Introduction
In recent years, a novel type of cancer
immunotherapy has arisen that involves a specific monoclonal
antibody (mAb) targeting immune checkpoint molecules, such as
cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and the
programmed death-1 (PD-1) receptor/PD-ligand 1 (PD-L1) interaction.
This type of therapy has been applied in many clinical trials
(1–5). The mechanisms responsible for the
antitumor activity of immune checkpoint blockade are thought to
involve a restoration of T cell suppression mediated by tumors
(6–8). The PD-1 and CTLA-4 pathways have
different roles in regulating T cell activity; CTLA-4 is involved
in the priming T-cell phase by interacting with antigen-presenting
cells (APC) expressing co-stimulatory molecules and PD-1 is
involved in the effector T cell phase by blocking cancer cells
expressing PD-L1 (9).
Ipilimumab is an anti-CTLA-4 mAb, and nivolumab is
an anti-PD-1 mAb; these antibodies were approved by the FDA in 2011
and 2014, respectively, for treating metastatic and unresectable
melanomas. The first phase I clinical trial for nivolumab involved
296 patients and reported that the objective response rates was 17,
32 and 29% for advanced non-small cell lung cancers (NSCLC),
melanoma and renal cell carcinoma (RCC), respectively, all of which
had been treated heavily prior to the study (3). Additionally, nivolumab demonstrated
an overall survival improvement over dacarbazine in a phase III
study on previously untreated metastatic melanoma patients
(10).
Despite the clinical trial success with nivolumab
against advanced cancers, few preclinical studies have focused on
PD-1 mAb. There are several reasons for this lack of preclinical
studies: i) mouse systems cannot accurately represent the human
immune response induced by the anti-PD-1 mAb, and ii) few efficient
mouse anti-PD-1 antibodies are available. Currently, only a few
in vivo studies have been published that used mouse tumor
cells and mouse anti-PD-1 antibodies and that verified the
antitumor response and specific CTL induction (11,12).
However, there are difficulties associated with replacing the mouse
immune system with a human system-like and then predicting the
clinical response in humans from those mouse in vivo
studies. In the future, the antitumor effect of the anti-PD-1
antibody may be investigated in a humanized mouse system that uses
human immune cells and tumor cells.
In this study, we manufactured an in-house anti-PD-1
mAb that is similar to nivolumab, and we investigated the
immunological effect of the antibody on the peripheral blood
mononuclear cells (PBMCs) of cancer patients. We obtained specific
in vitro immunological data by treating PBMCs with anti-PD-1
mAb. These results may contribute to the profiling of patients to
predict which patients are likely to respond to anti-PD-1 mAb.
Materials and methods
Cancer patient-derived PBMCs
PBMCs from malignant glioma patients were used for
in vitro experiments. The studies involving PBMCs derived
from glioma patients were approved by the Institutional Review
Board of Shizuoka Cancer Center, Shizuoka, Japan. All patients gave
written informed consent. PBMCs from 6 glioma patients were used
for in vitro cell-based assay (Table I).
 | Table IThe characteristics of high-grade
glioma patients. |
Table I
The characteristics of high-grade
glioma patients.
| Patient code | Age | Gender | HLA-typing | Pathology |
|---|
| GB-001 | 45 | M | A2 | GBM (IV)a |
| GB-002 | 37 | M | A2 | AA (III) |
| GB-003 | 53 | M | A24 | GBM (IV) |
| GB-004 | 63 | F | A2 | GBM (IV) |
| GB-005 | 56 | M | A24 | GB (IV) |
| GB-006 | 71 | M | A2 | GBM (IV) |
Antibodies and reagents
The following antibodies were used for flow
cytometric analyses: anti-CD3-PerCP, anti-CD4-PE, anti-CD8-FITC,
anti-CD11b-PE-Cy7, anti-CD14-PE, anti-CD19-FITC, anti-CD25-FITC,
anti-CD33-FITC, anti-CD45RO-PE, anti-CD56-biotin, anti-CCR7-biotin,
anti-FoxP3-PE, anti-PD-1-APC, anti-PD-L1-APC and anti-human
IFN-γ-PE. Anti-PD-1-APC and anti-PD-L1-APC antibodies were
purchased from BioLegend Inc. (San Diego, CA, USA). All other
antibodies were purchased from BD Pharmingen (San Diego, CA, USA).
No azide/low endotoxin (NA/LE) anti-CD3 mAb was also purchased from
BD Pharmingen and used for in vitro stimulation of human
PBMCs. The WST-1 assay reagent was purchased from Dojin Kagaku
Corp. (Kumamoto, Japan) and was used for cell proliferation assay.
Human recombinant PD-L1 and PD-1 proteins were purchased from Sino
Biotechnology (BDA, Beijing, China), and were used for a blocking
assay with the anti-PD-1 mAb and for surface plasmon resonance
(SPR) analysis, respectively. Commercially available unconjugated
anti-PD-1 mAb was purchased from BioLegend Inc. and used for SPR
assay.
Production and purification of the
in-house full-length anti-PD-1 monoclonal antibody
The amino acid sequence of nivolumab was downloaded
from the J-PlatPat data base from National Center for Industrial
Property Information and Training (INPIT) (https://www.j-platpat.inpit.go.jp/web/tokujitsu/tkbs/TKBS_GM401_ToPDF.action).
Nivolumab-derived VH and VL genes were synthesized according to
their cDNA sequences and were cloned into a pcDNA3.3 vector for IgH
and IgL co-expressions. Specifically, the VH gene was ligated with
human IgG4 fragment that was PCR-cloned from the human PBMC-derived
cDNA, and the product was finally inserted into pcDNA3.3. These IgH
and IgL vectors were expanded, purified by endotoxin-depletion and
co-transduced into expi293 cells using lipofection according to the
manufacturer’s instructions. The supernatant was harvested and
affinity-purified with a protein A prepacked column (GE
Healthcare). Finally anti-PD-1 mAb was purified as a biosimilar
antibody to nivolumab and was used for in vitro
experiments.
