Introduction
Pancreatic cancer is a highly aggressive and lethal
human malignancy, with a very low 5-year survival rate worldwide
(8%). The low survival rate is partly attributed to the fact that
over half of the cases are diagnosed at an advanced stage, for
which the 5-year survival may be as low as 3% (1). An estimated 53,670 new pancreatic
cancer cases were diagnosed in 2017, which may result in 43,090
cancer-related deaths in the United States (1). In China, pancreatic cancer is the
seventh most fatal disease, with an even lower 5-year survival rate
(4.1%) (2). The routine treatments
include surgery, radiation and chemotherapy (with gemcitabine,
5-fluorouracil or their combination). However, ~80% of pancreatic
cancer subjects are diagnosed when the tumor is unresectable and/or
has developed distant metastasis, and acquired resistance to
chemotherapeutic treatment always develops during the course of the
disease (3). Therefore, more
comprehensive and effective interventions are urgently required to
improve the treatment outcome of pancreatic cancer patients.
Curcumin is a natural polyphenol compound derived
from turmeric. It possesses several biological properties,
including anti-inflammatory and anti-oxidant properties, and also
plays a key role in inhibiting the initiation, progression and
metastasis of several tumors (4).
Curcumin exerts its anticancer effects by targeting multiple
intracellular signaling pathways, including mitogen-activated
protein kinase (MAPK), nuclear factor (NF)-κB, Akt and
Wnt/β-catenin, among others (5–7). In
addition, we recently demonstrated that curcumin inhibited
hypoxia-induced epithelial-to-mesenchymal transition (EMT) in
pancreatic cancer cells via blockade of the hedgehog signaling
pathway (8).
EMT has been recognized not only as a physiological
mechanism in mammalian embryonic development and tissue remodeling,
but also as an important phenomenon observed in tumorigenesis and
cancer development (9). During
EMT, epithelial cells lose their polarity, cell-cell tight
junctions and adhesive connections, and acquire mesenchymal
characteristics (10). The
hallmark of EMT is the loss of E-cadherin expression (an epithelial
marker) and the gain of vimentin and N-cadherin expression
(mesenchymal markers) (10). We
previously demonstrated that several factors could induce EMT in
pancreatic cancer cells, including hypoxic (8) and hyperglycemic environment (11), as well as the induction of
superoxide dismutase (SOD) (12).
Reactive oxygen species (ROS), including hydrogen
peroxide (H2O2), are a group of chemically
reactive molecules derived from oxygen. Intrinsic antioxidant
enzymes play a crucial role in the regulation of oxidative stress
in cells. SOD is one of the primary cellular antioxidants, and it
can catalyze the conversion of superoxide anion to
H2O2, which is cleared by catalase (CAT)
(13). Due to the cytoprotective
effects of SOD, its overexpression has been associated with
increased incidence of tumor metastasis (14). In our previous study, we verified
that the invasive ability and EMT of pancreatic cancer cells was
aggravated by SOD-dependent production of ROS via the extracellular
signal-regulated kinase (ERK)/NF-κB signaling pathway (12). However, whether curcumin can
repress the SOD-induced progression of pancreatic cancer, and the
possible underlying mechanisms, have not been fully elucidated.
The aim of the present study was to verify our
hypothesis and determine whether curcumin has the potential to
suppress SOD-driven H2O2-induced invasive and
migratory abilities and inhibit EMT in pancreatic cancer cells. We
also aimed to examine the effect of curcumin on SOD-induced
activation of the phosphoinositide-3 kinase (PI3K)/Akt signaling
pathway and the transcription factor NF-κB, to determine whether
using curcumin to inhibit the H2O2/Akt/NF-κB
axis may be a promising approach to the treatment of pancreatic
carcinoma.
Materials and methods
Cell culture and reagents
The human pancreatic cancer cell lines BxPC-3 and
Panc-1 were purchased from the American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM), which contains 10% dialyzed heat-inactivated
fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere of 5% CO2 at
37°C. The cells exponentially grew in complete medium and treated
with 400 U/ml SOD, with or without 20 µM curcumin, 400 U/ml
CAT or 10 µM LY 294002 (a PI3K inhibitor) for the indicated
time intervals, according to the experimental protocol. DMEM and
FBS were purchased from Gibco; Thermo Fisher Scientific (Grand
Island, NY, USA). Curcumin, CuZnSOD, CAT and LY294002 were
purchased from Sigma-Aldrich; Merck KGaA (St. Louis, MO, USA). The
hydrogen peroxide assay kit and the ROS assay kit were obtained
from Beyotime (Jinan, China). Millicell culture plate inserts were
purchased from Millipore (Bedford, MA, USA). Matrigel was purchased
from BD Biosciences (Bedford, MA, USA). Primary antibodies
[dilution 1:100 in phosphate-buffered saline (PBS)-Tween-20]
against E-cadherin (sc-52328), N-cadherin (sc-53488) and vimentin
(sc-66002) were obtained from Santa Cruz Biotechnology (Santa Cruz,
CA, USA). The anti-Akt (no. 9272), anti-phospho-Akt (anti-p-Akt,
Ser473, no. 4060), anti-NF-κB (no. 6956) and anti-phospho-NF-κB
(anti-p-NF-κB, Ser468, no. 3039) antibodies (dilution 1:200 in
PBS-Tween-20) were obtained from Cell Signaling Technology
(Beverly, MA, USA). Other reagents were purchased from common
commercial sources. All drug solutions were freshly prepared on the
day of testing.
Measurement of intracellular ROS
The level of intracellular ROS was examined using
the ROS assay kit according to the manufacturer's instructions.
Briefly, pancreatic cancer cells were incubated with
2,7-dichlorodihydrofluorescein diacetate (DCFDA) for 30 min. After
washing 3 times, fluorescence intensity was measured using a
fluorometer (Becton-Dickinson, Franklin Lakes, NJ, USA) with
excitation at 488 nm and emission at 525 nm.
Hydrogen peroxide assay
The level of intracellular
H2O2 was tested using a hydrogen peroxide
assay kit. In this kit, H2O2 oxidizes ferrous
(Fe2+) to ferric (Fe3+) ions; then,
Fe3+ ions combine with the indicator dye xylenol orange
to form a complex and produce a visible purple-colored complex,
which may be measured using a microplate reader at a wavelength of
560–590 nm (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transwell Matrigel invasion assay
The 8.0-µm pore inserts were covered with 25
µl Matrigel. BxPC-3 and Panc-1 cells were subjected to 24 h
serum starvation and were then suspended in DMEM supplemented with
1% FBS in the upper chamber at a density of 5×104, with
or without SOD, SOD with curcumin, CAT or LY 294002. In addition,
500 ml DMEM with 20% FBS were placed in the lower chamber. The
Matrigel invasion chamber was placed in a humidified tissue
incubator for 48 h. The non-invading cells were cleared away from
the top surface using a cotton swab and the filter was rinsed with
phosphate-buffered saline, fixed and stained with crystal violet.
