Introduction
Chemotherapeutic treatment efficacy of disseminated
cancer is limited by the indiscriminate toxicity of conventional
agents. The use of monoclonal antibodies (mAbs), which selectively
target cancer-specific antigens, increases treatment specificity in
comparison with conventional chemotherapy. However, the clinical
validation of therapeutic antibodies has revealed a modest efficacy
of unconjugated mAbs (1,2). A considerable number of patients were
inherently resistant to unconjugated mAbs and the majority acquired
resistance over time (3). The use
of immunotoxins, which consist of a cytotoxic molecule coupled to
an antibody or antibody fragment, selective for a cancer-specific
antigen, is a possible approach to improve the efficacy of mAb
therapy. The use of a potent cytotoxic drug and specific delivery
of the drug conjugate to the tumour creates preconditions to
improve the therapeutic outcome (4). The toxin PE38 (38 kDa), a truncated
version of Pseudomonas aeruginosa exotoxin A, has been
widely used in designing immunotoxins with high cytotoxic activity
(5). After translocation to the
cytosol, PE catalyses irreversible ribosylation of elongation
factor 2 (EF-2). This consequently inhibits protein synthesis and
leads to cell death. Derivatives of PE38 have been coupled to both
immunoglobulin- (6) and
non-immunoglobulin-based (7–10)
targeting agents for treatment of several types of cancer. Such
constructs showed an appreciable efficacy in preclinical models.
However, PE38 was found to trigger immune responses, which limited
repeated administration. Identification and removal of B- and
T-cell recognition epitopes resulted in development of less
immunogenic variants, such as PE38X8 (11–15).
The successful use of targeted toxins is hampered by
a number of physiological barriers (16). A precondition for effective
treatment is delivery of the cytotoxic agent to as many cancer
cells as possible, which is difficult with antibodies as targeting
vectors due to their large size. The antibody variable fragments,
scFv and dsFv, have been extensively studied as promising targeting
moieties for PE38-based immunotoxins in preclinical settings. Due
to their smaller size compared to full-length mAbs, these molecules
provided better penetration of solid tumours. Such constructs have
successfully been applied for targeting HER2-overexpressing breast
(17,18), ovarian (19), prostate (20) and gastric (21) cancers. However, these small,
toxin-conjugated domains had insufficient tumour localization due
to their short plasma half-life (low bioavailability) in
combination with a low maximum tolerated dose (MTD), which limited
the amount of drug that could be injected (22,23).
An alternative approach for the development of high
affinity, small-size tumour targeting agents is the use of
non-immunoglobulin scaffold proteins. Affibody molecules are the
first type of scaffold protein that has been applied for in
vivo targeting. They are based on a 58-amino acid (6.5 kDa)
scaffold derived from the B domain of staphylococcal protein A
(24). By randomization of 13
surface amino acids, combinatorial libraries have been created.
From these libraries, high-affinity Affibody variants binding with
exquisite selectivity and sub-nanomolar affinity to desired
molecular targets have been selected. These include HER2 (25), EGFR (26), IGF-1R (27), PDGFRβ (28), and HER3 (29). Affibody molecules have been
successfully labelled with radionuclides including
111In, 99mTc, 68Ga,
124I, and 18F (26,29–32)
or fluorophores (33,34) for in vivo imaging. The
β-emitting nuclides, 177Lu and 188Re
(35–37), and toxins (8,9) were
conjugated to Affibody molecules for therapeutic applications. The
radiolabelled anti-HER2 Affibody molecule ZHER2:2891,
showed specific tumour accumulation and high-contrast imaging in
clinics (38,39). Due to their small size, high
affinity and relatively easy recombinant production in prokaryotes,
Affibody molecules represent a promising alternative to
immunoglobulin-based fragments for toxin delivery. The feasibility
of using the anti-HER2 Affibody molecule (ZHER2:342)
genetically fused with the PE38-toxin has been demonstrated in
vivo by Zielinski et al (9). However the affitoxin construct
exhibited a short residence time in the bloodstream
(t1/2= 8.69±1.31 min) due to rapid renal clearance. This
made it necessary to inject the construct repeatedly in order to
achieve therapeutically meaningful doses in murine models. Methods
for prolongation of the in vivo half-life of Affibody-based
targeting agents were developed earlier in our laboratories
(35,36). The Affibody molecule was fused with
an albumin-binding domain (ABD) derived from the GA148-GA3 domain
of streptococcal protein G (40),
which resulted in binding to albumin in vivo and prevention
of glomerular filtration. In addition, binding to albumin enables
rescue by the FcRn-pathway from intracellular catabolism (41). The engineered ABD variant
ABD035 binds with femtomolar affinity to human serum
albumin as well as sub-nanomolar affinities to serum albumin from
rat, mouse and cynomolgus monkey (42). Binding of the construct
ZHER2:2891-ABD035 to serum albumin was
clearly demonstrated in animals (36). The elimination half-life of the
parental ZHER2:2891 Affibody molecule from blood was
increased 80-fold when conjugated to ABD035. In
addition, the capacity of specific targeting of HER2-expressing
xenografts was preserved in ABD-fused Affibody molecules (36).
The fusion toxin (ZHER2:342-PE38)
reported by Zielinski et al (9), showed an elevated accumulation in the
liver. That construct contained a Hexahistidine-tag to facilitate
purification by immobilized metal ion affinity chromatography
(IMAC). We have previously observed that the presence of an
N-terminally placed hexahistidine-tag in ZHER2:342 leads
to elevated unspecific uptake in the liver. Incorporation of
negatively charged glutamate residues into a
histidine-containing-tag (resulting in a tag with the amino acid
sequence HEHEHE or (HE)3) significantly reduced liver
uptake, while still allowing efficient purification by IMAC
(43). We have recently
constructed and evaluated in vitro a novel tripartite fusion
toxin (ZHER2:2891-ABD-PE38X8), which includes the
deimmunized PE38X8-toxin fused to the HER2-binding Affibody
molecule HEHEHE-ZHER2:2891 and the half-life extending
albumin binding domain ABD035 (10). The fusion toxin was successfully
produced in E. coli and all three components of the
construct preserved their functionality. Despite the lower affinity
of ZHER2:2891-ABD-PE38X8 compared to the parental
ZHER2:2891 molecule, the cytotoxic potency of the fusion
toxin was 1000-fold higher compared to a non-specific control.
In this study, we have evaluated the influence of
modifications in the molecular design of affitoxins on cellular
processing and biodistribution properties. For this purpose,
several affi-toxin-variants were labelled with the residualizing
radionuclide 111In. The internalization by living
HER2-expressing cells was measured, the influence of the
composition of the histidine-containing tag on biodistribution was
evaluated, and the effect of fusion to ABD on blood clearance rate
was investigated. The tumour targeting properties of the most
promising variant, ZHER2:2891-ABD-PE38X8, were
assessed.
Materials and methods
General
Affibody-based toxins
H6-ZHER2-PE38,
(HE)3-ZHER2-PE38X8,
(HE)3-ZHER2-ABD-PE38X8 and
(HE)3-Ztaq-ABD-PE38X8 were produced and
analyzed as described earlier (10). HER2-expressing SKOV-3 cells (ATCC)
were used in all experiments. For in vitro experiments
7×105 SKOV-3 cells per dish were seeded the day before
the experiment. An unpaired t-test was used to determine
significant difference (p<0.05) between measured values.
Conjugation and labelling
For labelling with 111In, affitoxins were
conjugated with a benzylisothiocyanate derivative of the
CHX-A″-DTPA chelator (Macrocyclics, Dallas, TX, USA) as described
earlier (44). For this purpose,
the protein (630–1,100 μg in 490 μl 0.07 M sodium borate, pH 9.3)
was mixed with 1.1-fold molar excess of CHX-A″-DTPA (1 mg/ml in the
same buffer). The mixture was vortexed and then incubated overnight
at ambient temperature. For removal of unreacted chelator and
buffer exchange, the mixture was passed through a NAP-5 column (GE
Healthcare, Uppsala, Sweden), equilibrated and eluted with 0.2 M
ammonium acetate, pH 5.5. The high molecular weight fraction was
collected according to the manufacturer’s instructions. The
conjugate was stored at −20°C. This methodology provides
conjugation of an average of one chelator per Affibody
molecule.
Labelling was performed by mixing the conjugate (200
μg in 200 μl 0.2 M ammonium acetate, pH 5.5) with 111In
chloride (15 MBq in 40 μl 0.05 M HCl). After 60-min incubation at
r.t., the radiolabelled conjugate was purified using a NAP-5 column
pre-equilibrated and eluted with PBS. Radiochemical yield and
purity of the conjugates were determined using silica-impregnated
ITLC strips (150-771 Dark Green Tec-Control Chromatography strips,
Biodex Medical Systems) eluted with 0.2 M citric acid. The relative
radioactivity associated with the affitoxins (Rf=0.0)
and free 111In (Rf =1.0) was measured using
the Cyclone Storage Phosphor System (Perkin-Elmer).
Cellular binding and processing by
HER2-expressing cells in vitro
An in vitro specificity test was performed
according to the method described earlier (45). Briefly, a solution of radiolabelled
affitoxin (10 nM) was added to the cell plates. For blocking, 1 μM
of non-labelled anti-HER2 Affibody molecule
(H6-ZHER2:342) was added 15 min before the
radiolabelled conjugates to saturate the receptors in some of
dishes. The cells were incubated during 1 h at 37°C. Thereafter,
the medium was collected, the cells were detached by a trypsin-EDTA
solution and cell-bound radioactivity was measured using an
automated γ-spectrometer (1480 Wizard; Wallac Oy). The experiment
was performed in triplicates.
