Introduction
With the development of modern industry, thyroid
cancer is becoming the most common endocrine malignancy, and its
incidence has increased by nearly 3-fold during the past several
years (1–4). Papillary thyroid cancer (PTC)
accounts for 85–90% of the predominant histological subtypes, and
>90% of patients have a 10 year survival rate (5,6).
Although the overall prognosis is good, 15–30% PTC patients will
have persistence or recurrence and 5–10% will suffer progressive,
distant metastasis, for whom 5 years of survival is only less than
half (7). As for these patients,
the available adjunctive therapies currently are often of limited
benefit (5). Therefore, it is
urgent to discover the molecular mechanisms by which PTC is
maintained and provide a new focus for the development of PTC
treatments.
The mechanisms underlying aggressive PTC continue to
be elucidated and may include tumor-mediated immune suppression
(8). Nowadays, immunotherapy has
gradually become a new therapeutic approach for patients with
recurrent or progressive PTC. There are studies showing that the
level of immunosuppressive molecules and specific patterns of tumor
infiltrating leukocytes (TIL) have predicted tumorigenesis and
recurrence in some solid malignancies (9–11).
In addition, immunotherapy regimens such as programmed cell death 1
ligand 1 (PD-L1) and IL-2 have induced remarkable durable cancer
regressions in patients with metastatic diseases, including lung,
prostate, and renal cell cancers (12,13).
It is now established that host immune cells can recognize and
eliminate malignant cells, but that abnormal antigens present in
tumors, neoplastic growths frequently evolve mechanisms to escape
immune destruction, including downregulation of antigen
recognition, the expression of immune-inhibitory ligands, and the
recruitment of suppressor cell populations (11,14,15).
In order to effectively treat cancer, immunotherapy could reverse
the tumor-driven immune dysfunction, further restore immune
responses and bring antigen specific reaction.
Long non-coding RNAs (lncRNAs) are a class of RNAs
that do not encode proteins, with length of >200 nt (16–18).
They are widely distributed in a variety of human tissues and play
an important role in physical processes, with increasing evidence
revealing that they are involved in cell-type feature and diverse
cellular events, including epigenetic regulation, gene
transcription and mRNA processing (19). LncRNAs are ubiquitous disorders in
cancers, especially resulting in aberrant proliferation, migration
and invasion, and apoptosis (20),
which contribute to the advance of human tumors and tumor outcomes
(21–23). As a kind of lncRNA, lncRNA nuclear
enrich abundant transcript 1 (NEAT1), a 4-kb lncRNA localized to
the nucleus (24), serves as a
crucial architectural component of a paraspeckle structure
(25–27). NEAT1 has been demonstrated to act
as a key role in various cancers, including acute promyelocytic
leukemia cells (28), prostate
cancer (29), breast cancer
(30), hepatocellular carcinoma
(31), ovarian carcinoma (32) and glioma (33). However, whether NEAT1 is associated
with the malignant progression of PTC remains unclear.
MicroRNAs (miRNAs, ~22 nt) are a group of small
non-coding RNAs with aberrant expression in various tumors
(34). miRNAs, as a major class of
well characterized, conserved and endogenous small interfering RNA
that regulate gene expression (35). It has been indicated that miRNAs
are involved in diverse biological processes, such as cell growth,
migration, apoptosis and differentiation through mainly binding to
the 3′-untranslated region (3′-UTR) of their target genes (34,36).
In addition, miRNAs can function as either oncogenes or tumor
suppressor genes via regulation of cell proliferation or cell death
(37). miRNA-214 also plays a
vital role as a tumor inhibitor by downregulating oncogenes, such
as GALNT7, Bcl2l2 and TFAM in cervical cancer (38–40)
and FGF-1 and ARL-2 in colorectal cancer (41,42).
However, miRNA-214 is highly expressed in melanoma (43), gastric cancers (44) and ovarian cancer (45), which suggesting that it may
function as an carcinogenic gene as well. These results are,
however, contradictory as the same miRNA may act as a carcinogen in
one cancer type and as a suppressor in another cancer. Therefore,
the expression and function of miRNA-214 in PTC need further
investigation.
In the present study, we sought to determine the
expression and function of NEAT1 and miRNA-214 in thyroid tumor
tissue and TPC-1 thyroid cancer cells. We also investigated the
interactions among them in the regulation of PTC malignant behavior
and the potential molecular pathways involved.
Materials and methods
Tissue collection
All the samples were collected from patients who had
undergone surgery and were diagnosed with thyroid cancer based on
pathological evaluation at the First Affiliated Hospital of
Zhengzhou University. Informed consent was gathered from patients,
and the research method was approved by the Ethics Committee of the
First Affiliated Hospital of Zhengzhou University. No local or
systemic treatment had been conducted in patients before the
operation. All the specimens were immediately snap-frozen and
preserved in liquid nitrogen until use in this study.
Cell lines and culture conditions
Human circulating blood monocytes (CBMs) and
tumor-associated macrophages (TAMs) were isolated from thyroid
cancer patients. Bone marrow derived macrophages (BMDMs) and
macrophages (RAW 264.7) were purchased from Shanghai Enzyme
Research Biotechnology Co., Ltd. (Shanghai, China). Thyroid cancer
cell TPC-1 was purchased from the ScienCell Research Laboratories
(Carlsbad, CA, USA). They were maintained in low-glucose Dulbecco's
modified Eagle's medium (DMEM-L) (Life Technologies Corp.,
Carlsbad, CA, USA) containing 10% FBS and 100 U/ml penicillin and
100 µg/ml streptomycin antibiotics in a humidified
atmosphere of 5% CO2 at 37°C.
Cell transfection
The NEAT1 knockdown (si-NEAT1) plasmid and the NEAT1
overexpression (Ad-NEAT1) plasmid, as well as the respective
non-targeting sequence (negative control, si-control and Ad-GFP)
were synthesized by GenePharma Co. (Shanghai, China). Cells were
transient transfected through the use of Lipofectamine 2000
transfection reagent (Life Technologies Corp., Shanghai, China)
according to the manufacturer's instructions when cells were at
50–70% confluence. The transfection efficiency was verified by
qRT-PCR analysis.
RNA extraction and qRT-PCR
Total RNA was extracted from clinical specimens and
cells with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. A One-Step SYBR Prime
Script RT-PCR kit was used for qRT-PCR. cDNA was generated from
miRNA with a TaqMan miRNA reverse transcription kit (Applied
Biosystems, Foster City, CA, USA). TaqMan Universal Master Mix II
was used to perform TaqMan miRNA assays for target genes on the ABI
7500 Fast Real-Time PCR system (Applied Biosystems). GAPDH and U6
were used as endogenous controls, respectively. The relative
expression level was calculated using the 2−ΔΔCt method.
The formula and its derivations were obtained from the ABI Prism
7300 sequence detection system user guide. Statistical analysis was
performed on the fold-change.
Western blotting
Total proteins were extracted from cells with RIPA
buffer containing protease inhibitors (Beyotime Institute of
Biotechnology) on ice, and these protein concentrations were
quantified by Bradford's method according to the manufacturer's
protocol. Samples were then subjected to SDS-PAGE and transferred
to PVDF membranes by semidry electroblotting. After non-specific
binding was blocked with 5% nonfat milk at room temperature,
membranes were incubated with primary antibodies anti-NEAT1 and
anti-ACTB (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Then, membranes were incubated with HRP-conjugated secondary
antibodies (Santa Cruz Biotechnology, Inc.) at room temperature.
Bands detected using the enhanced chemiluminescence kit (ECL kit;
Santa Cruz Biotechnology, Inc.) and analyzed by densitometry (Image
Lab; Bio-Rad Laboratories, Hercules, CA, USA). ACTB was used as an
internal control.
