Introduction
Podoplanin belongs to the family of type-I
transmembrane sialomucin-like glycoproteins and possesses
platelet-aggregating activity and metastasis-promoting ability
(1,2). Due to its selective expression by
lymphatic endothelial cells, it is widely used as a specific marker
for lymphangiogenesis in many species (3). Podoplanin is also expressed by normal
kidney podocytes (4), alveolar
type I cells (5), basal epidermal
keratinocytes (6), and mesothelial
cells (7,8). Moreover, various tumor types highly
express podoplanin, such as squamous cell carcinomas, brain tumors,
mesotheliomas, germ cell tumors and some subtypes of vascular
tumors (9–15).
Cancer cells undergo migration and invasion mainly
dependent on the single cell-migration or the collective
cell-migration (16). Generally,
the invasion of single cell or small groups of cells is often
correlated with dramatic changes in the expression and function of
adhesive (e.g., cadherins, immunoglobulin domain-containing cell
adhesion molecules) and regulatory proteins (e.g., Snail family
members, transforming growth factor-β) (17). These changes are reminiscent of
early developmental processes, in particular during neurulation and
gastrulation, when cells acquire a migratory, mesenchymal
phenotype. During this so-called epithelial-mesenchymal transition
(EMT) cells lose epithelial markers, such as E-cadherin and
Claudins, and gain the expression of mesenchymal markers, such as
N-cadherin and vimentin. EMT is thought to be particularly
important in cancers with single cell migration and early
dissemination of tumor cells (17,18).
In contrast, the migration of cell strands, cell sheets or
clusters, named as collective cell-migration, is also present when
epithelial tumors invade to neighboring tissue or migrate to
distant organs (16).
Human differentiated embryonic chondrocyte (DEC) 1
(BHLHE40/Stra13/Sharp2) and DEC2 (BHLHE41/Stra13/Sharp1) are basic
helix-loop-helix (bHLH) transcriptional factors that are involved
in the regulation of cell differentiation, apoptosis, circadian
rhythms, hypoxia responses, EMT and carcinogenesis. Our previous
report showed that TGF-β upregulated the expression of DEC1 in the
pancreatic adenocarcinoma cell line PANC-1 and described its close
correlation with EMT phenomena (19).
Esophageal carcinoma is one of the most frequent
cancers in the world. Squamous cell carcinoma and adenocarcinoma
are the two common types among all the cases. Adenocarcinoma
usually occurs in the lower esophagus or gastroesophageal junction,
which is called Barrett's adenocarcinoma. In contrast, most
squamous cell carcinomas locate in the middle or upper one-third of
the esophagus (20). High
expression level of podoplanin was reported to correlate with the
poor prognosis of ESCC patients (21). However, the effects of podoplanin
expression on EMT in ESCC have not been clarified. In this study,
we focused on the role of podoplanin in TGF-β-induced EMT and its
relation with DEC1 and DEC2 in TE-11 cells.
Materials and methods
Cell culture and treatment
Human esophagus carcinoma cell TE series (TE-10,
TE-11 and TE-5) and human squamous cell carcinoma cell line A431
were purchased from the Riken BRC through the National Bio-Resource
Project of the MEXT, Japan. The three cell lines of TE series were
from well differentiated to poorly differentiated. The cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum at 37°C in a humidified atmosphere of 95% air and 5%
CO2. A431 cells were cultured in DMEM medium
supplemented with 10% fetal bovine serum. In some experiments, the
cells were incubated with recombinant human TGF-β (R&D Systems,
Minneapolis, MN, USA) or SB431542 (R&D Systems, Tocris
Bioscience, UK) at various concentrations for 90 min.
Knockdown of podoplanin by RNA
interference
Short interference RNA (siRNA) against podoplanin
was purchased from Santa Cruz Biotechnology Inc. (TX, USA). For the
siRNA transfection experiments, the cells were seeded at
5×104 cells per 35-mm well. Scrambled siRNA and siRNA
against podoplanin were transfected into the cells 24 h later using
the Lipofectamine RNA iMAX reagent (Invitrogen, Carlsbad, CA, USA).
Following transfection, the cells were incubated for another 24 h
and subjected to various analyses.
DEC1 and DEC2 overexpression
Human DEC1 and DEC2 plasmids were a kind gift of Dr
Katsumi Fujimoto (Hiroshima University) (22). TE-11 cells were seeded at
5×104 cells per 35-mm well. DEC1 or DEC2 plasmid was
transiently transfected into the cells 24 h later using the
Lipofectamine LTX reagent (Invitrogen). Following transfection, the
cells were incubated for another 18 h and subjected to western blot
analyses.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Four independent RNA samples (n=4) from TE-11 and
A431 cells were prepared for RT-qPCR. Total RNA was isolated using
an RNeasy RNA isolation kit (Qiagen, Hilden, Germany). First-strand
cDNA was synthesized from 1 μg of total RNA using ReverTra Ace
(Toyobo, Osaka, Japan). Quantitative PCR was carried out using Taq
PCR Master Mix (Qiagen). The sequences, product sizes as well as
cycling conditions of the primer sets are shown in Table I.
 | Table ISequences of the primer sets and the
product sizes of RT-qPCR. |
Table I
Sequences of the primer sets and the
product sizes of RT-qPCR.
| Gene | Product size
(bp) | Cycles | Primer
sequences |
|---|
| TGF-βRI | 206 | 27 | F:
5′-TGACACCAACCAGAGCTGAG-3′
R: 5′-GCAAAGGTCGATTTGGAGAA-3′ |
|
TGF-βRII | 200 | 27 | F:
5′-CCAGAACCAAGCAGAGAAGG-3′
R: 5′-GTTCCATGGCCAGAAGAGAA-3′ |
| Slug | 331 | 26 | F:
5′-GAGCATTTGCAGACAGGTCA-3′
R: 5′-TGAATTCCATGCTCTTGCAG-3′ |
|
Podoplanin | 143 | 27 | F:
5′-AACGATGTGGAAGGTGTCAG-3′
R: 5′-TCCTGGAGTCACCACATCAT-3′ |
|
Vimentin | 301 | 25 | F:
5′-CTTCGCCAACTACATCGACAA-3′
R: 5′-CGCATTGTCAACATCCTGTC-3′ |
|
N-cadherin | 201 | 27 | F:
5′-ACAGTGGCCACCTACAAAGG-3′
R: 5′-CCGAGATGGGGTTGATAATG-3′ |
|
Claudin-4 | 234 | 27 | F:
5′-ATGGCCTCCATGGGGCTACA-3′
R: 5′-ACATTGTCACCTCGCAGAC-3′ |
|
E-cadherin | 200 | 25 | F:
5′-TGCCCAGAAAATGAAAAAGG-3′
R: 5′-GTGTATGTGGCAATGCGTTC-3′ |
| DEC1 | 534 | 28 | F:
5′-GTCTGTGAGTCACTCTTCAG-3′
R: 5′-GAGTCTAGTTCTGTTTGAAGG-3′ |
| DEC2 | 501 | 28 | F:
5′-CACCTTTGACGTCTTTGGAG-3′
R: 5′-GAGAGTGGGAATAGATGCAC-3′ |
| 18s
rRNA | 151 | 18 | F:
5′-GTAACCCGTTGAACCCATT-3′
R: 5′-CCATCCAATCGGTAGTAGCG-3′ |
Western blotting
Cells treated with TGF-β (final concentration: 5.0
ng/ml) or transfected with siRNA were harvested and protein were
extracted using M-PER lysis buffer (Thermo Scientific, Rockford,
IL, USA). The protein concentrations were determined using the
bicinchoninic acid (BCA method) assay. The obtained lysates (5 μg
protein) were subjected to SDS-PAGE, and the separated proteins
were transferred to PVDF membranes (Immobilon P, Millipore,
Billerica, MA, USA), followed by immunoblotting utilizing the
indicated antibodies. Signals were detected using Bio-Rad western
blotting systems (Bio-Rad, Hercules, CA, USA) with the ECL-prime or
ECL-select western blotting detection systems (GE Healthcare,
Wauwatosa, WI, USA).
