Introduction
Defect of B-cell apoptosis promotes initiation and
progression of autoimmune diseases like SLE and B-cell lymphoma
including multiple myeloma. The molecular definition of auto-immune
lymphoproliferative syndrome (ALPS) previously established that
defective apoptosis can lead to autoimmunity in humans (1). Systemic lupus erythematosus (SLE) is
a multi-system autoimmune disease characterized by B cell-defective
apoptosis and hyperproliferation (1,2).
Among many dysregulated molecular pathways and cell types,
apoptosis-defective B cells are the most important pathogenic
factor in that their capacity to produce pathogenic autoantibodies
and their contribution to tissue damage in SLE (1,3).
MRL/lpr mice bearing Fas/Fas ligand mutant genes are defective in
apoptosis of lymphocytes specifically B cells further to develop
autoimmunity and lymphoproliferation disease and are considered as
SLE mouse model (4,5). Similarly in autoimmune diseases,
alterations of apoptosis play an important role in tumor
development (6). Expansion of the
malignant clone of B-CLL cells appears to be due to an underlying
defect in its ability to undergo apoptosis (7–9).
These studies suggest that the defect of B-cell apoptosis plays an
important role in B-cell-mediated autoimmune diseases like SLE and
B-cell-related tumors.
Regulation of apoptosis is responsible for therapy
and new therapeutic approaches attempting to treat tumors and
autoimmune diseases (6,10–14).
B cell-activating factor (BAFF) has been regarded as a new
therapeutic target in SLE because it promotes B-cell survival and
development to block B-cell apoptosis (15–17).
Interfering BAFF-BAFF-R interaction promotes B-cell apoptosis with
small synthetic molecules (18).
March 9, 2011, belimumab, a fully human anti-BAFF mAb, was approved
by FDA to treat SLE. We and other researchers proved that
atacicept, a fusion protein of a BAFF receptor TACI and IgG Fc, had
similar clinical results to belimumab (19–21).
Aberrant BAFF expression protects malignant B-cells from
spontaneous or drug-induced apoptosis in B-cell non-Hodgkin
lymphoma (B-NHL) patients (22).
In addition, belimumab restores sensitivity of chronic lymphoid
leukemia cells to direct and Rituximab-induced NK lysis (23). These studies suggest that the
mechanisms underlying defect of B-cell apoptosis and therapeutic
target are similar in autoimmune diseases such as SLE and B-cell
lymphoma.
We further found that metabotropic glutamate
receptor 3 (Grm3) may be involved in autoreactive B-cell apoptosis
in atacicept-treated lupus-like mice and ligation of Grm3
ameliorates lupus-like disease by reducing B-cell numbers (24). Because both autoimmune diseases and
B-cell lymphoma including multiple myeloma share similar abnormal
apoptosis of B cells, we proposed that Grm3 may be involved in
apoptosis of B-cell lymphoma including multiple myeloma. Our data
demonstrated that Grm3, upregulated by apoptosis-induced agents,
effectively induced apoptosis of human B-cell leukemia cell line
Nalm-6 cells and mouse myeloma cell line SP 2/0 cells. Thus, Grm3
may be used as a potential target and effective therapeutic
indicator for B-cell lymphoma.
Materials and methods
Mice
Seven-to-eleven-week-old Balb/c and nude (Severe
combined immunodeficient, SCID) mice (Huafukang Technology Corp.,
Beijing, China), CD19cre mice and Foxo1F/F
(Nanjing Biomedical Research Institute of Nanjing University,
Nanjing, China) were bred in our animal facilities under specific
pathogen-free conditions. Mice with loxP sites flanking exon 2 of
Foxo1 (Foxo1F/F) were crossed to mice expressing Cre
recombinase under control of the CD19 promoter (CD19Cre)
to delete Foxo1 in B cells. Care, use and treatment of mice in the
present study were in strict agreement with international
guidelines for the care and use of laboratory animals. This study
was approved by the Animal Ethics Committee of the Beijing
Institute of Basic Medical Sciences.
Antibodies and reagents
APC-conjugated anti-mouse CD138 (281-2) was from BD
Biosciences (San Jose, CA, USA). FITC or PE conjugated anti-mouse
Grm3 (bs-12012R) was from Bioss Corp. (Woburn, MA, USA). Anti-Grm3
(ab13193) and anti-β-actin (ab8227) were from Abcam (Cambridge (MA,
USA). Anti-mouse Foxo1 (L27 and C29H4) was from Cell Signaling
Technology (Danvers, MA, USA). Apoptosis detection kits
(70-AP101-100) contained FITC or APC-conjugated Annexin V and PI
were from MultiSciences. Grm3 shRNA (MSH042669-HIVU6) and LV81/Grm3
(EX-Mm13188-Lv81) were from GeneCopoeia (Rockville, MD, USA). Foxo1
shRNA (sc-35383-V) was from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). FK506 (F4679), nocodazole (M1404), monastrol (M8515),
rapamycin (37094) and LPS (L2880) were from Sigma-Aldrich (St.
Louis, MO, USA). Bortezomib (M1686) was from Abmole Bioscience Inc.
(Houston, TX, USA).
Cell culture
Human B-cell leukemia cell line Nalm-6 cells and
mouse myeloma cell line SP 2/0 cells and human embryonic kidney HEK
293T cells were from the American Type Culture Collection (ATCC;
Rockville, MD, USA). Primary B cells were sorted from from
CD19cre/+Foxo1F/+ and
CD19cre/+Foxo1F/F mice by using B220
microbeads (autoMACS; Miltenyi Biotec, San Diego, CA, USA). All
cells were cultured in RPMI-1640 supplemented with 10% fetal calf
serum (FCS) and 50 μM 2-mercaptoethanol at 37°C under a 5%
CO2. In some experiments, different concentrations of
FK506, nocodazole, monastrol, rapamycin and LPS were added into the
culture.
Annexin V/PI staining
Annexin V/PI staining was performed as previously
described (25). Briefly, cells
were centrifuged at 335 × g for 10 min and resuspended in 2 ml 1X
phosphate-buffered saline (PBS) −/− (no calcium, no magnesium).
Cells were centrifuged at 335 × g for 10 min and resuspended in 1
ml 1X Annexin V binding buffer. A total of 5 μl Alexa Fluor
488-conjugated Annexin V was added and the tubes incubated in the
dark for 15 min at room temperature. A total of 100 μl of 1X
Annexin V binding buffer was added to each reaction tube (final
volume: ~200 μl). PI (4 μl) was diluted 1:10 in 1X Annexin V
binding buffer and a final PI concentration of 2 μg/ml was added in
each sample. Tubes were incubated in the dark for 15 min at room
temperature. 1X Annexin V binding buffer (500 μ) was added to wash
the cells. Then the samples were ready to be analyzed by flow
cytometry (FACS).
Cytometric analysis
Cytometric analysis has been described in our
previous studies (24,26). Briefly, cells (1×106
cells/sample) were washed with fluorescence-activated cell sorting
staining buffer (phosphate-buffered saline, 2% fetal bovine serum
or 1% bovine serum albumin, 0.1% sodium azide). All samples were
incubated with anti-Fc receptor Ab (BD Biosciences), prior to
incubation with other Abs diluted in fluorescence-activated cell
sorting buffer supplemented with 2% anti-Fc receptor Ab. The
samples were filtered immediately before analysis or cell sorting
to remove any clumps. Data collection and analyses were performed
on a FACSCalibur flow cytometer using CellQuest software.
Quantitative PCR analysis
Quantitative PCR analysis was described in our
previous studies (24). Briefly,
total RNA was extracted from B cells with TRIzol (Invitrogen-Life
Technologies, Carlsbad, CA, USA). The final RNA pellets were
dissolved in 0.1 mM EDTA (2 μl/mg original wet weight). Reverse
transcription reactions were carried out on 22 μl of sample using
sSuperScript II RNAse H-Reverse Transcriptase (Invitrogen-Life
Technologies) in a reaction volume of 40 μl. All samples were
diluted in 160 μl nuclease-free water. qPCR was employed to
quantify mouse Grm3 gene expression from the cDNA samples.
Sequences of primer pairs are available upon request. Mouse Grm3
mRNA expression was normalized to the levels of the β-actin
gene.
Western blot analysis
Western blot analysis was described in our previous
studies (24). Briefly, whole-cell
lysates were prepared for western blotting and blots were probed
with anti-mouse β-actin and anti-mouse Grm3 antibody. Preimmune
serum was used in parallel as controls and HRP-conjugated secondary
F(ab′)2 (Zymed Laboratories, San Francisco, CA, USA) were used in
concert with the ECL detection system (Amersham Life Science,
Arlington Heights, IL, USA).