Surface plasmon resonance (SPR) analysis
using an in-house full-length anti-PD-1 monoclonal antibody
SPR analysis was performed on a Biacore X100 (GE
Healthcare) as reported previously (15). All reagents and sensor chips were
purchased from GE Healthcare. Immobilization of anti-human IgG
antibody was performed at pH 5.0 on the CM5 sensor chip for
capturing anti-PD-1 antibody as ligand, and the amount targeted was
1,000 response units (RU). The analyte was a recombinant human PD-1
protein. Commercially available anti-PD-1 mAb was purchased from
BioLegend (clone EH12.2H7) and monitored as a control.
Cell proliferation assay
Cell proliferation was examined using the WST-1
assay (Dojin Kagaku Corp., Kumamoto, Japan). Briefly,
1–2×105 PBMCs were seeded into each well of a 96-well
micro-culture plate coated with anti-CD3 mAb at 5 μg/ml overnight
at 4°C. Anti-PD-1 mAb was added at various concentrations and cells
were cultured for 5 days. The WST-1 substrate was added to the
culture, and the optical density (OD) was measured at 450 and 620
nm using an immunoreader (Immuno Mini NJ-2300, Nalge Nunc
International, Roskilde, Denmark).
For the PD-L1 blocking assay, the anti-PD-1 mAb and
the PD-L1 recombinant protein were sequentially added to a 96-well
plate experiment. After a 5-day culture, the cell proliferation
assay was performed using a WST-1 reagent.
Human PBMC cultures stimulated with
anti-CD3 for FACS analysis
A 6-well culture plate was coated with NA/LE
anti-CD3 mAb at 5 μg/ml at 4°C overnight. Human PBMCs were seeded
at 8×106 per well in 4 ml of RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with L-glutamine
(2 mM), penicillin (100 U/ml), streptomycin (100 U/ml) and 10%
fetal bovine serum (FBS, Gibco, Paisley, UK). After 1 h, anti-PD-1
mAb was added at various concentrations and cells were cultured for
5 days. Then, FACS analysis was performed to analyze the T cell
markers, myeloid-derived suppressor cells (MDSCs) and IFN-γ
production.
For the PD-L1 blocking assay, 30 min after the
addition of anti-PD-1 mAb, PD-L1 recombinant protein was added for
a final concentration of 10 μg/ml in a 6-well culture plate and
cells were cultured for 5 days. For MDSC induction analysis,
CD33+CD11b+ MDSC and
CD14+CD11b+ monocyte MDSC fractions were
measured using a flow cytometry. In the case of IFN-γ production
analysis, after 5-day cultures with or without anti-PD-1 mAb and
PD-L1, cells were stimulated with PMA (Sigma-Aldrich Corp., St.
Louis, MO, USA) and ionomycin (Sigma) for 4 h. Finally, FACS
analysis was performed to measure IFN-γ+ T cells using
intracellular staining with anti-human IFN-γ-PE antibody.
Propidium iodide (PI) was used for detecting living
cells. Cell suspensions were harvested from cultured PBMCs and were
stained with various primary antibodies for 15 min at 4°C and then
washed with cold PBS+2% fetal bovine serum (FBS; Life
Technologies). Then, cells were stained with the secondary
antibodies for 15 min at 4°C. After washing, cells were fixed with
0.5% paraform aldehyde-containing PBS (−) and analyzed on a
FACSCanto II flow cytometer (BD Biosciences, San Diego, CA,
USA).
Peptide-pulsed dendritic cell (DC)-based
CTL induction assay using tetramer staining
CTL induction cultures were described previously
(13). Immature DCs were induced
by GM-CSF and IL-4, and mature type-1 DCs were induced by a
combination of cytokines, as reported previously (14). HLA-A2-positive PBMCs derived from
four glioma patients were used for CTL induction assay. Mature DCs
were pulsed with HLA-A2-restricted MART-1, GP100 or CMVpp65 peptide
and used for CTL induction cultures. An isotype control antibody or
anti-PD-1 mAb was added to CTL cultures at a concentration of 10
μg/ml. Two-rounds of co-culture of T cells and DCs were performed,
and CD8+tetramer+ cells fraction was measured
using flow cytometry. More than 0.1% of
CD8+tetramer+ cell frequency was rated as
positive after treatment with anti-PD-1 Ab. The tetramers for
MART-1, GP100 and CMVpp65 antigens were HLA-A2 restricted, and
HIV-tetramer was HLA-A24 restricted.
Regulatory T cell induction assay
During two-rounds of co-culture, T cells and mature
DCs were treated with or without anti-PD-1 mAb at a concentration
of 10 μg/ml in 6-well culture plate, cells were collected and used
also for Treg induction analysis. CD4+CD25+
fraction was gated and FoxP3+ cells were measured using
flow cytometry.
Statistical analysis
The significance of differences was analyzed using
Student’s t-test. Values of P<0.05 were considered statistically
significant.
Results
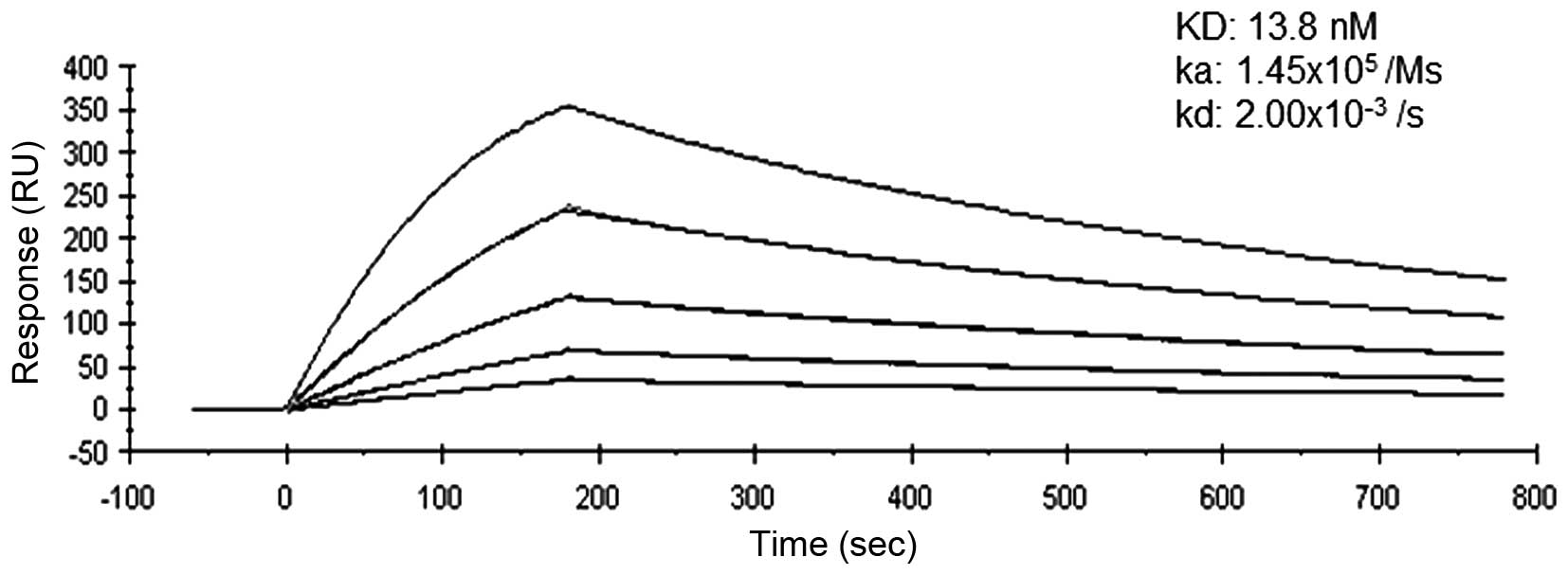
Binding affinity of anti-PD-1 mAb to
recombinant PD-1
SPR analysis confirmed the affinity of anti-PD-1 mAb
to recombinant PD-1 consistently and mean dissociation constants
were determined to be 13.8 nM (Fig.
