Introduction
Lung cancer is the most common cancer in men
worldwide, and is the fourth most frequent cancer in women
(1). The standard therapy of
intermediate and advanced lung cancer is based on the combination
of Cis-diamminedichloroplatinum (DDP, cisplatin) and other
chemotherapy agents (2,3). DDP is a DNA cross-linking agent,
which is used to treat cancers such as lung, ovarian and cervical
cancers (4–6). Cisplatin, carboplatin and oxaliplatin
induce cross-links between guanine bases. Cisplatin and carboplatin
form an identical cross-link, whereas the cross-link of oxaliplatin
is structurally different because of the bulky
1,2-diaminocyclohexane group. However, DDP resistance in lung
cancer has been widely reported (7–9).
Baicalin is a flavone glycoside found in several
species in the genus Scutellaria, such as Scutellaria
baicalensis and Scutellaria lateriflora. Baicalin and
its aglycone baicalein are positive allosteric modulator of
benzodiazepine and non-benzodiazepine sites of GABAA receptor
(10,11). Baicalin was shown to display
anxiolytic effects without sedative effects in mice (12,13).
Moreover, baicalin was revealed to inhibit prolyl endopeptidase
(14), and induce apoptosis in
pancreatic cancer cells (15).
Baicalin also inhibited proliferation of other malignant tumors,
such as hepatocellular carcinoma and glioma (16,17).
However, the effects of baicalin on DDP resistance in lung cancer
are unclear.
Microtubule affinity-regulating kinase 2 (MARK2) is
serine/threonine-protein kinase that is involved in the control of
cancer, microtubule stability, and cell polarity. MARK2 has been
shown to interact with Akt (18).
The phosphoinositide 3 kinase (PI3K)/Akt mammalian target of
rapamycin (mTOR) regulates cell cycling, and is associated with
cellular proliferation and the development of cancer. Once
activated, PI3K phosphorylates and activates Akt, which has
numerous downstream effects, including activating mTOR (19). Over-activation of the mTOR pathway
leads to increased cell proliferation and reduced levels of
cellular apoptosis involved in the pathogenesis of cancer. p-Akt is
the activated form of Akt that has biological function. Therefore,
we examined the expressions of MARK2 and p-Akt as a means of
exploring the effects of combination of baicalin and DDP on
proliferation and invasion of human lung cancer cells.
Materials and methods
Cells and reagents
Cells
A549 cells (human lung cancer cells) and A549/DDP
cells (DDP-resistant human lung cancer cells) were purchased from
MeiXuan Biological Science and Technology, Inc. (Shanghai, China),
and cultured at 37°C in F12K medium supplemented with 100 ml/l
fetal bovine serum (FBS), 100 kU/l penicillin, and 100 mg/l
chloramphenicol in a cell incubator with 5% CO2. Drugs:
the baicalin powder (Ronghe Inc., Shanghai, China) and cisplatin
(DDP; Macklin Inc., Shanghai, China) were dissolved in dimethyl
sulfoxide (DMSO). Both solutions were stored at −80°C.
Main reagents
F12K culture medium; RPMI-1640 culture medium; fetal
bovine serum (FBS); trypsin and antibodies (all from Gibco Inc.,
Grand Island, NY, USA); MTT cell proliferation and toxicity assay
kits (Aladdine Inc., Shanghai, China); primary antibodies against
GAPDH (Abcam Inc., Cambridge, MA, USA); primary antibodies against
Akt and p-Akt (Cell Signaling Technology Inc., Danvers, MA, USA);
primary antibodies against MARK2 (Proteintech Inc., Rosemont, IL,
USA); goat anti-rabbit antibody (Invitrogen Inc., Grand Island, NY,
USA); Martrigel (Becton-Dickinson Inc., Franklin Lakes, NJ, USA);
Transwell (Corning Inc., Corning, NY, USA); TRIzol (Invitrogen
Inc., Grand Island, NY, USA); Takara Reverse Transcriptase M-MLV;
qPCR kit (Tiangen Biotech, Inc., Beijing, China); and specific
primers for MARK2 and β-actin (Sangon Biotech Inc., Shanghai,
China).
Main pieces of equipment: light microscope (Olympus
Inc., Tokyo, Japan); microplate reader (Kehua Inc., Shanghai,
China); table-type refrigerated centrifuge (USTC Zonkia Inc.,
Hefei, China); cell incubator (Thermo Scientific Inc., Waltham, MA,
USA); vertical and horizontal electrophoresis system (Liuyi, Inc.,
Beijing, China); electric thermostatic drying oven (Huyue, Inc.,
Shangyu, China); PCR machine (Bio-Rad Inc., Irvine, CA, USA); and
Step One Plus quantitative PCR machine (Applied Biosystems, Inc.,
Waltham, MA, USA).
MTT assay
A549/DDP cells were cultured for 24 h before
baicalin was added. The final concentrations of baicalin were 1, 2,
4 and 8 µg/ml (n=3 wells/each concentration). The final
concentrations of DDP were 1, 2, 4 and 8 µg/ml. No drug was
added in control group. Meanwhile, other aliquots of cells were
treated with baicalin (8 µg/ml), DDP (1, 2, 4 and 8
µg/ml), and baicalin (8 µg/ml) combined with DDP (1,
2, 4 and 8 µg/ml) respectively (n=3 wells/each
concentration). Following addition of drugs, cells were cultured in
a 37°C incubator with 5% CO2 for periods of 24 and 48 h,
respectively. Following culture, 20 µl of MTT solution (5
mg/ml) was added to each well, and the cells were cultured at 37°C
for an additional 4 h. Following culture, the cell supernatants
were removed and discarded, and 150 µl of DMSO was added to
each well. The plates were then shaken for 15 min to dissolve
crystals, and the absorbance of each sample was detected at 570 nm
(A570) using an ELISA microplate reader. The degree of cell
proliferation inhibition in each sample was calculated using the
following formula: cell proliferation inhibition (%) =
(1-absorbance of the experimental group/absorbance of the control
group) ×100%. The probability sum method was utilized to look for
evidence of synergism achieved by combining baicalin with cisplatin
(20). The formula used for this
purpose was q=EAB/(EA+EB-EAxEB), where EAB is the effect achieved
(e.g., inhibition rate) when drug A and B are combined, and EA and
EB are the effects of drug A and B, respectively, when applied
separately. A q-value between 0.85 and 1.15 indicates that the
effects of drug A and B are additive. A q-value >1.15 indicates
that the effects of drug A and B are synergistic, while a q-value
<0.85 indicates that drug A and B have antagonistic effects.