Immunocytochemical staining
TE-11 and A431 cells were seeded in a 4-well chamber
slide glass and cultured with TGF-β at 5.0 ng/ml for 24 h. Cells
were fixed with 4% paraformaldehyde in PBS for 30 min, followed by
permeabilization with 0.2% Triton X-100 in PBS for 20 min. Normal
horse serum (5%) was continued for 30 min to minimize the
non-specific adsorption of antibodies. Subsequently, cells were
incubated with antibody against podoplanin or E-cadherin at 4°C
overnight. The cells were then incubated for 1 h with horseradish
peroxidase-conjugated secondary antibody (Immuno-Biological
Laboratories, Fujioka, Japan). Immunoreactivity was detected with a
ready-to-us DAB+ substrate-chromogen solution (1–3 min) (Dako
EnVision System; Dako Cytomation, Kyoto, Japan). Finally, the
slides were counterstained with Mayer's hematoxylin for nuclear
staining.
Cell proliferation assay
TE-11 cells seeded in the 96-well plate were
transfected with siRNA against podoplanin, followed by TGF-β for an
additional 24 h. Cells transfected with scrambled siRNA were used
as control. Cell proliferation rate was determined using Cell
Counting Kit-8 assay (CCK-8, Dojindo Molecular Technologies,
Kumamoto, Japan) according to the manufacturer's instructions.
Invasion assay and wound-healing
assay
The invasion assay was performed using a BD BioCoat
Matrigel invasion chamber kit (Becton-Dickinson, Franklin Lakes,
NJ, USA). TE-11 cells were separated using cell dissociation
solution (Sigma) and 5×104 cells/600 μl were added to
the top chamber of a cell culture insert in a 24-well companion
plate. After 48-h incubation, the cells that had invaded the lower
surface of the membrane were fixed with methanol and subjected to
Giemsa staining. The number of the migrated cells was quantified by
counting them in ten random distinct fields using a light
microscope.
For wound-healing assay, TE-11 cells were seeded in
a 4-well chamber slide glass, and an artificial ‘wound’ was
carefully created by scratching the confluent cell monolayer with
the tip of a P-200 pipette. Medium with the scratched out cells
were changed and then the scrambled siRNA and podoplanin siRNA were
transfected into the cells. Microphotographs were taken after 0, 24
and 48 h.
Results
Endogenous expression of podoplanin and
EMT-related markers in ESCC cells
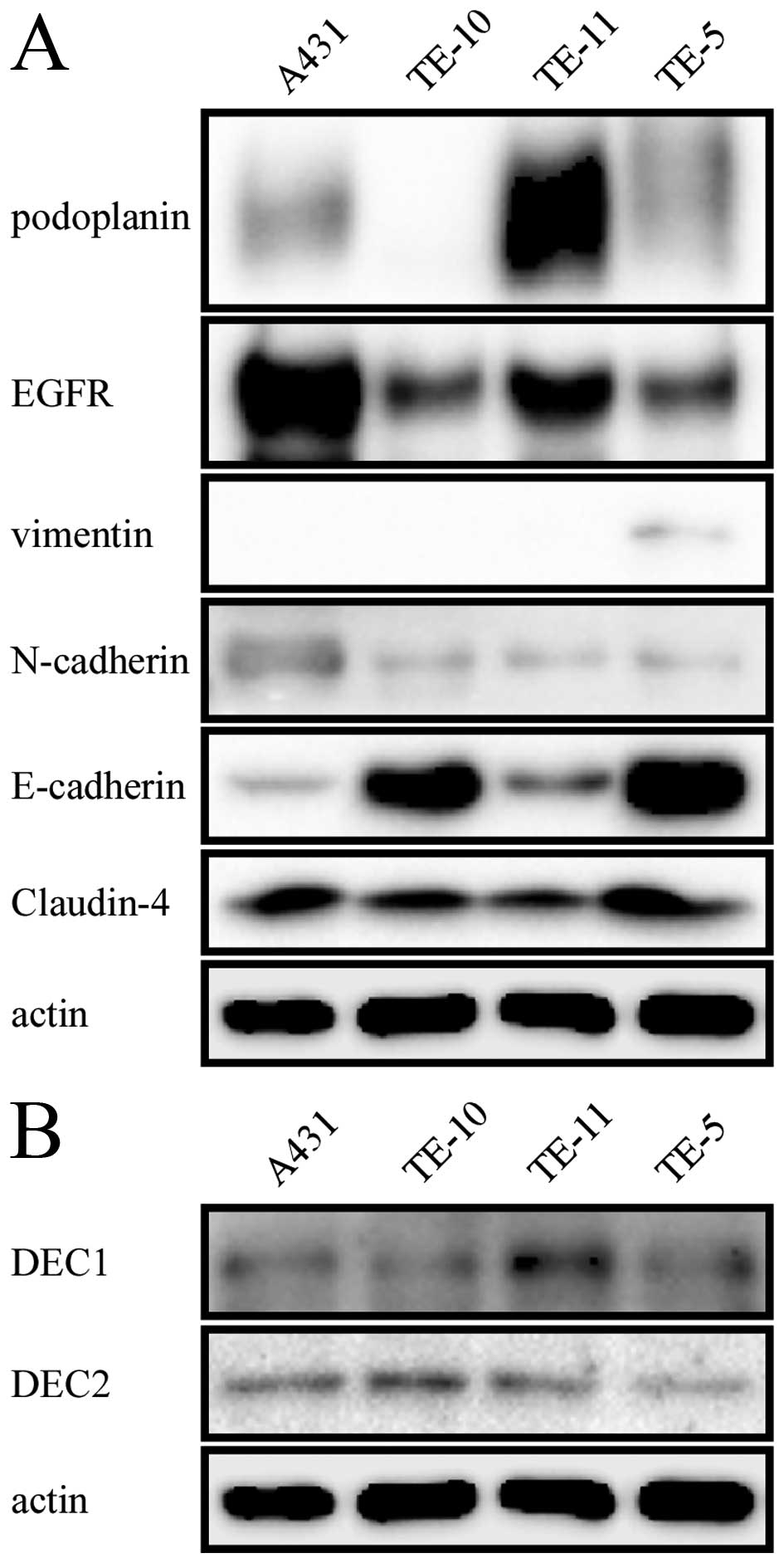
We investigated the level of podoplanin, EGFR,
vimentin, N-cadherin, E-cadherin, Claudin-4 (Fig. 1A) as well as DEC1 and DEC2
(Fig. 1B) in four cell lines A431,
TE-10, TE-11 and TE-5. Podoplanin showed an extremely strong
expression in TE-11 cells but weak expression in A431 and TE-5
cells. While little or no expression of this protein was observed
in the well differentiated ESCC TE-10 cells. A431 cells are
generally used as a positive control for EGFR, we found that TE-11
cells exhibited a relatively large amount of EGFR. When referred to
the mesenchymal markers, all of the four cell lines showed negative
or weak expression of vimentin and N-cadherin. In contrast, they
exhibited weak or strong expression of epithelial markers such as
E-cadherin and Claudin-4. Interestingly, the level of E-cadherin
and Claudin-4 was comparatively lower in TE-11 cells which had
strong expression of podoplanin. A weak endogenous expression of
both DEC1 and DEC2 was observed in all of the four cell lines.
