Introduction
Radio-gene therapy, which combined traditional
radiotherapy with gene therapy, was developed rapidly in recent few
years as a new strategy (1–3). It
has the advantage of reducing X-ray irradiation dose while
enhancing the efficacy of gene therapy. As the standard adjuvant
treatment, radiotherapy has played an important role in controlling
tumor growth, however, it may be sometimes ineffective and
resulting in unnecessarily severe side effects due to a significant
proportion of radiation resistance (4–7). As
for gene therapy, one of the most pivotal reasons that restricts
the clinical applications of it in treating tumors is poor target
activity. Therefore, for successful prediction of radiotherapy
resistance, it is crucial to find a radiosensitive tumor target and
to understand the mechanisms underlying the development of
radioresistance in tumors.
Slug (Snail 2), which belongs to the Snail family,
is a highly evolutionarily conserved zinc-finger transcription
factor. It presents an anti-apoptotic effect by regulation of the
transactivation of PUMA, Bcl-2 and Bax expression (8,9). The
members of the Slug/Snail superfamily share a similar zinc finger
domain and the same Snag domain (10). Slug-deficient cells were
radiosensitive to DNA damage and the function of Slug in response
to DNA damage seemed to be important for its function in both
normal development and cancer (11,12).
P53 upregulated modulator of apoptosis (PUMA), which
has a powerful pro-apoptotic effect, is a key protein in apoptosis
and might be a potential new target for radio-gene therapy
(13–15). It has been reported that PUMA could
increase sensitivity to radiation-induced apoptosis in certain
kinds of tumor cells both in vivo and in vitro
(15–19). As a suppressor of PUMA
transcription, Slug plays an important role in the tumorigenesis
and development of resistance to radiation therapy by suppressing
the expression of PUMA (15,20,21).
Zhang et al found that in cholangiocarcinomas Slug might be
a potential target as an inducer of PUMA and Slug inhibition could
upregulate radiation-induced PUMA activity leading to cell
apoptosis (15).
Although the role of Slug in cancer progression has
been well understood, Slug inhibition induced alteration in
radio-sensitivity of OSCC cells has not been analysed. Thus, the
aim of this study was to explore whether Slug inhibition could
increase radiosensitivity of oral squamous cell carcinoma HSC3 and
HSC6 cells by upregulating PUMA. The results indicated that after
the combined treatment of Slug siRNA transfection and X-ray
irradiation, the expression of Slug was reduced and PUMA expression
was upregulated, resulting in increased cell apoptosis. These
findings offer new insight into the relationship of Slug and PUMA
in OSCC cells and provide a new kind of radio-gene therapy of
OSCC.
Materials and methods
Patients and tissue acquisition
Surgically resected OSCC specimens were obtained
from 57 OSCC patients between September 2008 and April 2013 in the
Stomatology of Sun Yat-sen University with written informed
consents as a prospective study. All cases were divided into phase
I to IV according to the UICC (Union for International Cancer
Control) standard in 2002 and the experimental procedures were
approved by the Research Ethics Committee of Sun Yat-sen
University. A summary of the characteristics in the 57 OSCC cases
are presented in Table I. In
addition, 15 cases of normal tissue were collected as control.
 | Table IRelations of Slug and PUMA expression
with clinicopathological profiles. |
Table I
Relations of Slug and PUMA expression
with clinicopathological profiles.
| Clinicopathological
profiles | No. of tumor
specimens (n) | Slug expression | PUMA expression |
|---|
|
|
|---|
| n | χ2 | P-value | n | χ2 | P-value |
|---|
| Age (years) |
| >60 | 36 | 25 | 0.047 | 0.828 | 9 | 0.267 | 0.605 |
| ≤60 | 21 | 14 | | | 4 | | |
| Gender |
| Male | 25 | 16 | 0.403 | 0.526 | 5 | 0.199 | 0.655 |
| Female | 32 | 23 | | | 8 | | |
| Stage |
| I+II | 22 | 11 | 5.627 | 0.018 | 9 | 6.669 | 0.010 |
| III+IV | 35 | 28 | | | 4 | | |
|
Differentiation |
| Well | 15 | 11 | 5.956 | 0.051 | 4 | 0.201 | 0.904 |
| Moderately | 29 | 16 | | | 6 | | |
| Poorly | 13 | 12 | | | 3 | | |
| Lymph node
metastasis |
| Negative | 37 | 26 | 0.167 | 0.683 | 9 | 0.138 | 0.710 |
| Positive | 20 | 13 | | | 4 | | |
| Total | 57 | 39 | | | 13 | | |
Immunohistochemical staining
Immunohistochemistry was used to detect the
expression of Slug and PUMA in OSCC specimens. Ten percent
formalin-fixed and paraffin-embedded samples were cut into 4-μm
thick sections. The sections were deparaffinized using xylene and
rehydrated through an increased grades of ethanol. After antigen
retrieval, primary rabbit polyclonal antibodies to Slug and PUMA
both at 1:200 dilution were added for treatment at 4°C overnight.
Then immunohistochemical staining was performed according to the
recommended protocol. Sections were considered either as positive
or negative according to the presence or absence of brown staining
in OSCC epithelial or stromal cells.
Cell culture
The human oral squamous cell carcinoma cell lines
HSC3 and HSC6 were obtained from the American Type Culture
Collection and conserved in Guangdong Provincial Key Laboratory of
Stomatology of Sun Yat-sen University. Cells were cultured in DMEM
supplemented with fetal calf serum (10%) in a humidified 5%
CO2 incubator at 37°C. Cells were used in the
exponential growth phase in all experiments.
Slug siRNA transfections
A validated negative universal control was used as a
control for transfection. Three different strand Slug-targeting
siRNA oligonucleotides were used together and the siRNA
oligonucleotide that showed the highest knockdown efficiency of
Slug mRNA in HSC3 and HSC6 cell lines was used for the experiments.
The transfections were carried out using Lipofectamine™RNAi MAX
according to the recommended protocol.
Radiation treatment and clonogenic
survival assay
Irradiation was performed in a linear accelerator
(RS2000, Hong Kong) at a dose rate of 1.31 Gy/min at room
temperature. HSC3 and HSC6 cells were irradiated with five
single-radiation doses (0, 2, 4, 6 and 8 Gy) using
X-ray-irradiation equipment and then returned to the incubator.
Twenty-four hours after irradiation, cells were trypsinized and
plated in 60-mm dishes and incubated for 12–14 days to allow colony
growth to assay their colony-forming ability. The colonies were
stained with crystal violet and colonies containing ≥50 cells were
counted. Each experiment was repeated three times and the survival
curves were plotted by GraphPad Prism 3.0 software program. For the
treatment combination of Slug siRNA transfection and radiation,
cells were first transfected with Slug siRNA, and then irradiated
with 4 Gy X-ray according to previous description 24 h later. Both
HSC3 and HSC6 cells were divided into five groups, including
control group, scramble group, Slug siRNA group, radiation group
and Slug siRNA combination with radiation group which were used for
further analysis.