Finally, the stained cells on the bottom surface were counted to
evaluate the invasive capacity of cancer cells. Three random fields
were captured at a magnification of ×20 (n=3).
Wound healing assay
Cells were seeded in 24-well plates
(1.0×105 cells/500 µl). After the cells had grown
to a 90–100% confluence, a sterile pipette tip was used to create a
scratch wound. Cellular debris was cleared away and the remaining
cells were allowed to grow and migrate in the plate for 24 h.
Images were captured at time points 0 and 24 h post-wounding by a
Nikon Diaphot TMD inverted microscope (magnification, ×10). The
relative distance traveled by the leading edge from 0 to 24 h was
assessed using Photoshop software (n=5).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cancer cells using the
Fastgen200 RNA isolation system (Fastgen, Shanghai, China). cDNA
was reverse-transcribed from RNA using the Fermentas RevertAid™ Kit
(MBI Fermentas, Burlington, ON, Canada). The primer sequences were
as follows: E-cadherin, F: 5′-ATT CTGATTCTGCTGCTCTTG-3′ and R:
5′-AGTCCTGGTCCTC TTCTCC-3′; N-cadherin, F: 5′-TGTTTGACTATGAAGGCAG
TGG-3′ and R: 5′-TCAGTCATCACCTCCACCAT-3′; vimentin, F:
5′-AATGACCGCTTCGCCAAC-3′ and R: 5′-CCGCATCTCC TCCTCGTAG-3′; and
β-actin, F: 5′-GACTTAGTTGCGTTACAC CCTTTCT-3′ and R:
5′-GAACGGTGAAGGT GACAGCAGT-3′.
The PCR conditions were as follows: 30 sec at 95°C,
followed by 40 cycles at 95°C for 5 sec, 60°C for 30 sec and 72°C
for 30 sec. A dissociation curve analysis was applied after each
RT-qPCR experiment. The relative gene expression was calculated
using the previously described 2−ΔΔCq method (15).
Western blotting
Identical amounts of protein from cancer cells were
loaded on a denaturing sodium dodecyl sulfate-polyacrylamide gel.
After gel electrophoresis, the protein was transferred onto
nitrocellulose membranes. Subsequently, the membranes were
initially blocked with 5% non-fat dry milk in Tris-buffered saline
(TBS) for 2 h, followed by incubation with different primary
antibodies (E-cadherin, N-cadherin, vimentin, Akt, p-Akt, NF-κB,