Transwell invasion assay
A549 and A549/DDP cells were treated with baicalin
(8 µg/ml), DDP (4 µg/ml), and baicalin (8
µg/ml) combined with DDP (4 µg/ml) for 48 h,
respectively (n=3/group). No drugs were added in control groups.
Cells were digested by trypsin-ethylene diamine tetraacetic acid
(EDTA) solution (0.25% EDTA) and centrifuged. The cells were then
diluted to make concentration of 5×105/ml. The membrane
of the upper compartment was coated with 50 µl of Matrigel
(1 g/l), and incubated at 37°C for 1 h in order to reconstruct its
structure into basal membrane. Two hundred microliters of A549 and
A549/DPP cell suspension were incubated in upper compartment of
Transwell respectively, and 600 µl of 20% FBS were added
into lower compartment. Cells were incubated at a humid incubator
with 5% CO2 for 48 h. Following culture, 4%
paraformaldehyde was utilized to fix the microporous membrane.
Cells were stained with 0.05% crystal violet for 10 min, and washed
with phosphate-buffered saline (PBS) twice. Cells were then
observed under microscope (×400), and the number of cells that
penetrated the membrane were counted. The inhibition of tumor cell
invasion was calculated utilizing the following formula: inhibition
of cell invasion (%) = (1-the average number of cells that
penetrated the membrane in the experimental group/the average
number of cells that penetrated the membrane in the control group)
×100%.
Quantitative polymerase chain reaction
(qPCR)
A549/DDP cells were treated with different
concentrations of baicalin (0, 1, 2, 4, 8 and 10 µg/ml) for
48 h. Total RNA of A549/DDP cells was extracted and purified by
TRIzol according to manufacturer's instructions. A universal cDNA
synthesis kit (Tiangen Biotech, Inc.) was utilized for reverse
transcription. Each reaction contained 1 µl of random
hexamer primers (0.2 µg/µl) and 40 U M-MuLV reverse
transcriptase (20 U/µl). The specific primer for detection
of MARK2 gene was forward, ATGCTGCCCCAGAACTCTTC and reverse,
GTGCCTCTCTTGCTGGGATT. The specific primer for detection of β-actin
gene was forward, AGAAAATCTGGCACCACACC and reverse,
AGAGGGTACAGGGATAGCA. miRcute miRNA qPCR detection kit was used for
qPCR. PCR conditions were as follows: pre-denaturing at 95°C for 15
min; denaturing at 95°C for 10 sec; and annealing and
polymerization at 60–66°C for 20–32 sec. There were 40 PCR cycles.
PCR was performed in an ABI Step One Plus qPCR system. The
expression of MARK2 was determined as the ratio of relative optical
density of target gene to β-actin.
Western blot studies
Expression levels of AKT, p-AKT and MARK2 proteins
were detected by western blot analysis. A 549/DPP cells were
treated with different concentrations of baicalin (1, 2, 4 and 8
µg/ml) for 48 h. No drug was added in control group.
Cellular proteins were extracted and separated by electrophoresis
(120 V) on a 10% SDS-polyacrylamide gel. The separated proteins
were then electrophoretically (100 V for 120 min) transferred to
polyvinylidene fluoride (PVDF) membranes. After being blocked with
5% non-fat milk powder for 1 h, the membranes were incubated with
anti-Akt (1:1,000), anti-p-Akt (1:1,000), anti-MARK2 (1:2,000), and
anti-GAPDH antibodies (1:5,000) respectively at 4°C overnight.
Following incubation, membranes was washed three times (10 min
each) with a solution of Tris-buffered saline and Tween-20 (TBST).
The membranes were then incubated for 1 h at room temperature with
goat anti-rabbit secondary antibody labeled with horseradish
peroxidase (HRP) (1:3,000); after which, they were washed and
incubated for a short time period in electro-chemi-luminescence
(ECL) solution. The film was exposed in a dark room.
Statistical analysis
The statistical data were analyzed and the figures
were created using GraphPad Prism 5.0 software (GraphPad Software
Inc., La Jolla, CA, USA). All statistical results are expressed as
the mean ± SEM. Differences among 3 or more groups were compared by
analysis of variance (ANOVA), followed by the Bonferroni post-hoc
test for multiple comparisons. p-values ≤0.05 were considered
statistically significant.