Gene and protein expression changes when
treated with TGF-β
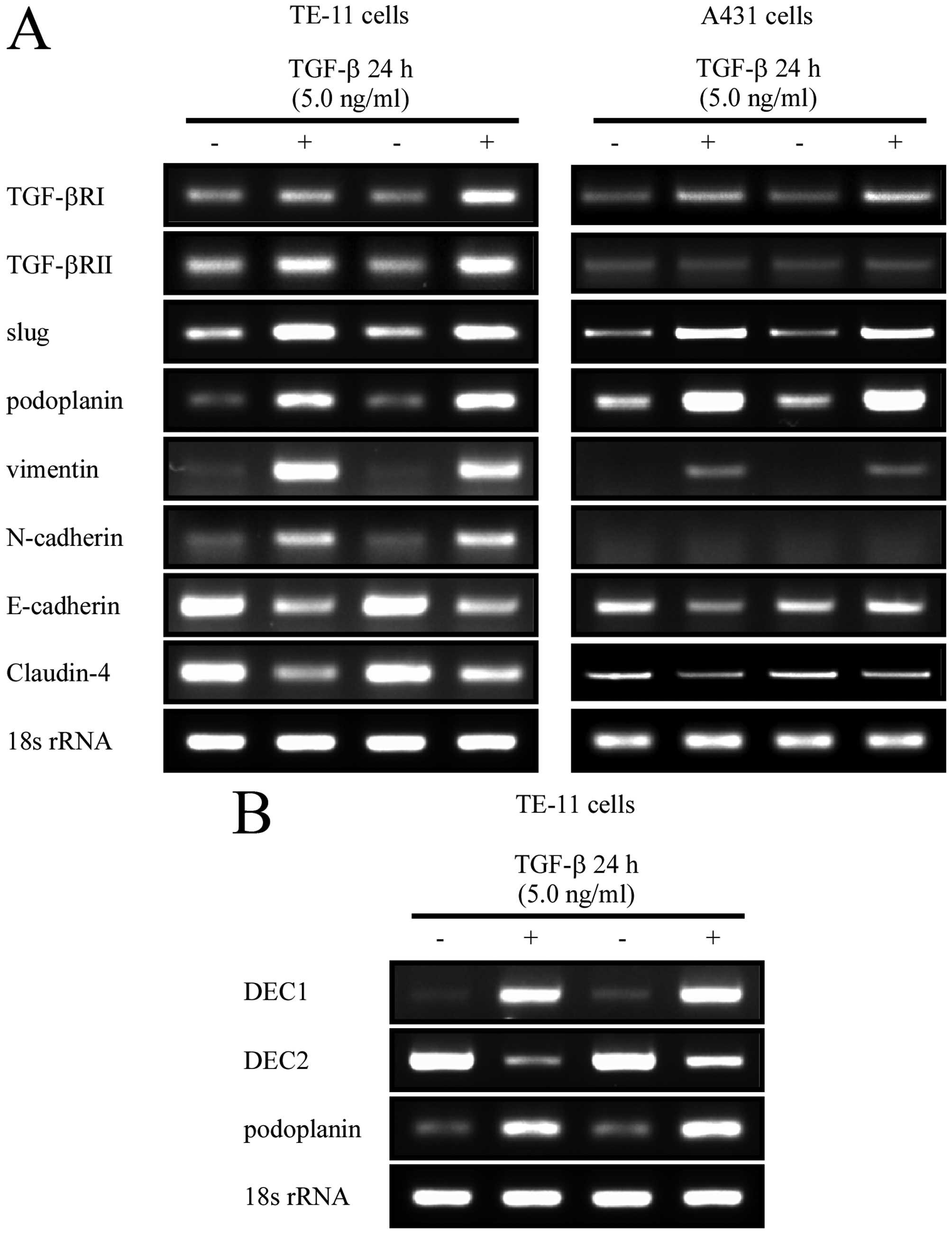
To analyze the roles of podoplanin in
epithelial-mesenchymal transition (EMT), TE-11 cell line with
positive expression of podoplanin was selected in our study, and
A431 cell line was used as control. Firstly, the two cell lines
were cultured in the medium containing TGF-β, one of the well-known
EMT inducers, at a final concentration of 5.0 ng/ml for 24 h.
RT-qPCR and western blot analysis were used to investigate gene
variation by TGF-β treatment. Both TGF-βRII and TGF-βRI were
induced as expectably by its ligand. Transcriptional factor slug
was involved in this process, however, snail was not activated by
TGF-β in the two cell lines (data not shown). Podoplanin was
sharply induced by TGF-β. Although a small amount of vimentin and
N-cadherin was detected in TE-11 and A431 cells, they were
upregulated by TGF-β in both the transcriptional and the
translational levels. In contrast with this, the epithelial markers
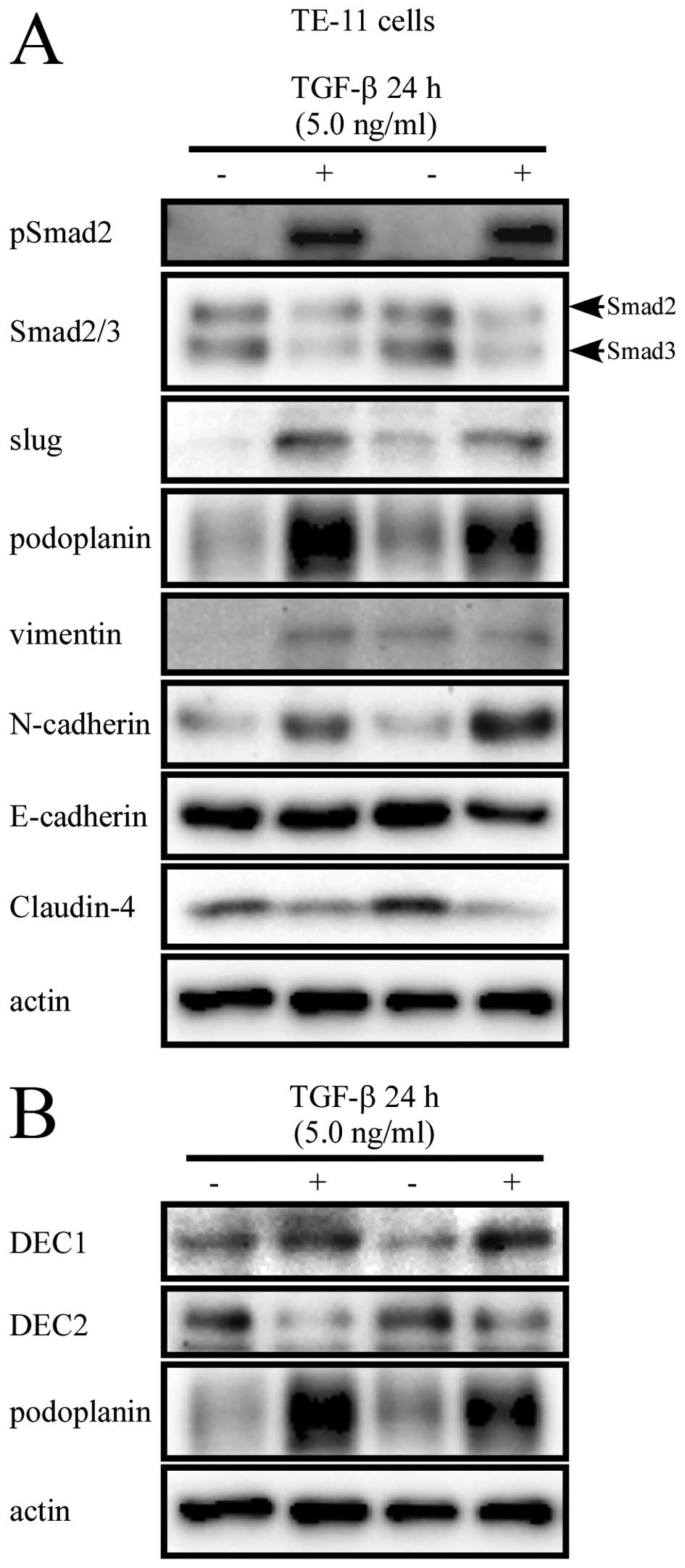
Claudin-4 and E-cadherin were downregulated (Fig. 2A). On the other hand, TGF-β caused
the phosphorylation of Smad2 (Fig.