p-NF-κB and β-actin antibodies) at 4°C overnight. The membranes
were then incubated with secondary goat anti-mouse or goat
anti-rabbit antibodies (Sigma-Aldrich; Merck KGaA) for 2 h at room
temperature. Immunopositive bands were developed using an enhanced
chemiluminescence (ECL) detection system (Amersham, Piscataway, NJ,
USA). All analyses were conducted in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS Inc., Chicago, IL, USA). Data are
presented as the means ± standard error of the mean of three
replicate assays. Differences between the groups were analyzed by
analysis of variance with Dunnett's post-hoc test. Statistical
significance was set at P<0.05. All experiments were repeated
independently at least three times.
Results
Curcumin inhibits SOD-induced oxidative
stress in pancreatic cancer cells
ROS caused by oxidative stress damage cellular DNA,
proteins and lipids, and produce toxic and highly mutagenic
metabolites that may modify tumor behavior (16). We previously demonstrated that SOD
stimulated the production of H2O2 in
pancreatic cancer cells (12). As
curcumin possess antioxidant properties, in the present study, we
first examined the effects of curcumin on the production of ROS and
H2O2 in BxPC-3 and Panc-1 cells following
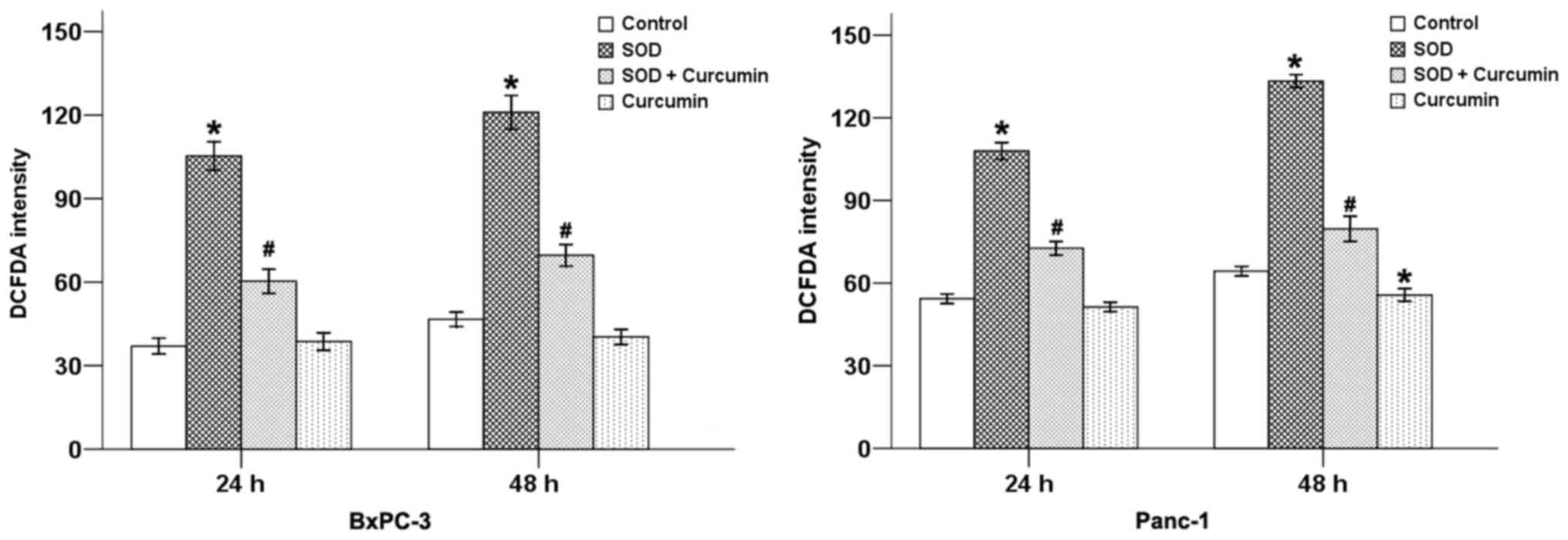
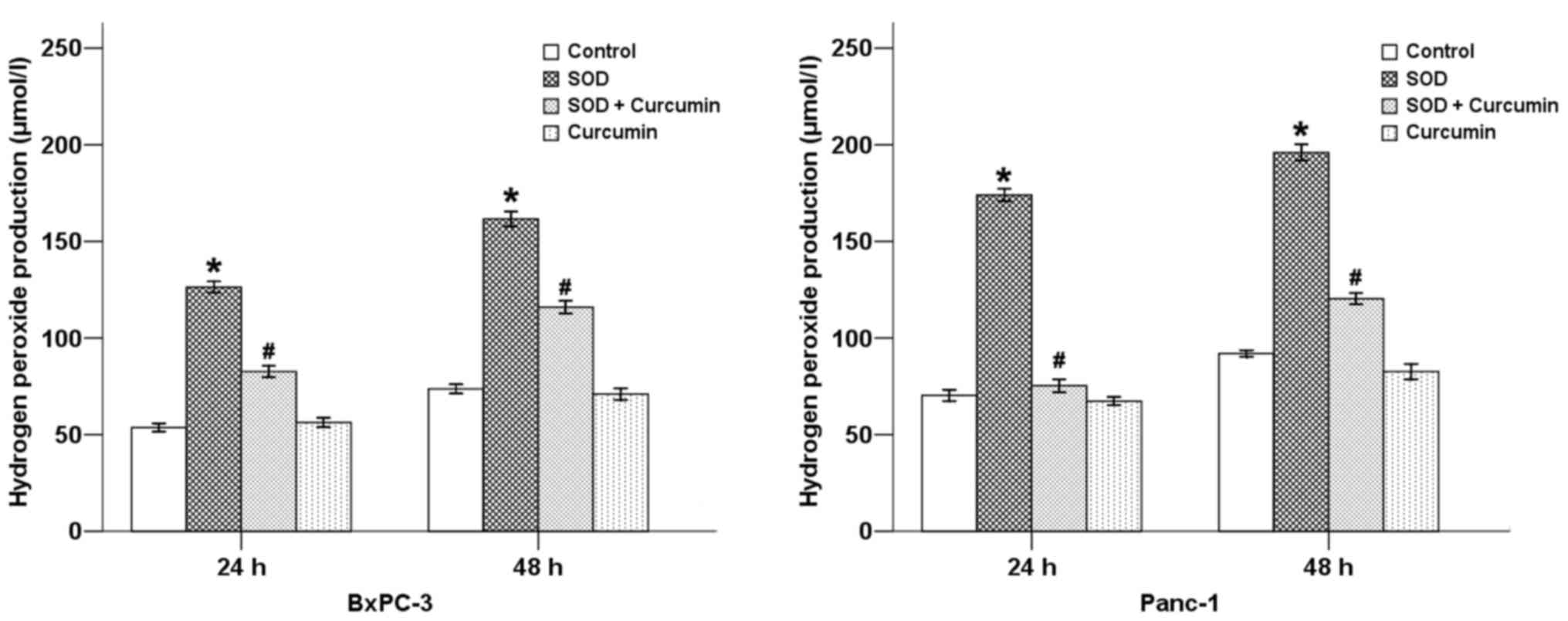
treatment with SOD. Our results revealed that SOD significantly
increased the DCFDA staining intensity (Fig. 1) and the level of
H2O2 (Fig.
2) in pancreatic cancer cells, while curcumin was able to
counterbalance these effects at 24 and 48 h in both cancer cell
types.
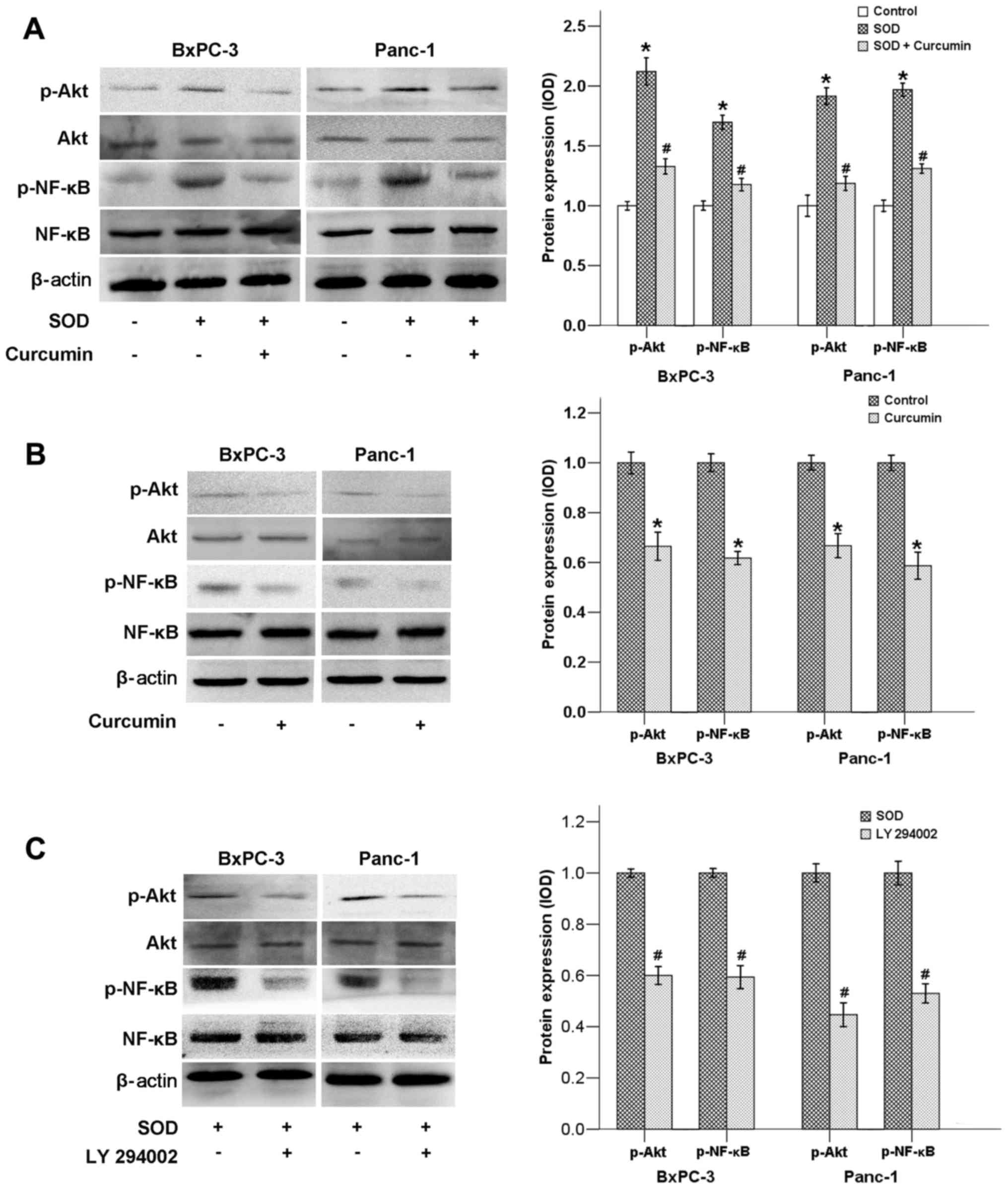
Curcumin downregulates the activation of
the Akt/NF-κB signaling pathway
The PI3K/Akt pathway plays an important role in
numerous cellular processes, such as cell metabolism, survival,
differentiation, proliferation, motility and angiogenesis (17). It has been proven that cancer cell
signaling is mediated by PI3K/Akt via activation of the
transcription factor NF-κB, which is associated with cell
proliferation, migration and invasion (18).