Cell survival and viability assay
After transfection efficacy was confirmed, cells
were dissociated with Accutase® Cell Dissociation buffer
(Life Technologies Corp.) resuspended and seeded in 96-well
micro-hole plates at 1.0×103 cells/well. Volume of 10
µl CCK-8 was added to each well at the time of harvest,
according to the manufacturer's instructions. The plate was
incubated for 2 h at 37°C and then recorded cell survival and
viability by measuring the absorbance of the converted dye at 450
nm on a SpectraMax M5 microplate reader (Molecular Devices,
Sunnyvale, CA, USA).
Cell migration and invasion assay
To measure cell migration, 8-mm pore size culture
inserts (Transwell; Costar, Cambridge, UK) were placed into the
24-well culture plates, separating the upper and the lower
chambers. In the lower chamber, DMEM containing 10% FBS was added.
Serum-free medium containing 5×104 cells were placed to
the upper chamber for migration and invasion assay. After
incubation at 37°C for 48 h, the cells on the upper membrane
surface were scraped off. The cells on the lower side of the member
were fixed and then stained with 0.1% crystal violet. The number of
cells that had migrated through the pores was quantified by
counting 10 independent visual fields under the microscope for
statistics. At magnification ×20. Each experiment was performed at
least 3 times.
RNA pull-down assay
NEAT1 and its antisense RNA were in vitro
transcribed and biotin-labeled using a biotin RNA labeling mix and
T7/SP6 RNA polymerase, treated with RNase-free DNase I and purified
using an RNeasy mini kit (Qiagen, Valencia, CA, USA) to detect
Argonaute 2 (Ago2) expression by western blotting. All these
procedures were according to the manufacturer's protocol. One
milligram of protein from cell extracts was mixed with 50 pmol
biotin labeled RNA, incubated with streptavidin-agarose beads, and
washed 3 times with NaCl/Pi at room temperature. The retrieved
proteins were detected using a standard western blotting
technique.
Tumor xenografts
Four-week-old nude C57BL/6 mice were purchased from
Henan Research Center of Laboratory Animals (Zhengzhou, China).
These mice were given free access to sterile food and water during
the experiment process. All animal experiments with nude mice were
performed strictly in accordance with a protocol approved by Henan
Research Center of Laboratory Animal. To establish thyroid cancer
xenograft model. The mice were subcutaneously injected with
5×104 TPC-1 cells (n=6 in each group). After 8 days of
transplantation, the transplanted nude mice were divided into two
groups: si-control, and si-NEAT1. Si-control or si-NEAT1 was
directly injected into the implanted tumors per mouse every 4 days
for 4 times. The subcutaneous tumor-bearing mice were sacrificed 2
weeks after injection. The tumor volume was calculated by the
formula: volume (mm3) = length ×
width2/2.
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). All experimental results were statistically analyzed with
Student's t-test or one-way analysis of variance (ANOVA). All
statistical analyses were performed with SPSS 18.0 statistical
software. A value of P<0.05 was considered to indicate
statistically significant difference.
Results
NEAT1 is upregulated while miR-214 is
downregulated in thyroid cancer cell TAMs
As previously reported, NEAT1 was upregulated in
glioblastoma tissues (46).
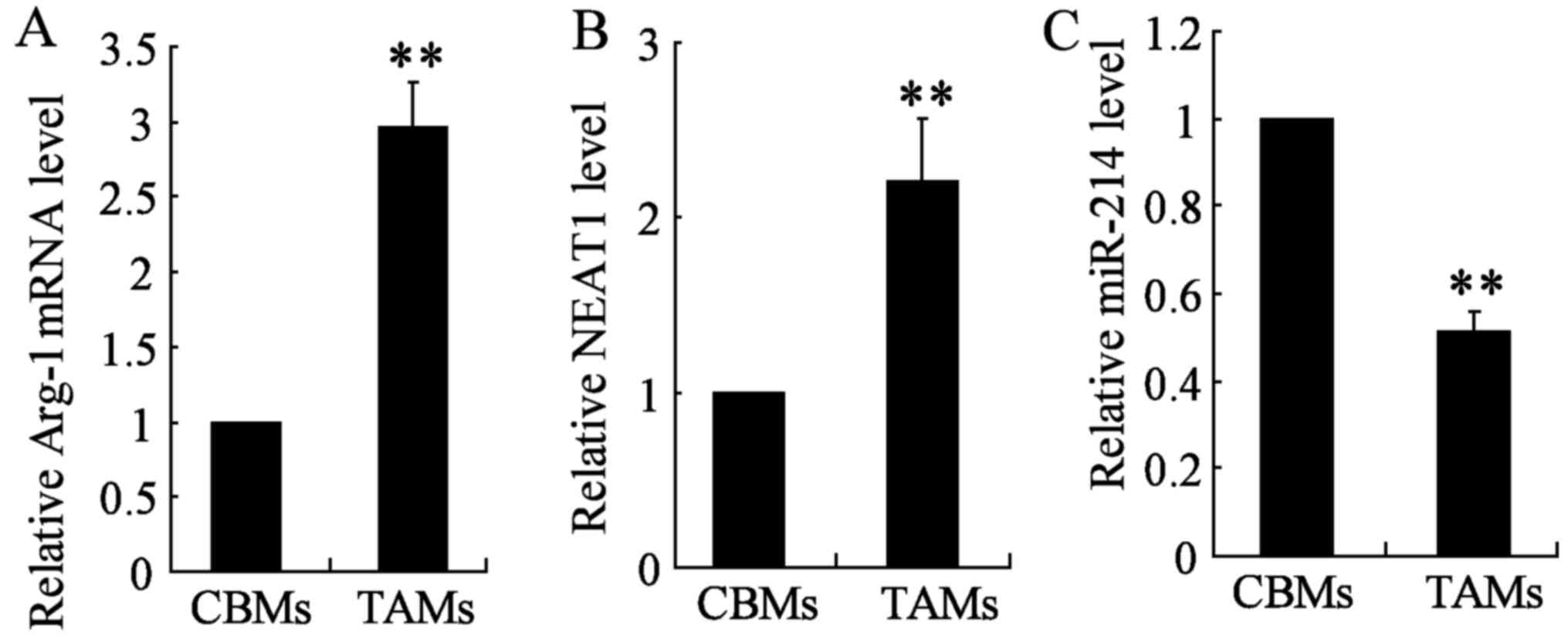
Similarly, we found NEAT1 was upregulated in TAM thyroid cancer
cell line TAMs compared with CBMs (Fig. 1B). In addition, quantitative
real-time PCR (qRT-PCR) was conducted to determine the expression
of Arg-1 and miR-214 in the two cell types TAM and CBM. Arg-1
expression was significantly upregulated in TAM cells, however,
miR-214 expression was significantly lower in TAM cell lines as
compared with those of CBMs (Fig. 1A
and C). These results suggested that NEAT1 promotes the
occurrence of tumor in TAMs, while miR-214 functions as a tumor
suppressor.