3A), but not that of Smad3 (data not shown). Consistent with
the mRNA expression, the protein levels of slug, podoplanin, and
mesenchymal markers were increased. However, the epithelial marker
Claudin-4 was decreased, but E-cadherin protein showed a small
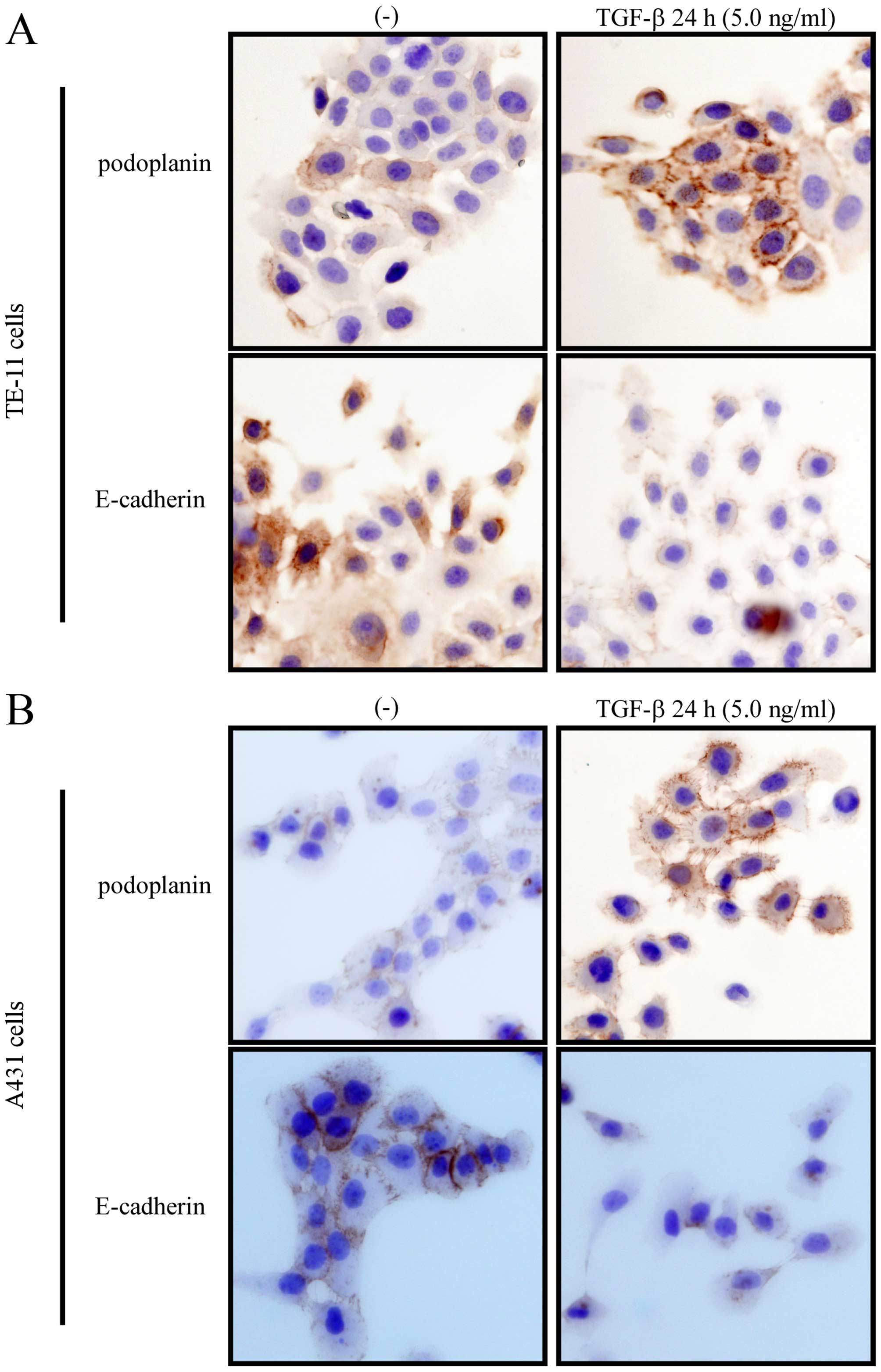
decrease when treated with TGF-β. Immunocytochemically, TGF-β
augmented the membrane or cytoplasmic expression of podoplanin, as
well as decreased the expression of E-cadherin in cell-cell
junction (Fig. 4). We also
investigated the expression of DEC, TGF-β exerted inverse effects
on DEC1 and DEC2. Moreover, podoplanin showed a similar expression
pattern with DEC1, but an opposite pattern with DEC2, with TGF-β
treatment (Figs. 2B and 3B).
Upregulation of podoplanin by TGF-β is
TGF-βRI/II-dependent
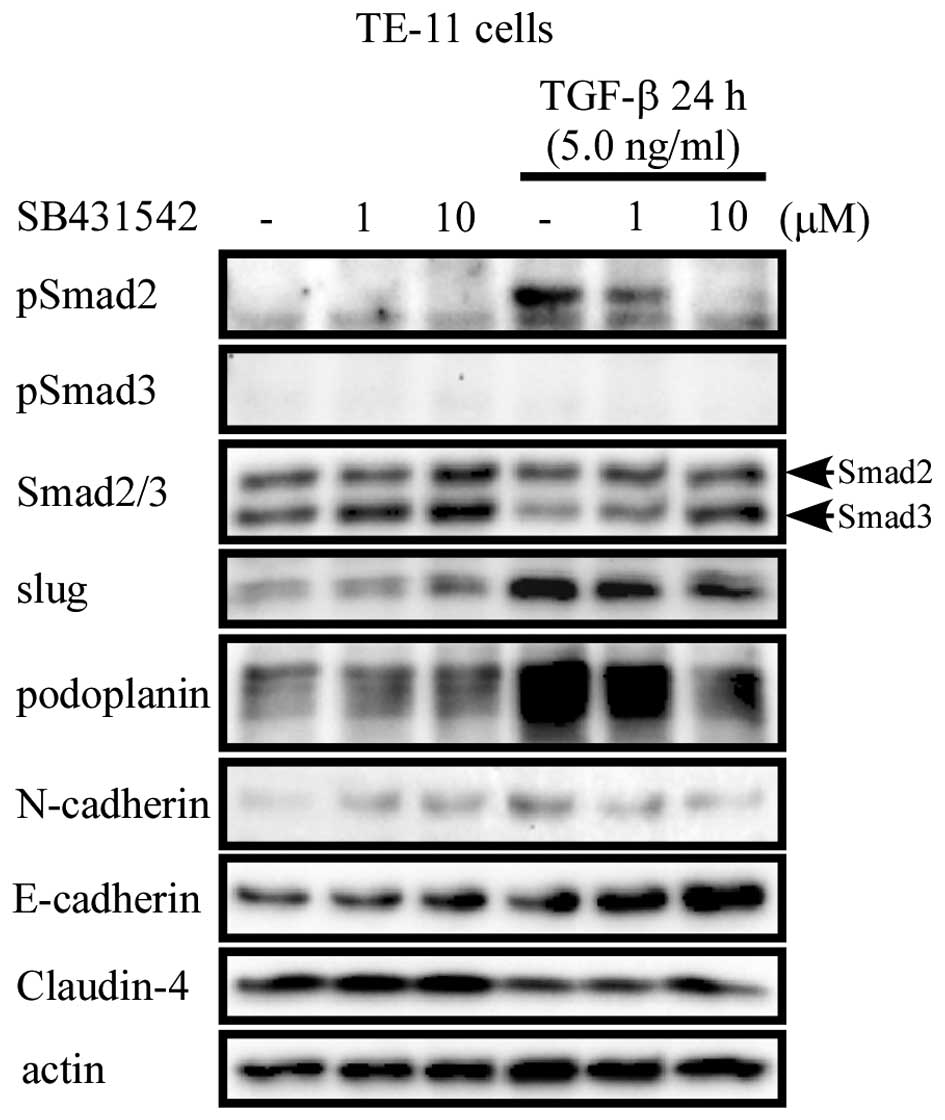
To investigate the mechanisms by which TGF-β
affected the expression of podoplanin, TE-11 cells were pre-treated
with a selective inhibitor of TGF-β receptor I/II, SB431542 (1 and
10 μM) for 90 min, followed by culture with or without TGF-β for 24
h. TGF-β-induced phosphorylation of Smad2 was gradually inhibited
by SB431542 in a dose-dependent manner. The expression of slug,
podoplanin and N-cadherin showed a similar pattern with that of
pSmad2. However, Claudin-4 and E-cadherin were upregulated when
TGF-β receptor was blocked (Fig.
