Introduction
Cancer is the leading cause of death worldwide, with
lung cancer being the most common type of cancer, with an estimated
1.8 million new cases in 2012 (1).
Even with administration of treatment, the 5-year survival rate for
lung cancer is very low (17.7%) in comparison to other leading
cancer sites such as colon (64.4%), breast (89.7%) and prostate
(98.9%) (2). While control for
early stage localized lung cancer has improved (3,4),
early stage diagnosis only accounts for ~16% of lung cancer
(2), with majority of patients
being diagnosed at an advanced or metastatic stage of disease.
Thus, it is of grave importance to further understand the molecular
mechanisms regulating lung carcinogenesis and to explore and
identify novel diagnostic biomarkers for treatment strategies.
MicroRNAs (miRNAs) are a subset of non-coding RNAs
of ~19–23 nucleotides in length, which post-transcriptionally
regulate gene expression (5).
miRNAs play a role in crucial biological processes including
proliferation (6,7), differentiation (8), chemosensitivity (9,10)
and apoptosis (11,12). Studies have shown that aberrations
in the expression of certain miRNAs may cause or contribute to
human diseases, including cancer (13). Evasion of apoptosis is a major
contributor to tumour progression, and past studies have elucidated
that manipulation of the apoptotic process is one way by which
miRNAs influence the development of lung cancer (11,14–17).
miR-608 is a novel prognostic marker in
carcinogenesis, its expression is dysregulated in various cancers
(18–21). A previous study by our group
demonstrated that downregulation of B-cell lymphocyte xL
(BCL-XL), the other major prototype of the anti-apoptotic
bcl-2 gene, dysregulates various miRNAs in lung adenocarcinoma cell
line A549, including miR-608. The study further shows that ectopic
expression of miR-608 was able to increase cell death in non-small
cell lung cancer (NSCLC) cells, and co-transfection of siRNA
targeting BCL-XL (siBCL-XL) followed by miR-608
inhibitors was able to block siBCL-XL induced cell death,
suggesting that miR-608 plays an important role in cell death
processes (22).
In the present study, we evaluated the role of
miR-608 NSCLC and the molecular mechanisms by which it regulates
apoptosis. Our data identified miR-608 as a tumour suppressor in
NSCLC, through identification of a novel direct target responsible
for mediating the activity of miR-608 in NSCLC.
Materials and methods
Bioinformatics analyses of miRNA gene
targets
In silico analyses was performed to identify
the putative miRNA targets using TargetScan Human V5.2 (http://www.targetscan.org/) (Whitehead Institute for
Biomedical Research, Cambridge, MA, USA), a database of conserved
3′UTR targets. TargetScan provides accurate ranking of the
predicted targets of miRNA based on total context+ score, which is
the sum of the contribution of six targeting factors including site
type, site number, site location, local AU content,
3′-supplementary pairing, target site abundance and seed-pairing
stability. The total context+score predicts the relative repression
of mRNA with 3′UTR, with low context scores being more favorable
(23). The web tool Database for
Annotation, Visualization and Integrated Discovery (DAVID) v6.7
(http://david.abcc.ncifcrf.gov/summary.jsp)
(SAIC-Frederick, Inc., Frederick, MD, USA), which is made up of an
integrated biological knowledgebase and analytic tools (24), was then employed, using default
parameters, to perform gene-annotation enrichment analyses on
TargetScan's predicted miRNA targets that has a total context+
score of <0. Data from TargetScan and DAVID were combined to
generate a hypothetical pathway of the relationship between the
miRNAs and their gene targets.
Cell lines and culture conditions
Human lung adenocarcinoma cell line A549 [Cancer
Research Initiative Foundation (CARIF), Subang Jaya Medical Centre,
Subang Jaya, Malaysia] was cultured in RPMI-1640 (SH30027.01;
HyClone Laboratories-GE Healthcare Life Sciences, Pittsburgh, PA,
USA) whereas SK-LU-1 cells (LA-HL-045; AseaCyte, Pvt. Ltd., Kuala
Lumpur, Malaysia) were cultured in MEM-α (32561037; Gibco, Waltham,
MA, USA). All cells were supplemented with 10% fetal bovine serum
(FBS) (SV30160.03; HyClone Laboratories-GE Healthcare Life
Sciences) and maintained at 37°C in a humidified incubator
containing 5% CO2.
miRNA transfection
Cells were seeded 24 h prior to transfection with
miR-608 mimics (C-300933-01-0010; GE Healthcare Dharmacon,
Lafayette, CO, USA), non-specific mimic controls (mimic NC)
(CN-001000-01-20; GE Healthcare Dharmacon), miR-608 inhibitors
(IH-300933-03-0010; GE Healthcare Dharmacon) or non-specific
antimiR controls (inhibitor NC) (IN-001005-01-20; GE Healthcare
Dharmacon) at a final concentration of 80.0 nM using DharmaFECT
reagent (T-2001-03; GE Healthcare Dharmacon), as per the
manufacturer's protocol.
Dual-luciferase reporter assay
system
Wild-type 3′UTR of AKT2 containing predicted
miR-608 binding sites and/or its corresponding mutant sequences
were cloned into the pmirGLO Dual-Luciferase miRNA expression
vector (E1330; Promega, Madison, WI, USA). A549 cells were plated
24 h prior to co-transfection with 40.0 ng of pmirGLO constructs
and 80.0 nM of miR-608 mimic/inhibitor or mimic NC/inhibitor NC
using DharmaFECT reagent. Luciferase activity was analyzed 48 h
post-transfection using the Dualluciferase reporter assay system
(E2920; Promega), as per the manufacturer's protocol and detected
on the GloMax Multi Luminescence Multimode Reader (Promega).
Relative luciferase activity was normalized against Renilla
luciferase activity.
Protein extraction and western
blotting
Protein was extracted using the NE-PER®
Nuclear and Cytoplasmic Extraction kit (78833; Thermo Fisher
Scientific, Waltham, MA, USA) 48 h post-transfection, as per the
manufacturer's protocol. Protein lysates were separated by
electrophoresis in 12% SDS-PAGE and then electrophoretically
transferred to nitrocellulose membranes. Membranes were blocked in
1X Tris-buffered saline (TBS) with 0.05% Tween-20 and 5% non-fat
skim milk powder (115363; Merck, Kenilworth, NJ, USA) for 1 h at
room temperature and then immunostained overnight at 4°C with
primary monoclonal rabbit antibodies: AKT (4691, 1:1,000 dilution;
Cell Signaling Technology, Danvers, MA, USA) or GAPDH (2118,
1:10,000 dilution; Cell Signaling Technology). The following day
membranes were washed and incubated with secondary goat anti-rabbit
IgG HRP-linked antibody (7074, 1:1,000 dilution; Cell Signaling
Technology) and anti-biotin HRP-linked antibody (7075, 1:1,000
dilution; Cell Signaling Technology). Bands were visualized using
WesternBright Quantum (K-12042-D10; Advansta, Inc., Menlo Park, CA,
USA) on the Fusion FX7 system (Vilber Lourmat GmbH, Eberhardzell,
Germany) and quantified using the ImageJ Analyst software (National
Institutes of Health, Bethesda, MD, USA), with band intensities
normalized to GAPDH.