NEAT1 is upregulated while miR-214 is
downregulated in BMDMs treated with IL-4
To further explore the oncogenic properties and
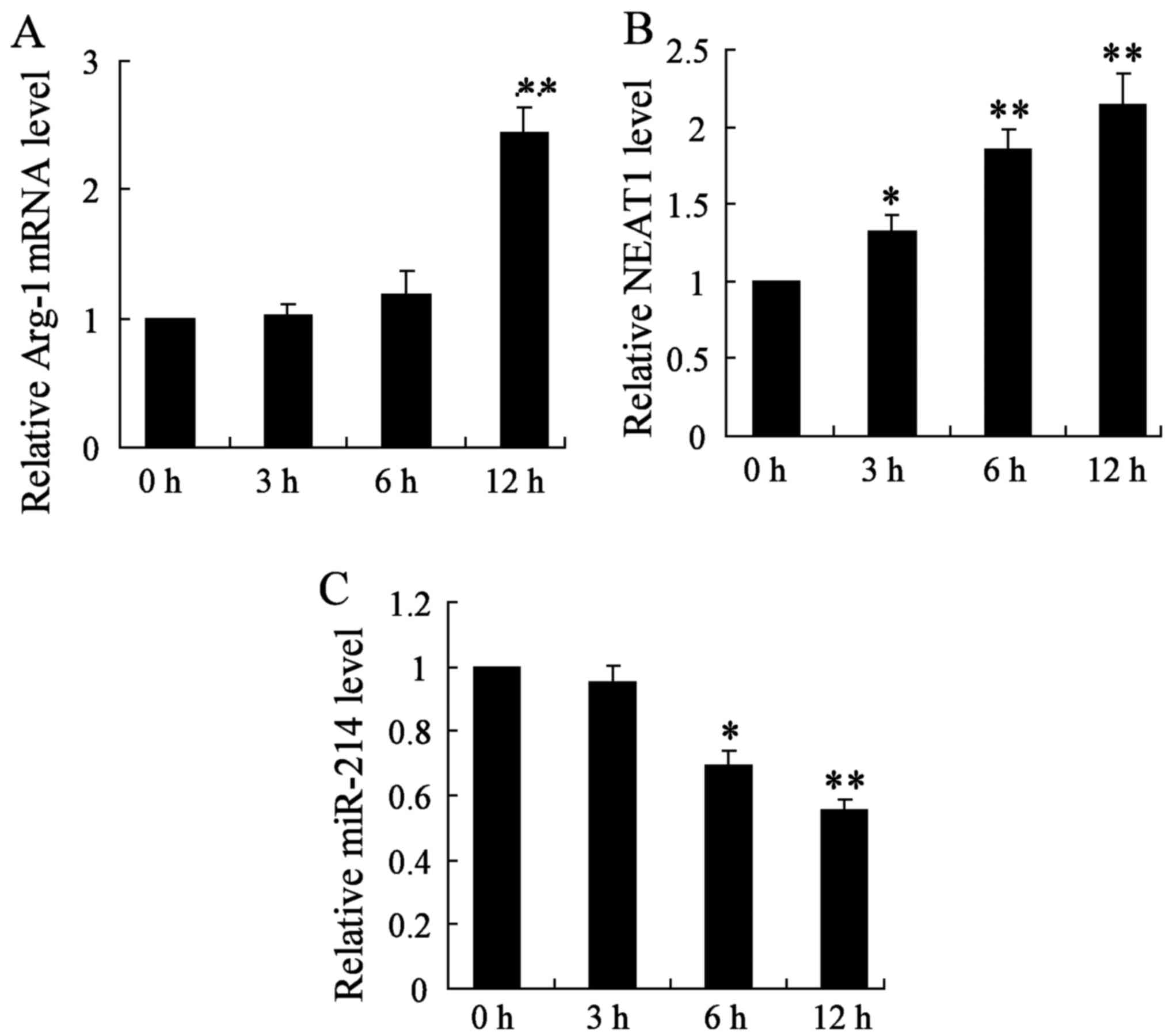
roles of NEAT1 on thyroid cancer in vitro, we established
thyroid cancer cell lines (BMDMs treated with 20 ng/ml IL-4), and
detected the mRNA expression of Arg-1, NEAT1 and miR-214 genes at
3, 6 and 12 h, respectively. In this study, we found that the
relative Arg-1 mRNA level was significantly increased after 12 h of
treatment in thyroid cancer cell lines (Fig. 2A). Moreover, qRT-PCR analysis for
the expression of NEAT1 showed that NEAT1 was obviously increased
in BMDM cells at 3, 6 and 12 h (Fig.
2B). On the contrary, miR-214 gene expression was significantly
decreased with 6 and 12 h treatment (Fig. 2C). The change of the above three
parameters all showed a time-dependent manner. These data
demonstrate that the upregulation of NEAT1 may play important roles
on thyroid cancer development and progression.
Transfection with si-NEAT1/Ad-NEAT1
attenuates/increases IL-4 induced Arg-1 expression in BMDMs and RAW
264.7 cells
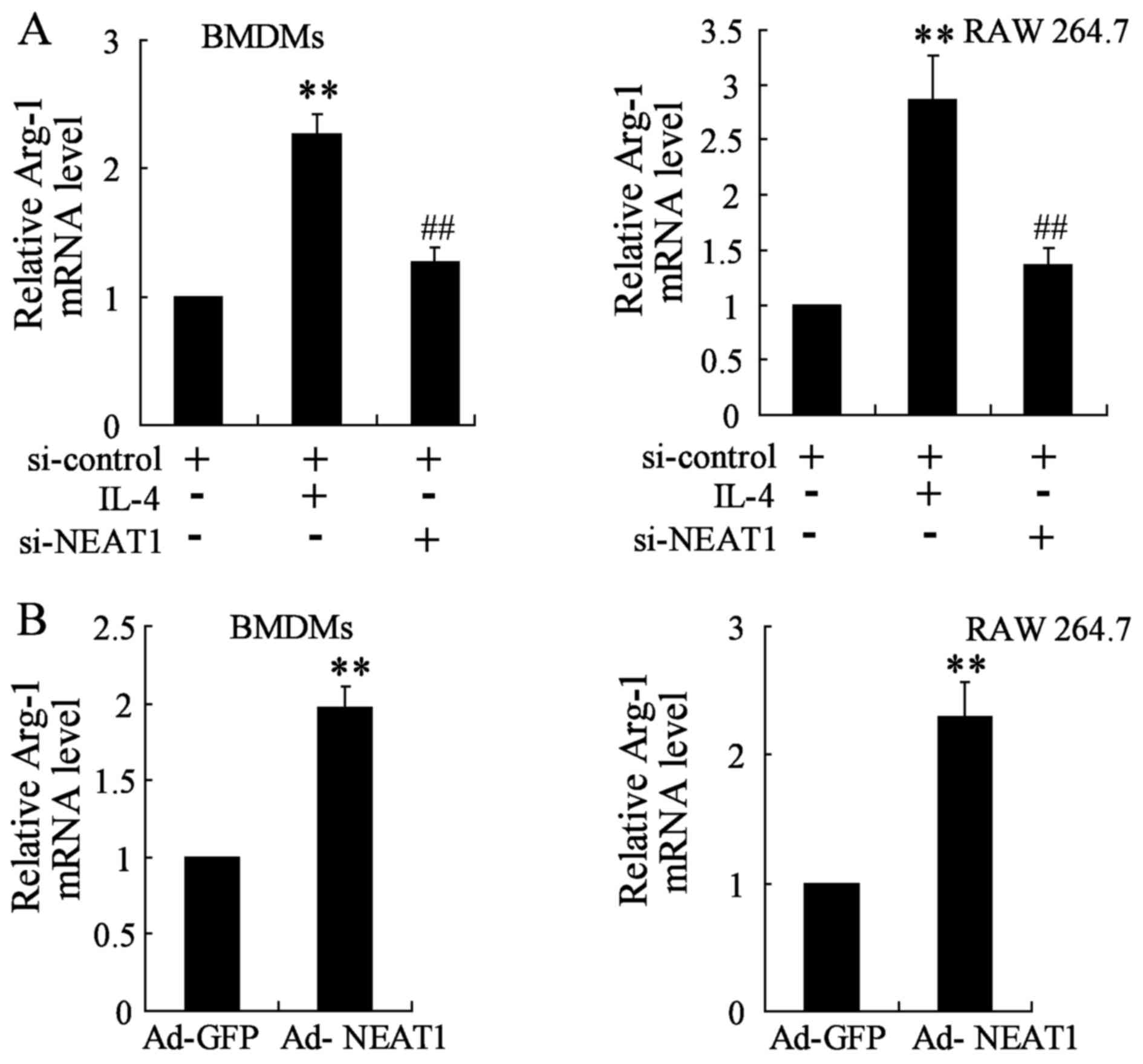
The expression of Arg-1 was assessed by qRT-PCR. As
mentioned above, NEAT1 expression was upregulated under IL-4
treatment, enforced NEAT1 silence was firstly performed and
analyzed by transfecting si-NEAT1 that targets NEAT1 transcript
into BMDMs and RAW 264.7 cells. Analysis for Arg-1 gene level
demonstrated to be strongly attenuated in NEAT1 silencing thyroid
cancer cell lines close to a basal expression level (Fig. 3A). Moreover, thyroid cancer cell
lines were transfected with Ad-NEAT1 to overexpress NEAT1. The
expression of Arg-1 was markedly promoted in BMDMs and RAW 264.7
cells transfected with Ad-NEAT1 compared to that in the control
group (Fig. 3B).