1). The binding affinity of anti-PD-1 mAb was greater than that
of the commercially available anti-PD-1 antibody (98.9 nM), as
judged by SPR analysis (data not shown).
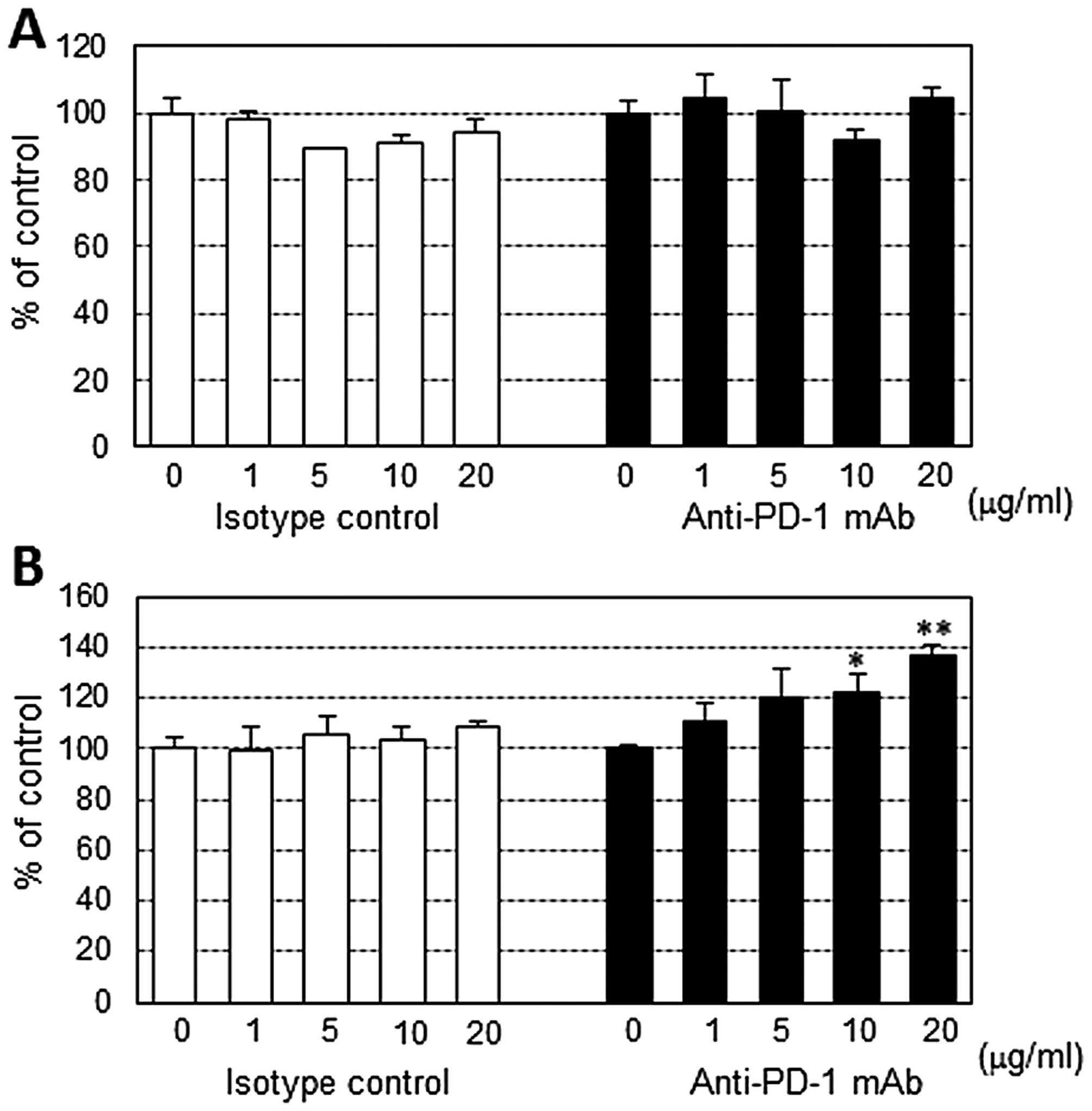
Effect of anti-PD-1 mAb on PBMC
proliferation stimulated by the anti-CD3 antibody
Anti-PD-1 mAb showed no stimulatory activity on
resting human PBMCs. However, high concentration of anti-PD-1 mAb
exhibited moderate stimulatory effect on CD3 antibody-stimulated
PBMCs from 2 of 6 patients (Fig.
2).