5).
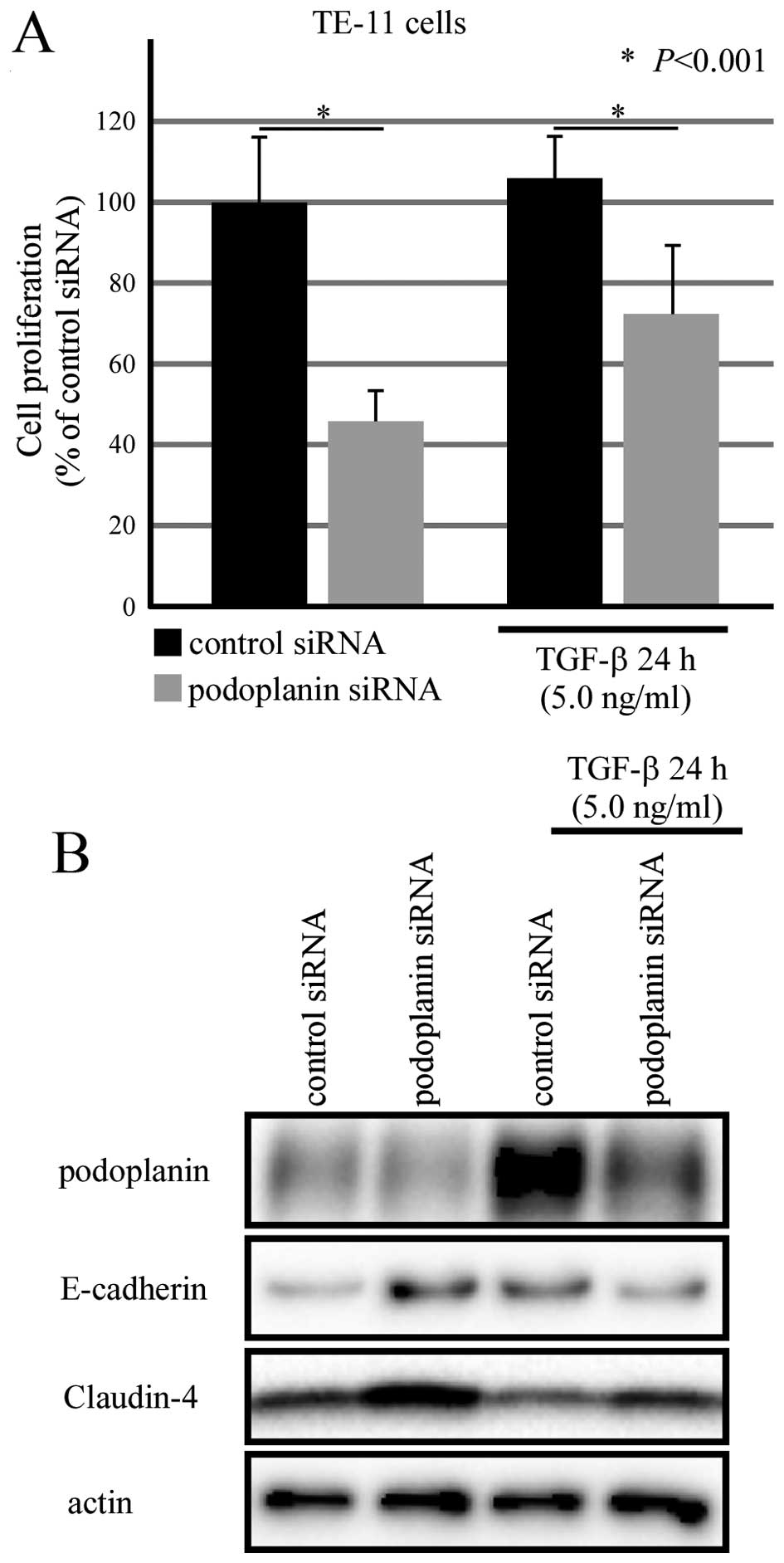
Podoplanin knockdown inhibited the cell
proliferation of TE-11 cells
To further clarify the roles of podoplanin in EMT,
small interference RNA was used to knock down the expression of
podoplanin. TE-11 cells cultured in 96-well plate were transfected
with podoplanin siRNA for 24 h, followed by TGF-β treatment for
another 24 h. Cell Counting Kit-8 was applied for analyzing the
cell proliferation rate. As Fig.
6A shows, podoplanin siRNA inhibited cell proliferation of
TE-11 cells in the presence and absence of TGF-β. Moreover,
decreased podoplanin correlated with an increased expression of
Claudin-4 regardless of TGF-β. However, unstable alterations of
E-cadherin was observed between the scrambled siRNA group and
podoplanin siRNA group (Fig.
6B).
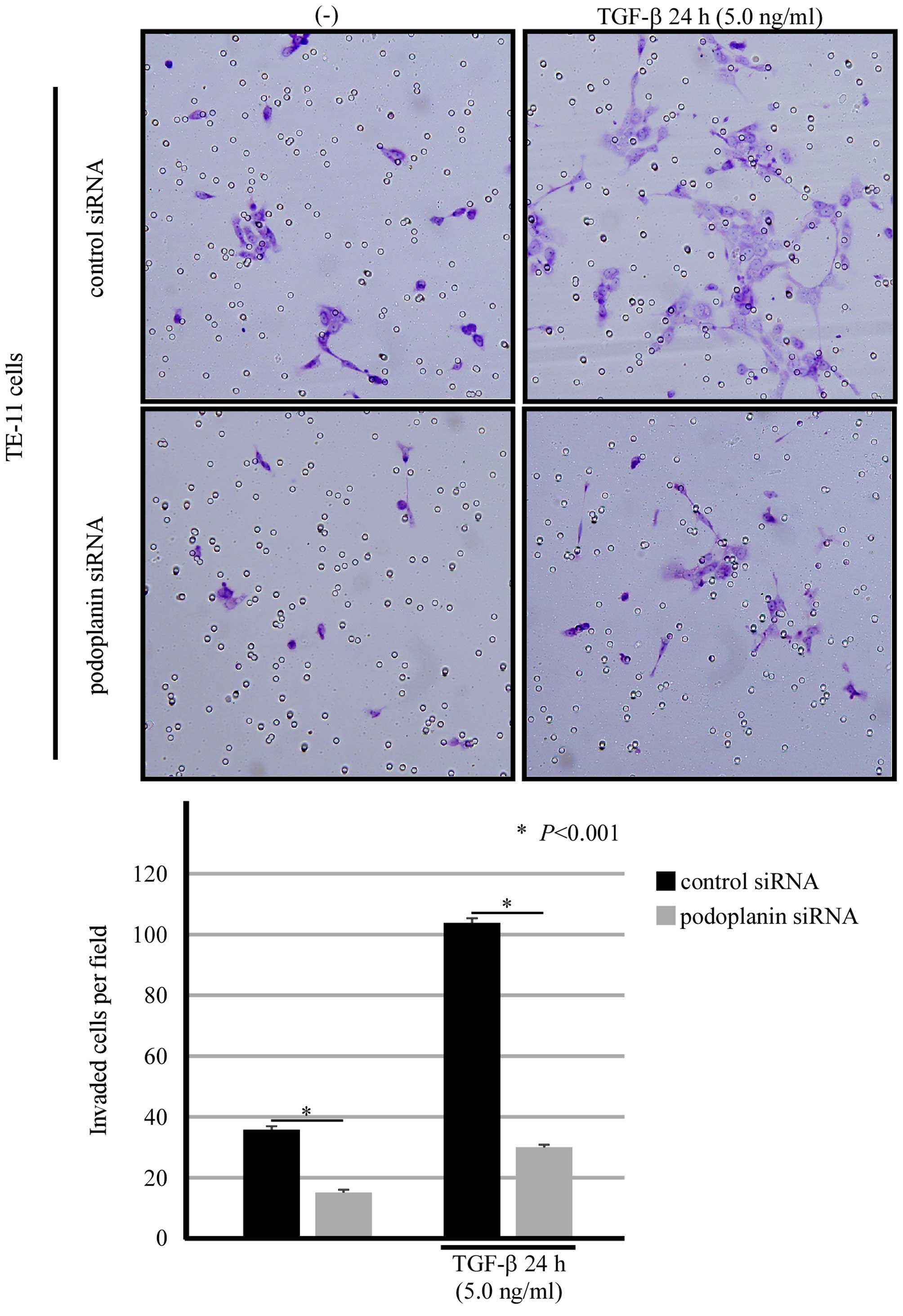
Podoplanin was closely involved in the
invasion and migration in TE-11 cells
The capacity of invasion and migration is the most
important indicator of cancer cells entering EMT. Then we carried
out an invasion assay in TE-11 cells which transiently transfected
with siRNA against podoplanin. TE-11 cells transfected with
scrambled siRNA showed weaker invasive ability, with the aid of
TGF-β, the power of invasion was significantly strengthened.
