Introduction
Solid tumors constitute approximately 90% of all
known types of cancer (1). The
rapid growth of such tumors alters the cellular microenvironment
because of an inadequate oxygen supply and results in hypoxia
(2,3). Tumor hypoxia is a potential
therapeutic problem because of its adverse impact on the
effectiveness of chemotherapy. Carbon dioxide (CO2)
therapy has historically been used for its therapeutic effect on
skin problems (4–6). The mechanisms of this beneficial
effect are an increase in blood flow and microcirculation, nitric
oxide-dependent neocapillary formation, and a partial increase in
oxygen pressure in the local tissue, known as the Bohr effect
(7). The anticancer effect of the
transcutaneous application or intra-arterial infusion of
CO2 has been reported (8–11).
In a recent study, the transcutaneous application of CO2
enhanced the therapeutic efficacy of doxorubicin for human
malignant fibrous histiocytoma (12). There is similarly a possibility
that the intra-arterial infusion of CO2 may enhance the
therapeutic effect of intra-arterial infusion chemotherapy, but
this is unknown.
We evaluated whether the intra-arterial infusion of
a CO2-saturated solution would sensitize the anticancer
effect of cisplatin, and we elucidated the mechanism of this
therapy in a rabbit VX2 liver tumor model.
Materials and methods
The VX2 liver tumor model
The present study was approved by the Institutional
Animal Care and Use Committee (Permission no. P-150202) on February
20, 2015 and was carried out according to the Kobe University
Animal Experimentation Regulations. Forty Japanese white rabbits
(age, ~3–4 months old; mean body weight, 2.62±0.03 kg) were
implanted with fresh VX2 tumors by injecting 0.1 ml of a VX2 tumor
tissue suspension (provided by Japan SLC, Inc., Shizuoka, Japan)
into their livers 3 weeks before the intra-arterial infusion.
Subsequently, the rabbits were randomly divided into four groups:
the control group, the CO2 group, the cisplatin group
and the combined group, with 10 in each group. Each material was
infused as follows: saline solution into the control group,
CO2-saturated solution into the CO2 group,
both cisplatin solution and saline solution into the cisplatin
group, and both CO2-saturated solution and cisplatin
solution into the combined group. For the following procedures,
each rabbit was anesthetized with sodium pentobarbital (maximum
dose of 50 mg/kg; Somnopenthyl; Kyoritsu Seiyaku, Tokyo, Japan) via
a marginal ear vein.
Preparation of materials for
infusion
The CO2-saturated solution at pH 4.0 was
prepared as previously described (8). The cisplatin solution was prepared by
dissolving DDP-H (a fine-powder formulation of cisplatin, IA-call;
Nippon Kayaku Co., Ltd., Tokyo, Japan) into the saline solution to
a concentration of 1.5 mg/ml. The dose of the
CO2-saturated solution and the saline solution was 50 ml
each, and the dose of cisplatin was 1.75 mg/kg (as determined from
the unpublished data of a DDP-H animal experiment by Nippon
Kayaku).
The CT examination
Contrast-enhanced CT (Toshiba Activion 16 TXS-031A;
Toshiba medical Systems, Tochigi, Japan or Rm-CT2; Rigaku, Tokyo,
Japan) was performed to evaluate the size of the tumor in the
liver. The CT scan was initiated 55 sec after the injection of the
contrast medium (Omnipaque 300; Daiichi Sankyo, Tokyo, Japan) at a
rate of 0.3 ml/sec via a marginal ear vein. The amount of contrast
medium used was set to 2.0 ml/kg. Contrast-enhanced CT was
performed before the procedure and 3 and 7 days after the
procedure.
Intra-arterial infusion procedure
After the CT examination, intra-arterial infusion
was performed with a C-arm device (SIREMOBIL Compact L; Siemens
Medical Solutions, Erlangen, Germany or ARCADIS Varic;
Siemens-Asahi Medical Technologies, Tokyo, Japan). The right
femoral artery was exposed and directly punctured with a 22-gauge
needle (SURFLO intravenous catheter; Terumo, Tokyo, Japan). A
0.018-inch nitinol guidewire (Cook Medical Japan, Tokyo, Japan) was
placed through the needle, and a 4-French introducer catheter
(Micropuncture introducer catheter; Cook Medical Japan) was
inserted over the guidewire. After the tip of a 2.4-French
microcatheter with a swan-neck shape (Nadeshiko; JMS, Co., Ltd.,
Hiroshima, Japan) was placed into the proper hepatic artery,
digital subtraction angiography was performed to confirm the
distribution of contrast medium to the liver by manually injecting
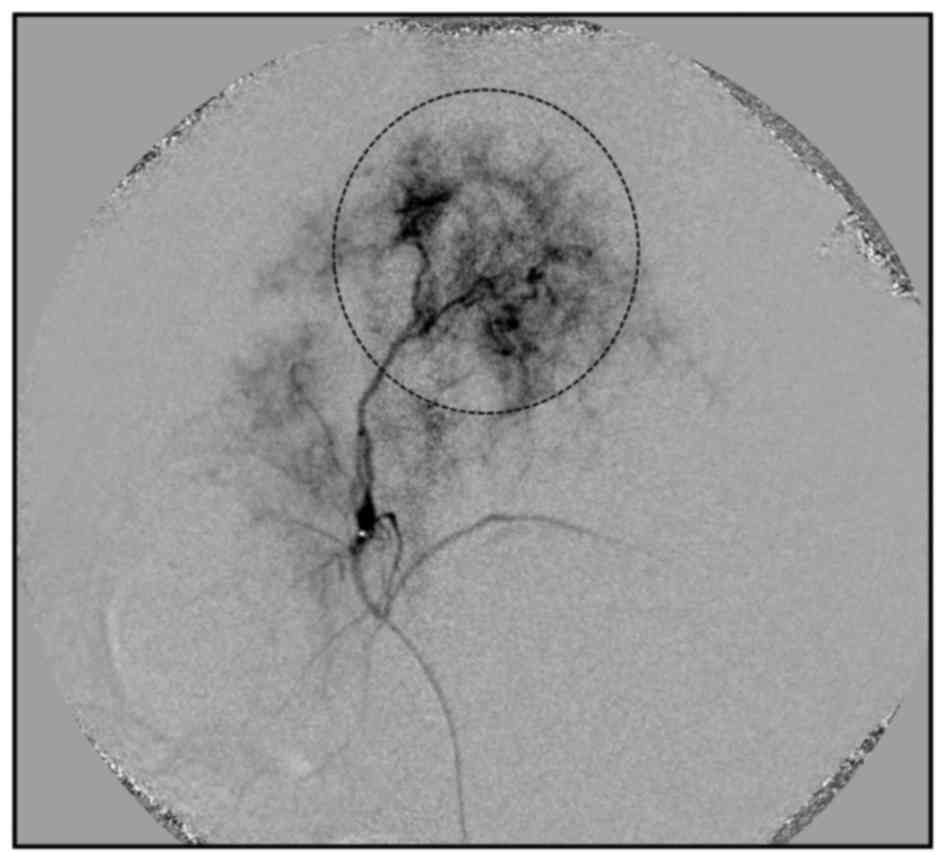
1 ml of contrast medium at a rate of 0.1 ml/s (Fig. 1). Each material of the group, as
described above, was infused after the angiography. The
CO2-saturated solution and the saline solution were
infused for 10 min, and the cisplatin solution was administered for
3 min through the catheter. After the injection of the solution,
the catheter was removed, and the right femoral artery was ligated
to achieve hemostasis. The incision wound was sutured, and the
rabbits were observed for 7 days while maintaining a normal feeding
regimen. All rabbits were euthanized after CT scanning on day 7,
and the liver of each rabbit was carefully excised and processed
for histological examination.