We previously demonstrated that SOD promotes
activation of the ERK/NF-κB signaling pathway (12). The findings of the present study
indicated that SOD could also upregulate the Akt/NF-κB signaling
pathway, as the phosphorylated levels of both Akt and NF-κB genes
were found to be significantly elevated following SOD addition
(Fig. 3A). When SOD was added to
the cell in culture medium along with curcumin, the expression of
p-Akt and p-NF-κB was strongly downregulated (Fig. 3A). Our results also demonstrated
that curcumin alone was able to inhibit the expression of p-AKT and
p-NF-κB (Fig. 3B). In addition, LY
294002, a PI3K inhibitor, also suppressed the expression of both
p-Akt and p-NF-κB, indicating that NF-κB is located downstream of
the PI3K/Akt pathway (Fig.
3C).
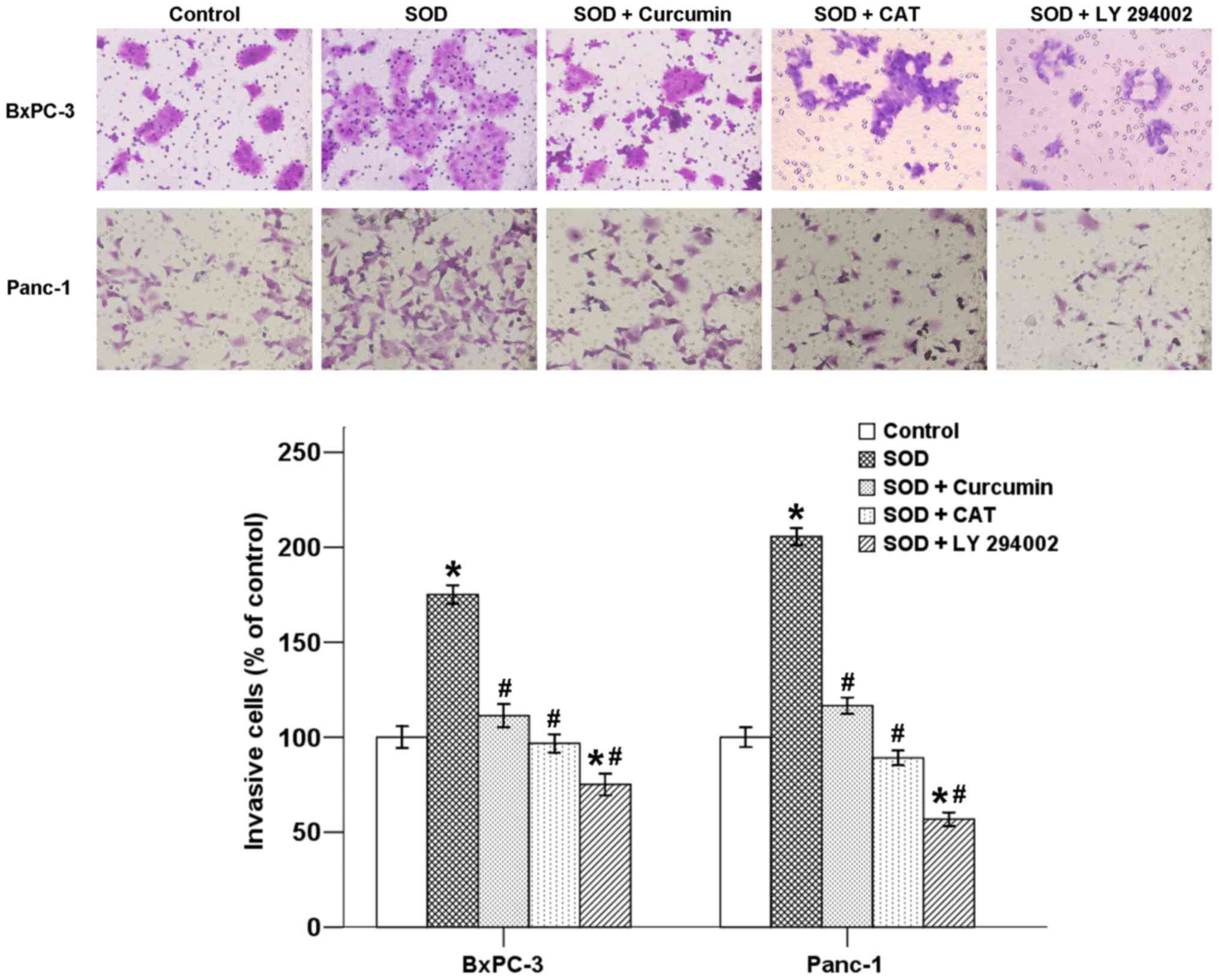
Curcumin inhibits SOD-induced invasion of
pancreatic cancer cells
Malignant tumors are often characterized by
metastasis, which is dissemination of cells from a primary site to
colonize distant organs. The ability of cancer cells to migrate and
invade other tissues are two important steps in the development of
metastasis. To verify whether curcumin is able to affect
SOD-induced cell invasion in pancreatic cancer cells, a Transwell
invasion assay was performed. As shown in Fig. 4, the mean number of cells invading
into the lower chamber increased in the presence of SOD after a
48-h incubation, and this increase was inhibited by co-treatment
with curcumin. In addition, CAT and LY 294002 also suppressed the
effect of SOD, indicating that SOD-induced invasion is associated
with the H2O2/Akt axis (Fig. 4).