Annexin V-FITC apoptosis assay
FITC Annexin V apoptosis detection kit (556547; BD
Biosciences, San Jose, CA, USA) was used to detect cell death 72 h
post-transfection, as per the manufacturer's protocol. Signals were
detected from 1.0×104 cell population using the BD
FACSCanto™ II flow cytometer (BD Biosciences) and examined on the
BD FACSDiva™ software (BD Biosciences).
Caspase-3/7 activity assay
Caspase-Glo 3/7 assay kit (G8090; Promega) was
utilized to analyze caspase-3 and -7 activity, 48 h
post-transfection as per the manufacturer's protocol. Samples were
incubated at 25°C for 1 h in the dark and luminescence was then
detected using the GloMax Multi Luminescence Multimode Reader.
Cell cycle analysis
Flow cytometry was used to analyze cell cycle using
the BD Cycletest™ Plus DNA kit assay (340242; BD Biosciences) 48 h
post-transfection, as per the manufacturer's protocol. Signals were
detected from 1.0×104 cell population using the BD
FACSCanto™ II flow cytometer and examined on the BD FACSDiva™
software. Results were then analyzed using the ModFit LT v3.2.1
(Verity Software House, Inc., Topsham, ME, USA) and the percentage
of the cells in G0/G1, S and G2/M phase were counted and
compared.
Zebrafish care and use
Experiments involving zebrafish were approved by the
University of Malaya, Faculty of Medicine, Institutional Care of
Use Committee (FOM IACUC) (Ethics reference number:
2015-181006/IBS/R/NO) and complied with all relevant animal welfare
laws, guidelines and policies. Wild-type Danio rerio
zebrafish embryos were cared for and maintained using standard
husbandry practices.
Zebrafish microinjection
Zebrafish embryos were injected with A549 cells
transfected with 80.0 nm miR-608 mimics, inhibitors, or their
corresponding negative controls at the superficial location of the
yolk near to the perivitelline space of the embryos using a
FemtoJet Microinjector (Eppendorf, Hamburg, Germany) and InjectMan
NI 2 Micromanipulator (Eppendorf) with constant injection pressure
and injection time. The injection volume and cell suspension was
calibrated to be ~100–200 cells/injection in each embryo. After
transplantation, embryos were immediately placed at 37°C
overnight.
Whole mount caspase-3
immunofluorescence
Embryos were fixed in 4% paraformaldehyde at 4°C
overnight followed by 2-h dehydration in methanol at −20°C.
Following rehydration, embryos were washed with 1% dimethyl
sulfoxide (DMSO), 0.1% Triton in PBS (1X PDT) and blocked with 10%
FBS, 2% BSA in PBST (blocking buffer) for 1 h at room temperature.
Embryos were then stained with purified rabbit anti-active
caspase-3 antibody (559565, 1:500 dilution; BD Biosciences) for 2 h
at room temperature followed by washes in PDT. Again embryos were
incubated with blocking buffer, and then stained with anti-rabbit
IgG Fab2 Alexa Fluor 647 Conjugate (4414, 1:500 dilution; Cell
Signaling Technology) overnight at 4°C. The following day, embryos
were washed with PDT before visualization and imaging using the
Leica confocal laser-scanning microscope SPII and Leica Application
Suite (LAS) software v5.0 (Leica Microsystems, Wetzlar, Germany).
Fluorescence was quantified using ImageJ Analyst software.
Threshold was set to eliminate background fluorescence and embryos
were analyzed to generate arbitrary fluorescence units.
siRNA silencing of AKT2
Silencing of the AKT2 gene was performed
using a set of three unique 27 mer siRNA duplexes at a final
concentration of 10.0 nM (siRNA A: GCAUCAUA AAUUGGUAGUUUCCUGC,
siRNA B: AGCGUGUGAAUA CAUCAAGACCTG, siRNA C: ACAGCAAAGCAGGAG
UAUAAGAAAG) (SR300144; Origene Technologies, Inc., Rockville, MD,
USA). A universal scrambled negative control siRNA (siRNA NC) was
used as a control. At 48 h post-transfection, silencing efficiency
was assessed by western blot analysis. Amongst the three siRNAs
utilized, the siRNA with the greatest silencing efficiency was
selected for further downstream work and referred to as
siAKT2. The effects of miR-608 mediated apoptosis via
AKT2 was validated through transfection of 80.0 nM miR-608
inhibitors, followed by transfection with 10.0 nM siAKT2
directed against the human AKT2 gene (siAKT2) 6 h later.
AKT2 protein levels were determined via western blot analysis 48 h
post-transfection while apoptosis was detected using the FITC
Annexin V apoptosis detection kit and Caspase-Glo 3/7 assay
kit.
Statistical analysis
All in vitro experiments were performed in
triplicate independent experiments. In vivo experiments were
performed with sample size of 15 zebrafish embryos per treatment
group. All data were presented as mean ± standard deviation
(25). Paired Student's t-test was
used to determine the statistical significance of results, whereby
a P-value of ≤0.05 was considered significant.