Tumor growth and volume measurement
All recorded CT volumetric data were transferred to
Ziostation software (Ziosoft, Inc., Tokyo, Japan) and reconstructed
in 3-mm thick slices. Two experienced radiologists, who were
blinded to the treatment group status, manually traced the contour
of the VX2 tumor area in each slice. All measurements were
independently performed twice, and the tumor area was determined as
the mean value of all measurements. The tumor volume (TV) was then
calculated using the following formula: TV = the total
circumscribed area in each slice × CT section thickness. The
relative tumor volume (RTV) was calculated as follows: RTV = (TV on
day 3 or 7)/(TV on day 0) × 100. We evaluated whether the
therapeutic effect of the combined group was antagonistic, additive
or synergistic by comparing with expected RTV for additive effect.
Expected RTV for additive effect was calculated based on the
following formula: expected RTV for additive effect = (RTV of the
CO2 group) × (RTV of the cisplatin group)/(RTV of the
control group), as reported (13).
Histology
Liver tissue was fixed in a 10% phosphate-buffered
formaldehyde solution, and 7-mm sections were obtained and embedded
in paraffin. Serial sections were then cut at 6-µm
thickness. One section was stained with hematoxylin and eosin
(H&E) and contiguous sections were immunofluorescently stained
using 4′,6-diamidino-2-phenylindole (DAPI) stain and APO-Direct kit
(Bay Bioscience, Co., Ltd., Kobe, Japan) to evaluate DNA
fragmentation. Immunofluorescence assay of DNA fragmentation is
described below. All histopathologic specimens were evaluated by a
pathologist under a light microscope (Keyence Corp., Osaka, Japan)
and the apoptotic area was described, based on the fluorescence
staining results.
Immunofluorescence assay of DNA
fragmentation
APO-Direct kit is a single-step staining method for
labeling DNA breaks to detect apoptotic cells by flow cytometry. A
method which is often used to detect fragmented DNA utilizes a
reaction catalyzed by exogenous TDT, often referred to as
'end-labeling' or 'TUNEL' (terminal deoxynucleotidyltransferase
dUTP nick end labeling). The APO-direct kit was used for DNA
fragmentation with immunofluorescence staining according to the
manufacturer's protocol. Solid specimens of tumors were minced and
filtered through a cell strainer (BD Falcon; BD Biosciences,
Bedford, MA, USA) to obtain a single cell suspension from implanted
tumors. Erythrocytes were lysed in BD Pharm Lyse™ lysing buffer
(Bay Bioscience), and the remaining cells were pelleted and
resuspended in phosphate-buffered saline (PBS) solution.
Single-cell suspensions were fixed with 1% (vol/vol)
paraformaldehyde and resuspended in 70% (vol/vol) ice-cold ethanol
at a concentration of 1×106 cells/ml. Each cell pellet
was resuspended in 50 ml of DNA labeling solution (reaction buffer,
10 µl; terminal deoxynucleotidyl transferase enzyme, 0.75
µl; fluorescein isothiocyanate, 2′-deoxyuridine,
5′-triphosphate, 0.8 µl; distilled H2O, 32.25
µl) and incubated for 60 min at 37°C.
Immunohistochemistory
The immunohistochemical expression of
hypoxia-inducible factor-1α (HIF-1α) and carbonic anhydrase IX (CA
IX) as intrinsic markers of tumor hypoxia were detected using
anti-HIF-1α antibody (1:100, H1alpha67; Abcam PLC, Cambridge, UK)
and anti-CA IX antibody (1:100; Novus Biologicals LLC, Littleton,
CO, USA). Deparaffinized sections were digested with proteinase
(Dako Retrieval Solution Ready-to-Use) for 10 min and treated
overnight at 4°C with the antibodies in Can Get Signal immunostain
solution. Following the treatment, sections were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
polyclonal antibody (Nichirei Bioscience, Tokyo, Japan) for 30 min
at room temperature. The signal was developed as a brown reaction
product using the peroxidase substrate 3,3′-diaminobenzidine
(Nichirei Bioscience). Sections were counter stained with
hematoxylin, and were captured under a microscope. Moreover, to
evaluate the quantification of HIF-1α expression, the secondary
antibody goat anti-mouse immunoglobulin Alexa Fluor 596 (1:200
dilution; Life Technologies, Carlsbad, CA, USA) was used for 100
min at room temperature. Immunofluorescence nuclear staining using
DAPI was performed to quantify HIF-1α expression. The numbers of
hypoxic cells were counted directly in four randomly selected
fields and averaged. Images were acquired using a fluorescence
microscope (BZ-X700; Keyence).