Effect of anti-PD-1 mAb on T cell subsets
in anti-CD3 antibody-stimulated PBMC
The anti-PD-1 mAb showed no significant effect on
the percentage (%) of CD3+CD4+ and
CD3+CD8+ subpopulations in anti-CD3
Ab-stimulated PBMCs. Additionally, the percentage (%) of effector
memory T cell subsets such as
CD4+CD45RO+CD95+CCR7−
were not affected by anti-PD-1 mAb (Table II).
 | Table IIEffect of anti-PD-1 antibody on T
cell subsets in PBMCs. |
Table II
Effect of anti-PD-1 antibody on T
cell subsets in PBMCs.
| Anti-PD-1 Ab
(μg/ml) |
CD3+ | T cell marker (%)
CD3+CD4+ |
CD3+CD8+ | Memory T cell
marker (%)
CD4+CD45RO+CD95+CCR7−a |
|---|
| 0 | 80.4±6.5 | 59.7±20.3 | 24.1±17.1 | 17.1±2.8 |
| 10 | 83.4±7.5 | 60.9±16.4 | 26.8±10.2 | 20.8±1.5 |
| 20 | 82.3±3.3 | 64.7±18.2 | 27.1±14.9 | 23.3±3.1 |
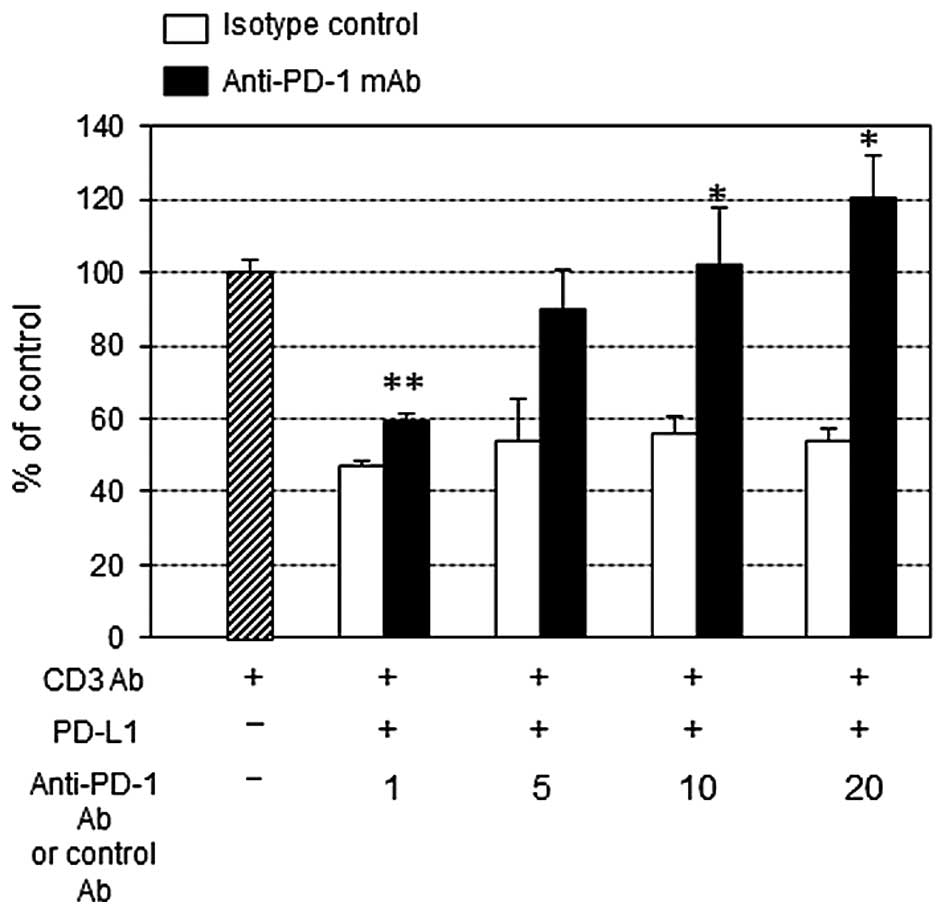
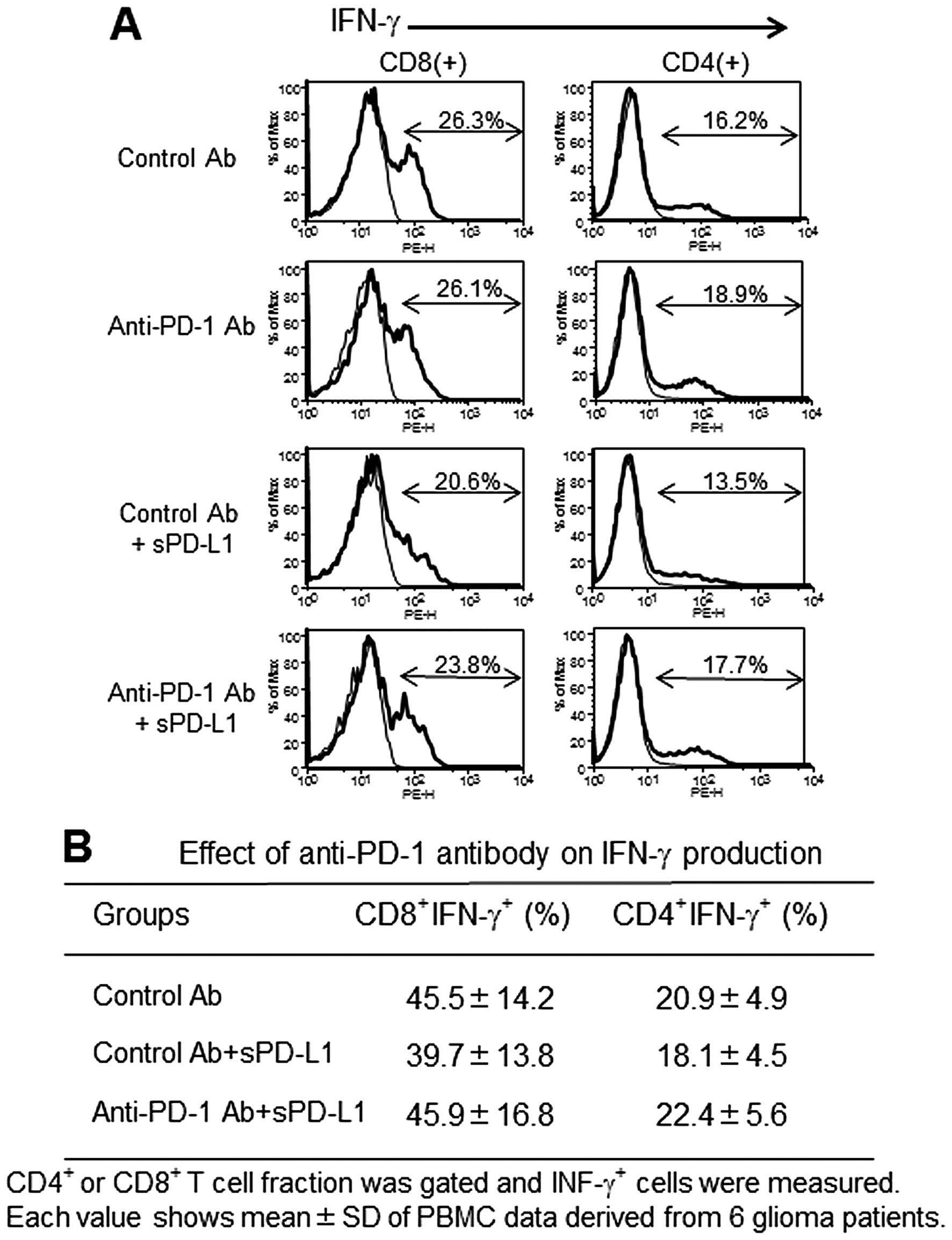
Effect of PD-L1-inhibited PBMC
proliferation and IFN-γ production by anti-PD-1 mAb
