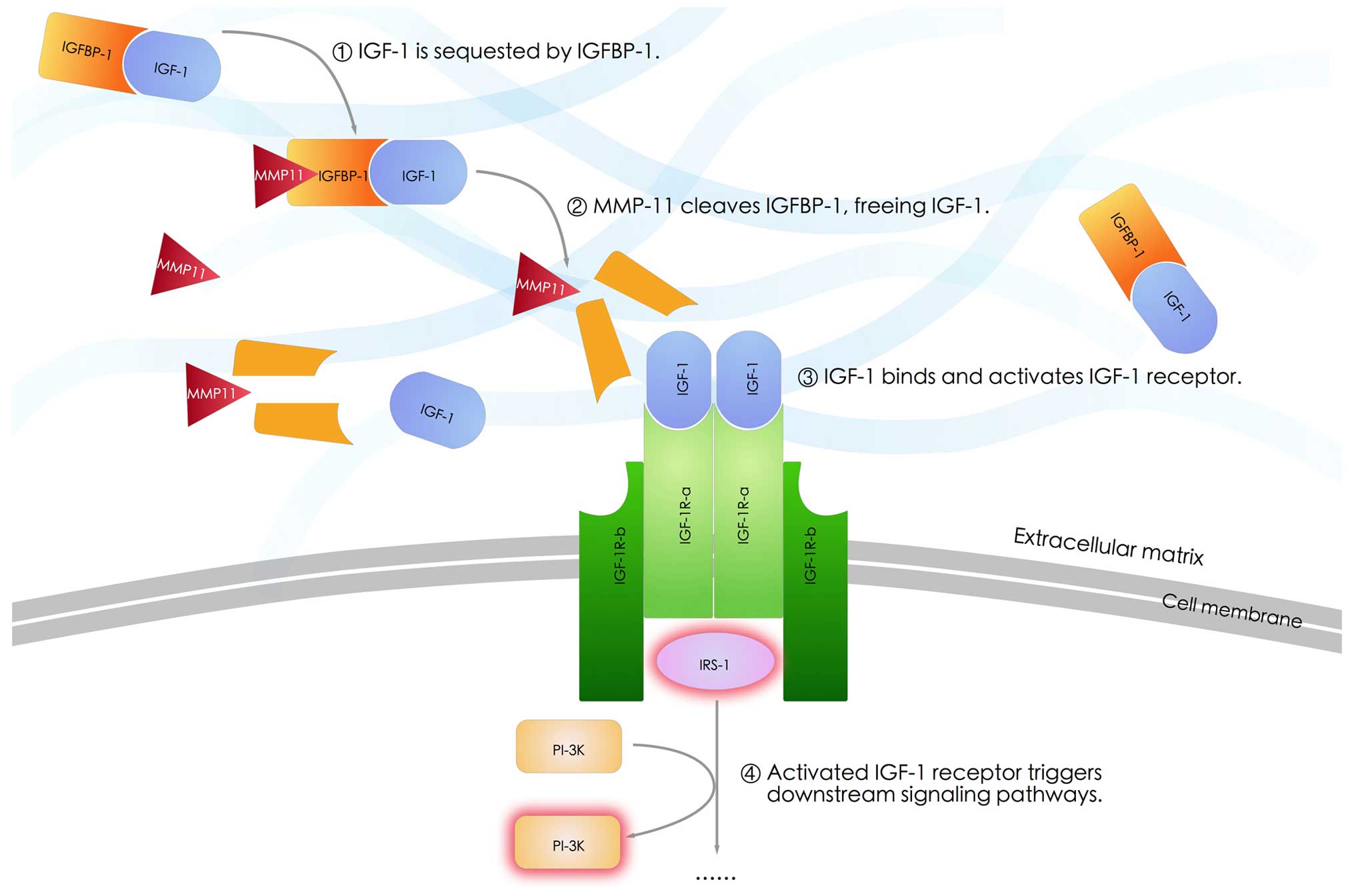
Insulin-like growth factor-1 (IGF-1) is an important
modulator of cancer metastasis. Upregulation of IGF-1 was reported
in invasive and metastatic cancer, playing a key role during tumor
proliferation and progression (62). In addition, IGF-1 receptor blockade
could promote radiation- and chemotherapy-induced apoptosis in
tumor-bearing mice (63). Some
studies showed that interactions between MMP-11 and IGF-1 are
closely related (64). Zhao et
al (36) further demonstrated
that MMP-11 expression was positively correlated with increased
expression of IGF-1. It is implied that MMP-11 protein is capable
of cleaving insulin-like growth factor binding protein 1 (IGFBP-1),
thereby freeing IGF-1 and activating IGF-1 signaling cascade,
triggering down-stage cascade reactions such as activation of PI-3K
(55,65); (Fig.
2).