Immunoblot analysis for caspase-3 and
caspase-9 assay
Immunoblot analysis was performed to evaluate the
apoptotic pathway of caspase-3 and caspase-9. Tumor lysates were
prepared from tumor tissues in whole-cell lysis buffer (Mammalian
Protein Extraction Reagent; Thermo Fisher Scientific, Rockford, IL,
USA). Samples were processed with standard western immunoblotting
procedures. Membranes were incubated overnight at 4°C with the
following antibodies in Can Get Signal Solution 1 (Toyobo, Co.,
Ltd., Osaka, Japan): caspase-3 antibody (1:1,000; Cell Signaling
Technology, Danvers, MA, USA), anti-cleaved caspase-9 antibody
(1:1,000; Cell Signaling Technology) and α-tubulin antibody
(1:2,000; Sigma-Aldrich, St. Louis, MO, USA). After washing, the
membranes were incubated with the appropriate secondary antibody
conjugated to horseradish peroxidase, and exposed using the ECL
Plus western blotting detection system (GE Healthcare Bio-Sciences,
Piscataway, NJ, USA). A chemilumino analyzer (LAS-3000 mini;
Fujifilm, Tokyo, Japan) was used to detect signals.
Statistical analysis
Statistical analyses were conducted using JMP 12.0.1
(SAS Institute, Inc., Cary, NC, USA). The data are presented as the
mean ± standard deviation, unless indicated otherwise. The
significance of differences between groups was evaluated using the
two-tailed Student's t-test, and by one-way analysis of variance
with post-hoc Tukey's honestly significant difference test for
multiple comparisons. P<0.05 was considered significant.
Results
All procedures were performed successfully, and all
rabbits survived for 1 week after the procedure. The rabbits were
euthanized on day 7, and the liver of each rabbit was excised and
processed for histological examination. The mean body weight on the
procedure day and on day 7 were, respectively, 2.76±0.18 and
2.78±0.19 kg in the control group; 2.66±0.23 and 2.70±0.23 kg in
the CO2 group; 2.62±0.16 and 2.64±0.16 kg in the
cisplatin group; and 2.58±0.08 and 2.59±0.07 kg in the combined
group. There were no significant differences among the four groups
(P>0.05).
Tumor growth and volume measurement
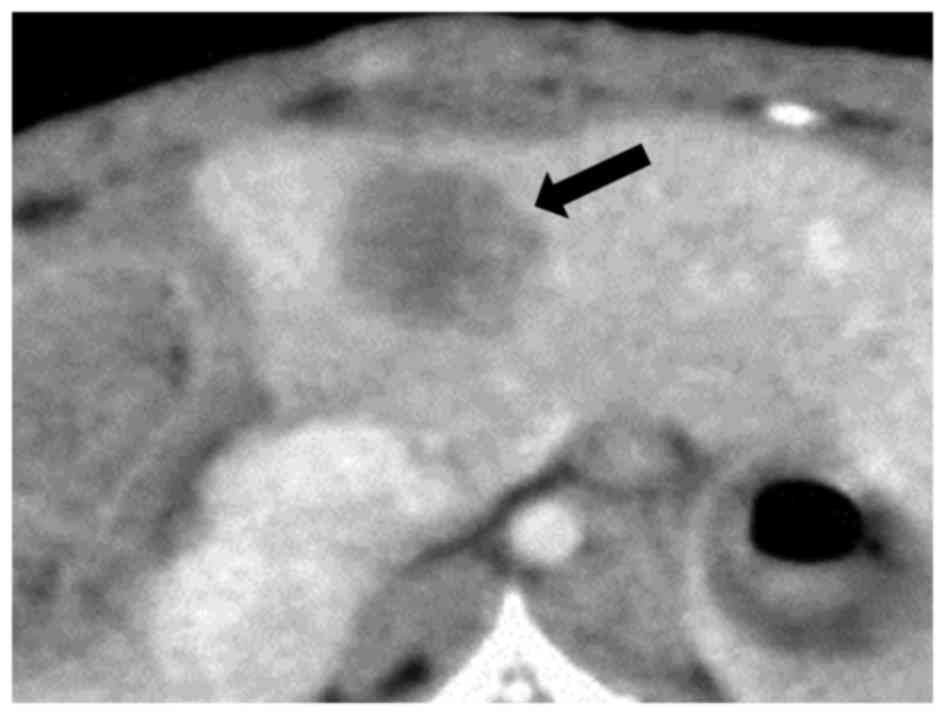
Contrast-enhanced CT of liver tumors demonstrated
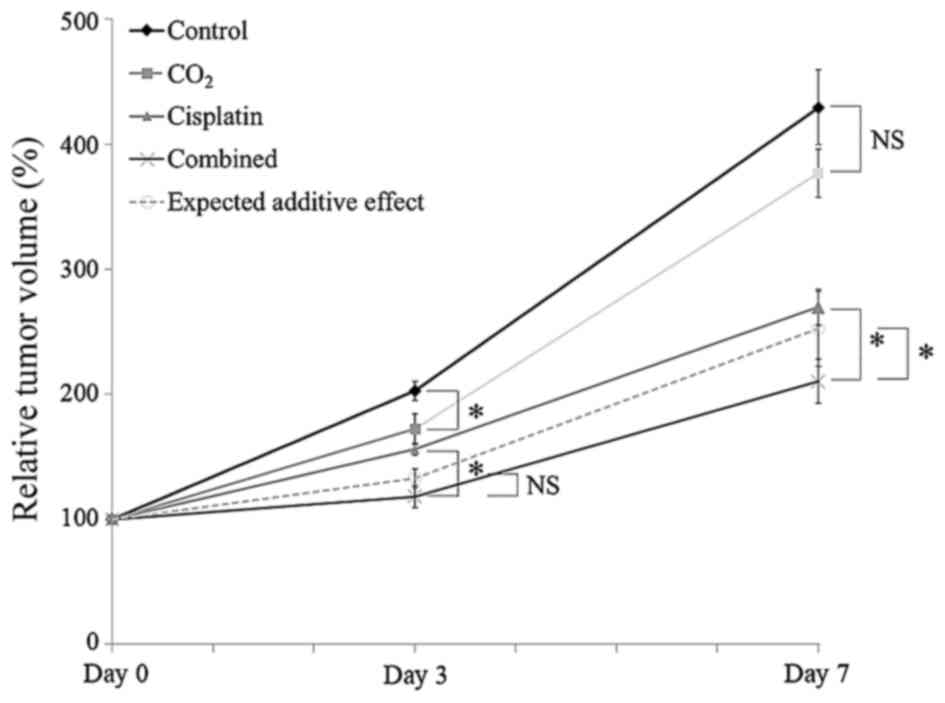
low-attenuation lesions with peripheral enhancement (Fig. 2). The mean TV and RTV on days 3 and
7 are shown in Table I. The line
graph of the mean RTV is shown in Fig.