However, podoplanin knockdown strikingly inhibited the invasive
ability of TE-11 cells (Fig. 7).
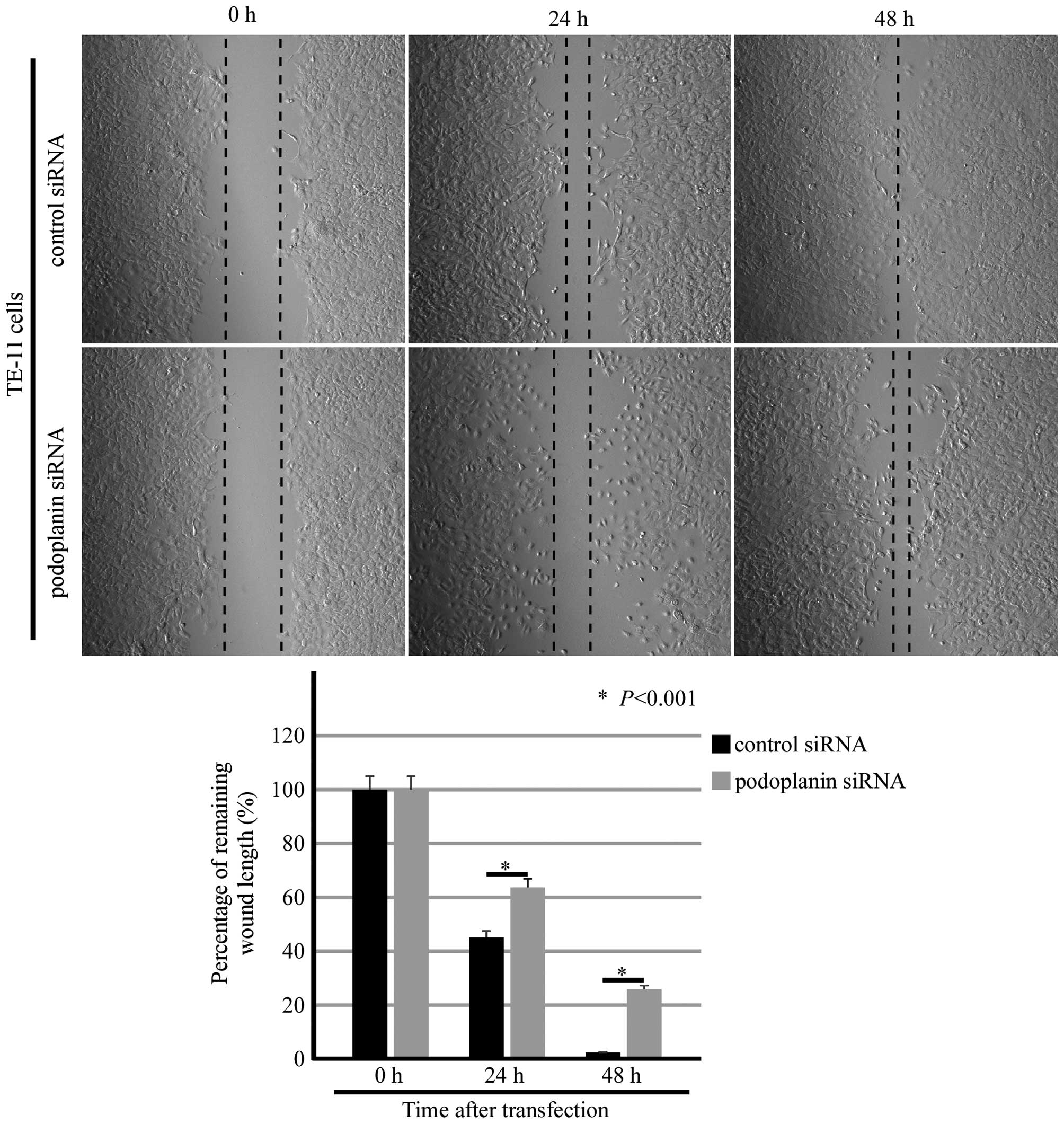
In order to evaluate the ability of migration, wound-healing assay
was introduced in podoplanin siRNA-transfected cells. Remaining
wound length was measured after 0, 24 and 48 h of podoplanin siRNA
transfection, and a significant difference between the control
siRNA-transfected group and the podoplanin siRNA-transfected group
was attained (Fig. 8).
Morphologically, a small amount of the TE-11 cells in the middle of
the chamber was dead in the podoplanin-siRNA transfected group,
especially at the 24-h time-point.
