Introduction
Pancreatic ductal adenocarcinoma (PDAC) is among the
most aggressive of types of cancer, and has a 5-year survival rate
of <6% (1). This poor prognosis
is due to a typically late-stage clinical diagnosis and the absence
of effective chemotherapeutic treatment regimens. Although the
5-year survival rate for early-stage PDAC is ~60%, only 2% of PDAC
cases are diagnosed at an early stage (1). Therefore, in order to improve the
prognosis of patients with PDAC, the development of novel
technologies for the early detection of the disease and effective
therapeutic strategies is required.
One characteristic of cancer cells is the ability to
reprogram their glucose metabolism. Differentiated tissues
(non-proliferating cells) tend to use oxidative phosphorylation and
anaerobic glycolysis under aerobic and anaerobic conditions,
respectively, as metabolic pathways (2). By contrast, tumor cells and
proliferative tissue tend to consume large amounts of glucose and
to produce lactate via glycolysis even in the presence of oxygen, a
phenomenon known as anaerobic glycolysis, or the Warburg effect.
Previous studies have revealed that the Warburg effect is
controlled by the pyruvate kinase (PK) M2 isoform (PKM2) (3,4). PK
regulates the final step of glycolysis by converting
phosphoenolpyruvate and adenosine diphosphate (ADP) into pyruvate
and adenosine triphosphate (ATP). PK has 4 isoforms (L, R, M1 and
M2) with tissue-specific and developmentally regulated expression
(4): PKL is expressed in the
liver, PKR in red blood cells, PKM1 in most differentiated tissues
(including muscle, heart and brain) and PKM2 in embryonic and tumor
cells (5).
PKM2 is strongly expressed in various types of human
cancer (6), including pancreatic
cancer (7). PKM2 is likely to
promote tumorigenesis by regulating the Warburg effect: The
downregulation of PKM2 increases oxygen consumption and decreases
glucose uptake and lactate production (3), and restoring PKM2 increases cell
proliferation and tumor formation in immunodeficient mice (8). In addition to its roles as a PK
enzyme, PKM2 also functions as a co-activator or a protein kinase
(9). For example, PKM2 interacts
with hypoxia-inducible factor (HIF)-1 (10) to reprogram glucose metabolism in
cancer cells, and it regulates cyclin D1 and c-Myc expression by
phosphorylating histone H3 at threonine 11 (H3-T11), leading to
G1-S phase transition, chromosome segregation, cell-cycle
progression and tumorigenesis (11). However, little is known about the
biological role of PKM2 in PDAC. In this study, to determine
whether PKM2 promotes the development of PDAC, we examined the
expression and function of PKM2 in human PDAC.
Our results clearly demonstrate that PKM2 is
upregulated in PDAC tissues, and that PKM2 knockdown reduces
tumorigenesis by altering the expression of genes that drive the
cell-cycle G1-S phase transition and the production of
intercellular metabolites, particularly spermine, which induces
transcription. These findings suggest that PKM2 regulates stable
cell division in PDAC and is a potential therapeutic target for
PDAC.
Materials and methods
Microdissection of tissue samples
A total of 10 PDAC tissue samples and non-tumor
tissue samples were obtained from patients who underwent surgical
resection at the Miyagi Cancer Center Hospital, Natori, Japan
during the period between 2010 and 2012. Each sample was examined
histologically and diagnosed by two pathologists who were unaware
of the present study. The non-tumor tissues obtained were adjacent
to the tumor tissue samples. The tissues were embedded in paraffin
and cut into 10-µm-thick sections, and ~10 sequential
regions were microdissected from the same paraffin block using a
Leica CIR MIC system (Leica Microsystems, Wetzlar, Germany) to
obtain samples of cancer cells (n=10) and normal pancreatic duct
cells (n=5). Total RNA was extracted using the Recover All™ Total
Nucleic Acid Isolation kit (Ambion, Austin, TX, USA) following the
manufacturer's instructions. This study was approved by the Ethics
Committee of Miyagi Cancer Center (2010-039), and informed consent
was obtained from each patient.
Cell culture
The PDAC cell lines, AsPC-1, BxPC-3, PANC-1 and
MIAPaCa, were obtained from the American Type Culture Collection
(Manassas, VA, USA), maintained in Dulbecco's modified Eagle's
medium (DMEM; Wako Pure Chemical Industries, Osaka, Japan)
containing 10% inactivated fetal bovine serum (FBS; EuroClone,
Milano, Italy) with 100 U/ml penicillin and 100 µg/ml
streptomycin (Nacalai Tesque, Kyoto, Japan) and cultured in a
humidified incubator at 37°C, 5% CO2.
RNA interference
PKM2 was transiently knocked down by introducing
siRNA against PKM2 (Nippongene, Tokyo, Japan) at a final
concentration of 100 nM; Universal Negative Control siRNA
(Nippongene) was used as a negative control. The siRNA was
introduced using Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with Opti-MEM I (Thermo Fisher
Scientific) according to the manufacturer's instructions. For
stable PKM2 knockdown, we used Knockout™ RNAi systems (Clontech
Laboratories, Inc., Mountain View, CA, USA) following the
manufacturer's instructions. Complementary shRNA oligonucleotides
were annealed and ligated into the pSIREN vector. Subsequently,
Plat-A packaging cells were transfected with shPKM2 or empty pSIREN
vector (control) to produce recombinant retroviruses. PDAC cells
stably infected with the recombinant retroviruses were selected
with puromycin. The PKM2 sequences targeted by siRNA (si27, si155
and si156) and shRNA (sh27) are described elsewhere (12).
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the PDAC cell lines
using the RNeasy Mini kit (Qiagen, Tokyo, Japan). First-Strand cDNA
was generated from 300–500 ng of total RNA using the
PrimeScript® 1st Strand cDNA Synthesis kit (Takara Bio,
Shiga, Japan). PCR was performed at 95°C for 10 min, followed by 45
cycles of 95°C for 10 sec and 60°C for 25 sec. For RT-qPCR, we used
a LightCycler® 480, a LightCycler® 480 probes
master kit, TaqMan probes from the Universal Probe Library (Roche,
Basel, Switzerland) and the following primers: PKM1 forward,
cagccaaaggggactatcct and reverse, gaggctcgcacaagttcttc; PKM2
forward, ctatcctctggaggctgtgc and reverse, gtggggtcgctggtaatg; and
β-actin forward, ccaaccgcgagaagatga and reverse,
tccatcacgatgccagtg.
Cell proliferation assay
Cell proliferation rates were assayed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The cells were seeded at 1×103–1×104
per well in 96-well plates in normal cell growth medium. MTT
solution (5 mg/m; Dojindo, Kumamoto, Japan) was added after 24, 48,
72 or 96 h, and the cells were incubated for a further 3 h at 37°C,
after which MTT-formazan production was estimated by measuring the
absorbance at 570 nm using a VersaMax microplate reader (Molecular
Devices LLC, Sunnyvale, CA, USA).
Cell migration assay
Cell migration was evaluated by a wound healing
scratch assay. Confluent PDAC cells in 6-well plates were incubated
with mitomycin C (Wako Pure Chemical Industries) for 2 h to inhibit
cellular proliferation, after which the cells were scratched with
the tip of a sterile P200 pipette. The medium was changed to a
normal cell growth medium, and changes in the scratched area were
measured following a 24-h incubation.