Transfection with si-NEAT1/Ad-NEAT1
reverses/decreases IL-4 induced miR-214 and β-catenin expression in
BMDMs and RAW 264.7 cells
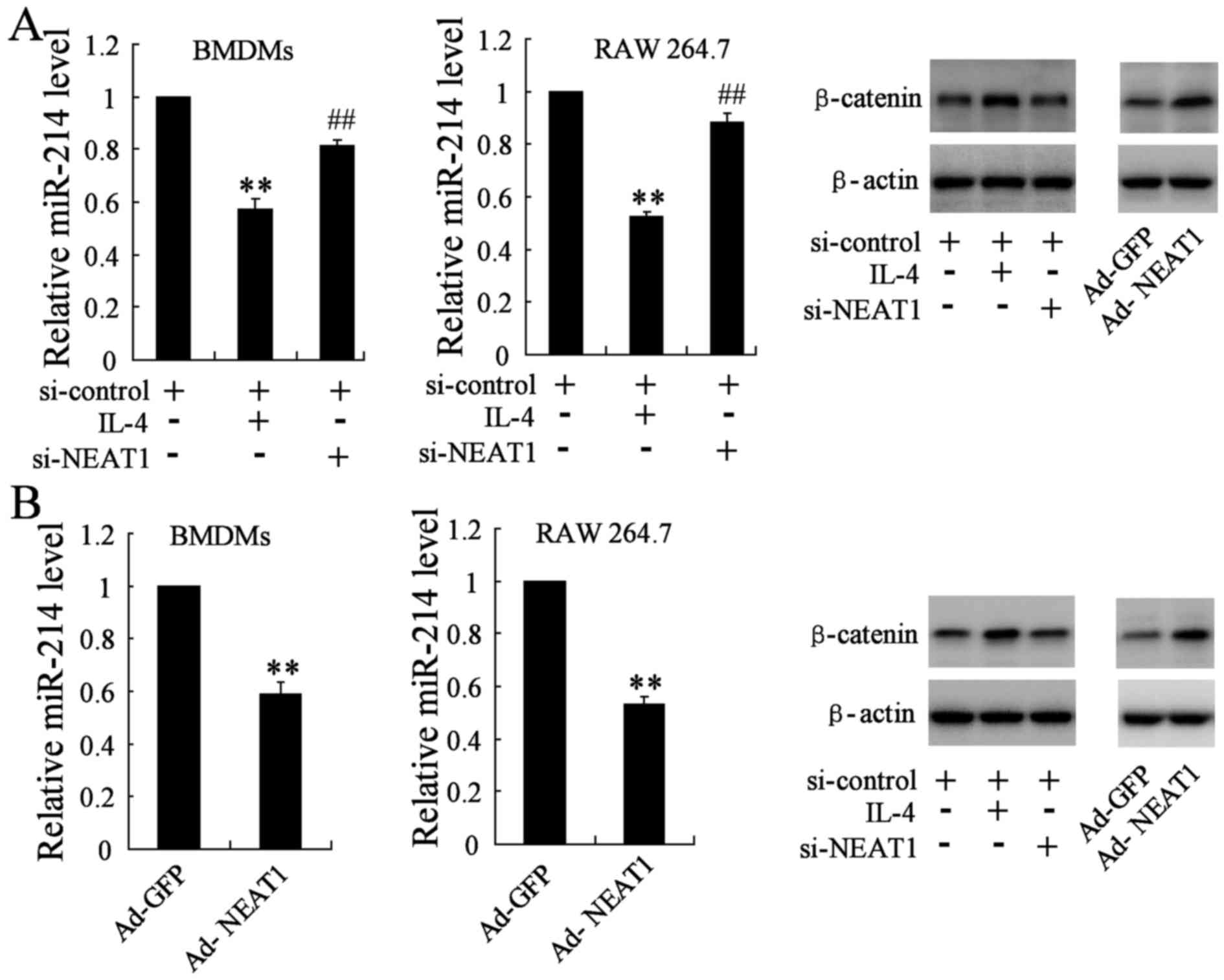
Similarly, in order to determine the effect of
si-NEAT1/Ad-NEAT1 on the expression of miR-214 and β-catenin, we
divided cells into three groups: si-control group (transfected with
si-control plasmid), si-NEAT1 group (transfected with si-control
plasmid and the si-NEAT1 plasmid) and the IL-4 group (transfected
with si-control plasmid and 20 ng/ml IL-4). The qRT-PCR assay
indicated that miR-214 gene level was reduced in IL-4 group, while
it was obviously reversed in NEAT1 silencing BMDMs and RAW 264.7
cells. β-catenin, as the direct target molecule of miR-214, was
also determined by western blotting. The protein level of β-catenin
was remarkably downregulated in BMDMs and RAW 264.7 cells
transfected with si-NEAT1 when compared with the IL-4 group
(Fig. 4A). BMDMs and RAW 264.7
were simultaneously transfected with the Ad-NEAT1 plasmid, the
change of miR-214 level and β-catenin expression was analyzed
accordingly, opposite results were observed in treated groups
(Fig. 4B).
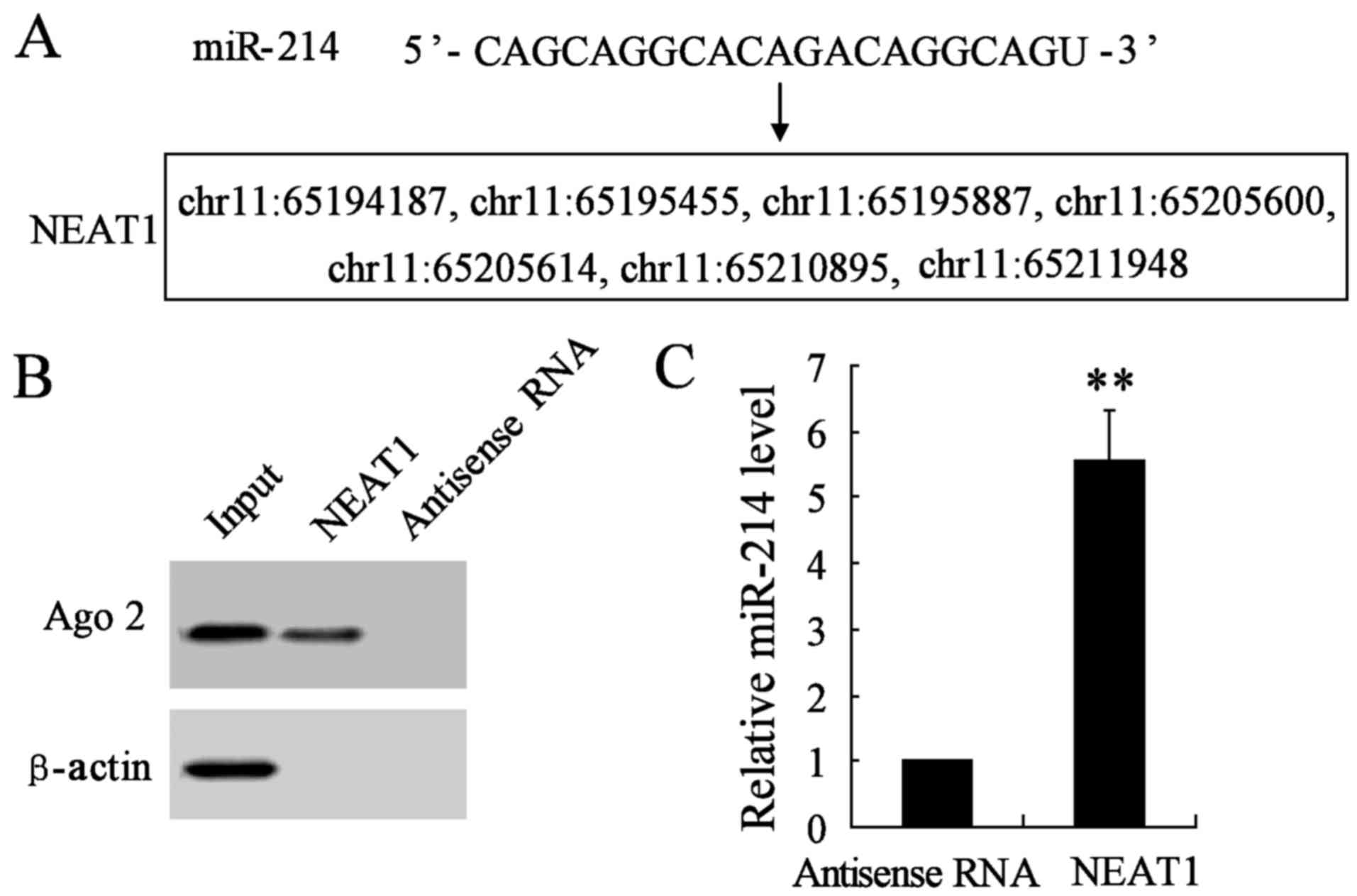
NEAT1 is a direct target of miR-214 in
thyroid cancer cell lines
Increasing evidence has demonstrated that lncRNAs
could act as a molecular sponge or a competition endogenous RNA
(ceRNA) in regulating the accumulation of miRNA and in turn
affecting its biological functions. Using a bioinformatics database
(StarBase), we determined that NEAT1 harbors seven putative binding
sites for miR-214 (Fig. 5A).
