Introduction
Cholangiocarcinoma (CCA) is a malignant cancer
arising from the neoplastic transformation of cholangiocytes, the
epithelial cells lining the intrahepatic and extrahepatic bile
ducts (1,2). In general, in most CCA cases, the
disease is at an advanced stage at the time of its diagnosis.
Although surgery including curative liver transplantation is a
therapeutic option for selected patients with CCA, 5-year survival
rate is very low (3,4). Therefore, developing a more effective
treatment of CCA would be desirable.
Angiotensin receptor blocker (ARB), telmisartan is
widely prescribed drug for the treatment of hypertension and heart
failure. It inhibited the growth of adult T-cell leukemia cell
(5), endometrial (6), and gastric cancer cells (7) in several studies. The main mechanism
associated with antitumor effect by telmisartan treatment has been
shown to inhibit cell proliferation by inducing apoptosis in
various cancer cell lines, including prostate (8), renal (9), endometrial (6) and colon (10) cancer lines. More recently,
telmisartan induced cell cycle arrest in hematologic and
non-hematologic malignancies including our study (5,11).
Cyclin and cyclin dependent kinase (CDKs) complexes
are key to the progression of cells through G1/S phase of the cell
cycle by inactivating Rb through phosphorylation (12). Over- expression of these molecules
are postulated to increase cell turnover and proliferative activity
(13). Especially, cyclin D1, and
CDKs are essential for driving cells to pass the restriction point
(14,15). These mechanisms are associated with
the restriction point control the order and timing of cell cycle
transition, whereas cell regulatory functions are usually impaired
in cancer cells. Overexpression of cyclin D1 has been shown to be a
poor prognostic factor in bile duct carcinoma (16). Therefore, repairing cell cycle
progression might be an effective strategy for treatment of CCA. In
our previous study, telmisartan inhibited human esophageal
adenocarcinoma cell proliferation and tumor growth, inducing cell
cycle arrest by regulating cell cycle-related molecules (11). However, the antitumor effect of
telmisartan and its mechanism of action in CCA are currently
unknown.
In the present study, we report on an investigation
of the anti-proliferative effect of telmisartan on CCA cells in
vitro, and on tumor growth in CCA xenograft model in
vivo. Cell cycle and cell cycle-related proteins were studied
in vitro. Furthermore, possible mechanism associated with
the anti-tumor effect of telmisartan were also explored, including
the activation of receptor tyrosine kinases, angiogenesis,
apoptosis and microRNAs (miRNAs).
Materials and methods
Drugs, chemicals and regents
The following were used: Telmisartan (Tokyo Chemical
Industry Co. Tokyo, Japan), Trypan Blue (Sigma-Aldrich, St. Louis,
MO, USA), RPMI-1640 (Gibco-Invitrogen, Carlsbad, CA, USA), Fetal
Bovine serum (FBS, Wako Pure Chemical Industries, Osaka, Japan),
penicillin-streptomycin (Invitrogen, Tokyo, Japan), Cell Counting
Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan), Cell Cycle
Phase Determination kit (Cayman Chemical, Ann Arbor, MI, USA),
Annexin V-FITC Early Apoptosis Detection kit (Cell Signaling
Technology, Boston, MA, USA), protease inhibitor cocktail
(Pro-Prep, complete protease inhibitor mixture; iNtRON
Biotechnology, Sungnam, Korea), M30 Apoptosense ELISA kit (PEVIVA
AB, Bromma, Sweden), Human Phospho-RTK Array kits and Angiogenesis
Antibody Array kits (R&D Systems, Minneapolis, MN, USA).
Telmisartan was prepared as a 10 mM stock solution in dimethyl
sulfoxide (DMSO). The stock solutions were stored at −20°C.
Cell lines and culture
The human CCA cell lines HuCCT-1 and TFK-1 were
obtained from the Japanese Cancer Research Resources Bank (JCRB).
All cell lines were grown in RPMI-1640 supplemented with 10% fetal
bovine serum and 100 mg/l of penicillin-streptomycin. Cells were
incubated at 37°C in a humidified atmosphere with 5% of
CO2. Passages were made when confluence reached 80–90%
by tripsinization with 0.05% (for HuCCT-1) or 0.25% (for TFK-1)
trypsin EDTA and gentle mechanical detachment.
Cell proliferation assays
Cell proliferation assays were conducted using CCK-8
according to the manufacturer's instructions, as described in our
previous studies (17,18). These assays were conducted in
HuCCT-1 and TFK-1 cell lines. Cells (5.0×103) from each
cell line were seeded into the wells of a 96-well plate and
cultured in 100 µl of RPMI-1640 supplemented with 10% FBS.
After 24 h, the seeded cells were treated with 0, 10, 50, 100
µM telmisartan or vehicle and then cultured for an
additional 48 h. CCK-8 reagent (10 µl) was added to each
well, and the plates were incubated at 37°C for 3 h. The absorbance
was measured at 450 nm in each well using an automated microplate
reader.
Cell cycle and apoptosis analysis
To evaluate the mechanism by which telmisartan
inhibits tumor growth, cell cycle and apoptosis analysis were
performed separately using a Cell Cycle Phase Determination kit and
an Annexin V-FITC Early Apoptosis Detection kit as described in our
previous studies (19). HuCCT-1
cells (1.0×106 cells in a 100-mm dish) were treated with
or without 100 µM telmisartan for 24–48 h. The cell cycle
distribution was analyzed by measuring the amount of propidium
iodide (PI)-labeled DNA in ethanol-fixed cells. The fixed cells
were washed with PBS and stored at −20°C until analysis by flow
cytometry. On the day of analysis, the cells were washed with cold
PBS, suspended in 100 µl of PBS plus 10 µl of RNase A
(250 µg/ml) and incubated for 30 min. A total of 110
µl of PI stain (100 µg/ml) was added to each tube and
incubated at 4°C for at least 30 min prior to analysis. Apoptotic
and necrotic cell death was analyzed by double staining with
FITC-conjugated Annexin V and PI, which is based on the binding of
Annexin V to apoptotic cells with exposed phosphatidylserines and
the PI labeling of late apoptotic/necrotic cells with membrane
damage. HuCCT-1 cells were treated with or without 100 µM
telmisartan for 24 h. Staining was performed according to the
manufacturer's instructions. Flow cytometry was conducted using a
Cytomics FC 500 flow cytometer (Beckman Coulter, Indianapolis, IN,
USA). The percentages of cells were analyzed using Kaluza software
(Beckman Coulter).
Gel electrophoresis and western
blotting
HuCCT-1 cells (1.0×106/dish) were seeded
in 100-mm culture dishes and cultured for 24 h. Then, 100 µM
telmisartan was added, and the cells were further cultured for
24–48 h. The cells were lysed using a protease inhibitor cocktail
(Pro-Prep, complete protease inhibitor mixture; iNtRON
Biotechnology) on ice for 20 min. Suspensions of lysed cells were
centrifuged at 13,000 × g at 4°C for 5 min; supernatants containing
soluble cellular proteins were collected and stored at −80°C until
use. Protein concentrations were measured using a NanoDrop 2000
fluorospectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
Then, the samples were subjected to sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) on 12% agarose gels,
and the proteins were transferred to nitrocellulose membranes.
After blocking, the membranes were incubated with primary
antibodies followed by secondary antibodies. The immunoreactive
proteins were visualized on X-ray film using an enhanced
chemiluminescence detection system (Perkin-Elmer Co., Waltham, MA,
USA). Following primary antibodies were used: anti-β-actin
monoclonal antibody (used at 1:10,000; Sigma-Aldrich) and
antibodies against cyclin D1 (used at 1:1,000; Thermo Fisher
Scientific), cyclin E (used at 1:1,000; Thermo Fisher Scientific),
Cdk6 (used at 1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA,
USA), Cdk4 (used at 1:1,000; Santa Cruz Biotechnology), Cdk2 (used
at 1:2,000; Santa Cruz Biotechnology), and phosphorylated
retinoblastoma protein (Rb; used at 1:1,000; BD Pharmingen), and
anti-Rb (used at 1:1,000; Santa Cruz Biotechnology). The secondary
antibodies included horseradish peroxidase (HRP)-linked anti-mouse
and anti-rabbit IgG (used at 1:2,000; GE Healthcare Life Sciences,
Chalfont, UK).
ELISA for apoptosis
Caspase-cleaved cytokeratin 18 (cCK18) expression
was evaluated using an M30 Apoptosense ELISA kit (20). HuCCT-1 cells (5×103
cells) were seeded in 96-well plates and cultured for 24 or 48 h
following the addition of 100 µM telmisartan. The subsequent
ELISA procedures were performed according to the manufacturer's
instructions. The amounts of antigen in the control and treated
samples were calculated via interpolation of a standard curve.
Antibody arrays of phosphorylated
receptor tyrosine kinases (p-RTKs)
Human p-RTKs were assayed using Human Phospho-RTK
Array Kits according to the manufacturer's instructions. Briefly,
p-RTK array membranes were blocked with 5% BSA/TBS (0.01 M
Tris-HCl, pH 7.6) for 1 h and incubated with 2 ml of lysate
prepared from the previously mentioned cells after normalization so
that the amounts of protein were equal. After 3 washes for 10 min
each with TBS plus 0.1% v/v Tween-20 and 2 washes for 10 min with
TBS alone to remove unbound materials, the membranes were incubated
with an HRP-conjugated anti-phosphotyrosine antibody for 2 h at
room temperature. The unbound HRP antibody was washed out with TBS
plus 0.1% Tween-20. Finally, each array membrane was exposed to
X-ray film using a chemiluminescence detection system (Perkin-Elmer
Co.). The density of the immunoreactive band obtained 118 on this
array was analyzed by densitometric scanning (TIc 119 scanner;
Shimizu Co, Ltd., Kyoto, Japan).