CD147 is a plasma membrane glycoprotein, which is
highly located on the surface of many malignant tumor cells and
facilitates tumor cells or stromal fibroblasts to generate MMPs
(12). Associations between CD147
expression with unfavorable prognosis as well as its
pro-angiogenesis effect have been shown in breast cancer and
glioblastoma (66,67). A recent study also demonstrated
that both CD147 and MMP-11 were involved in the progression of
colorectal cancer, which were proven to be independent prognostic
factors (68). As for potential
mechanisms, increased CD147 expression in hepatocarcinoma cells was
observed to significantly promote downregulation of MMP-11 and
VEGF-A at both mRNA and protein levels (69). Additionally, glycosylation status
of CD147 is associated with the expression level of MMP-11, which
affects the adhesive and invasive ability of tumor cells in
vitro (70).
Altogether, the exact roles of MMP-11 in
tumorigenesis and cancer progression seem to be complex and
controversial, depending on the location and status of solid
tumors. Current evidence demonstrates that it could facilitate
tumorigenesis, migration and invasion, homing of malignant
epithelial cells, and anti-apoptosis effect as a tumor enhancer,
while inhibiting metastatic development as a possible tumor
repressor.
4. Potential roles in cancer diagnosis,
staging and treatment
It has been shown that MMPs take part in nearly
every critical event during tumor development. Also, ample evidence
demonstrates that the members of MMPs could work as a possible
indicator for screening cancer at an early stage, monitoring tumor
progression and predicting outcomes. For example, MMP-2, MMP-7, and
MMP-9 have been reported to be related with the progression and
prognosis of endometrial carcinoma (71), colorectal cancer (72), gastric cancer (73,74),
and breast cancer (75). Herein,
we present some recent studies supporting the clinical value of
MMP-11 as a promising biomarker, prognosis predictor or a potential
target for immunotherapy against cancer.
Early diagnosis
Currently, invasion and metastasis status at the
early stage are major obstacles for cancer management such as
pancreatic cancer and gastric cancer. Thus, finding a potent
biomarker for clinical diagnosis is the premise for effective
treatment to malignant solid tumors. We have made great efforts in
exploring valid markers with high sensitivity and specificity. In
order to assist in early diagnosis and making the most optimal
treatment strategies, the potential marker must be easily acquired,
for example through serum or specimens.
MMP-11, which is a secreted protein, not only takes
effect in the cytoplasm, but is secreted to the extracellular space
as an important component in the microenvironment, affecting both
the tumors and the ECM. MMP-11 is highly expressed in cancer
tissues compared with adjacent normal tissues. Some evidence
indicates that MMP-11 plays significant roles in early
tumorigenesis, suggesting its potential to be a novel biomarker for
early detection of cancer. Serum MMP-11 efficacy of diagnosis has
been proved in several studies. It has been indicated that serum
levels of MMP-11 were significantly elevated in gastric cancer
patients compared with normal controls. Additionally, MMP-11 was
also observed elevated in the sera of intestinal metaplasia and
dysplasia patients (76).
Sensitivity for diagnosis of gastric cancer using MMP-11 was higher
when compared with other traditional tumor markers, such as CA199,
CEA, CA242 and MMP-9 (76). In
another study, a certain cut-off value of MMP-11 protein could
reach the optimal sensitivity and specificity according to ROC
analysis for diagnosis of gastric adenocarcinoma, 94% and 93.7%,
respectively (37).
Staging and prediction of prognosis
Identification of prognostic indicators has always
been one of the research hotspots in cancer research due to the aim
of making appropriate treatment strategies. The expression levels
of MMP-11 could be used to identify patients at greater risk for
cancer recurrence in breast carcinoma (77), pancreatic tumors (78), and colon cancer (79). Higher levels of MMP-11 expression
were observed in poorly differentiated high-grade thyroid carcinoma
(80) and in breast carcinoma
(81), correlating with less
survival time among patients with breast, non-small cell lung
cancer and colon cancer (77,82).
Overexpression of MMP-11 was reported to be
associated with several clinicopathological characteristics, such
as poor differentiation, lymph node metastasis and lack of
progesterone receptor, in oral squamous cell carcinoma (83) as well as in breast carcinoma
(34). Temporally increased MMP-11
expression can be considered as an early event, prior to lymph node
metastasis during breast cancer progression (34). In gastric cancer patients, some
evidence shows MMP-11 is not only correlated with advanced-stage
and high-grade tumors (36), but
significantly associated with metastasis (76), especially for lymph node metastasis
(84) rather than peritoneal
seeding and distant organ metastasis (37). Pedersen et al (85) found higher expression of MMP-11 in
colon carcinomas was associated with invasion depth, presence of
metastasis, and poor differentiation. In addition, expression of
MMP-11 and VEGF-C in colorectal adenocarcinoma is believed to be an
important index for predicting distant metastases and clinical
stages (86).
The prognostic significance of MMP-11 expression is
also reported in gastric carcinoma, indicating its role in
predicting outcomes and monitoring recurrence during follow-up. The
5-year survival rates of patients with high expression of both
MMP-11 and IGF-1 was significantly lower compared to the patients
with low expression levels of both MMP-11 and IGF-1 (36), which proved MMP-11 and IGF-1 to be
independent prognostic factors in patients with gastric carcinoma.
A study concerning gastric adenocarcinoma revealed that patients
with low expression levels of MMP-11 had longer median survival
time and one-year survival rate than those with high levels, while
the median TTP time was not significantly different from those with
high levels of MMP-11 (37).