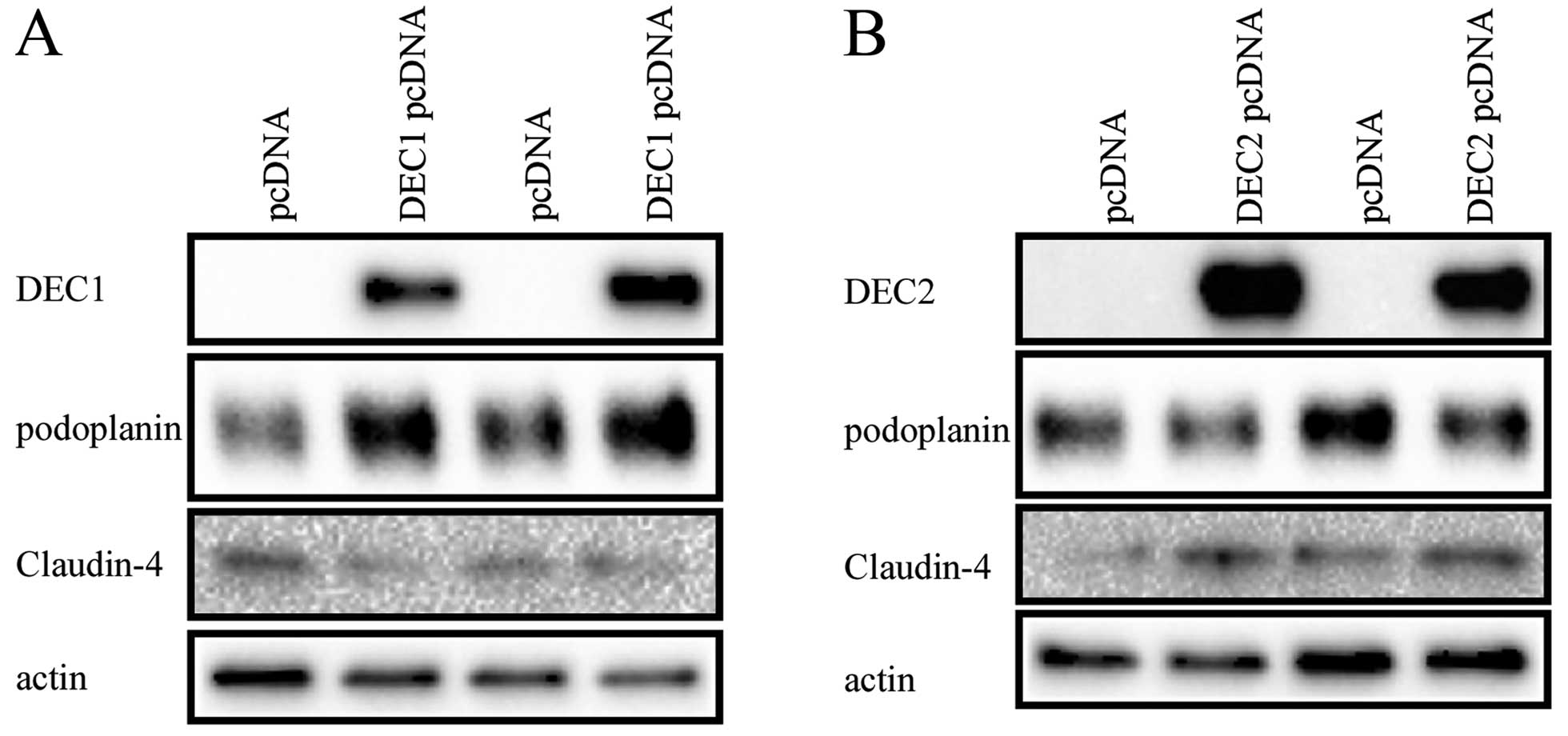
Overexpression of DEC1 and DEC2 has
distinct effects on podoplanin
To determine whether DEC1 or DEC2 directly regulated
podoplanin, the expression vector of DEC1 or DEC2 was transfected
into TE-11 cells. Podoplanin was induced by DEC1 overexpression,
but reduced by DEC2 overexpression. Furthermore, a weak but
inhibitory effect on Claudin-4 was observed in DEC1-overexpressed
TE-11 cells, whereas a slightly but inducible effect on Claudin-4
in DEC2-overexpressed TE-11 cells (Fig. 9).
Discussion
We focused on the function of podoplanin in EMT of
ESCC cells. Three cell lines among the human ESCC cell series,
TE-10, TE-11 and TE-5, from well to poorly differentiated, were
randomly chosen in our study, while A431 cells were used as
control. It was found that the expression of podoplanin is
independent of tumor differentiation, in which TE-11, a moderately
differentiated cell line, possessed the highest level of podoplanin
among the four cell lines. A reverse expression pattern of
podoplanin and the epithelial markers such as E-cadherin and
Claudin-4 was observed. In addition, the absence or weakly positive
of vimentin and N-cadherin, two of the well-known mesenchymal
markers was recorded in all of the four cell lines.
Upregulated podoplanin expression in squamous cell
carcinoma tissues has been confirmed in previous reports (6,9,12,15).
Although its expression was discussed in cancer cells, increasing
interest has been attracted to its function in the
cancer-associated fibroblasts (CAFs) which are located in the
stroma surrounding various cancerous cells. Kawase et al
suggested that stromal expression of podoplanin predicted a poor
prognosis in lung carcinoma (23),
while others showed an association between podoplanin expression
and a better prognosis in patients with uterine cervical carcinomas
and colorectal carcinomas (24,25).
Functions of podoplanin-positive CAFs may rely on the type of tumor
cells and the tissue from which the CAFs originate.
A variety of signaling agents and cytokines such as
basic fibroblast growth factor, tumor necrosis factor α, TGF-β,
IL-6, IL-22, or IFN-γ can induce podoplanin expression and cell
motility (15,26–29).
TGF-β is reported as a physiological regulator of podoplanin as
well as stimulating the platelet-aggregating ability of human
fibrosarcoma HT1080 cells (30).
To our limit knowledge, this is the first report on TGF-β and
podoplanin, involving their correlations with transcriptional
factors DEC1 and DEC2 in TE-11 cells. As an EMT inducer, TGF-β
induce pathological signaling in differentiated epithelial cells
and lead to fundamental changes in cellular phenotype. These
changes occurred in epithelial cells were accompanied by molecular
re-organization such as loss of E-cadherin and gain of N-cadherin.
This so-called cadherin switch is generally recognized as a
rate-limiting step in the transition from adenoma to carcinoma
(31,32).
In this study, we found an inverse expression
pattern of podoplanin and E-cadherin, and the opposite effects by
TGF-β treatment were observed in TE-11 cells. However, we failed to
find a direct influence of podoplanin on E-cadherin. Unlike
E-cadherin, the expression of Claudin-4 was upregulated when
suppressing podoplanin in TE-11 cells. It is not surprising since
podoplanin increased cell migration of MCF-7 cells and HaCaT
keratinocytes in the presence of E-cadherin expression (15,29).
In addition, invasion of podoplanin-expressing cells appeared to
rely on the activity of matrix metalloproteases (MMPs), as it was
repressed by TIMP2, an inhibitor of MMP. These data support the
viewpoint that podoplanin expression in human cancers promotes
migration and invasion of cancer cells without turning on the
cadherin switch, whereas, it has been reported that MDCK cells
demonstrated the expression of podoplanin leads to increased single
cell migration after loss of E-cadherin expression (33). Therefore, podoplanin may induce
cell invasion in both collective and single cell migration.
Detailed mechanisms should be further investigated on the governors
which decide the function of podoplanin to single cell-migration or
collective cell-migration.
Regarding the involvement of podoplanin in cell
movement, migration, and invasion, Martin-Villar et al
showed the podoplanin interaction with ezrin in its cytoplasmic
tail and with CD44 in its extracellular domain, which promote EMT
and directional cell migration (33). In contrast, Wicki et al
reported that podoplanin-induced collective cell migration and
invasion by filopodia formation via the downregulation of the
activities of RhoA GTPase, even in the absence of EMT (15). This study exhibited a negative
correlation between podoplanin and Claudin-4, a molecule widely
expressed at the tight junctions, affecting many cellular functions
such as migration and adhesion, and its downregulation are linked
to more invasive cancers. Since the cytoplasmic tail of podoplanin
is extremely short, we predicted the function might mediate through
ERM protein. However, further evidence is required to clarify
this.
The roles of DEC1 and DEC2 on podoplanin need
further clarification. DEC1 was reported to participate in
TGF-β-induced EMT of PANC-1 cells in our previous study (19). In this study, we found DEC1
overexpression upregulated podoplanin but decreased Claudin-4 in
TE-11 cells. It cannot be excluded that podoplanin might be a
downstream factor of DEC1 in TGF-β signaling pathway for EMT. It
seems that downregulation of podoplanin by DEC2 highlights another
possibility that DEC2 might play a role in lymphogenesis. DEC2 has
been confirmed to negatively regulate angiogenesis by inhibiting
the expression of VEGF (34).
Additionally, as a marker of lymphatic vessels, the function of
podoplanin in lymphogenesis cannot be ignored. It is possible that
DEC2 may inhibit lymphogenesis through regulating podoplanin. In
conclusion, podoplanin may be a key factor as shown by its roles in
EMT and lymphangiogenesis, and DEC2 as a new inhibitor of
podoplanin was found in our study. The detailed mechanisms by which
DEC2 regulate podoplanin should be further investigated.
Acknowledgements
This study was supported by Grants-in-Aid for
Science from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan; a Grant for Hirosaki University
Institutional Research; and the Fund for the Promotion of
International Scientific Research.