Angiogenic profile analysis using an
antibody array
A Human Angiogenesis Antibody Array was used
according to the manufacturer's protocol. This method is a
dot-based assay enabling the detection and comparison of 55
angiogenesis-specific cytokines. Each array membrane was exposed to
X-ray film using a chemiluminescence detection system (Perkin-Elmer
Co.). The immunoreactive bands were analyzed by densitometric
scanning.
miRNA arrays
HuCCT-1 cells were treated with 100 µM
telmisartan for 48 h and were stored in RNAprotect Reagent (Qiagen,
Venlo, The Netherlands). Total RNA was extracted from cancer cell
lines using a miRNeasy Mini kit (Qiagen, Hilden, Germany) according
to the manufacturer's instructions. RNA samples typically exhibited
A260/280 ratios of between 1.9 and 2.1, as determined using an
Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA,
USA). After RNA measurements were performed with an RNA 6000 Nano
kit (Agilent Technologies), the samples were labeled using a
miRCURYHy3/Hy5 Power Labeling kit and were subsequently hybridized
to a human miRNA Oligo chip (v. 21.0; Toray Industries, Tokyo,
Japan). The chips were scanned with a 3D-Gene Scanner 3000 (Toray
Industries), and the results were analyzed using 3D-Gene extraction
version 1.2 software (Toray Industries). To determine the
difference in miRNA expression between the telmisartan-treated and
control samples, the raw data were analyzed using GeneSpring GX
10.0 software (Agilent Technologies). Quantile normalization was
performed on the raw data that were above the background level.
Differentially expressed miRNAs were determined by the Mann-Whitney
U test. Hierarchical clustering was performed using the furthest
neighbor method with the absolute uncentered Pearson's correlation
coefficient as a metric. A heat map was produced with the relative
expression intensity for each miRNA, in which the base-2 logarithm
of the intensity was median-centered for each row.
Xenograft model analysis
Animal experiments were performed according to the
guidelines of the Committee on Experimental Animals of Kagawa
University, Kagawa, Japan. We purchased 21 male athymic mice
(BALB/c-nu/nu; 6 weeks old; 20–25 g) from Japan SLC (Shizuoka,
Japan). Moreover, the mice were maintained under specific
pathogen-free conditions using a laminar airflow rack. The mice had
continuous free access to sterilized (γ-irradiated) food (CL-2;
CLEA Japan, Inc.) and autoclaved water. Each mouse was
subcutaneously inoculated with HuCCT-1 cells (3×106
cells per animal) in the flank region. Then, when the xenografts
were identifiable as masses with a maximal diameter >3 mm, we
randomly assigned the animals to 3 groups. These groups were
treated with 50 µg per day of telmisartan (n=7), or with 100
µg per day of telmisartan (n=7) or diluent only (n=7). The
telmisartan-treated groups were intraperitoneally (i.p.) injected
telmisartan (50 or 100 µg) five times per week; the control
group was administered 5% DMSO alone. The tumor growth was
monitored daily by the same investigators (E. Samukawa and T.
Masaki), and the tumor size was measured two times per week. The
tumor volume (mm3) was calculated as the tumor length
(mm) × tumor width (mm)2/2 (21). All animals were sacrificed on day
31 after treatment, with all animals remaining alive during this
period.
Statistical analyses
All statistical analyses were performed using
GraphPad Prism software version 6.0 (GraphPad Software, San Diego,
CA, USA). Comparisons between treatment and control groups were
performed using the unpaired t-tests. A P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
Telmisartan inhibits cell proliferation
and viability of human CCA cells
To explore the role of telmisartan on the
proliferation of CCA cells, HuCCT-1 and TFK1 cells were treated
with different concentrations of telmisrtan for 24 to 48 h. HuCCT-1
and TFK-1 cells were grown in 10% FBS and treated with 0, 10, 50,
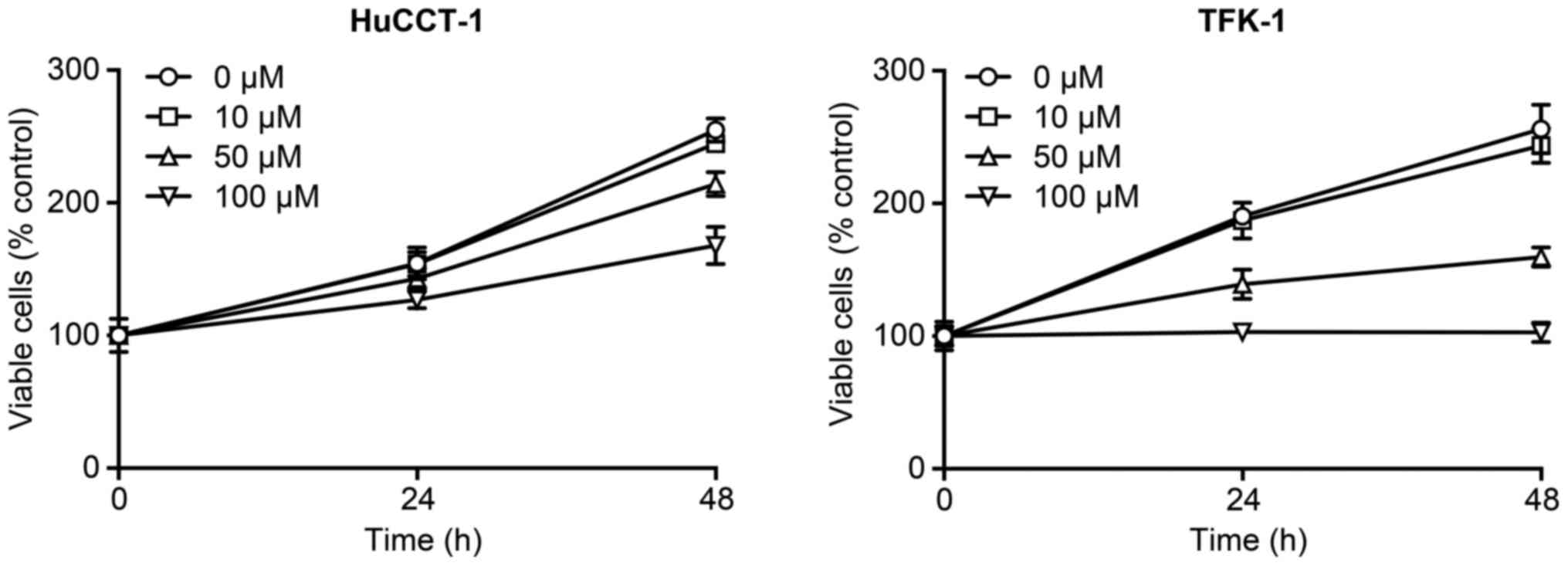
or 100 µM of telmisartan for 48 h. Telmisartan treatment
reduced the proliferation of HuCCT-1 and TFK-1 cells (Fig. 1). These results show that
telmisartan inhibits cell proliferation in CCA cell lines in a
dose-dependent manner.
Telmisartan induces
G0/G1 phase cell cycle arrest and regulates
cell cycle-related proteins in CCA cells
To investigate the effects of telmisartan in the CCA
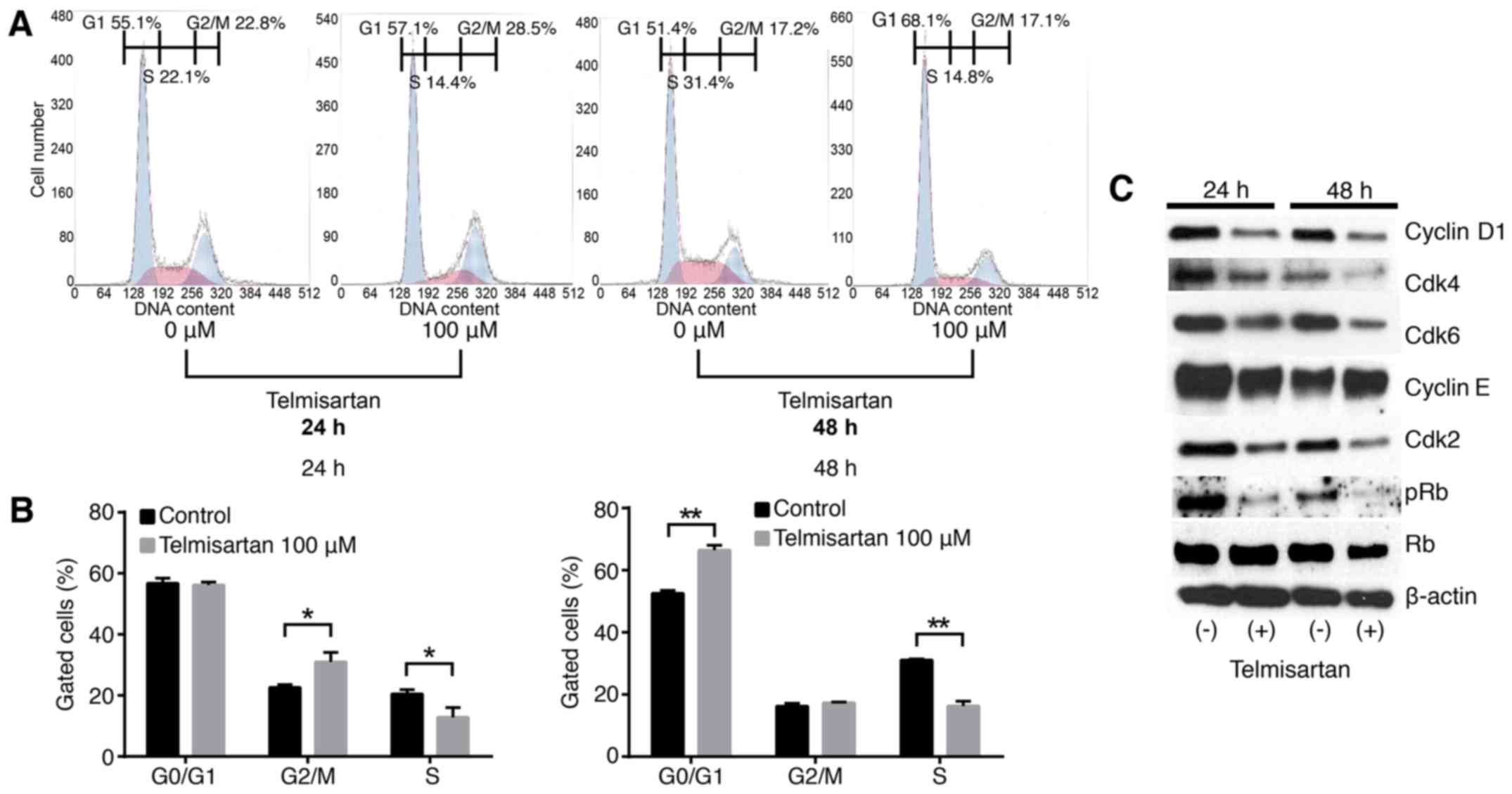
cell lines, we examined cell cycle progression using flow cytometry
analysis in HuCCT-1 cells. Flow cytometry was performed on PI
stained cells to determine the effects of telmisartan on the cell
cycle progression. Treatment with 100 µM of telmisartan
increased the population of cells in G1 phase and
reduced the populations of cells in the S phase for 48 h after
treatment (Fig. 2A and B).