Similarly in prostate cancer patients, high MMP-11 expression was
significantly correlated with poor differentiation degree in
Gleason grading, late tumor stage, and positive bone metastasis.
Overall survival time of patients with higher levels of MMP-11 was
significantly shorter than those with low levels (34). Furthermore, a recent study by Eiró
et al (87) found that
MMP-11 expression by mononuclear inflammatory cells (MICs) was a
potent prognostic factor for predicting outcomes of patients with
primary ductal invasive breast tumors. This finding implies
multiple functions of MMP-11 in various types of cells in the tumor
microenvironment, which may exert its influence upon cancer
development on different levels.
A promising therapeutic target
Stromal cells in tumor tissues are genetically more
stable compared with cancer cells, but differ from their
counterparts in normal tissues for types and levels of certain
proteins (10,88). This feature indicates that stromal
antigens may also work as antitumoral targets. Immunotherapy
regimens targeting stromal antigens may be shared by several tumor
types as stromal antigens are often expressed by a broad spectrum
of solid tumors (89). Several
preclinical and clinical studies have shown the possibility that
targeting tumor stroma is a promising therapeutic target. At
present, targets of immune interventions include cancer-associated
fibroblasts, infiltrating macrophages/histiocytes, and tumor
endothelial cells. Special antigens such as carbonic anhydrase IX
or fibroblast activation protein (FAP) α suggest that vaccination
against stromal antigens is a feasible therapeutic approach
(90). Immunologic targeting of
MMPs has been demonstrated by several studies. A vaccine against
MMP-2 was reported with an effective antitumoral function,
prolonging the survival time of cancer-bearing mice (91). MMP-7 was also identified as a
broadly expressed tumor-associated antigen target, which could be a
candidate for antitumoral vaccine (92). Moreover, a longer progression-free
survival (PFS) was observed in patients with recurrent and
progressive glioblastoma after a phase II clinical trial, who were
administered marimastat (a broad spectrum MMPs inhibitor) in
conjunction with an additional cytotoxic agent following standard
radiotherapy (93). These results
support that MMPs are promising targets for antigen-specific
immunotherapy.
Considering MMP-11 is expressed exclusively in most
primary solid cancer tissues and metastatic lesions, it is an ideal
antigen target for immunotherapy. The therapeutic potency of MMP-11
was also demonstrated in some studies. Peruzzi et al
(89) discovered that a genetic
vaccine against MMP-11 based on DNA electro-gene-transfer
technology was able to break immune tolerance and exert anti-tumor
effects in a colon-adenocarcinoma mouse model. This vaccine
significantly reduced tumor formation at all precancerous stages,
and inhibited tumor progression by reducing the number of lesions
at later periods. Therefore, targeting MMP-11 therapy may be
potentially efficient in controlling disease progression.
5. Conclusions and perspectives
A number of researches have shown that MMPs not only
participate in tumor invasion and metastasis or the late stages of
the carcinogenesis but are also implicated in early stages of
tumorigenesis in both favorable and unfavorable manners. MMPs are a
diverse group of enzymes with high heterogeneity, exerting various
functions based on different types and stages of solid tumors. Thus
the clinical use of different MMP subgroups and their inhibitors
must take into consideration of the type and stage of tumor.
According to current theory, MMPs take effects mainly through
reconstructing the tumor microenvironment, so MMP inhibitors would
be more effective in certain type of solid tumors where stroma
plays an important role, such as pancreatic cancer.
MMP-11, a member of MMP family, comes under the
spotlight due to its distinct characteristics. Overexpression of
MMP-11 can be detected among various malignant cancers including
breast cancer, colorectal cancer, gastric cancer, and pancreatic
cancer. Originally, the role of MMP-11 in tumorigenesis and cancer
progression was regarded as a tumor-facilitating factor by
promoting colony formation, remodeling extracellular matrix and
suppressing apoptosis, which is in accord with the proteolysis
activity of MMPs. However, recent evidence demonstrated MMP-11 may
work as a tumor repressor by inhibiting metastasis in certain
tumors. Therefore, we can preliminarily speculate that MMP-11 may
play a dual role, not only a tumor enhancer, but a repressor during
cancer development. Therefore, more efforts are required to clarify
the exact mechanisms. As MMP-11 expression is upregulated with the
progress of cancer development and exclusively located at cancer
tissues compared to normal controls, it is proved to be a potential
biomarker for prognosis analysis. The expression levels of MMP-11
are also found to be significantly associated with some important
clinicopathological characteristics. Additionally, considering the
relatively high specificity of MMP-11 expression in cancer tissues,
some initial trials evaluating the effects of anti-MMP-11
immunotherapy suggest that MMP-11 is a promising therapeutic target
for cancer treatment. While, limited by the single center study and
relative small number of patients, more well-designed clinical
trials are needed to evaluate the treatment efficacy of
MMP-11-monoclonal antibody therapy.
We have realized the importance of MMP-11 on
biological behaviors of cancer development. Nevertheless, at the
time of this review, no document proves an exact mechanism involved
in MMP-11-participated modulation process.