Results
Baicalin and DDP inhibit the
proliferation of human lung cancer cells when used alone
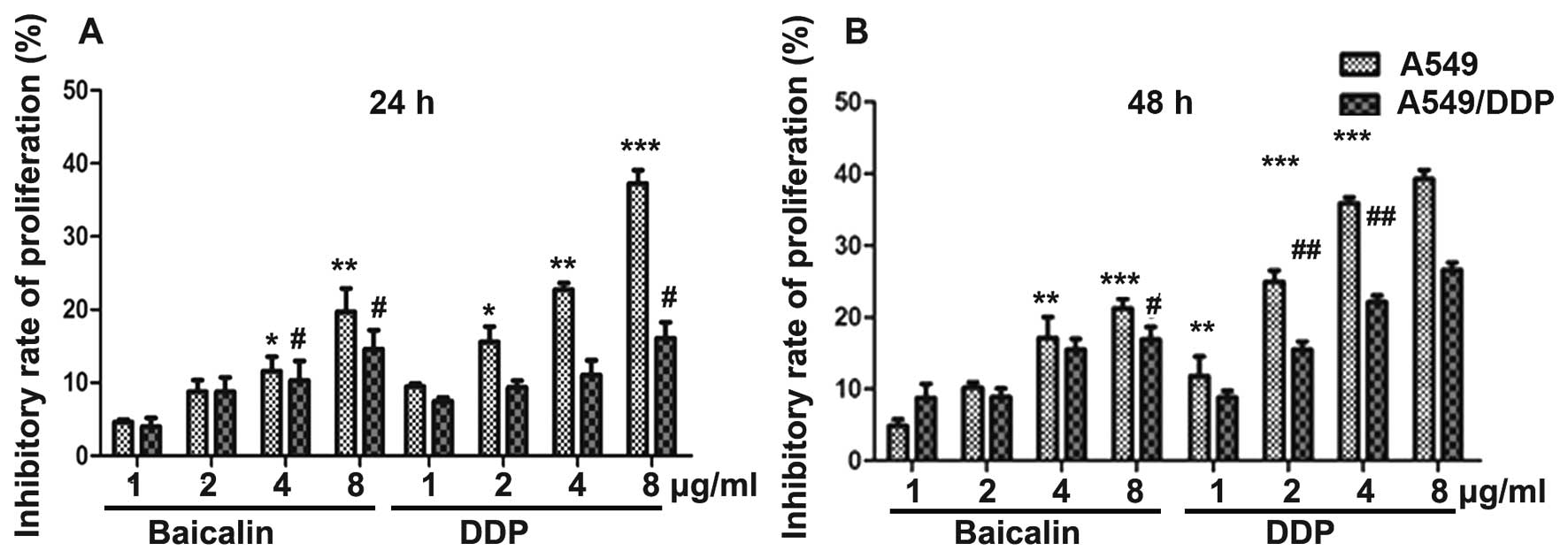
MTT assay was utilized to evaluate effects of
baicalin and DDP on the proliferation of A549 and A549/DPP human
lung cancer cells. Following addition of drugs, cells were cultured
for periods of 24 and 48 h, respectively. The degree of cell
proliferation inhibition was calculated as: inhibitory rate of cell
proliferation (%) = (1-absorbance of the experimental
group/absorbance of the control group) ×100%. Our results showed
that baicalin and DPP when used alone inhibited the proliferation
of A549 cells in a dose-dependent manner at 24 and 48 h,
respectively (Fig. 1). Similarly,
baicalin and DPP inhibited the proliferation of A549/DDP cells in a
dose-dependent manner at 24 and 48 h. The ability of baicalin to
inhibit tumor cell proliferation was similar between A549 and
A549/DDP cells, whereas the ability of DDP to inhibit cell
proliferation was lower in A549/DDP cells (Fig. 1).
Effects of combination of baicalin and
DDP on the proliferation of human lung cancer cells
MTT assay was utilized to evaluate effects of
combination of baicalin and DDP on the proliferation of A549 and
A549/DPP human lung cancer cells. Following addition of drugs,
cells were cultured for periods of 24 and 48 h, respectively. The
probability sum method was used to determine effects of the drug
combination. Formula: q=EAB/(EA+EB-EAxEB). The effects of drug A
and B are additive if q is between 0.85 and 1.15. Drug A and B are
synergistic if q>1.15, and antagonistic if q<0.85. Baicalin
(8 µg/ml) antagonized DDP when concentrations of DDP were 1,
2, 4 and 8 µg/ml at 24 h after A549 cells were treated
(q<0.85). The effects of baicalin and DDP were additive when the
concentration of DDP was 8 µg/ml (0.85<q<1.15), and
synergistic when the concentration of DDP was 4 µg/ml at 48
h after A549 cells were treated (q>1.15) (Table I). In addition, at 24 h after
A549/DPP cells were treated, baicalin (8 µg/ml) antagonized
DDP when concentrations of DDP were 1, 2 and 8 µg/ml
(q<0.85), whereas effects of baicalin and DDP were additive when
the concentration of DDP was 4 µg/ml (0.85<q<1.15). At
48 h after A549/DDP cells were treated, effects of baicalin and DDP
were additive when the concentration of DDP was 8 µg/ml
(0.85<q<1.15), and synergistic when the concentration of DDP
was 4 µg/ml (q>1.15) (Table
II). In conclusion, synergistic effects of baicalin and DDP on
proliferation of both A549 and A549/DDP cells were observed when
concentrations of baicalin and DPP were 8 and 4 µg/ml
respectively. Therefore, we used the dosages to examine tumor
invasion.
 | Table IEffects of baicalin and DDP on the
proliferation of A549 cells. |
Table I
Effects of baicalin and DDP on the
proliferation of A549 cells.
| A549 cells | Baicalin
(8a) | DDP
(1) | DDP
(2) | DDP
(4) | DDP
(8) | Baicalin (8) + DDP (1) | Baicalin (8) + DDP (2) | Baicalin (8)+ DDP (4) | Baicalin (8) + DDP (8) |
|---|
| A549 cells/24
h | | | | | | | | | |
| EA | 0.20 | | | | | | | | |
| EB | | 0.13 | 0.20 | 0.22 | 0.31 | | | | |
| EAB | | | | | | 0.10 | 0.18 | 0.28 | 0.36 |
|
q=(EAB/(EA+EB-EAxEB) | | | | | | 0.31 | 0.50 | 0.74 | 0.80 |
| A549 cells/48
h | | | | | | | | | |
| EA | 0.21 | | | | | | | | |
| EB | | 0.17 | 0.21 | 0.26 | 0.41 | | | | |
| EAB | | | | | | 0.26 | 0.29 | 0.49 | 0.50 |
|
q=(EAB/(EA+EB-EAxEB) | | | | | | 0.76 | 0.76 | 1.20b | 0.94c |
 | Table IIEffects of baicalin and DDP on the
proliferation of A549/DDP cells. |
Table II
Effects of baicalin and DDP on the
proliferation of A549/DDP cells.