To assess the rate of internalization of the
radiolabelled affitoxins by SKOV-3 cells, a modified acid wash
method was used (45). Briefly,
the cells were incubated with the radiolabelled affitoxins (10 nM)
at 37°C. At 1, 2, 4 and 6 h after incubation start, the medium was
removed. To collect membrane-bound radioactivity, the cells were
treated with 0.2 M glycine buffer containing 4 M urea, pH 2.5, for
5 min on ice. To collect radioactivity internalized by the cells,
treatment with 1 M NaOH at 37°C for 0.5 h was performed. The
radioactivity of the collected fractions was measured. The
experiment was performed in triplicates.
The affinity between radiolabelled affitoxins and
living HER2 expressing SKOV-3 cells was measured using a
LigandTracer Yellow instrument (Ridgeview Instruments, Vänge,
Sweden) as described earlier (46). To measure the kinetics during
association, three different concentrations (0.7, 2 and 6 nM) of
the affitoxins,
111In-(HE)3-ZHER2-P38X8 and
111In-(HE)3-ZHER2-ABD-P38X8, were
used.
Animal studies
The animal experiments were planned and performed in
accordance with national legislation on protection of laboratory
animals. The animal studies were approved by the Local Ethics
Committee for Animal Research in Uppsala.
The influence of the composition of the
histidine-containing tag on hepatic uptake
To evaluate the influence of the composition of the
histidine-containing tag, one group of four mice was injected with
2.4 μg (51 pmol) of the hexahistidine-tag containing affitoxin,
111In-H6-ZHER2-PE38 (20 kBq in 100
μl PBS per mouse). Another group of mice was injected with 2.4 μg
(51 pmol) of
111In-(HE)3-ZHER2-PE38X8
containing the HEHEHE-tag (20 kBq in 100 μl PBS per mouse). The
mice were sacrificed 4 h after injection by an overdose of
anaesthesia solution (30 μl of solution per gram body weight;
Ketalar: 10 mg/ml; Rompun: 1 mg/ml). Blood was withdrawn by heart
puncture. Blood, heart, lung, salivary gland, liver, spleen,
pancreas, stomach, kidney, colon, skin, muscle, bone,
gastrointestinal tract (with its content) and remaining carcass
were collected and weighed. The radioactivity of the organs and
standards of injected solutions was measured using an automated
gamma-spectrometer (1480 Wizard; Wallac Oy). Tissue uptake values
were calculated as percent of injected dose per gram tissue weight
(%ID/g) except for the gastrointestinal tract (with its content)
and remaining carcass, which was calculated as %ID per whole tissue
sample.
The influence of ABD-fusion on
biodistribution
To compare the biodistribution of
111In-(HE)3-ZHER2-ABD-P38X8 and
111In-(HE)3-ZHER2-P38X8,
thirty-two female NMRI mice (27.3±2 g) were randomised to eight
groups with four mice each. Four groups were intravenously injected
with 2.4 μg (51 pmol) of
111In-(HE)3-ZHER2-P38X8, and four
groups with 2.4 μg (48 pmol)
111In-(HE)3-ZHER2-ABD-P38X8. The
injected radioactivity was 20 kBq per mouse. At 1, 4, 24 and 48 h
after injection, the distribution of each conjugate was measured in
one group of mice, as described above.
Biodistribution of
111In-(HE)3-ZHER2-ABD-P38X8 in
tumour bearing mice
For tumour implantation, female Balb/c nu/nu mice
(Charles River Laboratories) were subcutaneously injected with
8×106 SKOV-3 cells on the right hind leg. At the time of
experiment the average tumour weight was 0.52±0.15 g and the
average animal weight was 18.6±0.98 g.
The mice were randomized into four groups (n=4).
Three groups were intravenously injected with 2 μg (40 pmol)
HER2-specific
111In-(HE)3-ZHER2-ABD-P38X8 (20
kBq in 100 μl of PBS per mouse). Mice were euthanized at 1, 4 and
24 h after injection, and the biodistribution was measured as
described above. To confirm that the tumour accumulation of
111In-ZHER2-ABD-PE38X8 is HER2-specific, an
additional group of mice was injected with 2 μg (40 pmol)
111In-(HE)3-Ztaq-ABD-PE38X8 (20
kBq in 100 μl of PBS per mouse). The mice were euthanized at 24 h
after injection and the biodistribution was measured.
Toxicity of
(HE)3-ZHER2-ABD-P38X8
Eighteen female Balb/c mice (Taconic M&B) were
randomized into three groups (n=6). The average animal weight was
21.4±0.5 g. The mice received five intravenous injections of
(HE)3-ZHER2-ABD-P38X8 (0.137, 0.275 or 0.55
mg/kg, every fourth day). The mice were followed for 9 days after
the last injection (total of 25 days after the start of the
experiment) to observe any dose-dependent signs of morbidity or
mortality. The response to treatment was assessed according to the
Guidelines for Pain and Distress in Laboratory Animals from the
National Institute of Cancer (NIH, USA) adopted by Uppsala
University. Assessment parameters included exterior, general
conditions, behaviour, stress, pain, ataxia, appetite, sores and
blistering, eye’s inflammation, porphyria, function of urinary and
gastrointestinal systems, respiration and body weight. After the
mice were sacrificed the heart, liver and kidneys were collected,
fixated and further investigated.
Results
Production and initial
characterization
Essentially pure affitoxins (purity >95%) could
be obtained after expression in Escherichia coli followed by
purification by IMAC, anion exchange chromatography and gel
filtration. The molecular mass of each construct was verified by
mass spectrometry. The equilibrium dissociation constant between
the affitoxins and HER2 was measured by a biosensor and was found
to be between 2 and 5 nM for ZHER2-containing constructs
following a 1:1 binding model. The Ztaq-containing
construct has no measurable affinity to HER2.
Conjugation and labelling
The labelling yield was >99% for
111In-H6-ZHER2-PE38 and
111In-(HE)3-ZHER2-PE38X8. For
111In-(HE)3-ZHER2-ABD-P38X8, the
yield was in the range of 84–95%. Purification using NAP-5
size-exclusion columns provided a radiochemical purity of >99%
for all labelled affitoxins. The labelling yield of the control
Affibody (HE)3-Ztaq-ABD-PE38X8 was 90.5% and
its radiochemical purity was 99.9%.
Cellular binding and processing by
HER2-expressing cells in vitro
Binding of 111In-labelled
H6-ZHER2-PE38,
(HE)3-ZHER2-PE38X8, and
(HE)3-ZHER2-ABD-PE38X8 to SKOV-3 cells was
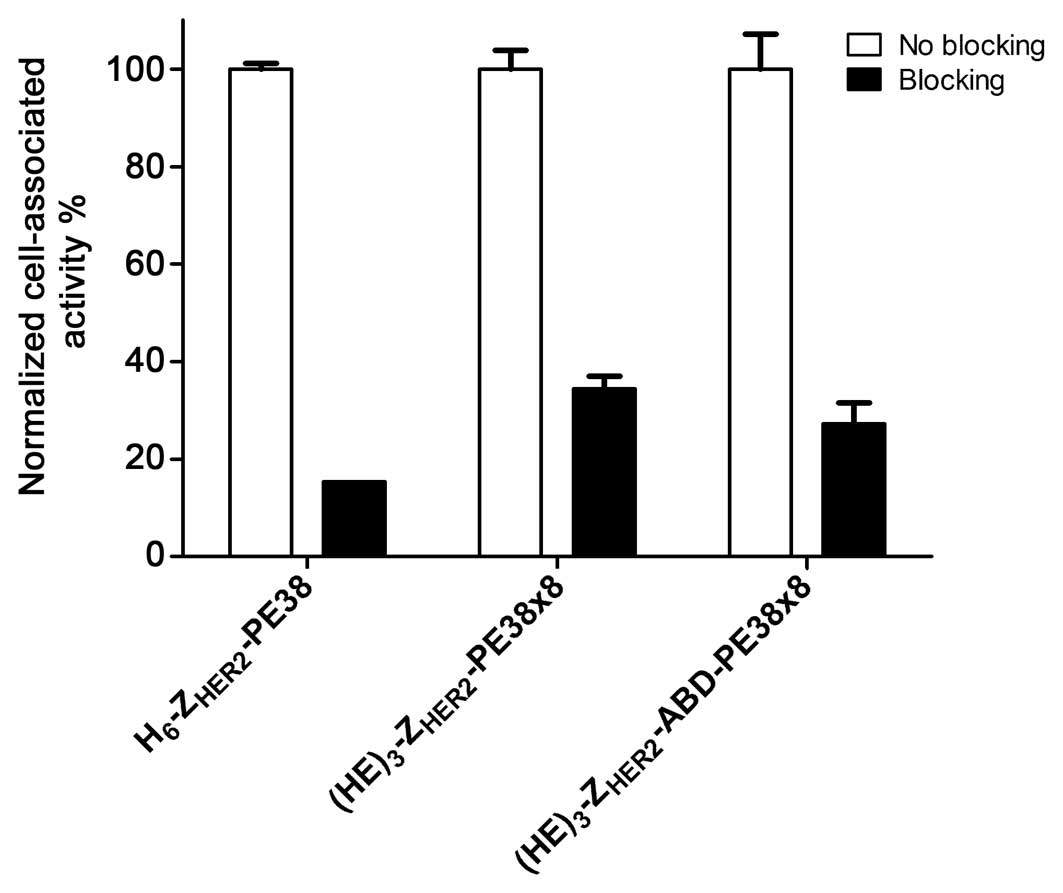
HER2 specific. The cell associated radioactivity was significantly
reduced when the receptors were pre-saturated with a large excess
of non-labelled anti-HER2 Affibody molecule
(H6-ZHER2:342) (Fig. 1).
