Introduction
The herbicide atrazine (2-chloro- 4-(ethylamino)-6
-(isopropylamino)-S-triazine; ATR) is a pesticide widely used in
agriculture worldwide to control broadleaf weeds in crops such as
corns, sugarcane, and sorghum (1).
In the United States alone, the annual use of ATR is ~65–80 million
pounds (2). Gerecke et al
demonstrated that atrazine is the most frequently detected
pesticide in soil and surface water even in urban areas (3). In China, atrazine has been reported
in Guanting reservoir and Taihu Lake (4,5).
Previous in vivo toxicological studies mostly focused on the
effects of atrazine on endocrine and reproductive systems,
demonstrating that it overtly disrupts the hypothalamic control of
pituitary-ovarian function, which results in altered pituitary
prolactin and luteinizing hormone secretion (6–8). In
addition, atrazine is associated with global DNA hypomethylation
(9), and induces apoptosis of
splenocytes (10). In terms of
relevance between atrazine and tumors, Boffetta et al
insisted that ATR has no causal association with tumor development
and should be considered an agent unlikely to pose a cancer risk to
humans (11); however, Van Leeuwen
et al reported that ATR contamination levels are positively
associated with stomach cancer incidence (12). It should be noted that the above
views are mostly based on epidemiological studies.
In this study, the RM1 prostate cancer cell line was
used to study the effects of atrazine in vitro, and a
subcutaneous tumor model was established in mice to explore the
impacts of atrazine on tumors in vivo as well as the
underlying mechanisms.
Materials and methods
Cell lines and reagents
RM1 cells were obtained from the Shanghai Institute
of Cell Biology, Chinese Academy of Sciences (Shanghai, China).
They were cultured in IMDM supplemented with 10% fetal bovine serum
(Gibco BRL, Gaithersburg, MD, USA), in a humidified environment
with 5% CO2 at 37°C. Atrazine (99% purity), SDS, TEMED,
Acrylamide, N, N-Dimethyl-bis-acrylamide, DTT and PMSF were
obtained from Sigma Chemical Co. (USA). ATR stock solutions
(10−2 mol/l) were prepared in corn oil (13), and kept at 4°C for a maximum of 1
week. Anti-Grim-19, Stat3, MMP9, MMP2, VEGF, PCNA, P53, c-myc,
Cyclin B1, Cyclin D1, Bcl-2, Bax, and Caspase-3 primary antibodies,
as well as secondary antibodies were acquired from Proteintech
Group (USA). Pierce ECL Plus kit was purchased from Thermo Fisher
Scientific Inc. (USA).
Tumor xenograft models
C57BL6 mice (18–22 g) were purchased from the
Beijing Institute for Experimental Animals, and housed at constant
room temperature (23±1°C) and relative humidity (50%) under a 12-h
light/dark cycle, with food and water ad libitum. Mice were
transplanted subcutaneously with 2×106 RM1 cells on the
right flank and randomly divided into three groups for 15 daily
oral treatments with 0, 20 and 100 mg/kg atrazine, respectively
(14). The animals were sacrificed
by ether and transplanted tumors were removed, fixed in 10%
formalin or stored at −80°C. The animal experiments were conducted
following internationally recognized guidelines, and approved by
the Animal Research Committee of Norman Bethune College of
Medicine, Jilin University.
MTT assay
The effects of atrazine on RM1 cell proliferation
in vitro was measured using MTT (Sigma-Aldrich, St. Louis,
MO, USA). Briefly, RM1 cells were seeded in 96-well plates and
incubated at 37°C in the presence of variable concentrations of
atrazine, including 0 μM (control), 0.1 and 0.01 μM for 24 h. Then,
10 μl of 5 mg/ml MTT in PBS was added to each well then incubated
for 4 h at 37°C. After careful removal of the culture medium, the
formazan crystals were solubilized in 100 μl DMSO (Sigma) with
shaking for 15 min. Absorption was measured at 570 nm on a
microplate reader (Bio-Rad). Each assay was performed nine times
and results are expressed as mean ± SE (15).
Colony formation assay
Cells were seeded in 6-well culture plates at
104 cells/well. After 10 days of incubation, they were
stained with Giemsa. Colonies with >50 cells were counted, and
colony formation rate acquired as the number of colonies formed to
that of seeded cells (16).
Invasion assay
Cell invasion was measured using a Matrigel-coated
film insert (8-μm pore size) fitting into 24-well invasion chambers
(Millipore, GE, USA). A total of 5×104 cells were
suspended in 200 μl IMDM, and added into the upper compartment of
the invasion chamber; after the medium was removed, cells on the
lower surface of the filter were stained and counted under a
microscope (Olympus) at a magnification of ×200 (17). The cells in the lower chamber were
incubated at 37°C for another 24 h; MTT was added to a final
concentration of 1 mM. After 4 h, formazan crystals were dissolved
by addition of DMSO. Optical densities were measured on a
microplate reader at 490 nm. Data are mean ± standard error from at
least 3 independent experiments.
In vitro wound-healing assay
Cells were cultured in a 6-well plate. Confluent
monolayers were scratched with a 200-μl pipette tip to generate a
wound. The monolayer was then washed twice with PBS to remove the
detached cells, supplemented with different doses of atrazine, and
incubated for 48 h at 37°C. Images were captured on a Nikon Eclipse
TS-100 microscope fitted with a digital camera to monitor the cell
movement into the wounded area. All micrographs were taken at the
same time and magnification (18).
Real-time quantitative PCR
Gene expression levels of Stat3 and its downstream
genes were determined using real-time quantitative PCR (qPCR).
Cells were collected after 48 h, and total RNA was extracted using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Reverse
transcription was performed with 2 μg total RNA treated with DNAse
I, using a commercially available RT-PCR kit (Promega, Madison, WI,
USA) according to the manufacturer's instructions. Quantitative-PCR
primers are shown in Table I. Each
reaction (25 μl) was carried out under the following cycling
conditions: initialization for 10 min at 95°C; 40 cycles of
amplification, with 15 sec at 95°C (denaturation), and 1 min at
60°C (annealing and elongation). A standard curve was plotted for
each primer probe by using a serial dilution of pooled cDNA from
cells. All standards and samples were assessed in triplicate.
Quantification was carried out by normalizing gene expression
levels to total cDNA amounts obtained for the ubiquitously
expressed β-actin as a standard (19).
 | Table IPrimer sets used for real-time
qRT-PCR. |
Table I
Primer sets used for real-time
qRT-PCR.