The effects of telmisartan on the expression of
various cell cycle-related proteins in HuCCT-1 cells were evaluated
by western blotting. Cells were treated with 0 or 100 µM
telmisartan for 24–48 h. The strongest reduction was observed in
cyclin D1, key proteins involved in the transition from
G0 to G1 phase, by telmisartan in a
time-dependent manner (Fig. 2C).
Additionally, analysis of other proteins associated with the
G0 to G1 transition indicated that Cdk4 and
Cdk6, the catalytic subunits of cyclin D1 were decreased in HuCCT-1
cells 24–48 h after the addition of telmisartan (Fig. 2C). With the treatment of
telmisartan, although cyclin E that is the key cyclin for the G1/S
transition was unchanged, its catalytic subunit of Cdk2 was
decreased (Fig. 2C). Cyclin
D1/Cdk4, cyclin D1/Cdk6 and cyclin E/Cdk2 are responsible for the
phosphorylation of retinoblastoma (pRB) (22–24).
In the present study, pRB was reduced in CCA cells with treatment
of telmisartan.
These findings suggest that telmisartan inhibits
cell cycle progression from G0/G1 to S-phase
by decreasing cyclin D1 and CDKs levels, which results in
G1 cell cycle arrest in CCA cells.
No effects of telmisartan on the
apoptosis in HuCCT-1 cells
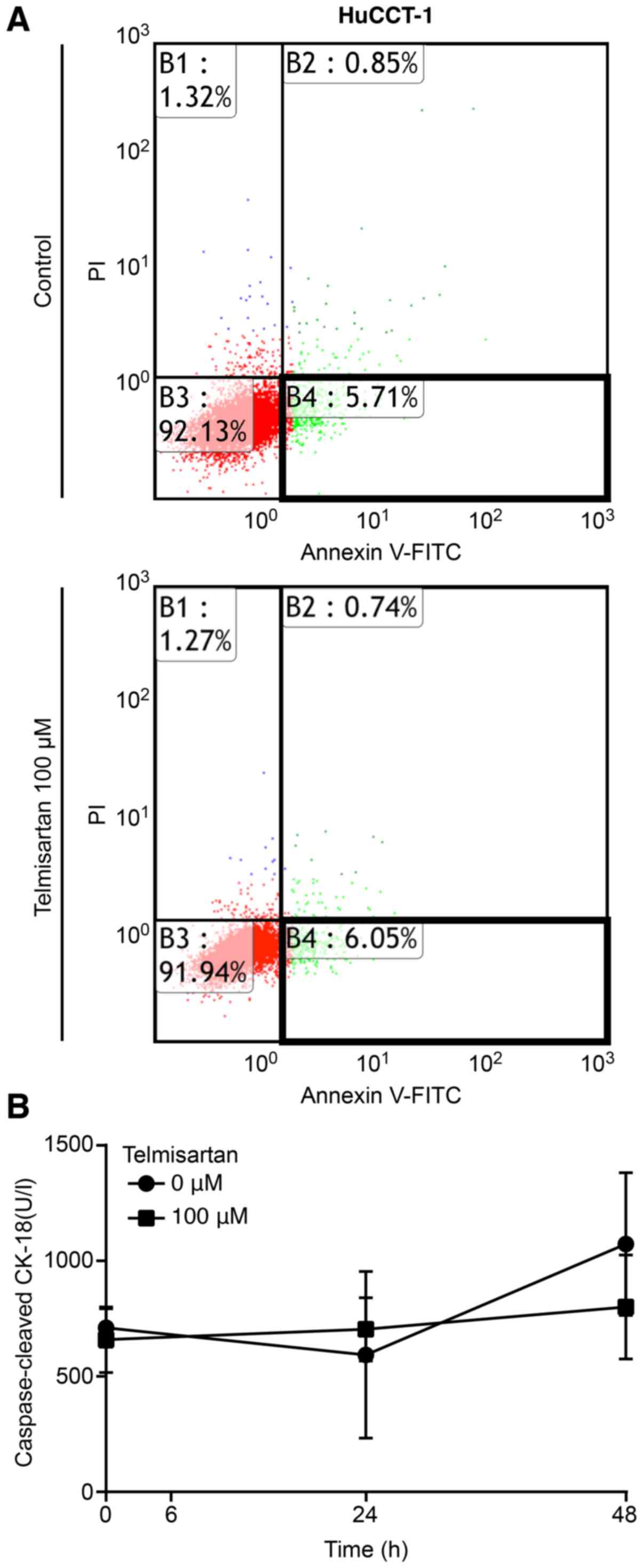
To determine whether telmisartan induced apoptosis,
CCA cells were treated with or without 100 µM telmisartan
for 24 h and analyzed using Annexin V-FITC/PI staining and flow
cytometry. Telmisartan did not change the proportion of apoptotic
cancer cells 24 h after treatment in HuCCT-1 cells (Fig. 3A, enclosed areas in bold squares).
Additionally, the levels of cCK18 following telmisartan treatment
were measured using an M30 ELISA kit. The results showed that
telmisartan did not increased the levels of cCK-18 in HuCCT-1 cells
(Fig. 3B). These results indicate
that telmisartan inhibits CCA cell proliferation without inducing
apoptosis.
Effects of p-RTK in HuCCT-1 cells with
telmisartan treatment
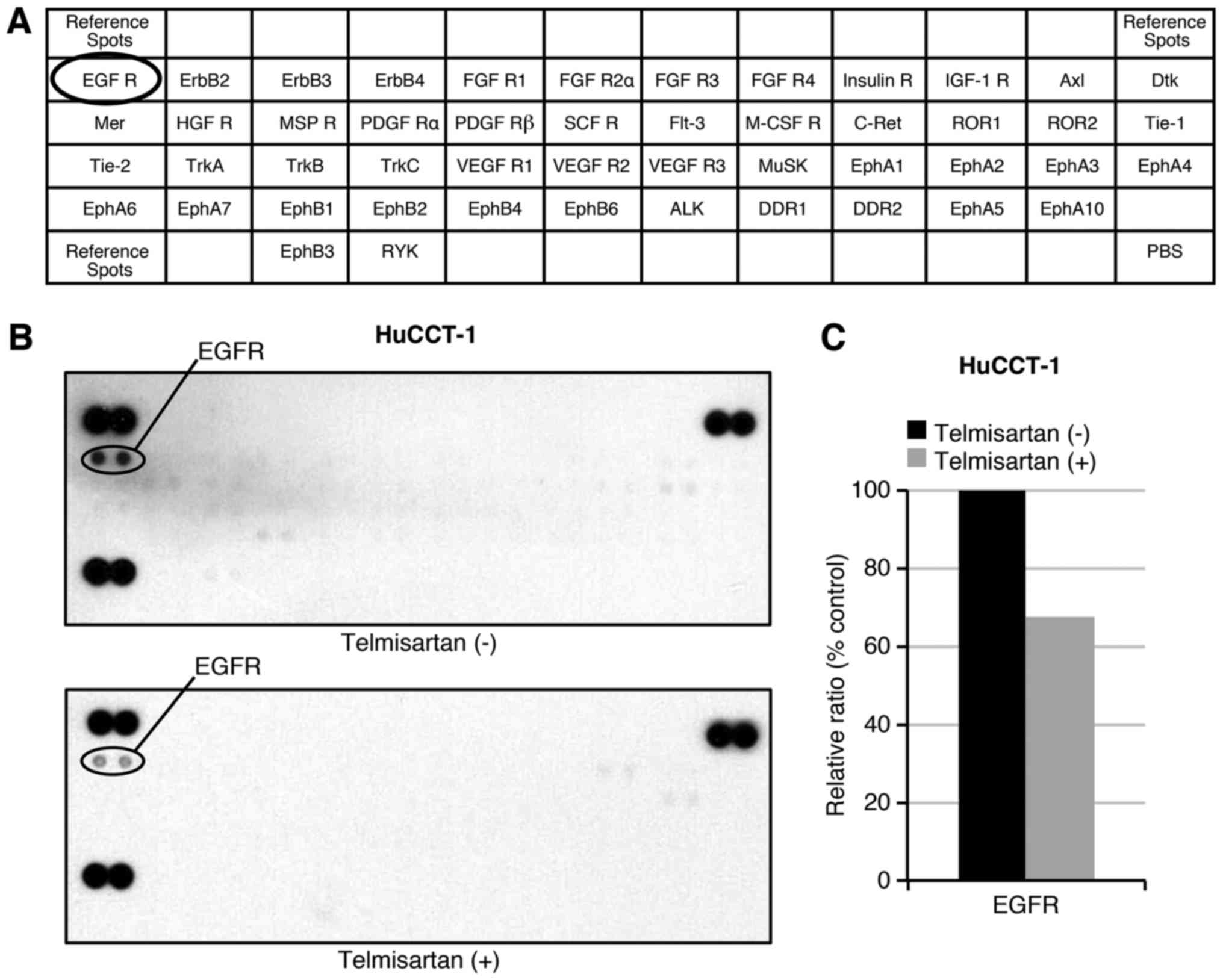
We used a p-RTK array to identify the key RTKs
associated with the antitumor effects of telmisartan. Using an
antibody array (Fig. 4A), we
simultaneously analyzed the expression of 46 different activated
RTKs in HuCCT-1 cells 48 h after telmisartan administration.