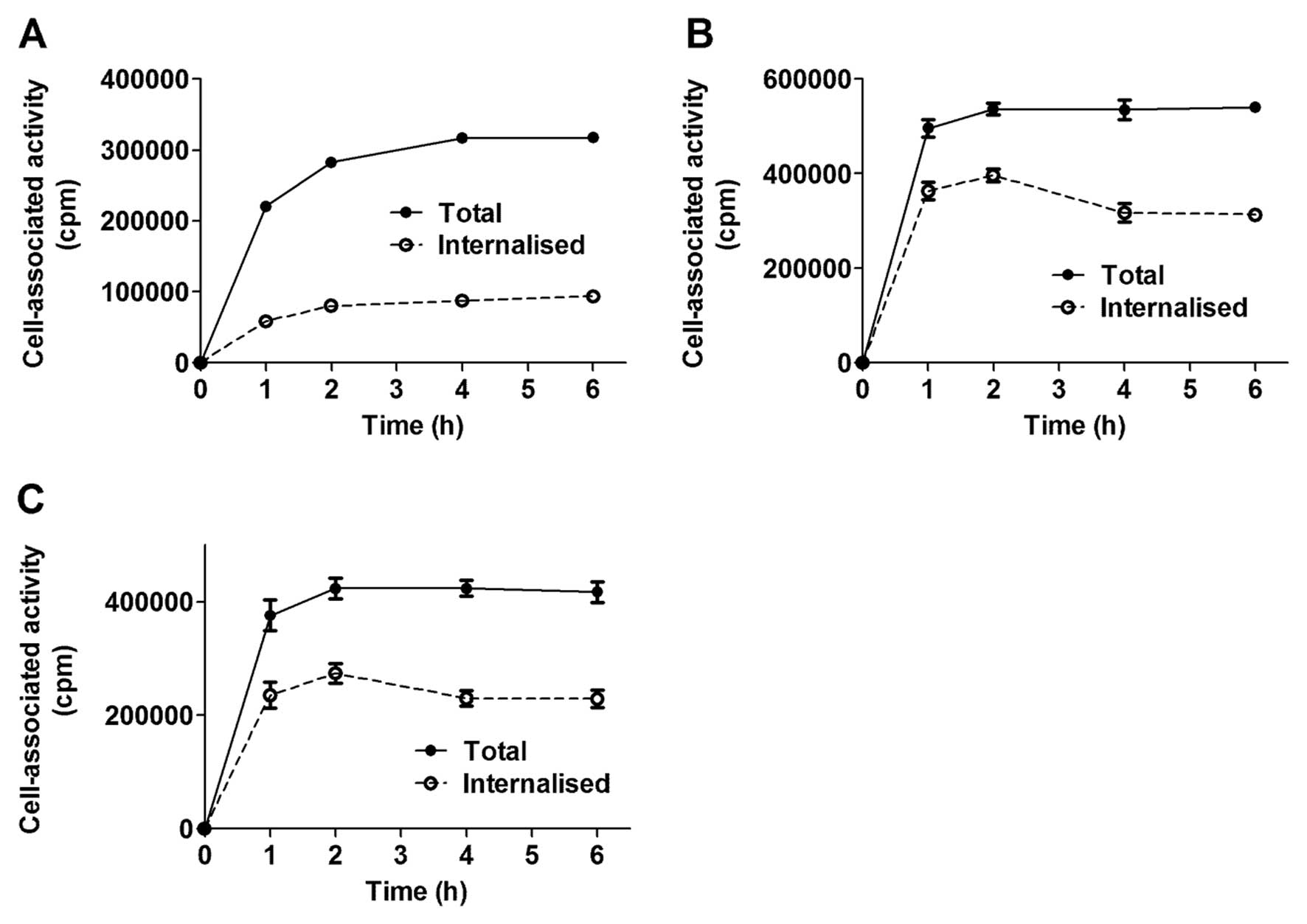
All conjugates demonstrated rapid binding to
HER2-expressing cells and cell associated radioactivity reached a
plateau after 1–2 h of incubation (Fig. 2). Cell counting demonstrated that 6
h after incubation with a 10 nM solution of conjugate, the amount
of cells per dish decreased marginally. The internalisation pattern
of 111In-H6-ZHER2-PE38 differed
from that of
111In-(HE)3-ZHER2-PE38X8 and
111In-(HE)3-ZHER2-ABD-P38X8. The
111In-H6-ZHER2-PE38 affitoxin had
an internalisation pattern similar to its parental molecule
111In-DOTA-ZHER2:342 (45), characterised by slow
internalisation. After 6 h of continuous incubation, <30% of
cell associated radioactivity was internalised, but neither cell
uptake nor the internalised fraction increased after 2 h of
incubation. The internalisation pattern of
111In-(HE)3-ZHER2-PE38X8 and
111In-(HE)3-ZHER2-ABD-P38X8
demonstrated very rapid internalisation and after 2 h of continuous
incubation, 65–70% cell associated radioactivity was internalised.
Further incubation led to slight decrease of the internalised
fraction.