| Gene | Primers |
|---|
| Stat3 |
5′-TTGCCAGTTGTGGTGATC-3′
5′-AGAACCCAGAAGGAGAAGC-3′ |
| Grim19 |
5′-GTGGTGTAGGCACTGCAGGTTC-3′
5′-GAACTTCGTCGATCGACCTCGA-3′ |
| Bax |
5-′AGGGTTTCATCCAGGATCGAGC-3′
5-′AGGCGGTGAGGACTCCAGCC-3′ |
| Bcl-2 |
5′-ACTTGACAGAAGATCATGCC-3′
5′-GGTTATCATACCCTGTTCTC-3′ |
| Caspase-3 |
5′-TGGACTGTGGCATTGAGAC-3′
5′-AGGAATAGTAACCAGGTGCTG-3′ |
| VEGF |
5′-AGTCCCATGAAGTGATCAAGTTC-3′
5′-ATCCGCATGATCTGCATGG-3′ |
| c-myc |
5′-AGTTGGACAGTGGCAGGG-3′
5′-ACAGGATGTAGGCGGTGG-3′ |
| p53 |
5′-GGCCATCTACAAGCAGTCACAG-3′
5′-AGCAAATCTACAAGCAGTCACAG-3′ |
| p21 |
5′-TAGCAGCGGAACAAGGAG-3′
5′-AAACGGGAACCAGGACAC-3′ |
| Cyclin D1 |
5′-CGCCTTCCGTTTCTTACTTCA-3′
5′-AACTTCTCGGCAGTCAGGGGA-3′ |
| MMP2 |
5′-GGAAGCATCAAATCGGACTG-3′
5′-CACCCTCTTAAATCTGAAATCACC-3′ |
| MMP9 |
5′-CCCACTTACTTTGGAAACG-3′
5′-GAAGATGAATGGAAATACGC-3′ |
| Cyclin B1 |
5′-GCGTGTGCCTGTGACAGTTA-3′
5′-CCTAGCGTTTTTGCTTCCCTT-3′ |
| β-actin |
5′-ATATCGCTGCGCTGGTCGTC-3′
5′-AGGATGGCGTGAGGGAGAGC-3′ |
Apoptosis and cell cycle assessment
RM1 cells (1×106) were treated with the
indicated drugs for up to 48 h, collected, and resuspended in 100
μl PBS. Then, 5 μl PI (Beckman Coulter, Fullerton, CA, USA) was
added for 30 min at room temperature in the dark. The cells were
assessed by flow cytometry to measure apoptosis rates on an
Epics-XL-MCL flow cytometer (Beckman Coulter). Cell cycle
distribution was analyzed by measuring the DNA fragments stained
with PI, as described by the manufacturer. RM1 cells grown in
6-well plates were harvested and centrifuged at 48 h after
transfection. Cells were counted and washed twice with pre-cooled
PBS. Then, cells were fixed and permeabilized overnight by adding 1
ml of 70% pre-cooled ethanol to each tube at 4°C. After
centrifugation, the fixatives were decanted and cells were
resuspended in 0.5 ml of staining solution containing 50 μg/ml PI
and 100 μg/ml DNase-free RNase (Sigma-Aldrich) followed by 30 min
of incubation at room temperature in the dark. Finally, cells were
analyzed immediately by flow cytometry on a FACScan™ system using
CellQuest™ software (version 3.3) (BD Biosciences, San Jose, CA,
USA).
H&E staining and
immunohistochemistry
Tumor specimens were fixed in 10% formalin,
paraffin-embedded, and cut into 4-μm thick sections. The slides
were dewaxed, and stained routinely with hematoxylin-eosin for
histological assessments.
For immunohistochemistry, tumor tissue blocks were
fixed in formalin, cut into 5-μm-thick sections, stained with
H&E, and processed for routine histological examination. For
immunohistochemistry, slices were dewaxed; endogenous peroxidase
activity was quenched by incubation in methanol containing 3%
hydrogen peroxide for 10 min. The sections were immunostained with
rabbit anti-mouse MMP-2 and MMP-9 polyclonal antibodies (Santa Cruz
Biotechnology Inc., USA). As a negative control, rabbit
immunoglobulins (Vector Laboratories, Burlingame, CA, USA) were
used to replace the primary antibodies. Goat anti-rabbit IgG
conjugated with horseradish peroxidase was used as secondary
antibody. Immunohistochemical staining was performed at room
temperature using the avidin-biotin-peroxidase complex method
(Vectastain Elite ABC kit; Vector Laboratories).
Western blot analysis
Cell lysis, protein quantification and western blot
assays were performed as previously described (20). The protein bands were visualized by
SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford,
IL, USA), and membranes were subjected to X-ray autoradiography.
Band intensities were determined with the Quantity One software
(Bio-Rad). Experiments were performed in triplicate, and data are
mean ± SE.
Statistical analysis
Data are mean ± SE from three independent
experiments. Statistical analyses were performed with SPSS17.0; the
different groups were compared by one-factor analysis of variance
(ANOVA). P<0.05 was considered statistically significant.
Results
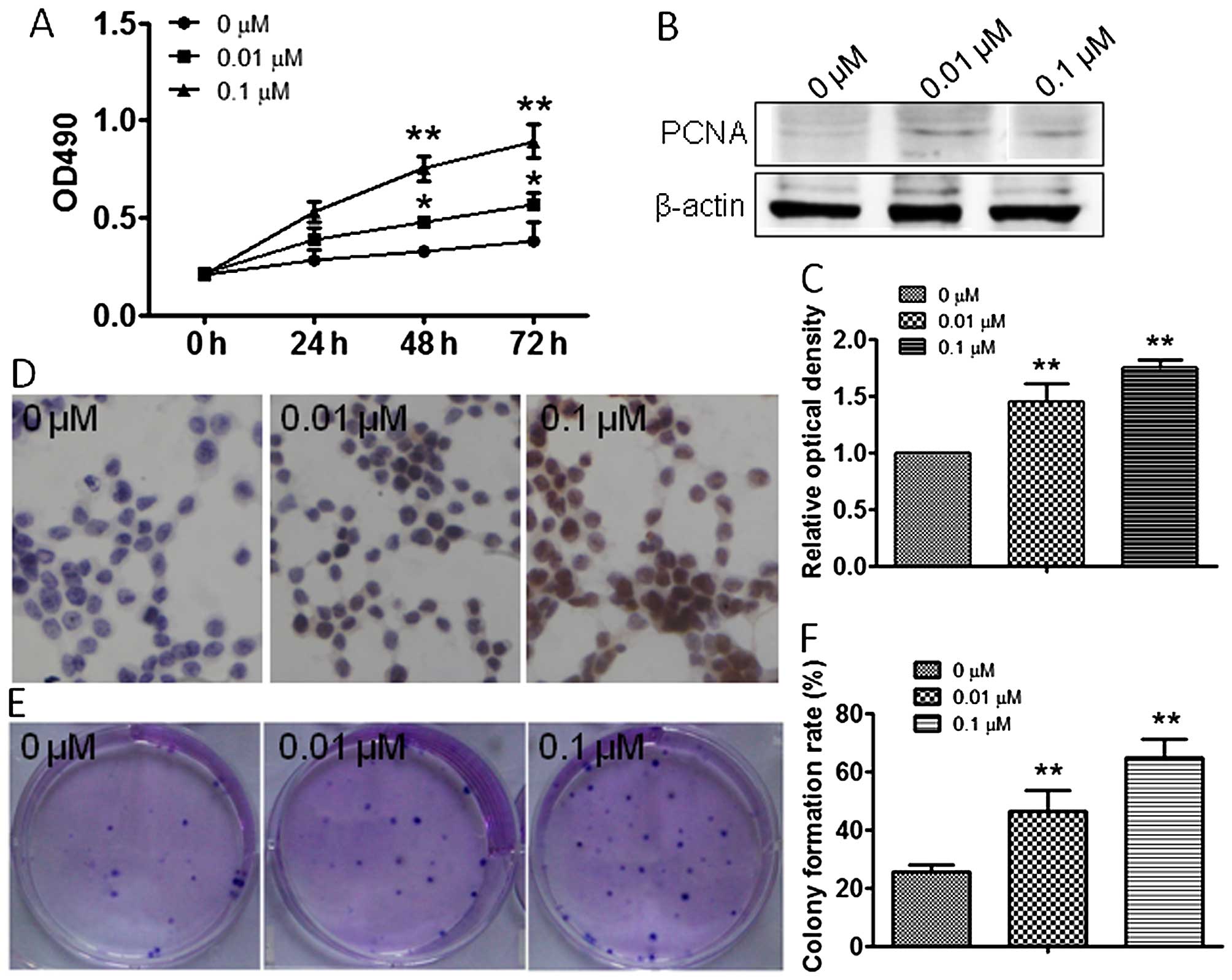
Atrazine promotes RM1 cell
proliferation
To explore the effect of atrazine on RM1 cell
growth, MTT and colony formation assays were employed in
vitro. MTT data showed significantly increased cell viability
after treatment with 0.01 and 0.1 μM atrazine compared with the
control group, in a dose-dependent manner (Fig. 1A). To further assess the effects of
atrazine on cell division, the expression levels of factors related
to cell proliferation were assessed. Western blotting and
immunocytochemistry were used to evaluate the expression of PCNA
in vivo and in vitro. Compared with the control
group, PCNA expression was dose-dependently higher after treatment
with 0.01 and 0.1 μM atrazine (Fig.