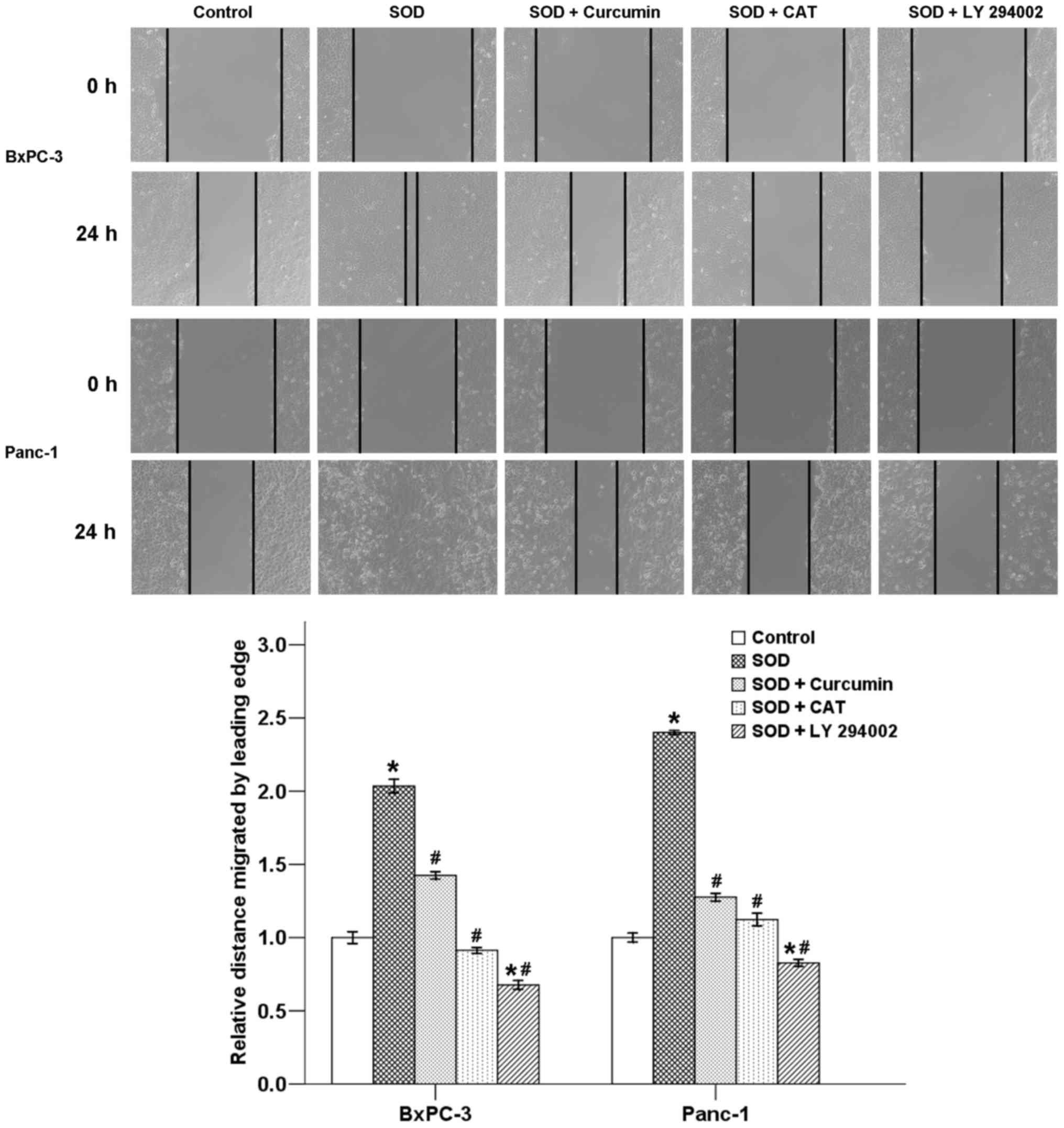
Curcumin suppresses SOD-induced migration
of pancreatic cancer cells
A classic wound healing assay was next conducted to
assess the effect of curcumin on SOD-induced cell motility in
pancreatic cancer cells. Our results revealed that the migration of
cancer cells was significantly promoted by SOD after a 24-h
incubation. Delayed wound closure was observed following treatment
with curcumin in the two cancer cell types. The increase in cell
migration was also inhibited by co-treatment with CAT and LY
294002. Therefore, curcumin likely exerts its inhibitory effects on
cancer cell motility through suppression of the
H2O2/Akt/NF-κB axis (Fig. 5).
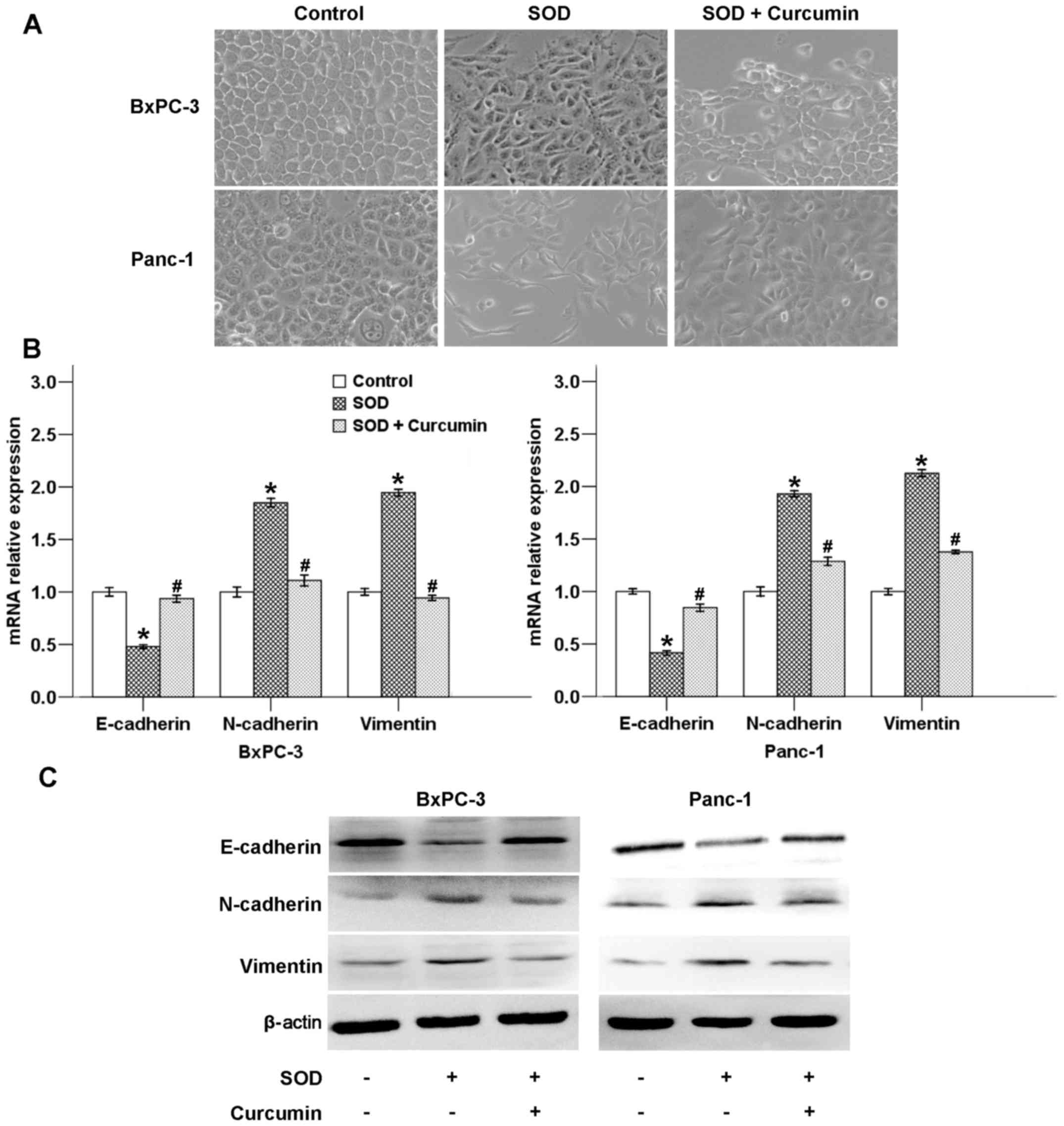
Curcumin inhibits SOD-promoted EMT in
pancreatic cancer cells
EMT is considered a prerequisite for cells to adopt
a motile and invasive phenotype and eventually become metastatic.