PD-L1 at 10 μg/ml significantly suppressed the
anti-CD3 Ab-stimulated PBMC proliferation (Fig. 3). The addition of anti-PD-1 mAb at
concentration >10 μg/ml restored the growth inhibition, and
interestingly at 20 μg/ml anti-PD-1 mAb surpassed the control
growth level (in the absence of PD-L1). On the other hand, IFN-γ
production measured by intracellular staining showed a tendency of
restoration in anti-PD-1 mAb compared to isotype control cultures
containing PD-L1, however it was not statistically significant
(Fig. 4).
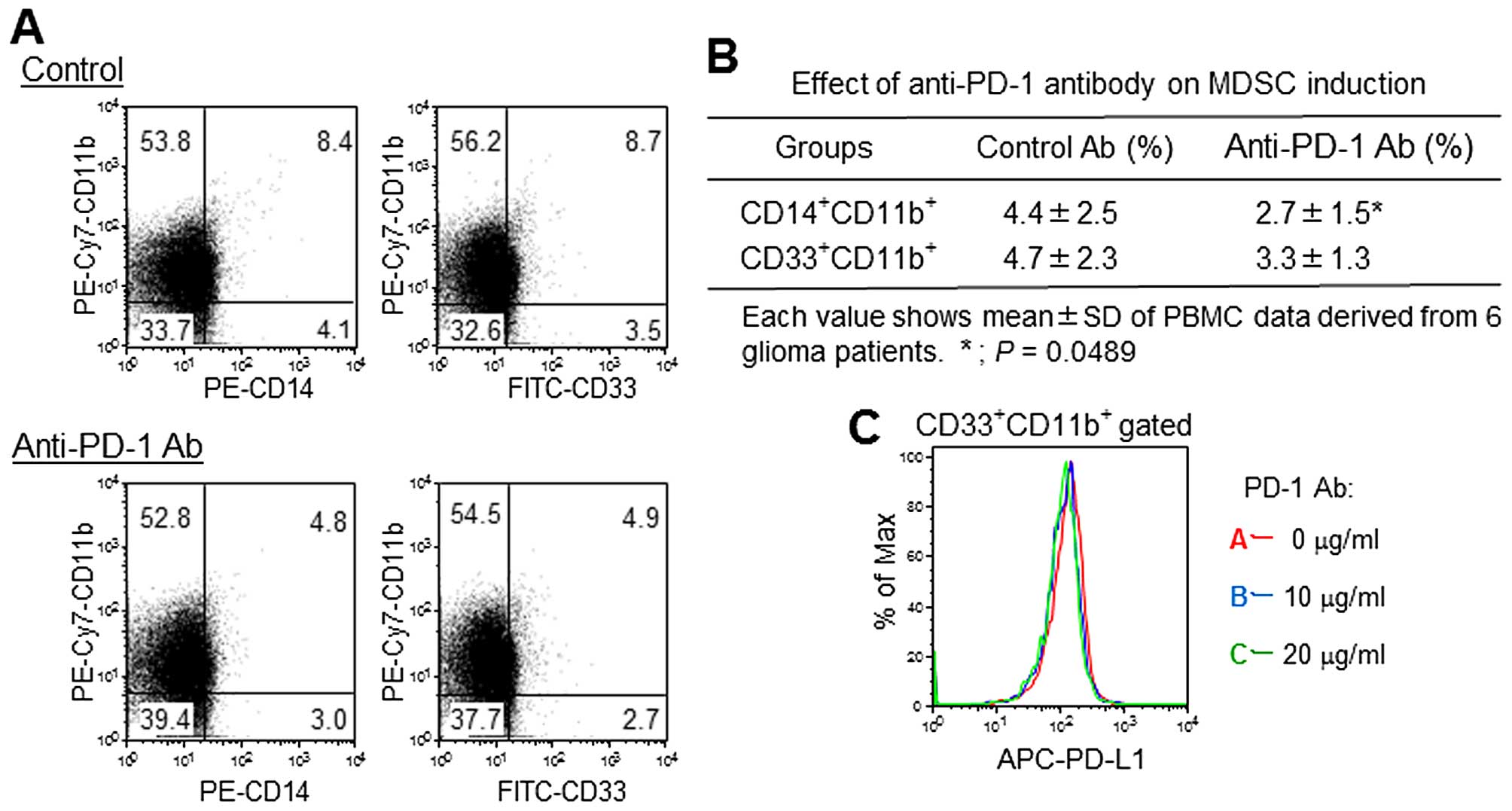
Effect of anti-PD-1 mAb on MDSC
induction
A small number of CD33+CD11b+
MDSCs and CD14+CD11b+ monocyte MDSCs were
identified in anti-CD3 antibody-stimulated PBMC cultures. The
addition of anti-PD-1 mAb inhibited monocyte MDSC induction by ~40%
compared to the control (Fig. 5A and
B). PD-L1 expression was observed in 60% of
CD33+CD11b+ MDSCs; however, anti-PD-1 mAb did
not show significant effects on PD-L1 expression (Fig. 5C).