Further efforts should be made to explore the
comprehensive mechanisms and associated signal molecules, as well
as to find a promising therapy target to reduce the
pro-tumorigenesis effect of MMP-11. Moreover, the potential
prognostic value of MMP-11 in the serum should be verified in
large-scale multi-center clinical studies.
Acknowledgements
This work was funded by National High Technology
Research and Development Program (863 Program, 2014AA020609),
China.
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frisch SM and Francis H: Disruption of
epithelial cell-matrix interactions induces apoptosis. J Cell Biol.
124:619–626. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei L and Shi YB: Matrix metalloproteinase
stromelysin-3 in development and pathogenesis. Histol Histopathol.
20:177–185. 2005.
|
|
4
|
Wieczorek E, Jablonska E, Wasowicz W and
Reszka E: Matrix metalloproteinases and genetic mouse models in
cancer research: a mini-review. Tumour Biol. 36:163–175. 2015.
View Article : Google Scholar :
|
|
5
|
Geho DH, Bandle RW, Clair T and Liotta LA:
Physiological mechanisms of tumor-cell invasion and migration.
Physiology (Bethesda). 20:194–200. 2005. View Article : Google Scholar
|
|
6
|
Motrescu ER and Rio MC: Cancer cells,
adipocytes and matrix metalloproteinase 11: A vicious tumor
progression cycle. Biol Chem. 389:1037–1041. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vazquez-Ortiz G, Pina-Sanchez P, Vazquez
K, Duenas A, Taja L, Mendoza P, Garcia JA and Salcedo M:
Overexpression of cathepsin F, matrix metalloproteinases 11 and 12
in cervical cancer. BMC Cancer. 5:682005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartolomé RA, Ferreiro S, Miquilena-Colina
ME, Martínez-Prats L, Soto-Montenegro ML, García-Bernal D, Vaquero
JJ, Agami R, Delgado R, Desco M, et al: The chemokine receptor
CXCR4 and the metalloproteinase MT1-MMP are mutually required
during melanoma metastasis to lungs. Am J Pathol. 174:602–612.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Chen Y, Huang L and Yu J:
Evaluation of heparanase and matrix metalloproteinase-9 in patients
with cutaneous malignant melanoma. J Dermatol. 39:339–343. 2012.
View Article : Google Scholar
|
|
10
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folgueras AR, Pendás AM, Sánchez LM and
López-Otín C: Matrix metalloproteinases in cancer: From new
functions to improved inhibition strategies. Int J Dev Biol.
48:411–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Basset P, Bellocq JP, Wolf C, Stoll I,
Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC and Chambon
P: A novel metalloproteinase gene specifically expressed in stromal
cells of breast carcinomas. Nature. 348:699–704. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pei D and Weiss SJ: Furin-dependent
intracellular activation of the human stromelysin-3 zymogen.
Nature. 375:244–247. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deng H, Guo RF, Li WM, Zhao M and Lu YY:
Matrix metalloproteinase 11 depletion inhibits cell proliferation
in gastric cancer cells. Biochem Biophys Res Commun. 326:274–281.
2005. View Article : Google Scholar
|
|
15
|
Andarawewa KL, Motrescu ER, Chenard MP,
Gansmuller A, Stoll I, Tomasetto C and Rio MC: Stromelysin-3 is a
potent negative regulator of adipogenesis participating to cancer
cell-adipocyte interaction/crosstalk at the tumor invasive front.
Cancer Res. 65:10862–10871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motrescu ER, Blaise S, Etique N, Messaddeq
N, Chenard MP, Stoll I, Tomasetto C and Rio MC: Matrix
metalloproteinase-11/ stromelysin-3 exhibits collagenolytic
function against collagen VI under normal and malignant conditions.
Oncogene. 27:6347–6355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soni S, Mathur M, Shukla NK, Deo SV and
Ralhan R: Stromelysin-3 expression is an early event in human oral
tumorigenesis. Int J Cancer. 107:309–316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arora S, Kaur J, Sharma C, Mathur M,
Bahadur S, Shukla NK, Deo SV and Ralhan R: Stromelysin 3, Ets-1,
and vascular endothelial growth factor expression in oral
precancerous and cancerous lesions: correlation with microvessel
density, progression, and prognosis. Clin Cancer Res. 11:2272–2284.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Denys H, De Wever O, Nusgens B, Kong Y,
Sciot R, Le AT, Van Dam K, Jadidizadeh A, Tejpar S, Mareel M, et
al: Invasion and MMP expression profile in desmoid tumours. Br J
Cancer. 90:1443–1449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kettunen E, Anttila S, Seppänen JK,
Karjalainen A, Edgren H, Lindström I, Salovaara R, Nissén AM, Salo
J, Mattson K, et al: Differentially expressed genes in nonsmall
cell lung cancer: Expression profiling of cancer-related genes in
squamous cell lung cancer. Cancer Genet Cytogenet. 149:98–106.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hourihan RN, O'Sullivan GC and Morgan JG:
Transcriptional gene expression profiles of oesophageal
adenocarcinoma and normal oesophageal tissues. Anticancer Res.