1B–D). Colony formation assay results showed significantly
enhanced proliferation ability of cells in the 0.01 and 0.1 μM
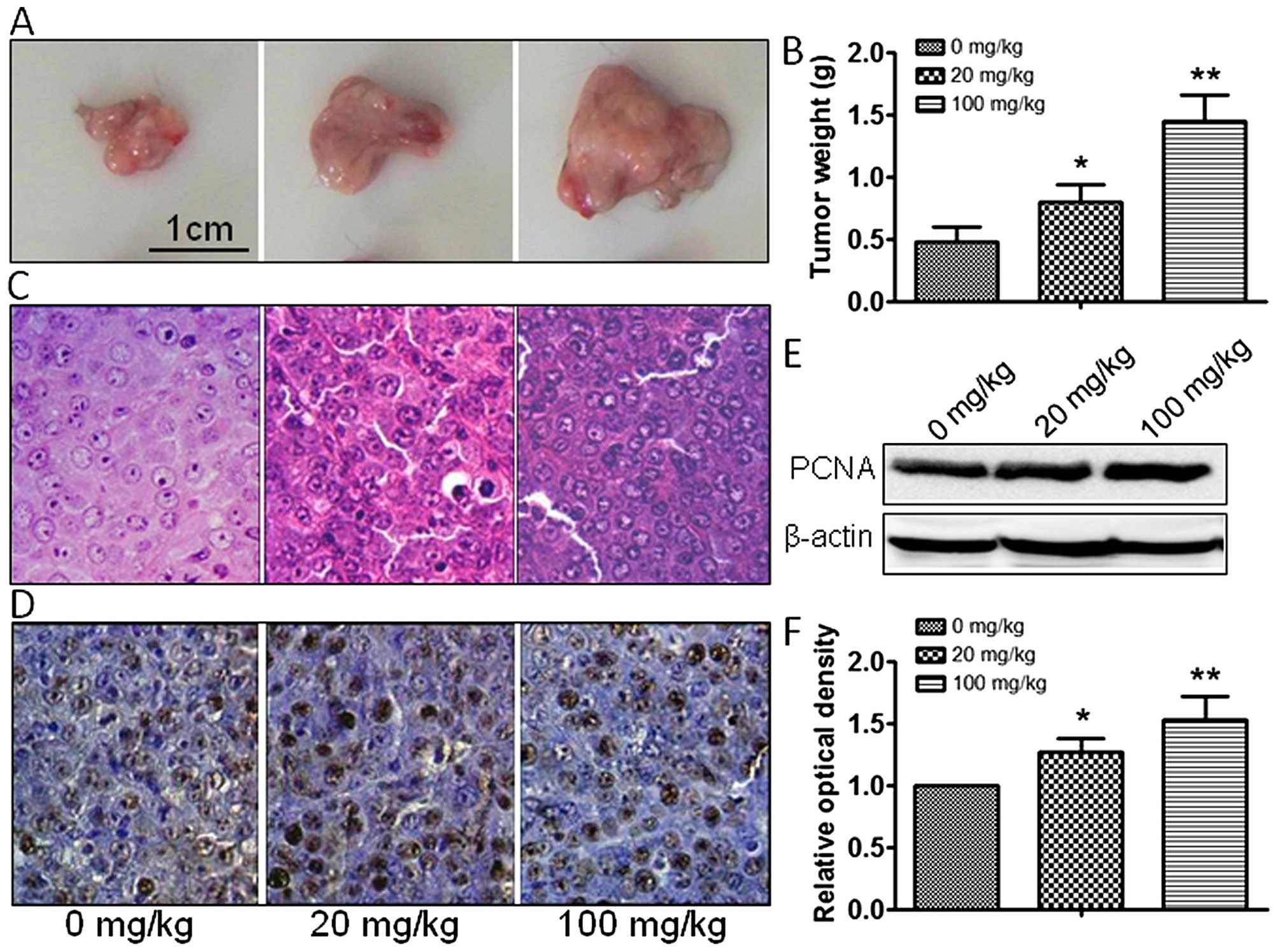
atrazine groups, compared with the control group (Fig. 1E and 1F). In vivo, tumor
size and weight were measured, and atrazine was found to accelerate
the growth of tumor cells in a dose-dependent manner (Fig. 2A–C). In addition, PCNA expression
was dose-dependently increased by 20 and 100 mg/kg atrazine
(Fig. 2D–F). Taken together, these
data indicated that atrazine promoted RM1 cell proliferation.
Together, these data suggested that atrazine promoted RM1 cell
proliferation.
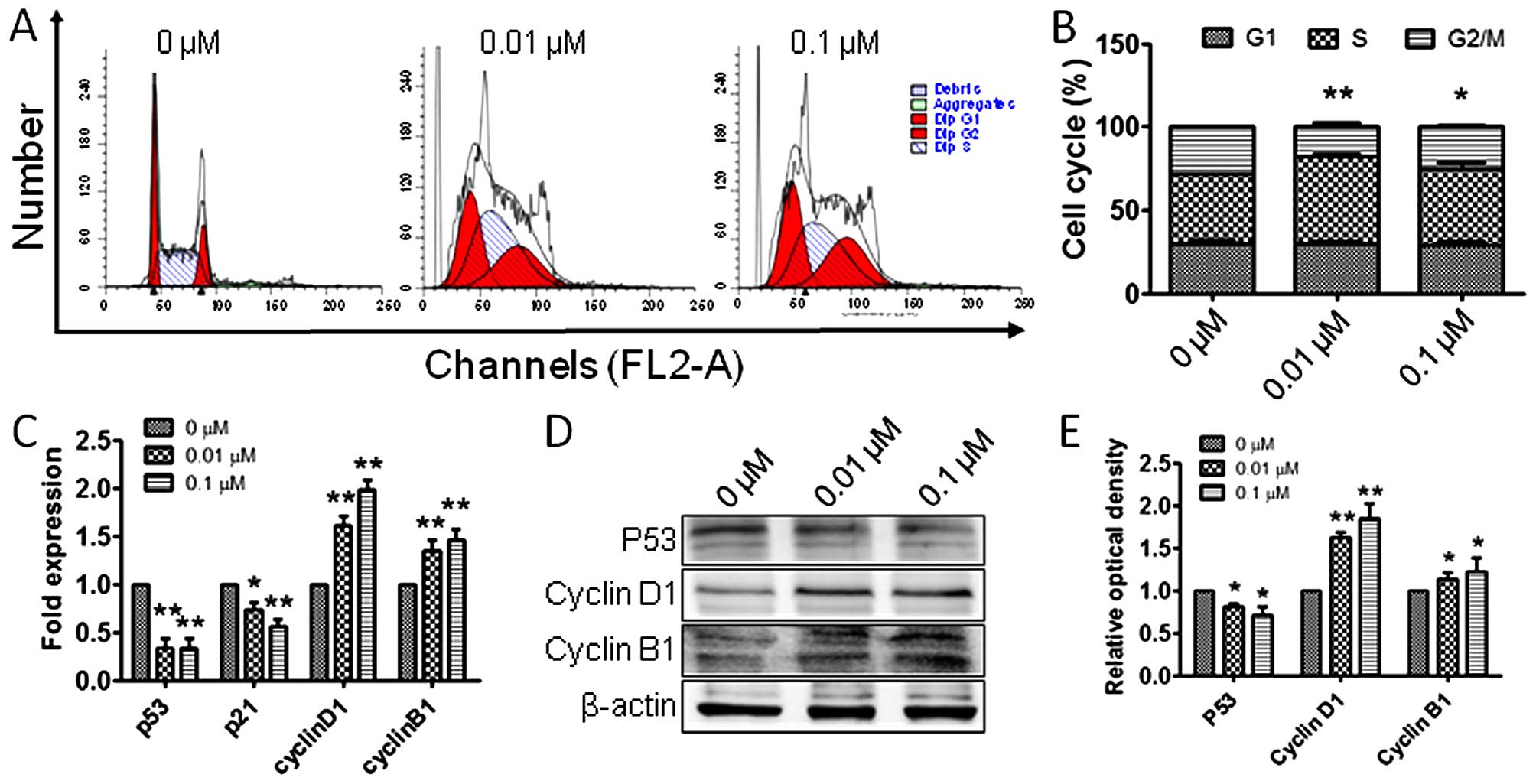
Atrazine accelerates cell cycle
progression in RM1 cells
To explore whether the observed proliferation of RM1
cells is related to cell cycle alterations, flow cytometry was
employed to detect the cell populations in the G1, S and G2 phases.