Western blot analysis
Cell lysates were extracted at 72 h after
transfection with PKM2 siRNA or negative control. The cells were
lysed with RIPA buffer supplemented with protease and phosphatase
inhibitors (Roche). The total cell lysates were fractionated by
5–20% SDS-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% non-fat milk in Tris-buffered saline (TBS)
containing 0.1% Tween-20. The membranes were incubated overnight at
4°C with primary antibodies (anti-PKM2 and anti-α-tubulin
antibodies and the details are described below), washed with TBS 3
times, and incubated with the horseradish peroxidase (HRP) linked
secondary antibodies. Reactive bands were detected using Super
Signal West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific). Anti-PKM2 rabbit polyclonal antibodies used as primary
antibody were originally generated by our group against synthetic
peptides. The amino acid sequences of antigen peptides were
described previously (3). The
α-tubulin (sc-23948; Santa Cruz Biotechnology, Santa Cruz, CA, USA)
primary antibody was used as the internal standard. Anti-rat
HRP-linked antibody (#7077) and anti-mouse IgG HRP-linked antibody
(#7076) (both from Cell Signaling Technology, Danvers, MA, USA)
were used as secondary antibodies. The dilution ratio of all the
antibodies was 1:1,000.
Cell cycle analysis
Cell cycle regulation was analyzed by
fluorescence-activated cell sorting (FACS). The cells were fixed
with 70% ethanol, stored overnight at −30°C, and washed twice with
FACS buffer (PBS, 0.5% BSA and 0.1% NaN3). The cells
were incubated with an anti-Ki-67 antibody (BioLegend, San Diego,
CA, USA) at a dilution of 1:60 for 1.5 h at room temperature and
then with 20 µg/ml of RNase for 1 h at 37°C. The samples
were stained with 5 mg/ml propidium iodide (PI) and analyzed with a
FACSCanto™ II flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Glucose consumption assay
Extracellular glucose was measured using a Glucose
Assay kit (BioVision Technologies, Chester Springs, PA, USA). The
cells were incubated in DMEM without FBS for 12 h prior to the
assay, and glucose levels were quantified using a VersaMaxPLUS ROM
plate reader (Molecular Devices LLC) according to the
manufacturer's instructions.
Extracellular flux analyzer
The oxygen consumption rate (OCR, pmoles/min) and
extracellular acidification rate (ECAR, mpH/min) were measured
using Seahorse XFe96 extracellular flux analyzers (Seahorse
Bioscience, North Billerica, MA, USA) according to the
manufacturer's instructions. The cells in which PKM2 was knocked
down or the control cells were seeded in an XF96 cell-culture
microplate at 24 h prior to the assay. On the day of the
experiment, the initial culture medium was changed to Agilent
Seahorse XF base medium (102353-100; Agilent Technologies, Santa
Clara, CA, USA) supplemented with GlutaMAX™ (Thermo Fisher
Scientific) with or without 25 mM glucose, and the cells were
cultured for 1 h in a CO2-free 37°C incubator and then
subjected to a Seahorse XF Cell Mito Stress Test (103015-100) or
Seahorse XF Glycolysis Stress Test (103020-100) (both from Agilent
Technologies). The following drugs at the indicated concentrations
were used for the tests: 3 µM oligomycin A, 3 µM
FCCP, 2.78 µM antimycin and 2.78 µM rotenone A for
the glycolysis test (all drugs were included in the Seahorse XF
Glycolysis Stress Test; 103020-100; Agilent Technologies), and 10
mM glucose, 3 µM oligomycin A and 100 mM 2-DG for the Mito
test (all drugs were included in the Seahorse XF Cell Mito Stress
Test; 103015-100; Agilent Technologies). Measurements were recorded
at the intervals prescribed by the test protocols.
Metabolome analysis
Intracellular metabolites were extracted from the
MIAPaCa-2 cells (2×106) in which PKM2 was knocked down
or the MIAPaCa-2 control cells. The culture medium was aspirated
from the dish, and the cells were washed twice with a 5% mannitol
solution. The cells were lysed with 1,300 µl of 60% methanol
containing 10 µM commercial Internal Standard Solution 1
(Solution ID: H3304-1002; Human Metabolome Technologies, Inc.,
Tokyo, Japan) to inactivate enzymes and then harvested with a cell
scraper. Tissue samples (50 mg) were plunged into 1,500 µl
of a 50/50 mixture of acetonitrile and Milli-Q water containing the
internal standards, 50 µM each of methionine sulfone
(MetSul) and CSA, homogenized at 9,000 × g at 4°C for 30 sec twice
with zirconia beads using a Precellys-24 Homogenizer (Bertin
Technologies, Washington, DC, USA), and centrifuged again at 2,300
× g for 5 min. The upper aqueous layer was passed through a filter
with a 5-kDa cut-off (UFC3LCCNB-HMT; Human Metabolome Technologies
Inc.) to remove the proteins. The filtrate was dried under reduced
pressure and suspended in 50 µl of Milli-Q water. The
extracted metabolites were measured by a capillary electrophoresis
time-of-flight mass spectrometer (CE-TOF/MS) with a commercial
electrophoresis buffer (Solution ID H3302-1011and H3302-1021; Human
Metabolome Technologies Inc.).
Microarray analysis
To identify the molecular target of PKM2 in the PDAC
cells, comprehensive gene expression profiles were analyzed in the
PDAC ells (MIAPaCa-2, PANC-1 and AsPC1) in which PKM2 was knocked
down and the control (empty vector-transfected) cells using the
Agilent Gene Expression Hybridization kit (Agilent Technologies).
RNA concentration and purity were determined spectrophotometrically
using the NanoDrop ND-1000 (Thermo Fisher Scientific) for all
samples. Targets were prepared and the microarrays processed
according to the manufacturer's instructions. Chips were scanned by
the Agilent Feature Extraction 10.9 to assess the raw probe signal
intensities.
Gene set enrichment analysis (GSEA)
GSEA was performed using the Broad Institute
platform. Gene sets with a false discovery rate (FDR) value
<0.25 after 1,000 permutations were considered significantly
enriched.
Gene Expression Omnibus (GEO)
PKM expression was analyzed in PDAC tissue samples
using the Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) datasets (GDS4336
and GDS4103).
Xenograft tumor experiments
A total of 15 female NOD/Shi-scid-IL2Rγnull (NOG)
mice aged 6–10 weeks (~20–25 g) were obtained from the Central
Institute for Experimental Animals (CIEA, Kawasaki, Japan). The
mice were housed in a cleanroom in the animal care facility at
Miyagi Cancer Research Center, with standard temperature, humidity,
and timed lighting conditions, and were fed mouse chow/water ad
libitum. All animal experiments were approved by the Animal
Welfare Committee of Miyagi Cancer Center (MCC-AE-2016-6). Tumor
xenografts were generated by subcutaneously injecting
1×104–5 PDAC cells in 100 µl of PBS. Tumor volume
was measured once a week for 9 weeks. Tumor volume was calculated
using the following formula: [(long diameter) + (short
diameter)2] ×1/2. The mice were sacrificed at 9–10 weeks
after the injection of the PDAC cells. At that time, the mean
weight of the mice was ~30 g. In addition, no mice developed
multiple tumors.