Control, Grm3 or Foxo1-specific shRNA
infected SP 2/0 cells
SP 2/0 cells were infected with control, Grm3 or
Foxo1-specific shRNA using standard methods as described in our
previous studies (24,26). Briefly, in a 6-well tissue culture
plate, 2×105 cells/well were seeded in 2 ml
antibiotic-free normal growth medium supplemented with FBS. Cells
were incubated for 24 h at 37°C in a CO2 incubator until
60–80% confluent. 1× 106 infectious units of virus (IFU)
of control, Grm3 or Foxo1-specific shRNA-expressing lentivirus and
10 μg/ml polybrene were added into the culture. On day 1 after the
infection, the transfection mixture was removed and 1X normal
growth medium was added into the culture.
EGFP+ cell sorting
On days 3 after the infection, cells were sorted
using multicolor flow cytometry. All flow cytometry data were
acquired with FACSCanto, FACSCanto II, or FACSAria (BD
Biosciences), gated on live lymphocyte-sized cells on the basis of
forward and side scatter, and analyzed using FlowJo software (Tree
Star, Inc., Ashland, OR, USA).
SP 2/0 xenograft mouse model
To evaluate tumor growth in mouse models, 200 μl of
cell suspension from 5×106 SP 2/0 expressing GFP/Grm3
shRNA and SP 2/0 cells expressing GFP/control shRNA were
subcutaneously injected into the left and right sides of the back
of each Balb/c or nude mouse. Mice were sacrificed on day 10 after
the injection. Tumor volumes were determined by measuring the major
(L) and minor (W) diameters with an electronic caliper. The tumor
volume was calculated according to the following formula: Tumor
volume = π/6 × L × W2.
Plasmids and cell transfection
cDNA encoding Foxo1 (1-149), Foxo1 (1-400) and Foxo1
(1-655) corresponding to amino acids 1-149, 1-400, 1-655 of Foxo1
was amplified from cDNA encoding Foxo1 (Addgene, Cambridge, MA,
USA) by PCR and subcloned into plasmid pEGFP-N1. SP 2/0 cells were
transfected with the appropriate amount of plasmid (5–20 μg) using
Lipofectamine 2000 according to the manufacturer’s
instructions.
Statistical analysis
Statistics were generated using t-test in GraphPad
Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) and
values are represented as mean ± standard error of the mean (SEM).
Results were considered statistically significant at P<0.05.
Results
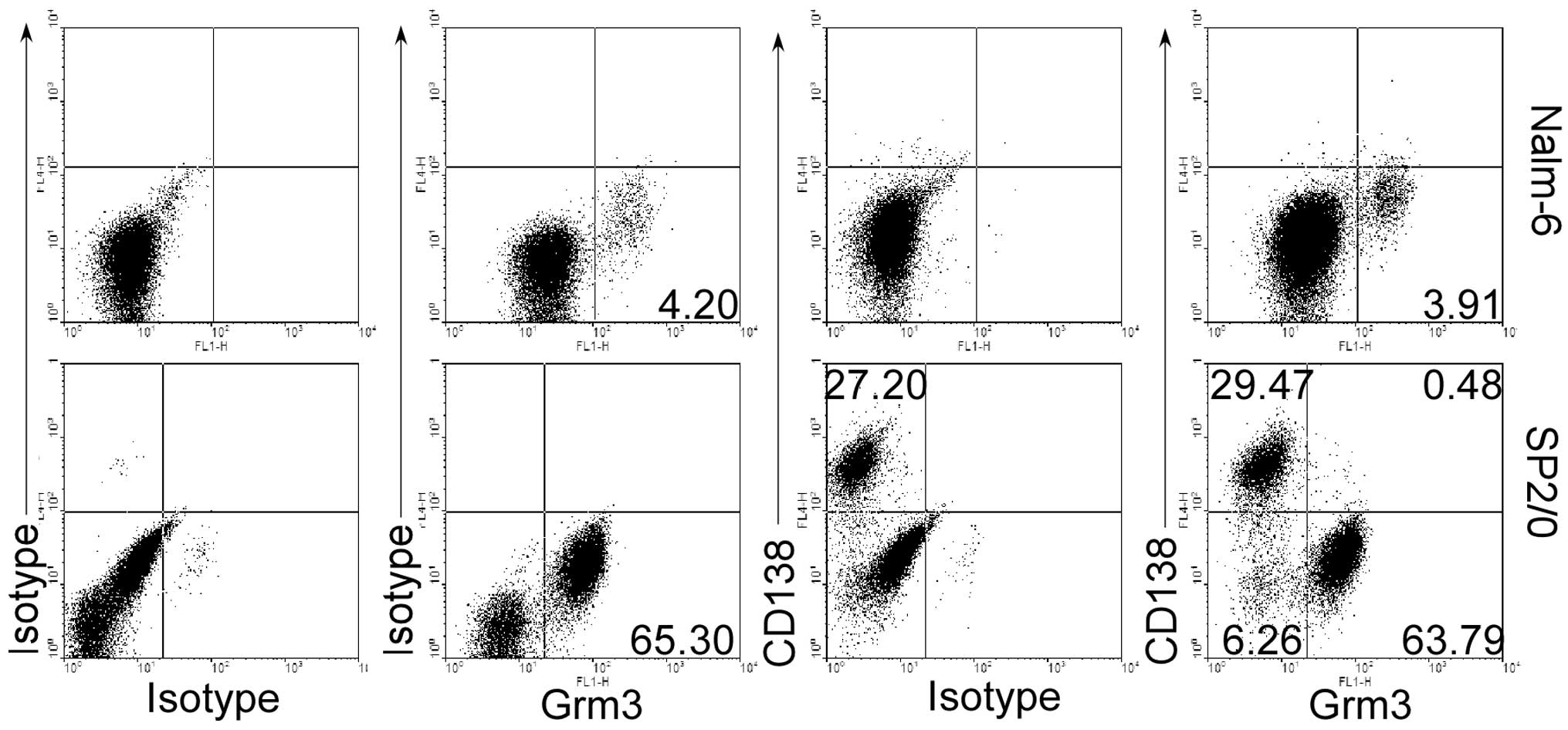
Grm3 expresses on the surface of
B-cell-related tumor cells
To explore whether Grm3 play an important role in
the apoptosis in B-cell-related tumor, we first determined whether
B-cell-related tumor cell lines can express Grm3. Human B-cell
leukemia Nalm-6 cells and mouse myeloma SP 2/0 cells were selected
because Pre-B Nalm-6 cells and plasma SP 2/0 cells represent two
different stages of B-cell development and two different species
(human and mouse). This is proved by our data suggesting that SP
2/0 cells but not Nalm-6 cells express CD138, a marker on the
surface of plasma cells (Fig. 1).
As expected, we found that both Nalm-6 cells and SP 2/0 cells could
express Grm3 by flow cytometry (FACS) (Fig. 1). However, Grm3 was not expressed
in CD138+ SP 2/0 cells (Fig. 1), making us to propose that Grm3
would be expressed in apoptotic cells.
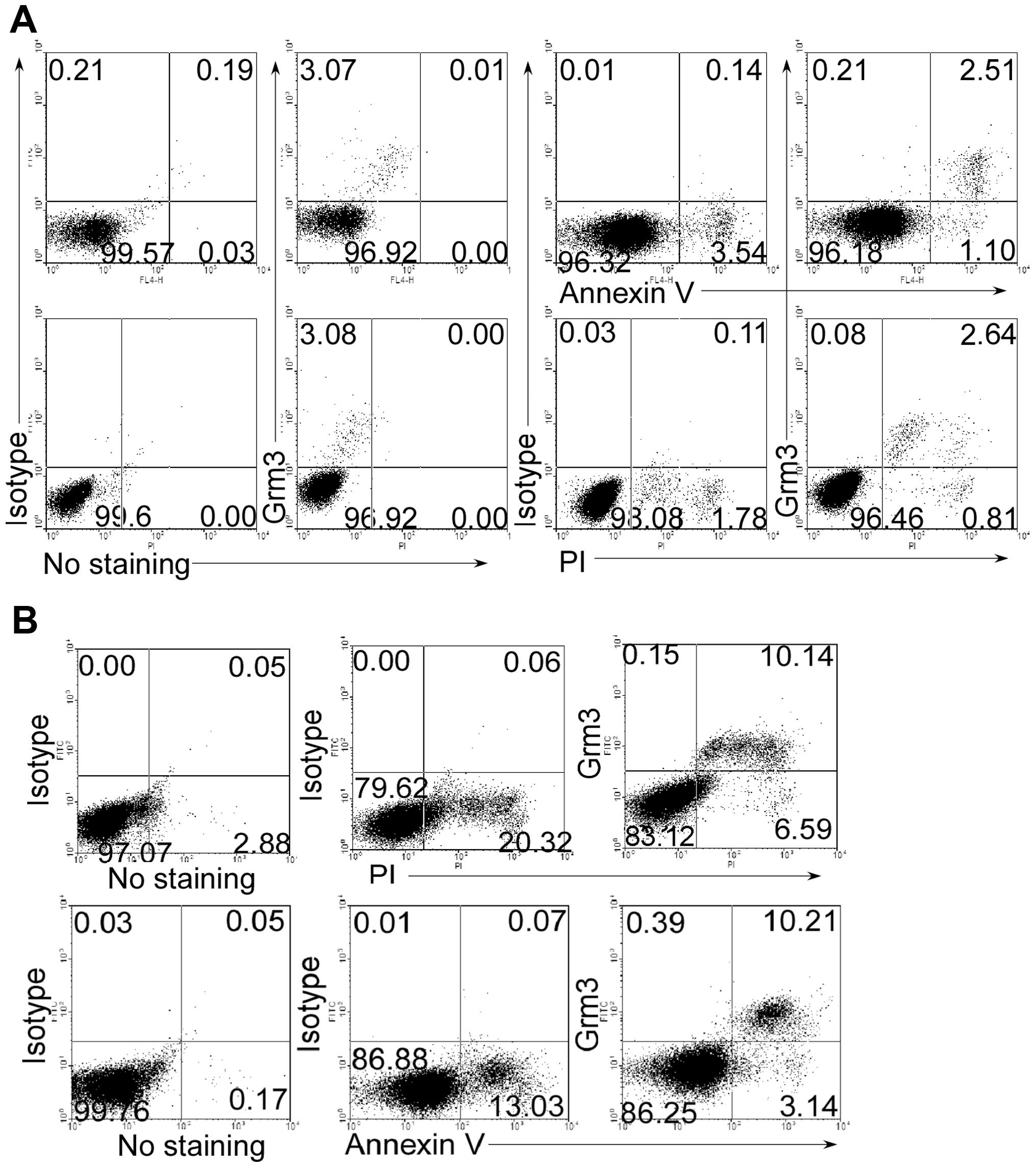
Grm3 is expressed in apoptotic cells
To determine whether Grm3 is expressed in apoptotic
cells, we used Annexin V and phospholine iodide (PI) staining to
mark the apoptotic cells. As expected, we found that Grm3 was
expressed in Annexin V+ and PI+ cells of SP
2/0 (Fig. 2A) and Nalm-6 (Fig. 2B) cells, whereas live cells did not
express Grm3. These results suggest that Grm3 is expressed in
apoptotic cells.