RNA isolation and real-time qRT-PCR
Total RNA was isolated from HSC3 and HSC6 cell lines
in all treatment conditions using TRIzol reagent according to the
manufacturer's instructions. Real-time qRT-PCR analysis was
performed to validate mRNA expression with the one-step RT-PCR kit
and glyceral-dehyde-3-phosphate dehydrogenase (GAPDH) was used as
an internal control. The nucleotide sequences of specific primers
for mRNA amplification were designed using Beacon Designer Software
and its sequences were as follows: the sequence of Slug: sense,
CCTCCAAAAAGCCAAACTA CTA; antisense, GTGTGCTACACAGCAGCC; the
sequence of PUMA: sense, CCAGGAAAGGCTGTTGTGCTG; antisense, TCACTC
GGTTTGCACTGGTGA; the sequence of GAPDH: sense,
TGATGGGTGTGAACCACGAG; antisense, TTGAAG TCGCAGGAGACAACC. The RT-PCR
conditions were as follows: 95°C for 5 min, followed by 40 cycles
of 95°C for 15 sec, 60°C for 15 sec and 72°C for 1 min and the
final extension was 72°C for 5 min. Relative gene expression levels
were calculated using the 2−ΔΔCt method.
Western blot analysis
Western blot analysis was used to investigate the
expression of Slug and PUMA protein according to the manufacturer's
protocol. Cells in different groups were lysed in protein lysis
buffer supplemented with 1 mM of PMSF (phenylmethylsulfonyl
fluoride) and protease inhibitor mixture. Protein concentration was
measured by BCA protein assay kit and then diluted with 5X loading
buffer and denatured for 10 min at 99°C. Thirty grams of protein
per lane in all treatment conditions were separated on 12% SDS-PAGE
and subsequently transferred to a PVDF (polyvinylidene difluoride)
membrane by electro-blotting. The membranes were blocked with 5%
non-fat dry milk and incubated with the primary antibodies against
Slug, PUMA, Caspase-3, Bax, Bcl-2 and GAPDH (1:1,000 dilution) at
4°C for 6 h. Then the membranes were washed with TBST and incubated
with the secondary antibody for 1.5 h at room temperature.
Membranes were detected according to an ECL (enhanced
chemiluminescence) reagent kit instruction and visualized in the
AlphaView SA system. Quantity One software was used for
analysis.
Immunofluorescence
HSC3 and HSC6 cells were seeded in laser-scanning
confocal Petri dishes and incubated at 37°C overnight. Firstly,
after different treatments for 48 h, cells were washed with PBS for
three times and fixed in 4% paraformaldehyde for 20 min and then
permeabilized with 1% Triton X-100 for 30 min and sequentially
blocked in 1% BSA for 20 min. Secondly, the treated cells were
incubated with primary antibodies (Slug and PUMA) according to
manufacturer's protocols for 18 h and then incubated with the
corresponding secondary antibody for 1 h. Lastly, DAPI
(4′,6-diamidino-2-phenylindole) was used to stain nuclei. Images
were captured by confocal laser scanning microscopy.
Cell viability assay
HSC3 and HSC6 cells were seeded into 96-well plates
at 4,000 cells/well. After treatment as described above, 20 μl MTT
(3-(4,5-dimethyl-2-thiazolyl)-2,5 -diphenyl-2-H-tetrazolium
bromide, 5 μg/μl) was added per well and incubated for 4 h at 37°C
in a humidified environment at 24, 48, 72, 96 and 120 h
post-transfection. The supernatant was discarded and 200 μl DMSO
(dimethyl sulphoxide) was added to each well to dissolve the
precipitate. Optical density (OD) value was measured at the
wavelength of 570 nm. Each test was repeated in eight wells and
performed daily for five days.
Cell cycle assay
Cells in different groups were washed twice with
PBS, harvested, fixed with 70% ethanol and then stained with
propidium iodide (PI, 20 μg/ml) for 30 min at 4°C. Samples were
analyzed by fluorescence-activated cell-sorting (FACS) flow
cytometer and the data were elaborated using Modfit software. Each
test was repeated in triplicate.
Annexin V staining
The Annexin V staining was performed to measure cell
apoptosis according to the manufacturer's protocol. After
treatment, HSC3 and HSC6 cells in the log phase of growth were
collected and resuspended in binding buffer at a density of
106 cells/ml. An Annexin V-FITC labeled Apoptosis
Detection kit was used for the apoptosis assay and the percentage
of apoptotic cells was quantified by flow cytometry. Each test was
repeated in triplicate.
Statistical analysis
All data were performed using the SPSS17.0 software
and the results are presented as mean ± SD and determined by
one-way analysis of variance (ANOVA) or t-test of three replicate
assays. P-value <0.05 was considered to indicate statistical
significance.
Results
The expression of Slug and PUMA in OSCC
samples
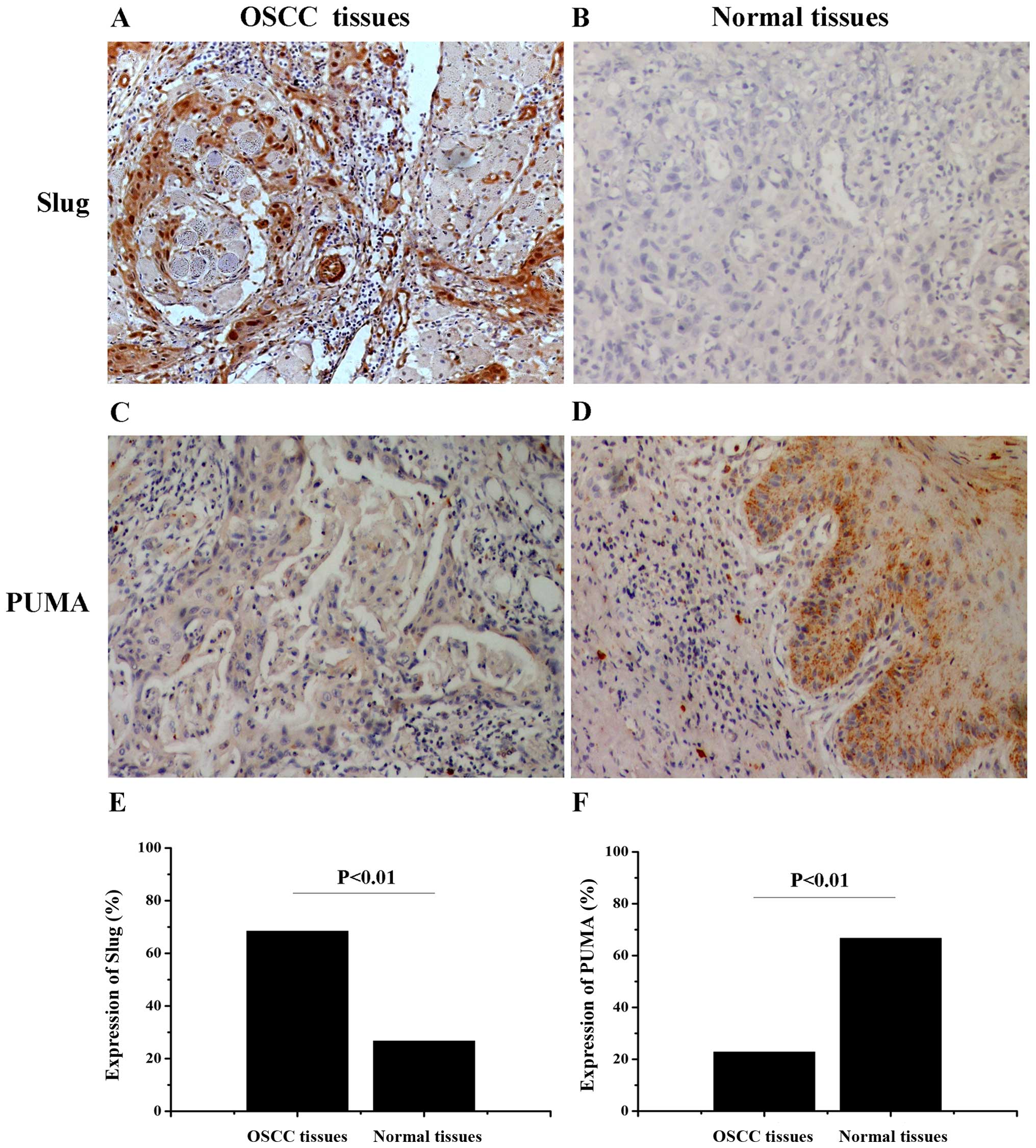
First, the expression of Slug and PUMA were examined
by the immunohistochemical staining respectively in human OSCC
tissues and normal tissues (Fig.