Furthermore, an RNA pull-down experiment was conducted to determine
whether NEAT1 and Ago2 were in the expected RNA-induced silencing
complex (RISC). A significant enrichment was found of Ago2 in the
presence of NEAT1 compared with antisense RNA (negative control)
(Fig. 5B). In addition, the
miR-214 gene expression was remarkably promoted in NEAT1 interfered
group compared with antisense RNA group (Fig. 5C). These results revealed miR-214
could directly bind to NEAT1 at the miRNA recognition site.
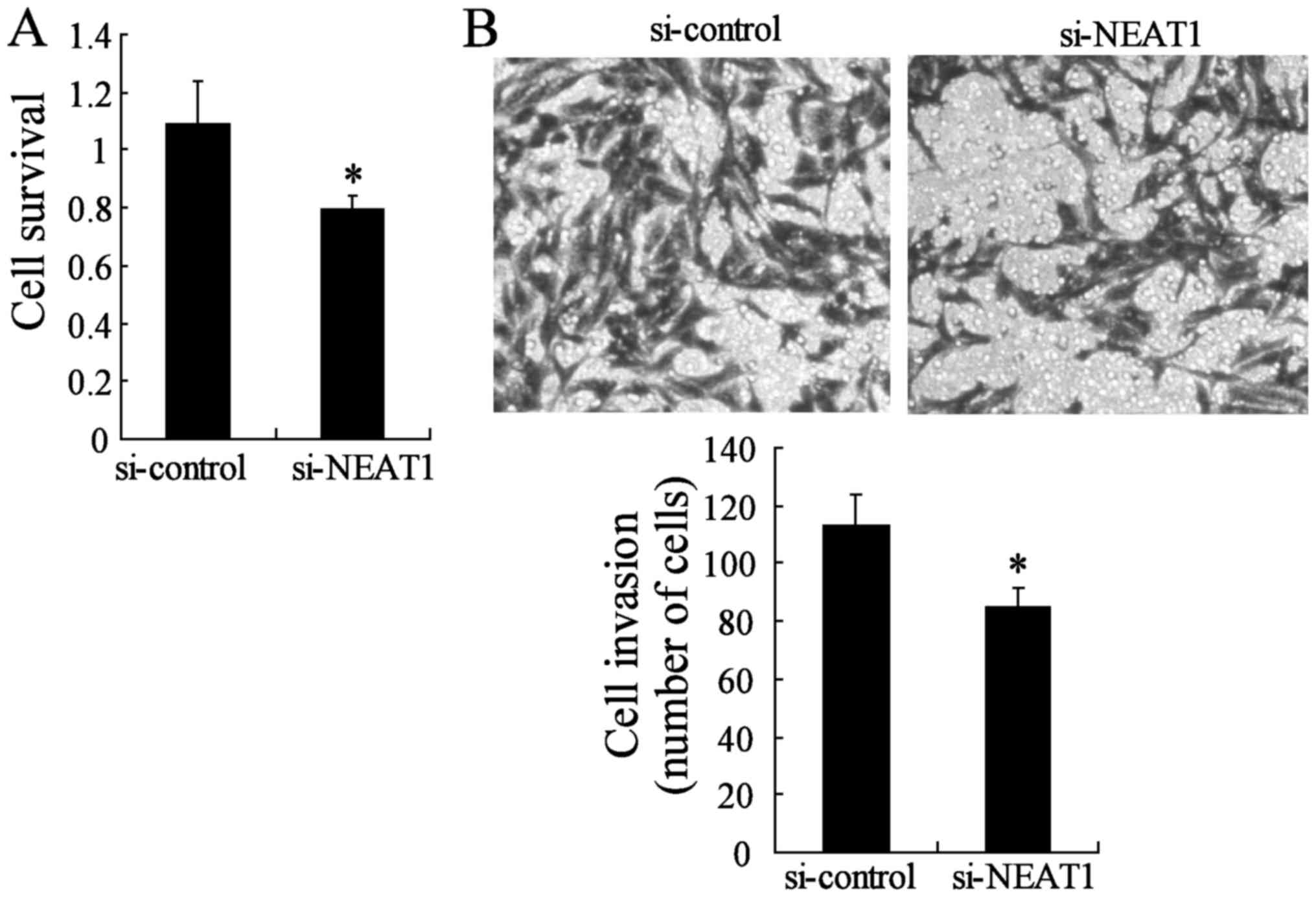
Knockdown of NEAT1 impairs the malignant
progression of thyroid papillary carcinoma-1
To investigate whether the NEAT1 silencing can block
cell survival and invasion, we used two different approaches to
evaluate the role of NEAT1 on TPC-1 cell malignant progression. The
CCK-8 assay indicated that TPC-1 survival was lower in the si-NEAT1
group than in the si-control group (Fig. 6A). We also evaluated cancer cell
migration and invasion through Transwell assays. The migration and
invasion of TPC-1 were also significantly impeded in the
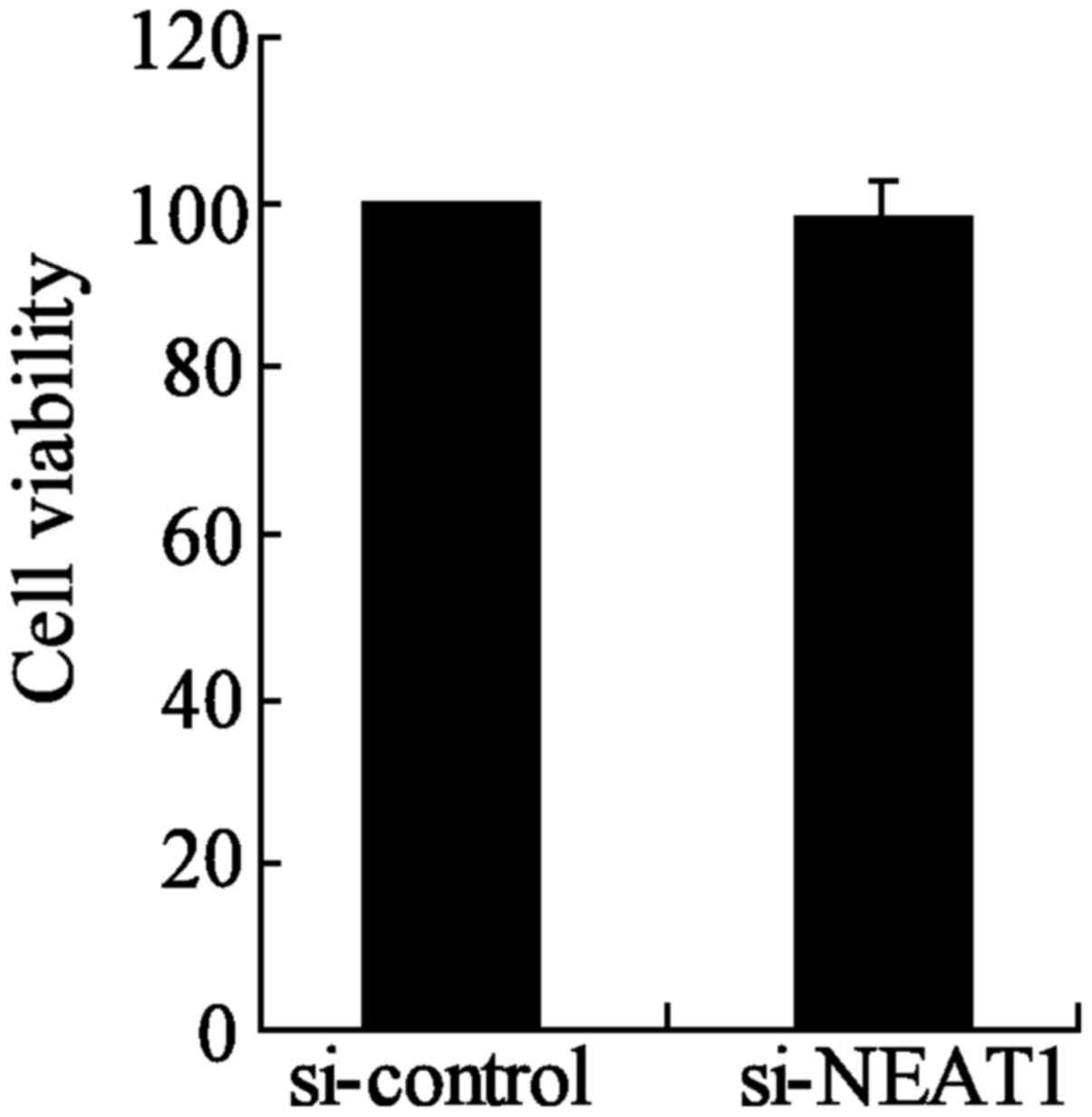
NEAT1-knockdown group compared to the si-control group (Fig. 6B). Moreover, the inhibition of
NEAT1 had no significant effect on the cell viability of TPC-1
cells between si-NEAT1 and si-control groups (Fig. 7). These results indicated that
NEAT1 may act as a oncogene in TPC-1 cells.
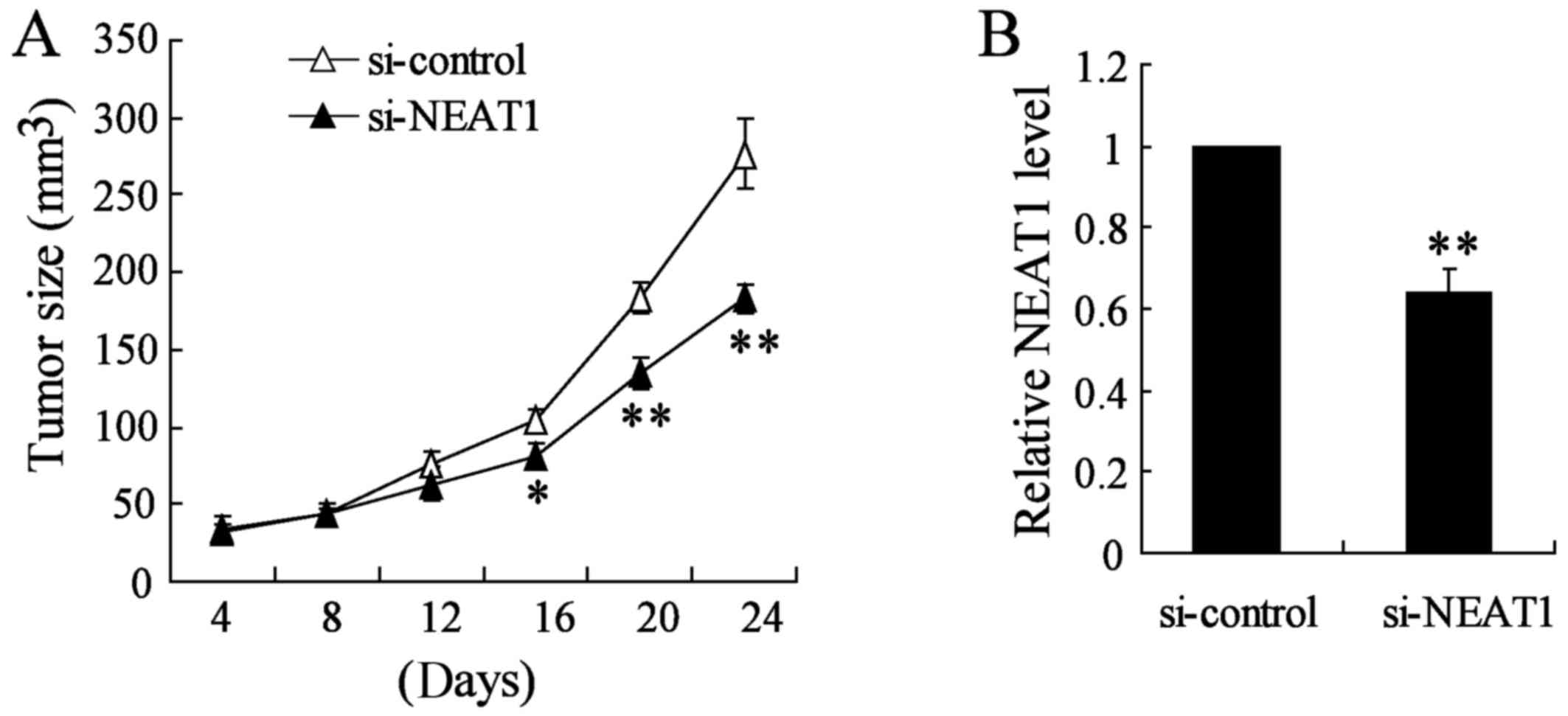