23(1A): 161–165. 2003.PubMed/NCBI
|
|
22
|
von Marschall Z, Riecken EO and Rosewicz
S: Stromelysin 3 is over expressed in human pancreatic carcinoma
and regulated by retinoic acid in pancreatic carcinoma cell lines.
Gut. 43:692–698. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Perret AG, Duthel R, Fotso MJ, Brunon J
and Mosnier JF: Stromelysin-3 is expressed by aggressive
meningiomas. Cancer. 94:765–772. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mueller J, Brebeck B, Schmalfeldt B, Kuhn
W, Graeff H and Höfler H: Stromelysin-3 expression in invasive
ovarian carcinomas and tumours of low malignant potential. Virchows
Arch. 437:618–624. 2000. View Article : Google Scholar
|
|
25
|
Wlodarczyk J, Stolte M and Mueller J:
E-cadherin, beta-catenin and stromelysin-3 expression in de novo
carcinoma of the colorectum. Pol J Pathol. 52:119–124. 2001.
|
|
26
|
Wolf C, Rouyer N, Lutz Y, Adida C, Loriot
M, Bellocq JP, Chambon P and Basset P: Stromelysin 3 belongs to a
subgroup of proteinases expressed in breast carcinoma fibroblastic
cells and possibly implicated in tumor progression. Proc Natl Acad
Sci USA. 90:1843–1847. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Basset P, Okada A, Chenard MP, Kannan R,
Stoll I, Anglard P, Bellocq JP and Rio MC: Matrix
metalloproteinases as stromal effectors of human carcinoma
progression: therapeutic implications. Matrix Biol. 15:535–541.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Laurell H, Bouisson M, Berthelemy P,
Rochaix P, Dejean S, Besse P, Susini C, Pradayrol L, Vaysse N and
Buscail L: Identification of biomarkers of human pancreatic
adenocarcinomas by expression profiling and validation with gene
expression analysis in endoscopic ultrasound-guided fine needle
aspiration samples. World J Gastroenterol. 12:3344–3351. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barrasa JI, Olmo N, Santiago-Gómez A,
Lecona E, Anglard P, Turnay J and Lizarbe MA: Histone deacetylase
inhibitors upregulate MMP11 gene expression through Sp1/Smad
complexes in human colon adenocarcinoma cells. Biochim Biophys
Acta. 1823:570–581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Genestie C, Zafrani B, Asselain B,
Fourquet A, Rozan S, Validire P, Vincent-Salomon A and Sastre-Garau
X: Comparison of the prognostic value of Scarff-Bloom-Richardson
and Nottingham histological grades in a series of 825 cases of
breast cancer: Major importance of the mitotic count as a component
of both grading systems. Anticancer Res. 18:571–576.
1998.PubMed/NCBI
|
|
31
|
Min KW, Kim DH, Do SI, Pyo JS, Kim K, Chae
SW, Sohn JH, Oh YH, Kim HJ, Choi SH, et al: Diagnostic and
prognostic relevance of MMP-11 expression in the stromal
fibroblast-like cells adjacent to invasive ductal carcinoma of the
breast. Ann Surg Oncol. 20(Suppl 3): S433–S442. 2013. View Article : Google Scholar
|
|
32
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
33
|
Fu J, Khaybullin R, Zhang Y, Xia A and Qi
X: Gene expression profiling leads to discovery of correlation of
matrix metalloproteinase 11 and heparanase 2 in breast cancer
progression. BMC Cancer. 15:4732015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Roscilli G, Cappelletti M, De Vitis C,
Ciliberto G, Di Napoli A, Ruco L, Mancini R and Aurisicchio L:
Circulating MMP11 and specific antibody immune response in breast
and prostate cancer patients. J Transl Med. 12:542014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lustosa SA, Saconato H, Atallah AN, Lopes
Filho Gde J and Matos D: Impact of extended lymphadenectomy on
morbidity, mortality, recurrence and 5-year survival after
gastrectomy for cancer. Meta-analysis of randomized clinical
trials. Acta Cir Bras. 23:520–530. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao ZS, Chu YQ, Ye ZY, Wang YY and Tao
HQ: Overexpression of matrix metalloproteinase 11 in human gastric
carcinoma and its clinicopathologic significance. Hum Pathol.
41:686–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yan D, Dai H and Liu JW: Serum levels of
MMP-11 correlate with clinical outcome in Chinese patients with
advanced gastric adenocarcinoma. BMC Cancer. 11:1512011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Asano T, Tada M, Cheng S, Takemoto N,
Kuramae T, Abe M, Takahashi O, Miyamoto M, Hamada J, Moriuchi T, et
al: Prognostic values of matrix metalloproteinase family expression
in human colorectal carcinoma. J Surg Res. 146:32–42. 2008.