3. The mean RTV of the CO2 group on day 3 was
significantly decreased, compared with the control group
(P<0.05); however, this ratio showed no significant difference
on day 7 (P=0.16). The mean RTV on days 3 and 7 of the combined
group was significantly lower than that in the cisplatin group
(P<0.05). The mean RTV on day 7 was also significantly lower
than expected RTV for additive effect (P<0.05).
 | Table IThe tumor volumes (mm3)
and the relative tumor volume (%) on days 0, 3 and 7. |
Table I
The tumor volumes (mm3)
and the relative tumor volume (%) on days 0, 3 and 7.
| Group | Day 0 | Day 3 | Day 7 |
|---|
| Control | 2,669.2±496.7 | 5,378.0±1034.5 |
11,267.4±2344.7 |
| (n=10) | | (202.6±23.7%) | (429.2±94.8%) |
| CO2 | 2,704.3±59.22 | 4,670.3±1500.6 |
10,215.5±3092.6 |
| (n=10) | | (172.2±38.1%) | (376.5±61.1%) |
| Cisplatin | 2,849.4±918.4 | 4,442.8±1565.4 | 7,786.0±3242.6 |
| (n=10) | | (156.1±15.1%) | (269.6±45.2%) |
| Combined | 2,981.1±873.5 | 3,445.9±968.0 | 6,005.1±1409.0 |
| (n=10) | | (118.3±28.1%) | (210.3±55.1%) |
| Expected | | (132.9±27.3%) | (252.3±99.2%) |
| RTV | | | |
Evaluation of apoptosis
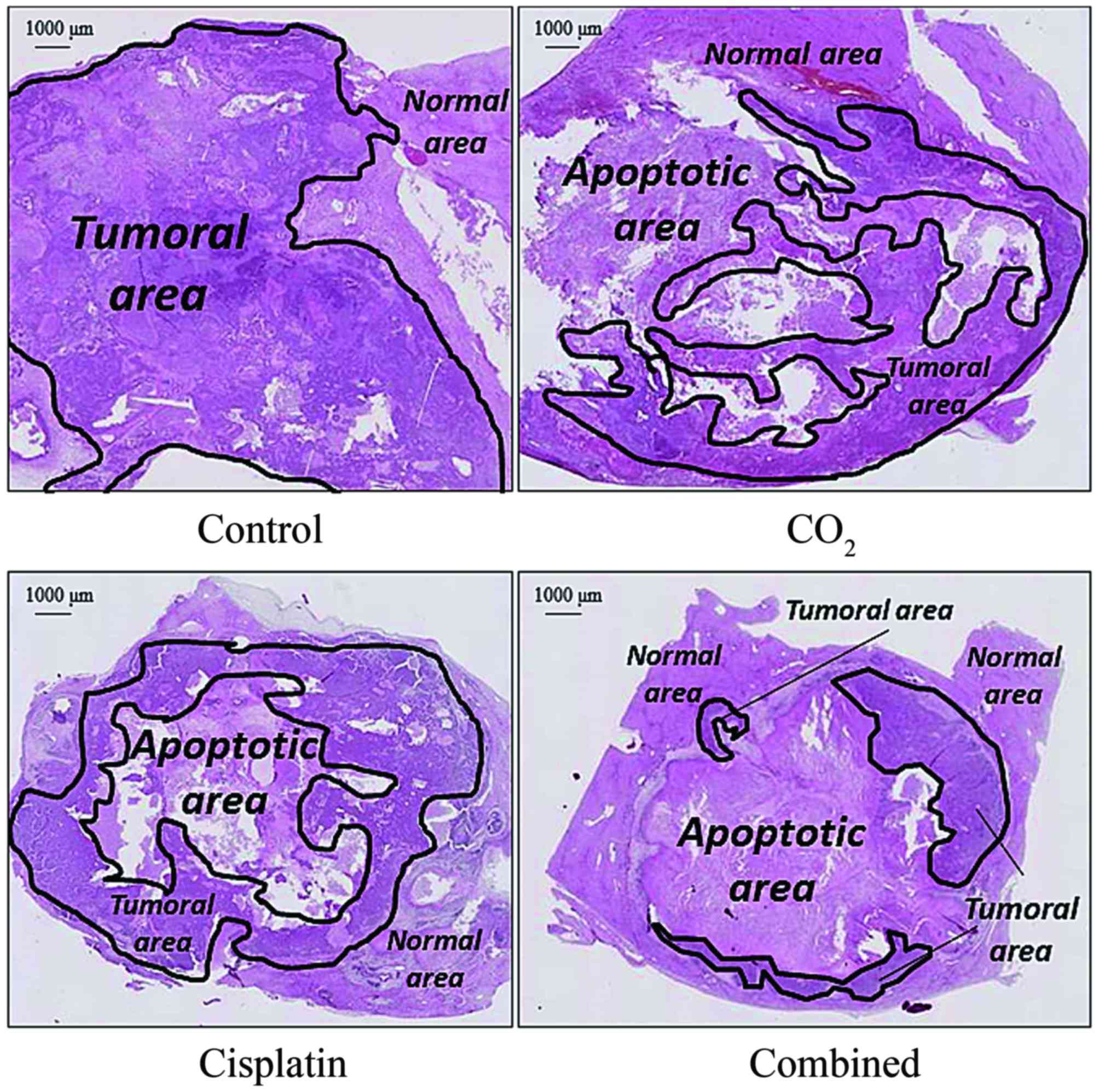
Representative H&E-stained liver sections
demonstrated an increased apoptotic area and decreased tumor area
in the CO2 group, cisplatin group, and combined group,
compared with the control group (Fig.