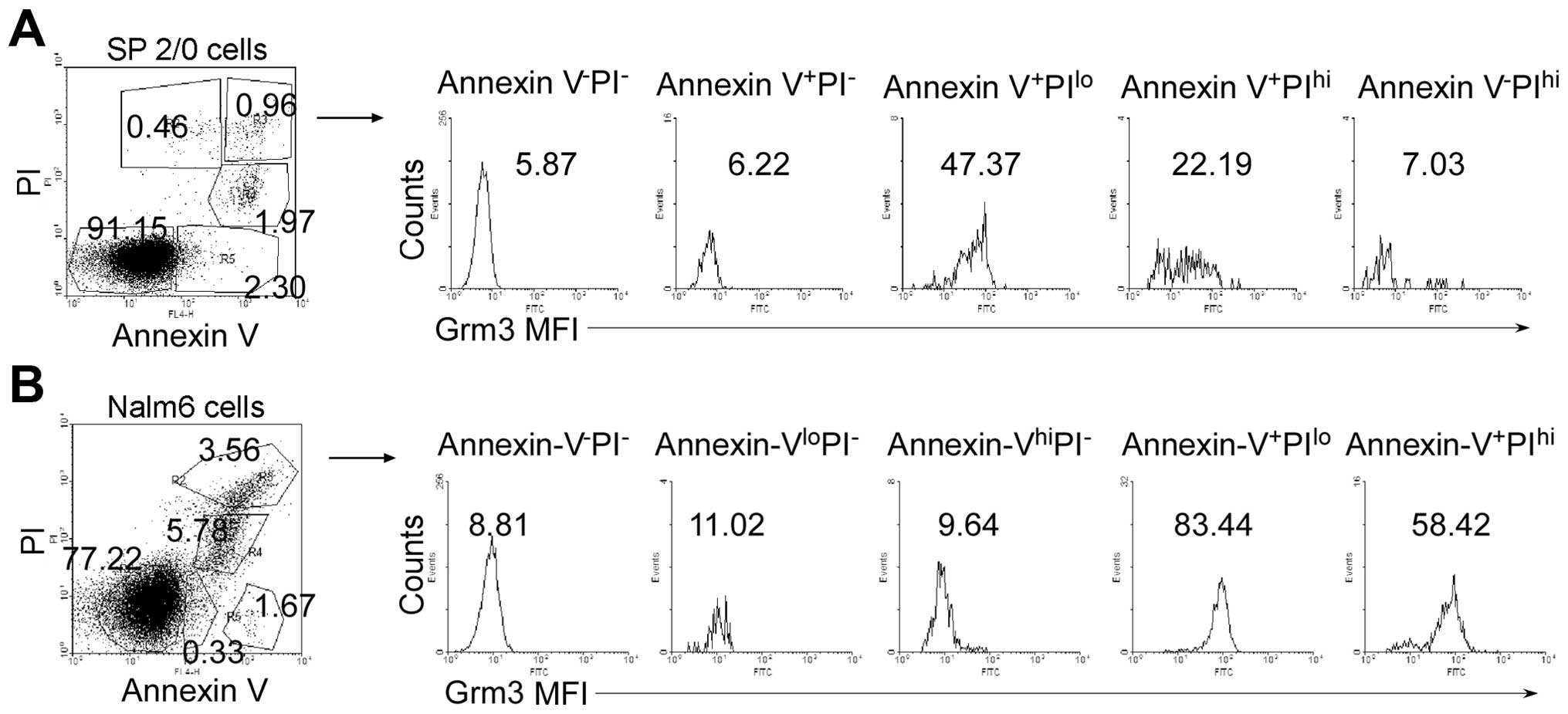
To examine which apoptotic stage Grm3 expression
emerged, we used Annexin V and PI co-staining assay to distinguish
different apoptotic stages. We analyzed mean fluorescence intensity
(MFI) of Grm3 expression in Annexin V−PI−,
Annexin V+PI−, Annexin
V+PIlo, Annexin V+PIhi,
Annexin V-PIhi cell subpopulations (Fig. 3, left panel). The data demonstrated
that Grm3 MFIis the highest in Annexin V+PIlo
cell subpopulation of SP 2/0 (Fig.
3A) and Nalm-6 (Fig. 3B)
cells. These results suggest that Grm3 is expressed mainly in the
middle stage of apoptosis.
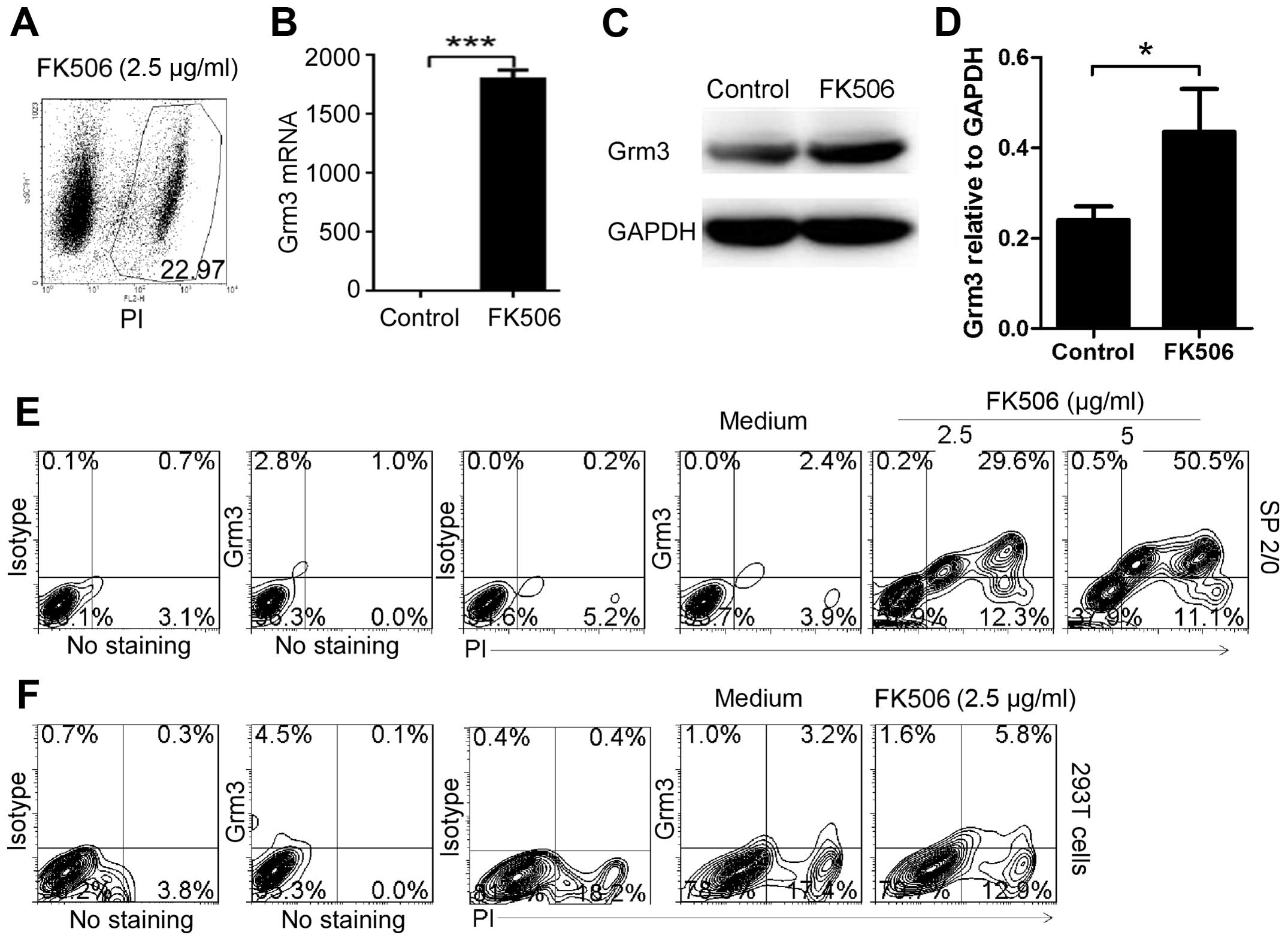
Apoptosis-induced drugs can effectively
induce Grm3 expression
To determine whether apoptosis-induced drugs can
effectively induce Grm3 expression, we first used FK506
(tacrolimus), a generally applied immunosuppressant in organ
transplantation and recently reported to induce apoptosis in
prostate cancer (27). We found
that 2.5 μg/ml FK506 could effectively induce SP 2/0 cell apoptosis
(Fig. 4A). Furthermore, qPCR
(Fig. 4B) and western blot
analysis (Fig. 4C and D) assays
demonstrated that apoptosis significantly upregulated Grm3 mRNA and
protein expression in SP 2/0 cells. In addition, FACS analysis
showed that FK506 dose-dependently induced Grm3 expression in SP
2/0 cells (Fig. 4E). Compared with
SP 2/0 cells, human embryonic kidney 293T cells could not
effectively be induced to express Grm3 (Fig. 4F). These results are in line with
our previous study suggesting that Grm3 expression was induced
mainly in B cells (24).