Telmisartan reduced the expression of phosphorylated EGFR in
vitro (Fig. 4B). The
densitometric analyses of p-EGFR showed decreases of 67.4%
(Fig. 4C). Thus, telmisartan may
inhibit the activation of EGFR and decreased the cell cycle
regulatory molecules partially through the in CCA cells.
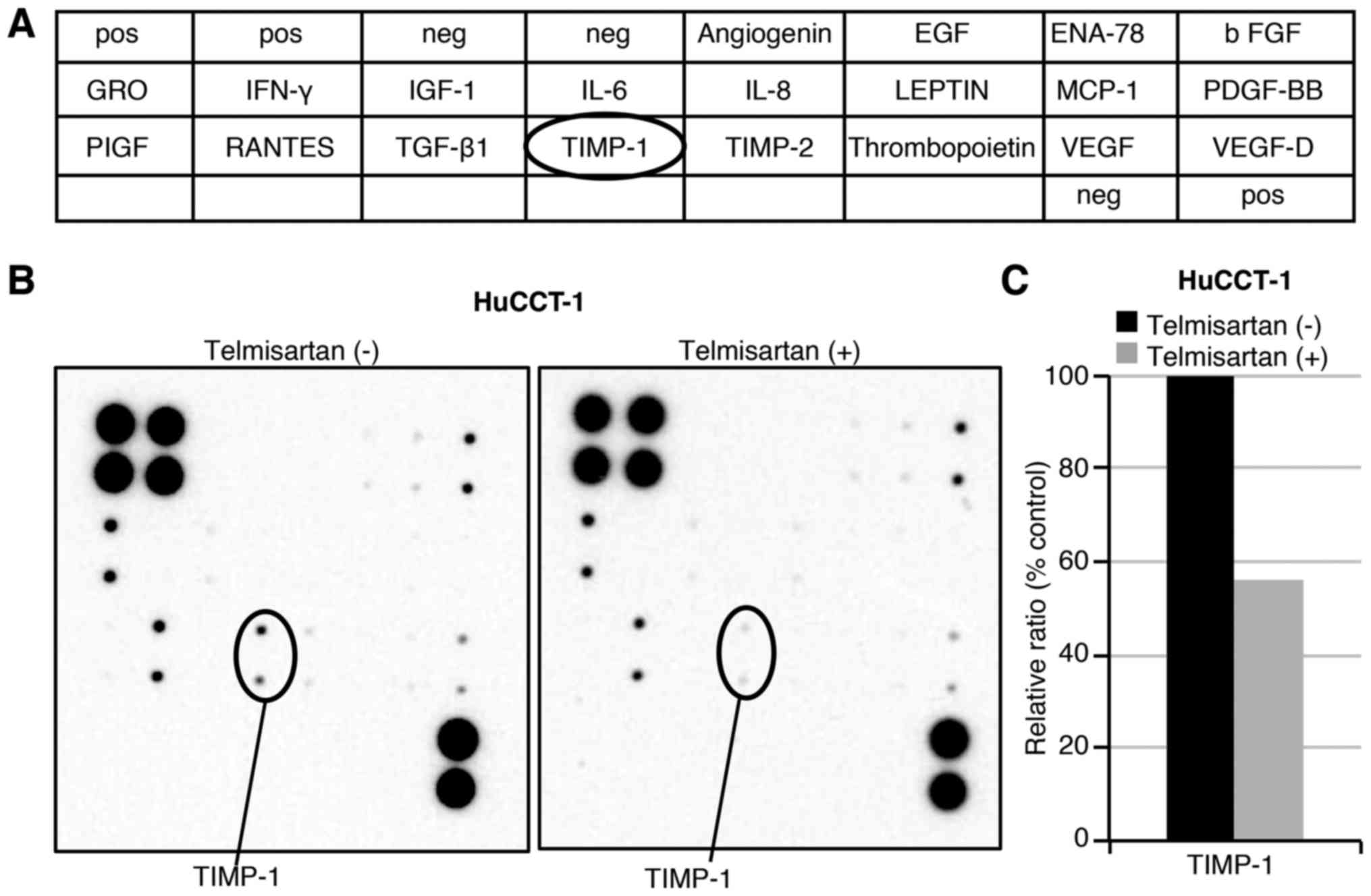
Effects of telmisartan on angiogenesis in
HuCCT-1 cells
To examine the relationship between angiogenesis and
telmisartan, angiogenesis antibody analysis was conducted regarding
the antitumor effects of telmisartan (Fig. 5A). Using the antibody array, we
simultaneously screened the expression levels of 20 different
angiogenesis-related proteins in HuCCT-1 cells with or without
telmisartan. The expression levels of TIMP-1 was induced by
telmisartan treatment in HuCCT-1 cells as detected by the protein
array (Fig. 5B). Densitometric
analyses indicated that the ratios of the TIMP-1 spots of the
telmisartan-treated cells to those of the untreated cells was 56.0%
(Fig. 5C).
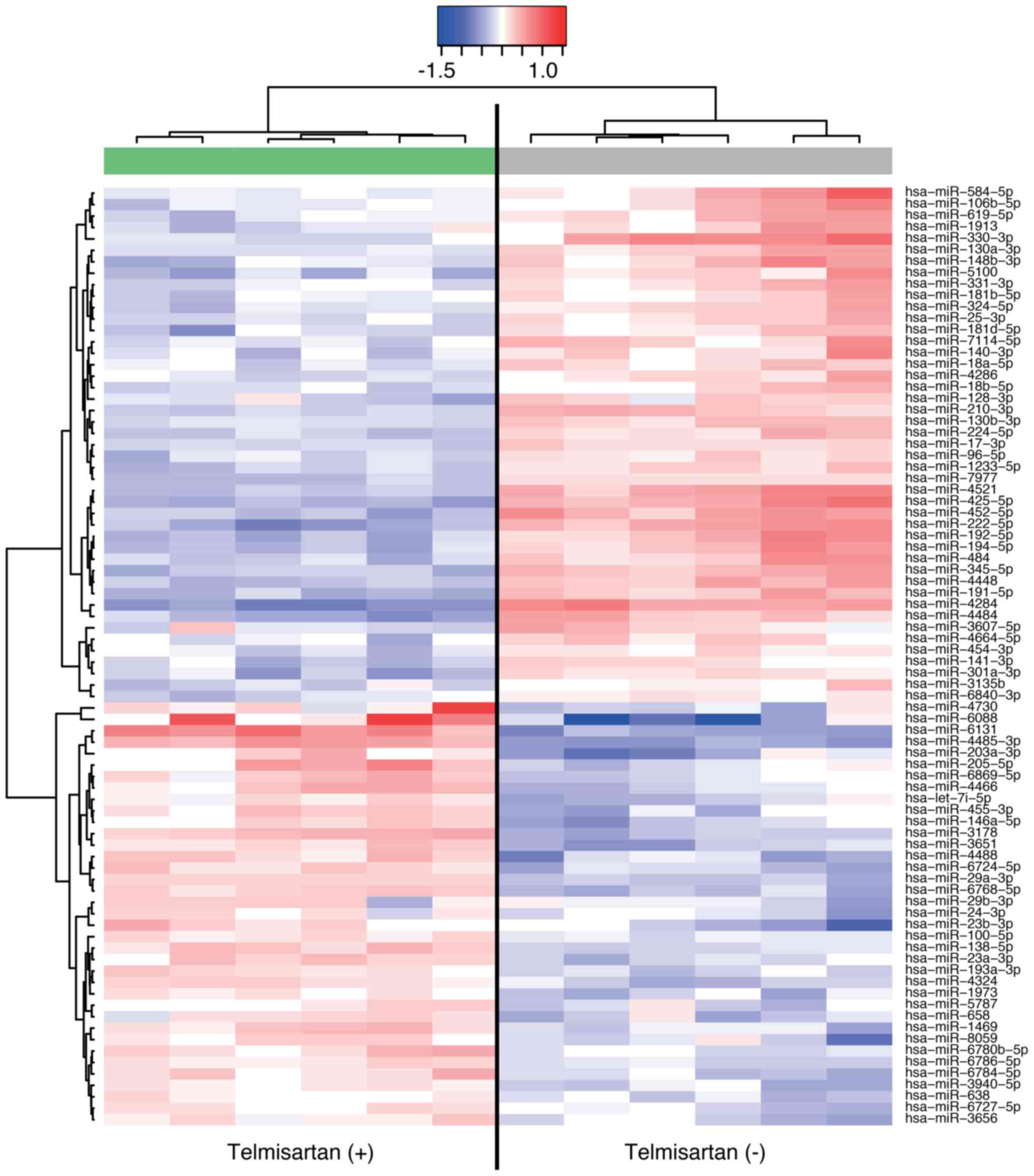
Telmisartan affects miRNA expression in
HuCCT-1 cells
Using a custom microarray platform, we analyzed the
expression levels of 2555 miRNA probes in the cell lines in the
presence and absence of telmisartan. Treatment with 100 µM
telmisartan for 48 h resulted in the upregulation of 37 miRNAs and
the downregulation of 45 miRNAs in HuCCT-1 cells (Table I). Unsupervised hierarchical
clustering analysis was conducted using Pearson's correlation, and
the results indicated that the telmisartan-treated group clustered
separately from the non-treated group (Fig. 6).