EMT includes four important steps: Loss of epithelial cell
adhesion, expression of mesenchymal proteins and acquisition of a
mesenchymal-like phenotype, degradation of the basement membrane,
and enhanced cell migration and invasion, which facilitate tumor
cell invasion into the stroma and entrance into the circulation
(10). Our previous study
demonstrated that SOD-induced H2O2 production
is able to promote EMT in pancreatic cancer cells, resulting in
increased cell motility and invasion via activation of the ERK
signaling pathway (12). In the
present study, we demonstrated that cancer cell morphology changed
from a typical epithelial phenotype to a mesenchymal phenotype
following SOD treatment for 48 h, which was counterbalanced by
curcumin (Fig. 6A).
A hallmark of EMT includes a marked decline in
E-cadherin expression (a cell-cell adhesion molecule) and increase
in vimentin and N-cadherin expression (mesenchymal markers)
(11). To further verify the
effect of curcumin on SOD-induced EMT, the mRNA and protein
expression levels of several EMT-related factors (E-cadherin,
N-cadherin and vimentin) were assessed after the cells were treated
with SOD in the presence or absence of curcumin. As shown in
Fig. 6B, SOD decreased the mRNA
level of E-cadherin and increased the mRNA level of N-cadherin and
vimentin, whereas curcumin significantly reversed these effects. As
shown in Fig. 6C, SOD exposure
could modulate the protein levels of EMT-related factors, while
curcumin suppressed these effects of SOD.
To summarize the abovementioned results, SOD was
able to induce EMT progression and facilitate tumor invasion and
migration via the production of H2O2 in
BxPC-3 and Panc-1 pancreatic cancer cells, whereas these effects
were counterbalanced by curcumin treatment in both types of
cells.
Discussion
An increasing volume of evidence suggests that EMT
plays a key role in cancer progression, cancer stem cell
intravasation, establishment of metastasis and treatment
resistance, resulting in a marked increase in disease
aggressiveness and poorer disease outcome and overall patient
survival (19). Pancreatic cancer,
an aggressive and lethal malignant disease, is predicted to become
the leading cause of cancer-related mortality in the USA by 2050
(20). The poor prognosis of
pancreatic cancer patients is mainly attributed to the metastatic
predilection of cancer cells. Although some tumors appear to be
resectable, in reality surgery is not curative, due to the
microscopic systemic spread that occurs prior to surgical resection
(21). Our previous studies
demonstrated that several factors may induce EMT in pancreatic
cancer cells, thereby promoting tumor progression, including a
hypoxic environment (8), a
hyperglycemic environment (11),
as well as the induction SOD (12). The aim of the present study was to
determine whether curcumin has the potential to inhibit SOD-induced
cell invasion and migration and EMT in the BxPC-3 and Panc-1 cell
lines, and elucidate the possible related mechanism.
Our findings demonstrated that curcumin
significantly decreased SOD-induced production of ROS and
H2O2 in pancreatic cancer cells. Curcumin,
CAT (a scavenger of H2O2) and LY 294002 (a
PI3K inhibitor) were able to suppress SOD-induced migration and
invasion of BxPC-3 and Panc-1 cells. SOD-modulated cancer cell
morphology and the expression of E-cadherin, N-cadherin and
vimentin were markedly affected by curcumin. In addition,
SOD-induced activation of the PI3K/Akt signaling pathway and the
transcription factor NF-κB were also suppressed by curcumin. It is
known that H2O2 induces ERK and Akt
activation through a number of mechanisms. For example, Nrf2 is a
transcription factor that plays a key role in controlling the
response to oxidative stress by regulating antioxidant enzymes. The
activation of Nrf2 may also be mediated by additional signal
transduction pathways, such as ERK, AMPK or PI3K/Akt, exerting
antioxidant effects, which mediate enhanced resistance to oxidative
stress (22). The
thioredoxin/thioredoxin reductase/TXNIP system is also involved in
oxidative stress and the ERK pathway, as it was previously proven
that hyperglycemia-induced TXNIP expression is involved in
diabetes-mediated oxidative stress in pancreatic cancer via the
p38, MAPK and ERK pathways (23).
Previous studies demonstrated that H2O2
contributes to both copper-zinc SOD (CuZnSOD)- and manganese SOD
(MnSOD)-induced cancer progression through activation of Akt and
ERK (12,24,25),
while curcumin suppresses H2O2-induced
migration and invasion of pancreatic cancer cells (26). Our results revealed that curcumin
suppresses SOD-induced EMT in pancreatic cancer, which may be
associated with the H2O2/Akt/NF-κB axis.
ROS and the activation of redox-sensitive signaling
pathways are important participants in the development of neoplasms
(27). SOD is a type of primary
cellular antioxidant catalyzing the conversion of superoxide to
H2O2, which has been proven to favor tumor
progression (12). There are three
members of the SOD family present in mammals, namely CuZnSOD, MnSOD
and extracellular SOD (EC-SOD), which are located in different
places inside or outside the cell (28). Epidemiological evidence has
indicated that increased levels of SOD were observed in several
tumor types along with tumor progression from early-stage
(non-invasive) to late-stage (metastatic) (29). SOD polymorphism is associated with
increased risk of prostate cancer, esophageal cancer, non-Hodgkin
lymphoma, lung cancer and colorectal cancer (30,31).
The MnSOD-1221G>A AA genotype carriers exhibited a significantly
increased risk of pancreatic cancer among those with a low dietary
vitamin E intake (32). In
addition, both in vitro and in vivo studies
demonstrated that cancer cells containing elevated levels of SOD
exhibit a propensity for metastasis, proliferation and resistance
to apoptosis. Hart et al (33) revealed that SOD-induced
H2O2 sustained the Warburg effect through
AMPK-dependent signaling, enabling cancer cell survival. Chronic
inflammation is a major activator of the metastatic cascade.
Tumor-associated inflammation also participates in the regulation
of EMT, which contributes to cancer invasion and metastasis. Yi
et al (34) recently
reported that SOD may favor inflammation-mediated EMT and migration
of tumor cells in AFG1-induced lung adenocarcinoma. We have
previously reported that SOD-dependent production of ROS promoted
the invasion of pancreatic cancer cells (12). The present study demonstrated that
curcumin effectively inhibited SOD-induced EMT in the BxPC-3 and
Panc-1 pancreatic cancer cell lines.