Low dose of bortezomib, first-line drug in patients
with newly diagnosed multiple myeloma, could effectively induce
apoptosis and Grm3 expression (Fig.
5). Antineoplastic agents such as nocodazole and monastrol
could also effectively induce apoptosis and Grm3 expression
(Fig. 5). However, rapamycin, a
drug which prevents activation of T cells and B cells by inhibiting
the production of interleukin-2 (IL-2) in organ transplantation,
could not induce SP 2/0 cell apoptosis (Fig. 5). Therefore, it could not
effectively induce Grm3 expression (Fig. 5). Together, our data suggest that
apoptosis-induced drugs can effectively induce Grm3 expression in
SP 2/0 cells.
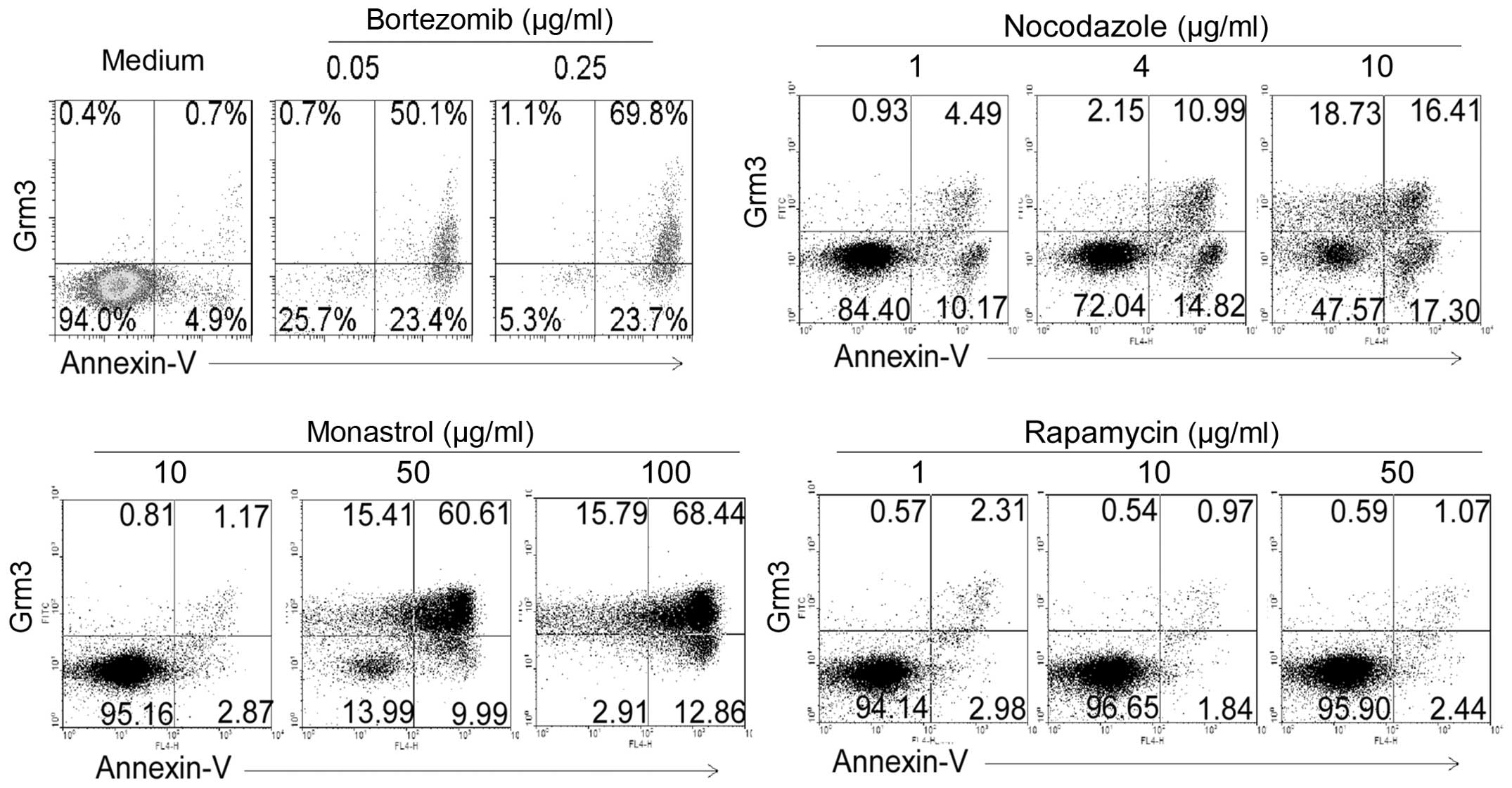 | Figure 5Bortezomib, nocodazole, monastrol and
rapamycin induce apoptosis and Grm3 expression. SP 2/0 cells were
cultured for 24 h in the medium contained with 0, 0.05 and 0.25
μg/ml bortezomib, 1, 4 and 10 μg/ml nocodazole, 10, 50 and 100
μg/ml monastrol, and 1, 10 and 50 μg/ml rapamycin. Cells were
stained with fluorescence-conjugated anti-mouse Grm3 antibody and
Annexin V, and analyzed by FACS. Quadrants indicate percentage of
Grm3-expressing and Annexin V-staining cells. Data are
representative of six independent experiments. |
Grm3 deficiency promotes SP 2/0 xenograft
tumor progression by suppressing cell apoptosis
To explore the role of Grm3 in B-cell-related tumor
cell apoptosis, we first used Grm3-specific shRNA to knock down the
Grm3 expression in SP2/0 cells. We found that the level of EGFP
expression is negatively associated with cell apoptosis in Grm3
shRNA-infected SP 2/0 cells, whereas the level of EGFP expression
is positively associated with cell apoptosis in control
shRNA-infected SP 2/0 cells (Fig.
6A). In addition, FACS analysis demonstrated that Grm3 shRNA
effectively depleted Grm3 expression in EGFP+ cells
(Fig. 6B). Together, our data
suggest that Grm3 deficiency suppresses SP 2/0 cell apoptosis.
Furthermore, we found that Grm3 deficiency could effectively reduce
FK506-, rapamycin-, monastrol- or nocodazole-induced SP 2/0 cell
apoptosis (Fig. 6C–E).