1). As showed in Table II,
Slug expression was observed in 68.4% (39/57) of OSCC samples and
in 26.7% (4/15) of normal tissues (χ2=8.607, P=0.003).
In contrast, PUMA expression exhibited an opposite trend compared
with the expression of Slug in OSCC samples (22.8%, 13/57) and
normal tissues (66.7%, 10/15) (χ2=10.508, P=0.001). The
results shoewed that the expression of PUMA was significantly
higher than Slug in OSCC samples. The relationship between Slug and
PUMA expression and clinico-pathological profiles was evaluated
(Table I). The expression of Slug
was significantly lower in early stages (I+II) than in advanced
stages (III+IV) (χ2=5.627, P=0.018). In contrast, PUMA
expression was higher in early stages than in advanced stages
(χ2=6.669, P=0.010). The data showed that Slug and PUMA
expression was positively correlated with tumor stage (P<0.05)
and negatively correlated with patient age, gender, tumor
differentiation and lymph node metastases (P>0.05), suggesting
that they play an important role in the progression of OSCC.
 | Table IIThe expression of Slug and PUMA in
OSCC tissues and normal tissues. |
Table II
The expression of Slug and PUMA in
OSCC tissues and normal tissues.
| Group | No. of tumor
specimens (n) | Slug
expression | PUMA
expression |
|---|
|
|
|---|
| n | χ2 | P-value | n | χ2 | P-value |
|---|
| OSCC tissues | 57 | 39 | 8.607 | 0.003 | 13 | 10.508 | 0.001 |
| Normal tissues | 15 | 4 | | | 10 | | |
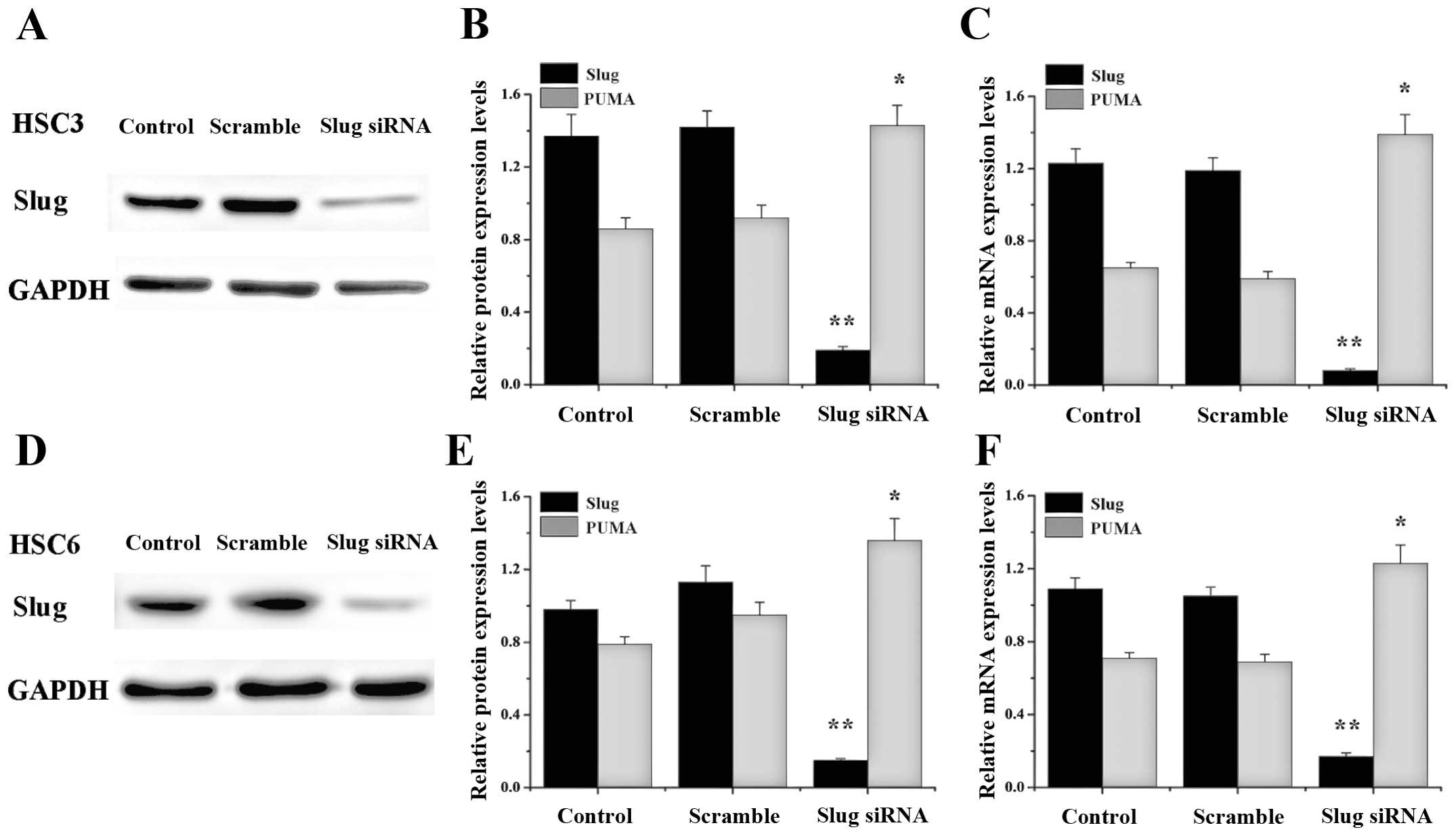
Slug siRNA transfection inhibits the
expression of Slug at both mRNA and protein levels in OSCC cell
lines
We analyzed the effect of Slug siRNA on HSC3 and
HSC6 cell lines (Fig. 2). RT-PCR
and western blot analysis showed that Slug siRNA transfection
resulted in a reduction at Slug mRNA (90–95%) and protein (80–90%)
level in both cell lines compared with control or scramble group
(**P<0.01).