Measurement of reactive oxygen species
(ROS)
Hydrogen peroxide (H2O2) and
ROS levels were measured with the ROS-Glo™
H2O2 assay (Promega, Madison, WI, USA)
according to the manufacturer's instructions.
Statistical analysis
Significant differences between 2 groups were
determined using the Student's t-test, and differences between
>2 groups were evaluated by analysis of variance (ANOVA) and
Dunnett's multiple comparisons test. A P-value <0.05 at the 95%
confidence level was considered significant. All statistical
analyses were performed using GraphPad Prism 6.0 software (GraphPad
Software Inc., San Diego, CA, USA).
Results
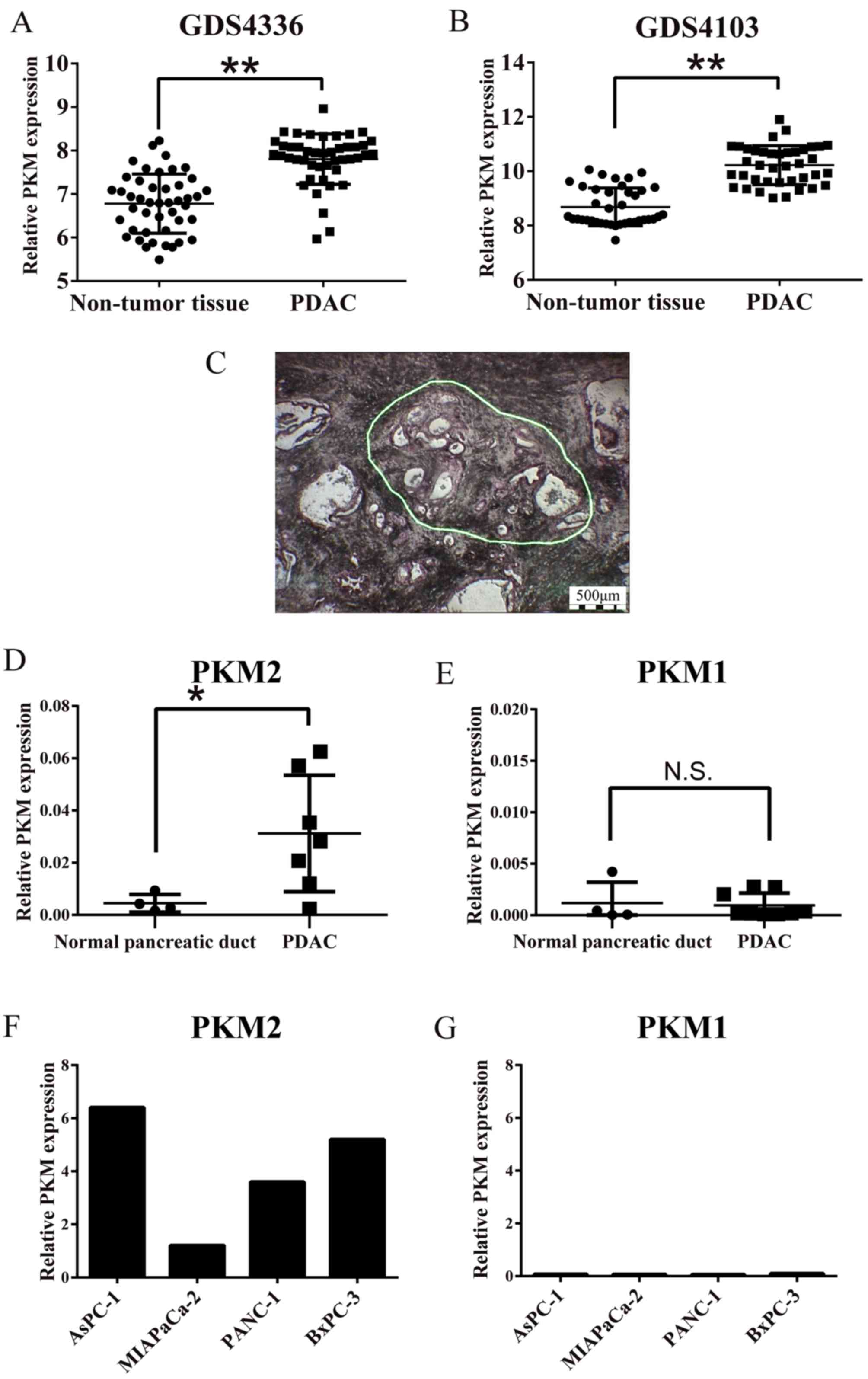
PKM2 expression is enhanced in PDAC
tissues and cell lines
We first analyzed the expression of whole PKM,
including the M1 and M2 isoforms, in PDAC tissues using GEO
data-sets (Fig. 1A and B), since
the probe used in this dataset coded for the common sequence of
PKM1 and PKM2, and found that PKM was significantly overexpressed
in the PDAC compared with the non-tumorous pancreatic tissues
(P<0.01). We then examined the expression of PKM1 and PKM2 by
RT-qPCR using specific primers in the microdissected pancreatic
lesions (Fig. 1C). As shown in
Fig. 1D, the cancer cells
expressed significantly more PKM2 than the normal pancreatic duct
cells (P<0.01). On the other hand, there was no difference in
PKM1 expression levels between the carcinoma and normal cells
(Fig. 1E). PKM2 was predominantly
expressed compared to PKM1 in the cultured PDAC cells (Fig. 1F and G). Taken together, these
results indicated that PKM2, but not PKM1, was upregulated in the
PDAC cells.