Results
miR-608 is predicted to bind to AKT2
3′UTR
A previous study conducted by our laboratory
determined that the expression of miR-608 was significantly
downregulated following the silencing of BCL-XL in lung
adenocarcinoma cell line A549. Results also indicated that miR-608
played a tumour suppressor role in regulating the apoptotic
properties of A549 and a secondary lung adenocarcinoma cell line
SK-LU-1 (22). To determine the
molecular mechanism by which miR-608 regulates the apoptotic
properties in NSCLC cell lines, we performed an in silico
bioinformatics analysis to identify the putative miR-608 gene
targets through the use of the TargetScan Human v5.2 algorithm,
followed by functional annotation using the web tool DAVID v6.7,
which lists the predicted targets of miR-608 according to their
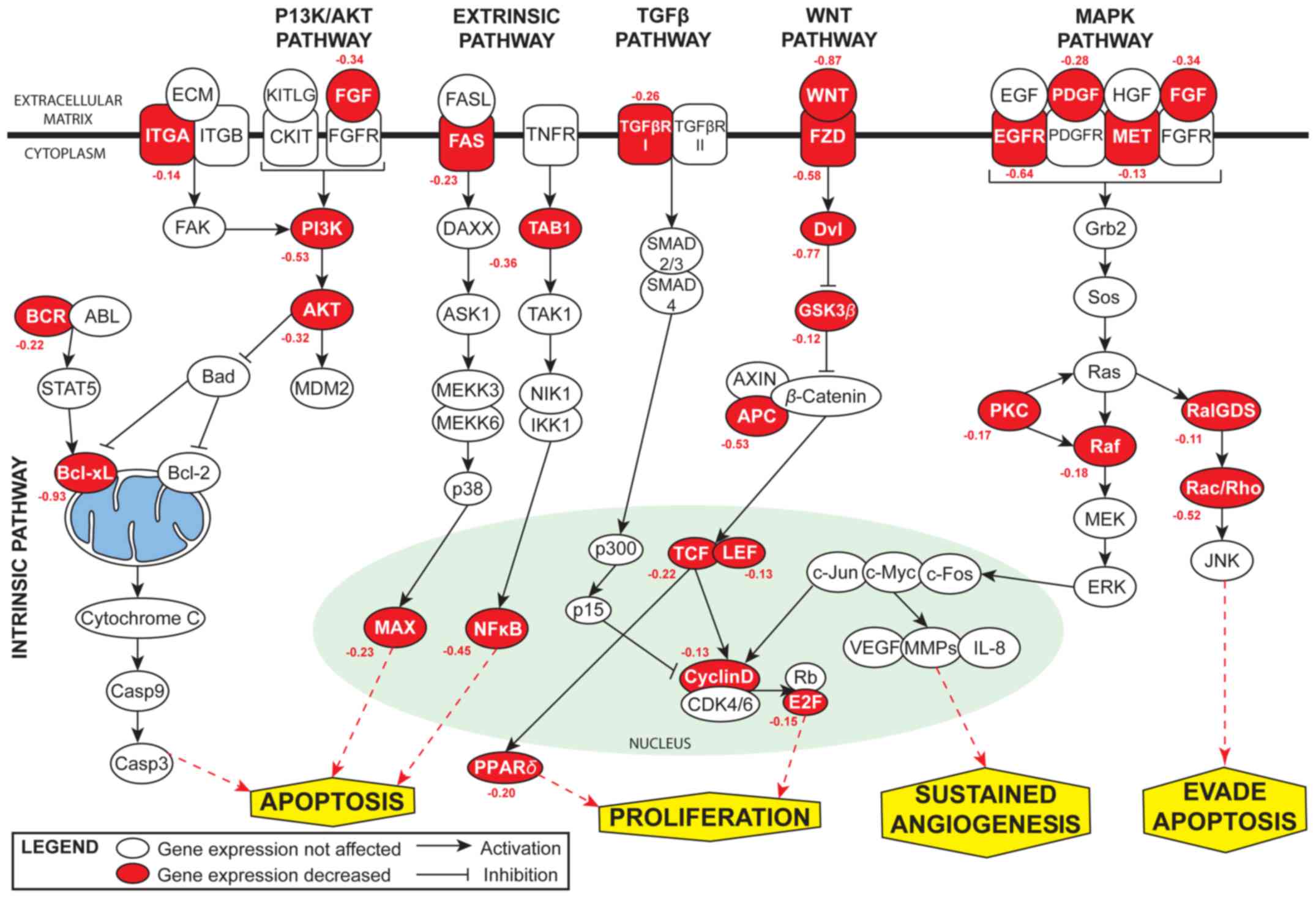
apoptosis-related pathways. miR-608 was found to be associated with
various signaling pathways involved in cancer, including the
phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT),
wingless-type MMTV integration site family (WNT), transforming
growth factor (TGF-β), mitogen activated protein kinase (MAPK) and
the intrinsic and extrinsic pathway (Fig. 1).
Identification of AKT2 as a direct target
of miR-608 in NSCLC cells
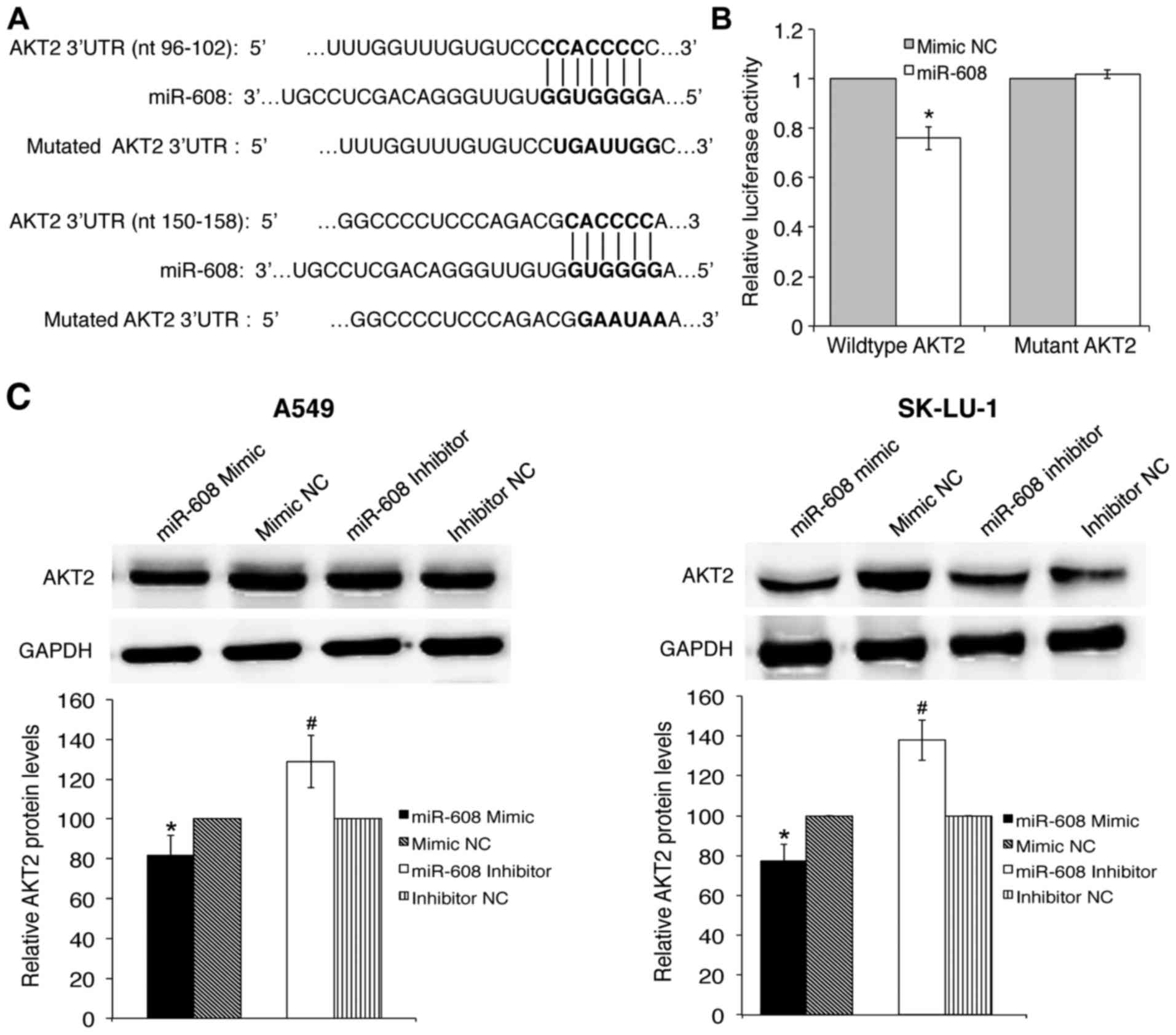
The 3′UTR of V-Akt Murine Thymoma Viral Oncogene
Homolog 2 (AKT2) contains two miR-608 binding sites, and is
involved with apoptosis and proliferation and was thus chosen for
further validation. To verify whether AKT2 3′UTR was a
direct target of miR-608, the wild-type and mutated AKT2
3′UTR were cloned into the pmirGLO Dual-luciferase miRNA target
expression vector (Fig. 2A).