View Article : Google Scholar
|
|
39
|
Skoglund J, Emterling A, Arbman G, Anglard
P and Sun XF: Clinicopathological significance of stromelysin-3
expression in colorectal cancer. Oncology. 67:67–72. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bakkevold KE, Arnesjø B and Kambestad B:
Carcinoma of the pancreas and papilla of Vater: Presenting
symptoms, signs, and diagnosis related to stage and tumour site. A
prospective multi-centre trial in 472 patients Norwegian Pancreatic
Cancer Trial. Scand J Gastroenterol. 27:317–325. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rouyer N, Wolf C, Chenard MP, Rio MC,
Chambon P, Bellocq JP and Basset P: Stromelysin-3 gene expression
in human cancer: An overview. Invasion Metastasis. 14:269–275.
1995.
|
|
42
|
Andarawewa KL, Boulay A, Masson R,
Mathelin C, Stoll I, Tomasetto C, Chenard MP, Gintz M, Bellocq JP
and Rio MC: Dual stromelysin-3 function during natural mouse
mammary tumor virus-ras tumor progression. Cancer Res.
63:5844–5849. 2003.PubMed/NCBI
|
|
43
|
Noël A, Boulay A, Kebers F, Kannan R,
Hajitou A, Calberg-Bacq CM, Basset P, Rio MC and Foidart JM:
Demonstration in vivo that stromelysin-3 functions through its
proteolytic activity. Oncogene. 19:1605–1612. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Masson R, Lefebvre O, Noël A, Fahime ME,
Chenard MP, Wendling C, Kebers F, LeMeur M, Dierich A, Foidart JM,
et al: In vivo evidence that the stromelysin-3 metalloproteinase
contributes in a paracrine manner to epithelial cell malignancy. J
Cell Biol. 140:1535–1541. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kwon YJ, Hurst DR, Steg AD, Yuan K, Vaidya
KS, Welch DR and Frost AR: Gli1 enhances migration and invasion via
up-regulation of MMP-11 and promotes metastasis in ERα negative
breast cancer cell lines. Clin Exp Metastasis. 28:437–449. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fromigue O, Louis K, Wu E, Belhacène N,
Loubat A, Shipp M, Auberger P and Mari B: Active stromelysin-3
(MMP-11) increases MCF-7 survival in three-dimensional Matrigel
culture via activation of p42/p44 MAP-kinase. Int J Cancer.
106:355–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boulay A, Masson R, Chenard MP, El Fahime
M, Cassard L, Bellocq JP, Sautès-Fridman C, Basset P and Rio MC:
High cancer cell death in syngeneic tumors developed in host mice
deficient for the stromelysin-3 matrix metalloproteinase. Cancer
Res. 61:2189–2193. 2001.PubMed/NCBI
|
|
48
|
Krüger A, Soeltl R, Sopov I, Kopitz C,
Arlt M, Magdolen V, Harbeck N, Gänsbacher B and Schmitt M:
Hydroxamate-type matrix metalloproteinase inhibitor batimastat
promotes liver metastasis. Cancer Res. 61:1272–1275.
2001.PubMed/NCBI
|
|
49
|
Zucker S, Cao J and Chen WT: Critical
appraisal of the use of matrix metalloproteinase inhibitors in
cancer treatment. Oncogene. 19:6642–6650. 2000. View Article : Google Scholar
|
|
50
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Overall CM and López-Otín C: Strategies
for MMP inhibition in cancer: Innovations for the post-trial era.
Nat Rev Cancer. 2:657–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kanharat N and Tuamsuk P: Correlation
between microvascular density and matrix metalloproteinase 11
expression in prostate cancer tissues: A preliminary study in
Thailand. Asian Pac J Cancer Prev. 16:6639–6643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rio MC: From a unique cell to metastasis
is a long way to go: Clues to stromelysin-3 participation.
Biochimie. 87:299–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Brasse D, Mathelin C, Leroux K, Chenard
MP, Blaise S, Stoll I, Tomasetto C and Rio MC: Matrix
metalloproteinase 11/ stromelysin-3 exerts both activator and
repressor functions during the hematogenous metastatic process in
mice. Int J Cancer. 127:1347–1355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kasper G, Reule M, Tschirschmann M,
Dankert N, Stout-Weider K, Lauster R, Schrock E, Mennerich D, Duda
GN and Lehmann KE: Stromelysin-3 over-expression enhances
tumourigenesis in MCF-7 and MDA-MB-231 breast cancer cell lines:
Involvement of the IGF-1 signalling pathway. BMC Cancer. 7:122007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jones L, Ghaneh P, Humphreys M and
Neoptolemos JP: The matrix metalloproteinases and their inhibitors
in the treatment of pancreatic cancer. Ann NY Acad Sci.
880:288–307. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Johansson N, Ahonen M and Kähäri VM:
Matrix metalloproteinases in tumor invasion. Cell Mol Life Sci.