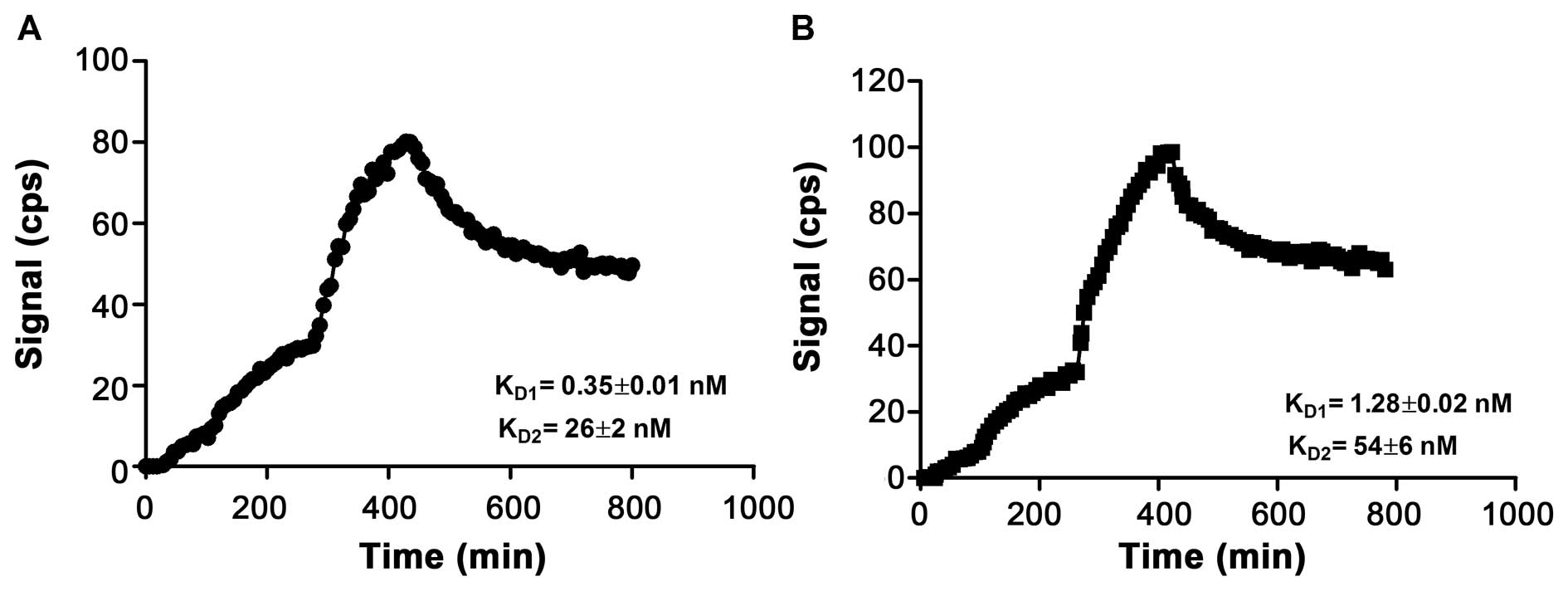
The results from the real-time radiotracer-receptor
interaction measurement, using a LigandTracer, are shown in
Fig. 3. The association and
dissociation phases of 111In-labeled
(HE)3-ZHER2-ABD-P38X8 and
(HE)3-ZHER2-P38X8 with SKOV-3 cells were best
fitted to a 1:2 interaction model, suggesting that each conjugate
has two dissociation constants: KD1 = 0.35±0.01 nM and
KD2 = 26±2 nM for
111In-(HE)3-ZHER2-ABD-P38X8, and
KD1 = 1.28±0.02 nM and KD2 = 54±6 nM for
111 In-(HE)3-ZHER2-P38X8.
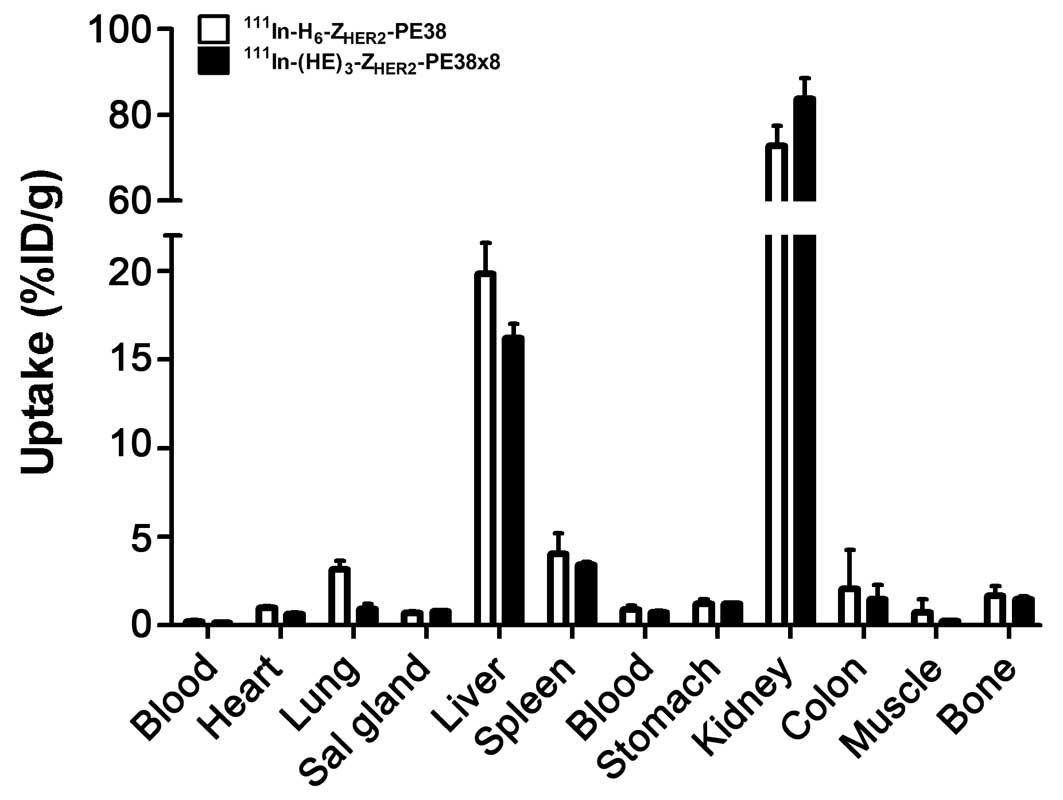
The influence of the composition of the
histidine-tag on hepatic uptake
Data concerning the influence of the composition of
the purification tag on biodistribution of the anti-HER2 Affibody
fused-toxins are presented in Fig.
4. The HEHEHE-containing variant
111In-(HE)3-ZHER2-PE38X8
demonstrated significantly (p<0.05) lower uptake in the liver
compared to 111In-H6-ZHER2-PE38
(16.2±0.8 vs 20±2%ID/g). It has to be noted, that the hepatic
uptake remained high even for
111In-(HE)3-ZHER2-PE38X8. The
radioactivity concentration of
111In-(HE)3-ZHER2-PE38X8 was also
significantly (p<0.05) lower in blood, heart and lung. The renal
uptake was significantly higher for
111In-(HE)3-ZHER2-PE38X8
(84±5%ID/g) compared to
111In-H6-ZHER2-PE38
(73±5%ID/g).
Influence of ABD-fusion on
biodistribution
The comparison of biodistribution of
111In-(HE)3-ZHER2-ABD-PE38X8 and
111In-(HE)3-ZHER2-PE38X8 in female
NMRI mice is summarized in Table
I. The data showed prominently higher concentration of
111In-(HE)3-ZHER2-ABD-PE38X8 in
the blood compared to the non-ABD fused
111In-(HE)3-ZHER2-PE38X8 variant,
at all time-points. This resulted in overall 2-fold-higher body
retention of
111In-(HE)3-ZHER2-ABD-PE38X8. The
data also demonstrated a 5-fold-lower radioactivity uptake in the
kidneys for
111In-(HE)3-ZHER2-ABD-PE38X8.
There was similar uptake of both conjugates in the liver and
gastrointestinal tract at all time-points. The longer residence
time in blood of
111In-(HE)3-ZHER2-ABD-PE38X8 was
not directly translated to elevated uptake in the studied
organs.
 | Table IComparative biodistribution of
111 In-labeled
(HE)3-ZHER2-ABD-PE38x8
and(HE)3-ZHER2-PE38x8 in female NMRI mice up
to 72 h after intravenous injection.a |
Table I
Comparative biodistribution of
111 In-labeled
(HE)3-ZHER2-ABD-PE38x8
and(HE)3-ZHER2-PE38x8 in female NMRI mice up
to 72 h after intravenous injection.a
| 1 h | 4 h | 24 h | 48 h |
|---|
|
|
|
|
|
|---|
|
(HE)3-ZHER2-ABD-
PE38x8 |
(HE)3-ZHER2-PE38x8 |
(HE)3-ZHER2-ABD-PE38x8 |
(HE)3-ZHER2-
PE38x8 |
(HE)3-ZHER2-ABD-PE38x8 |
(HE)3-ZHER2-PE38x8 |
(HE)3-ZHER2-ABD-PE38x8 |
(HE)3-ZHER2-PE38x8 |
|---|
| Blood | 11±2 | 0.6±0.1 | 4.8±0.6 | 0.13±0.01 | 1.2±0.1 | 0.04±0.01 | 0.53±0.04 | 0.02±0.01 |