Curcumin is a bioactive natural compound, which has
been proven to restrain initiation, progression and metastasis of
multifarious tumors, including pancreatic cancer (8). More importantly, curcumin is
associated with minimal toxicity and it is safe at a high dose, as
demonstrated by human clinical trials, in contrast with
conventional cytotoxic drugs (35). Curcumin exerts its anticancer
effects via multiple signaling pathways, such as Notch, mammalian
target of rapamycin (mTOR), MAPK and NF-κB (5,36–39).
Zhou et al (36) proved
that curcumin suppressed pancreatic cancer cell growth, induced
apoptosis and cell cycle arrest, weakened clonogenic potential, and
inhibited migration and invasion via suppression of YAP/TAZ and
Notch signaling. Treatment with curcumin also effectively
attenuated tobacco smoke-induced activation of ERK and JNK MAPK
pathways, AP-1 proteins and EMT alterations in mouse liver
(5). Curcumin was also found to be
able to induce autophagy and activate lysosomal function via its
inhibitory effects on the Akt-mTOR signaling pathway and via direct
targeting and activation of TFEB (37). In addition, curcumin exerted its
anticancer effects both alone and in combination with other
anticancer drugs. It has been proven that curcumin promotes the
anticancer effects of gemcitabine via suppression of cancer cell
proliferation, angiogenesis and inhibition of the NF-κB pathway in
a pancreatic cancer model (38). A
recent study indicated that co-treatment with metformin and
curcumin not only induced apoptosis of hepatocellular carcinoma
cells through activating the mitochondrial pathways, but also
suppressed the invasion and metastasis of cancer cells and
angiogenesis of human umbilical vein endothelial cells via
suppression of PI3K/Akt/mTOR/NF-κB and EGFR/STAT3 signaling
(39). Our recent study
demonstrated that curcumin was able to suppress hypoxia-induced
pancreatic cancer EMT and metastasis by inhibiting the hedgehog
signaling pathway (8). It was also
proven that curcumin was able to suppress cell migration and
invasion through suppression of the ROS/ERK/NF-κB signaling pathway
(26). It was previously
demonstrated that curcumin inhibited pancreatic cancer cell
migration and invasion and suppressed NEDD4 expression (40). In our previous study, we also
demonstrated that LY294002 exposure for 24 h reduced the migration
of pancreatic cancer cells without the addition of SOD (41). The present study revealed that
curcumin inhibited the effects of SOD in pancreatic cancer through
suppression of the PI3K/Akt/NF-κB signaling pathway in pancreatic
cancer cells.
The PI3K/Akt signaling pathway is one of the most
frequently changing signaling networks in human cancer, and has
long been identified as being implicated in cancer metastasis. Akt
is hyperactivated in cancer cells through multiple mechanisms,
including the loss of phosphatase and tensin homolog, mutations
that activate the catalytic subunit of PI3K, mutations that
activate Akt isoforms, and amplification of the genes encoding the
catalytic subunit of PI3K and Akt (42). Increased activation of PI3K/Akt
signaling has been found in ~50% of pancreatic cancers, which is
usually associated with a low grade of tumor differentiation and is
correlated with a poor prognosis. PI3K/Akt signaling modulates a
series of cellular functions, including cell transformation,
proliferation, growth, motility and survival (43). Our previous study demonstrated that
resveratrol was highly efficient in inhibiting the proliferation,
migration and invasion of pancreatic cancer cells in vitro
by regulating EMT-related factors via the PI3K/Akt/NF-κB signaling
pathway (41). Recent studies
demonstrated that both CuZnSOD and MnSOD can activate PI3K/Akt
signaling in cancer cells (24,25).
The results of the present study revealed that curcumin has great
therapeutic potential, as it can restrain SOD-induced activation of
p-Akt and p-NF-κB. Following suppression of the PI3K/Akt signaling
pathway by LY 249002, the expression of p-Akt and NF-κB decreased,
and the invasive and migratory abilities of pancreatic cancer cells
were weakened.
In conclusion, the present study demonstrated that
curcumin inhibited SOD-induced invasion and migration of pancreatic
cancer cells by regulating EMT-related factors via the
PI3K/Akt/NF-κB signaling pathway. Therefore, inhibition of the
H2O2/Akt/NF-κB axis by curcumin may represent
a promising option for the treatment of patients with pancreatic
cancer.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by the Natural Science
Basic Research Project of Shaanxi Province (grant no.
2017JM8037).
[2] Availability
of data and materials
The analysed data sets generated during the study
are available from the corresponding authors on reasonable
request.
[3] Author
contributions
QM and LC conceived the study; WL, ZWu and LC
designed the study; WL, ZJ and XX conducted the experiments; and
ZWa and LC performed the data analysis. All authors have read and
approved this manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shanmugam MK, Rane G, Kanchi MM, Arfuso F,
Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP and Sethi G:
The multifaceted role of curcumin in cancer prevention and
treatment. Molecules. 20:2728–2769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang Z, Wu R, Xie W, Xie C, Wu J, Geng S,
Li X, Zhu M, Zhu W, Zhu J, et al: Effects of curcumin on tobacco
smoke-induced hepatic MAPK pathway activation and
epithelial-mesenchymal transition in vivo. Phytother Res.
31:1230–1239. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu H, Wang C, Yang D, Wei Z, Xu J, Hu Z,
Zhang Y, Wang W, Yan R and Cai Q: Curcumin regulates proliferation,
autophagy, and apoptosis in gastric cancer cells by affecting PI3K
and P53 signaling. J Cell Physiol. Sep 19–2017.Epub ahead of print.
View Article : Google Scholar
|
|
7
|
Liang Z, Lu L, Mao J, Li X, Qian H and Xu
W: Curcumin reversed chronic tobacco smoke exposure induced
urocystic EMT and acquisition of cancer stem cells properties via
Wnt/β-catenin. Cell Death Dis. 8:e30662017. View Article : Google Scholar
|
|
8
|
Cao L, Xiao X, Lei J, Duan W, Ma Q and Li
W: Curcumin inhibits hypoxia-induced epithelial mesenchymal
transition in pancreatic cancer cells via suppression of the
hedgehog signaling pathway. Oncol Rep. 35:3728–3734. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatia S, Monkman J, Toh AKL, Nagaraj SH
and Thompson EW: Targeting epithelial-mesenchymal plasticity in
cancer: Clinical and preclinical advances in therapy and
monitoring. Biochem J. 474:3269–3306. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Ma Q, Liu J, Han L, Ma G, Liu H,
Shan T, Xie K and Wu E: Hyperglycemia as a mechanism of pancreatic
cancer metastasis. Front Biosci (Landmark Ed). 17:1761–1774. 2012.