 | Table IStatistical analysis results and
chromosomal locations of miRNAs in HuCCT-1 cells treated with and
without telmisartan. |
Table I
Statistical analysis results and
chromosomal locations of miRNAs in HuCCT-1 cells treated with and
without telmisartan.
| MicroRNAs | Fold-changea | P-value | Chromosomal
localization |
|---|
| Upregulated | | | |
| hsa-miR-6088 | 2.41 | 0.0087 | 19q13.32 |
| hsa-miR-6131 | 2.27 | 0.0050 | 5p15.2 |
|
hsa-miR-4485-3p | 2.02 | 0.0050 | 11 |
| hsa-miR-3178 | 1.63 | 0.0050 | 16p13.3 |
|
hsa-miR-6768-5p | 1.54 | 0.0049 | 16 |
| hsa-miR-4488 | 1.51 | 0.0050 | 11 |
| hsa-miR-3651 | 1.48 | 0.0022 | 9 |
| hsa-miR-4730 | 1.48 | 0.0260 | 17 |
|
hsa-miR-203a-3p | 1.48 | 0.0200 | 14q32.33 |
|
hsa-miR-23b-3p | 1.46 | 0.0114 | 9q22.32 |
|
hsa-miR-29a-3p | 1.45 | 0.0025 | 7q32.1 |
|
hsa-miR-205-5p | 1.43 | 0.0129 | 1q32.2 |
| hsa-miR-4466 | 1.43 | 0.0049 | 6 |
|
hsa-miR-6724-5p | 1.42 | 0.0047 | |
| hsa-miR-4324 | 1.39 | 0.0050 | 19 |
|
hsa-miR-138-5p | 1.39 | 0.0049 | |
|
hsa-miR-455-3p | 1.39 | 0.0101 | 9q32 |
|
hsa-miR-146a-5p | 1.38 | 0.0103 | 5q33.3 |
|
hsa-miR-6784-5p | 1.37 | 0.0063 | 17 |
| hsa-miR-1469 | 1.37 | 0.0022 | 15q26.2 |
|
hsa-miR-23a-3p | 1.34 | 0.0056 | 19p13.13 |
|
hsa-miR-6869-5p | 1.34 | 0.0129 | 20 |
| hsa-miR-8059 | 1.31 | 0.0159 | 17 |
| hsa-let-7i-5p | 1.31 | 0.0123 | 12q14.1 |
|
hsa-miR-193a-3p | 1.31 | 0.0050 | 17q11.2 |
|
hsa-miR-6780b-5p | 1.3 | 0.0043 | 17 |
| hsa-miR-658 | 1.3 | 0.0124 | 22q13.1 |
| hsa-miR-1973 | 1.29 | 0.0049 | 4 |
|
hsa-miR-6786-5p | 1.29 | 0.0050 | 17 |
|
hsa-miR-3940-5p | 1.29 | 0.0050 | 19 |
| hsa-miR-3656 | 1.27 | 0.0127 | 11 |
|
hsa-miR-29b-3p | 1.23 | 0.0484 | 3 |
|
hsa-miR-6727-5p | 1.23 | 0.0046 | 1 |
| hsa-miR-5787 | 1.23 | 0.0235 | 3 |
|
hsa-miR-100-5p | 1.22 | 0.0043 | 11q24.1 |
| hsa-miR-24-3p | 1.22 | 0.0354 | 8 |
| hsa-miR-638 | 1.22 | 0.0043 | 19p13.2 |
| Downregulated | | | |
| hsa-miR-4284 | 0.43 | 0.0044 | 7 |
|
hsa-miR-425-5p | 0.5 | 0.0049 | 3p21.31 |
|
hsa-miR-222-5p | 0.51 | 0.0050 | Xp11.3 |
| hsa-miR-4521 | 0.56 | 0.0049 | 1 |
|
hsa-miR-452-5p | 0.57 | 0.0022 | Xq28 |
|
hsa-miR-192-5p | 0.57 | 0.0050 | 11q13.1 |
| hsa-miR-4484 | 0.58 | 0.0050 | 10 |
|
hsa-miR-330-3p | 0.58 | 0.0046 | 19q13.32 |
|
hsa-miR-194-5p | 0.6 | 0.0022 | |
|
hsa-miR-191-5p | 0.61 | 0.0048 | 3p21.31 |
|
hsa-miR-345-5p | 0.61 | 0.0047 | 14q32.2 |
| hsa-miR-4448 | 0.62 | 0.0022 | 3q27.1 |
| hsa-miR-484 | 0.65 | 0.0022 | 16p13.11 |
| hsa-miR-5100 | 0.66 | 0.0048 | 10q11.21 |
|
hsa-miR-210-3p | 0.66 | 0.0049 | 11p15.5 |
|
hsa-miR-148b-3p | 0.67 | 0.0064 | 12q13.13 |
|
hsa-miR-584-5p | 0.69 | 0.0050 | 5q32 |
|
hsa-miR-224-5p | 0.7 | 0.0050 | Xq28 |
|
hsa-miR-1233-5p | 0.71 | 0.0050 | 15 |
|
hsa-miR-301a-3p | 0.71 | 0.0050 | 17q22 |
|
hsa-miR-619-5p | 0.71 | 0.0050 | 12q24.11 |
| hsa-miR-7977 | 0.71 | 0.0023 | 3 |
|
hsa-miR-130a-3p | 0.71 | 0.0035 | 11q12.1 |
|
hsa-miR-7114-5p | 0.72 | 0.0022 | 9 |
|
hsa-miR-130b-3p | 0.72 | 0.0049 | 22 |
|
hsa-miR-106b-5p | 0.73 | 0.0048 | 7q22.1 |
|
hsa-miR-324-5p | 0.73 | 0.0022 | 17p13.1 |
|
hsa-miR-181d-5p | 0.74 | 0.0064 | 19p13.13 |
| hsa-miR-1913 | 0.74 | 0.0087 | 6 |
|
hsa-miR-140-3p | 0.75 | 0.0087 | 16q22.1 |
| hsa-miR-17-3p | 0.75 | 0.0048 | 13q31.3 |
|
hsa-miR-128-3p | 0.76 | 0.0043 | |
|
hsa-miR-141-3p | 0.76 | 0.0100 | 12p13.31 |
|
hsa-miR-331-3p | 0.76 | 0.0129 | 12q22 |
| hsa-miR-25-3p | 0.76 | 0.0077 | 7q22.1 |
| hsa-miR-96-5p | 0.76 | 0.0048 | 7q32.2 |
| hsa-miR-4286 | 0.77 | 0.0048 | 8 |
|
hsa-miR-18b-5p | 0.77 | 0.0049 | Xq26.2 |
|
hsa-miR-181b-5p | 0.78 | 0.0064 | |
|
hsa-miR-18a-5p | 0.78 | 0.0063 | 13q31.1 |
|
hsa-miR-3607-5p | 0.78 | 0.0303 | 5q14.3 |
|
hsa-miR-4664-5p | 0.79 | 0.0087 | 8 |
|
hsa-miR-454-3p | 0.81 | 0.0049 | 17q22 |
|
hsa-miR-6840-3p | 0.82 | 0.0079 | 7 |
| hsa-miR-3135b | 0.82 | 0.0367 | 6 |
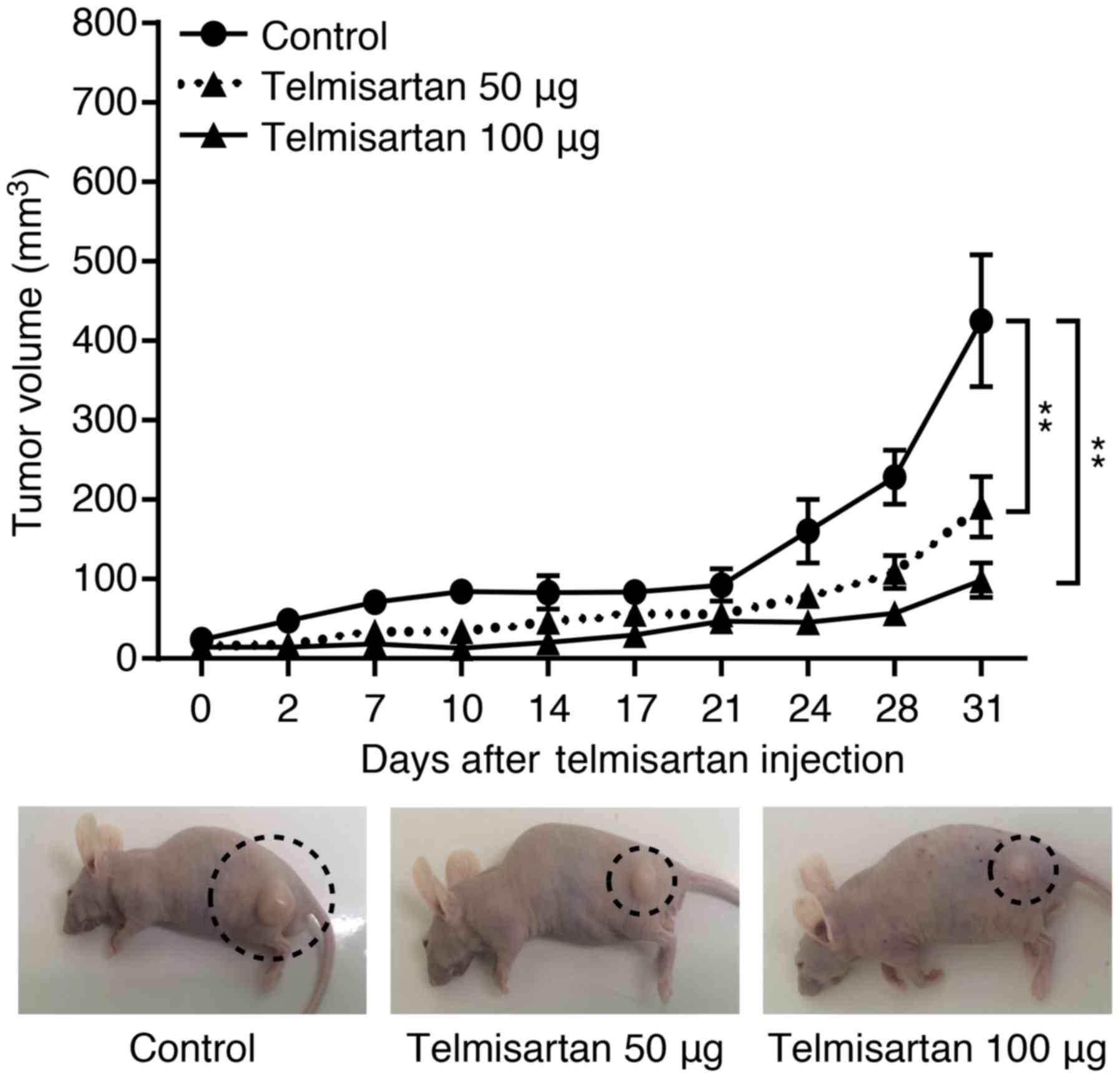
Telmisartan inhibits tumor proliferation
in vivo
To determine whether telmisartan could affect tumor
growth in vivo, we subcutaneously injected nude mice with
HuCCT-1 cells, followed by i.p. injection of telmisartan. The
telmisartan treatment significantly inhibited tumor growth by 55.1%
(with 50 µg) and 76.8% (with 100 µg), as determined
by integrated tumor growth curves (Fig. 7), compared with that of the
untreated control mice (**P<0.001). In addition, 100
µg telmisartan treatment did not inhibit tumor growth
significantly compared with 50 µg telmisartan-treated mice.
Telmisartan had no apparent toxic effects on the mice and no effect
on body weight during the study. Furthermore, all animals survived
to the end of the experiment.
Discussion
Angiotensin receptor blockers (ARBs) including
telmisartan are widely prescribed drugs for the treatment of
hypertension. Various cancer cells have recently been reported to
express AT1R such as a breast, renal cancer, and hepatocellular
carcinoma (25–27). Some epidemiologic studies revealed
that the use of ARBs may increase the risk of cancer (28). Meanwhile, several studies indicated
that ARB treatment of hypertensive patients was associated with
lower cancer incidence and mortality rates (29,30).
According to previously controversial clinical studies, the
antitumor effect of telmisartan remains inadequately known.
Moreover, in previous studies, telmisartan has been shown to
inhibit proliferation of various cancer cells (31–33)
in vitro and tumor growth in vivo (5–7).
However, there are no any studies on antitumor effects of
telmisartan CCA date. Therefore, in the present study, we focused
on the antitumor effects of telmisartan in CCA. This report is the
first study showing that telmisartan inhibits CCA cells in
vitro and in vivo.
In the present study, telmisartan induced cell cycle
arrest at the G0/G1 phase, which was
correlated with a remarkable decrease in the expression of cyclinD1