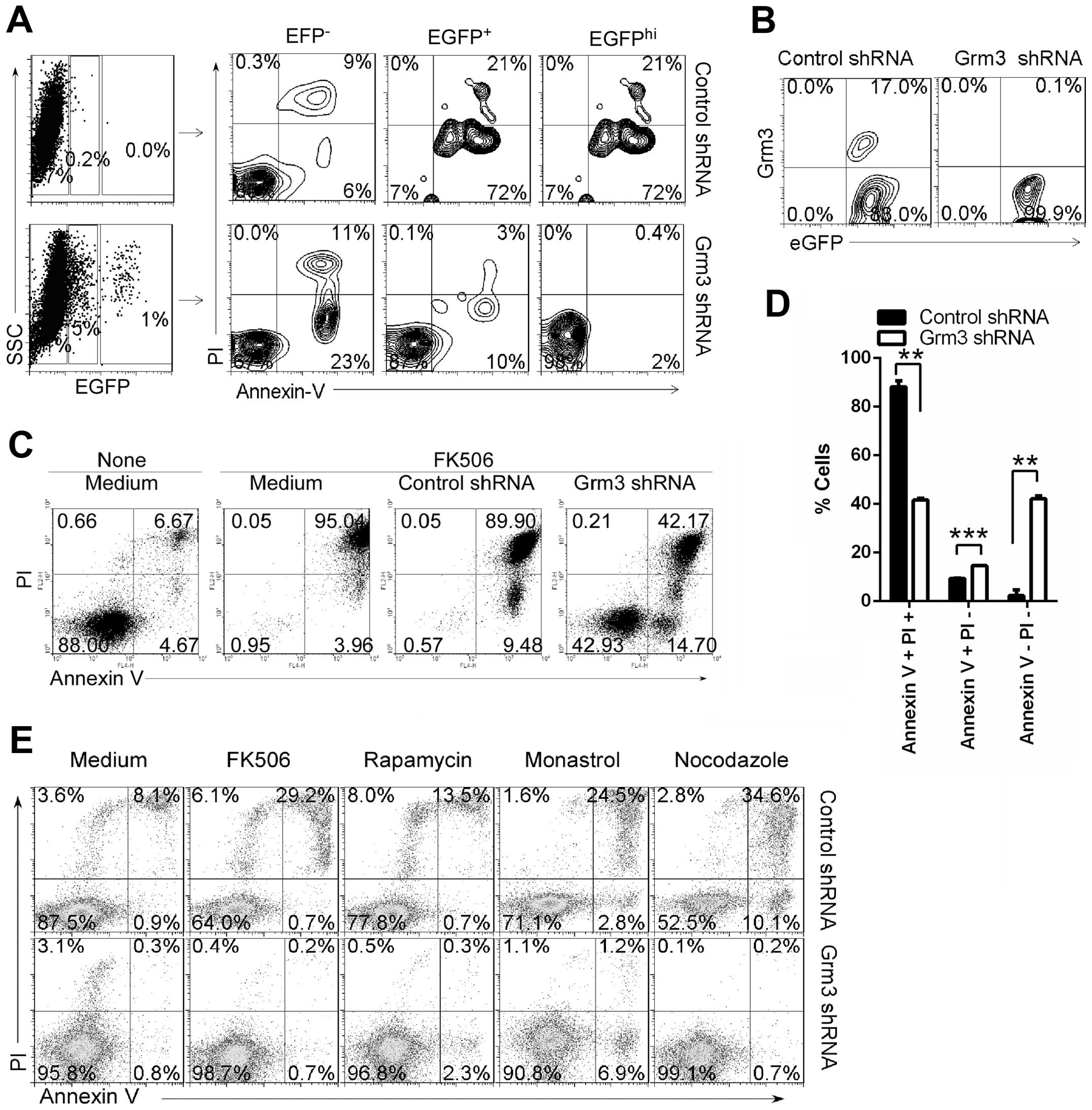 | Figure 6Grm3 deficiency suppresses cell
apoptosis. SP 2/0 cells were infected with control- or
Grm3-specific shRNA (with EGFP)-expressing lentivirus. On day 3
after infection, cells were collected. (A) Cells were stained with
Annexin V and PI, and analyzed by FACS. Quadrants indicate
percentage of EGFP−, EGFPlo and
EGFPhi-expressing cells (left panel), Annexin V- and
PI-staining cells in EGFP−, EGFPlo and
EGFPhi cell subpopulations (right panel). (B) Cells were
stained with fluorescence-conjugated anti-mouse Grm3 antibody, and
analyzed by FACS. Quadrants indicate percentage of Grm3-expressing
cells in EGFP+ cells. (C–E) EGFP+ cells were
sorted by FACS and cultured in the medium contained with 10 μg/ml
FK506 (C and D), 2.5 μg/ml FK506, 50 μg/ml rapamycin, 25 μg/ml
monastrol, 10 μg/ml nocodazole (E). Quadrants indicate percentage
of Annexin V- and PI-staining cells. (A–E) Data are representative
of three independent experiments. (D) Data are shown as mean ± SEM
(n=3) of three independent experiments. **P<0.01,
***P<0.001, (two tailed Student’s t-test). |
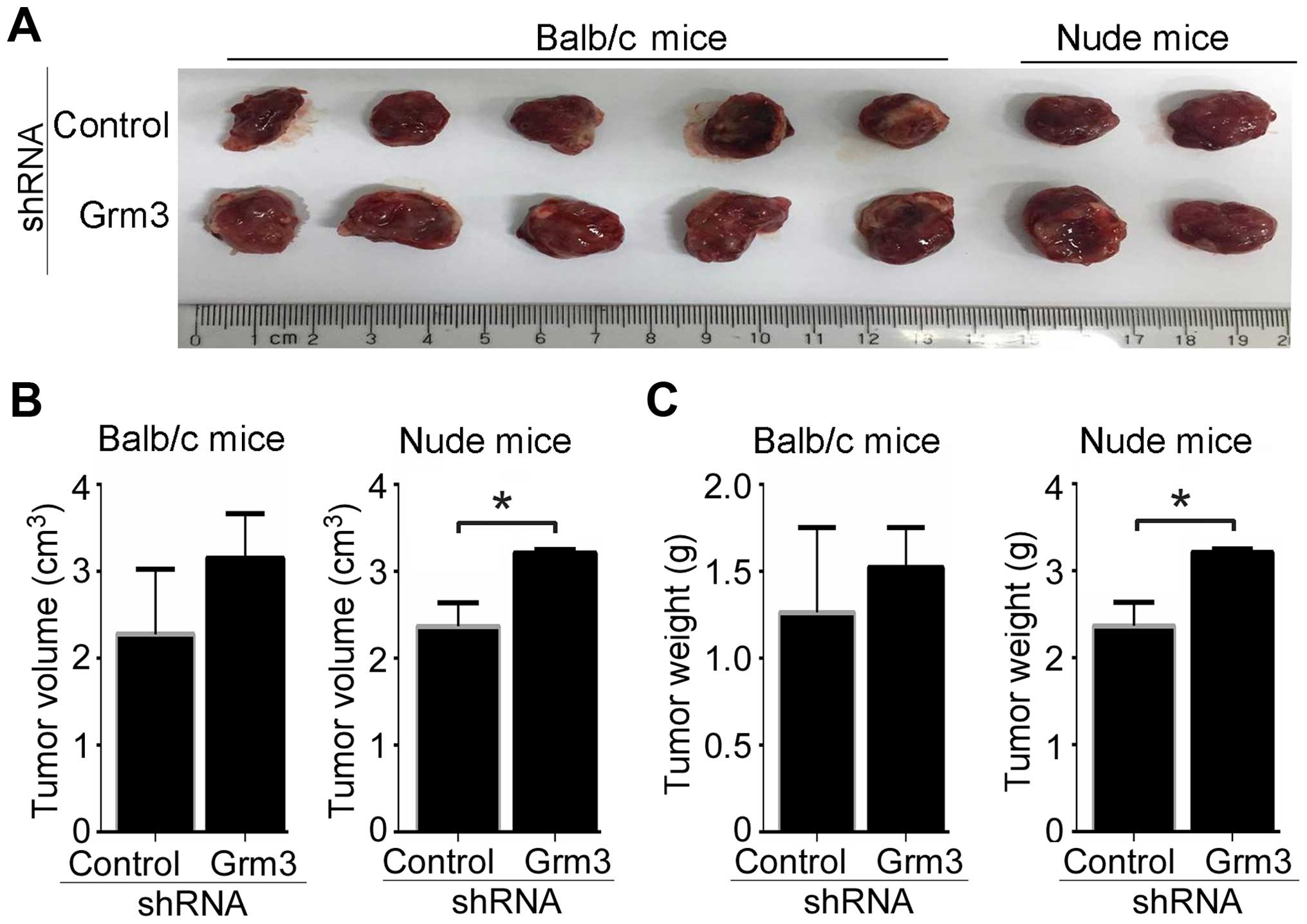
To further determine the effect of Grm3 deficiency
on in vivo tumor progression, SP 2/0 xenograft mouse models
were developed in Balb/c and nude mice. SP 2/0 cells were infected
with control- or Grm3-specific shRNA (with EGFP)-expressing
lentivirus and sorted by FACS based on EGFP expression.
EGFP+ cells/mouse (5×106) were subcutaneously
injected into Balb/C (5 mice/group) or nude (2 mice/group) mice.