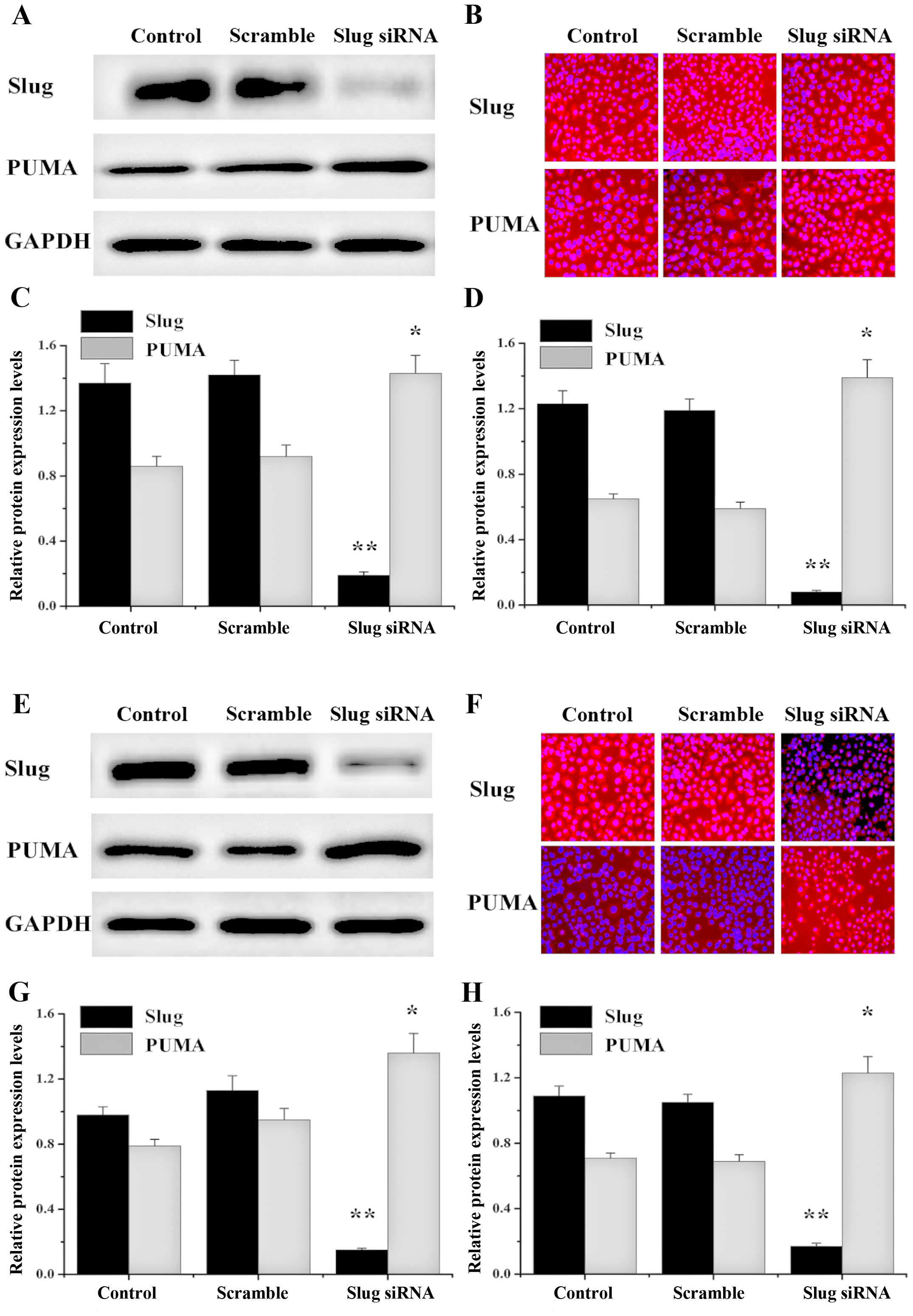
Knockdown of Slug upregulates PUMA
expression in OSCC cell lines
PUMA is a common tumor suppressor gene and exhibited
a very low expression in OSCC cell lines. In contrast, Slug was
highly expressed in many tumors as an oncogene and has been shown
to be involved in cell apoptosis by regulation of PUMA. Therefore,
we intended to evaluate whether silencing of Slug plays an
important role in upregulating PUMA in HSC3 and HSC6 cells. To
assess the effect of endogenous Slug on PUMA expression, we
transfected Slug siRNA into HSC3 and HSC6 cells and analyzed the
expression of PUMA mRNA by RT-PCR and analyzed PUMA protein
expression by western blot and immunofluorescence analyses. The
data showed that Slug expression was decreased and PUMA was
upregulated in Slug siRNA group and no significant change of Slug
and PUMA expression was detected in control or scramble group
(Fig. 3).