Interestingly, RM1 cells in G2 phase were significantly increased
after treatment with 0.01 and 0.1 μM atrazine, respectively,
compared with the control group (Fig.
3A and B), indicating that atrazine may promote RM1 cell
proliferation by increasing the G2 population. To determine the
molecular mechanisms by which atrazine increases cells in G2 phase,
RT-PCR and western blot analyses were employed to assess the
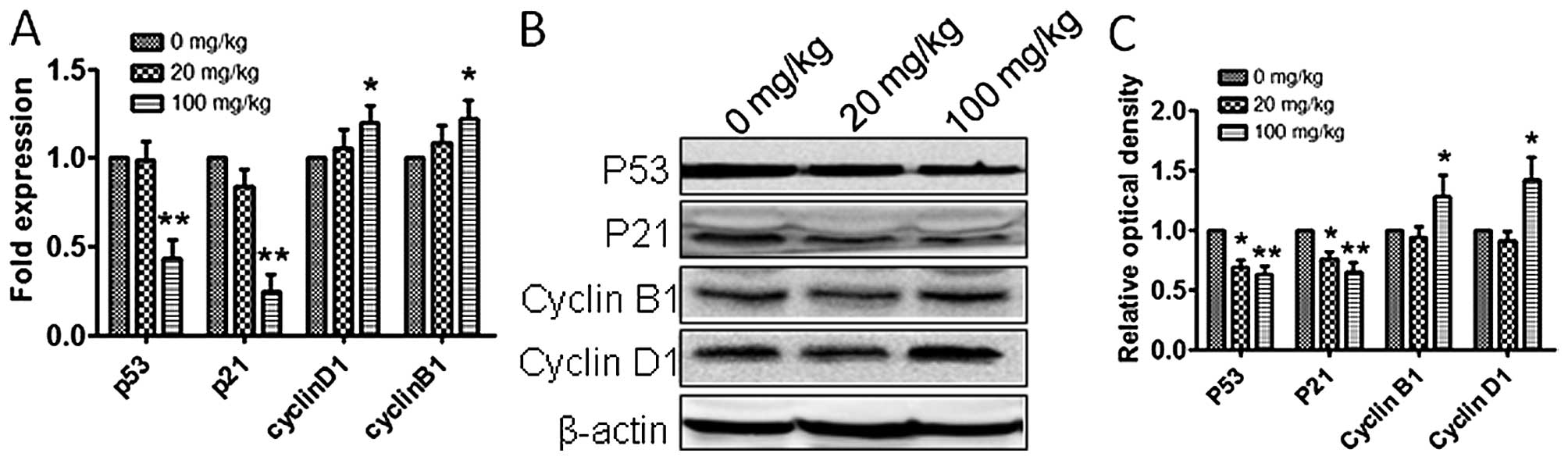
expression levels of p53, cyclin B1 and cyclin D1, in vitro
and in vivo. The expression of p53 was downregulated and
cyclin B1 and cyclin D1 amounts were upregulated (Figs. 3C–E and 4), which suggested that atrazine can
accelerate the cell cycle by helping RM1 cells go through the G1-S
and G2-M checkpoints.
Atrazine has no remarkable impact on
apoptosis in RM1 cells
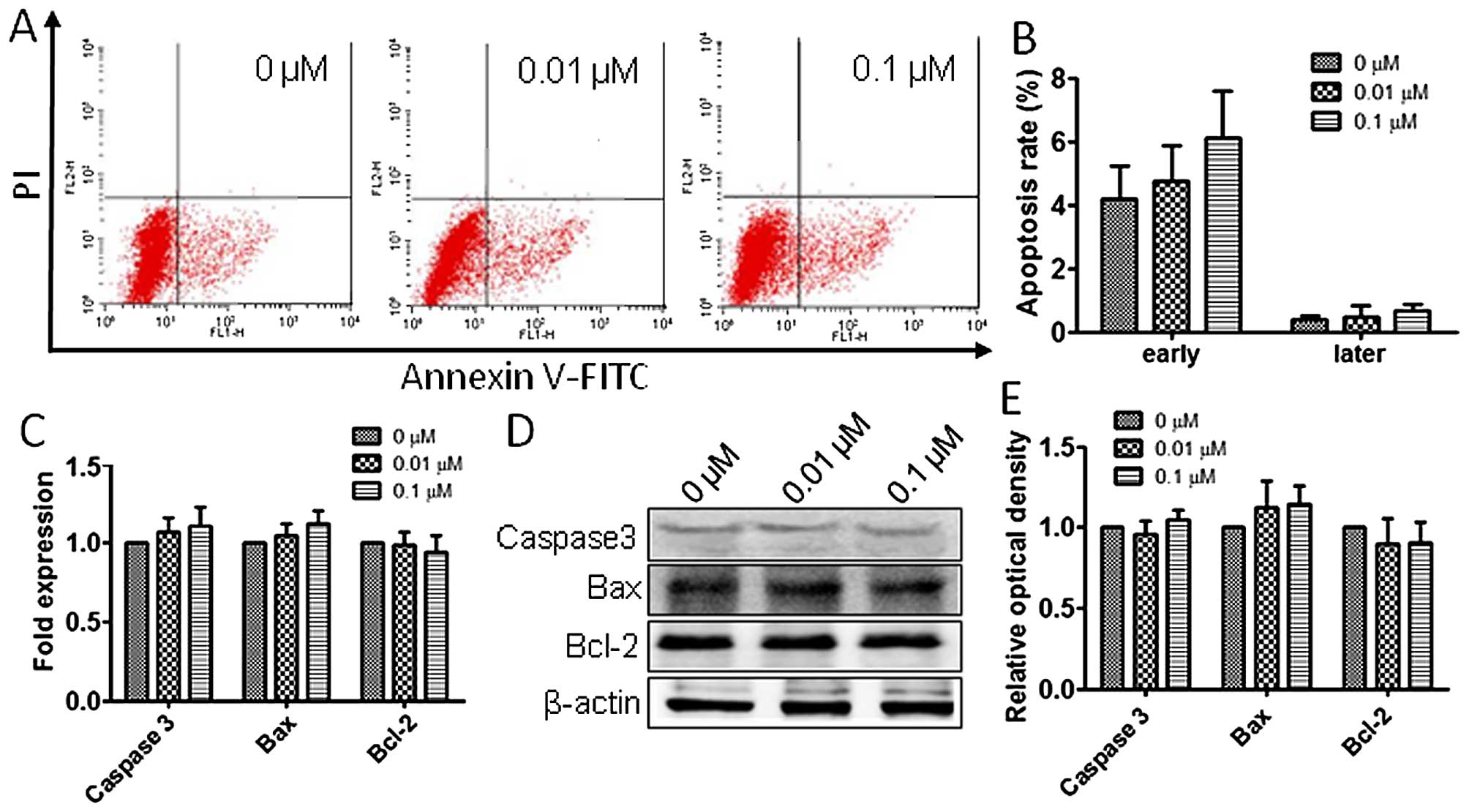
To assess the atrazine effects on RM1 cell
apoptosis, flow cytometry analysis was employed as described above.