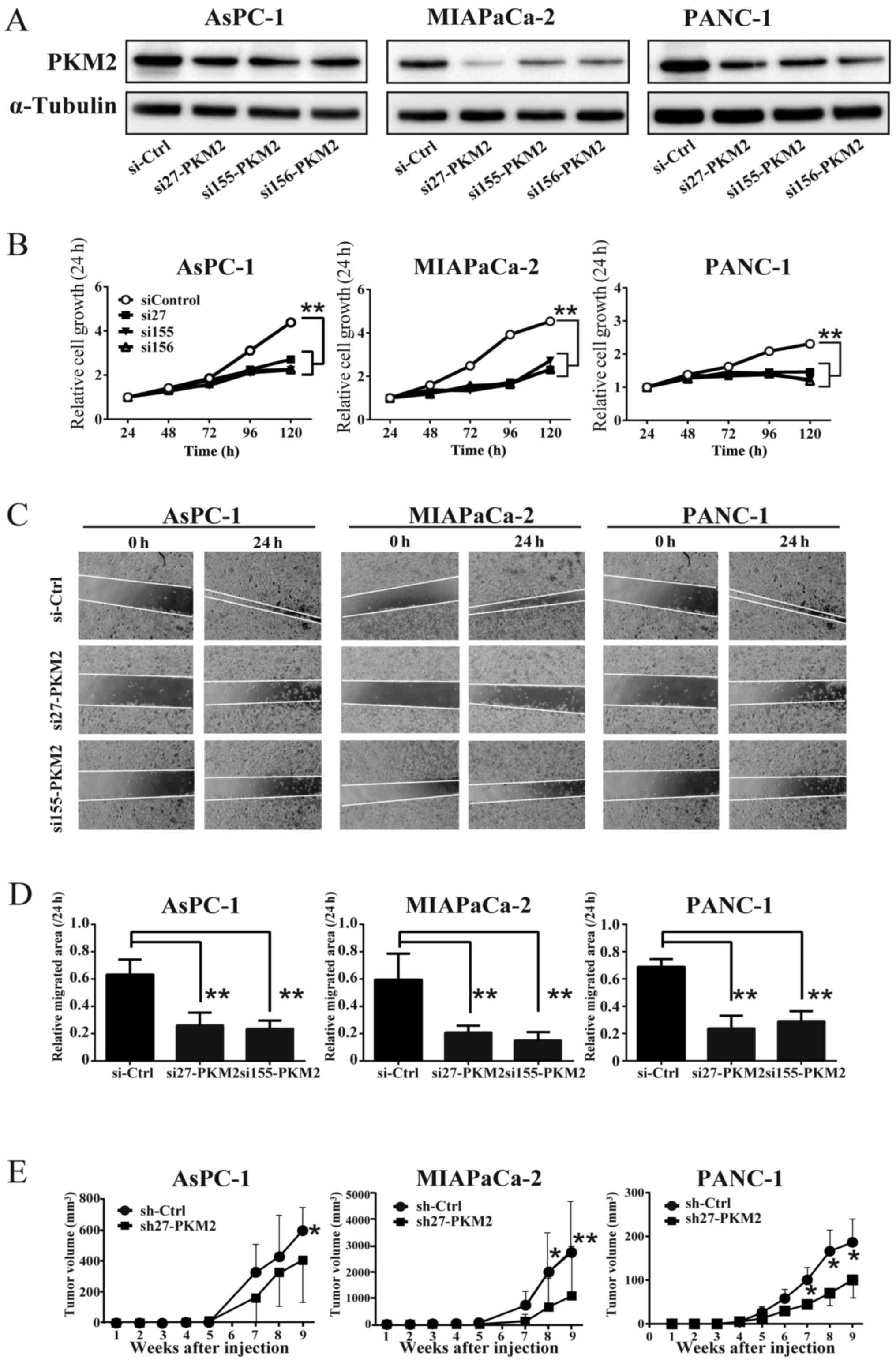
PKM2 knockdown in PDAC cells reduced cell
growth, migration, and tumorigenecity
To clarify the role of PKM2 in PDAC, we introduced
siRNA (in vitro) and shRNA (in vivo) into the PDAC
cells to generate cells in which PKM2 was knocked down (PKM2-KD
group). The results of western blot analysis confirmed the
reduction of PKM2 expression in each PDAC cell line (Fig. 2A). The knockdown of PKM2 (PKM2-KD)
also suppressed the growth and migration of the PDAC cells
(Fig. 2B–D). Consistent with these
results in vitro, PKM2-KD significantly decreased the growth
of PDAC cell-derived tumors (PDAC cells were injected
subcutaneously into NOG mice) (Fig.
2E). These data thus suggest that PKM2 enhances cellular
growth, migration and tumorigenesis in PDAC. The xenograft and the
following experiments were performed using sh27 or si27 RNA which
downregulated PKM2 expression most effectively.
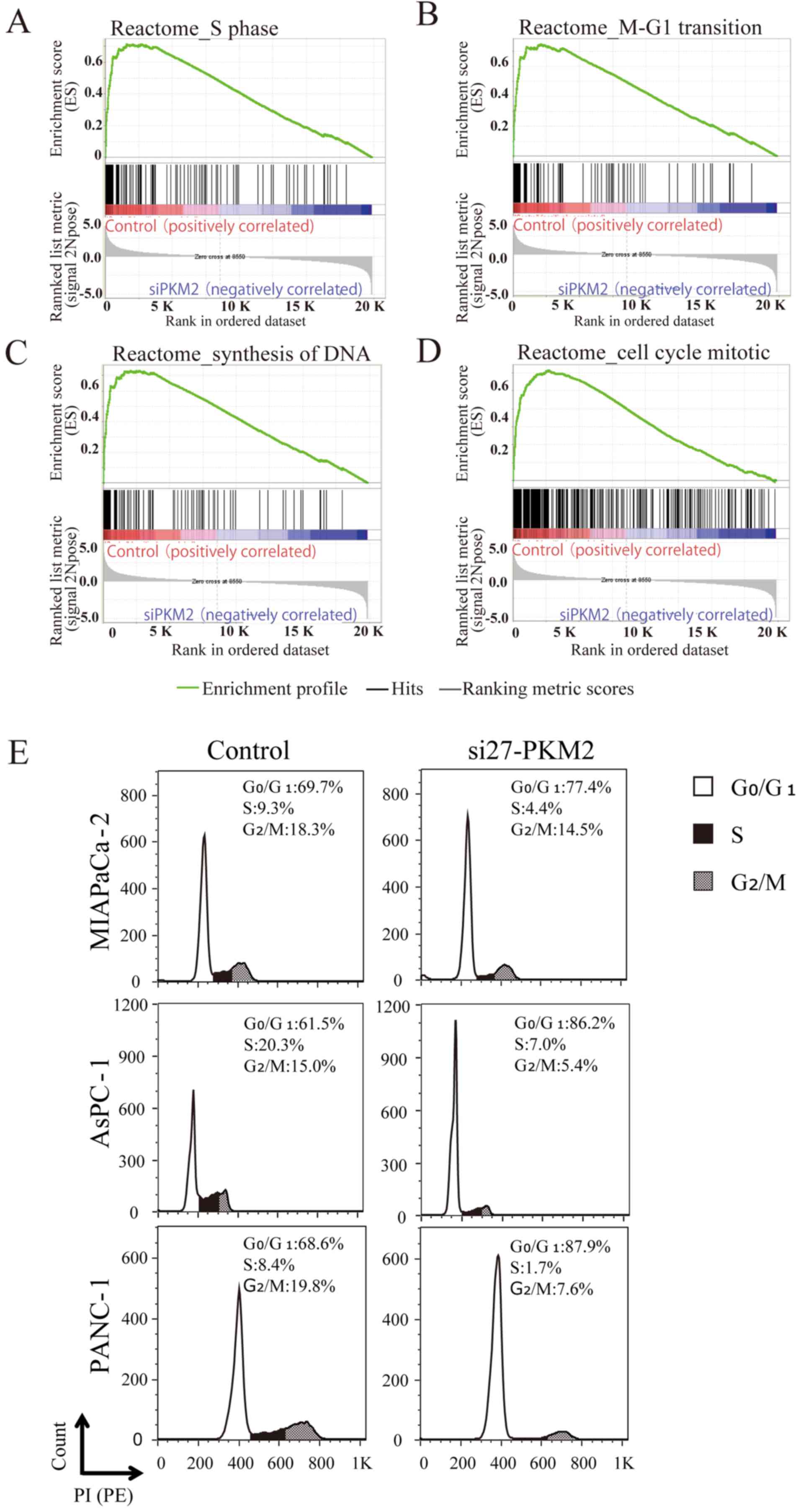
The expression of cell cycle-related
genes is downregulated and the cell cycle is arrested in the PDAC
cells in which PKM2 is downregulated
To determine whether the PKM2 expression level in
PDAC was associated with the expression of molecules involved in
tumor promotion, we used GSEA to identify gene expression
signatures associated with PKM2 expression, and identified 146 gene
sets that were significantly enriched in the PDA
C control cells compared to the PDAC cells in which
PKM2 was knocked down (PKM2-KD group), with a P-value of <0.01
and a FDR of <25%. Among these gene sets, the expression levels
of cell cycle signature genes were markedly downregulated in the
siRNA-transfected PDAC cells compared to the PDAC cells in the
PKM2-KD group, as shown by the enrichment plots for the activated
genes related to the different phases of the cell cycle (Fig. 3A–D). In particular, the expression
of MCM2-6, the gene that encodes Mcm2-6 (which makes up part
of the hexameric Mcm2-7 complex) (13), was downregulated in the PDAC cells
in the PKM2-KD group. The expression levels of ORC1 and
ORC6, which encode proteins in the origin recognition
complex (ORC), a multi-subunit DNA binding complex that binds to
origins of replication, were also downregulated. Consistent with
these affected genes, the cell cycle was arrested in the
PKM2-deficient PDAC cells (Fig.