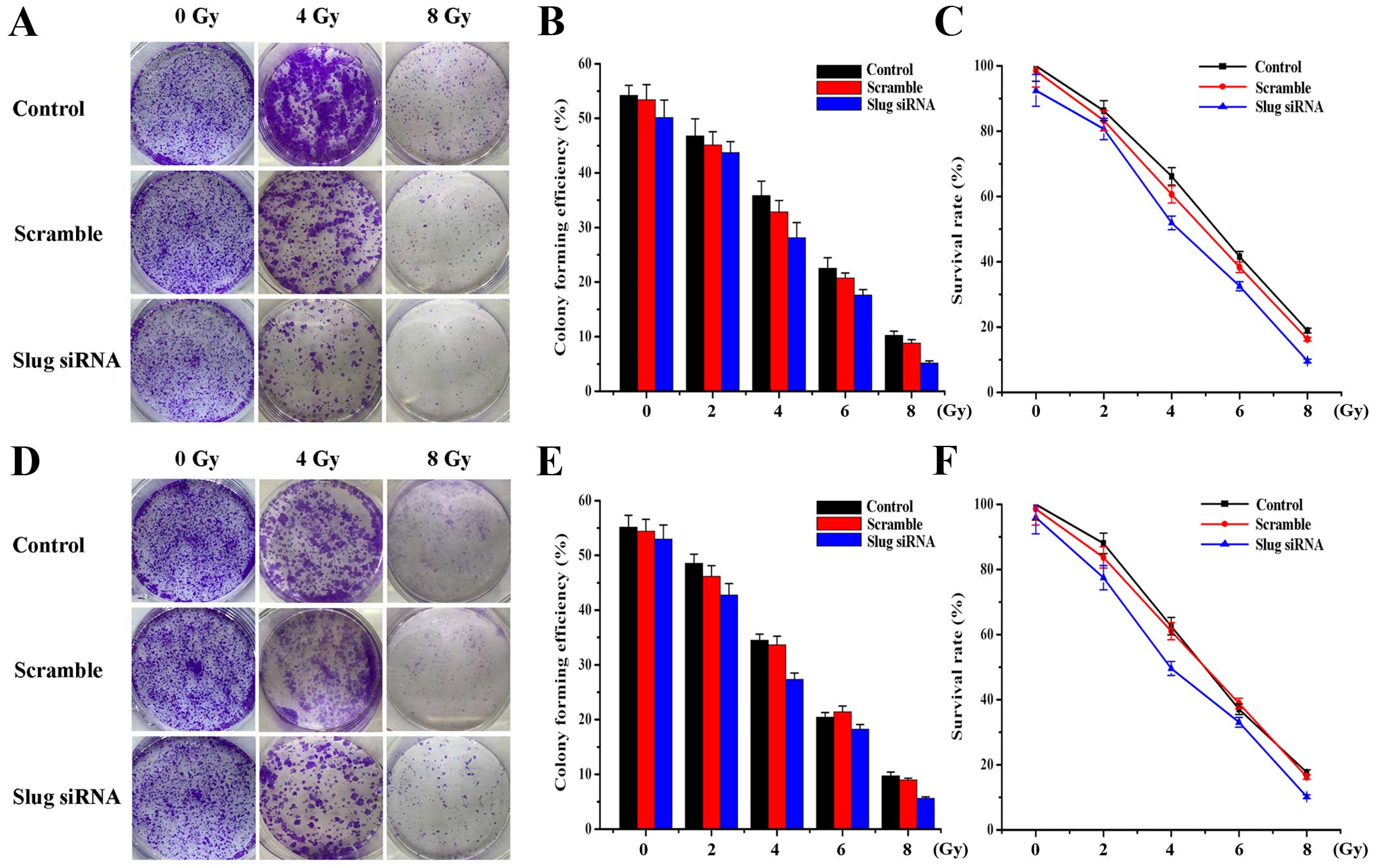
Effect of Slug siRNA transfection on OSCC
cell survival and radiosensitivity
To investigate the potential effects of Slug siRNA
transfection alone or in combination with X-ray irradiation on HSC3
and HSC6 cell survival, clonogenic survival assay was applied. The
results suggested that Slug inhibition could decrease cell
proliferation and increase cell sensitivity to X-ray irradiation
(Fig. 4). Statistical analysis
showed that cell colony forming efficiency (Fig. 4B and E) and survival rate (Fig. 4C and F) were gradually decreased
with the increasing radiation dose in control group (P<0.01),
scramble group (P<0.01) and Slug siRNA group (P<0.01) of HSC3
and HSC6 cells. Compared with the other two groups, cell colony
forming efficiency and survival rate in Slug siRNA radiation group
was significantly reduced at the same radiation dose (P<0.05)
and there was no significant difference between control group and
scramble group (P>0.05). A dose of 4-Gy was selected for
subsequent experiments according to the survival rate (50–70%) of
OSCC cells.
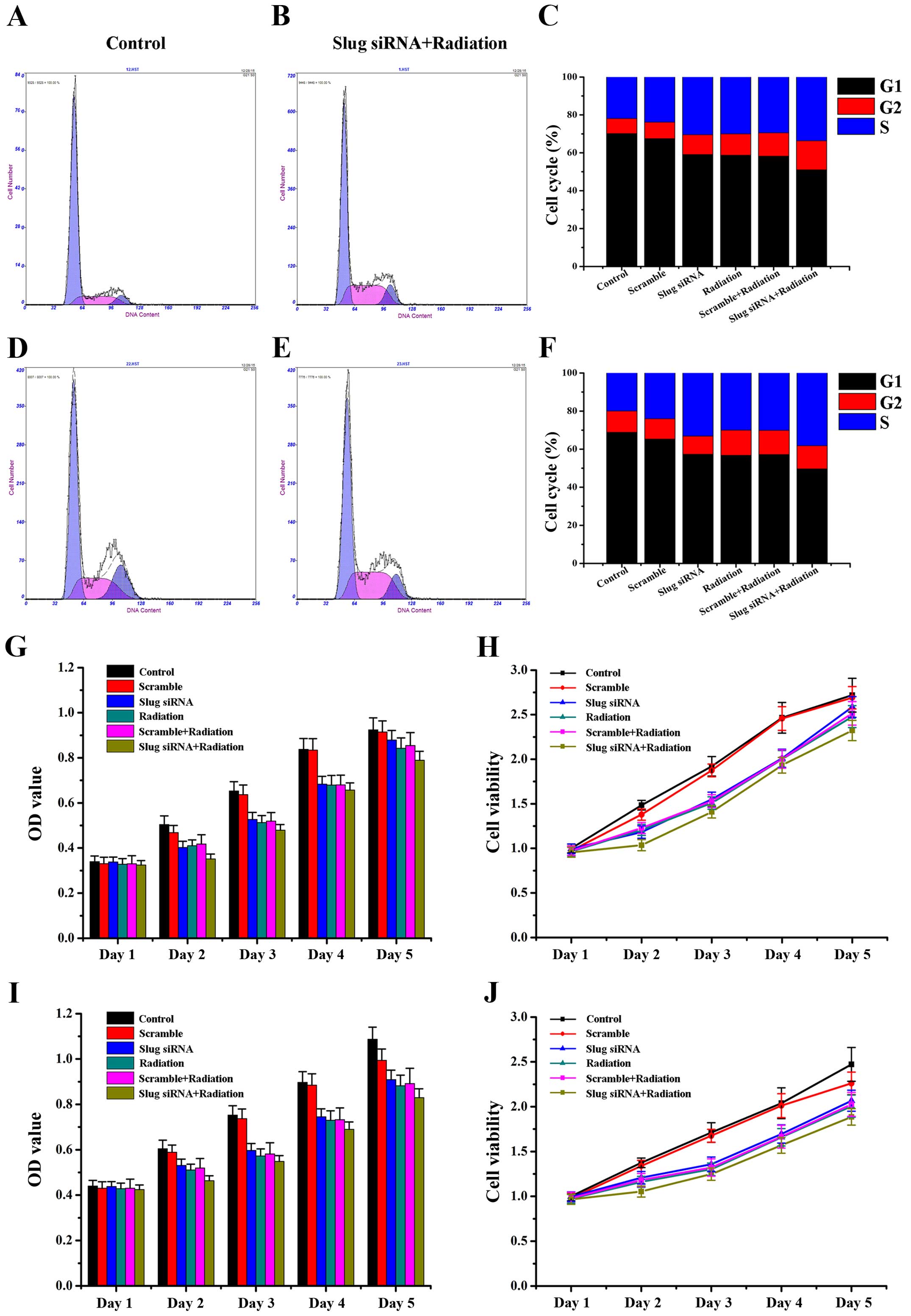
Effect of Slug inhibition combined with
X-ray irradiation on OSCC cell cycle and cell proliferation
We assessed whether Slug inhibition or/and X-ray
irradiation treatment modulated cell cycle progress. Slug siRNA and
X-ray irradition induced a significant increase in the percentage
of S phase cells in HSC3 (Fig.