| A549 cells | Baicalin
(8a) | DDP
(1) | DDP
(2) | DDP
(4) | DDP
(8) | Baicalin (8) + DDP (1) | Baicalin (8) + DDP (2) | Baicalin (8) + DDP (4) | Baicalin (8) + DDP (8) |
|---|
| A549/DDP cells/24
h | | | | | | | | | |
| EA | 0.11 | | | | | | | | |
| EB | | 0.10 | 0.12 | 0.13 | 0.21 | | | | |
| EAB | | | | | | 0.07 | 0.09 | 0.21 | 0.21 |
|
q=(EAB/(EA+EB-EAxEB) | | | | | | 0.36 | 0.40 | 0.89b | 0.73 |
| A549/DDP cells/48
h | | | | | | | | | |
| EA | 0.19 | | | | | | | | |
| EB | | 0.11 | 0.12 | 0.19 | 0.29 | | | | |
| EAB | | | | | | 0.13 | 0.24 | 0.40 | 0.41 |
|
q=(EAB/(EA+EB-EAxEB) | | | | | | 0.48 | 0.84 | 1.17c | 0.95b |
Combination of baicalin and DDP enhances
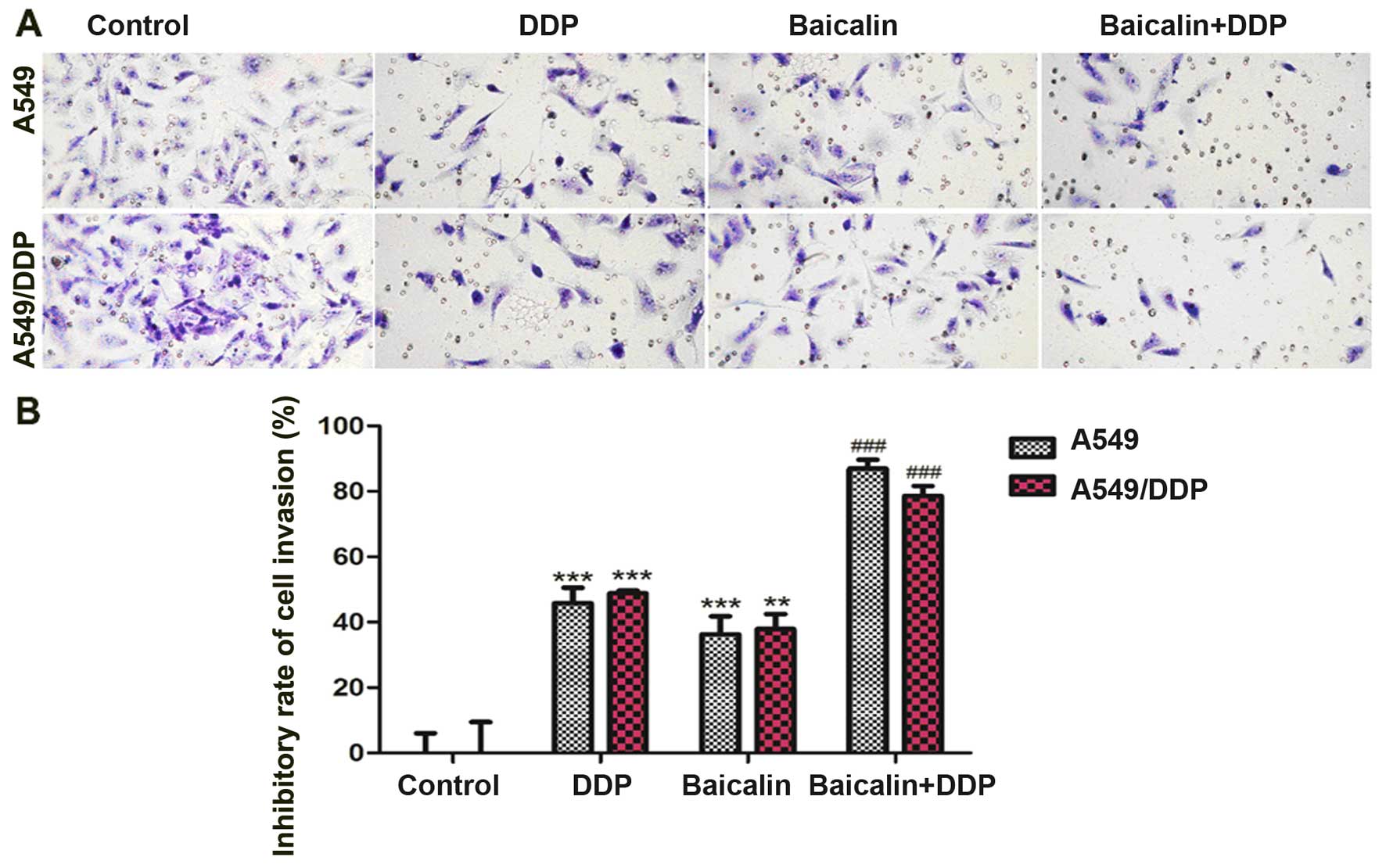
the invasion of human lung cancer cells
A549 and A549/DDP cells were treated with baicalin
(8 µg/ml), DDP (4 µg/ml), and baicalin (8
µg/ml) combined with DDP (4 µg/ml) for 48 h,
respectively. Transwell invasion assay was used to detect the
invasion of A549 and A549/DPP human lung cancer cells. When used
alone, DDP and baicalin significantly inhibited the invasion of
A549 (p<0.001) and A549/DDP cells (DDP, p<0.001; baicalin,
p<0.01) (Fig. 2) as compared to
the control group. When DDP and baicalin were combined, the
inhibitory rate increased markedly as compared to DPP or baicalin
single treatment groups (p<0.001) (Fig. 2).