57:5–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Selvey S, Haupt LM, Thompson EW, Matthaei
KI, Irving MG and Griffiths LR: Stimulation of MMP-11
(stromelysin-3) expression in mouse fibroblasts by cytokines,
collagen and co-culture with human breast cancer cell lines. BMC
Cancer. 4:402004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Itoh H, Kishore AH, Lindqvist A, Rogers DE
and Word RA: Transforming growth factor β1 (TGFβ1) and progesterone
regulate matrix metalloproteinases (MMP) in human endometrial
stromal cells. J Clin Endocrinol Metab. 97:E888–E897. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Buache E, Thai R, Wendling C, Alpy F, Page
A, Chenard MP, Dive V, Ruff M, Dejaegere A, Tomasetto C, et al:
Functional relationship between matrix metalloproteinase-11 and
matrix metalloproteinase-14. Cancer Med. 3:1197–1210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu D, Li M, Wang L, Zhou Y, Zhou J, Pan H
and Qu P: microRNA-145 inhibits cell proliferation, migration and
invasion by targeting matrix metallopeptidase-11 in renal cell
carcinoma. Mol Med Rep. 10:393–398. 2014.PubMed/NCBI
|
|
62
|
Jiang Y, Wang L, Gong W, Wei D, Le X, Yao
J, Ajani J, Abbruzzese JL, Huang S and Xie K: A high expression
level of insulin-like growth factor I receptor is associated with
increased expression of transcription factor Sp1 and regional lymph
node metastasis of human gastric cancer. Clin Exp Metastasis.
21:755–764. 2004. View Article : Google Scholar
|
|
63
|
Min Y, Adachi Y, Yamamoto H, Imsumran A,
Arimura Y, Endo T, Hinoda Y, Lee CT, Nadaf S, Carbone DP, et al:
Insulin-like growth factor I receptor blockade enhances
chemotherapy and radiation responses and inhibits tumour growth in
human gastric cancer xenografts. Gut. 54:591–600. 2005. View Article : Google Scholar
|
|
64
|
Sharma R, Chattopadhyay TK, Mathur M and
Ralhan R: Prognostic significance of stromelysin-3 and tissue
inhibitor of matrix metalloproteinase-2 in esophageal cancer.
Oncology. 67:300–309. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mañes S, Mira E, Barbacid MM, Ciprés A,
Fernández-Resa P, Buesa JM, Mérida I, Aracil M, Márquez G and
Martínez-A C: Identification of insulin-like growth factor-binding
protein-1 as a potential physiological substrate for human
stromelysin-3. J Biol Chem. 272:25706–25712. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liang Q, Xiong H, Gao G, Xiong K, Wang X,
Zhao Z, Zhang H and Li Y: Inhibition of basigin expression in
glioblastoma cell line via antisense RNA reduces tumor cell
invasion and angiogenesis. Cancer Biol Ther. 4:759–762. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tang Y, Nakada MT, Rafferty P, Laraio J,
McCabe FL, Millar H, Cunningham M, Snyder LA, Bugelski P and Yan L:
Regulation of vascular endothelial growth factor expression by
EMMPRIN via the PI3K-Akt signaling pathway. Mol Cancer Res.
4:371–377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tian X, Ye C, Yang Y, Guan X, Dong B, Zhao
M and Hao C: Expression of CD147 and matrix metalloproteinase-11 in
colorectal cancer and their relationship to clinicopathological
features. J Transl Med. 13:3372015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Jia L, Cao J, Wei W, Wang S, Zuo Y and
Zhang J: CD147 depletion down-regulates matrix
metalloproteinase-11, vascular endothelial growth factor-A
expression and the lymphatic metastasis potential of murine
hepatocarcinoma Hca-F cells. Int J Biochem Cell Biol. 39:2135–2142.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jia L, Zhou H, Wang S, Cao J, Wei W and
Zhang J: Deglycosylation of CD147 down-regulates matrix
metalloproteinase-11 expression and the adhesive capability of
murine hepatocarcinoma cell HcaF in vitro. IUBMB Life. 58:209–216.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Honkavuori M, Talvensaari-Mattila A, Soini
Y, Turpeenniemi-Hujanen T and Santala M: MMP-2 expression
associates with CA 125 and clinical course in endometrial
carcinoma. Gynecol Oncol. 104:217–221. 2007. View Article : Google Scholar
|
|
72
|
Ogawa M, Ikeuchi K, Watanabe M, Etoh K,
Kobayashi T, Takao Y, Anazawa S and Yamazaki Y: Expression of
matrix metalloproteinase 7, laminin and type IV collagen-associated
liver metastasis in human colorectal cancer: Immunohistochemical
approach. Hepatogastroenterology. 52:875–880. 2005.PubMed/NCBI
|
|
73
|
Zheng H, Takahashi H, Murai Y, Cui Z,
Nomoto K, Niwa H, Tsuneyama K and Takano Y: Expressions of MMP-2,
MMP-9 and VEGF are closely linked to growth, invasion, metastasis
and angiogenesis of gastric carcinoma. Anticancer Res.
26:3579–3583. 2006.PubMed/NCBI
|
|
74
|
Wu CY, Wu MS, Chiang EP, Chen YJ, Chen CJ,
Chi NH, Shih YT, Chen GH and Lin JT: Plasma matrix
metalloproteinase-9 level is better than serum matrix
metalloproteinase-9 level to predict gastric cancer evolution. Clin
Cancer Res. 13:2054–2060. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mylona E, Nomikos A, Magkou C, Kamberou M,
Papassideri I, Keramopoulos A and Nakopoulou L: The
clinicopathological and prognostic significance of membrane type 1
matrix metalloproteinase (MT1-MMP) and MMP-9 according to their
localization in invasive breast carcinoma. Histopathology.