5A–C; P<0.05) and HSC6 (Fig.
5D–F; P<0.01) cells. To evaluate the synergistic effect of
Slug siRNA and X-ray irradiation on cell proliferation, we used
CCK8 assay to compare the growth of HSC3 and HSC6 cells when
treated with Slug siRNA alone or with radiation. As shown in
Fig. 5, HSC3 and HSC6 cells
proliferated at a significantly lower rate in Slug siRNA/radiation
group than did other groups at day 2 (HSC3 F=4.199, P=0.019; HSC6
F=3.623, P=0.031), day 3 (HSC3 F=4.121, P=0.021; HSC6 F=4.751,
P=0.013) and day 4 (HSC3 F=3.464, P=0.036; HSC6 F=3.391, P=0.039)
and there was no obvious difference of cells proliferation at day 1
(HSC3 F=2.092, P=0.137; HSC6 F=0.586, P=0.711) and day 5 (HSC3
F=1.888, P=0.170; HSC6 F=1.944, P=0.160). Thus, the results showed
that Slug inhibition combined with X-ray irradiation could inhibit
cell proliferation through increasing cells in S phase.
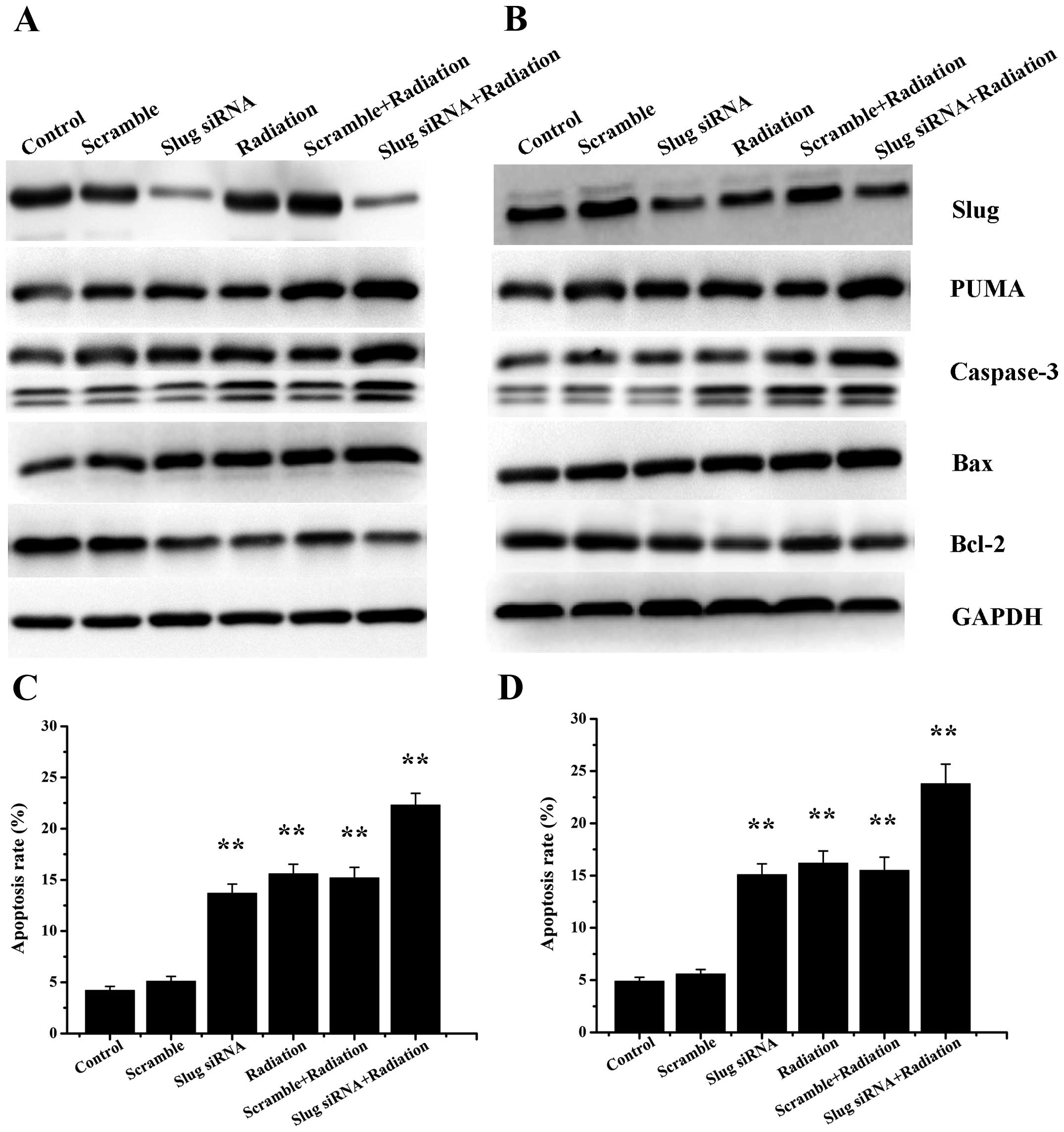
Radiation treatment affects the
expression of proteins involved in apoptotic processes
To assess the effect of X-ray irradiation on the
expression of Slug and PUMA, we analyzed Slug and PUMA expression
at both mRNA and protein levels in HSC3 and HSC6 cells that
received 4-Gy X-ray irradiation. Our data showed that the
expression of Slug were strikingly higher in radiation group than
control group, however, there was no obvious Slug upregulation in
Slug siRNA group compared with radiation group. It was indicated
that X-ray irradiation could improve Slug expression in HSC3 and
HSC6 cells.
As for PUMA, the results showed that PUMA expression
increased significantly after X-ray irradiation or Slug siRNA
transfection compared with control group. To investigate the effect
of Slug siRNA transfection in combination with X-ray irradiation on
PUMA activity, we measured PUMA expression after the above
treatment in HSC3 and HSC6 cells. RT-PCR and western blot analysis
showed that cells treated with Slug siRNA transfection and
radiation displayed notable increased PUMA expression when compared
with radiation group or Slug siRNA group. The data indicated that
both Slug siRNA transfection and radiotherapy could upregulate the
expression of PUMA. In addition, Caspase-3 and Bax were observed to
be overexpressed in Slug siRNA, radiation, scramble/radiation and
Slug siRNA/radiation groups. In contrast, Bcl-2 exhibited an
opposite trend (Fig. 6A and
B).