Our data demonstrated that Grm3 deficiency promoted in vivo
tumor progression in Balb/c and nude mice (Fig. 7A). Tumor volumes and average tumor
weights from each group were also measured. The results showed that
Grm3 deficiency upregulated tumor volumes and weights in Balb/c,
although the difference was not significant, whereas Grm3
deficiency significantly upregulated in vivo tumor volumes
and weights in nude mice (Fig. 7B and
C). Together, our results suggest that Grm3 deficiency promotes
SP 2/0 xenograft tumor progression.
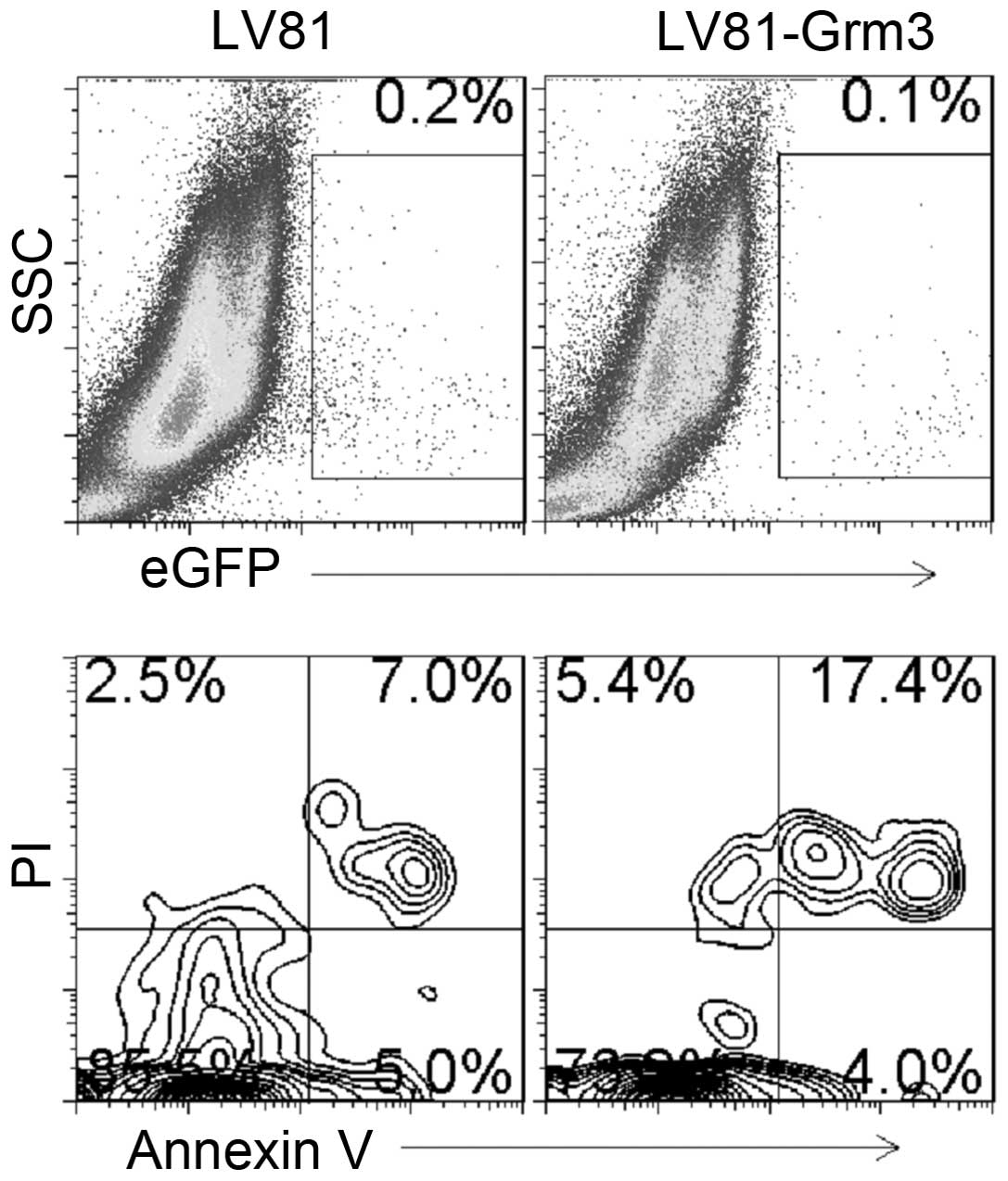
To further explore the role of Grm3 in SP 2/0 cell
apoptosis, we overexpressed Grm3 in SP 2/0 cells. We used control-
or Grm3-expressing Lv81 (with EGFP) lentivirus to infect SP 2/0
cells. We analyzed cell apoptosis in EGFP+ cells by
FACS. The results demonstrated that Grm3 overexpression upregulates
cell apoptosis (Fig. 8).
Grm3 mediates cell apoptosis by
Foxo1
Grm3 are linked to the inhibition of the cyclic AMP
cascade (24) and cAMP-PKA has
been shown to phosphorylate FoxO1 (28). These results suggest that Grm3 may
promote Foxo1 expression by suppressing Foxo1 phosphorylation.
Thus, we proposed that Foxo1 mediated mechanisms underlie
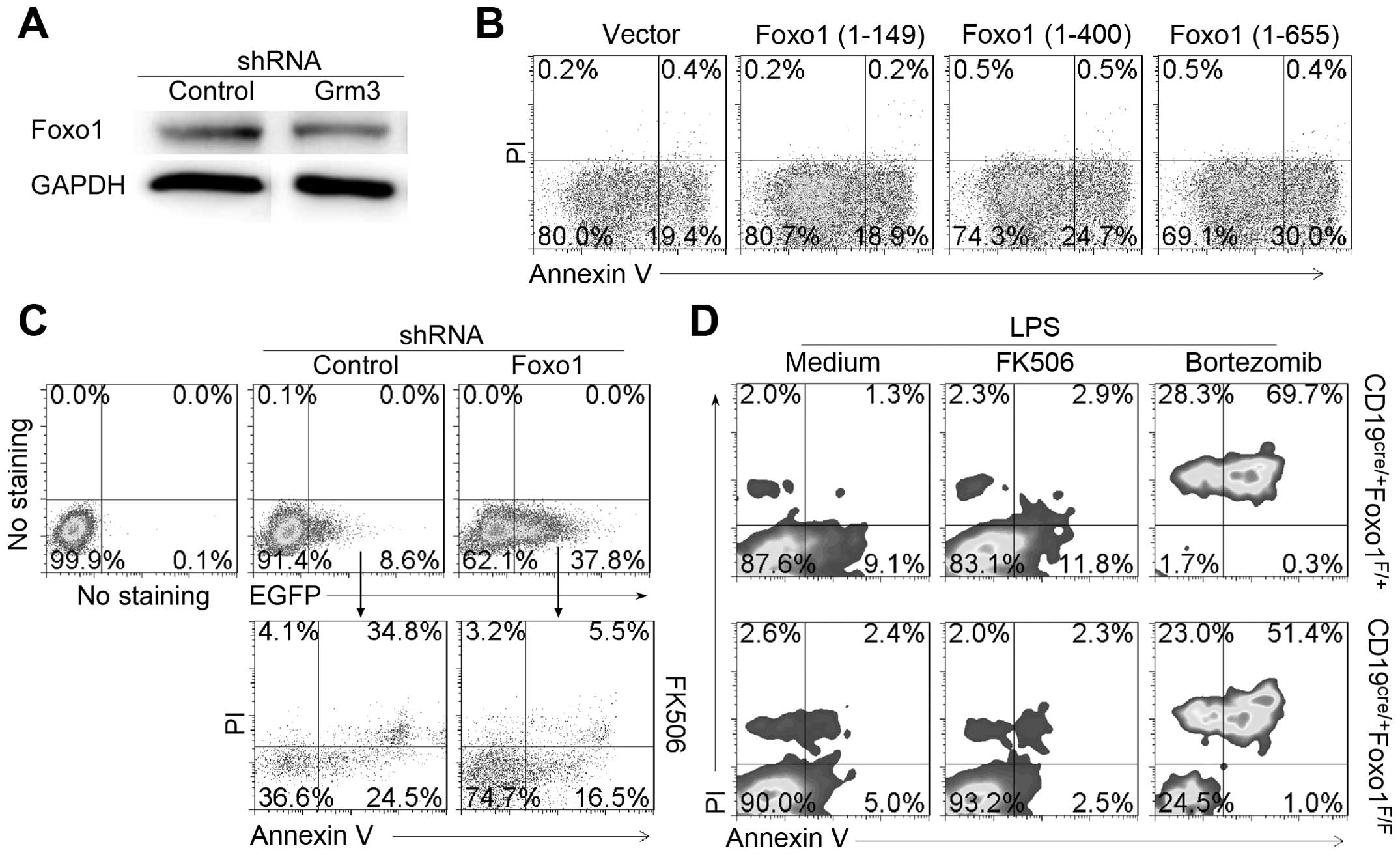
Grm3-induced SP 2/0 cell apopotosis. We first examined the effect
of Grm3 deficiency on Foxo1 expression in SP 2/0 cells. The data
demonstrated that compared with control, or Grm3-specific
shRNA-infected SP 2/0 cells reduced Foxo1 expression in SP 2/0
cells (Fig. 9A). The results
suggest that Grm3 deficiency reduce Foxo1 expression in SP 2/0
cells. To explore direct effect of Foxo1 on SP2/0 cells, SP 2/0
cells were transfected with plasmids pEGFP-N1 expressing Foxo1
(1-149), Foxo1 (1-400) or Foxo1 (1-655). The data showed that
full-length Foxo1 could upregulate SP 2/0 cell apoptosis (Fig. 9B). In addition, we used
Foxo1-specific shRNA to reduce Foxo1 expression in SP 2/0 cells.