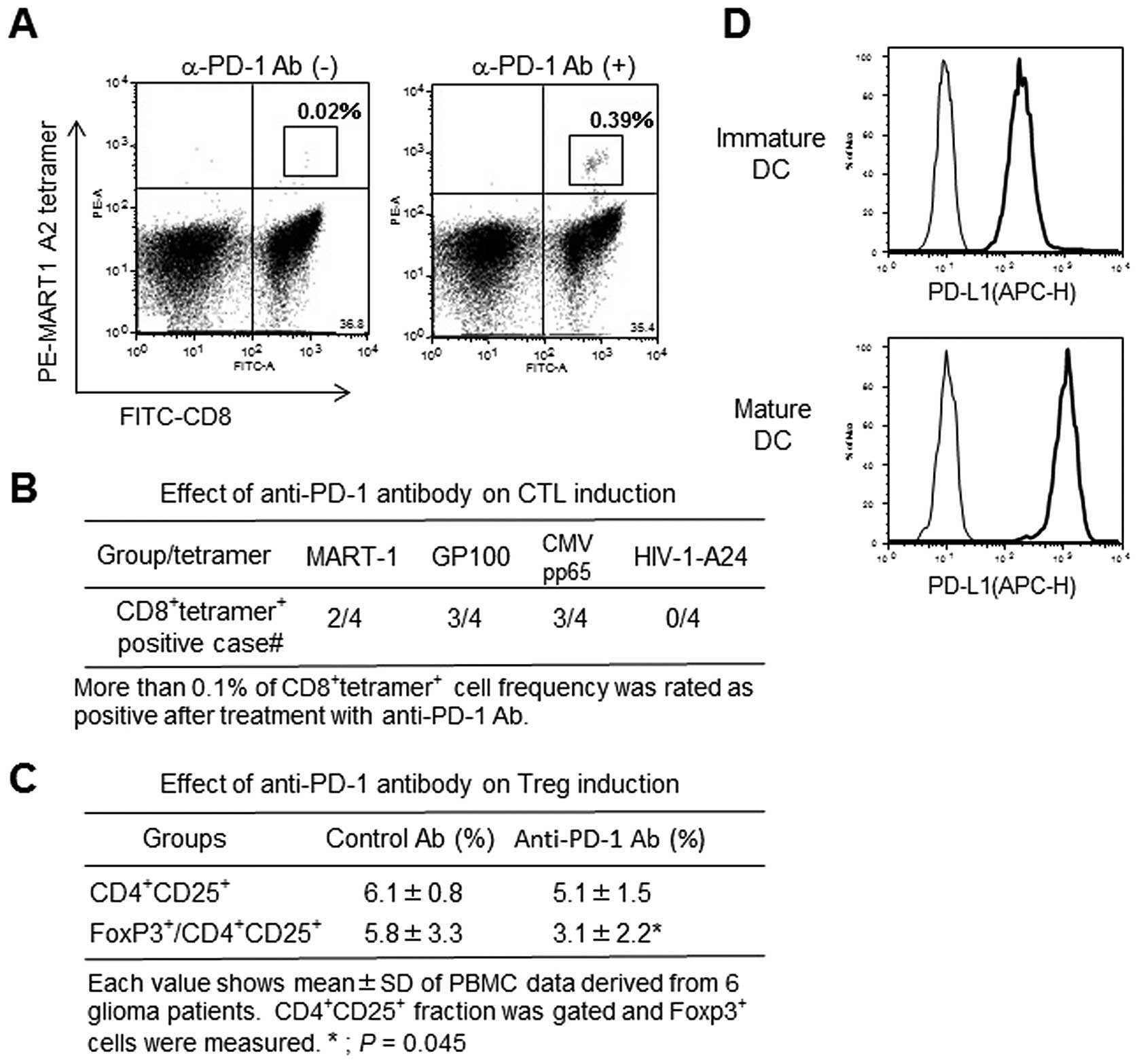
Stimulatory effect of anti-PD-1 mAb on
antigen-specific CTL induction
Antigen peptide (MRAT-1, GP100, CMVpp65)-specific
CTLs with HLA-A2 restriction significantly increased in anti-PD-1
Ab-treated CTL cultures compared to control Ab-treated cultures
(Fig. 6A and B and Table III). In contrast, no CTLs
responded to HLA-A24-restricted HIV peptide. Interestingly, mature
DCs demonstrated higher PD-L1 expression than immature DCs
(Fig. 6D).
 | Table IIIEffect of anti-PD-1 Ab on tumor
antigen-specific CTL inductions. |
Table III
Effect of anti-PD-1 Ab on tumor
antigen-specific CTL inductions.
| CD8+
tetramer+ cell (%) |
|---|
|
|
|---|
|
Patient/tetramer | MART-1 | GP100 | CMVpp65 | A2 HIV A24 |
|---|
| GB-001 | 0.02b/0.39a,c | 0.16/0.26a | 0.01/0.01 | 0/0.01 |
| GB-002 | 0.01/0.01 | 0.02/0.74a | 0.02/0.11a | 0/0.02 |
| GB-004 | 0.02/0.12a | 0.15/0.27a | 0.09/0.17a | 0/0.02 |
| GB-006 | 0.04/0.05 | 0.05/0.06 | 0.38/1.13a | ND |
Effect of anti-PD-1 mAb on Treg
induction
Regulatory T cell induction was investigated after
two-rounds of CMVpp65 peptide-pulsed DC-mediated CTL stimulation.
The frequency of CD4+CD25+ fraction was not
different between control and anti-PD-1 Ab treated cultures.
However, the frequency of FoxP3+ cells in gated
CD4+CD25+ fraction was inhibited in anti-PD-1
Ab-treated cultures (Fig. 6C).
Discussion
Since the development of anti-CTLA-4 Ab, ipilimumab
has been administered to metastatic melanoma patients as an
anti-check-point Ab (1,16). More immunomodulatory Abs such as
anti-PD-1 (3,17), anti-PD-L1 (4), anti-CD137 (18) and anti-CD40 (19,20)
have been developed and may be applicable to various advanced
cancers. Ipilimumab treatment resulted in >20% responders; in
addition, the antibody resulted in long-term survival in metastatic
melanoma patients despite adverse effects (1,2).