and its catalytic subunits, Cdk2 and Cdk4. Complexes of Cdk4 and
Cdk6 with cyclin D1 are required for G1 phase
progression (22), the expression
of various cell cycle-related molecules is related to cancer
progression and prognosis (22,34).
These data indicate that the major cell cycle regulators may be
intracellular targets of telmisartan in human CCA cells. In our
previous studies, in esophageal adenocarcinoma cells, telmisartan
treatment led to a dose-dependent inhibition of proliferation
through a decrease in cyclin D1 levels, correlated with blocking
cell cycle in the G0/G1 phase (11). Our results also demonstrated that
telmisartan induce cell cycle arrest in the
G0/G1 phases by decreasing cyclin D1, Cdk4,
Cdk6, Cdk2. Moreover, telmisartan induces antiproliferative effects
by phosphorylation of AMPKα in EAC cells, suggesting that the
activation of the AMPKα/mTOR pathway inhibits cell cycle regulatory
proteins (11). However, in our
present study, telmisartan did not induce the phosphorylation of
AMPKα in CCA cells (data not shown). Therefore, these results
indicate that telmisartan may reduce the expression of cell
cycle-related proteins and induced cell cycle arrest without
AMPKα/mTOR pathway in CCA cells.
Our in vitro study used a higher dose of
telmisartan than that used in human treatments (1–10 µM)
(35–37). However, telmisartan of higher doses
used in the present study has been criticized in similar studies
examining other cancer cell types, such as esophagus (11), stomach (7), and prostate cancer cells (8). Our in vivo study used a
slightly higher dose of telmisartan than that used in human
administration. Our results revealed that a slightly higher dose of
telmisartan also markedly suppressed the growth of subcutaneous CCA
tumors in athymic nude mice. These data (in vivo study)
suggest that the use of telmisartan in CCA treatment may be
effective in combination therapy with other anticancer drugs
(38).
Telmisartan has been shown to inhibit cell
proliferation by inducing apoptosis in various cancer cell lines,
namely, prostate (8), renal
(9), endometrial (6) and colon (10) cancer lines. To determine whether
telmisartan induced apoptosis, HuCCT-1 cells were treated with or
without 100 µM telmisartan and analyzed using Annexin
V-FITC/PI staining and measuring the levels of cCK18, but it did
not induce apoptosis and cell death after telmisartan-treatment in
HuCCT-1 cells. These data indicated that telmisartan inhibits CCA
cell proliferation without inducing apoptosis. This discrepancy in
our data and previous other studies (6,8–10)
could due to the differences in the properties of different types
of cancers.
Our previous studies evaluated the existence of an
association between telmisartan and the inhibition of esophageal
adenocarcinoma cell proliferation through p-RTK regulation
(11). We demonstrated that
telmisartan reduces the phosphorylation of EGFR in CCA cells using
p-RTK arrays.
EGFR activation was detected in some human cancers,
and EGFR activation could induce cyclin D1, a protein that is
important in cell cycle progression (15,39,40,41).
In addition, EGFR might act as a hub for transmitting downstream
signals to activate some proliferation signals, such as, RAS-MAPK,
JAK-STAT, and PI3K-Akt-mTOR pathways (42,43).
In short, the EGFR pathway is important in controlling cell cycle.
Thus, telmisartan may partially inhibit cell cycle regulatory
proteins via decreasing the phosphorylation of EGFR to regulate
cell proliferation in CCA cells.
TIMP-1 was overexpressed in the progression of
chorangiocarcinoma (44). In our
present study, telmisartan also reduced TIMP-1 levels in HuCCT-1
cells. These data suggest that telmisartan may play an important
role in angiogenesis and tumor development through reduction of
TIMP-1.
MicroRNAs are small, endogenous, noncoding RNA
sequences that can modulate protein expression by regulating
translational efficiency or the cleavage of target mRNA molecules
(45). It has become apparent that
miRNAs regulate the development and progression of various cancers
(46). To identify the miRNAs
associated with the antitumor effects of telmisartan, we used miRNA
expression arrays. Several miRNAs were significantly altered
following telmisartan treatment in vitro. Among these
miRNAs, let-7i was significantly upregulated in HuCCT-1 cells
treated with telmisartan. The let-7 family contains 13 members and
is widely recognized as a class of miRNAs that produce
tumor-suppressing effects. Reduction of let-7 family members have
been reported in various cancers, including colorectal cancer
(47), hepatocellular carcinoma
(48), lung cancer (49) and breast cancer (50). The let-7 family members act as
tumor suppressor molecules by binding its target oncogenes, such as
HMGA2 (51), Ras (52) and c-Myc (53). Moreover, Sun et al reported
that in breast cancer, let-7 directly inhibits cyclin D1
expression, resulting in low phosphorylation of Akt1, which is
critical for the let-7 induced inhibition of mammosphere numbers
(54).
In addition, as shown in Table I, miR-3178 was also significantly
upregulated in HuCCT-1 cells treated with telmisartan. miR-3178 has
lower expression in human pancreatic cancer tissues than in healthy
control tissues (55), and
hepatocellular carcinoma (HCC) tumor endothelial cells (TECs) than
in hepatic sinusoidal endothelial cells (HSECs) (56). miR-3178 acts as a tumor suppressor
to inhibit the proliferation, migration, invasion, and angiogenesis
and promoted the apoptosis and G1 phase arrest of HCC TECs in
vitro (57). On the other
hand, in the present study, miR-425-5p was remarkably down
regulated in HuCCT-1 cells treated with telmisartan. miR-425-5p has
recently been reported to be upregulated and to promote
tumourigenesis in various cancer types (58–60).