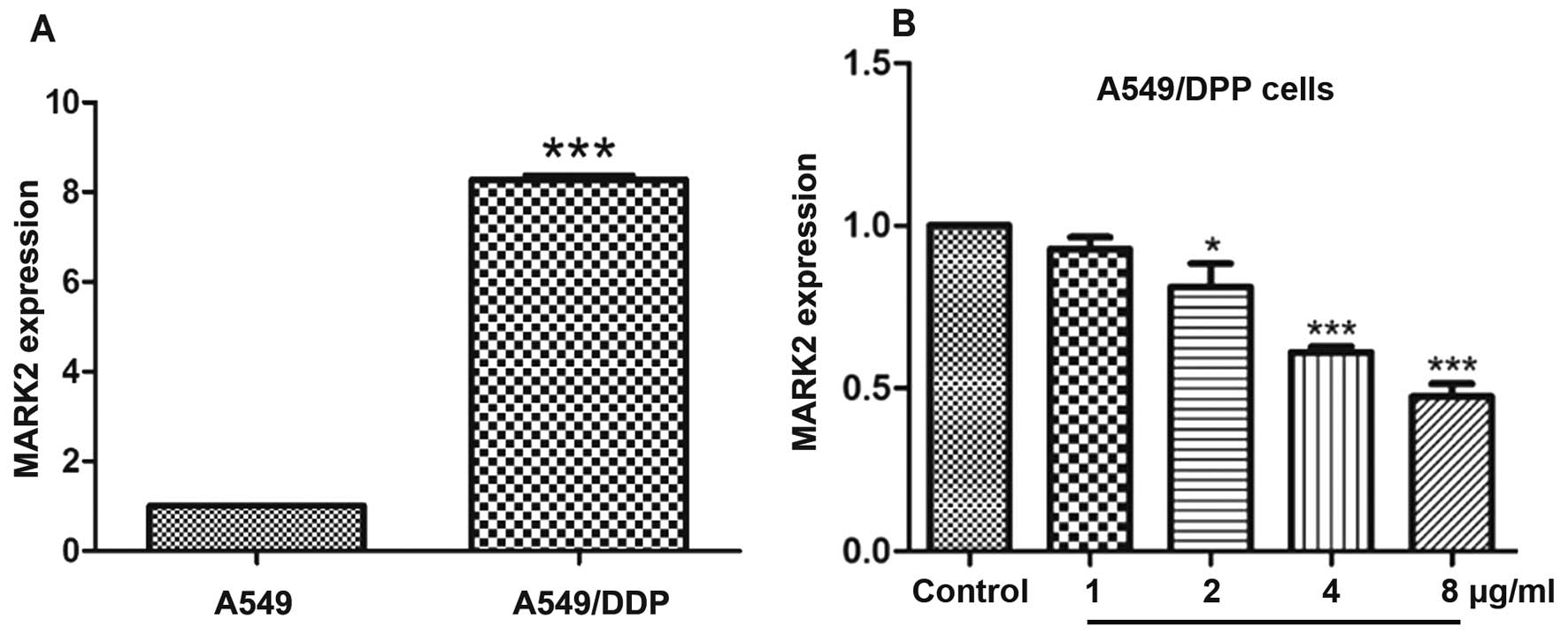
mRNA expression of MARK2 in human lung
cancer cells
The mRNA expression of MARK2 in A549 and A549/DPP
human lung cancer cells was detected by qPCR. A549/DDP cells had
markedly higher MARK2 mRNA levels compared to A549 cells
(p<0.001) (Fig. 3A). Therefore,
we chose A549/DDP cells to examine effects of different
concentrations of baicalin on MARK2 mRNA expression. Baicalin
decreased MARK2 mRNA levels in A549/DDP cells dose-dependently, and
higher doses of baicalin (2, 4 and 8 µg/ml) markedly
inhibited MARK2 mRNA expression (p<0.05, p<0.001 and
p<0.001, respectively) (Fig.
3B) when compared to the control group.
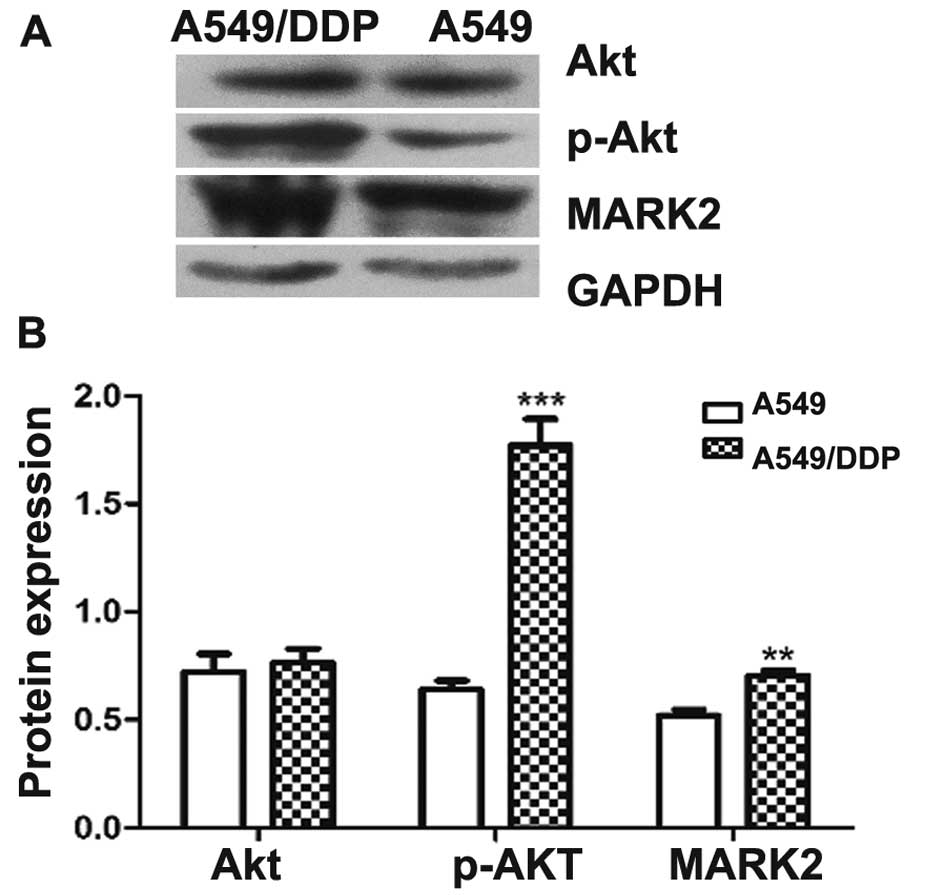
Protein expression of Akt, p-Akt and
MARK2 in human lung cancer cells
Protein expression of Akt, p-Akt and MARK2 was
detected by western blot analysis. Relative protein expression of
Akt, p-Akt and MARK2 to GAPDH was calculated. There were no
differences in Akt expression between A549 and A549/DDP cells. The
protein expression of p-Akt and MARK2 was markedly higher in
A549/DDP cells as compared to A549 cells (p-Akt: p<0.001; MARK2:
p<0.01) (Fig. 4). Therefore, we
chose A549/DDP cells to examine effects of different doses of
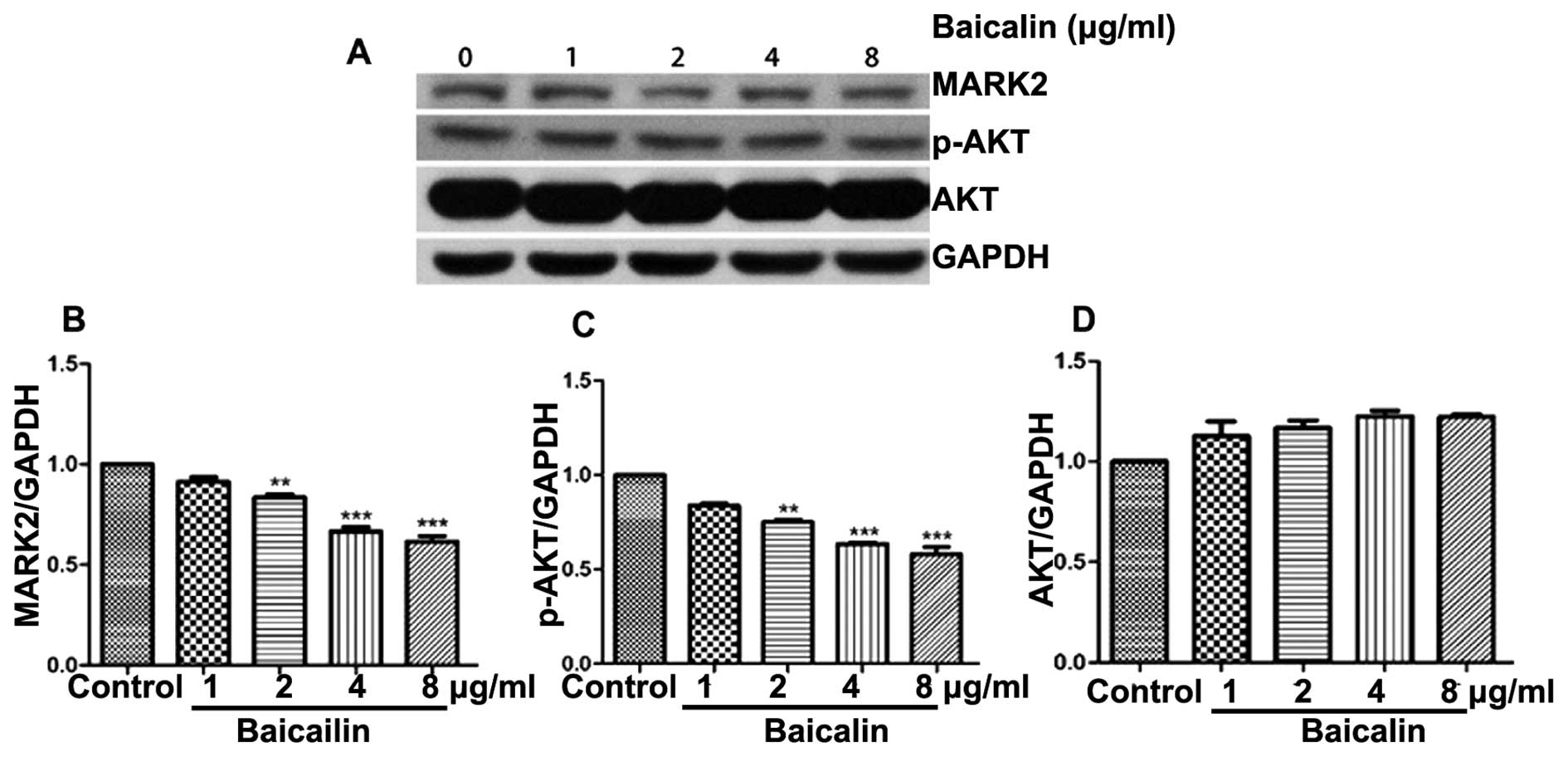
baicalin on protein expression of MARK2, p-Akt, and Akt. Baicalin
decreased protein expression of MARK2 and p-Akt in A549/DDP cells
in a dose-dependent manner, whereas it did not alter protein
expression of Akt. At higher doses (2, 4 and 8 µg/ml),
baicalin significantly inhibited protein expression of MARK2 and
p-Akt in A549/DDP cells as compared to the control group
(p<0.01, p<0.001 and p<0.001, respectively) (Fig. 5).
Discussion
We demonstrated that baicalin and DDP were