Luciferase reporter assay confirmed that miR-608 mimics had a
significant inhibitory effect on wild-type 3′UTR but not on the
mutant 3′UTR of AKT2 luciferase activity, while mimic NC had
no effect on either the wild-type or mutant luciferase activity
(Fig. 2B). This result suggests
that miR-608 directly binds to the binding sequence of AKT2
3′UTR, and this was further verified by a decrease in AKT2 protein
levels in response to miR-608 mimic transfection, as analyzed by
western blot analysis. Conversely, the expression of AKT2 was
significantly increased when miR-608 was inhibited (Fig. 2C).
siRNA-mediated silencing of AKT2 restores
miR-608 induced effects in NSCLC cells
We have previously demonstrated that miR-608 plays
an important role in the regulation of apoptosis, and presently
identified miR-608 as a direct regulator of AKT2. It was
thus hypothesized that low expression of miR-608 in NSCLC may
result in suppression of its inhibitory effects towards AKT2
causing AKT2 expression to be upregulated, which in turn blocks
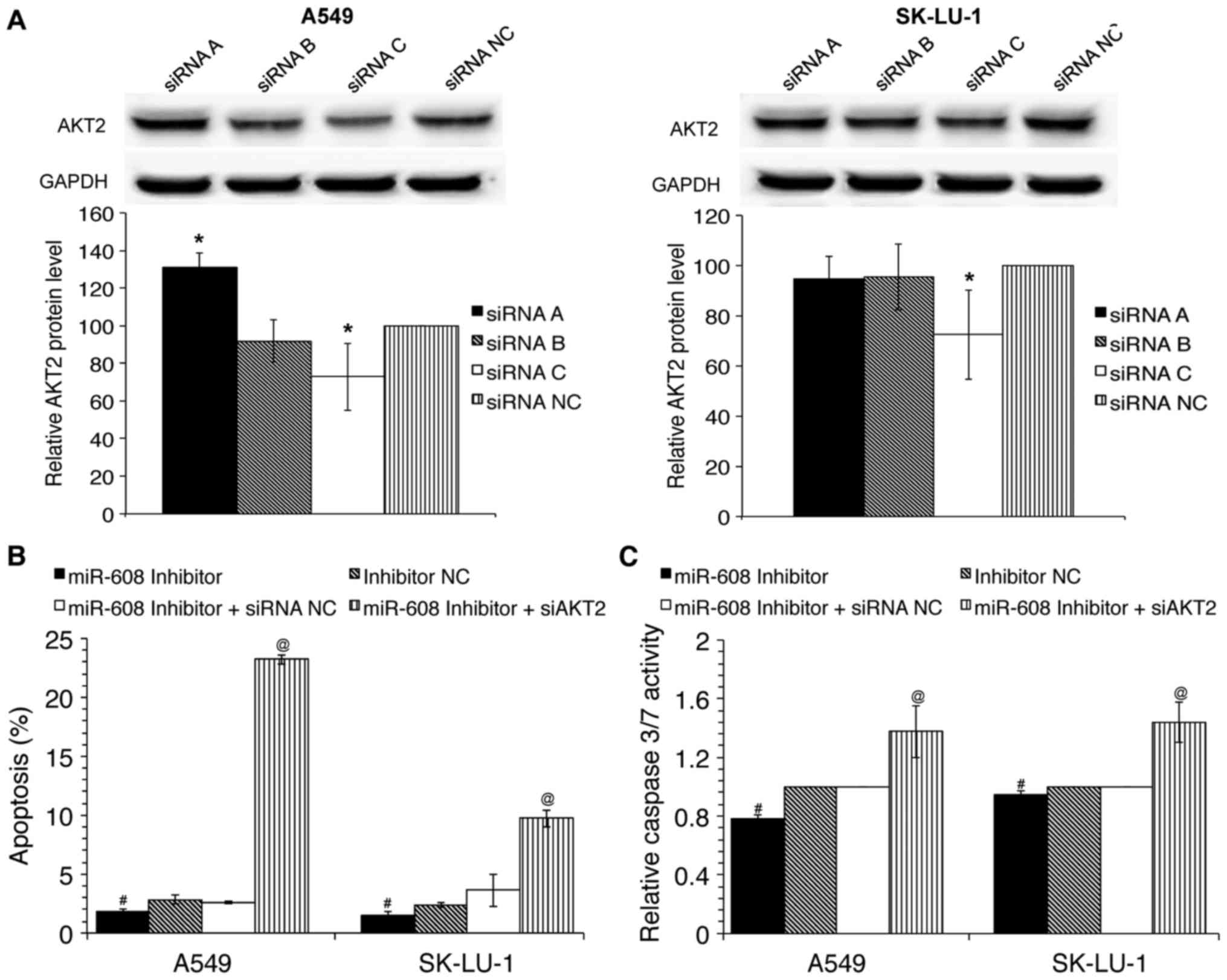
apoptosis. To investigate this hypothesis, co-transfection of
miR-608 inhibitors and siRNA inhibiting AKT2 was performed
in A549 and SK-LU-1 cells. siRNAs were provided as a set of three
siRNA duplexes; therefore to evaluate the silencing efficiency of
the siRNAs, densitometry analysis of western blot bands was
performed to evaluate the AKT2 protein expression in
siRNA-transfected in comparison to siRNA NC transfected cells.