As shown in Fig. 5A and B,
apoptosis rates in the three groups showed no statistically
significant difference, suggesting that atrazine has no significant
impact on apoptosis in RM1 cells. Also, RT-PCR and western blot
analyses were employed to evaluate the mRNA and protein levels of
Caspase-3, Bax and Bcl-2 in vitro, and no significant
differences among groups treated by different concentrations of
atrazine and controls were obtained (Fig. 5C–E).
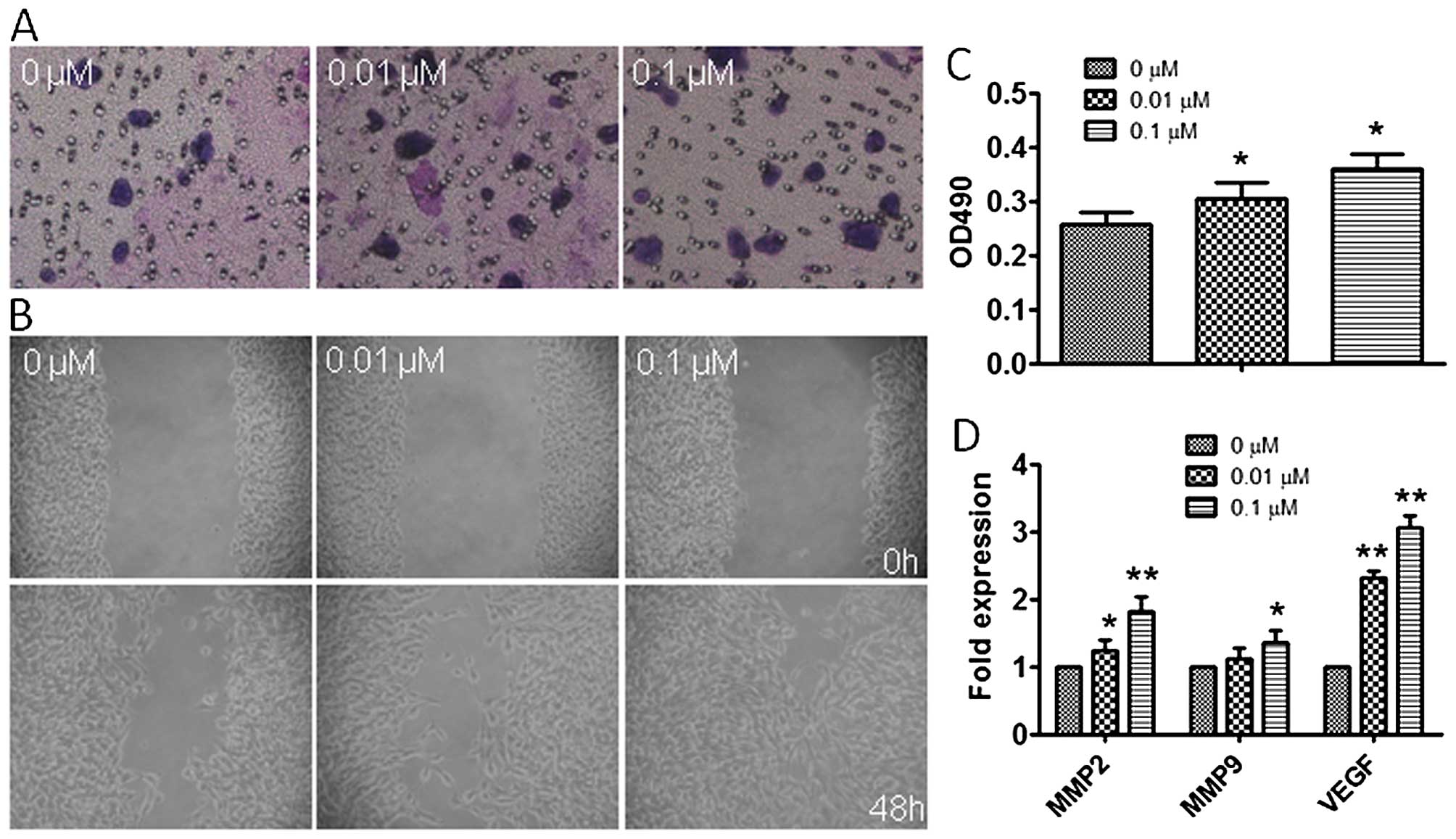
Atrazine enhances invasion and migration
in RM1 cells
To explore the effects of atrazine on migration and
invasion in RM1 cells, wound healing and transwell migration assays
were employed. Interestingly, migration of cells in the 0.01 and
0.1 μM atrazine groups were significantly enhanced compared to
controls in wound closure (Fig.