Abbreviations:
|
TGF-β
|
transforming growth factor-β
|
|
EMT
|
epithelial-mesenchymal transition
|
|
DEC1
|
differentiated embryonic chondrocyte
1
|
|
DEC2
|
differentiated embryonic chondrocyte
2
|
References
|
1
|
Kato Y, Fujita N, Kunita A, Sato S, Kaneko
M, Osawa M and Tsuruo T: Molecular identification of Aggrus/T1alpha
as a platelet aggregation-inducing factor expressed in colorectal
tumors. J Biol Chem. 278:51599–51605. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaneko M, Kato Y, Kunita A, Fujita N,
Tsuruo T and Osawa M: Functional sialylated O-glycan to platelet
aggregation on Aggrus (T1alpha/Podoplanin) molecules expressed in
Chinese hamster ovary cells. J Biol Chem. 279:38838–38843. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Breiteneder-Geleff S, Soleiman A, Kowalski
H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler
E, Alitalo K, et al: Angiosarcomas express mixed endothelial
phenotypes of blood and lymphatic capillaries: Podoplanin as a
specific marker for lymphatic endothelium. Am J Pathol.
154:385–394. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsui K, Breitender-Geleff S, Soleiman A,
Kowalski H and Kerjaschki D: Podoplanin, a novel 43-kDa membrane
protein, controls the shape of podocytes. Nephrol Dial Transplant.
14(Suppl 1): 9–11. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rishi AK, Joyce-Brady M, Fisher J, Dobbs
LG, Floros J, VanderSpek J, Brody JS and Williams MC: Cloning,
characterization, and development expression of a rat lung alveolar
type I cell gene in embryonic endodermal and neural derivatives.
Dev Biol. 167:294–306. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schacht V, Dadras SS, Johnson LA, Jackson
DG, Hong YK and Detmar M: Up-regulation of the lymphatic marker
podoplanin, a mucin-type transmembrane glycoprotein, in human
squamous cell carcinomas and germ cell tumors. Am J Pathol.
166:913–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ordóñez NG: The diagnostic utility of
immunohistochemistry and electron microscopy in distinguishing
between peritoneal mesotheliomas and serous carcinomas: A
comparative study. Mod Pathol. 19:34–48. 2006. View Article : Google Scholar
|
|
8
|
Wicki A and Christofori G: The potential
role of podoplanin in tumour invasion. Br J Cancer. 96:1–5. 2007.
View Article : Google Scholar
|
|
9
|
Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo
T and Osawa M: Enhanced expression of Aggrus (T1alpha/podoplanin),
a platelet-aggregation-inducing factor in lung squamous cell
carcinoma. Tumour Biol. 26:195–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato Y, Sasagawa I, Kaneko M, Osawa M,
Fujita N and Tsuruo T: Aggrus: A diagnostic marker that
distinguishes seminoma from embryonal carcinoma in testicular germ
cell tumors. Oncogene. 23:8552–8556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kimura N and Kimura I: Podoplanin as a
marker for mesothelioma. Pathol Int. 55:83–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin-Villar E, Scholl FG, Gamallo C,
Yurrita MM, Muñoz-Guerra M, Cruces J and Quintanilla M:
Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a
small membrane mucin induced in oral squamous cell carcinomas. Int
J Cancer. 113:899–910. 2005. View Article : Google Scholar
|
|
13
|
Mishima K, Kato Y, Kaneko MK, Nishikawa R,
Hirose T and Matsutani M: Increased expression of podoplanin in
malignant astrocytic tumors as a novel molecular marker of
malignant progression. Acta Neuropathol. 111:483–488. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naqvi J, Ordonez NG, Luna MA, Williams MD,
Weber RS and El-Naggar AK: Epithelioid hemangioendothelioma of the
head and neck: Role of podoplanin in the differential diagnosis.
Head Neck Pathol. 2:25–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wicki A, Lehembre F, Wick N, Hantusch B,
Kerjaschki D and Christofori G: Tumor invasion in the absence of
epithelial-mesenchymal transition: Podoplanin-mediated remodeling
of the actin cytoskeleton. Cancer Cell. 9:261–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Sato F, Yamada T, et al: The BHLH
transcription factor DEC1 plays an important role in the
epithelial-mesenchymal transition of pancreatic cancer. Int J
Oncol. 41:1337–1346. 2012.PubMed/NCBI
|
|
20
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rahadiani N, Ikeda J, Makino T, Tian T,
Qiu Y, Mamat S, Wang Y, Doki Y, Aozasa K and Morii E: Tumorigenic
role of podoplanin in esophageal squamous-cell carcinoma. Ann Surg
Oncol. 17:1311–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Honma S, Kawamoto T, Takagi Y, Fujimoto K,
Sato F, Noshiro M, Kato Y and Honma K: Dec1 and Dec2 are regulators
of the mammalian molecular clock. Nature. 419:841–844. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawase A, Ishii G, Nagai K, Ito T, Nagano
T, Murata Y, Hishida T, Nishimura M, Yoshida J, Suzuki K, et al:
Podoplanin expression by cancer associated fibroblasts predicts
poor prognosis of lung adenocarcinoma. Int J Cancer. 123:1053–1059.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamanashi T, Nakanishi Y, Fujii G,
Akishima-Fukasawa Y, Moriya Y, Kanai Y, Watanabe M and Hirohashi S:
Podoplanin expression identified in stromal fibroblasts as a
favorable prognostic marker in patients with colorectal carcinoma.
Oncology. 77:53–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carvalho FM, Zaganelli FL, Almeida BG,
Goes JC, Baracat EC and Carvalho JP: Prognostic value of podoplanin
expression in intratumoral stroma and neoplastic cells of uterine
cervical carcinomas. Clinics (Sao Paulo). 65:1279–1283. 2010.
View Article : Google Scholar
|
|
26
|
Gandarillas A, Scholl FG, Benito N,
Gamallo C and Quintanilla M: Induction of PA2.26, a cell-surface
antigen expressed by active fibroblasts, in mouse epidermal
keratinocytes during carcinogenesis. Mol Carcinog. 20:10–18. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Honma M, Minami-Hori M, Takahashi H and
Iizuka H: Podoplanin expression in wound and hyperproliferative
psoriatic epidermis: Regulation by TGF-beta and STAT-3 activating
cytokines, IFN-gamma, IL-6, and IL-22. J Dermatol Sci. 65:134–140.
2012. View Article : Google Scholar
|
|
28
|
Nose K, Saito H and Kuroki T: Isolation of
a gene sequence induced later by tumor-promoting
12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells
(MC3T3-E1) and expressed constitutively in ras-transformed cells.
Cell Growth Differ. 1:511–518. 1990.PubMed/NCBI
|
|
29
|
Scholl FG, Gamallo C, Vilaro S and
Quintanilla M: Identification of PA2.26 antigen as a novel
cell-surface mucin-type glycoprotein that induces plasma membrane
extensions and increased motility in keratinocytes. J Cell Sci.
112:4601–4613. 1999.PubMed/NCBI
|
|
30
|
Suzuki H, Kato Y, Kaneko MK, Okita Y,
Narimatsu H and Kato M: Induction of podoplanin by transforming
growth factor-beta in human fibrosarcoma. FEBS Lett. 582:341–345.
2008. View Article : Google Scholar
|
|
31
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li G and Herlyn M: Dynamics of
intercellular communication during melanoma development. Mol Med
Today. 6:163–169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin-Villar E, Megias D, Castel S,
Yurrita MM, Vilaro S and Quintanilla M: Podoplanin binds ERM
proteins to activate RhoA and promote epithelial-mesenchymal
transition. J Cell Sci. 119:4541–4553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sato F, Bhawal UK, Kawamoto T, Fujimoto K,
Imaizumi T, Imanaka T, Kondo J, Koyanagi S, Noshiro M, Yoshida H,
et al: Basic-helix-loop-helix (bHLH) transcription factor DEC2
negatively regulates vascular endothelial growth factor expression.
Genes Cells. 13:131–144. 2008. View Article : Google Scholar : PubMed/NCBI
|