Knockdown of NEAT1 inhibits thyroid tumor
growth in vivo
To verify the effects of NEAT1 on tumorigenesis
in vivo, TPC-1 cells or appropriate control cells were
subcutaneously injected into nude mice. Si-NEAT1 was directly
injected into the implanted tumor at the 8th day after injection
every 4 days. Knockout of NEAT1 significantly decreased tumor size
and tumor growth in vivo compared with si-control group
(Fig. 8A). Using qRT-PCR analysis,
we confirmed the NEAT1 knockout in the xenograft tumors generated
from thyroid cancer TAM cells (Fig.
8B). Taken together, these results demonstrated that NEAT1
plays a crucial role in thyroid cancer TAM cell progression.
Discussion
Previous studies have explored the functional roles
of lncRNAs (18,47,48),
and indicated new insights into the underlying molecular mechanisms
by which lncRNAs take effect in various human cancers (49–51).
However, the mechanisms of NEAT1 in thyroid cancer have not been
thoroughly revealed. In the present study, we provided new evidence
that highly expressed NEAT1 in thyroid cancer cells acted as an
oncogenic biomarker. NEAT1 was found to be a direct target of
miR-214 and there was a negative correlation between them. NEAT1
served as an oncogene to promote tumor process partiallu due to its
ability to depress the expression of miR-214. Hence, our findings
may help to improve the literature supporting the importance of
lncRNA species in cancer treatment.