| Heart | 3.1±0.5 | 0.8±0.2 | 2.4±0.2 | 0.65±0.08 | 1.6±0.1 | 0.5±0.1 | 1.1±0.2 | 0.4±0.05 |
| Lung | 4.2±1.0 | 1±0.1 | 2.6±0.03 | 0.9±0.3 | 1.4±0.3 | 0.4±0.03 | 0.7±0.1 | 0.3±0.04 |
| Salivary gland | 1.4±0.5 | 0.8±0.1 | 1.2±0.3 | 0.8±0.04 | 1±0.1 | 0.6±0.1 | 0.7±0.1 | 0.5±0.07 |
| Liver | 17±2.6 | 15±5 | 21±2 | 16±0.8 | 16±1 | 12±1.4 | 11±0.5 | 8.5±0.8 |
| Spleen | 6±1 | 4±1 | 7.2±0.8 | 3.4±0.2 | 5±0.8 | 2.8±0.2 | 3.7±0.4 | 2.3±0.5 |
| Pancreas | 1.1±0.2 | 0.9±0.1 | 0.6±0.1 | 0.8±0.1 | 0.5±0.1 | 0.6±0.1 | 0.4±0.04 | 0.8±0.4 |
| Stomach | 1.0±0.1 | 1.4±0.2 | 0.9±0.1 | 1.2±0.1 | 0.7±0.1 | 0.8±0.1 | 0.5±0.1 | 0.6±0.3 |
| Kidney | 13±1 | 84±12 | 13.3±1.7 | 84±5 | 11±0.6 | 51±5 | 7.6±0.7 | 41±6 |
| Colon | 0.8±0.2 | 1±0.2 | 1.1±0.2 | 1.5±0.8 | 0.7±0.2 | 0.6±0.1 | 0.5±0.06 | 0.6±0.02 |
| Skin | 0.7±0.2 | 0.8±0.2 | 0.8±0.1 | 0.6±0.1 | 1.2±0.3 | 0.4±0.04 | 0.9±0.1 | 0.3±0.04 |
| Muscle | 0.5±0.1 | 0.3±0.04 | 0.5±0.03 | 0.3±0.03 | 0.4±0.02 | 0.2±0.02 | 0.4±0.2 | 0.14±0.02 |
| Bone | 1.9±0.2 | 1.7±0.4 | 1.6±0.2 | 1.5±0.14 | 1.6±0.1 | 0.9±0.2 | 1.1±0.5 | 0.7±0.1 |
| GI tractb | 4±0.3 | 4.5±0.3 | 4.5±1.5 | 4.2±1.4 | 2.2±0.3 | 1.3±0.2 | 1.3±0.1 | 0.8±0.2 |
| Carcassb | 16±3 | 8±0.3 | 15±2.2 | 6.4±0.5 | 12±0.8 | 6±0.8 | 8.6±0.8 | 4±0.5 |
Biodistribution of
111In-(HE)3-Z HER2-ABD-PE38X8 in
tumour bearing mice
Data concerning the biodistribution of
111In-(HE)3-ZHER2-ABD-PE38X8 in
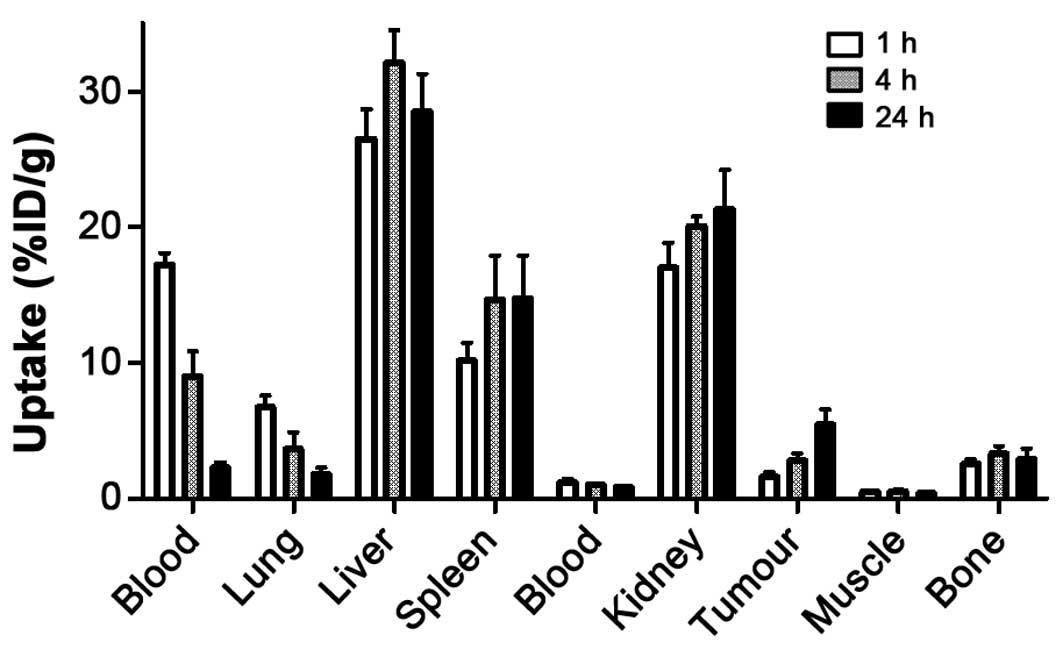
mice bearing SKOV-3 xenografts are presented in Fig. 5. The data were in good agreement
with the biodistribution data in healthy NMRI mice except for
elevated uptake in blood, liver, spleen and the kidneys, which can
be explained by a difference in weight and volume. The tumour
accumulation of
111In-(HE)3-ZHER2-ABD-PE38X8 was
1.6±0.4%ID/g at 1 h after injection and continued to increase with
time up to 5.5±1%ID/g at 24 h after injection. By 24 h after
injection, the uptake in the tumour was higher than the uptake in
any other studied organ except for the liver, spleen and the
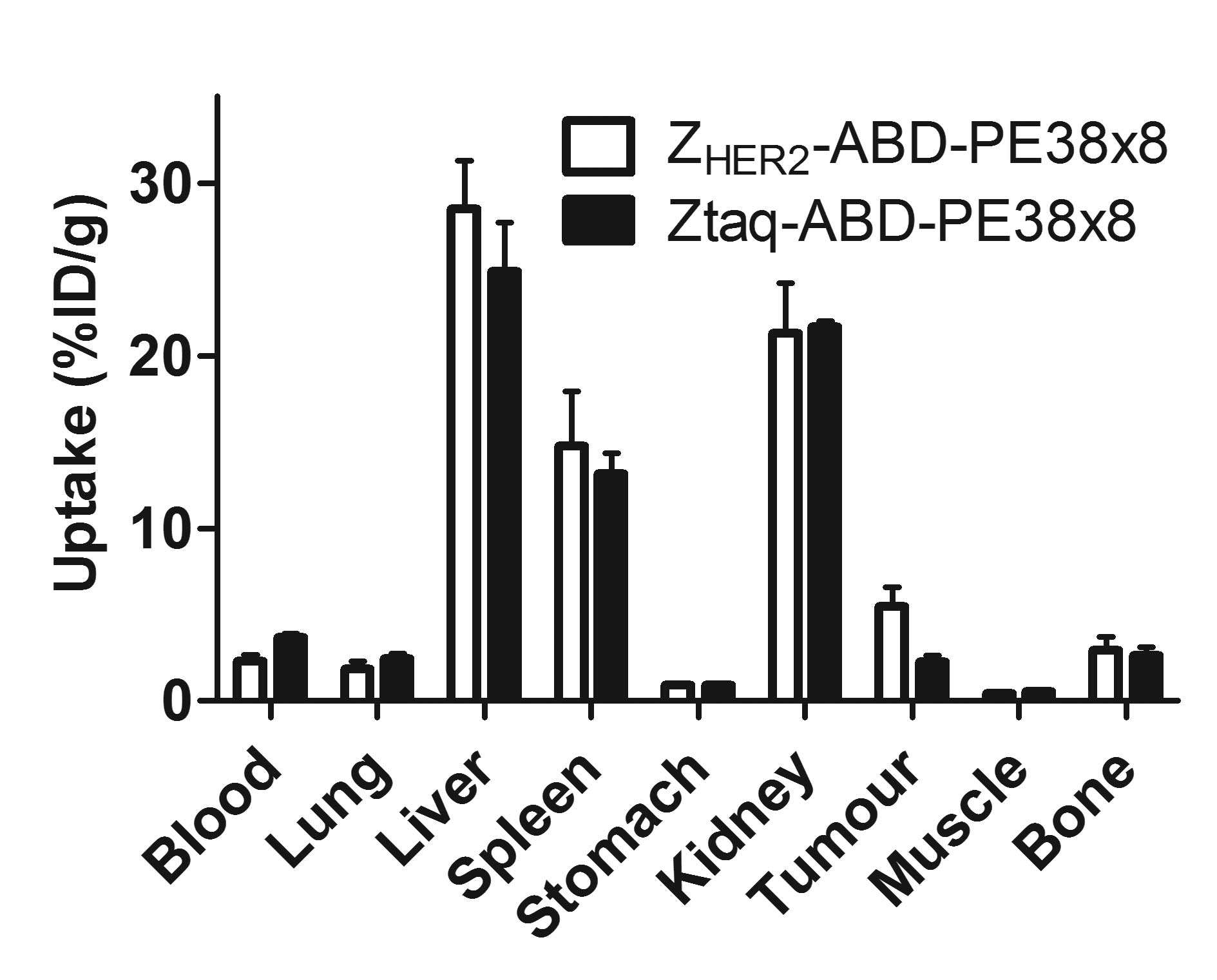
kidneys. The results of the targeting specificity test are
presented in Fig. 6. The
radioactivity concentration in the tumour was 2.7-fold higher for
111In-(HE)3-ZHER2-ABD-PE38X8 than
for the control fusion toxin
111In-(HE)3-Ztaq-ABD-PE38X8, which
does not bind to HER2 (p<0.05). There was no significant
difference in the radioactivity concentrations in any other organs
and tissue samples.
Toxicity of
(HE)3-ZHER2-ABD-PE38X8
No morbidity (change in weight, appearance or
behaviour) or mortality was observed in the groups injected with
0.137 and 0.275 mg/kg (HE)3-ZHER2-ABD-PE38X8.
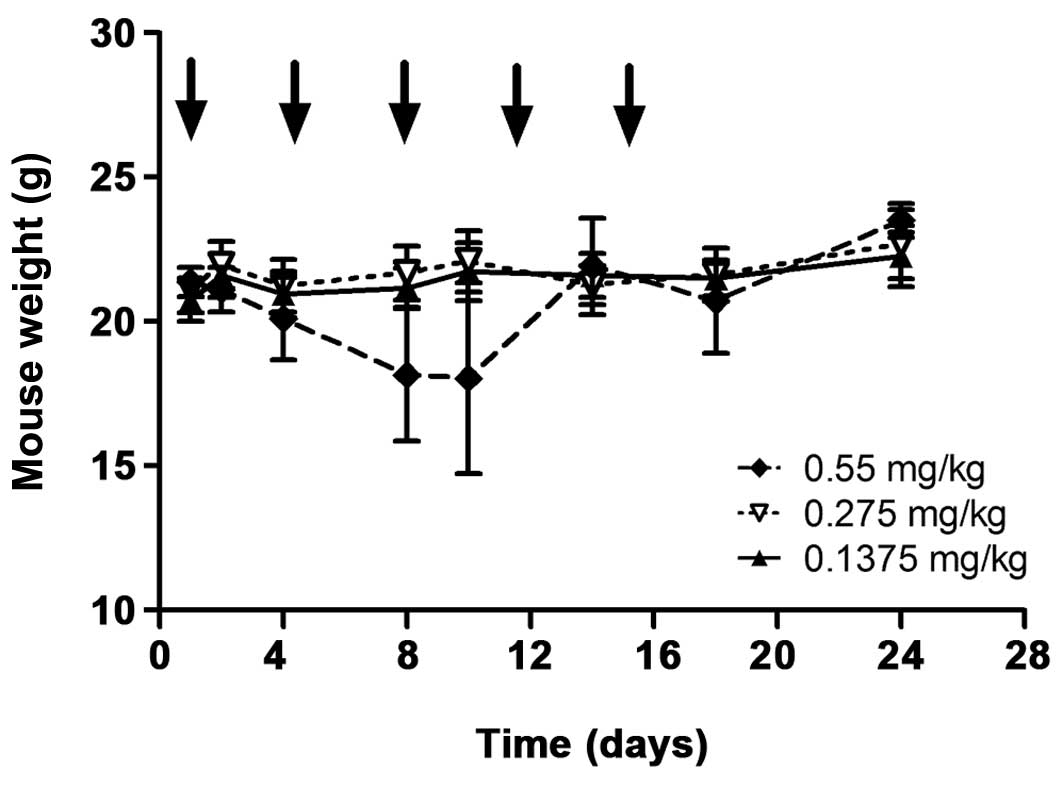
One mouse in the group injected with 0.55 mg/kg affitoxin had a
critical weight loss and was euthanized after the third injection.