Amongst the three siRNAs utilized, siRNA C was able to
significantly decrease AKT2 protein levels in A549 and SK-LU-1
cells (Fig. 3A). As siRNA C
(referred to as siAKT2 henceforth) had the greatest
silencing efficiency amongst the three siRNAs, it was selected for
further downstream work. Results indicated that silencing of
AKT2 was able to partially rescue the inhibition of
apoptosis and caspase-3/7 activation that was induced by miR-608
inhibitors (Fig. 3B and C).
Collectively, these results demonstrate the tumour suppressor role
of miR-608 in NSCLC is at least partially through its inhibition of
AKT2.
Transfection of miR-608 increases
caspase-3 detection in zebrafish embryo animal model
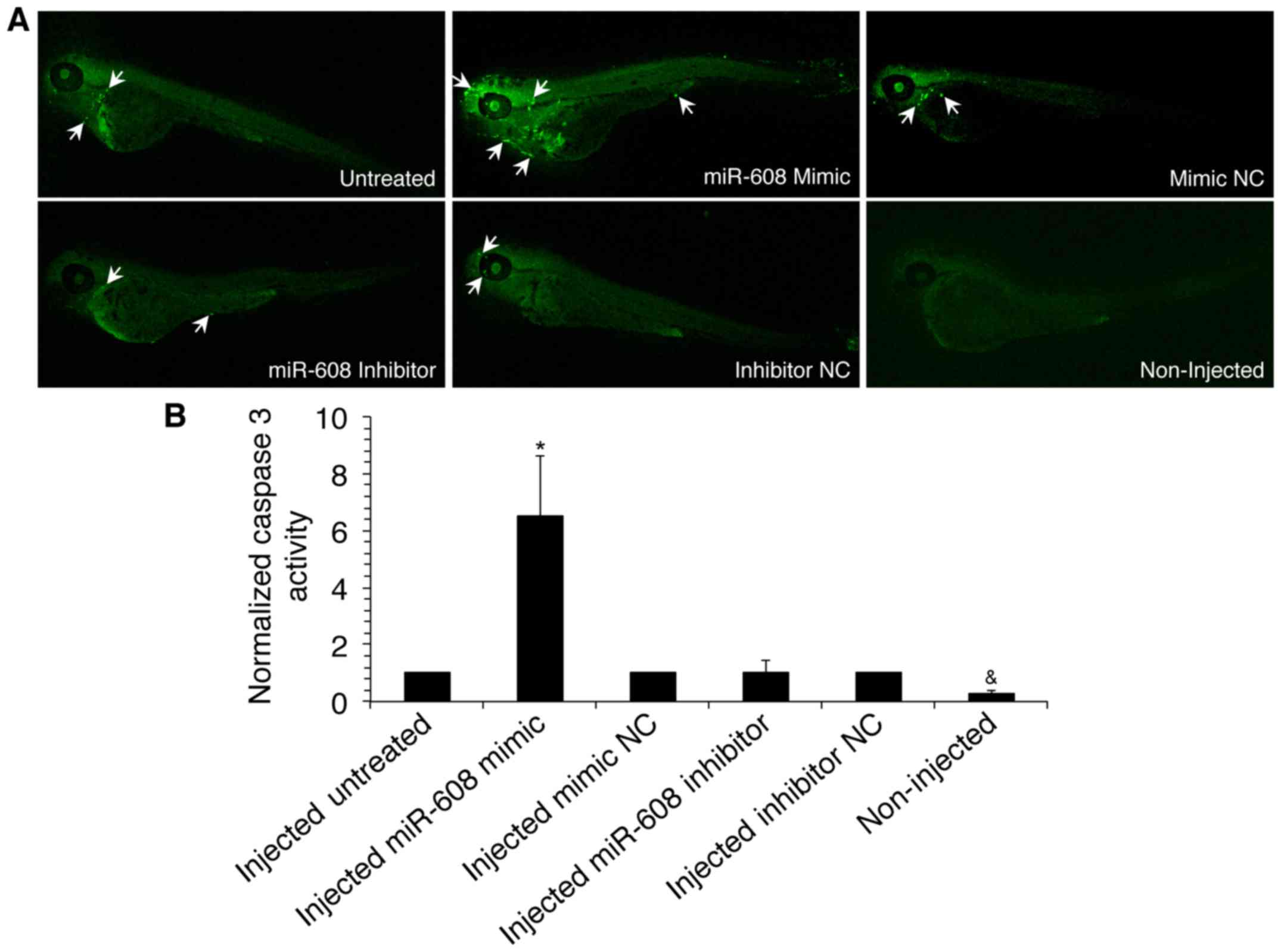
The in vivo effect of miR-608 on apoptosis
was determined through utilization of zebrafish embryos as an
animal model. miR-608 mimic, inhibitor or negative control
transfected A549 cells were microinjected into zebrafish. Embryos
were then visualized using a Leica confocal microscope following
immunostaining with anti-active caspase-3 monoclonal antibodies
(Fig. 4A). Results of fluorescent
image analysis using the ImageJ software indicated that detection
of caspase-3 was significantly increased in zebrafish injected with
miR-608 in comparison to negative control injected zebrafish
(Fig. 4B). This suggests that
miR-608 is able to induce apoptosis in vivo through caspase
activation.