PTEN is one of the major target genes for miR-425-5p, and it has
been reported as a tumour suppressor (61,62).
For this reason, it was suggested that the downregulation of
miR-425-5p led to antitumor effect. In the present study, miR-222
was also down-regulated in HuCCT-1 cells treated with telmisartan.
miR-222 was upregulated in several cancers, and also recognized as
oncogenic miRNA (63–67). The cell cycle-dependent kinase
inhibitor, p27Kip1 is the target gene of miR-222 (63,64,67).
Therefore, the downregulation of miR-222 led to G1 arrest. Thus,
telmisartan may regulate cell cycle regulatory molecules through
miR-425-5p or miR-222 to regulate cell proliferation in HuCCT-1
cells.
Collectively, our data suggest that telmisartan
induced inhibition of human CCA cell proliferation, in part, by the
tumor suppressor activities caused by downregulation of above
described miRNAs (miR-425-5p and miR-222). Furthermore, in the
present study, miR-6088 and miR-6131 were remarkably upregulated in
cells treated with telmisartan comparerd with control cells.
However, the target gene of these miRNAs remains unknown.
Therefore, we need to elucidate further the function of these
microRNAs.
Telmisartan is a drug widely used for the treatment
of hypertension with limited side effect. Thus, telmisartan of a
long-term management of CCA may become effective, and benefits of
low costs therapy for the treatment in the patients with
hypertension. In conclusion, our results revealed that telmisartan
inhibits human CCA cell proliferation by inducing cell cycle arrest
and certain miRNAs.
Acknowledgments
The authors would like to thank Ms. Kayo Hirose, Ms.
Kana Ogawa, Ms. Keiko Fujikawa, Ms. Miwako Watanabe, Ms. Megumi
Okamoto, and Ms. Fuyuko Kokado for their skillful technical
assistance.
Glossary
Abbreviations
Abbreviations:
|
CCA
|
cholangiocarcinoma
|
|
ARBs
|
angiotensin II type 1 receptor
blockers
|
|
AT1
|
angiotensin II type 1
|
|
AMPK
|
AMP-activated protein kinase
|
|
EGFR
|
epidermal growth factor receptor
|
|
DMSO
|
dimethyl sulfoxide
|
|
CCK-8
|
Cell Counting Kit-8
|
References
|
1
|
Blechacz B and Gores GJ:
Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and
treatment. Hepatology. 48:308–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Welzel TM, McGlynn KA, Hsing AW, O'Brien
TR and Pfeiffer RM: Impact of classification of hilar
cholangiocarcinomas (Klatskin tumors) on the incidence of intra-
and extrahepatic cholangiocarcinoma in the United States. J Natl
Cancer Inst. 98:873–875. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Everhart JE and Ruhl CE: Burden of
digestive diseases in the United States Part III: Liver, biliary
tract, and pancreas. Gastroenterology. 136:1134–1144. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tyson GL and El-Serag HB: Risk factors for
cholangiocarcinoma. Hepatology. 54:173–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kozako T, Soeda S, Yoshimitsu M, Arima N,
Kuroki A, Hirata S, Tanaka H, Imakyure O, Tone N, Honda S, et al:
Angiotensin II type 1 receptor blocker telmisartan induces
apoptosis and autophagy in adult T-cell leukemia cells. FEBS Open
Bio. 6:442–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koyama N, Nishida Y, Ishii T, Yoshida T,
Furukawa Y and Narahara H: Telmisartan induces growth inhibition,
DNA double-strand breaks and apoptosis in human endometrial cancer
cells. PLoS One. 9:e930502014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okazaki M, Fushida S, Harada S, Tsukada T,
Kinoshita J, Oyama K, Tajima H, Ninomiya I, Fujimura T and Ohta T:
The angiotensin II type 1 receptor blocker candesartan suppresses
proliferation and fibrosis in gastric cancer. Cancer Lett.
355:46–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Funao K, Matsuyama M, Kawahito Y, Sano H,
Chargui J, Touraine JL, Nakatani T and Yoshimura R: Telmisartan is
a potent target for prevention and treatment in human prostate
cancer. Oncol Rep. 20:295–300. 2008.PubMed/NCBI
|
|
9
|
Funao K, Matsuyama M, Kawahito Y, Sano H,
Chargui J, Touraine JL, Nakatani T and Yoshimura R: Telmisartan as
a peroxisome proliferator-activated receptor-γ ligand is a new
target in the treatment of human renal cell carcinoma. Mol Med Rep.
2:193–198. 2009.PubMed/NCBI
|
|
10
|
Lee LD, Mafura B, Lauscher JC, Seeliger H,
Kreis ME and Gröne J: Antiproliferative and apoptotic effects of
telmisartan in human colon cancer cells. Oncol Lett. 8:2681–2686.
2014.PubMed/NCBI
|
|
11
|
Fujihara S, Morishita A, Ogawa K, Tadokoro
T, Chiyo T, Kato K, Kobara H, Mori H, Iwama H and Masaki T: The
angiotensin II type 1 receptor antagonist telmisartan inhibits cell
proliferation and tumor growth of esophageal adenocarcinoma via the
AMPKα/mTOR pathway in vitro and in vivo. Oncotarget. 8:8536–8549.
2017.PubMed/NCBI
|
|
12
|
Briggs CD, Neal CP, Mann CD, Steward WP,
Manson MM and Berry DP: Prognostic molecular markers in
cholangiocarcinoma: A systematic review. Eur J Cancer. 45:33–47.
2009. View Article : Google Scholar
|
|
13
|
Sharma PS, Sharma R and Tyagi R:
Inhibitors of cyclin dependent kinases: Useful targets for cancer
treatment. Curr Cancer Drug Targets. 8:53–75. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kato K, Gong J, Iwama H, Kitanaka A, Tani
J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, et al:
The anti-diabetic drug metformin inhibits gastric cancer cell
proliferation in vitro and in vivo. Mol Cancer Ther. 11:549–560.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hui AM, Cui X, Makuuchi M, Li X, Shi YZ
and Takayama T: Decreased p27(Kip1) expression and cyclin D1
overexpression, alone and in combination, influence recurrence and
survival of patients with resectable extrahepatic bile duct
carcinoma. Hepatology. 30:1167–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kobayashi K, Morishita A, Iwama H, Fujita
K, Okura R, Fujihara S, Yamashita T, Fujimori T, Kato K, Kamada H,
et al: Galectin-9 suppresses cholangiocarcinoma cell proliferation
by inducing apoptosis but not cell cycle arrest. Oncol Rep.
34:1761–1770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tadokoro T, Morishita A, Fujihara S, Iwama
H, Niki T, Fujita K, Akashi E, Mimura S, Oura K, Sakamoto T, et al:
Galectin-9: An anticancer molecule for gallbladder carcinoma. Int J
Oncol. 48:1165–1174. 2016.PubMed/NCBI
|
|
19
|
Fujita K, Iwama H, Sakamoto T, Okura R,
Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et
al: Galectin-9 suppresses the growth of hepatocellular carcinoma
via apoptosis in vitro and in vivo. Int J Oncol. 46:2419–2430.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schutte B, Henfling M, Kölgen W, Bouman M,
Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et
al: Keratin 8/18 breakdown and reorganization during apoptosis. Exp
Cell Res. 297:11–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
D'Incalci M, Colombo T, Ubezio P,
Nicoletti I, Giavazzi R, Erba E, Ferrarese L, Meco D, Riccardi R,
Sessa C, et al: The combination of yondelis and cisplatin is
synergistic against human tumor xenografts. Eur J Cancer.
39:1920–1926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masaki T, Shiratori Y, Rengifo W, Igarashi
K, Yamagata M, Kurokohchi K, Uchida N, Miyauchi Y, Yoshiji H,
Watanabe S, et al: Cyclins and cyclin-dependent kinases:
Comparative study of hepatocellular carcinoma versus cirrhosis.
Hepatology. 37:534–543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Masaki T, Shiratori Y, Rengifo W, Igarashi
K, Matsumoto K, Nishioka M, Hatanaka Y and Omata M: Hepatocellular
carcinoma cell cycle: Study of Long-Evans cinnamon rats.
Hepatology. 32:711–720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Igarashi K, Masaki T, Shiratori Y, Rengifo
W, Nagata T, Hara K, Oka T, Nakajima J, Hisada T and Hata E:
Activation of cyclin D1-related kinase in human lung
adenocarcinoma. Br J Cancer. 81:705–711. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Itabashi H, Maesawa C, Oikawa H, Kotani K,
Sakurai E, Kato K, Komatsu H, Nitta H, Kawamura H, Wakabayashi G,
et al: Angiotensin II and epidermal growth factor receptor
cross-talk mediated by a disintegrin and metalloprotease
accelerates tumor cell proliferation of hepatocellular carcinoma
cell lines. Hepatol Res. 38:601–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Molina Wolgien MC, Guerreiro da Silva ID,
Pinto Nazário AC, Nakaie CR, Correa-Noronha SA, Ribeiro de Noronha
SM and Facina G: Genetic association study of angiotensin II
receptor types 1 (A168G) and 2 (T1247G and A5235G) polymorphisms in
breast carcinoma among Brazilian women. Breast Care (Basel).
9:176–181. 2014. View Article : Google Scholar
|
|
27
|
Miyajima A, Kosaka T, Asano T, Asano T,
Seta K, Kawai T and Hayakawa M: Angiotensin II type I antagonist
prevents pulmonary metastasis of murine renal cancer by inhibiting
tumor angiogenesis. Cancer Res. 62:4176–4179. 2002.PubMed/NCBI
|
|
28
|
Sipahi I, Debanne SM, Rowland DY, Simon DI
and Fang JC: Angiotensin-receptor blockade and risk of cancer:
Meta-analysis of randomised controlled trials. Lancet Oncol.