Other mice in this group started to lose weight after the second
injection (day 8) but recovered by day 14 (Fig. 7). However tissue viability analysis
of the heart, liver and kidneys from this group did not show any
morphological differences or signs of injury compared to tissues
from the other two groups (data not shown).
Discussion
The successful therapeutic implementation of
Affibody-based toxin is hampered by their short residence time in
the circulation and uptake in normal tissues, particularly the
liver (9). Previous data suggest
that small changes in the physio-chemical properties of
Affibody-based targeting agents may influence tumour targeting
properties and overall biodistribution considerably (47). In this study, an adequate toxin
delivery was achieved by modification in the molecular design of
the affitoxin molecule. This involved the use of the deimmunized
toxin PE38X8, prolongation of the plasma half-life by conjugation
to ABD and minimization of the uptake in normal tissues
(particularly liver) using the HEHEHE-tag.
Zielinski and co-workers, developed and studied a
recombinant protein combining a HER2-specific Affibody and the PE38
toxin (H6-ZHER2-PE38) (9). Their HER2-Affitoxin was labelled with
DyLight 750 dye and characterization was done using near-infrared
optical imaging. A main disadvantage with optical imaging is its
lower sensitivity because of the high photon absorption by tissues.
This makes the technique more suitable for image guided diagnosis
or surgery but does not permit exact quantification of uptake in
different tissues i.e., it is semi-quantitative (33,48).
On the other hand, the use of radioactive labels enables more
sensitive tracking of the studied constructs and accurate
quantification of tissue distribution. Therefore, our affitoxin
variants were labelled with the radiometal 111In to
permit exact measurement of the biodistribution. We expect that
after internalization, the internalized radiometal accumulates
inside the cells i.e., residualises. This would allow estimation of
the affitoxin amounts internalized by different tissues. Kobayashi
and co-workers have earlier compared the biodistribution of the
anti-Tac(Fv)-PE38 immunotoxin labelled with 111In
(residualizing) or I (non-residualizing) (49). They observed significant
differences in uptake of the immunotoxin in the tissues that
internalize it (liver and kidneys) with different labels. The
biodistribution of 125I-labeled anti-Tac(Fv)-PE38 showed
low renal accumulation of the immunotoxin. However, the
biodistribution of the 111In-labeled variant suggests
that the critical organ of anti-Tac(Fv)-PE38 toxicity should be the
kidney. They finally concluded that in the case of a radiolabelled
PE38-fused immunotoxin, 111In is a more appropriate
label to reflect conjugate delivery to tissue.
All three constructs were efficiently labelled with
111In using the CHX-A″-DTPA chelator under the selected
labelling conditions with high stability of the radiolabel. The
three conjugates were capable of specific binding to
HER2-expressing cells, indicating no apparent influence on the
binding capacity of the affitoxins to HER2 after labelling
(Fig. 1).
Interestingly, the novel constructs containing the
HEHEHE-tag demonstrated more rapid internalization by SKOV-3 cells
in comparison with the H6-tag containing variant
developed by Zelienski and co-workers and the parental Affibody
molecule DOTA-ZHER2:342 (45) (Fig.
2). Part of the explanation can likely be attributed to the
noticeable modifications on both its N- (incorporation of the
HEHEHE-tag) and C- (the use of modified PE38X8 derivative) termini
in comparison with H6-ZHER2-PE38 and
DOTA-ZHER2:342. These changes influence the overall
structure and the local charge concentration of the affitoxin,
which might interfere in different ways with Affitoxin-HER2
receptor interaction. We have earlier observed similar effects on
the internalization rate when Affibody molecules were modified
using different chelating moieties and even when the same chelator
was used for labelling Affibody molecules with different
radionuclides (47,50). Regardless of the underlying
reasons, the higher internalization (rate and total amount) of the
HEHEHE-containing constructs may improve efficacy of cancer
treatment because of enhanced delivery of toxins inside the
malignant cells. The measured affinity of 111In-labeled-
(HE)3-ZHER2-ABD-PE38X8 and
(HE)3-ZHER2-ABD-PE38X8 to the HER2-receptor
on cells was in the low nanomolar range and in agreement with
previously reported data for the same conjugates (Fig. 3) (10). This demonstrates no alteration of
the constructs’ affinity by radiolabelling. An interesting finding
from the LigandTracer experiment was that the interaction of the
new conjugates with SKOV-3 cells most resembled a 1:2 interaction.
Earlier we have found that binding of anti-HER2 Affibody molecules
to HER2-expressing cell lines in vitro is influenced by the
interaction of the target receptors with other co-expressed HER
family receptors (51). The change
in receptor conformation due to heterodimerization might result in
weaker interactions of the affitoxin with a sub-set of the receptor
molecules, which would lead to that the 1:2 model best describes
the affitoxin/SKOV3 interaction. A biosensor analysis of the
interaction between the affitoxins and the HER2 receptor alone,
lacking dimerization partners, shows that the interaction follows a
1:1 model.
The substitution of the hexahistidine-tag in
111In-H6-ZHER2-PE38 with the more
hydrophilic HEHEHE-tag in
111In-(HE)3-ZHER2-PE38X8 reduced
the liver uptake by almost 20% (from 19.9±1.8 to 16.2±0.8%ID/g, at
4 h after injection (Fig. 4). This
is in agreement with our previous results (43). Despite the reduction in hepatic
uptake achieved by (HE)3-ZHER2-PE38X8, the
liver accumulated radioactivity remains high compared to non-toxin
fused Affibody molecules (43).
This indicates that the mechanism of uptake of the affitoxin in
liver is mainly driven by the PE38X8-part. This assumption is
supported by studies involving the anti-Tac Fv fragment targeting
the interleukin-2-receptor (IL-2Rα pr Tac) (49,52).
When the anti-Tac Fv fragment was conjugated to PE38, liver uptake
increased several-fold compared to the non-toxin fused fragment
(12.42±0.58 vs. 3.4±0.7%ID/g 15 min after injection for
125I-anti-Tac-Fv-PE38 and 125I-anti-Tac-dsFv
respectively). In this study, the reduction in hepatic uptake of
the HEHEHE-tag containing construct was accompanied with a
significantly increased renal uptake. After glomerular filtration,
it is likely that the radiolabelled affitoxin will be reabsorbed by
the proximal tubular cells where it will be prone to enzymatic
degradation (49,53). This catabolic process may lead to
the conversion of PE38X8 to a less toxic molecule. This may have a
positive impact on the maximum tolerated dose of the affitoxin that
can be injected without toxic implications.
In order to extend the plasma half-life of the
affitoxin, we fused it with albumin-binding domain (ABD). The
results from the comparative biodistribution study between the ABD-
and non-ABD-fused affitoxins demonstrated a clear effect on the
half-life when the affitoxin was conjugated to ABD (Table I). The retention of
111In-(HE)3-ZHER2-ABD-PE38X8 in
the blood was on average 28-fold higher than that of
111In-(HE)3-ZHER2-PE38X8. A
surprising finding of this study is the more rapid blood clearance
of 111In-(HE)3-ZHER2-ABD-PE38X8 in
comparison to previously studied ABD-fused Affibody molecules
(35,36). Interestingly
111In-(HE)3-ZHER2-ABD-PE38X8
showed also several-fold higher accumulation in the liver compared
to other ABD-fused Affibody molecules (35,36).
The main difference between these fusion-molecules is the presence
of the toxin PE38X8. As pointed out earlier, we expect that the
elevated hepatic uptake is mainly a toxin-dependent effect.
Therefore, it would be reasonable to assume that the more rapid
clearance of the ABD-fused affitoxin from circulation is due to
accumulation and trapping of the toxin-conjugate by the
hepatocytes. As a result of longer retention in blood,
111In-(HE)3-ZHER2-ABD-PE38X8
showed >5-fold reduced renal accumulation in comparison with
111In-(HE)3-ZHER2-PE38X8. The
higher renal excretion of
111In-(HE)3-ZHER2-PE38X8 may
explain the significantly lower accumulation of this construct in
the liver.
111In-(H
E)3-ZHER2-ABD-PE38X8 targeted HER2-expressing
xenografts in mice specifically (Fig.
6). The uptake of the radiolabelled control fusion toxin
111In-(HE)3-Ztaq-ABD-PE38X8 in the
xenografted tumour was significantly (p<0.05) lower than the
uptake of the HER2-binding construct. The uptake of the
HER2-affitoxin (HE)3-ZHER2-ABD-PE38X8 in the
tumour (5.5±1%ID/g at 24 h after injection) was lower in comparison
to the parental HER2-Affibody ZHER2:2891 (11±4%ID/g at
24 h after injection) (54). A
similar difference was reported by Zilienski et al (9). They observed a non-even distribution
of the affitoxin, H6-ZHER2-PE38 in BT-474
xenografts (with high HER2-expression), in comparison with
non-toxin fused ZHER2 Affibody molecules. They
attributed this to the large size of the affitoxin, which decreases
the ability of the construct to diffuse deep into the tumour
tissue. However, this size effect was more profound in xenografts
with larger volumes, while tumours with relatively small sizes
responded efficaciously to the HER2-Affitoxin treatment. It would
be expected that the even bigger construct
(HE)3-ZHER2-ABD-PE38X8, associated with the
serum albumin will have a relatively lower tumour penetration when
compared to the parental ZHER2 Affibody molecule.