6A); in addition, the number of invasive cells exposed to
atrazine were remarkably increased compared with values obtained
for the control group, especially at high dose (Fig. 6B and C). Moreover, western
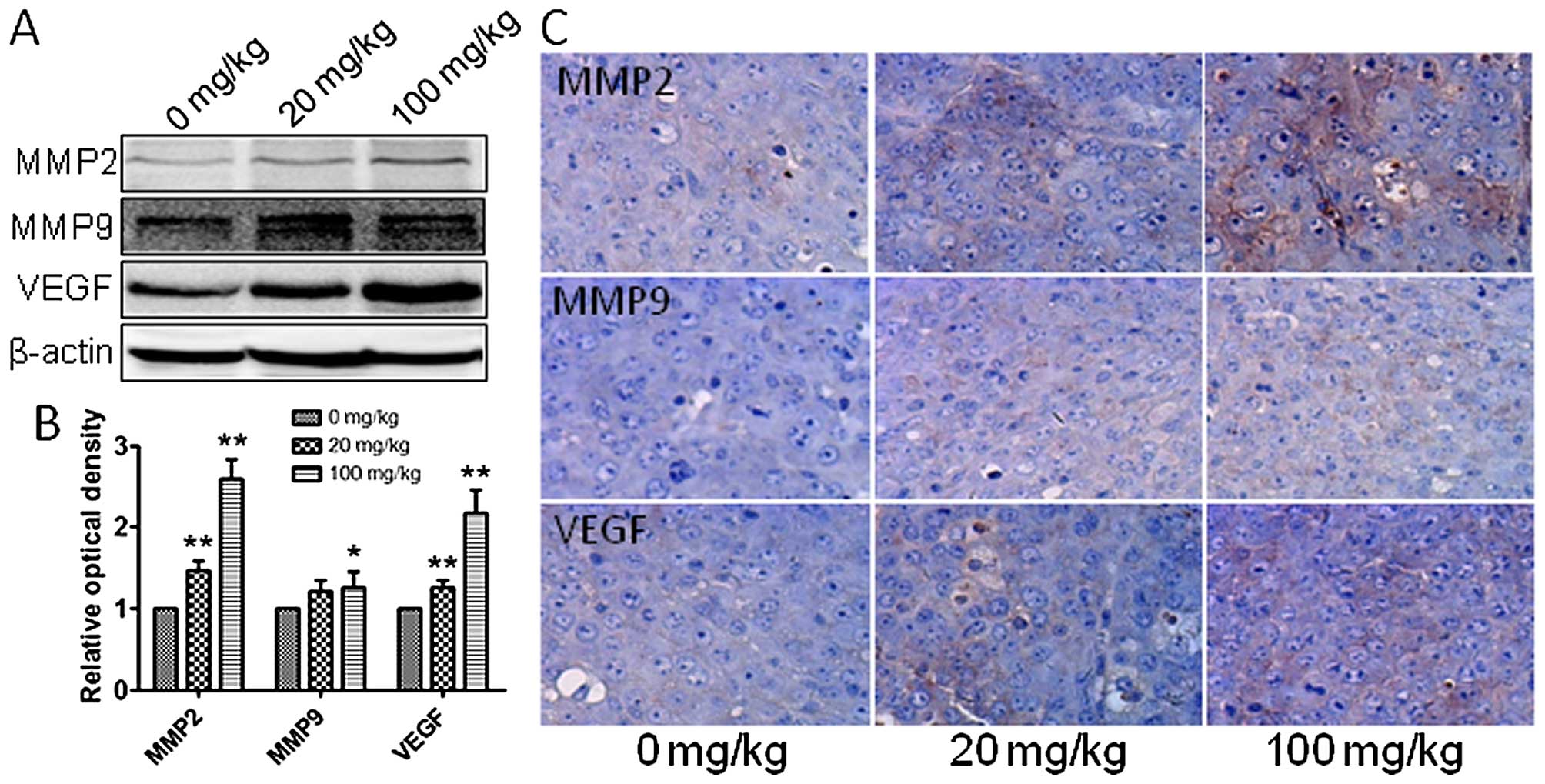
blotting, qRT-PCR and immunohistochemistry were employed to assess
the in vivo expression of MMP2, MMP9 and VEGF, which are
related to tumor migration and invasion. Gene and protein levels of
MMP2, MMP9 and VEGF were significantly increased after exposure to
atrazine in a dose-dependent manner (Figs. 6D and 7), indicating starkly enhanced migration
and invasion abilities. This indicated that atrazine can enhance
invasion and migration ability of RM1 cells.
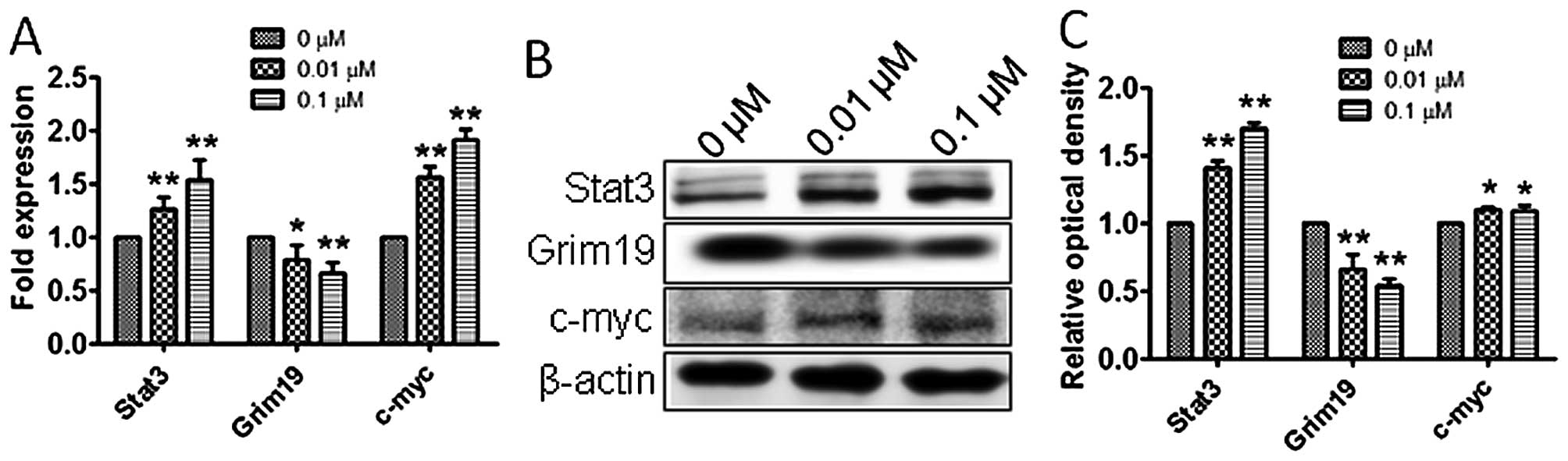
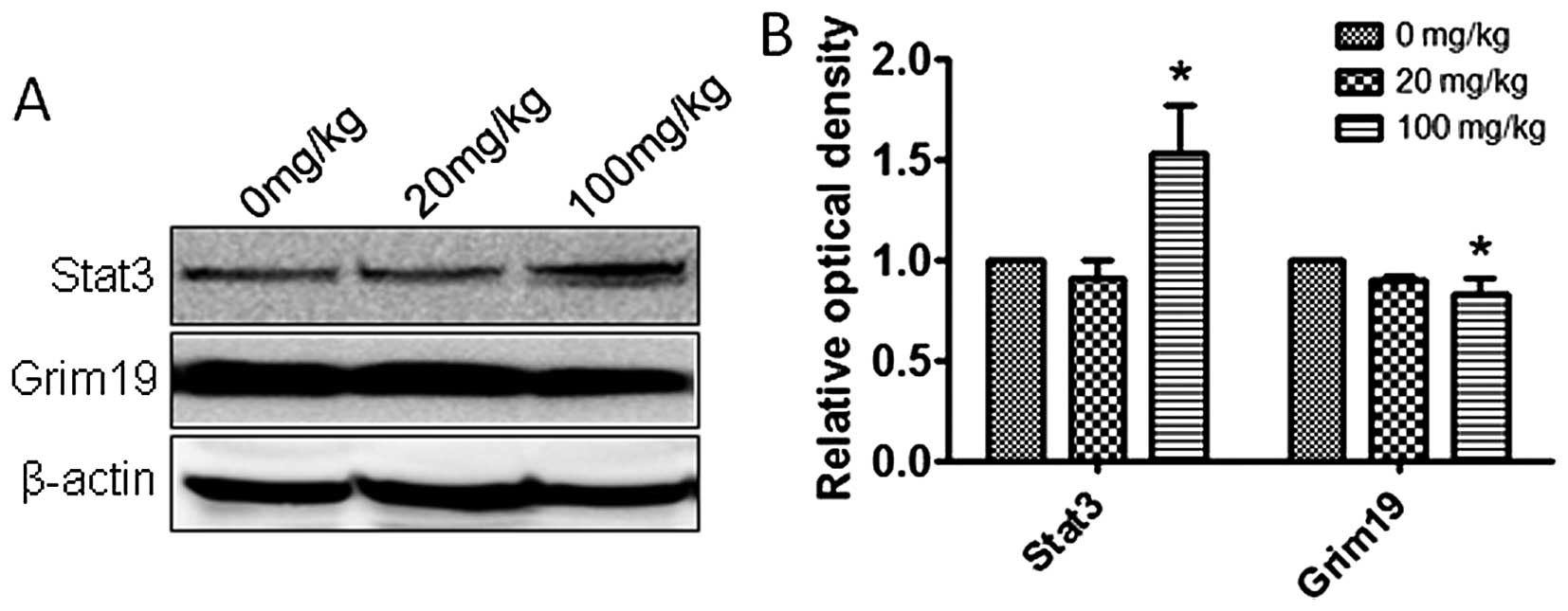
Atrazine activates the STAT3 signaling
pathway
To explore the molecular mechanisms by which
atrazine promotes proliferation, accelerates cell cycle, and
enhances cell migration and invasion abilities, qRT-PCR and western
blotting were employed to detect the expression levels of Stat3,
c-myc and Grim-19 in vitro and in vivo.