In this study, we demonstrated that NEAT1 was
upregulated in thyroid cancer cells. NEAT1 inhibition impaired the
malignant process of thyroid papillary carcinoma-1 and attenuated
β-catenin expression. On the contrary, miR-214 expression was
downregulated in thyroid cancer TAMs, BMDMs and RAW 264.7 cells.
Moreover, miR-214 was found to bind to NEAT1 in a specific manner,
and showed a reciprocal repression correlation. β-catenin was
considered to be a direct target of miR-214 and was involved in the
NEAT1-induced malignant behavior of thyroid cancer. Collectively,
our observations indicated NEAT1 may serve as an oncogene and play
an important role in thyroid cancer initiation, development and
progression.
Emerging evidence presented that lncRNAs are
abnormally expressed in various cancers (46). Because they are involved in the
occurrence and progression of cancer, lncRNAs could be used as
diagnostic or prognostic markers and potential therapeutic targets.
Previous study indicated that NEAT1 was upregulated in glioma, and
promoted cell proliferation, migration and invasion while
inhibiting apoptosis in glioma cell lines (33). Similar findings were observed in
this study, knockout NEAT1 suppressed the malignant progression of
thyroid papillary carcinoma-1 cells. In addition, we also further
determined the expression of β-catenin and found that NEAT1
knockout significantly reduced β-catenin level in thyroid cancer
cells. However, whether β-catenin is also involved in the
NEAT1-induced enhancement of thyroid cancer progression needs to be
further investigated.
So far, NEAT1 has been considered to act as a cancer
inducing gene, but the deep mechanism by which NEAT1-regulated gene
expression remains to be clarified (52). It has been demonstrated that NEAT1
played an important role in numerous biological processes,
including cellular differentiation and stress response through
paraspeckles pathway (53).
Evaluation of the effect of NEAT1 on transcriptional regulation by
sequestering SFPQ from the RNA-specific adenosine deaminase, RNA
specific B2 (ADARB2) gene in response to proteasome inhibition
effect has been reported (54). In
the present study, we intended to discover another underlying
molecular mechanism of NEAT1 on thyroid cancer progression, that
is, functioning as 'molecular sponges' to regulate miRNAs. A
previous study proved that in a variety of cell processes, lncRNAs
played a vital role through acting as ceRNAs to mediate the miRNAs
(17). Various lncRNAs have been
investigated in cancer research, including lncRNA GAS5 (54) and CCAT1 (55). In this study, we evaluated the
effect ofNEAT1 on thyroid cancer cells and discovered that NEAT1
participated in the development of ceRNA regulatory networks and
acted as an endogenous miRNA sponge, binding to miR-214 and
regulating its function. There are studies indicating miR-214 tumor
inhibitory effect in various acute or chronic diseases. Study of
Xiong et al found that downregulation of miR-214 induced G1
cell cycle arrest in gastric cancer cells by upregulating PTEN
(56). Yang et al reported
that miR-214 induces cell survival and cisplatin resistance through
targeting the 3′-UTR of PTEN (45). miR-214 was downregulated in
cervical cancer tissue and could negatively regulate HeLa cell
growth (38,57), while its role on thyroid cancer
cells have not been investigated. In this experiment, we found that
miR-214 expresson was low in thyroid cancer cells. In addition, the
expression of NEAT1 and miR-214 showed a significantly negative
correlation in thyroid cancer cell lines. Knockout of NEAT1
remarkably increased miR-214 expression, while overexpression of
NEAT1 decreased its expression. Moreover, a pull-down assay showed
NEAT1 could pull-down miR-214.
In conclusion, our results indicate that highly
expressed NEAT1 is an oncogenic lncRNA that promotes tumorigenesis
and progression of thyroid cancer through regulating the expression
of miR-214. The significance of the correlation among NEAT1,
miR-214 and thyroid cancer was highlighted for the first time.
Thus, NEAT1 could be a useful marker and the combination of
NEAT1/miR-214 may be a promising option for the treatment of human
thyroid cancer.
Acknowledgments
We would like to thank all the members participated
in the research. This study was supported by Henan Provincial
Science and Technology Department of Foundation and Frontier
Technology Research Fund (No. 162300410114).
References
|
1
|
Amphlett B, Lawson Z, Abdulrahman GO Jr,
White C, Bailey R, Premawardhana LD and Okosieme OE: Recent trends
in the incidence, geographical distribution, and survival from
thyroid cancer in Wales, 1985–2010. Thyroid. 23:1470–1478. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen AY, Jemal A and Ward EM: Increasing
incidence of differentiated thyroid cancer in the United States,
1988–2005. Cancer. 115:3801–3807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F,
Schlumberger M, et al American Thyroid Association (ATA) Guidelines
Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer:
Revised American Thyroid Association management guidelines for
patients with thyroid nodules and differentiated thyroid cancer.
Thyroid. 19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hay ID, Thompson GB, Grant CS, Bergstralh
EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL,
et al: Papillary thyroid carcinoma managed at the Mayo Clinic
during six decades (1940–1999): Temporal trends in initial therapy
and long-term outcome in 2444 consecutively treated patients. World
J Surg. 26:879–885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grebe SK and Hay ID: Thyroid cancer nodal
metastases: Biologic significance and therapeutic considerations.
Surg Oncol Clin N Am. 5:43–63. 1996.PubMed/NCBI
|
|
8
|
Angell TE, Lechner MG, Jang JK, Correa AJ,
LoPresti JS and Epstein AL: BRAF V600E in papillary thyroid
carcinoma is associated with increased programmed death ligand 1
expression and suppressive immune cell infiltration. Thyroid.
24:1385–1393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galon J, Pagès F, Marincola FM, Thurin M,
Trinchieri G, Fox BA, Gajewski TF and Ascierto PA: The immune score
as a new possible approach for the classification of cancer. J
Transl Med. 10:12012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Russell S, Angell T, Lechner M, Liebertz
D, Correa A, Sinha U, Kokot N and Epstein A: Immune cell
infiltration patterns and survival in head and neck squamous cell
carcinoma. Head Neck Oncol. 5:242013.PubMed/NCBI
|
|
11
|
Stewart TJ and Abrams SI: How tumours
escape mass destruction. Oncogene. 27:5894–5903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McDermott DF: Improving the therapeutic
index of IL-2. Clin Adv Hematol Oncol. 8:862–864. 2010.
|
|
13
|
Shablak A, Sikand K, Shanks JH,
Thistlethwaite F, Spencer-Shaw A and Hawkins RE: High-dose
interleukin-2 can produce a high rate of response and durable
remissions in appropriately selected patients with metastatic renal
cancer. J Immunother. 34:107–112. 2011. View Article : Google Scholar
|
|
14
|
Lechner MG, Russell SM, Bass RS and
Epstein AL: Chemokines, costimulatory molecules and fusion proteins
for the immunotherapy of solid tumors. Immunotherapy. 3:1317–1340.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lechner MG, Karimi SS, Barry-Holson K,
Angell TE, Murphy KA, Church CH, Ohlfest JR, Hu P and Epstein AL:
Immunogenicity of murine solid tumor models as a defining feature
of in vivo behavior and response to immunotherapy. J Immunother.
36:477–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar
|
|
17
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ørom UA, Derrien T, Beringer M, Gumireddy
K, Gardini A, Bussotti G, Lai F, Zytnicki M, Notredame C, Huang Q,
et al: Long noncoding RNAs with enhancer-like function in human
cells. Cell. 143:46–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y,
Chen N, Sun F and Fan Q: CREB up-regulates long non-coding RNA,
HULC expression through interaction with microRNA-372 in liver
cancer. Nucleic Acids Res. 38:5366–5383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao X, Wang P, Liu J, Zheng J, Liu Y,
Chen J and Xue Y: Gas5 exerts tumor-suppressive functions in human
glioma cells by targeting miR-222. Mol Ther. 23:1899–1911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clemson CM, Hutchinson JN, Sara SA,
Ensminger AW, Fox AH, Chess A and Lawrence JB: An architectural
role for a nuclear noncoding RNA: NEAT1 RNA is essential for the
structure of paraspeckles. Mol Cell. 33:717–726. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen LL and Carmichael GG: Altered nuclear
retention of mRNAs containing inverted repeats in human embryonic
stem cells: Functional role of a nuclear noncoding RNA. Mol Cell.