synergistic at inhibiting proliferation and invasion of human lung
cancer cells at appropriate dosages and incubation time in the
presence or absence of DDP resistance. In addition, the attenuation
of DDP resistance was associated with downregulation of MARK2 and
p-Akt.
Lung cancer is the leading cause of cancer-related
death worldwide (21). DDP was the
first member of a class of platinum-containing anticancer drugs.
These platinum complexes react in vivo and cause DNA
cross-linking, which ultimately triggers cell apoptosis (22). Like other chemotherapeutic agents,
resistance to DDP is inevitable and frequently occurs after several
cycles of treatment. DDP resistance has been reported to be
associated with mechanisms such as DNA damage/repair proteins, drug
retention such as increased influx or decreased uptake, increased
drug inactivation or prevention of drug to reach DNA target, growth
signaling via different pathways or increase in anti-apoptotic
proteins, and hypoxia-induced autophagy (23–28).
Studies have proposed measures to decrease DDP
resistance. mTOR inhibitor (CCI-779) was revealed to be able to
restore sensitivity to DDP in lung cancer (29). Inhibition of miR-196a reversed DDP
resistance of A549/DDP cell lines, which may be linked to
inhibition of drug efflux, downregulation of drug-resistant protein
expression, cell apoptosis, and suppression of cell proliferation
(30). Moreover, a fusion protein
based on two tumstatin-derived sequences named recombinant VBMDMP
(rVBMDMP) decreased cancer cell resistance to DDP in A549/DDP cell
xenograft model of nude mice (31). Epigallocatechin-3-gallate (EGCG),
the major polyphenol in green tea, was also shown to resensitize
non-small cell lung cancer cells to DDP via demethylation of
candidate genes.