The results suggest that Foxo1-deficiency reduced FK506-induced SP
2/0 cell apoptosis (Fig. 9C). The
effect of Foxo1-deficiency on cell apoptosis was approved by using
B cells from CD19cre/+Foxo1F/+ and
CD19cre/+Foxo1F/F (Fig. 9D). Together, our data suggest that
Grm3 mediates cell apoptosis by regulating Foxo1 expression.
Discussion
Cancer is a multifaceted disease comprising a
combination of genetic, metabolic and signalling aberrations, which
severely disrupt the normal homeostasis of cell growth and death
(29). Cell apoptosis is closely
associated with tumor resistance and susceptibility to various
therapeutic agents (30). We found
here that apoptosis-induced drugs could effectively induce
apoptosis-controlled Grm3 expression in human B-cell leukemia cell
line Nalm-6 cells and mouse myeloma cell line SP 2/0 cells.
Compared with anticancer drugs, immunosuppressor rapamycin could
not effectively induce Grm3 expression. Thus, Grm3 may be used as a
susceptible indicator to various therapeutic agent-induced
apoptosis of B-cell-related tumor. The impairment of cell death
function is also often the reason for the development of
chemotherapeutic resistance encountered during treatment (29). Undoubtedly, the mechanism
underlying cell apoptosis will lead to novel anticancer agents
(30). Our data suggest that
apoptosis-controlled Grm3 may be a potential target for treatment
of B-cell-related tumor.
Grm3 belongs to the metabotropic glutamate G
protein-coupled receptor family that has been divided into 3 groups
on the basis of sequence homology, putative signal transduction
mechanisms and pharmacologic properties. GRM3 gene has been found
to be associated with bipolar affective disorder (31). In addition, GRM3 expression is also
reported in various types of human malignancies including glioma,
ganglioglioma, laryngeal cell carcinoma and adrenocortical tumor
and considered a key regulator of cell proliferation in these
cancers. Furthermore, activating mutations in Grm3 was also
identified in melanoma (32). We
showed here that Grm3 could be induced on the surface of
B-cell-related tumor Nalm-6 cells and SP 2/0 cells by
apoptosis-induced drugs. The study provides evidence for Grm3
expression in the middle stage of B-cell-related tumor cell
apoptosis.
Grm3, a group II receptor, is linked to the
inhibition of the cyclic AMP cascade. Some literature demonstrates
that cAMP/protein kinase A (PKA) induces apoptosis such as in
immature T cells by inducing pro-apoptotic protein BIM (33), in aluminum chloride-treated
lymphocytes by inhibiting NF-κB (34). Other studies suggest that elevation
of cAMP levels inhibits apoptosis such as in doxorubicin-treated
Nalm-6 cells through induction of BAD phosphorylation and
inhibition of p53 accumulation (35), in arsenic trioxide-treated acute
promyelocytic leukemia cells by blocking caspase-3 activation
(36). These results suggest that
cAMP/PKA signaling pathway played a suppressive role in tumor cell
apoptosis such as leukemia cells and Nalm-6. Thus, Grm3 may
suppress cAMP/PKA signaling pathway to promote drug-induced Nalm-6
and SP 2/0 cell apoptosis.
cAMP/PKA reduces the nuclear localization of Foxo1
by phosphorylation (28,37) and phosphorylated Foxo1 protein are
ubiquitin-dependently de-gradated (38). In accordance with these studies,
our data showed here that Grm3, the inhibitor of the cyclic AMP
cascade, induced Foxo1 expression. Recently, Foxo1 has been
reported as a tumor suppressor in cervical cancer (39). In addition, Foxo1 downregulation
contributes to the oncogenic program of primary mediastinal B-cell
lymphoma (40). Substantial
literature demonstrates that Foxo1 suppressed tumor by inducing
apoptosis (41,42). In accordance with these studies,
our data suggest that Grm3 limits multiple myeloma SP2/0 cell
growth by inducing Foxo1-mediated apoptosis.
The majority of multiple myeloma patients relapse
with the current treatment strategies, raising the need for
alternative therapeutic approaches. Cellular immunotherapy is a
rapidly evolving field and currently being translated into clinical
trials with encouraging results in several cancer types, including
multiple myeloma (43). Remarkable
progress in gene expression profiling of B-cell lymphoma have led
to the development of a variety of tumor-specific regimens. Novel
agents target directly the pathways involved in signal
transduction, leads to cancer cell apoptosis (44). Bortezomib is the first therapeutic
proteasome inhibitor approved by the US FDA for treating relapsed
multiple myeloma. We found here that compared with other anticancer
drugs such as nocodazole and monastrol, bortezomib was the most
effective drug in inducing SP 2/0 cell apoptosis and Grm3
expression. It is worth to further explore whether Grm3 can be used
as an indicator in B-cell-related tumor susceptibility to
therapeutic agents and a potential target in the treatment of
B-cell-related tumor including multiple myeloma.
In conclusion, apoptosis-induced agents such as
FK506, bortezomib, nocodazole, monastrol effectively induced Grm3
expression in human B-cell leukemia cell line Nalm-6 cells and
mouse myeloma cell line SP 2/0 cells. Critically, Grm3 deficiency
promotes SP 2/0 xenograft tumor progression by suppressing cell
apoptosis, whereas Grm3 overexpression effectively upregulates SP
2/0 cell apoptosis. Finally, we showed that Grm3 mediated cell
apoptosis by Foxo1. Together, our results suggest that Grm3 may be
an indicator in B-cell-related tumor susceptibility to various
therapeutic agents. It is worth further study whether Grm3 can be
used as a potential target in the treatment of B-cell-related
tumors including multiple myeloma and B-cell leukemia.
Acknowledgements
The present study was supported by the National
Basic Research Program 973 Grants (2013CB530506 and 2015CB553704),
the National Nature and Science Fund (81471529, 81401332, 81272320,
81471540 and 81472647), the Key Program of the Beijing Natural
Science Foundation (7141007) and the Service Industry Scientific
Research of National Health and Family Planning Commission of China
(2015SQ00192).
References
|
1
|
Belot A, Kasher PR, Trotter EW, Foray AP,
Debaud AL, Rice GI, Szynkiewicz M, Zabot MT, Rouvet I, Bhaskar SS,
et al: Protein kinase cδ deficiency causes mendelian systemic lupus
erythematosus with B cell-defective apoptosis and
hyperproliferation. Arthritis Rheum. 65:2161–2171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahman A and Isenberg DA: Systemic lupus
erythematosus. N Engl J Med. 358:929–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anolik JH: B cell biology and dysfunction
in SLE. Bull NYU Hosp Jt Dis. 65:182–186. 2007.PubMed/NCBI
|
|
4
|
Furukawa F: Animal models of cutaneous
lupus erythematosus and lupus erythematosus photosensitivity.
Lupus. 6:193–202. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen PL and Eisenberg RA: Lpr and gld:
Single gene models of systemic autoimmunity and lymphoproliferative
disease. Annu Rev Immunol. 9:243–269. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany, NY). 4:330–349. 2012. View Article : Google Scholar
|
|
7
|
Reed JC: Molecular biology of chronic
lymphocytic leukemia. Semin Oncol. 25:11–18. 1998.PubMed/NCBI
|
|
8
|
Zhang Y, Dawson MI, Mohammad R, Rishi AK,
Farhana L, Feng KC, Leid M, Peterson V, Zhang XK, Edelstein M, et
al: Induction of apoptosis of human B-CLL and ALL cells by a novel
retinoid and its nonretinoidal analog. Blood. 100:2917–2925. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitada S, Andersen J, Akar S, Zapata JM,
Takayama S, Krajewski S, Wang HG, Zhang X, Bullrich F, Croce CM, et
al: Expression of apoptosis-regulating proteins in chronic
lymphocytic leukemia: Correlations with In vitro and In vivo
chemoresponses. Blood. 91:3379–3389. 1998.PubMed/NCBI
|
|
10
|
Burz C, Berindan-Neagoe I, Balacescu O and
Irimie A: Apoptosis in cancer: Key molecular signaling pathways and
therapy targets. Acta Oncol. 48:811–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jazirehi AR: Regulation of
apoptosis-associated genes by histone deacetylase inhibitors:
Implications in cancer therapy. Anticancer Drugs. 21:805–813. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neznanov N, Komarov AP, Neznanova L,
Stanhope-Baker P and Gudkov AV: Proteotoxic stress targeted therapy
(PSTT): Induction of protein misfolding enhances the antitumor
effect of the proteasome inhibitor bortezomib. Oncotarget.