Interestingly, mRNA and protein levels of Stat3 and c-myc were
higher and Grim-19 lower in the 0.01 and 0.1 μM atrazine groups,
compared with the control group, in a dose-dependent manner
(Figs. 8 and 9), suggesting that atrazine may activate
STAT3 signaling to enhance proliferation, migration and invasion in
RM1 cells.
Discussion
Atrazine, a pesticide widely used to control
broadleaf weeds in agriculture worldwide and especially in America,
has been detected frequently in surface and ground water, where it
tends to persist for months (21).
This alarms medical researchers and environmentalists. Studies have
reported that atrazine affects the endocrine and reproductive
systems; however, whether it impacts the growth of tumor cells
remains unclear. Prostate cancer is the most common malignancy in
men, and epidemiologists have reported that pesticides can cause
prostate cancer. Here, we used the RM1 cell line to explore the
contribution of atrazine to the development of prostate cancer.
In this study, MTT and colony formation assays were
employed in vitro to demonstrate that atrazine promoted RM1
cell proliferation.
PCNA is a known molecular marker for cell
proliferation, and a regulator of the essential cellular function
of cell growth. Upregulated expression of PCNA indicates the
enhanced proliferation ability of cells (22). Western blotting and
immunohistochemistry data obtained both in vitro and in
vivo showed that PCNA levels were higher after exposure to
atrazine, which suggested that atrazine can enhance RM1 cell
proliferation.
Increased cell proliferation indicates induced
mitosis and/or inhibited apoptosis. To assess how atrazine promotes
RM1 cell proliferation, flow cytometry was employed to detect the
changes in cell cycle distribution and apoptosis of RM1 cells in
vitro; interestingly, the rates of G2 phase cells were
increased, while apoptosis rates were unchanged after exposure to
atrazine, which suggested that atrazine promotes mitosis by
accelerating the cell cycle, but has no significant impact on
apoptosis. To further clarify the molecular mechanisms by which
atrazine affects cell cycle and apoptosis in RM1 cells, RT-PCR and
western blot analyses were employed to detect the mRNA and protein
levels of factors related to cell cycle and apoptosis. The cell
cycle has many checkpoints, and p53 and p21 play important roles in
cell cycle arrest through the Chk2-p53-Cdc25A-p21-MCM4 signaling
pathway, and finally induce G1 cell cycle arrest; overexpression of
p21 induces cell cycle arrest (23). In this study, p53 and p21 mRNA and
protein levels were downregulated in vitro and in
vivo after exposure to atrazine, which indicated that atrazine
inhibits cell cycle arrest. Cyclin B1 (G2/mitotic-specific, cyclin
B1; CCNB1) is essential for cell cycle control at the G2/M
transition, and interacts with the CDK1 protein kinase to form a
serine/threonine kinase holoenzyme complex also known as MPF;
cyclin D1 binds and activates Cdk4 to regulate G1→S phase
transition (24,25). In this study, cyclin B1 and cyclin
D1 were upregulated after atrazine treatment, which suggested that
atrazine can help RM1 cells go through the G2-M and G1-S
checkpoints. Gene and protein levels of factors related to
apoptosis, such as Bax, Bcl-2 and Caspase-3, were also detected by
RT-PCR and western blot analyses: no significant differences were
noted after treatment with atrazine (26–28).
It is well known that tumor migration and invasion
are the main factors that promote cancer progression. To assess
whether atrazine effects RM1 cell migration and invasion in mouse
prostate cancer, in vitro invasion and wound-healing assays
were employed. As shown above, migration and invasion abilities of
RM1 cells were enhanced after treatment with atrazine. To clarify
the underlying molecular mechanisms, RT-PCR and western blot
analyses were employed to assess mRNA and protein amounts of a
series of genes, including MMP2, MMP9 and VEGF. It is known that
VEGF is crucial for vascular development and neovascularization in
physiological and pathological cancer progression (29). In addition, VEGF is essential for
cell migration (30). MMP2 and
MMP9 are frequently highly expressed in various malignant tumors
and appear to increase with development stages; indeed, these
proteins are essential for cancer invasion and metastasis. Li et
al reported that MMP2 and MMP9 upregulation promotes the
migration and invasion of tumor cells (31). In this study, VEGF, MMP2 and MMP9
were upregulated by atrazine in vitro and in vivo,
indicating that atrazine can promote metastasis and migration in
RM1 cells.
It is well known that proto-oncogene and
anti-oncogene unbalance induces tumor occurrence and development.
RT-PCR and western blot analyses were employed to assess mRNA and
protein levels of STAT3, Grim-19 and c-myc in vitro and
in vivo. Grim-19 participates in multiple pathways affecting
cell growth as a tumor suppressor; therefore, its deregulation is
advantageous to cancer development (32). STAT3, an oncogene essential for
tumor growth (33), is
constitutively active in a number of human cancers, affecting
autocrine growth factors. Activated STAT3 induces the expression of
a number of cellular proto-oncogenes, including c-myc, c-fos and
c-met, as well as cell cycle regulating proteins such as cyclin D1
and cyclin B1, which are all known to promote tumor growth. Grim-19
directly interacts with STAT3, inhibiting its gene function through
Grim-19-depentment inhibitory and stimulatory pathways (34,35).
In this study, STAT3 and c-myc were upregulated, and Grim-19
downregulated, indicating that atrazine may upregulate STAT3 by
downregulating Grim-19, promoting RM1 cell proliferation.
This study hinted that atrazine may activate the
STAT3 signaling pathway by reducing the production of ROS, which
induce the cell pro-oncogene c-myc as well as cell cycle regulators
such as cyclin D1 and cyclin B1. Upregulation of c-myc, cyclin B1
and cyclin D1 accelerates the cell cycle, promoting the
proliferation of tumor cells. Moreover, VEGF, MMP1 and MMP9
upregulation enhances the migration and invasion of tumor cells.
These effects promote tumor malignancy. Therefore, prostate cancer
patients should stay away from atrazine, and farmers should be
regularly screened for prostate cancer.
References
|
1
|
Dong X, Zhu L, Wang J, Wang J, Xie H, Hou
X and Jia W: Effects of atrazine on cytochrome P450 enzymes of
zebrafish (Danio rerio). Chemosphere. 77:404–412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin Z, Fisher JW, Ross MK and Filipov NM:
A physiologically based pharmacokinetic model for atrazine and its
main metabolites in the adult male C57BL/6 mouse. Toxicol Appl
Pharmacol. 251:16–31. 2011. View Article : Google Scholar
|
|
3
|
Gerecke AC, Schärer M, Singer HP, Müller
SR, Schwarzenbach RP, Sägesser M, Ochsenbein U and Popow G: Sources
of pesticides in surface waters in Switzerland: Pesticide load
through waste water treatment plants - current situation and
reduction potential. Chemosphere. 48:307–315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren J, Jiang K and Zhou H: The
concentration and source of atrazine residue in water of Guanting
reservoir. Huan Jing Ke Xue. 23:126–128. 2002.In Chinese.