3E).
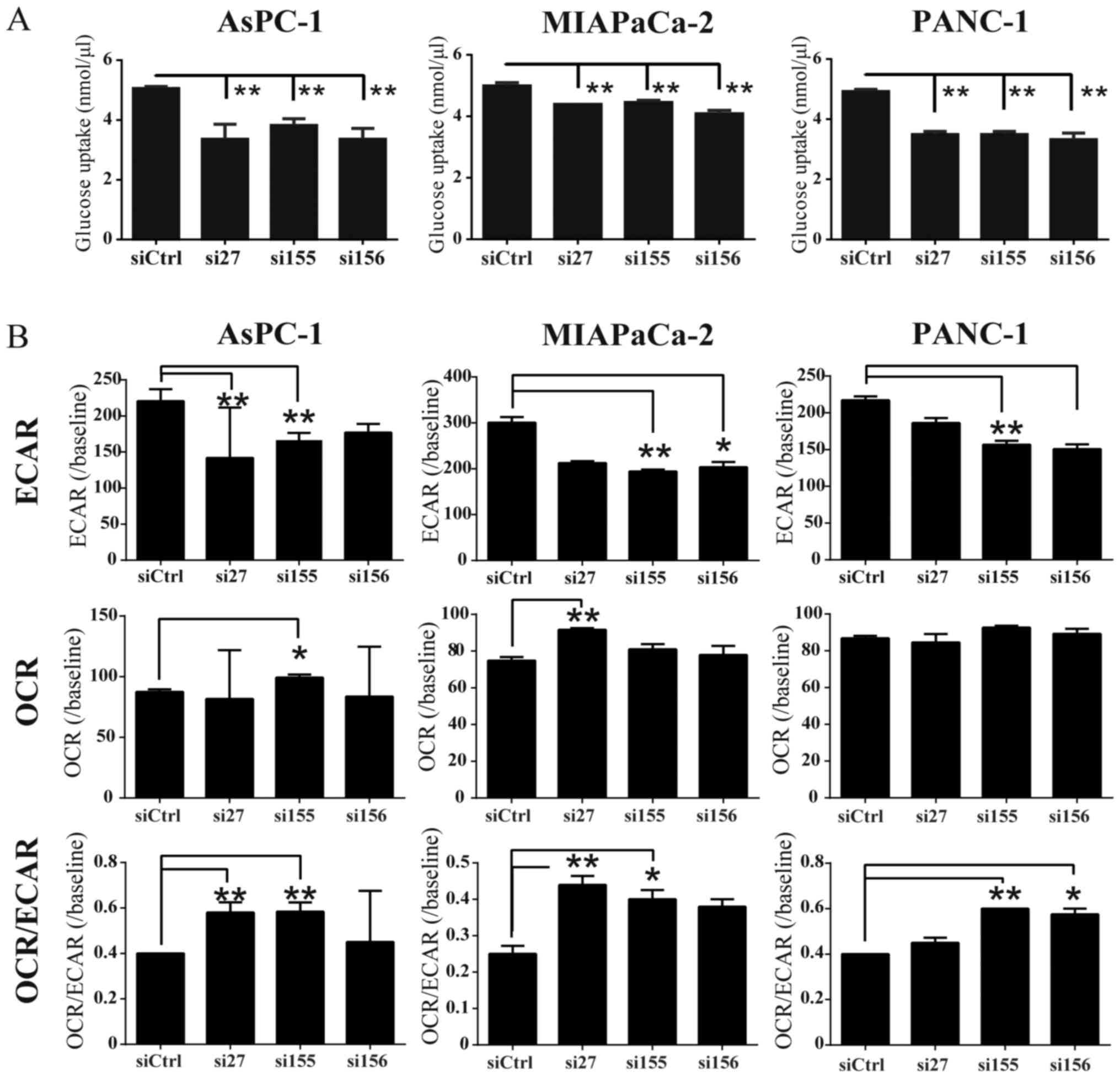
Glycolysis and the production of some
metabolites are attenuated in PDAC cells in which PKM2 is
downregulated
To determine whether PKM2 KD influences glucose
uptake, we examined glucose consumption in PDAC cell lines, and
found that it was decreased in the siPKM2-transfected PDAC cells
(Fig. 4A). To examine whether PKM2
expression was associated with glycolysis and oxidative
phosphorylation, we used extracellular flux analyzers to monitor
ECAR and OCR, which indicate the glycolytic pathway and
mitochondrial respiration, respectively. ECAR was significantly
reduced in the cells in the PKM2-KD group compared to the controls
(Fig. 4B). PKM2 deficiency had
little effect on OCR; however, the OCR/ECAR ratio was increased in
the cells in the PKM2-KD group, indicating that their energy was
mainly generated by mitochondrial respiration. We then evaluated
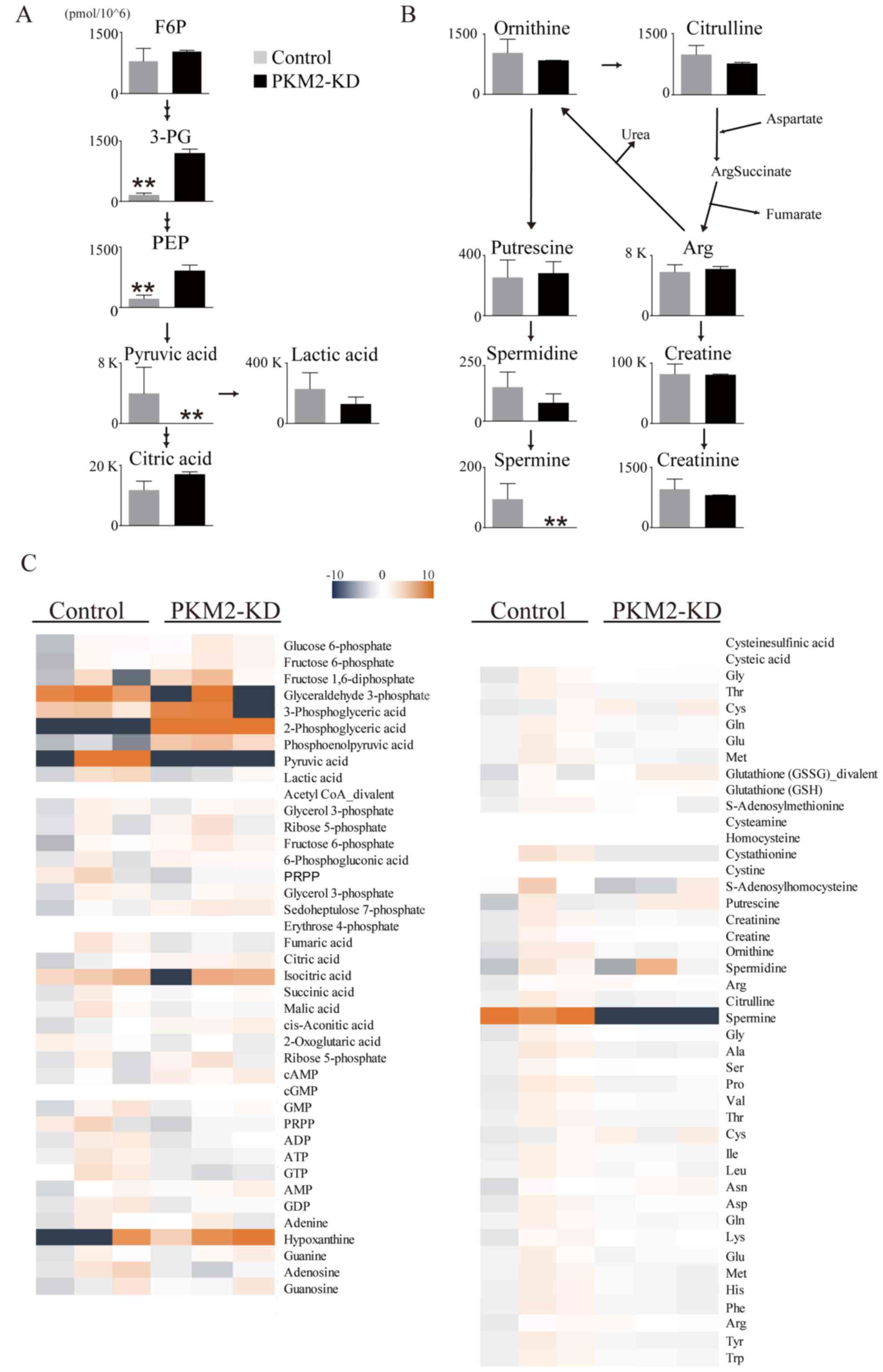
changes in PDAC intercellular metabolites following PKM2 knockdown
(Fig. 5A and B). CE-TOF/MS
revealed that the levels of some intracellular metabolites were