Remarkably, combination therapy with ipilimumab and nivolumab has
shown great success in phase III clinical trials, with >50%
response rate (21,22).
Intensive search for suitable biomarkers has been
performed to enable responder prediction prior to treatment. This
search uncovered blood biomarkers such as an increase in lymphocyte
number, a decrease in LDH and MDSC numbers, and intratumoral
biomarkers such as an increase in infiltrating CD8+ T
cell numbers and granzyme and a decrease in regulatory T cell
numbers (23,24).
Similarly to ipilimumab, nivolumab has been reported
to show a remarkable antitumor response and survival benefit in
advanced melanoma and non-small cell lung cancer patients; however,
the antibody was also found to induce autoimmune effects in thyroid
and lung cancer patients in a phase III clinical trial (22,25,26).
With regard to biomarkers, genetic and cytokine markers have been
intensively investigated using T cells and monocytes derived from
cancer patients who have been treated with a combination of
ipilimumab and nivolumab. These investigations demonstrated an
increase in T cell proliferation, as well as upregulation of
chemokine and NK cell function-associated genes (27).
Clinical trial studies demonstrated that a
heterogeneous response to antibody therapy is expected for
individuals, and the prediction of response will be difficult in
spite of strong biomarkers. For this reason, the direct observation
of the PBMC response to Ab treatment on an individual basis would
be helpful for predicting the immunological response for the
patients in question. A substantial amount of evidence from many
clinical trials has accumulated on this subject. However, in
vitro research using nivolumab has not been extensively
performed. In this study, we manufactured a biosimilar mAb to
nivolumab in-house and evaluated its biological function using
specific immunological assays. SPR analysis showed that our
anti-PD-1 antibody had a KD value of 13.8 nM, and that
of nivolumab is 3.06 nM (28). Our
in-house anti-PD-1 antibody seems to have a greater affinity than
other commercially available anti-PD-1 monoclonal antibodies (98.9
nM, data not shown).
Wang et al (28) demonstrated that nivolumab showed
simulatory activity in in vitro experiments, such as in MLR
assays and cytokine production experiments; three positive
observations were verified: i) T cell growth was stimulated and
IFN-γ production increased in co-culture with allogeneic dendritic
cells (DCs), ii) regulatory T cell-mediated T cell growth
inhibition was reversed, and iii) a synergistic increase in
specific antibody titer after antigen vaccination in non-human
primate resulted. However, no in vitro antibody-dependent
cell cytotoxicity was detected when nivolumab was used against
activated T cells.
In this study, our anti-PD-1 antibody stimulated the
proliferation of T cells activated with anti-CD3 antibody even at
high antibody concentration in only a few cases (20 μg/ml). In
particular, anti-PD-1 antibody restored the PD-L1-mediated T cell
growth inhibition. These observations are consistent with those in
previous studies.
Regarding the MDSC induction by anti-CD3 antibody
stimulation, anti-PD-1 antibody inhibited MDSC induction in
response to anti-CD3 antibody-stimulated PBMCs. The MDSC
populations, CD33+CD11b+ and
CD14+CD11b+, are reported to be induced in
the peripheral blood of cancer patients treated with chemotherapy
(29,30). The inhibitory effect shown by the
anti-PD-1 antibody on MDSC induction represents the first report in
the study of the immuno-logical function of the anti-PD-1 antibody
using human PBMCs.
In the near future, the effect of anti-PD-1 antibody
on the MDSC inhibitory action on immune cells should be
investigated to clarify the mechanism of antitumor effect of
anti-PD-1 antibody.
Similarly, regulatory T cell induction mediated by
mature DCs was suppressed by the addition of anti-PD-1 antibody in
CTL induction cultures. Wang et al, Klein et al and
Wong et al emphasized the restorative activity of the
anti-PD-1 antibody on the regulatory T cell-mediated inhibition of
effector T cell activation and cytokine production (7,28,31,32).
These observations that the anti-PD-1 antibody reversed the
immunological inhibition by regulatory T cells and MDSCs in
vitro, suggest that the anti-PD-1 antibody induces antitumor
activity by restoring the immunosuppressive state and by activating
T cell function.
Importantly, DC-mediated antigen-specific CTL
induction was potentiated more efficiently in the presence of the
anti-PD-1 antibody. Therefore, this observation suggests that it
may be beneficial to develop a combined anti-PD-1 antibody and DC
vaccine as a therapy (33,34).
Finally, to develop an anti-PD-1 antibody therapy
model that is more significant than in vitro studies, we
have developed an autologous immunotherapy in vivo model
based on humanized NOG mice (35),
in which both the autologous immune system and autologous tumors
can be established. This autologous immunotherapy in vivo
model will next be used to predict the immunological effect in
cancer patients who will receive anti-PD-1 antibody therapy.
Acknowledgements
This study was supported by a grant to Yasuto
Akiyama by JSPS KAKENHI (grant no. 25430166), Japan.
Abbreviations:
|
CTL
|
cytotoxic T lymphocytes
|
|
CTLA-4
|
cytotoxic T-lymphocyte-associated
antigen-4
|
|
DC
|
dendritic cell
|
|
MDSC
|
myeloid-derived suppressor cell
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PD-1
|
programmed death-1
|
|
Treg
|
regulatory T cells
|
References
|
1
|
Weber JS, O‘Day S, Urba W, Powderly J,
Nichol G, Yellin M, Snively J and Hersh E: Phase I/II study of
ipilimumab for patients with metastatic melanoma. J Clin Oncol.
26:5950–5956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hodi FS, O‘Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pericord VA, Montalvo W, Leiner IM and
Allison JP: Single dose of anti-CTLA-4 enhances CD8 T-cell memory
formation, function, and maintenance. Proc Natl Acad Sci USA.
108:261–271. 2011.
|
|
7
|
Wang W, Lau R, Yu D, Zhu W, Korman A and
Weber J: PD1 blockade reverses the suppression of melanoma
antigen-specific CTL by CD4+ CD25(Hi) regulatory T
cells. Int Immunol. 21:1065–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sznol M and Chen L: Antagonist antibodies
to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human
cancer. Clin Cancer Res. 19:1021–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okazaki T, Chikuma S, Iwai Y, Fagarasan S
and Honjo T: A rheostat for immune responses: The unique properties
of PD-1 and their advantages for clinical application. Nat Immunol.