Discussion
While progress has been made in molecular targeted
therapies and early diagnosis of lung cancer, the 5-year survival
rate of patients is still very low due to most patients being
diagnosed at an advanced stage (2). It is therefore essential for
identification of novel diagnostic biomarkers and to explore more
effective and safe treatment tools. Recent studies have shown that
dysregulation of miRNA expression contributes to the development
and progression of cancer (26–28).
miRNA profiles can also be used as biomarkers for detection of
cancer (29–34) and to predict chemotherapeutic
response (35–38). It is therefore of particular
interest to investigate the therapeutic application of miRNAs in
lung cancer.
miR-608 is a novel prognostic marker in
carcinogenesis with its expression downregulated in various cancers
including chordoma (18), colon
cancer (19), glioblastoma
(20) and osteosarcoma (21). Single-nucleotide polymorphisms in
miR-608 have also been associated with several cancers such as
nasopharyngeal carcinoma (39),
colorectal adenocarcinoma (40–42),
breast (43,44) and bladder cancer (45). In recent years evidence has emerged
illustrating the role miR-608 plays as a tumour suppressor. In
chordoma cancer, miR-608 induces apoptosis and inhibits cell
proliferation via regulation of epidermal growth factor receptor
(EGFR) and B-cell lymphoma-extra large (BCL-XL)
(18). miR-608 has also been shown
to directly target macrophage migration inhibitory factor
(MIF), inhibiting proliferation, migration and invasion, and
inducing apoptosis in both osteosarcoma cell lines (21) and glioma stem cells (20). Furthermore, miR-608 has been
demonstrated to repress tumorigenesis of colon cancer cells both
in vitro and in vivo through the regulation of
N-a-acetyltransferase 10 protein (NAA10) (19).
Our previous study revealed that downregulation of
anti-apoptotic BCL-XL in lung adenocarcinoma cell line A549
resulted in a decrease in cell proliferation, an increase in
apoptosis as well as dysregulation of various miRNAs, including
upregulation of miR-608. It was further demonstrated via
overexpression and knockdown studies that miR-608 plays a role in
BCL-XL induced apoptosis in A549 cells (22). To identify the molecular mechanism
by which miR-608 regulates apoptosis in NSCLC cells, in the present
study bioinformatics analysis was performed, which predicted
AKT2 as a novel target with two regions containing perfect
complementary miR-608 binding sites in its 3′UTR. Measurement of
relative firefly luciferase activity, indicative of translation
from the plasmid, and quantification of protein levels via western
blot analysis validated AKT2 as a direct target of
miR-608.
AKT2 is a serine/threonine protein kinase that plays
an essential role in various signaling pathways including
metabolism, proliferation, cell survival, growth and angiogenesis
(45,46). Increasing evidence suggests that
hyperactivation of AKT2 plays an important role in human
malignancy, with amplification and overexpression being reported in
several cancers including breast (47,48),
pancreatic (49), hepatocellular
(50), ovarian (51,52),
thyroid (53), glioma (54,55),
colorectal (55) and non-small
cell lung cancer (56–58). AKT2 has been reported to play a
role in cell cycle progression in breast cancer cell line
MDA-MB-231, with silencing of AKT2 leading to cell cycle
arrest through downregulation of Cdk2 and cyclin D and upregulation
of p27. Prolonged inhibition of AKT2 was also shown to lead
to an increase in the mitochondrial volume, eventually leading to
cell death by autophagy (48).
Another study indicates that silencing of AKT2 in
neuroblastoma disrupts cell migration and invasion and also
decreases metastasis in the liver (59). Downregulation of AKT2 has
also been demonstrated to lead to MCL-1 cleavage, collapse of the
mitochondrial membrane potential, release of cytochrome c
into the cytosol, followed by activation of the caspase cascade in
NSCLC (56). Similarly, in glioma
cell lines, knockdown of AKT2 was able to induce apoptosis
via dephosphorylation of BAD and the activation of caspase-9 and
caspase-3 (55).
As AKT2 is a well-established pro-survival
factor, we hypothesized that targeting of AKT2 could be a
mechanism by which miR-608 functions as a tumour suppressor in
NSCLC. This was further validated when cells were co-transfected
with miR-608 inhibitors and siAKT2 to partially rescue
inhibition of apoptosis induced by miR-608 inhibitors. In a
recently published study, the relationship between miR-608 and the
AKT pathway in bladder cancer further supports the tumour
suppressive role of miR-608. Liang and colleagues (60) demonstrated that upregulation of
miR-608 was able to suppress cell cycle progression through direct
inhibition of FLOT1 3′UTR, which is an upstream regulator of
the AKT/FOXO3a signaling pathway.
To summarize, in the present study we identified a
tumour suppressive role of miR-608 in non-small cell lung cancer
(NSCLC). Its role as a tumour suppressor was attributed to
identification of a novel direct target, AKT2. In
vivo studies using the zebrafish animal model also confirmed
that miR-608 could significantly induce activation of caspase-3, a
major apoptotic effector. AKT2 has been illustrated to have
significant roles in tumour progression; therefore selective
targeting of AKT2 via miR-608 may be developed and used as a
strategic treatment for NSCLC cancer.
Acknowledgments
The present study was supported by the High Impact
Research Grant under Grant UM.C/625/1/HIR/MOE/CHAN/016; and the
University of Malaya Postgraduate Research Grant under Grant
PG019-2016A.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M, Forman
D, Bray F, Dikshit R, Elser S, Mathers C, Rebelo M and Parkin DM:
GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC
CancerBase No. 11. International Agency for Research on Cancer;
Lyon, France: 2013, Available from: http://globocan.iarc.fr,
accessed on day/month/year.
|
|
2
|
Howlader N, Noone A, Krapcho M, Miller D,
Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et
al: SEER Cancer Statistics Review, 1975–2013. National Cancer
Institute; Bethesda, MD: 2017
|
|
3
|
Ginsberg RJRL and Rubinstein LV; Lung
Cancer Study Group. Randomized trial of lobectomy versus limited
resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg.
60:615–622; discussion 622–623. 1995. View Article : Google Scholar
|
|
4
|
Timmerman R, Paulus R, Galvin J, Michalski
J, Straube W, Bradley J, Fakiris A, Bezjak A, Videtic G, Johnstone
D, et al: Stereotactic body radiation therapy for inoperable early
stage lung cancer. JAMA. 303:1070–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng B, Wang B, Fang J, Zhu X, Cao Z, Lin
Q, Zhou L and Sun X: MiRNA-203 suppresses cell proliferation,
migration and invasion in colorectal cancer via targeting of
EIF5A2. Sci Rep. 6:283012016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Ma B, Ji X, Deng Y, Zhang T, Zhang
X, Gao H, Sun H, Wu H, Chen X, et al: MicroRNA-378-5p suppresses
cell proliferation and induces apoptosis in colorectal cancer cells
by targeting BRAF. Cancer Cell Int. 15:402016. View Article : Google Scholar
|
|
8
|
Shivdasani RA: MicroRNAs: Regulators of
gene expression and cell differentiation. Blood. 108:3646–3653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phuah NH, In LLA, Azmi MN, Ibrahim H,
Awang K and Nagoor NH: Alterations of MicroRNA expression patterns
in human cervical carcinoma cells (Ca Ski) toward
10S-10-acetoxychavicol acetate and cisplatin. Reprod Sci.