decreased in the cells in the PKM2-KD group, particularly pyruvate
and spermine (Fig. 5C). These data
thus indicate that PKM2 plays an important role in maintaining
glycolysis in PDAC cells.
PKM2 knockdown results in the
downregulation of spermidine synthase (SRM) and the upregulation of
spermidine N1-acetyltransferase 1 (SAT1)
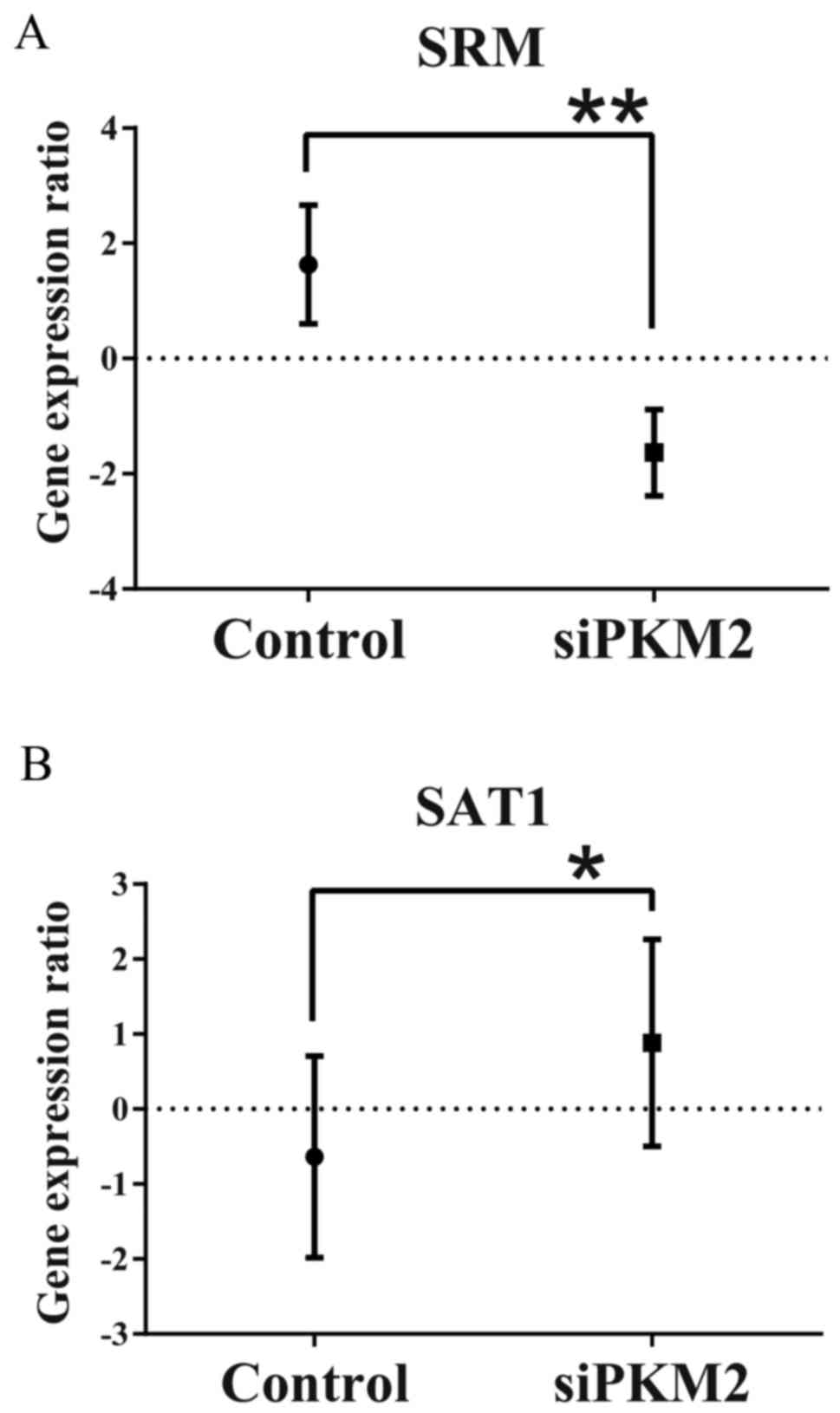
To assess the molecular mechanisms responsible for
the decrease in spermine production in the PDAC cells in the
PKM2-KD group, we evaluated the differences in gene expression
profiles between the PDAC control cells and those in the PKM2-KD
and group. We found a significant decrease in SRM levels in the
PDAC cells in the PKM2-KD group (Fig.
6A). In addition, the expression of SAT1, which catalyzes the
acetylation of spermidine and spermine to lead to rapid depletion
of spermidine and spermine (14)
was significantly increased in the PDAC cells in the PKM2-KD group
(Fig. 6B). In this context, the
attenuated production of spermine was due to the alteration of the
expression of these enzymes by PKM2 suppression in the PDAC
cells.