50:338–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang YH, Deng H, Li WM, Zhang QY, Hu XT,
Xiao B, Zhu HH, Geng PL and Lu YY: Identification of matrix
metalloproteinase 11 as a predictive tumor marker in serum based on
gene expression profiling. Clin Cancer Res. 14:74–81. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Cheng CW, Yu JC, Wang HW, Huang CS, Shieh
JC, Fu YP, Chang CW, Wu PE and Shen CY: The clinical implications
of MMP-11 and CK-20 expression in human breast cancer. Clin Chim
Acta. 411:234–241. 2010. View Article : Google Scholar
|
|
78
|
Jones LE, Humphreys MJ, Campbell F,
Neoptolemos JP and Boyd MT: Comprehensive analysis of matrix
metalloproteinase and tissue inhibitor expression in pancreatic
cancer: increased expression of matrix metalloproteinase-7 predicts
poor survival. Clin Cancer Res. 10:2832–2845. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Thewes M, Pohlmann G, Atkinson M, Mueller
J, Pütz B and Höfler H: Stromelysin-3 (ST-3) mRNA expression in
colorectal carcinomas. Localization and clinicopathologic
correlations. Diagn Mol Pathol. 5:284–290. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ito Y, Yoshida H, Kakudo K, Nakamura Y,
Kuma K and Miyauchi A: Inverse relationships between the expression
of MMP-7 and MMP-11 and predictors of poor prognosis of papillary
thyroid carcinoma. Pathology. 38:421–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Mellick AS, Blackmore D, Weinstein SR and
Griffiths LR: An assessment of MMP and TIMP gene expression in cell
lines and stroma - tumour differences in microdissected breast
cancer biopsies. Tumour Biol. 24:258–270. 2003. View Article : Google Scholar
|
|
82
|
Têtu B, Trudel D and Wang CS: Proteases by
reactive stromal cells in cancer: An attractive therapeutic target.
Bull Cancer. 93:944–948. 2006.In French.
|
|
83
|
Hsin CH, Chen MK, Tang CH, Lin HP, Chou
MY, Lin CW and Yang SF: High level of plasma matrix
metalloproteinase-11 is associated with clinicopathological
characteristics in patients with oral squamous cell carcinoma. PLoS
One. 9:e1131292014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation-related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pedersen G, Saermark T, Kirkegaard T and
Brynskov J: Spontaneous and cytokine induced expression and
activity of matrix metalloproteinases in human colonic epithelium.
Clin Exp Immunol. 155:257–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Xu CJ and Xu F: MMP-11 and VEGF-C
expression correlate with clinical features of colorectal
adenocarcinoma. Int J Clin Exp Med. 7:2883–2888. 2014.PubMed/NCBI
|
|
87
|
Eiró N, Fernandez-Garcia B, Vázquez J, Del
Casar JM, González LO and Vizoso FJ: A phenotype from tumor stroma
based on the expression of metalloproteases and their inhibitors,
associated with prognosis in breast cancer. Oncoimmunology.
4:e9922222015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mari BP, Anderson IC, Mari SE, Ning Y,
Lutz Y, Kobzik L and Shipp MA: Stromelysin-3 is induced in
tumor/stroma cocultures and inactivated via a tumor-specific and
basic fibroblast growth factor-dependent mechanism. J Biol Chem.
273:618–626. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Peruzzi D, Mori F, Conforti A, Lazzaro D,
De Rinaldis E, Ciliberto G, La Monica N and Aurisicchio L: MMP11: a
novel target antigen for cancer immunotherapy. Clin Cancer Res.
15:4104–4113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hofmeister V, Schrama D and Becker JC:
Anti-cancer therapies targeting the tumor stroma. Cancer Immunol
Immunother. 57:1–17. 2008. View Article : Google Scholar
|
|
91
|
Yi T, Wei YQ, Tian L, Zhao X, Li J, Deng
HX, Wen YJ, Zou CH, Tan GH, Kan B, et al: Humoral and cellular
immunity induced by tumor cell vaccine based on the chicken
xenogeneic homologous matrix metalloproteinase-2. Cancer Gene Ther.
14:158–164. 2007. View Article : Google Scholar
|
|
92
|
Yokoyama Y, Grünebach F, Schmidt SM,
Lazzaro D, De Rinaldis E, Ciliberto G, La Monica N and Aurisicchio
L: Matrilysin (MMP-7) is a novel broadly expressed tumor antigen
recognized by antigen-specific T cells. Clin Cancer Res.
14:5503–5511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Groves MD, Puduvalli VK, Hess KR, Jaeckle
KA, Peterson P, Yung WK and Levin VA: Phase II trial of
temozolomide plus the matrix metalloproteinase inhibitor,
marimastat, in recurrent and progressive glioblastoma multiforme. J
Clin Oncol. 20:1383–1388. 2002. View Article : Google Scholar : PubMed/NCBI
|