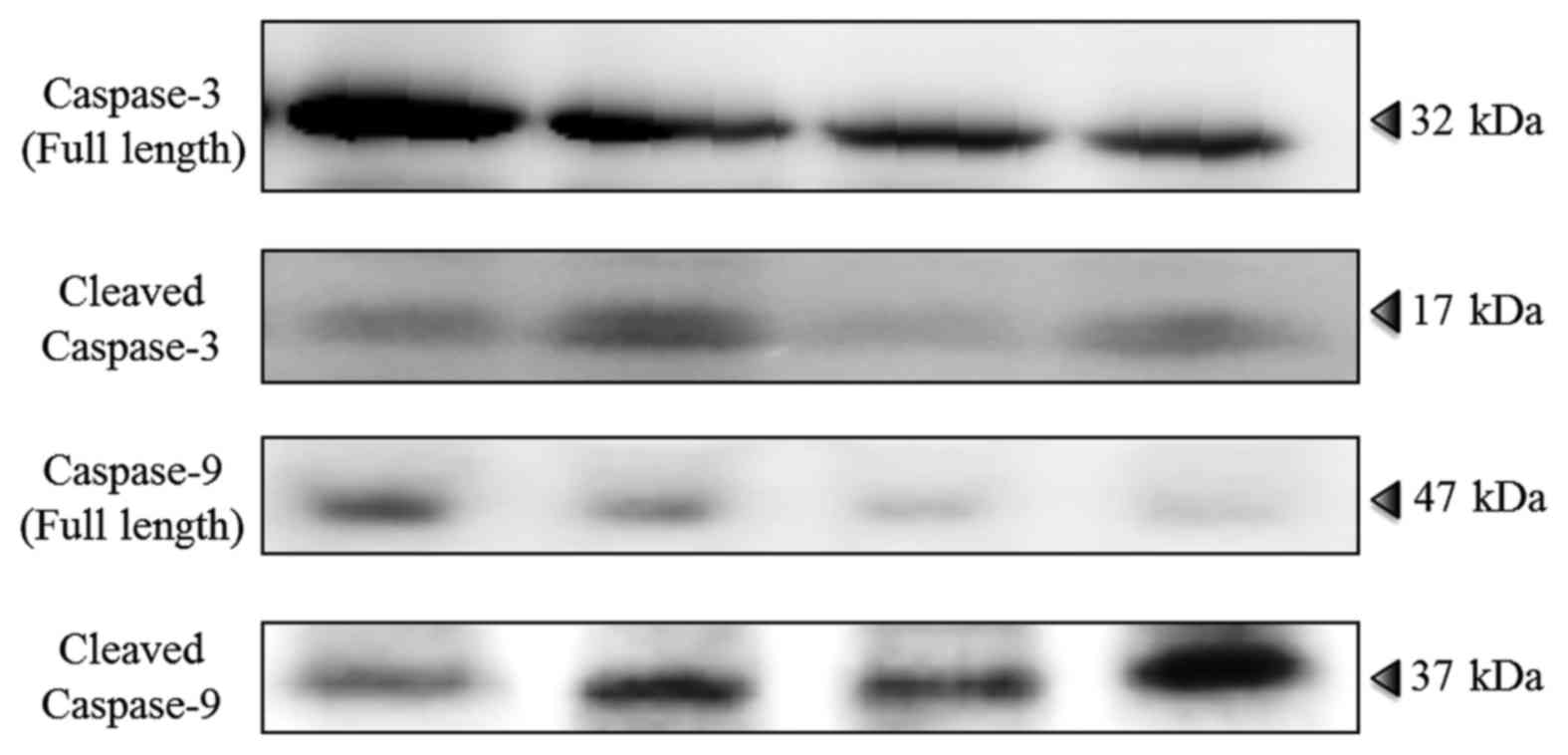
4). Immunoblot analyses showed higher expression of cleaved
caspase-3 and caspase-9 in the CO2 and combined groups
than in the control and cisplatin groups (Fig. 5).