ROS levels increase in PDAC cells in
which PKM2 is knocked down
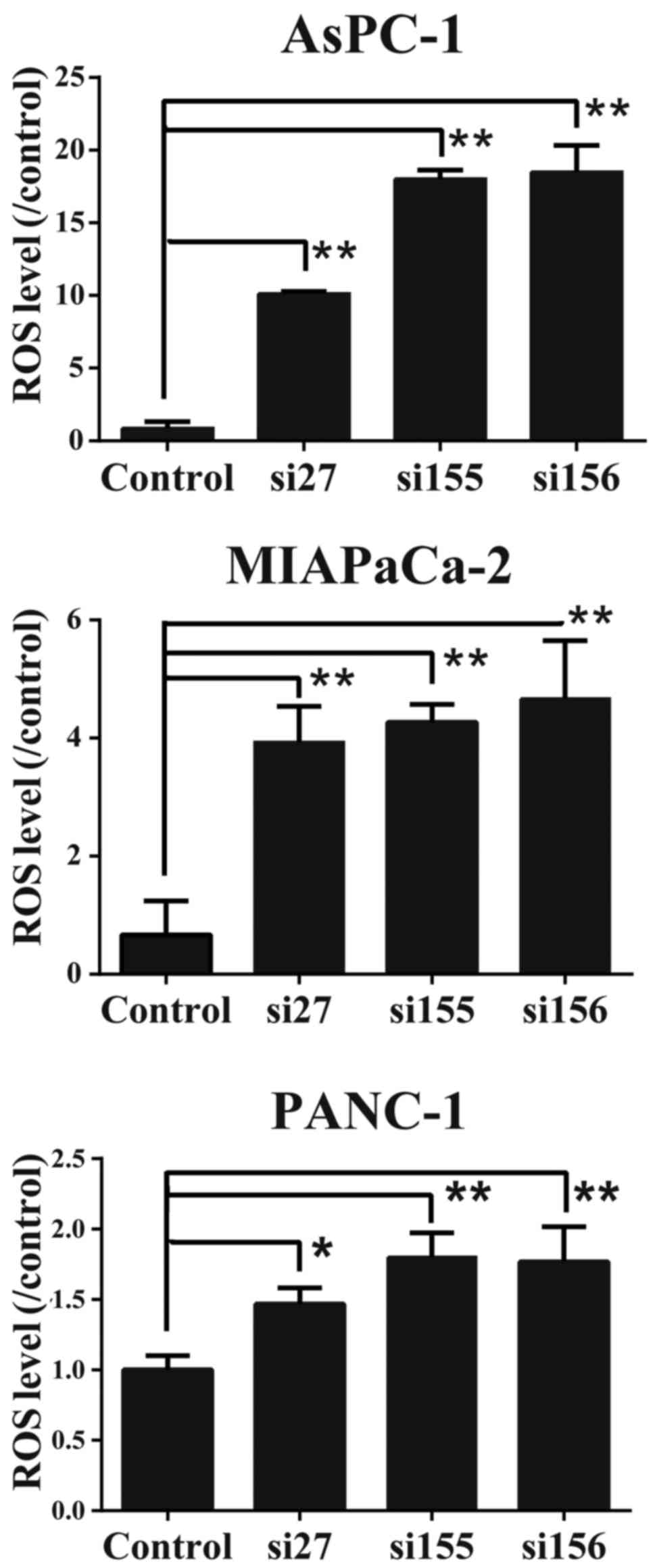
To determine whether PKM2 downregulation affects the
oxidative stress level, we examined the intercellular ROS
concentrations in the PDAC cells. As shown in Fig. 7, ROS concentration was elevated in
the PDAC cells in which PKM2 was knocked down. These data suggest
that the presence of PKM2 regulates ROS production or concentration
in PDAC cells.
Discussion
In this study, we demonstrated that PKM2, but not
PKM1 expression was upregulated in PDAC cells compared to
non-tumorous pancreatic tissue, and decreasing the PKM2 expression
in PDAC cells suppressed cell proliferation, migration and tumor
growth, and attenuated the Warburg effect, indicating that PKM2
contributes to carcinogenesis and development of PDAC. We also
showed that when PKM2 expression was downregulated in the PDAC
cells, the expression levels of genes that drive the cell cycle
were also downregulated, leading to cell cycle arrest. In addition,
PKM2 knockdown impeded the production of intercellular metabolites,
particularly spermine, and increased the ROS concentration.
Cancer metabolism has been extensively researched,
and some studies have suggested that PKM2 contributes to
tumorigenesis in various types of cancer by regulating cancer
metabolism (4). The PKM2 level is
associated with overall survival in PDAC (15), and a decreased PKM2 expression
suppresses cancer cell survival and invasion by altering the
Warburg effect (7). Similar
results were observed in this study, suggesting that PKM2 regulates
the Warburg effect in the development of PDAC. In addition, our
microarray analysis revealed that suppression of PKM2 expression
resulted in the change in gene sets of glycolysis and glucose
metabolism (data not shown), indicating that PKM2 is involved in
more metabolic pathway facilitating cancer development other than
expected.
Spermine is one of the polyamines, which are small
basic molecules that are essential to normal cell growth and DNA
stability (16–18). The polyamine content in the cell is
highly regulated, and its metabolic alteration is implicated in
many diseases. Studies have indicated correlations between
polyamines and tumor progression in breast, colon, prostate, and
skin cancers (19). Furthermore,
depleting polyamines inhibits skin-tumor growth (20) and breast-cancer migration (21). The spermine concentration is
increased in the urine of humans with pancreatic cancer (22). Spermine also accelerates
hypoxia-initiated cancer-cell migration (23). In this study, we found that
spermine was decreased in the cells in the PKM2-KD group compared
to the control PDAC cells. To elucidate the molecular mechanisms
responsible for the alteration of spermine production, we examined
the changes in the expression of polyamine-related genes between
the PDAC cells in the PKM2-KD group and the control PDAC cells by
microarray analysis and found the SRM reduction and SAT1 induction,
both of which cause the attenuation of spermine production, in the
PDAC cells in the PKM2-KD group. Taken together, these data suggest
the possibility that PKM2 facilitates PDAC cell growth and
migration by enhancing spermine production.
We also found that the ROS levels were elevated in
the cells in the PKM2-KD group. Increased ROS levels cause
oxidative stress and promote tumorigenesis and tumor migration
(24,25). On the other hand, excessive
increases in ROS levels have been shown to induce cell cycle arrest
and cell death (26,27). In breast cancer, cancer stem cells
contain lower ROS concentrations than those found in corresponding
non-tumorigenic cells (28). Taken
together, these findings suggest that elevated ROS in levels in
PDAC cells in which PKM2 is knocked down may inhibit the cell cycle
and cell survival, along with tumor migration and growth.
In conclusion, in this study, we demonstrated that
PKM2 was upregulated in PDAC cells compared to non-cancerous cells
and that the knockdown of PKM2 decreased the of cell growth and
migratory abilities that contribute to cancer cell development via
the alteration of cancer-specific metabolism and the acceleration
of ROS production, suggesting that PKM2 may be a biological marker
and an effective therapeutic target in PDAC.