20:567–578. 2012. View Article : Google Scholar
|
|
10
|
Lin L, Tu HB, Wu L, Liu M and Jiang GN:
MicroRNA-21 regulates non-small cell lung cancer cell invasion and
chemosensitivity through SMAD7. Cell Physiol Biochem. 38:2152–2162.
2016. View Article : Google Scholar
|
|
11
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tagscherer KE, Fassl A, Sinkovic T,
Richter J, Schecher S, Macher-Goeppinger S and Roth W: MicroRNA-210
induces apoptosis in colorectal cancer via induction of reactive
oxygen. Cancer Cell Int. 16:422016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wiemer EA: The role of microRNAs in
cancer: No small matter. Eur J Cancer. 43:1529–1544. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu C, Zhang L, Li H, Liu Z, Duan L and Lu
C: MiRNA-1469 promotes lung cancer cells apoptosis through
targeting STAT5a. Am J Cancer Res. 5:1180–1189. 2015.PubMed/NCBI
|
|
15
|
Wu T, Chen W, Kong D, Li X, Lu H, Liu S,
Wang J, Du L, Kong Q, Huang X, et al: miR-25 targets the modulator
of apoptosis 1 gene in lung cancer. Carcinogenesis. 36:925–935.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu C, Li H, Zhang L, Jia T, Duan L and Lu
C: MicroRNA-1915-3p prevents the apoptosis of lung cancer cells by
downregulating DRG2 and PBX2. Mol Med Rep. 13:505–512. 2016.
View Article : Google Scholar
|
|
17
|
Jiang J, Huang J, Wang XR and Quan YH:
MicroRNA-202 induces cell cycle arrest and apoptosis in lung cancer
cells through targeting cyclin D1. Eur Rev Med Pharmacol Sci.
20:2278–2284. 2016.PubMed/NCBI
|
|
18
|
Zhang Y, Schiff D, Park D and Abounader R:
MicroRNA-608 and microRNA-34a regulate chordoma malignancy by
targeting EGFR, Bcl-xL and MET. PLoS One. 9:e915462014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Li Q, Niu J, Li B, Jiang D, Wan Z,
Yang Q, Jiang F, Wei P and Bai S: microRNA-342-5p and miR-608
inhibit colon cancer tumorigenesis by targeting NAA10. Oncotarget.
7:2709–2720. 2016. View Article : Google Scholar :
|
|
20
|
Wang Z, Xue Y, Wang P, Zhu J and Ma J:
MiR-608 inhibits the migration and invasion of glioma stem cells by
targeting macrophage migration inhibitory factor. Oncol Rep.
35:2733–2742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Sun J, Gu J, Liu G and Huizhu S:
MicroRNA-608 inhibits the cell proliferation in osteosarcoma by
macrophage migration inhibitory factor. Int J Clin Exp Pathol.
9:9166–9174. 2016.
|
|
22
|
Othman N, In LLA, Harikrishna JA and
Hasima N: Bcl-xL Silencing induces alterations in hsa-miR-608
expression and subsequent cell death in A549 and SKLU1 human lung
adenocarcinoma cells. PLoS One. 10:e817352013. View Article : Google Scholar
|
|
23
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: Determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatic resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar
|
|
25
|
Reynisdóttir I, Polyak K, Iavarone A and
Massagué J: Kip/Cip and Ink4 Cdk inhibitors cooperate to induce
cell cycle arrest in response to TGF-beta. Genes Dev. 9:1831–1845.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calin GA, Liu CG, Sevignani C, Ferracin M,
Felli N, Dumitru CD, Shimizu M, Cimmino A, Zupo S, Dono M, et al:
MicroRNA profiling reveals distinct signatures in B cell chronic
lymphocytic leukemias. Proc Natl Acad Sci USA. 101:11755–11760.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciafrè SA, Galardi S, Mangiola A, Ferracin
M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM and Farace MG:
Extensive modulation of a set of microRNAs in primary glioblastoma.
Biochem Biophys Res Commun. 334:1351–1358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wozniak MB, Scelo G, Muller DC, Mukeria A,
Zaridze D and Brennan P: Circulating microRNAs as non-invasive
biomarkers for early detection of non-small-cell lung cancer. PLoS
One. 10:e01250262015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nadal E, Truini A, Nakata A, Lin J, Reddy
RM, Chang AC, Ramnath N, Gotoh N, Beer DG and Chen G: A novel serum
4-microRNA signature for lung cancer detection. Sci Rep.
5:124642015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Wang Y, Zhang Q, Tang L, Liu X, Dai
Y, Xiao L, Huang S, Chen L, Guo Z, et al: MicroRNA-486 as a
biomarker for early diagnosis and recurrence of non-small cell lung
cancer. PLoS One. 10:e01342202015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bianchi F: Lung cancer early detection:
The role of circulating microRNAs. EBioMedicine. 2:1278–1279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su Y, Fang H and Jiang F: Integrating DNA
methylation and microRNA biomarkers in sputum for lung cancer
detection. Clin Epigenetics. 8:1092016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu C, Zheng Y, Lian D, Ye S, Yang J and
Zeng Z: Analysis of microRNA expression profile identifies novel
biomarkers for non-small cell lung cancer. Tumori. 101:104–110.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei J, Liu LK, Gao W, Zhu C-J, Liu Y-Q,
Cheng T and Shu YQ: Reduction of plasma microRNA-21 is associated
with chemotherapeutic response in patients with non-small cell lung
cancer. Chin J Cancer Res. 23:123–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Enfield KS, Stewart GL, Pikor LA, Alvarez
CE, Lam S, Lam WL and Chari R: MicroRNA gene dosage alterations and
drug response in lung cancer. J Biomed Biotechnol. 2011:4746322011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saito M, Shiraishi K, Matsumoto K,
Schetter AJ, Ogata-Kawata H, Tsuchiya N, Kunitoh H, Nokihara H,
Watanabe S, Tsuta K, et al: A three-microRNA signature predicts
responses to platinum-based doublet chemotherapy in patients with
lung adenocarcinoma. Clin Cancer Res. 20:4784–4793. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pedroza-Torres A, Fernández-Retana J,
Peralta-Zaragoza O, Jacobo-Herrera N, Cantú de Leon D, Cerna-Cortés
JF, Lopez-Camarillo C and Pérez-Plasencia C: A microRNA expression
signature for clinical response in locally advanced cervical
cancer. Gynecol Oncol. 142:557–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng J, Deng J, Xiao M, Yang L, Zhang L,
You Y, Hu M, Li N, Wu H, Li W, et al: A sequence polymorphism in
miR-608 predicts recurrence after radiotherapy for nasopharyngeal
carcinoma. Cancer Res. 73:5151–5162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin M, Gu J, Eng C, Ellis LM, Hildebrandt
MA, Lin J, Huang M, Calin GA, Wang D, Dubois RN, et al: Genetic
polymorphisms in microRNA-related genes as predictors of clinical
outcomes in colorectal adenocarcinoma patients. Clin Cancer Res.