2:209–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eberhard Y, Gronda M, Hurren R, Datti A,
MacLean N, Ketela T, Moffat J, Wrana JL and Schimmer AD: Inhibition
of SREBP1 sensitizes cells to death ligands. Oncotarget. 2:186–196.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldberg AA, Beach A, Davies GF, Harkness
TA, Leblanc A and Titorenko VI: Lithocholic bile acid selectively
kills neuroblastoma cells, while sparing normal neuronal cells.
Oncotarget. 2:761–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vincent FB, Morand EF and Mackay F: BAFF
and innate immunity: New therapeutic targets for systemic lupus
erythematosus. Immunol Cell Biol. 90:293–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vincent FB, Morand EF, Schneider P and
Mackay F: The BAFF/APRIL system in SLE pathogenesis. Nat Rev
Rheumatol. 10:365–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma N, He Y, Xiao H, Han G, Chen G, Wang Y,
Wang K, Hou C, Yang X, Marrero B, et al: BAFF maintains T-cell
survival by inducing OPN expression in B cells. Mol Immunol.
57:129–137. 2014. View Article : Google Scholar
|
|
18
|
Moon EY, Yi KY and Lee S: An increase in B
cell apoptosis by interfering BAFF-BAFF-R interaction with small
synthetic molecules. Int Immunopharmacol. 11:1523–1533. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nestorov I, Munafo A, Papasouliotis O and
Visich J: Pharmacokinetics and biological activity of atacicept in
patients with rheumatoid arthritis. J Clin Pharmacol. 48:406–417.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma N, Xing C, Xiao H, He Y, Han G, Chen G,
Hou C, Marrero B, Wang Y, Zhang S, et al: BAFF suppresses IL-15
expression in B cells. J Immunol. 192:4192–4201. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carbonatto M, Yu P, Bertolino M, Vigna E,
Steidler S, Fava L, Daghero C, Roattino B, Onidi M, Ardizzone M, et
al: Nonclinical safety, pharmacokinetics, and pharmacodynamics of
atacicept. Toxicol Sci. 105:200–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang S, Li JY and Xu W: Role of
BAFF/BAFF-R axis in B-cell non-Hodgkin lymphoma. Crit Rev Oncol
Hematol. 91:113–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wild J, Schmiedel BJ, Maurer A, Raab S,
Prokop L, Stevanović S, Dörfel D, Schneider P and Salih HR:
Neutralization of (NK-cell-derived) B-cell activating factor by
Belimumab restores sensitivity of chronic lymphoid leukemia cells
to direct and Rituximab-induced NK lysis. Leukemia. 29:1676–1683.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma N, Liu X, Xing C, Wang X, Wei Y, Han G,
Chen G, Hou C, Shen B, Li Y, et al: Ligation of metabotropic
glutamate receptor 3 (Grm3) ameliorates lupus-like disease by
reducing B cells. Clin Immunol. 160:142–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rieger AM, Nelson KL, Konowalchuk JD and
Barreda DR: Modified annexin V/propidium iodide apoptosis assay for
accurate assessment of cell death. J Vis Exp.
50:25972011.PubMed/NCBI
|
|
26
|
Wang X, Wei Y, Xiao H, Liu X, Zhang Y, Han
G, Chen G, Hou C, Ma N, Shen B, et al: A novel IL-23p19/Ebi3
(IL-39) cytokine mediates inflammation in Lupus-like mice. Eur J
Immunol. 46:1343–1350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawahara T, Kashiwagi E, Ide H, Li Y,
Zheng Y, Ishiguro H and Miyamoto H: The role of NFATc1 in prostate
cancer progression: Cyclosporine A and tacrolimus inhibit cell
proliferation, migration, and invasion. Prostate. 75:573–584. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee JW, Chen H, Pullikotil P and Quon MJ:
Protein kinase A-alpha directly phosphorylates FoxO1 in vascular
endothelial cells to regulate expression of vascular cellular
adhesion molecule-1 mRNA. J Biol Chem. 286:6423–6432. 2011.
View Article : Google Scholar
|
|
29
|
Long JS and Ryan KM: New frontiers in
promoting tumour cell death: Targeting apoptosis, necroptosis and
autophagy. Oncogene. 31:5045–5060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horwacik I and Rokita H: Targeting of
tumor-associated ganglio-sides with antibodies affects signaling
pathways and leads to cell death including apoptosis. Apoptosis.
20:679–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kandaswamy R, McQuillin A, Sharp SI,
Fiorentino A, Anjorin A, Blizard RA, Curtis D and Gurling HM:
Genetic association, mutation screening, and functional analysis of
a Kozak sequence variant in the metabotropic glutamate receptor 3
gene in bipolar disorder. JAMA Psychiatry. 70:591–598. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Prickett TD, Wei X, Cardenas-Navia I, Teer
JK, Lin JC, Walia V, Gartner J, Jiang J, Cherukuri PF, Molinolo A,
et al: Exon capture analysis of G protein-coupled receptors
identifies activating mutations in GRM3 in melanoma. Nat Genet.
43:1119–1126. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zambon AC, Wilderman A, Ho A and Insel PA:
Increased expression of the pro-apoptotic protein BIM, a mechanism
for cAMP/protein kinase A (PKA)-induced apoptosis of immature T
cells. J Biol Chem. 286:33260–33267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu F, Wang J, Cao Z, Song M, Fu Y, Zhu Y
and Li Y: cAMP/PKA signaling pathway induces apoptosis by inhibited
NF-κB in aluminum chloride-treated lymphocytes in vitro. Biol Trace
Elem Res. 170:424–431. 2016. View Article : Google Scholar
|
|
35
|
Fatemi A, Kazemi A, Kashiri M and Safa M:
Elevation of cAMP levels inhibits doxorubicin-induced apoptosis in
Pre- B ALL NALM-6 cells through induction of BAD phosphorylation
and inhibition of P53 accumulation. Int J Mol Cell Med. 4:94–102.
2015.PubMed/NCBI
|
|
36
|
Safa M, Mousavizadeh K, Noori S,
Pourfathollah A and Zand H: cAMP protects acute promyelocytic
leukemia cells from arsenic trioxide-induced caspase-3 activation
and apoptosis. Eur J Pharmacol. 736:115–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cochran BJ, Bisoendial RJ, Hou L, Glaros
EN, Rossy J, Thomas SR, Barter PJ and Rye KA: Apolipoprotein A-I
increases insulin secretion and production from pancreatic β-cells
via a G-protein-cAMP-PKA-FoxO1-dependent mechanism. Arterioscler
Thromb Vasc Biol. 34:2261–2267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vogt PK, Jiang H and Aoki M: Triple layer
control: Phosphorylation, acetylation and ubiquitination of FOXO
proteins. Cell Cycle. 4:908–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang B, Gui LS, Zhao XL, Zhu LL and Li
QW: FOXO1 is a tumor suppressor in cervical cancer. Genet Mol Res.
14:6605–6616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie L, Ritz O, Leithäuser F, Guan H,
Färbinger J, Weitzer CD, Gehringer F, Bruederlein S, Holzmann K,
Vogel MJ, et al: FOXO1 downregulation contributes to the oncogenic
program of primary mediastinal B-cell lymphoma. Oncotarget.
5:5392–5402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hwang KE, Park DS, Kim YS, Kim BR, Park
SN, Lee MK, Park SH, Yoon KH, Jeong ET and Kim HR: Prx1 modulates
the chemosensitivity of lung cancer to docetaxel through
suppression of FOXO1-induced apoptosis. Int J Oncol. 43:72–78.
2013.PubMed/NCBI
|
|
42
|
Marshall AD, Picchione F, Geltink RI and
Grosveld GC: PAX3-FOXO1 induces up-regulation of Noxa sensitizing
alveolar rhabdomyosarcoma cells to apoptosis. Neoplasia.
15:738–748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Binsfeld M, Fostier K, Muller J, Baron F,
Schots R, Beguin Y, Heusschen R and Caers J: Cellular immunotherapy
in multiple myeloma: Lessons from preclinical models. Biochim
Biophys Acta. 1846:392–404. 2014.PubMed/NCBI
|
|
44
|
Witkowska M and Smolewski P: Emerging
immunotherapy and strategies directly targeting B cells for the
treatment of diffuse large B-cell lymphoma. Immunotherapy. 7:37–46.
2015. View Article : Google Scholar : PubMed/NCBI
|