Downregulation of Slug plus radiation
induces OSCC cell apoptosis
RT-PCR and western blot analysis above displayed the
upregulation of PUMA expression in Slug siRNA group, radiation
group, scramble plus X-ray irradiation group and Slug siRNA
combination with radiation group. The impact of Slug siRNA
transfection and X-ray irradiation on HSC3 and HSC6 cell apoptosis
was evaluated by Annexin V assay. The apoptosis rate were
statistically significant in each group both of HSC3 (F=24.344,
P<0.01) and HSC6 cells (F=32.843, P<0.01). The data showed
that the apoptotic cells were notably increased in cells treated
with Slug siRNA and 4 Gy X-ray irradiation (HSC3: 22.03±1.23%;
HSC6: 24.06±1.67%) compared with cells in Slug siRNA group (HSC3:
13.43±1.29%, P=0.001; HSC6: 15.25±1.01%, P=0.006), radition group
(HSC3: 15.77±1.21%, P=0.008; HSC6: 16.36±1.28%, P=0.016) and
scramble plus X-ray irradiation group (HSC3: 15.11±1.67%, P=0.004;
HSC6: 15.50±1.57%, P=0.007), suggesting that cell apoptosis was
obviously induced when treated with Slug siRNA and X-ray
irradiation. There was no remarkable changes of cell apoptosis in
control (HSC3: 4.08±0.56%, HSC6: 4.75±0.91%) or scramble group
(HSC3: 5.06±0.75%, P=0.626; HSC6: 5.53±0.85%, P=1.000) (Fig. 6C and D).
Discussion
Oral squamous cell carcinoma (OSCC) is the most
commom type of oral cancer and accounts for >90% of it. Despite
advances in the common treatments such as surgery, chemotherapy,
radiotherapy or a combination of them for OSCC patients, the
overall survival rate has not been drastically improved over the
past few years (22–25). Radiotherapy played an important
role in the treatment of OSCC, however, its efficacy was still
limited mainly due to some patients exhibit tolerance to
radiotherapy. Therefore, reducing the radiation tolerance and
increasing sensitivity to radiotherapy has become a breakthrough to
improve the efficacy of OSCC patients.
Slug is involved in the chemoresistance and
radioresistance of several types of cancers (26,27).
As an E-cadherin repressor and a suppressor of PUMA, and Slug has
been proved to play an important role in controlling cell apoptosis
recently. Zhang et al (15)
found that Slug is a considerable modulator of the therapeutic
response of cholangiocarcinoma cells and may be potentially useful
as a sensitizer in cholangiocarcinoma therapy. One of the
mechanisms is the regulation of PUMA by Slug. Xu et al
(8) found that Slug overexpression
in CNE-2-RES cells may result in the radioresistance of cells and
Slug mediates CNE-2 radioresistance via downregulation of PUMA in
both a p53-dependent and p53-independent manner. Some studies
(28) confirmed the survival
function of Slug-PUMA axis in human breast cancer cells that Slug
knockdown increased PUMA expression and inhibited lung colonization
and demonstrated a pivotal role for Slug in carcinoma cell
survival, which implied that disruption of the Slug-PUMA axis may
impinge on the survival of metastatic cells. To assess the effect
of endogenous Slug on PUMA expression in HSC3 and HSC6 cells, we
transfected Slug siRNA into cells and analyzed the expression of
PUMA mRNA and protein expression. In agreement with this notion,
our data showed that Slug expression was decreased and PUMA was
upregulated in Slug siRNA group which evaluated that silencing of
Slug played an important role in upregulating PUMA expression in
HSC3 and HSC6 cells.
As a member of the Bcl-2 family, PUMA was discovered
in 2001 and identified as an essential mediator of p53-independent
and p53-dependent apoptosis (29,30).
PUMA was localized in the mitochondria and could kill a variety of
human cancer cells by activating caspases through mitochondrial
dysfunction (13,31). PUMA also functioned through other
Bcl-2 family members, such as Bcl-2, Bcl-XL and Bax (32). Although the specific mechanism for
PUMA inducing apoptosis needs further investigation, it has been
shown to be a promising new terget in gene therapy (33,34).
The goal of this study was to explore the effect of combining Slug
siRNA with X-ray irradiation on HSC3 and HSC6 cells. We found that
downregulation of Slug expression was correlated with the
sensitivity of OSCC cells to radiotherapy. However, further studies
are needed to investigate the mechanism that Slug silencing exerts
its anti-survival and pro-apoptotic effect in OSCC cells.
In this study, the inhibition of Slug was efficient
in suppression of proliferation and induction of apoptosis in HSC3
and HSC6 cells. Surprisingly, the combination of Slug siRNA and
X-ray irradiation induced a relatively higher apoptosis as compared
with Slug siRNA or X-ray irradiation alone in OSCC cells. We
performed the clonogenic survival assay which demonstrated that the
treatment with Slug downregulation and X-ray irradiation
synergistically reduced clonogenia survival to address that the
induction of apoptosis may lead to long-term response to
radiotherapy. The data revealed that Slug down-regulation
potentially enhanced radiosensitivity of HSC3 and HSC6 cells in
vitro by increasing PUMA expression.
In conclusion, this study indicates that Slug may be
a potential target as an inducer of PUMA and inhibition of Slug by
Slug siRNA may be a strategy to overcome radioresistance by
upregulation of PUMA. These findings provided new information for
novel combinational therapies using Slug siRNA to cooperate with
X-ray irradiation in patients with OSCC.
Acknowledgements
This study was supported by the National Natural
Sciences Foundation of China (nos. 81272554 and 81472526), the
Guangdong Sciences and Technology Project (no. 2016A020216007).
References
|
1
|
Zhao Y, Li Z, Sheng W, Miao J and Yang J:
Radiosensitivity by ING4-IL-24 bicistronic adenovirus-mediated gene
cotransfer on human breast cancer cells. Cancer Gene Ther.
20:38–45. 2013. View Article : Google Scholar
|
|
2
|
Wu J, Zhang JY, Yin L, Wu JZ, Guo WJ, Wu
JF, Chen M, Xia YY, Tang JH, Ma YC, et al: HAP1 gene expression is
associated with radiosensitivity in breast cancer cells. Biochem
Biophys Res Commun. 456:162–166. 2015. View Article : Google Scholar
|
|
3
|
Finnon P, Kabacik S, MacKay A, Raffy C,
A'Hern R, Owen R, Badie C, Yarnold J and Bouffler S: Correlation of
in vitro lymphocyte radiosensitivity and gene expression with late
normal tissue reactions following curative radiotherapy for breast
cancer. Radiother Oncol. 105:329–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganapathy S, Xiao S, Yang M, Qi M, Choi
DE, Ha CS, Little JB and Yuan ZM: A low-dose arsenic-induced p53
protein-mediated metabolic mechanism of radiotherapy protection. J
Biol Chem. 289:5340–5347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agra IM, Filho JG, Martins EP and Kowalski
LP: Second salvage surgery for re-recurrent oral cavity and
oropharynx carcinoma. Head Neck. 32:997–1002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brun SN, Markant SL, Esparza LA, Garcia G,
Terry D, Huang JM, Pavlyukov MS, Li XN, Grant GA, Crawford JR, et
al: Survivin as a therapeutic target in Sonic hedgehog-driven
medulloblastoma. Oncogene. 34:3770–3779. 2015. View Article : Google Scholar :
|
|
7
|
Markant SL, Esparza LA, Sun J, Barton KL,
McCoig LM, Grant GA, Crawford JR, Levy ML, Northcott PA, Shih D, et
al: Targeting sonic hedgehog-associated medulloblastoma through
inhibition of Aurora and Polo-like kinases. Cancer Res.