Together this may explain the relatively lower tumour accumulation
of (HE)3-ZHER2-ABD-PE38X8. It has to be
noted, that (HE)3-ZHER2-ABD-PE38X8 adduct to
albumin would still have a smaller size compared to a full IgG mAb
and thus would have better tissue penetration.
In the toxicity experiment, one mouse in the group
(n=6) injected with the highest dose of
(HE)3-ZHER2-ABD-PE38X8 had a critical loss of
weight and was terminated. Other mice in this group had a
significant reduction in the average weight, but recovered
successfully and survived until termination of the study (Fig. 7). When mice were injected with
lower doses (0.275 and 0.1375 mg/kg, equivalent doses providing a
clear therapeutic effect in the study reported by Zielinski and
co-workers (9), mice did not
experience significant weight loss during the study and did not
show any other signs of toxicity.
In conclusion, the use of the HEHEHE-tag on the
N-terminal end of the affitoxin molecule reduced hepatic uptake,
however the effect was relatively small, presumably due to the
presence of PE38X8. Fusion to the ABD is associated with longer
residence in circulation and would permit less frequent injections
of the affitoxins. The novel tripartite fusion toxin
(HE)3-ZHER2-ABD-PE38X8 is capable of specific
targeting of HER2-expressing xenografts in vivo. Multiple
injections of therapeutic doses of
(HE)3-ZHER2-ABD-PE38X8 had no significant
side effects. The results of this study emphasize the importance of
careful design to improve the properties of therapeutic agents.
Acknowledgements
This study was supported by grants from the Swedish
Research Council (Vetenskapsrådet) and the Swedish Cancer Society
(Cancerfonden). The authors express their gratitude to Mrs.
Christina Atterby for her assistance in biodistribution
studies.
Abbreviations:
|
ABD
|
albumin binding domain
|
|
mAbs
|
monoclonal antibodies
|
|
Fv
|
variable fragment
|
|
HER2
|
human epidermal growth factor
receptor 2
|
|
HSA
|
human serum albumin
|
|
IMAC
|
immobilized metal-ion affinity
chromatography
|
|
KD
|
equilibrium dissociation constant
|
|
MSA
|
mouse serum albumin
|
|
PBS
|
phosphate-buffered saline
|
|
scFv
|
single-chain variable fragment
|
|
PE38
|
truncated exotoxin A from
Pseudomonas aeruginosa
|
References
|
1
|
Pohlmann PR, Mayer IA and Mernaugh R:
Resistance to trastuzumab in breast cancer. Clin Cancer Res.
15:7479–7491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenzweig SA: Acquired resistance to
drugs targeting receptor tyrosine kinases. Biochem Pharmacol.
83:1041–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim EG and Kim KM: Strategies and
advancement in antibody-drug conjugate optimization for targeted
cancer therapeutics. Biomol Ther (Seoul). 23:493–509. 2015.
View Article : Google Scholar
|
|
5
|
Kreitman RJ: Recombinant immunotoxins
containing truncated bacterial toxins for the treatment of
hematologic malignancies. BioDrugs. 23:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alewine C, Hassan R and Pastan I: Advances
in anticancer immunotoxin therapy. Oncologist. 20:176–185. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin-Killias P, Stefan N, Rothschild S,
Plückthun A and Zangemeister-Wittke U: A novel fusion toxin derived
from an EpCAM-specific designed ankyrin repeat protein has potent
antitumor activity. Clin Cancer Res. 17:100–110. 2011. View Article : Google Scholar
|
|
8
|
Zielinski R, Lyakhov I, Jacobs A, Chertov
O, Kramer-Marek G, Francella N, Stephen A, Fisher R, Blumenthal R
and Capala J: Affitoxin - a novel recombinant, HER2-specific,
anticancer agent for targeted therapy of HER2-positive tumors. J
Immunother. 32:817–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zielinski R, Lyakhov I, Hassan M, Kuban M,
Shafer-Weaver K, Gandjbakhche A and Capala J: HER2-affitoxin: A
potent therapeutic agent for the treatment of HER2-overexpressing
tumors. Clin Cancer Res. 17:5071–5081. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Seijsing J, Frejd FY, Tolmachev V
and Gräslund T: Target-specific cytotoxic effects on
HER2-expressing cells by the tripartite fusion toxin
ZHER2:2891-ABD-PE38X8, including a targeting affibody molecule and
a half-life extension domain. Int J Oncol. 47:601–609.
2015.PubMed/NCBI
|
|
11
|
Mazor R, Vassall AN, Eberle JA, Beers R,
Weldon JE, Venzon DJ, Tsang KY, Benhar I and Pastan I:
Identification and elimination of an immunodominant T-cell epitope
in recombinant immunotoxins based on Pseudomonas exotoxin A. Proc
Natl Acad Sci USA. 109:E3597–E3603. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Onda M, Beers R, Xiang L, Nagata S, Wang
Q-C and Pastan I: An immunotoxin with greatly reduced
immunogenicity by identification and removal of B cell epitopes.
Proc Natl Acad Sci USA. 105:11311–11316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Onda M, Lee B, Kreitman RJ, Hassan
R, Xiang L and Pastan I: Recombinant immunotoxin engineered for low
immunogenicity and antigenicity by identifying and silencing human
B-cell epitopes. Proc Natl Acad Sci USA. 109:11782–11787. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onda M, Beers R, Xiang L, Lee B, Weldon
JE, Kreitman RJ and Pastan I: Recombinant immunotoxin against
B-cell malignancies with no immunogenicity in mice by removal of
B-cell epitopes. Proc Natl Acad Sci USA. 108:5742–5747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
King C, Garza EN, Mazor R, Linehan JL,
Pastan I, Pepper M and Baker D: Removing T-cell epitopes with
computational protein design. Proc Natl Acad Sci USA.
111:8577–8582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vuhai-Luuthi MT, Jolivet A, Jallal B,
Salesse R, Bidart JM, Houllier A, Guiochon-Mantel A, Garnier J and
Milgrom E: Monoclonal antibodies against luteinizing hormone
receptor. Immunochemical characterization of the receptor.
Endocrinology. 127:2090–2098. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reiter Y, Brinkmann U, Jung SH, Lee B,
Kasprzyk PG, King CR and Pastan I: Improved binding and antitumor
activity of a recombinant anti-erbB2 immunotoxin by disulfide
stabilization of the Fv fragment. J Biol Chem. 269:18327–18331.
1994.PubMed/NCBI
|
|
18
|
Bera TK, Viner J, Brinkmann E and Pastan
I: Pharmacokinetics and antitumor activity of a bivalent
disulfide-stabilized Fv immunotoxin with improved antigen binding
to erbB2. Cancer Res. 59:4018–4022. 1999.PubMed/NCBI
|
|
19
|
Schmidt M, McWatters A, White RA, Groner
B, Wels W, Fan Z and Bast RC Jr: Synergistic interaction between an
anti-p185HER-2 pseudomonas exotoxin fusion protein [scFv(FRP5)-ETA]
and ionizing radiation for inhibiting growth of ovarian cancer
cells that overexpress HER-2. Gynecol Oncol. 80:145–155. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Liu B, Schmidt M, Lu Y, Wels W and
Fan Z: Antitumor effect of an HER2-specific antibody-toxin fusion
protein on human prostate cancer cells. Prostate. 47:21–28. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shinohara H, Morita S, Kawai M, Miyamoto
A, Sonoda T, Pastan I and Tanigawa N: Expression of HER2 in human
gastric cancer cells directly correlates with antitumor activity of
a recombinant disulfide-stabilized anti-HER2 immunotoxin. J Surg
Res. 102:169–177. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kreitman RJ, Wilson WH, Bergeron K, Raggio
M, Stetler-Stevenson M, FitzGerald DJ and Pastan I: Efficacy of the
anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant
hairy-cell leukemia. N Engl J Med. 345:241–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thurber GM, Schmidt MM and Wittrup KD:
Antibody tumor penetration: Transport opposed by systemic and
antigen-mediated clearance. Adv Drug Deliv Rev. 60:1421–1434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Löfblom J, Feldwisch J, Tolmachev V,
Carlsson J, Ståhl S and Frejd FY: Affibody molecules: Engineered
proteins for therapeutic, diagnostic and biotechnological
applications. FEBS Lett. 584:2670–2680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orlova A, Magnusson M, Eriksson TLJ,
Nilsson M, Larsson B, Höidén-Guthenberg I, Widström C, Carlsson J,
Tolmachev V, Ståhl S, et al: Tumor imaging using a picomolar
affinity HER2 binding affibody molecule. Cancer Res. 66:4339–4348.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tolmachev V, Rosik D, Wållberg H, Sjöberg
A, Sandström M, Hansson M, Wennborg A and Orlova A: Imaging of EGFR
expression in murine xenografts using site-specifically labelled
anti-EGFR 111In-DOTA-Z EGFR:2377 Affibody molecule:
aspect of the injected tracer amount. Eur J Nucl Med Mol Imaging.