11:627–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhaskaran K, Douglas I, Evans S, van Staa
T and Smeeth L: Angiotensin receptor blockers and risk of cancer:
Cohort study among people receiving antihypertensive drugs in UK
General Practice Research Database. BMJ. 344:e26972012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makar GA, Holmes JH and Yang YX:
Angiotensin-converting enzyme inhibitor therapy and colorectal
cancer risk. J Natl Cancer Inst. 106:djt3742014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kinoshita J, Fushida S, Harada S, Yagi Y,
Fujita H, Kinami S, Ninomiya I, Fujimura T, Kayahara M, Yashiro M,
et al: Local angiotensin II-generation in human gastric cancer:
Correlation with tumor progression through the activation of
ERK1/2, NF-κB and survivin. Int J Oncol. 34:1573–1582. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okamoto K, Tajima H, Ohta T, Nakanuma S,
Hayashi H, Nakagawara H, Onishi I, Takamura H, Ninomiya I, Kitagawa
H, et al: Angiotensin II induces tumor progression and fibrosis in
intrahepatic cholangiocarcinoma through an interaction with hepatic
stellate cells. Int J Oncol. 37:1251–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Du N, Feng J, Hu LJ, Sun X, Sun HB, Zhao
Y, Yang YP and Ren H: Angiotensin II receptor type 1 blockers
suppress the cell proliferation effects of angiotensin II in breast
cancer cells by inhibiting AT1R signaling. Oncol Rep. 27:1893–1903.
2012.PubMed/NCBI
|
|
34
|
Matsuda Y: Molecular mechanism underlying
the functional loss of cyclin dependent kinase inhibitors p16 and
p27 in hepatocellular carcinoma. World J Gastroenterol.
14:1734–1740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Benson SC, Pershadsingh HA, Ho CI,
Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA and
Kurtz TW: Identification of telmisartan as a unique angiotensin II
receptor antagonist with selective PPARgamma-modulating activity.
Hypertension. 43:993–1002. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stangier J, Su CA and Roth W:
Pharmacokinetics of orally and intravenously administered
telmisartan in healthy young and elderly volunteers and in
hypertensive patients. J Int Med Res. 28:149–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Scalera F, Martens-Lobenhoffer J, Bukowska
A, Lendeckel U, Täger M and Bode-Böger SM: Effect of telmisartan on
nitric oxide - asymmetrical dimethylarginine system: Role of
angiotensin II type 1 receptor gamma and peroxisome proliferator
activated receptor gamma signaling during endothelial aging.
Hypertension. 51:696–703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sukumaran S, Patel HJ and Patel BM:
Evaluation of role of telmisartan in combination with
5-fluorouracil in gastric cancer cachexia. Life Sci. 154:15–23.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Perry JE, Grossmann ME and Tindall DJ:
Epidermal growth factor induces cyclin D1 in a human prostate
cancer cell line. Prostate. 35:117–124. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Herbst RS and Shin DM: Monoclonal
antibodies to target epidermal growth factor receptor-positive
tumors: A new paradigm for cancer therapy. Cancer. 94:1593–1611.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Masaki T, Hatanaka Y, Nishioka M, Tokuda
M, Shiratori Y, Reginfo W and Omata M: Activation of epidermal
growth factor receptor kinase in gastric carcinoma: A preliminary
study. Am J Gastroenterol. 95:2135–2136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han W and Lo HW: Landscape of EGFR
signaling network in human cancers: Biology and therapeutic
response in relation to receptor subcellular locations. Cancer
Lett. 318:124–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Geynisman DM and Catenacci DV: Toward
personalized treatment of advanced biliary tract cancers. Discov
Med. 14:41–57. 2012.PubMed/NCBI
|
|
44
|
Jo Chae K, Rha SY, Oh BK, Koo JS, Kim YJ,
Choi J, Park C and Park YN: Expression of matrix
metalloproteinase-2 and -9 and tissue inhibitor of
metalloproteinase-1 and -2 in intraductal and nonintraductal growth
type of cholangiocarcinoma. Am J Gastroenterol. 99:68–75. 2004.
View Article : Google Scholar
|
|
45
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Morishita A and Masaki T: miRNA in
hepatocellular carcinoma. Hepatol Res. 45:128–141. 2015. View Article : Google Scholar
|
|
47
|
Akao Y, Nakagawa Y and Naoe T: let-7
microRNA functions as a potential growth suppressor in human colon
cancer cells. Biol Pharm Bull. 29:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu XM, Wu LJ, Xu J, Yang R and Wu FS:
Let-7c microRNA expression and clinical significance in
hepatocellular carcinoma. J Int Med Res. 39:2323–2329. 2011.
View Article : Google Scholar
|
|
49
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong
C, Huang Y, Hu X, Su F, Lieberman J, et al: let-7 regulates self
renewal and tumorigenicity of breast cancer cells. Cell.
131:1109–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee YS and Dutta A: The tumor suppressor
microRNA let-7 represses the HMGA2 oncogene. Genes Dev.
21:1025–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Johnson SM, Grosshans H, Shingara J, Byrom
M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D and Slack
FJ: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Osada H and Takahashi T: let-7 and
miR-17-92: Small-sized major players in lung cancer development.
Cancer Sci. 102:9–17. 2011. View Article : Google Scholar
|
|
54
|
Sun H, Ding C, Zhang H and Gao J: Let-7
miRNAs sensitize breast cancer stem cells to radiation-induced
repression through inhibition of the cyclin D1/Akt1/Wnt1 signaling
pathway. Mol Med Rep. 14:3285–3292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin MS, Chen WC, Huang JX, Gao HJ and
Sheng HH: Aberrant expression of microRNAs in serum may identify
individuals with pancreatic cancer. Int J Clin Exp Med.
7:5226–5234. 2014.
|
|
56
|
Cui ZH, Shen SQ, Chen ZB and Hu C: Growth
inhibition of hepatocellular carcinoma tumor endothelial cells by
miR-204-3p and underlying mechanism. World J Gastroenterol.
20:5493–5504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li W, Shen S, Wu S, Chen Z, Hu C and Yan
R: Regulation of tumorigenesis and metastasis of hepatocellular
carcinoma tumor endothelial cells by microRNA-3178 and underlying
mechanism. Biochem Biophys Res Commun. 464:881–887. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ueda T, Volinia S, Okumura H, Shimizu M,
Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: A microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar
|
|
59
|
Sun L, Jiang R, Li J, Wang B, Ma C, Lv Y
and Mu N: MicoRNA-425-5p is a potential prognostic biomarker for
cervical cancer. Ann Clin Biochem. 54:127–133. 2017. View Article : Google Scholar
|
|
60
|
Di Leva C, Piovan C, Gasparini P, Ngankeu
A, Taccioli C, Briskin D, Cheung DG, Bolon B, Anderlucci L, Alder
H, et al: Estrogen mediated-activation of miR-191/425 cluster
modulates tumorigenicity of breast cancer cells depending on
estrogen receptor status. PLoS Genet. 9:e10033112013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yu M, Trobridge P, Wang Y, Kanngurn S,
Morris SM, Knoblaugh S and Grady WM: Inactivation of TGF-β
signaling and loss of PTEN cooperate to induce colon cancer in
vivo. Oncogene. 33:1538–1547. 2014. View Article : Google Scholar
|
|
62
|
Ma J, Liu J, Wang Z, Gu X, Fan Y, Zhang W,
Xu L, Zhang J and Cai D: NF-kappaB-dependent microRNA-425
upregulation promotes gastric cancer cell growth by targeting PTEN
upon IL-1β induction. Mol Cancer. 13:402014. View Article : Google Scholar
|
|
63
|
Yang Y-F, Wang F, Xiao J-J, Song Y, Zhao
YY, Cao Y, Bei YH and Yang CQ: MiR-222 overexpression promotes
proliferation of human hepatocellular carcinoma HepG2 cells by
downregulating p27. Int J Clin Exp Med. 7:893–902. 2014.PubMed/NCBI
|
|
64
|
Sun C, Li N, Zhou B, Yang Z, Ding D, Weng
D, Meng L, Wang S, Zhou J, Ma D, et al: miR-222 is upregulated in
epithelial ovarian cancer and promotes cell proliferation by
downregulating p27kip1. Oncol Lett. 6:507–512.
2013.PubMed/NCBI
|
|
65
|
Saito Y, Suzuki H, Matsuura M, Sato A,
Kasai Y, Yamada K, Saito H and Hibi T: MicroRNAs in hepatobiliary
and pancreatic cancers. Front Genet. 2:662011. View Article : Google Scholar
|
|
66
|
Zhang C-Z, Han L, Zhang A-L, Fu Y-C, Yue
X, Wang G-X, Jia Z-F, Pu P-Y, Zhang Q-Y and Kang C-S: MicroRNA-221
and microRNA-222 regulate gastric carcinoma cell proliferation and
radioresistance by targeting PTEN. BMC Cancer. 10:3672010.
View Article : Google Scholar
|
|
67
|
Visone R, Russo L, Pallante P, De Martino
I, Ferraro A, Leone V, Borbone E, Petrocca F, Alder H, Croce CM, et
al: MicroRNAs (miR)-221 and miR-222, both overexpressed in human
thyroid papillary carcinomas, regulate p27Kip1 protein levels and
cell cycle. Endocr Relat Cancer. 14:791–798. 2007. View Article : Google Scholar : PubMed/NCBI
|