73:6310–6322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu T, Fan B, Lv C and Xiao D: Slug
mediates nasopharyngeal carcinoma radioresistance via
downregulation of PUMA in a p53-dependent and -independent manner.
Oncol Rep. 33:2631–2638. 2015.PubMed/NCBI
|
|
9
|
Lee SH, Kim DY, Jing F, Kim H, Yun CO, Han
DJ and Choi EY: Del-1 overexpression potentiates lung cancer cell
proliferation and invasion. Biochem Biophys Res Commun. 468:92–98.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiang C and Ayyanathan K:
Characterization of the E-box binding affinity to snag-zinc finger
proteins. Mol Biol (Mosk). 46:907–914. 2012. View Article : Google Scholar
|
|
11
|
Pérez-Losada J, Sánchez-Martín M,
Pérez-Caro M, Pérez-Mancera PA and Sánchez-García I: The
radioresistance biological function of the SCF/kit signaling
pathway is mediated by the zinc-finger transcription factor Slug.
Oncogene. 22:4205–4211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pérez-Caro M, Bermejo-Rodríguez C,
González-Herrero I, Sánchez-Beato M, Piris MA and Sánchez-García I:
Transcriptomal profiling of the cellular response to DNA damage
mediated by Slug (Snai2). Br J Cancer. 98:480–488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu J, Zhang L, Hwang PM, Kinzler KW and
Vogelstein B: PUMA induces the rapid apoptosis of colorectal cancer
cells. Mol Cell. 7:673–682. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Meng C and Xing D: Aβ induces PUMA
activation: A new mechanism for Aβ-mediated neuronal apoptosis.
Neurobiol Aging. 36:789–800. 2015. View Article : Google Scholar
|
|
15
|
Zhang K, Zhang B, Lu Y, Sun C, Zhao W,
Jiao X, Hu J, Mu P, Lu H and Zhou C: Slug inhibition upregulates
radiation-induced PUMA activity leading to apoptosis in
cholangiocarcinomas. Med Oncol. 28(Suppl 1): S301–S309. 2011.
View Article : Google Scholar
|
|
16
|
Kuribayashi K, Finnberg N, Jeffers JR,
Zambetti GP and El-Deiry WS: The relative contribution of
pro-apoptotic p53-target genes in the triggering of apoptosis
following DNA damage in vitro and in vivo. Cell Cycle.
10:2380–2389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gauger KJ and Schneider SS: Tumour
supressor secreted frizzled related protein 1 regulates
p53-mediated apoptosis. Cell Biol Int. 38:124–130. 2014. View Article : Google Scholar
|
|
18
|
Wang R, Wang X, Li B, Lin F, Dong K, Gao P
and Zhang HZ: Tumor-specific adenovirus-mediated PUMA gene transfer
using the survivin promoter enhances radiosensitivity of breast
cancer cells in vitro and in vivo. Breast Cancer Res Treat.
117:45–54. 2009. View Article : Google Scholar
|
|
19
|
Yu J, Yue W, Wu B and Zhang L: PUMA
sensitizes lung cancer cells to chemotherapeutic agents and
irradiation. Clin Cancer Res. 12:2928–2936. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu WS, Heinrichs S, Xu D, Garrison SP,
Zambetti GP, Adams JM and Look AT: Slug antagonizes p53-mediated
apoptosis of hematopoietic progenitors by repressing PUMA. Cell.
123:641–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arienti C, Tesei A, Carloni S, Ulivi P,
Romeo A, Ghigi G, Menghi E, Sarnelli A, Parisi E, Silvestrini R, et
al: SLUG silencing increases radiosensitivity of melanoma cells in
vitro. Cell Oncol (Dordr). 36:131–139. 2013. View Article : Google Scholar
|
|
22
|
Dillon JK, Brown CB, McDonald TM, Ludwig
DC, Clark PJ, Leroux BG and Futran ND: How does the close surgical
margin impact recurrence and survival when treating oral squamous
cell carcinoma? J Oral Maxillofac Surg. 73:1182–1188. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inhestern J, Oertel K, Stemmann V,
Schmalenberg H, Dietz A, Rotter N, Veit J, Görner M, Sudhoff H,
Junghanss C, et al: Prognostic role of circulating tumor cells
during induction chemotherapy followed by curative surgery combined
with postoperative radiotherapy in patients with locally advanced
oral and oropharyngeal squamous cell cancer. PLoS One.
10:e01329012015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang SH and O'Sullivan B: Oral cancer:
Current role of radiotherapy and chemotherapy. Med Oral Patol Oral
Cir Bucal. 18:e233–e240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lo WL, Kao SY, Chi LY, Wong YK and Chang
RC: Outcomes of oral squamous cell carcinoma in Taiwan after
surgical therapy: Factors affecting survival. J Oral Maxillofac
Surg. 61:751–758. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang P, Liu H, Xia F, Zhang QW, Zhang YY,
Zhao Q, Chao ZH, Jiang ZW and Jiang CC: Epithelial-mesenchymal
transition is necessary for acquired resistance to cisplatin and
increases the metastatic potential of nasopharyngeal carcinoma
cells. Int J Mol Med. 33:151–159. 2014.
|
|
27
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Yao J, Suyama K, Qian X, Qian BZ,
Bandyopadhyay S, Loudig O, De Leon-Rodriguez C, Zhou ZN, Segall J,
et al: Slug promotes survival during metastasis through suppression
of Puma-mediated apoptosis. Cancer Res. 74:3695–3706. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu J and Zhang L: No PUMA, no death:
Implications for p53-dependent apoptosis. Cancer Cell. 4:248–249.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Akhter R, Sanphui P and Biswas SC: The
essential role of p53-up-regulated modulator of apoptosis (Puma)
and its regulation by FoxO3a transcription factor in
β-amyloid-induced neuron death. J Biol Chem. 289:10812–10822. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu J, Wang Z, Kinzler KW, Vogelstein B and
Zhang L: PUMA mediates the apoptotic response to p53 in colorectal
cancer cells. Proc Natl Acad Sci USA. 100:1931–1936. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen L, Willis SN, Wei A, Smith BJ,
Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM and Huang DC:
Differential targeting of prosurvival Bcl-2 proteins by their
BH3-only ligands allows complementary apoptotic function. Mol Cell.
17:393–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Funamizu N, Lacy CR, Kamada M, Yanaga K
and Manome Y: MicroRNA-203 induces apoptosis by upregulating Puma
expression in colon and lung cancer cells. Int J Oncol.
47:1981–1988. 2015.PubMed/NCBI
|