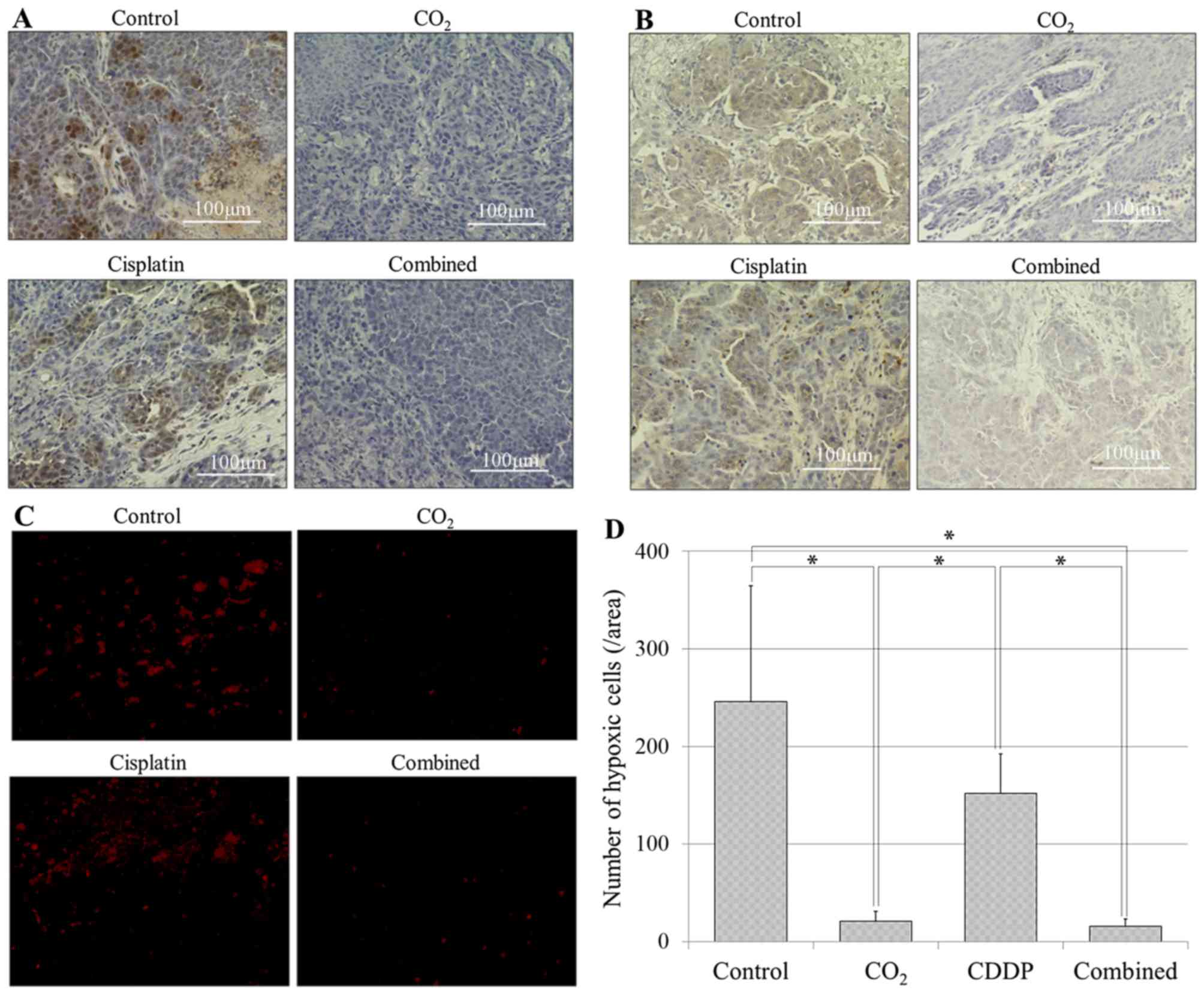
Evaluation of hypoxia
HIF-1α and CA IX staining demonstrated suppression
of HIF-1α and CA IX in the CO2 and combined group
(Fig. 6A and 6B). As for the quantification of HIF-1α
expression, the numbers of hypoxic cells per area were 246±118 in
the control group, 21±10.4 in the CO2 group, 152±40.7 in
the cisplatin group and 16±7.3 in the combined group. Hypoxic cells
were significantly more in the control and cisplatin groups
compared with the CO2 and combined groups (Fig. 6C and 6d; P<0.05).
Discussion
There was a significant difference in tumor growth
between the control group and the CO2 group, and between
the cisplatin group and the combined group. The intra-arterial
infusion of the CO2-saturated solution inhibited tumor
growth and sensitized the anticancer effect of cisplatin. The
results of this study will contribute to improving the therapeutic
effect of intra-arterial chemotherapy using cisplatin.
CO2 effect of improving hypoxia and
inducing apoptosis have been explained by some mechanisms. The
first mechanism is direct antitumor effect of CO2. There
are several reports showing that intracellular calcium (i.e.,
Ca2+) concentrations increased by CO2 induces
the expression of peroxisome proliferator-activated receptor gamma
coactivator-1 alpha and mitochondrial biogenesis (14–19).
In vivo study of a human malignant fibrous hystiocytoma
tumor model, transcutaneous CO2 treatment increased
intracellular Ca2+ concentrations and induced
mitochondrial DNA apoptosis (12).
In vitro study of a human neuroblastoma cell model,
intracellular reactive oxygen species induced by CO2
intracellular reactive oxygen species, lead to proapoptotic p53
signal stimulation, DNA damage, and cell death through the
mitochondrial pathway (20). The
second and third possible mechanisms are related to oxygenation and
pH in the tumor microenvironment. The present study did not show
evidence of increased partial pressure of oxygen or oxygen
saturation and decreased pH in VX2 tumor tissue during the
procedure. However, a previous study (8) demonstrated that transcutaneous
CO2 application significantly lowers intracellular pH,
decreases oxyhemoglobin, and increases deoxyhemoglobin in treated
muscle.
CO2 therapy is considered to improve
tumor hypoxia and induce the mitochondrial pathway of apoptosis as
described above. Moreover, this therapy was reported to suppress
vascular endothelial growth factor and HIF-1α (21). In this study, there was less HIF-1α
expression and more cleaved caspase-3 and caspase-9 expression in
the CO2 and combined groups than in the control and
cisplatin groups. This result revealed that intra-arterial
CO2 infusion could improve hypoxia and induce apoptosis
in tumors. Caspase-3 is activated in apoptotic cells by the
extrinsic (i.e., death ligand) and intrinsic (i.e., mitochondrial)
pathways and caspase-9 reflects mitochondrial apoptosis. Cleaved
caspase-3 and cleaved caspase-9 are activated forms of caspase-3
and caspase-9, and are commonly used to detect apoptosis (22). HIF-1α is a basic
helix-loop-helix-PAS (bHLH-PAS) transcription factor that has an
essential role in O2 homeostasis (6,7,9,10),
and has recently emerged as a major factor influencing tumor
proliferation and malignant progression (23,24).
Hypercapnia was reported to counter-regulate the activation of the
HIF pathway by reducing the intracellular pH (25).