PubMed/NCBI
|
|
5
|
Na T, Fang Z, Zhanqi G, Ming Z and Cheng
S: The status of pesticide residues in the drinking water sources
in Meiliangwan Bay, Taihu Lake of China. Environ Monit Assess.
123:351–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodriguez VM, Thiruchelvam M and
Cory-Slechta DA: Sustained exposure to the widely used herbicide
atrazine: Altered function and loss of neurons in brain monoamine
systems. Environ Health Perspect. 113:708–715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coban A and Filipov NM: Dopaminergic
toxicity associated with oral exposure to the herbicide atrazine in
juvenile male C57BL/6 mice. J Neurochem. 100:1177–1187. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cooper RL, Stoker TE, Tyrey L, Goldman JM
and McElroy WK: Atrazine disrupts the hypothalamic control of
pituitary-ovarian function. Toxicol Sci. 53:297–307. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xing H, Wang C, Wu H, Chen D, Li S and Xu
S: Effects of atrazine and chlorpyrifos on DNA methylation in the
brain and gonad of the common carp. Comp Biochem Physiol C Toxicol
Pharmacol. 168:11–19. 2015. View Article : Google Scholar
|
|
10
|
Zhang X, Wang M, Gao S, Ren R, Zheng J and
Zhang Y: Atrazine-induced apoptosis of splenocytes in BALB/C mice.
BMC Med. 9:1172011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boffetta P, Adami HO, Berry SC and Mandel
JS: Atrazine and cancer: A review of the epidemiologic evidence.
Eur J Cancer Prev. 22:169–180. 2013. View Article : Google Scholar
|
|
12
|
Van Leeuwen JA, Waltner-Toews D, Abernathy
T, Smit B and Shoukri M: Associations between stomach cancer
incidence and drinking water contamination with atrazine and
nitrate in Ontario (Canada) agroecosystems, 1987–1991. Int J
Epidemiol. 28:836–840. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kandori H, Suzuki S, Asamoto M, Murasaki
T, Mingxi T, Ogawa K and Shirai T: Influence of atrazine
administration and reduction of calorie intake on prostate
carcinogenesis in probasin/SV40 T antigen transgenic rats. Cancer
Sci. 96:221–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan W, Yanase T, Morinaga H, Gondo S,
Okabe T, Nomura M, Komatsu T, Morohashi K, Hayes TB, Takayanagi R,
et al: Atrazine-induced aromatase expression is SF-1 dependent:
Implications for endocrine disruption in wildlife and reproductive
cancers in humans. Environ Health Perspect. 115:720–727. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, Niu X, Zhang W, Caldwell JT,
Edwards H, Chen W, Taub JW, Zhao L and Ge Y: Synergistic antitumor
interactions between MK-1775 and panobinostat in preclinical models
of pancreatic cancer. Cancer Lett. 356B:656–668. 2015. View Article : Google Scholar
|
|
16
|
Zhang Y, Hao H, Zhao S, Liu Q, Yuan Q, Ni
S, Wang F, Liu S, Wang L and Hao A: Downregulation of GRIM-19
promotes growth and migration of human glioma cells. Cancer Sci.
102:1991–1999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao H, Liu J, Liu G, Guan D, Yang Y, Zhang
X, Cao X and Liu Q: Depletion of GRIM-19 accelerates hepatocellular
carcinoma invasion via inducing EMT and loss of contact inhibition.
J Cell Physiol. 227:1212–1219. 2012. View Article : Google Scholar
|
|
18
|
Nallar SC, Kalakonda S, Sun P, Ohmori Y,
Hiroi M, Mori K, Lindner DJ and Kalvakolanu DV: Identification of a
structural motif in the tumor-suppressive protein GRIM-19 required
for its antitumor activity. Am J Pathol. 177:896–907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Yang M, Yang H and Zeng Z:
Upregulation of the GRIM-19 gene suppresses invasion and metastasis
of human gastric cancer SGC-7901 cell line. Exp Cell Res.
316:2061–2070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Gao L, Li Y, Lin G, Shao Y, Ji K,
Yu H, Hu J, Kalvakolanu DV, Kopecko DJ, et al: Effects of
plasmid-based Stat3-specific short hairpin RNA and GRIM-19 on PC-3M
tumor cell growth. Clin Cancer Res. 14:559–568. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graymore M, Stagnitti F and Allinson G:
Impacts of atrazine in aquatic ecosystems. Environ Int. 26:483–495.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang SC: PCNA: A silent housekeeper or a
potential therapeutic target? Trends Pharmacol Sci. 35:178–186.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yun HJ, Hyun SK, Park JH, Kim BW and Kwon
HJ: Widdrol activates DNA damage checkpoint through the signaling
Chk2-p53-Cdc25A-p21-MCM4 pathway in HT29 cells. Mol Cell Biochem.
363:281–289. 2012. View Article : Google Scholar
|
|
24
|
Schick V, Majores M, Fassunke J, Engels G,
Simon M, Elger CE and Becker AJ: Mutational and expression analysis
of CDK1, cyclinA2 and cyclinB1 in epilepsy-associated glioneuronal
lesions. Neuropathol Appl Neurobiol. 33:152–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee Y, Dominy JE, Choi YJ, Jurczak M,
Tolliday N, Camporez JP, Chim H, Lim JH, Ruan HB, Yang X, et al:
Cyclin D1-Cdk4 controls glucose metabolism independently of cell
cycle progression. Nature. 510:547–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lamb HM and Hardwick JM: Unlatched BAX
pairs for death. Cell. 152:383–384. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Volkmann N, Marassi FM, Newmeyer DD and
Hanein D: The rheostat in the membrane: BCL-2 family proteins and
apoptosis. Cell Death Differ. 21:206–215. 2014. View Article : Google Scholar :
|
|
28
|
Li SQ, Hu ZH, Zhu S, Wang DM, Han HM and
Lu HJ: The effect of ADAM8 on the proliferation and apoptosis of
hepatocytes and hepatoma carcinoma cells. J Biochem Mol Toxicol.
29:440–448. 2015. View Article : Google Scholar
|
|
29
|
Baka S, Clamp AR and Jayson GC: A review
of the latest clinical compounds to inhibit VEGF in pathological
angiogenesis. Expert Opin Ther Targets. 10:867–876. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cross MJ, Dixelius J, Matsumoto T and
Claesson-Welsh L: VEGF-receptor signal transduction. Trends Biochem
Sci. 28:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li G, Zhang Y, Qian Y, Zhang H, Guo S,
Sunagawa M, Hisamitsu T and Liu Y: Interleukin-17A promotes
rheumatoid arthritis synoviocytes migration and invasion under
hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α
pathway. Mol Immunol. 53:227–236. 2013. View Article : Google Scholar
|
|
32
|
Kalvakolanu DV, Nallar SC and Kalakonda S:
Cytokine-induced tumor suppressors: A GRIM story. Cytokine.
52:128–142. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moreira S, Correia M, Soares P and Máximo
V: GRIM-19 function in cancer development. Mitochondrion.
11:693–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu YB, Zhang L, Guo YX, Gao LF, Liu XC,
Zhao LJ, Guo BF, Zhao LJ, Zhao XJ and Xu DQ: Plasmid-based Survivin
shRNA and GRIM-19 carried by attenuated Salmonella suppresses tumor
cell growth. Asian J Androl. 14:536–545. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou T, Chao L, Rong G, Wang C, Ma R and
Wang X: Down-regulation of GRIM-19 is associated with STAT3
overexpression in breast carcinomas. Hum Pathol. 44:1773–1779.
2013. View Article : Google Scholar : PubMed/NCBI
|