Abbreviations:
|
ADP
|
adenosine diphosphate
|
|
ATP
|
adenosine triphosphate
|
|
cDNA
|
complementary DNA
|
|
CE-TOF/MS
|
capillary electrophoresis
time-of-flight mass spectrometry
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
siRNA
|
small interfering ribonucleic acid
|
|
shRNA
|
short hairpin ribonucleic acid
|
|
PBS
|
phosphate-buffered saline
|
|
PVDF
|
polyvinylidene difluoride
|
|
RT-PCR
|
reverse-transcription polymerase chain
reaction
|
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by Grants-in-Aid for
Scientific Research (KAKENHI) (nos. 15K19080 and 15K09055) to MY
and KS, respectively.
[2] Availability
of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
[3] Authors'
contributions
M.Y., N.T., K.T. and K.S. designed the study,
interpreted the results, analyzed the data and wrote and edited the
manuscript. M.A., K.Y., K.M. and I.S. provided the surgical
specimens and provided the histopathological findings. M.Y., R.S.
and T.S. performed the in vivo experiments involving
animals. M.Y., K.Y. and K.T performed the in vitro
experiments. All authors are aware of the content in this
manuscript and have read and edited the manuscript.
[4] Ethics
approval and consent to participate
For the use of human samples, this study was
approved by the Ethics Committee of Miyagi Cancer Center
(2010-039), and informed consent was obtained from each patient. In
addition, all animal experiments were approved by the Animal
Welfare Committee of Miyagi Cancer Center (MCC-AE-2016-6).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma J, Siegel R and Jemal A: Pancreatic
cancer death rates by race among US men and women, 1970–2009. J
Natl Cancer Inst. 105:1694–1700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Christofk HR, Vander Heiden MG, Harris MH,
Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL and
Cantley LC: The M2 splice isoform of pyruvate kinase is important
for cancer metabolism and tumour growth. Nature. 452:230–233. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mazurek S: Pyruvate kinase type M2: A key
regulator of the metabolic budget system in tumor cells. Int J
Biochem Cell Biol. 43:969–980. 2011. View Article : Google Scholar
|
|
5
|
Marie J, Levin MJ, Simon MP and Kahn A:
Genetic and epigenetic control of the pyruvate kinase isozymes in
mammals. Isozymes Curr Top Biol Med Res. 7:221–240. 1983.PubMed/NCBI
|
|
6
|
Dong G, Mao Q, Xia W, Xu Y, Wang J, Xu L
and Jiang F: PKM2 and cancer: The function of PKM2 beyond
glycolysis. Oncol Lett. 11:1980–1986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Zhao Z, Zhou Z and Liu R: PKM2
promotes cell survival and invasion under metabolic stress by
enhancing Warburg effect in pancreatic ductal adenocarcinoma. Dig
Dis Sci. 61:767–773. 2016. View Article : Google Scholar
|
|
8
|
Yang W, Xia Y, Cao Y, Zheng Y, Bu W, Zhang
L, You MJ, Koh MY, Cote G, Aldape K, et al: EGFR-induced and PKCε
monoubiquitylation-dependent NF-κB activation upregulates PKM2
expression and promotes tumorigenesis. Mol Cell. 48:771–784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong N, Ojo D, Yan J and Tang D: PKM2
contributes to cancer metabolism. Cancer Lett. 356:184–191. 2015.
View Article : Google Scholar
|
|
10
|
Luo W, Hu H, Chang R, Zhong J, Knabel M,
O'Meally R, Cole RN, Pandey A and Semenza GL: Pyruvate kinase M2 is
a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell.
145:732–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang W, Xia Y, Hawke D, Li X, Liang J,
Xing D, Aldape K, Hunter T, Alfred Yung WK and Lu Z: PKM2
phosphorylates histone H3 and promotes gene transcription and
tumorigenesis. Cell. 150:685–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldberg MS and Sharp PA: Pyruvate kinase
M2-specific siRNA induces apoptosis and tumor regression. J Exp
Med. 209:217–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bell SP and Dutta A: DNA replication in
eukaryotic cells. Annu Rev Biochem. 71:333–374. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pegg AE:
Spermidine/spermine-N(1)-acetyltransferase: A key metabolic
regulator. Am J Physiol Endocrinol Metab. 294:E995–E1010. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lockney NA, Zhang M, Lu Y, Sopha SC,
Washington MK, Merchant N, Zhao Z, Shyr Y, Chakravarthy AB and Xia
F: Pyruvate kinase muscle isoenzyme 2 (PKM2) expression is
associated with overall survival in pancreatic ductal
adenocarcinoma. J Gastrointest Cancer. 46:390–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pegg AE: Polyamine metabolism and its
importance in neoplastic growth and a target for chemotherapy.
Cancer Res. 48:759–774. 1988.PubMed/NCBI
|
|
17
|
Pegg AE: Mammalian polyamine metabolism
and function. IUBMB Life. 61:880–894. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Childs AC, Mehta DJ and Gerner EW:
Polyamine-dependent gene expression. Cell Mol Life Sci.
60:1394–1406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nowotarski SL, Woster PM and Casero RA Jr:
Polyamines and cancer: Implications for chemotherapy and
chemoprevention. Expert Rev Mol Med. 15:e32013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takigawa M, Verma AK, Simsiman RC and
Boutwell RK: Inhibition of mouse skin tumor promotion and of
promoter-stimulated epidermal polyamine biosynthesis by
alpha-difluoromethylornithine. Cancer Res. 43:3732–3738.
1983.PubMed/NCBI
|
|
21
|
Gupta ED, Pachauri M, Ghosh PC and Rajam
MV: Targeting polyamine biosynthetic pathway through RNAi causes
the abrogation of MCF 7 breast cancer cell line. Tumour Biol.
37:1159–1171. 2016. View Article : Google Scholar
|
|
22
|
Russell DH: Increased polyamine
concentrations in the urine of human cancer patients. Nat New Biol.
233:144–145. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krüger A, Vowinckel J, Mülleder M, Grote
P, Capuano F, Bluemlein K and Ralser M: Tpo1-mediated spermine and
spermidine export controls cell cycle delay and times antioxidant
protein expression during the oxidative stress response. EMBO Rep.
14:1113–1119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cross CE, Halliwell B, Borish ET, Pryor
WA, Ames BN, Saul RL, McCord JM and Harman D: Oxygen radicals and
human disease. Ann Intern Med. 107:526–545. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chio IIC and Tuveson DA: ROS in cancer:
The burning question. Trends Mol Med. 23:411–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Galadari S, Rahman A, Pallichankandy S and
Thayyullathil F: Reactive oxygen species and cancer paradox: To
promote or to suppress? Free Radic Biol Med. 104:144–164. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng B, Peng J, Mollayup A, Bakri A, Guo
L, Zheng J and Xu H: Construction of a prognostic prediction system
for pancreatic ductal adenocarcinoma to investigate the key
prognostic genes. Mol Med Rep. 17:216–224. 2018.
|
|
30
|
Badea L, Herlea V, Dima SO, Dumitrascu T
and Popescu I: Combined gene expression analysis of whole-tissue
and microdissected pancreatic ductal adenocarcinoma identifies
genes specifically overexpressed in tumor epithelia.
Hepatogastroenterology. 55:2016–2027. 2008.
|