Minimizing hypoxia and suppression of HIF-1α
expression in tumors also has the potential to enhance
chemotherapeutic effects (26). In
this study, the combined group achieved a higher tumor growth
inhibition rate, compared with the other groups and expected
additive effect. We expected that a CO2-saturated
solution would sensitize the tumor to the antitumor effect of
cisplatin by suppressing HIF-1α expression. Previous reports
support our hypothesis: Ai et al (27) revealed that the genetic knockdown
of HIF-1α or pharmacological promotion of HIF-1α degradation
enhanced the response of ovarian cancer cells to cisplatin, and
diindolylmethane is reportedly a cisplatin sensitizer that exerts
its effect by targeting signal transducer and activator of
transcription 3, which suppresses HIF-1α and vascular endothelial
growth factor (28).
Intra-arterial infusion of CO2 is
well-known to interventional radiologists as a negative contrast
medium. CO2 has advantages over other treatments, such
as its lack of nephrotoxic and allergenic effects on the human
body. Moreover, it is markedly less expensive than other drugs (the
typical cost for CO2 is 3 cents per 100 ml) (29). Thus, we believe that a
CO2-saturated solution is the ideal material for
sensitizing the anticancer effect of intra-arterial cisplatin
infusions. The infusion volume of the CO2-saturated
solution was set to 50 ml; approximately 38 ml of CO2
gas was dissolved in the CO2-saturated solution
(calculated using a solubility limit of 1,508 parts per million at
1 atm, 25°C). Thus, the CO2 gas was injected at a dose
of 12.6–16.2 ml/kg without severe complications, although hepatic
isozymes were not measured. A previous report described a rabbit
experimental model of intra-arterial CO2 gas injection
at a dose of 10 ml/kg in which no subacute hepatic adverse effects
were observed (30).
There are several limitations to the present study.
First, VX2 tumors can easily be implanted into other organs,
allowing for the investigation of many interventional procedures.
However, this model does not represent the complexity or size of
human liver cancer (31). VX2
tumors grow rapidly and have been reported to contain large areas
of liquefaction necrosis beyond 15 days after implantation
(32). We assume that the large
standard deviation of TV in this study was mainly caused by this
VX2 tumor characteristic. Larger sample sizes may be needed to
decrease the standard deviation. Functional imaging, such as
perfusion CT and magnetic resonance spectroscopy, have been used to
evaluate tumor angiogenesis and necrotic changes of VX2 tumors
(33,34); however, these examinations were not
performed because our laboratory lacks these facilities. This study
was a pilot animal investigation to assess the effect of a
CO2-saturated solution; therefore, only a single
infusion and a single dose were used. More CO2 doses may
need to be assessed to achieve a stronger antitumor effect, which
was suggested by a previous report in which CO2 was
administered twice weekly by transcutaneous application (9).
In conclusions, intra-arterial infusion of a
CO2-saturated solution should inhibit tumor growth and
sensitize the anticancer effect of cisplatin by suppressing HIF-1α
expression in a rabbit VX2 liver tumor model.
Abbreviations:
|
CO2
|
carbon dioxide
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
CA IX
|
carbonic anhydrase IX
|
Acknowledgments
The authors would like to thank Editage (www.editage.jp) for English language editing. Dr M.
Yamaguchi reports grants from Grant-in-Aid for Scientific Research
(C) from the Japan Society for the Promotion of Science.
References
|
1
|
Mees G, Dierckx R, Vangestel C and Van de
Wiele C: Molecular imaging of hypoxia with radiolabelled agents.
Eur J Nucl Med Mol Imaging. 36:1674–1686. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laking G and Price P: Radionuclide imaging
of perfusion and hypoxia. Eur J Nucl Med Mol Imaging. 37(Suppl 1):
S20–S29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9(Suppl 5): 10–17. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartmann BR, Bassenge E, Pittler M and
Hartmann BR: Effect of carbon dioxide-enriched water and fresh
water on the cutaneous microcirculation and oxygen tension in the
skin of the foot. Angiology. 48:337–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang J, Kang D, Wang Y, Yu Y, Fan J and
Takashi E: Carbonate ion-enriched hot spring water promotes skin
wound healing in nude rats. PLoS One. 10:e01171062015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jensen FB: Red blood cell pH, the Bohr
effect, and other oxygenation-linked phenomena in blood
O2 and CO2 transport. Acta Physiol Scand.
182:215–227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakai Y, Miwa M, Oe K, Ueha T, Koh A,
Niikura T, Iwakura T, Lee SY, Tanaka M and Kurosaka M: A novel
system for transcutaneous application of carbon dioxide causing an
'artificial Bohr effect' in the human body. PLoS One. 6:e241372011.
View Article : Google Scholar
|
|
9
|
Onishi Y, Kawamoto T, Ueha T, Kishimoto K,
Hara H, Fukase N, Toda M, Harada R, Minoda M, Sakai Y, et al:
Transcutaneous application of carbon dioxide (CO2)
induces mitochondrial apoptosis in human malignant fibrous
histiocytoma in vivo. PLoS One. 7:e491892012. View Article : Google Scholar
|
|
10
|
Harada R, Kawamoto T, Ueha T, Minoda M,
Toda M, Onishi Y, Fukase N, Hara H, Sakai Y, Miwa M, et al:
Reoxygenation using a novel CO2 therapy decreases the
metastatic potential of osteosarcoma cells. Exp Cell Res.
319:1988–1997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ueshima E, Yamaguchi M, Ueha T, Muradi A,
Okada T, Idoguchi K, Sofue K, Akisue T, Miwa M, Fujii M, et al:
Inhibition of growth in a rabbit VX2 thigh tumor model with
intraarterial infusion of carbon dioxide-saturated solution. J Vasc
Interv Radiol. 25:469–476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Onishi Y, Kawamoto T, Ueha T, Hara H,
Fukase N, Toda M, Harada R, Sakai Y, Miwa M, Nishida K, et al:
Transcutaneous application of carbon dioxide (CO2)
enhances chemosensitivity by reducing hypoxic conditions in human
malignant fibrous histiocytoma. J Cancer Sci Ther. 04:174–181.