18:3982–3991. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ying HQ, Peng HX, He BS, Pan YQ, Wang F,
Sun HL, Liu X, Chen J, Lin K and Wang SK: MiR-608, pre-miR-124-1
and pre-miR26a-1 polymorphisms modify susceptibility and
recurrence-free survival in surgically resected CRC individuals.
Oncotarget. 7:75865–75873. 2016.PubMed/NCBI
|
|
42
|
Ryan BM, McClary AC, Valeri N, Robinson D,
Paone A, Bowman ED, Robles AI, Croce C and Harris CC: rs4919510 in
hsa-mir-608 is associated with outcome but not risk of colorectal
cancer. PLoS One. 7:e363062012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huang AJ, Yu KD, Li J, Fan L and Shao ZM:
Polymorphism rs4919510:C>G in mature sequence of human
microRNA-608 contributes to the risk of HER2-positive breast cancer
but not other subtypes. PLoS One. 7:e352522012. View Article : Google Scholar :
|
|
44
|
Hashemi M, Bizhani F, Danesh Hiva D,
Narouie B, Sotoudeh M, Radfar MH, Ramezani MH, Bahari G, Taheri M
and Ghavami S: MiR-608 rs4919510 C>G polymorphism increased the
risk of bladder cancer in an Iranian population. AIMS Genet.
3:212–218. 2016. View Article : Google Scholar
|
|
45
|
Hashemi M, Sanaei S, Rezaei M, Bahari G,
Hashemi SM, Mashhadi MA, Taheri M and Ghavami S: miR-608 rs4919510
C>G polymorphism decreased the risk of breast cancer in an
Iranian subpopulation. Exp Oncol. 38:57–59. 2016.PubMed/NCBI
|
|
45
|
Testa JR and Bellacosa A: AKT plays a
central role in tumorigenesis. Proc Natl Acad Sci USA.
98:10983–10985. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martelli AM, Tabellini G, Bressanin D,
Ognibene A, Goto K, Cocco L and Evangelisti C: The emerging
multiple roles of nuclear Akt. Biochim Biophys Acta.
1823:2168–2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Arboleda MJ, Lyons JF, Kabbinavar FF, Bray
MR, Snow BE, Ayala R, Danino M, Karlan BY and Slamon DJ:
Overexpression of AKT2/protein kinase Bbeta leads to up-regulation
of beta1 integrins, increased invasion, and metastasis of human
breast and ovarian cancer cells. Cancer Res. 63:196–206.
2003.PubMed/NCBI
|
|
48
|
Santi SA and Lee H: Ablation of Akt2
induces autophagy through cell cycle arrest, the downregulation of
p70S6K, and the deregulation of mitochondria in MDA-MB-231 cells.
PLoS One. 6:e146142011. View Article : Google Scholar
|
|
49
|
Altomare DA, Tanno S, De Rienzo A,
Klein-Szanto AJ, Tanno S, Skele KL, Hoffman JP and Testa JR:
Frequent activation of AKT2 kinase in human pancreatic carcinomas.
J Cell Biochem. 87:470–476. 2002. View Article : Google Scholar
|
|
50
|
Xu X, Sakon M, Nagano H, Hiraoka N,
Yamamoto H, Hayashi N, Dono K, Nakamori S, Umeshita K, Ito Y, et
al: Akt2 expression correlates with prognosis of human
hepatocellular carcinoma. Oncol Rep. 11:25–32. 2004.
|
|
51
|
Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X,
Jiang C, Coppola D, Nicosia SV and Cheng JQ: Frequent activation of
AKT2 and induction of apoptosis by inhibition of
phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer.
Oncogene. 19:2324–2330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheng JQ, Godwin AK, Bellacosa A, Taguchi
T, Franke TF, Hamilton TC, Tsichlis PN and Testa JR: AKT2, a
putative oncogene encoding a member of a subfamily of
protein-serine/threonine kinases, is amplified in human ovarian
carcinomas. Proc Natl Acad Sci USA. 89:9267–9271. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ringel MD, Hayre N, Saito J, Saunier B,
Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD and Saji M:
Overexpression and overactivation of Akt in thyroid carcinoma.
Cancer Res. 61:6105–6111. 2001.PubMed/NCBI
|
|
54
|
Cui Y, Wang Q, Wang J, Dong Y, Luo C, Hu G
and Lu Y: Knockdown of AKT2 expression by RNA interference inhibits
proliferation, enhances apoptosis, and increases chemosensitivity
to the anticancer drug VM-26 in U87 glioma cells. Brain Res.
1469:1–9. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mure H, Matsuzaki K, Kitazato KT,
Mizobuchi Y, Kuwayama K, Kageji T and Nagahiro S: Akt2 and Akt3
play a pivotal role in malignant gliomas. Neuro Oncol. 12:221–232.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lee MW, Kim DS, Lee JH, Lee BS, Lee SH,
Jung HL, Sung KW, Kim HT, Yoo KH and Koo HH: Roles of AKT1 and AKT2
in non-small cell lung cancer cell survival, growth, and migration.
Cancer Sci. 102:1822–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Balsara BR, Pei J, Mitsuuchi Y, Page R,
Klein-Szanto A, Wang H, Unger M and Testa JR: Frequent activation
of AKT in non-small cell lung carcinomas and preneoplastic
bronchial lesions. Carcinogenesis. 25:2053–2059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Attoub S, Arafat K, Hammadi NK, Mester J
and Gaben AM: Akt2 knock-down reveals its contribution to human
lung cancer cell proliferation, growth, motility, invasion and
endothelial cell tube formation. Sci Rep. 5:127592015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Qiao J, Lee S, Paul P, Qiao L, Taylor CJ,
Schlegel C, Colon NC and Chung DH: Akt2 regulates metastatic
potential in neuroblastoma. PLoS One. 8:e563822013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liang Z, Wang X, Xu X, Xie B, Ji A, Meng
S, Li S, Zhu Y, Wu J, Hu Z, et al: MicroRNA-608 inhibits
proliferation of bladder cancer via AKT/FOXO3a signaling pathway.
Mol Cancer. 16:962017. View Article : Google Scholar : PubMed/NCBI
|