14:1212–1218. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weber JS, D‘Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
John LB, Devaud C, Duong CP, Yong CS,
Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH and Darcy PK:
Anti-PD-1 antibody therapy potently enhances the eradication of
established tumors by gene-modified T cells. Clin Cancer Res.
19:5636–5646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mangsbo SM, Sandin LC, Anger K, Korman AJ,
Loskog A and Tötterman TH: Enhanced tumor eradication by combining
CTLA-4 or PD-1 blockade with CpG therapy. J Immunother. 33:225–235.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura Y, Tai S, Oshita C, Iizuka A,
Ashizawa T, Saito S, Yamaguchi S, Kondo H, Yamaguchi K and Akiyama
Y: Analysis of HLA-A24-restricted peptides of carcinoembryonic
antigen using a novel structure-based peptide-HLA docking
algorithm. Cancer Sci. 102:690–696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akiyama Y, Komiyama M, Nakamura Y, Iizuka
A, Oshita C, Kume A, Nogami M, Miyata H, Ashizawa T, Yoshikawa S,
et al: Identification of novel MAGE-A6- and MAGE-A12-derived
HLA-A24-restricted cytotoxic T lymphocyte epitopes using an in
silico peptide-docking assay. Cancer Immunol Immunother.
61:2311–2319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iizuka A, Komiyama M, Oshita C, Kume A,
Ashizawa T, Mitsuya K, Hayashi N, Nakasu Y, Yamaguchi K and Akiyama
Y: Anti-vascular endothelial growth factor receptor (VEGFR) 2
autoantibody identification in glioblastoma patient using single B
cell-based antibody gene cloning. Immunol Lett. 159:15–22. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Callahan MK, Wolchok JD and Allison JP:
Anti-CTLA-4 antibody therapy: Immune monitoring during clinical
development of a novel immunotherapy. Semin Oncol. 37:473–484.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Topalian SL, Sznol M, McDermott DF, Kluger
HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB,
Powderly JD, et al: Survival, durable tumor remission, and
long-term safety in patients with advanced melanoma receiving
nivolumab. J Clin Oncol. 32:1020–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ascierto PA, Simeone E, Sznol M, Fu YX and
Melero I: Clinical experiences with anti-CD137 and anti-PD1
therapeutic antibodies. Semin Oncol. 37:508–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vonderheide RH, Flaherty KT, Khalil M,
Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ,
Gallagher M, Kramer A, et al: Clinical activity and immune
modulation in cancer patients treated with CP-870,893, a novel CD40
agonist monoclonal antibody. J Clin Oncol. 25:876–883. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Melero I, Grimaldi AM, Perez-Gracia JL and
Ascierto PA: Clinical development of immunostimulatory monoclonal
antibodies and opportunities for combination. Clin Cancer Res.
19:997–1008. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahoney KM, Freeman GJ and McDermott DF:
The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in
melanoma. Clin Ther. 37:764–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Larkin J, Chiarion-Sileni V, Gonzaliz R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ascierto PA, Kalos M, Schaer DA, Callahan
MK and Wolchok JD: Biomarkers for immunostimulatory monoclonal
antibodies in combination strategies for melanoma and other tumor
types. Clin Cancer Res. 19:1009–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simeone E, Gentilcore G, Giannarelli D,
Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M,
Cavalcanti E, et al: Immunological and biological changes during
ipilimumab treatment and their potential correlation with clinical
response and survival in patients with advanced melanoma. Cancer
Immunol Immunother. 63:675–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brahmer J, Reckamp KL, Baas P, Crino L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Das R, Verma R, Sznol M, Boddupalli CS,
Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R,
Dhodapkar MV, et al: Combination therapy with anti-CTLA-4 and
anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol.
194:950–959. 2015. View Article : Google Scholar
|
|
28
|
Wang C, Thudium KB, Han M, Wang XT, Huang
H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al: In
vitro characterization of the anti-PD-1 antibody nivolumab,
BMS-936558, and in vivo toxicology in non-human primates. Cancer
Immunol Res. 2:846–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang J, Guo W and Liang X: Phenotypes,
accumulation, and functions of myeloid-derived suppressor cells and
associated treatment strategies in cancer patients. Hum Immunol.
75:1128–1137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Markowitz J, Brooks TR, Duggan MC, Paul
BK, Pan X, Wei L, Abrams Z, Luedke E, Lesinski GB, Mundy-Bosse B,
et al: Patients with pancreatic adenocarcinoma exhibit elevated
levels of myeloid-derived suppressor cells upon progression of
disease. Cancer Immunol Immunother. 64:149–159. 2015. View Article : Google Scholar :
|
|
31
|
Klein O, Ebert LM, Nicholaou T, Browning
J, Russell SE, Zuber M, Jackson HM, Dimopoulos N, Tan BS, Hoos A,
et al: Melan-A-specific cytotoxic T cells are associated with tumor
regression and autoimmunity following treatment with anti-CTLA-4.
Clin Cancer Res. 15:2507–2513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong RM, Scotland RR, Lau RL, Wang C,
Korman AJ, Kast WM and Weber JS: Programmed death-1 blockade
enhances expansion and functional capacity of human melanoma
antigen-specific CTLs. Int Immunol. 19:1223–1234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weber JS, Kudchadkar RR, Yu B, Gallenstein
D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, et
al: Safety, efficacy, and biomarkers of nivolumab with vaccine in
ipilimumab-refractory or -naïve melanoma. J Clin Oncol.
31:4311–4318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ribas A, Comin-Anduix B, Chmielowski B,
Jalil J, de la Rocha P, McCannel TA, Ochoa MT, Seja E, Villanueva
A, Oseguera DK, et al: Dendritic cell vaccination combined with
CTLA4 blockade in patients with metastatic melanoma. Clin Cancer
Res. 15:6267–6276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Inoue M, Senju S, Hirata S, Irie A, Baba H
and Nishimura Y: An in vivo model of priming of antigen-specific
human CTL by Mo-DC in NOD/Shi-scid IL2rgamma(null) (NOG) mice.
Immunol Lett. 126:67–72. 2009. View Article : Google Scholar : PubMed/NCBI
|