2012. View Article : Google Scholar
|
|
13
|
Nagano T, Yasunaga M, Goto K, Kenmotsu H,
Koga Y, Kuroda J, Nishimura Y, Sugino T, Nishiwaki Y and Matsumura
Y: Synergistic antitumor activity of the SN-38-incorporating
polymeric micelles NK012 with S-1 in a mouse model of non-small
cell lung cancer. Int J Cancer. 127:2699–2706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vadász I, Dada LA, Briva A, Trejo HE,
Welch LC, Chen J, Tóth PT, Lecuona E, Witters LA, Schumacker PT, et
al: AMP-activated protein kinase regulates CO2-induced
alveolar epithelial dysfunction in rats and human cells by
promoting Na,K-ATPase endocytosis. J Clin Invest. 118:752–762.
2008.
|
|
15
|
Summers BA, Overholt JL and Prabhakar NR:
CO2 and pH independently modulate L-type Ca2+
current in rabbit carotid body glomus cells. J Neurophysiol.
88:604–612. 2002.PubMed/NCBI
|
|
16
|
Iwabu M, Yamauchi T, Okada-Iwabu M, Sato
K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata
M, et al: Adiponectin and AdipoR1 regulate PGC-1α and mitochondria
by Ca2+ and AMPK/SIRT1. Nature. 464:1313–1319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Irrcher I, Adhihetty PJ, Sheehan T, Joseph
AM and Hood DA: PPARgamma coactivator-1α expression during thyroid
hormone- and contractile activity-induced mitochondrial
adaptations. Am J Physiol Cell Physiol. 284:C1669–C1677. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ojuka EO, Jones TE, Han DH, Chen M and
Holloszy JO: Raising Ca2+ in L6 myotubes mimics effects
of exercise on mitochondrial biogenesis in muscle. FASEB J.
17:675–681. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oe K, Ueha T, Sakai Y, Niikura T, Lee SY,
Koh A, Hasegawa T, Tanaka M, Miwa M and Kurosaka M: The effect of
transcutaneous application of carbon dioxide (CO2) on
skeletal muscle. Biochem Biophys Res Commun. 407:148–152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montalto AS, Currò M, Russo T, Visalli G,
Impellizzeri P, Antonuccio P, Arena S, Borruto FA, Scalfari G,
Ientile R, et al: In vitro CO2-induced ROS production
impairs cell cycle in SH-SY5Y neuroblastoma cells. Pediatr Surg
Int. 29:51–59. 2013. View Article : Google Scholar
|
|
21
|
Takeda D, Hasegawa T, Ueha T, Imai Y,
Sakakibara A, Minoda M, Kawamoto T, Minamikawa T, Shibuya Y, Akisue
T, et al: Transcutaneous carbon dioxide induces mitochondrial
apoptosis and suppresses metastasis of oral squamous cell carcinoma
in vivo. PLoS One. 9:e1005302014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan Y and Bergmann A: The
cleaved-caspase-3 antibody is a marker of caspase-9-like DRONC
activity in Drosophila. Cell Death Differ. 17:534–539. 2010.
View Article : Google Scholar :
|
|
23
|
Wang W, Lee NY, Georgi JC, Narayanan M,
Guillem J, Schöder H and Humm JL: Pharmacokinetic analysis of
hypoxia 18F-fluoromisonidazole dynamic PET in head and
neck cancer. J Nucl Med. 51:37–45. 2010. View Article : Google Scholar
|
|
24
|
Janssen HL, Haustermans KM, Balm AJ and
Begg AC: Hypoxia in head and neck cancer: How much, how important?
Head Neck. 27:622–638. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Selfridge AC, Cavadas MA, Scholz CC,
Campbell EL, Welch LC, Lecuona E, Colgan SP, Barrett KE, Sporn PH,
Sznajder JI, et al: Hypercapnia suppresses the HIF-dependent
adaptive response to hypoxia. J Biol Chem. 291:11800–11808. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Onnis B, Rapisarda A and Melillo G:
Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med.
13:2780–2786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ai Z, Lu Y, Qiu S and Fan Z: Overcoming
cisplatin resistance of ovarian cancer cells by targeting
HIF-1-regulated cancer metabolism. Cancer Lett. 373:36–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kandala PK and Srivastava SK:
Diindolylmethane suppresses ovarian cancer growth and potentiates
the effect of cisplatin in tumor mouse model by targeting signal
transducer and activator of transcription 3 (STAT3). BMC Med.
10:92012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caridi JG, Cho KJ, Fauria C and Eghbalieh
N: Carbon dioxide digital subtraction angiography (CO2
DSA): A comprehensive user guide for all operators. Vasc Dis
Manage. 11:E221–E256. 2014.
|
|
30
|
Mladinich CR, Hawkins IF Jr, Heaton-Jones
TG, Shiroma JT, Weingarten K, Kiehl A, Mays MB and Kublis P:
Effects of carbon dioxide arterial infusion on hepatic biochemistry
and histology in a rabbit model. Invest Radiol. 30:192–195. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pascale F, Ghegediban SH, Bonneau M,
Bedouet L, Namur J, Verret V, Schwartz-Cornil I, Wassef M and
Laurent A: Modified model of VX2 tumor overexpressing vascular
endothelial growth factor. J Vasc Interv Radiol. 23:809–817.e2.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Buijs M, Vossen JA, Geschwind JF, Salibi
N, Pan L, Ventura VP, Liapi E, Lee KH and Kamel IR: Quantitative
proton MR spectroscopy as a biomarker of tumor necrosis in the
rabbit VX2 liver tumor. J Vasc Interv Radiol. 22:1175–1180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Zheng LF, Feng Y, Xie XQ, Yang XM
and Zhang GX: CTA combined with CT perfusion for assessing the
efficacy of anti-angiogenic therapy in rabbit VX2 tumors. Acad
Radiol. 19:358–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Winter JD, Akens MK and Cheng HL:
Quantitative MRI assessment of VX2 tumour oxygenation changes in
response to hyperoxia and hypercapnia. Phys Med Biol. 56:1225–1242.
2011. View Article : Google Scholar : PubMed/NCBI
|