35:467–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasaki YT, Ideue T, Sano M, Mituyama T and
Hirose T: MENepsilon/beta noncoding RNAs are essential for
structural integrity of nuclear paraspeckles. Proc Natl Acad Sci
USA. 106:2525–2530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sunwoo H, Dinger ME, Wilusz JE, Amaral PP,
Mattick JS and Spector DL: MEN epsilon/beta nuclear-retained
non-coding RNAs are up-regulated upon muscle differentiation and
are essential components of paraspeckles. Genome Res. 19:347–359.
2009. View Article : Google Scholar :
|
|
28
|
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L,
Chen S and Li Y: Inhibition of long non-coding RNA NEAT1 impairs
myeloid differentiation in acute promyelocytic leukemia cells. BMC
Cancer. 14:6932014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chakravarty D, Sboner A, Nair SS,
Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K,
Kossai M, et al: The oestrogen receptor alpha-regulated lncRNA
NEAT1 is a critical modulator of prostate cancer. Nat Commun.
5:5383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choudhry H, Albukhari A, Morotti M, Haider
S, Moralli D, Smythies J, Schödel J, Green CM, Camps C, Buffa F, et
al: Tumor hypoxia induces nuclear paraspeckle formation through
HIF-2α dependent transcriptional activation of NEAT1 leading to
cancer cell survival. Oncogene. 34:45462015. View Article : Google Scholar
|
|
31
|
Guo S, Chen W, Luo Y, Ren F, Zhong T, Rong
M, Dang Y, Feng Z and Chen G: Clinical implication of long
non-coding RNA NEAT1 expression in hepatocellular carcinoma
patients. Int J Clin Exp Pathol. 8:5395–5402. 2015.PubMed/NCBI
|
|
32
|
Kim YS, Hwan JD, Bae S, Bae DH and Shick
WA: Identification of differentially expressed genes using an
annealing control primer system in stage III serous ovarian
carcinoma. BMC Cancer. 10:5762010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Liu YH, Diao HY, Ma J and Yao YL:
Long noncoding RNA NEAT1 promotes glioma pathogenesis by regulating
miR-449b-5p/c-Met axis. Tumour Biol. 37:673–683. 2016. View Article : Google Scholar
|
|
34
|
Frixa T, Donzelli S and Blandino G:
Oncogenic MicroRNAs: key players in malignant transformation.
Cancers (Basel). 7:2466–2485. 2015. View Article : Google Scholar
|
|
35
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang P, Liu YH, Yao YL, Li Z, Li ZQ, Ma J
and Xue YX: Long non-coding RNA CASC2 suppresses malignancy in
human gliomas by miR-21. Cell Signal. 27:275–282. 2015. View Article : Google Scholar
|
|
37
|
Mardente S, Mari E, Massimi I, Fico F,
Faggioni A, Pulcinelli F, Antonaci A and Zicari A: HMGB1-induced
cross talk between PTEN and miRs 221/222 in thyroid cancer. Biomed
Res Int. 2015:5120272015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Z, Chen S, Luan X, Li Y, Liu M, Li X,
Liu T and Tang H: MicroRNA-214 is aberrantly expressed in cervical
cancers and inhibits the growth of HeLa cells. IUBMB Life.
61:1075–1082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wen Z, Lei Z, Ma JA, Li XZ, Zheng XN and
Deng XW: The inhibitory role of miR-214 in cervical cancer cells
through directly targeting mitochondrial transcription factor A
(TFAM). Eur J Gynaecol Oncol. 35:676–682. 2014.
|
|
41
|
Chen DL, Wang ZQ, Zeng ZL, Wu WJ, Zhang
DS, Luo HY, Wang F, Qiu MZ, Wang DS, Ren C, et al: Identification
of MicroRNA-214 as a negative regulator of colorectal cancer liver
metastasis by way of regulation of fibroblast growth factor
receptor 1 expression. Hepatology. 60:598–609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Long LM, He BF, Huang GQ, Guo YH, Liu YS
and Huo JR: microRNA-214 functions as a tumor suppressor in human
colon cancer via the suppression of ADP-ribosylation factor-like
protein 2. Oncol Lett. 9:645–650. 2015.PubMed/NCBI
|
|
43
|
Penna E, Orso F, Cimino D, Vercellino I,
Grassi E, Quaglino E, Turco E and Taverna D: miR-214 coordinates
melanoma progression by upregulating ALCAM through TFAP2 and
miR-148b downmodulation. Cancer Res. 73:4098–4111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang TS, Yang XH, Wang XD, Wang YL, Zhou B
and Song ZS: MiR-214 regulate gastric cancer cell proliferation,
migration and invasion by targeting PTEN. Cancer Cell Int.
13:682013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV and Cheng JQ:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targeting PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gong W, Zheng J, Liu X, Ma J, Liu Y and
Xue Y: Knockdown of NEAT1 restrained the malignant progression of
glioma stem cells by activating microRNA let-7e. Oncotarget. Aug
19–2016.Epub ahead of print. View Article : Google Scholar
|
|
47
|
Geisler S, Lojek L, Khalil AM, Baker KE
and Coller J: Decapping of long noncoding RNAs regulates inducible
genes. Mol Cell. 45:279–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Barnhill LM, Williams RT, Cohen O, Kim Y,
Batova A, Mielke JA, Messer K, Pu M, Bao L, Yu AL, et al: High
expression of CAI2, a 9p21-embedded long noncoding RNA, contributes
to advanced-stage neuroblastoma. Cancer Res. 74:3753–3763. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nie FQ, Zhu Q, Xu TP, Zou YF, Xie M, Sun
M, Xia R and Lu KH: Long non-coding RNA MVIH indicates a poor
prognosis for non-small cell lung cancer and promotes cell
proliferation and invasion. Tumour Biol. 35:7587–7594. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang F, Huo XS, Yuan SX, Zhang L, Zhou WP,
Wang F and Sun SH: Repression of the long noncoding RNA-LET by
histone deacetylase 3 contributes to hypoxia-mediated metastasis.
Mol Cell. 49:1083–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sun C, Li S, Zhang F, Xi Y, Wang L, Bi Y
and Li D: Long non-coding RNA NEAT1 promotes non-small cell lung
cancer progression through regulation of miR-377-3p-E2F3 pathway.
Oncotarget. Jun 16–2016.Epub ahead of print.
|
|
53
|
Naganuma T and Hirose T: Paraspeckle
formation during the biogenesis of long non-coding RNAs. RNA Biol.
10:456–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tani H, Torimura M and Akimitsu N: The RNA
degradation pathway regulates the function of GAS5 a non-coding RNA
in mammalian cells. PLoS One. 8:e556842013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY,
Gong W and Quan ZW: Long non-coding RNA CCAT1 promotes gallbladder
cancer development via negative modulation of miRNA-218-5p. Cell
Death Dis. 6:e1583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiong X, Ren HZ, Li MH, Mei JH, Wen JF and
Zheng CL: Down-regulated miRNA-214 induces a cell cycle G1 arrest
in gastric cancer cells by up-regulating the PTEN protein. Pathol
Oncol Res. 17:931–937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qiang R, Wang F, Shi LY, Liu M, Chen S,
Wan HY, Li YX, Li X, Gao SY and Sun BC: Plexin-B1 is a target of
miR-214 in cervical cancer and promotes the growth and invasion of
HeLa cells. Int J Biochem Cell Biol. 43:632–641. 2011. View Article : Google Scholar : PubMed/NCBI
|