37:613–622. 2010. View Article : Google Scholar
|
|
27
|
Tolmachev V, Malmberg J, Hofström C,
Abrahmsén L, Bergman T, Sjöberg A, Sandström M, Gräslund T and
Orlova A: Imaging of insulinlike growth factor type 1 receptor in
prostate cancer xenografts using the affibody molecule
111In-DOTA-ZIGF1R:4551. J Nucl Med. 53:90–97. 2012.
View Article : Google Scholar
|
|
28
|
Tolmachev V, Varasteh Z, Honarvar H,
Hosseinimehr SJ, Eriksson O, Jonasson P, Frejd FY, Abrahmsen L and
Orlova A: Imaging of platelet-derived growth factor receptor β
expression in glioblastoma xenografts using affibody molecule
111In-DOTA-Z09591. J Nucl Med. 55:294–300. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orlova A, Malm M, Rosestedt M, Varasteh Z,
Andersson K, Selvaraju RK, Altai M, Honarvar H, Strand J, Ståhl S,
et al: Imaging of HER3-expressing xenografts in mice using a (99m)
Tc(CO) 3-HEHEHE-Z HER3:08699 affibody molecule. Eur J Nucl Med Mol
Imaging. 41:1450–1459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kramer-Marek G, Shenoy N, Seidel J,
Griffiths GL, Choyke P and Capala J: 68Ga-DOTA-affibody molecule
for in vivo assessment of HER2/neu expression with PET. Eur J Nucl
Med Mol Imaging. 38:1967–1976. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orlova A, Wållberg H, Stone-Elander S and
Tolmachev V: On the selection of a tracer for PET imaging of
HER2-expressing tumors: Direct comparison of a 124I-labeled
affibody molecule and trastuzumab in a murine xenograft model. J
Nucl Med. 50:417–425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heskamp S, Laverman P, Rosik D, Boschetti
F, van der Graaf WT, Oyen WJ, van Laarhoven HW, Tolmachev V and
Boerman OC: Imaging of human epidermal growth factor receptor type
2 expression with 18F-labeled affibody molecule ZHER2:2395 in a
mouse model for ovarian cancer. J Nucl Med. 53:146–153. 2012.
View Article : Google Scholar
|
|
33
|
Lee SB, Hassan M, Fisher R, Chertov O,
Chernomordik V, Kramer-Marek G, Gandjbakhche A and Capala J:
Affibody molecules for in vivo characterization of HER2-positive
tumors by near-infrared imaging. Clin Cancer Res. 14:3840–3849.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lundberg E, Höidén-Guthenberg I, Larsson
B, Uhlén M and Gräslund T: Site-specifically conjugated anti-HER2
Affibody molecules as one-step reagents for target expression
analyses on cells and xenograft samples. J Immunol Methods.
319:53–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tolmachev V, Orlova A, Pehrson R, Galli J,
Baastrup B, Andersson K, Sandström M, Rosik D, Carlsson J,
Lundqvist H, et al: Radionuclide therapy of HER2-positive
microxenografts using a 177Lu-labeled HER2-specific Affibody
molecule. Cancer Res. 67:2773–2782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Orlova A, Jonsson A, Rosik D, Lundqvist H,
Lindborg M, Abrahmsen L, Ekblad C, Frejd FY and Tolmachev V:
Site-specific radiometal labeling and improved biodistribution
using ABY-027, a novel HER2-targeting affibody
molecule-albumin-binding domain fusion protein. J Nucl Med.
54:961–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altai M, Wållberg H, Honarvar H, Strand J,
Orlova A, Varasteh Z, Sandström M, Löfblom J, Larsson E, Strand SE,
et al: 188Re-ZHER2:V2, a promising affibody-based targeting agent
against HER2-expressing tumors: preclinical assessment. J Nucl Med.
55:1842–1848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sörensen J, Sandberg D, Sandström M,
Wennborg A, Feldwisch J, Tolmachev V, Åström G, Lubberink M,
Garske-Román U, Carlsson J, et al: First-in-human molecular imaging
of HER2 expression in breast cancer metastases using the
111In-ABY-025 affibody molecule. J Nucl Med. 55:730–735.
2014. View Article : Google Scholar
|
|
39
|
Sörensen J, Velikyan I, Sandberg D,
Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M,
Lubberink M, Olofsson H, et al: Measuring HER2-receptor expression
in metastatic breast cancer using [(68)Ga]ABY-025 Affibody PET/CT.
Theranostics. 6:262–271. 2016. View Article : Google Scholar
|
|
40
|
Makrides SC, Nygren PA, Andrews B, Ford
PJ, Evans KS, Hayman EG, Adari H, Uhlén M and Toth CA: Extended in
vivo half-life of human soluble complement receptor type 1 fused to
a serum albumin-binding receptor. J Pharmacol Exp Ther.
277:534–542. 1996.PubMed/NCBI
|
|
41
|
Andersen JT, Pehrson R, Tolmachev V, Daba
MB, Abrahmsén L and Ekblad C: Extending half-life by indirect
targeting of the neonatal Fc receptor (FcRn) using a minimal
albumin binding domain. J Biol Chem. 286:5234–5241. 2011.
View Article : Google Scholar :
|
|
42
|
Jonsson A, Dogan J, Herne N, Abrahmsén L
and Nygren P-A: Engineering of a femtomolar affinity binding
protein to human serum albumin. Protein Eng Des Sel. 21:515–527.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tolmachev V, Hofström C, Malmberg J,
Ahlgren S, Hosseinimehr SJ, Sandström M, Abrahmsén L, Orlova A and
Gräslund T: HEHEHE-tagged affibody molecule may be purified by
IMAC, is conveniently labeled with [99(m)Tc(CO)3](+), and shows
improved biodistribution with reduced hepatic radioactivity
accumulation. Bioconjug Chem. 21:2013–2022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Orlova A, Rosik D, Sandström M, Lundqvist
H, Einarsson L and Tolmachev V: Evaluation of
[(111/114m)In]CHX-A″-DTPA-ZHER2:342, an affibody ligand coniugate
for targeting of HER2-expressing malignant tumors. Q J Nucl Med Mol
Imaging. 51:314–323. 2007.PubMed/NCBI
|
|
45
|
Wållberg H and Orlova A: Slow
internalization of anti-HER2 synthetic affibody monomer
111In-DOTA-ZHER2:342-pep2: implications for development of labeled
tracers. Cancer Biother Radiopharm. 23:435–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Varasteh Z and Orlova A: Comparing the
measured affinity of 111In-labeled ligands for cellular
receptors by monitoring gamma, beta, or X-ray radiation with three
different LigandTracer® devices. J Radioanal Nucl Chem.
304:823–828. 2015. View Article : Google Scholar
|
|
47
|
Altai M, Strand J, Rosik D, Selvaraju RK,
Eriksson Karlström A, Orlova A and Tolmachev V: Influence of
nuclides and chelators on imaging using affibody molecules:
Comparative evaluation of recombinant affibody molecules
site-specifically labeled with 68Ga and 111In via
maleimido derivatives of DOTA and NODAGA. Bioconjug Chem.
24:1102–1109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gong H, Kovar J, Little G, Chen H and
Olive DM: In vivo imaging of xenograft tumors using an epidermal
growth factor receptor-specific affibody molecule labeled with a
near-infrared fluorophore. Neoplasia. 12:139–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kobayashi H, Kao CH, Kreitman RJ, Le N,
Kim MK, Brechbiel MW, Paik CH, Pastan I and Carrasquillo JA:
Pharmacokinetics of 111In- and 125I-labeled
antiTac single-chain Fv recombinant immunotoxin. J Nucl Med.
41:755–762. 2000.PubMed/NCBI
|
|
50
|
Tolmachev V, Velikyan I, Sandström M and
Orlova A: A HER2-binding Affibody molecule labelled with 68Ga for
PET imaging: Direct in vivo comparison with the
111In-labelled analogue. Eur J Nucl Med Mol Imaging.
37:1356–1367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Barta P, Malmberg J, Melicharova L,
Strandgård J, Orlova A, Tolmachev V, Laznicek M and Andersson K:
Protein interactions with HER-family receptors can have different
characteristics depending on the hosting cell line. Int J Oncol.
40:1677–1682. 2012.
|
|
52
|
Kobayashi H, Yoo TM, Kim IS, Kim MK, Le N,
Webber KO, Pastan I, Paik CH, Eckelman WC and Carrasquillo JA:
L-lysine effectively blocks renal uptake of 125I- or
99mTc-labeled anti-Tac disulfide-stabilized Fv fragment. Cancer
Res. 56:3788–3795. 1996.PubMed/NCBI
|
|
53
|
Altai M, Varasteh Z, Andersson K, Eek A,
Boerman O and Orlova A: In vivo and in vitro studies on renal
uptake of radio-labeled affibody molecules for imaging of HER2
expression in tumors. Cancer Biother Radiopharm. 28:187–195. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ahlgren S, Orlova A, Wållberg H, Hansson
M, Sandström M, Lewsley R, Wennborg A, Abrahmsén L, Tolmachev V and
Feldwisch J: Targeting of HER2-expressing tumors using
111In-ABY-025, a second-generation affibody molecule
with a fundamentally reengineered scaffold. J Nucl Med.
51:1131–1138. 2010. View Article : Google Scholar : PubMed/NCBI
|