Some traditional Chinese medicines were revealed to
protect cancer patients against treatment-related complications and
reduce toxicity of conventional therapy (32–34).
Baicalin, a flavone glycoside, was reported to inhibit
proliferation of malignant tumors including hepatocellular
carcinoma and glioma (16,17). However, effects of baicalin on DDP
resistance in lung cancer were unclear. The main principle of lung
cancer therapy is to induce cell death or inhibit cell survival
(35). Therefore, we explored
effects of combination of baicalin and DDP on proliferation and
invasion of human lung cancer cells.
We demonstrated in the present study that effects of
baicalin and DDP on proliferation inhibition of A549 and A549/DDP
cells were synergistic when concentrations of baicalin and DDP were
8 and 4 µg/ml at 48 h after incubation. At these dosages,
the inhibitory rate of tumor cell invasion increased significantly
compared to DPP or baicalin alone groups in both A549 and A549/DDP
cells. These findings indicate that baicalin increases the
sensitivity and decreases resistance of DDP in lung cancer cells,
no matter whether lung cancer cells already are resistant to DDP or
not. These findings provide another novel approach to decrease DDP
resistance in human lung cancer.
We then unveiled that baicalin dose-dependently
decreased expression of MARK2 and p-Akt in A549/DDP cells.
Interestingly, we showed that DDP-resistant A549 cells had
significantly higher expression of MARK2 and p-Akt as compared to
non-DDP-resistant A549 cells. Hence, the decreased expression of
MARK2 and p-Akt after baicalin treatment may be associated with
decreased DDP resistance in human lung cancer cells.
The role of MARK2 in lung cancer was recently
identified. MARK2 was shown to activate cell cycle and DNA repair.
High MARK2 expression levels correlated with resistance to DDP
(36). In addition, Akt is an
essential kinase enzyme component of the PI3K/Akt/mTOR pathway, and
is a downstream effector of PI3K (37). The PI3K/Akt/mTOR pathway is an
important intracellular signaling pathway related to cellular
quiescence, proliferation and cancer. Over-activation of the
PI3K/Akt/mTOR pathway reduces apoptosis and stimulates
proliferation, and both of these processes are involved in the
pathogenesis of cancer. Akt amplification was revealed to increase
DDP resistance in human lung cancer cells through the mTOR/p70S6K1
pathway (38). IL-6 signaling
contributed to cisplatin resistance in non-small cell lung cancer
via upregulation of anti-apoptotic molecules including Akt
(39). Meanwhile, DDP resistance
due to loss of fragile histidine triad (FHIT) was reported to be
conquered by Akt inhibitor perifosine in xenografts of non-small
cell lung cancer (28).
Furthermore, sorafenib reversed resistance of human gastric cancer
cell line to DDP through downregulating expression MDR1 and Akt
(40). As a result, baicalin is
able to decrease DDP resistance, and inhibit proliferation and
invasion of human lung cancer cells by downregulating MARK2 and
p-Akt expression.
In conclusion, we demonstrated for the first time
that baicalin and DDP were synergistic at inhibiting proliferation
and invasion of human lung cancer cells at appropriate dosages and
incubation time in the presence or absence of DDP resistance. The
attenuation of DDP resistance was associated with downregulation of
MARK2 and p-Akt. Although future research is needed to elucidate
more underlying cellular and molecular mechanisms, baicalin appears
to be a promising agent for reducing DDP resistance.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Belani CP: Chemotherapy regimens in
advanced non-small-cell lung cancer: Recent randomized trials. Clin
Lung Cancer. 3(Suppl 1): S5–S9. 2002. View Article : Google Scholar
|
|
3
|
Suehisa H and Toyooka S: Adjuvant
chemotherapy for completely resected non-small-cell lung cancer.
Acta Med Okayama. 63:223–230. 2009.PubMed/NCBI
|
|
4
|
Li CH, Cai L, Chen XS, Meng QW and Sui GJ:
DDP-sensitivity-related genes in 10 lung cancer cell lines.
Zhonghua Zhong Liu Za Zhi. 30:418–421. 2008.In Chinese. PubMed/NCBI
|
|
5
|
Shen Y, Ren M, Shi Y, Zhang Y and Cai Y:
Octreotide enhances the sensitivity of the SKOV3/DDP ovarian cancer
cell line to cisplatin chemotherapy in vitro. Exp Ther Med.
2:1171–1176. 2011.
|
|
6
|
Weng Y, Wang Y, Shi Y, Zhou W, Wang H and
Wang C: TLR9 expression and its role in chemosensitivity to DDP in
human cervical cancer cells in vitro. J Huazhong Univ Sci Technolog
Med Sci. 31:550–554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Solomides C, Parekh H, Simpkins F
and Simpkins H: Cisplatin resistance in human cervical, ovarian and
lung cancer cells. Cancer Chemother Pharmacol. 75:1217–1227. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Gao Y, Zhang K, Li C, Pan Y, Chen
J, Wang R and Chen L: MicroRNAs as regulators of cisplatin
resistance in lung cancer. Cell Physiol Biochem. 37:1869–1880.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Müller CB, De Bastiani MA, Becker M,
França FS, Branco MA, Castro MA and Klamt F: Potential crosstalk
between cofilin-1 and EGFR pathways in cisplatin resistance of
non-small-cell lung cancer. Oncotarget. 6:3531–3539. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Hui KM, Xu S, Chen Y, Wong JT and
Xue H: Two flavones from Scutellaria baicalensis Georgi and their
binding affinities to the benzodiazepine site of the GABAA receptor
complex. Pharmazie. 57:857–858. 2002.
|
|
11
|
Hui KM, Wang XH and Xue H: Interaction of
flavones from the roots of Scutellaria baicalensis with the
benzodiazepine site. Planta Med. 66:91–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Z, Wang F, Tsang SY, Ho KH, Zheng H,
Yuen CT, Chow CY and Xue H: Anxiolytic-like effect of baicalin and
its additivity with other anxiolytics. Planta Med. 72:189–192.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao JF, Hung WY and Chen CF:
Anxiolytic-like effects of baicalein and baicalin in the Vogel
conflict test in mice. Eur J Pharmacol. 464:141–146. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tarragó T, Kichik N, Claasen B, Prades R,
Teixidó M and Giralt E: Baicalin, a prodrug able to reach the CNS,
is a prolyl oligopeptidase inhibitor. Bioorg Med Chem.
16:7516–7524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et
al: Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 downregulation in human pancreatic cancer cells.
Biochim Biophys Acta. 1813:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Z, Lv J, Lei X, Li S, Zhang Y, Meng
L, Xue R and Li Z: Baicalein reduces the invasion of glioma cells
via reducing the activity of p38 signaling pathway. PLoS One.
9:e903182014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
downregulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar
|
|
18
|
Dickey CA, Koren J, Zhang YJ, Xu YF,
Jinwal UK, Birnbaum MJ, Monks B, Sun M, Cheng JQ, Patterson C, et
al: Akt and CHIP coregulate tau degradation through coordinated
interactions. Proc Natl Acad Sci USA. 105:3622–3627. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peltier J, O'Neill A and Schaffer DV:
PI3K/Akt and CREB regulate adult neural hippocampal progenitor
proliferation and differentiation. Dev Neurobiol. 67:1348–1361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin ZJ: About the evaluation of drug
combination. Acta Pharmacol Sin. 25:146–147. 2004.PubMed/NCBI
|
|
21
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Apps MG, Choi EH and Wheate NJ: The
state-of-play and future of platinum drugs. Endocr Relat Cancer.
22:R219–R233. 2015.PubMed/NCBI
|
|
23
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murata T, Haisa M, Uetsuka H, Nobuhisa T,
Ookawa T, Tabuchi Y, Shirakawa Y, Yamatsuji T, Matsuoka J,
Nishiyama M, et al: Molecular mechanism of chemoresistance to
cisplatin in ovarian cancer cell lines. Int J Mol Med. 13:865–868.
2004.PubMed/NCBI
|
|
25
|
Wu HM, Jiang ZF, Ding PS, Shao LJ and Liu
RY: Hypoxia-induced autophagy mediates cisplatin resistance in lung
cancer cells. Sci Rep. 5:122912015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Im JY, Lee KW, Won KJ, Kim BK, Ban HS,
Yoon SH, Lee YJ, Kim YJ, Song KB and Won M: DNA damage-induced
apoptosis suppressor (DDIAS), a novel target of NFATc1, is
associated with cisplatin resistance in lung cancer. Biochim
Biophys Acta. 1863:40–49. 2016. View Article : Google Scholar
|
|
27
|
Yang Y, Zhang P, Zhao Y, Yang J, Jiang G
and Fan J: Decreased MicroRNA-26a expression causes cisplatin
resistance in human non-small cell lung cancer. Cancer Biol Ther.
17:515–525. 2016. View Article : Google Scholar :
|
|
28
|
Wu DW, Lee MC, Hsu NY, Wu TC, Wu JY, Wang
YC, Cheng YW, Chen CY and Lee H: FHIT loss confers cisplatin
resistance in lung cancer via the AKT/NF-κB/Slug-mediated PUMA
reduction. Oncogene. 34:3882–3883. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu C, Wangpaichitr M, Feun L, Kuo MT,
Robles C, Lampidis T and Savaraj N: Overcoming cisplatin resistance
by mTOR inhibitor in lung cancer. Mol Cancer. 4:252005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li JH, Luo N, Zhong MZ, Xiao ZQ, Wang JX,
Yao XY, Peng Y and Cao J: Inhibition of microRNA-196a may reverse
cisplatin resistance of A549/DDP non-small-cell lung cancer cell
line. Tumour Biol. 37:2387–2394. 2016. View Article : Google Scholar
|
|
31
|
Wang CK, Zhang Y, Zhang ZJ, Qiu QW, Cao JG
and He ZM: Effects of VBMDMP on the reversal of cisplatin
resistance in human lung cancer A549/DDP cells. Oncol Rep.
33:372–382. 2015.
|
|
32
|
Li X, Yang G, Li X, Zhang Y, Yang J, Chang
J, Sun X, Zhou X, Guo Y, Xu Y, et al: Traditional Chinese medicine
in cancer care: A review of controlled clinical studies published
in Chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong J, Su SY, Wang MY and Zhan Z: Shenqi
fuzheng, an injection concocted from Chinese medicinal herbs,
combined with platinum-based chemotherapy for advanced non-small
cell lung cancer: A systematic review. J Exp Clin Cancer Res.
29:1372010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lichti-Kaiser K and Staudinger JL: The
traditional Chinese herbal remedy tian xian activates pregnane X
receptor and induces CYP3A gene expression in hepatocytes. Drug
Metab Dispos. 36:1538–1545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sui X, Chen R, Wang Z, Huang Z, Kong N,
Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and
chemotherapy resistance: A promising therapeutic target for cancer
treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hubaux R, Thu KL, Vucic EA, Pikor LA, Kung
SH, Martinez VD, Mosslemi M, Becker-Santos DD, Gazdar AF, Lam S, et
al: Microtubule affinity-regulating kinase 2 is associated with DNA
damage response and cisplatin resistance in non-small cell lung
cancer. Int J Cancer. 137:2072–2082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu LZ, Zhou XD, Qian G, Shi X, Fang J and
Jiang BH: AKT1 amplification regulates cisplatin resistance in
human lung cancer cells through the mammalian target of
rapamycin/p70S6K1 pathway. Cancer Res. 67:6325–6332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duan S, Tsai Y, Keng P and Chen Y, Lee SO
and Chen Y: IL-6 signaling contributes to cisplatin resistance in
non-small cell lung cancer via the upregulation of anti-apoptotic
and DNA repair associated molecules. Oncotarget. 6:27651–27660.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang YS, Xue Z and Zhang H: Sorafenib
reverses resistance of gastric cancer to treatment by cisplatin
through downregulating MDR1 expression. Med Oncol. 32:4702015.
View Article : Google Scholar
|