View Article : Google Scholar
|
|
11
|
Li W, Zhang L, Chen X, Jiang Z, Zong L and
Ma Q: Hyperglycemia promotes the epithelial-mesenchymal transition
of pancreatic cancer via hydrogen peroxide. Oxid Med Cell Longev.
2016:51903142016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Cao L, Han L, Xu Q and Ma Q:
Superoxide dismutase promotes the epithelial-mesenchymal transition
of pancreatic cancer cells via activation of the
H2O2/ERK/NF-κB axis. Int J Oncol.
46:2613–2620. 2015. View Article : Google Scholar
|
|
13
|
Costa A, Scholer-Dahirel A and
Mechta-Grigoriou F: The role of reactive oxygen species and
metabolism on cancer cells and their microenvironment. Semin Cancer
Biol. 25:23–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo L, Tan K, Wang H and Zhang X:
Pterostilbene inhibits hepatocellular carcinoma through
p53/SOD2/ROS-mediated mitochondrial apoptosis. Oncol Rep.
36:3233–3240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Martinez-Useros J, Li W, Cabeza-Morales M
and Garcia-Foncillas J: Oxidative stress: A new target for
pancreatic cancer prognosis and treatment. J Clin Med. 6:E292017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mayer IA and Arteaga CL: The PI3K/AKT
pathway as a target for cancer treatment. Annu Rev Med. 67:11–28.
2016. View Article : Google Scholar
|
|
18
|
Yu X, Wu Q, Wang L, Zhao Y, Zhang Q, Meng
Q, Pawan and Wang S: Silencing of ST6GalNAc I suppresses the
proliferation, migration and invasion of hepatocarcinoma cells
through PI3K/AKT/NF-κB pathway. Tumour Biol. 37:12213–12221. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaianigo N, Melisi D and Carbone C: EMT
and treatment resistance in pancreatic cancer. Cancers (Basel).
9:92017. View Article : Google Scholar
|
|
20
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castellanos EH, Cardin DB and Berlin JD:
Treatment of early-stage pancreatic cancer. Oncology (Williston
Park). 25:182–189. 2011.
|
|
22
|
Claudia L, Jette R, Rudolf L, Ludger AW
and Barbara Sr: Hydrogen peroxide - production, fate and role in
redox signaling of tumor cells. Cell Commun Signal. 13:392015.
View Article : Google Scholar
|
|
23
|
Li W, Wu Z, Ma Q, Liu J, Xu Q, Han L, Duan
W, Lv Y, Wang F, Reindl KM, et al: Hyperglycemia regulates
TXNIP/TRX/ROS axis via p38 MAPK and ERK pathways in pancreatic
cancer. Curr Cancer Drug Targets. 14:348–356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li F, Wang H, Huang C, Lin J, Zhu G, Hu R
and Feng H: Hydrogen peroxide contributes to the manganese
superoxide dismutase promotion of migration and invasion in glioma
cells. Free Radic Res. 45:1154–1161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Damiano S, Petrozziello T, Ucci V, Amente
S, Santillo M and Mondola P: Cu-Zn superoxide dismutase activates
muscarinic acetylcholine M1 receptor pathway in neuroblastoma
cells. Mol Cell Neurosci. 52:31–37. 2013. View Article : Google Scholar
|
|
26
|
Cao L, Liu J, Zhang L, Xiao X and Li W:
Curcumin inhibits H2O2-induced invasion and
migration of human pancreatic cancer via suppression of the
ERK/NF-κB pathway. Oncol Rep. 36:2245–2251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schieber M and Chandel NS: ROS function in
redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Griess B, Tom E, Domann F and
Teoh-Fitzgerald M: Extracellular superoxide dismutase and its role
in cancer. Free Radic Biol Med. 112:464–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hempel N, Carrico PM and Melendez JA:
Manganese superoxide dismutase (Sod2) and redox-control of
signaling events that drive metastasis. Anticancer Agents Med Chem.
11:191–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun GG, Wang YD, Lu YF and Hu WN:
Different association of manganese superoxide dismutase gene
polymorphisms with risk of prostate, esophageal, and lung cancers:
Evidence from a meta-analysis of 20,025 subjects. Asian Pac J
Cancer Prev. 14:1937–1943. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang SW: Superoxide dismutase 2 gene and
cancer risk: Evidence from an updated meta-analysis. Int J Clin Exp
Med. 8:14647–14655. 2015.PubMed/NCBI
|
|
32
|
Tang H, Dong X, Day RS, Hassan MM and Li
D: Antioxidant genes, diabetes and dietary antioxidants in
association with risk of pancreatic cancer. Carcinogenesis.
31:607–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hart PC, Mao M, de Abreu AL,
Ansenberger-Fricano K, Ekoue DN, Ganini D, Kajdacsy-Balla A,
Diamond AM, Minshall RD, Consolaro ME, et al: MnSOD upregulation
sustains the Warburg effect via mitochondrial ROS and
AMPK-dependent signalling in cancer. Nat Commun. 6:60532015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yi L, Shen H, Zhao M, Shao P, Liu C, Cui
J, Wang J, Wang C, Guo N, Kang L, et al: Inflammation-mediated
SOD-2 upregulation contributes to epithelial-mesenchymal transition
and migration of tumor cells in aflatoxin G1-induced lung
adenocarcinoma. Sci Rep. 7:79532017. View Article : Google Scholar :
|
|
35
|
Yang C, Su X, Liu A, Zhang L, Yu A, Xi Y
and Zhai G: Advances in clinical study of curcumin. Curr Pharm Des.
19:1966–1973. 2013.
|
|
36
|
Zhou X, Su J, Feng S, Wang L, Yin X, Yan J
and Wang Z: Antitumor activity of curcumin is involved in
down-regulation of YAP/TAZ expression in pancreatic cancer cells.
Oncotarget. 7:79076–79088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Wang J, Xu J, Lu Y, Jiang J, Wang
L, Shen HM and Xia D: Curcumin targets the TFEB-lysosome pathway
for induction of autophagy. Oncotarget. 7:75659–75671.
2016.PubMed/NCBI
|
|
38
|
Kunnumakkara AB, Guha S, Krishnan S,
Diagaradjane P, Gelovani J and Aggarwal BB: Curcumin potentiates
antitumor activity of gemcitabine in an orthotopic model of
pancreatic cancer through suppression of proliferation,
angiogenesis, and inhibition of nuclear factor-kappaB-regulated
gene products. Cancer Res. 67:3853–3861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao
ZQ, Yu LG and Guo XL: Metformin incombination with curcumin
inhibits the growth, metastasis, and angiogenesis of hepatocellular
carcinoma in vitro and in vivo. Mol Carcinog. 57:44–56. 2018.
View Article : Google Scholar
|
|
40
|
Su J, Zhou X, Yin X, Wang L, Zhao Z, Hou
Y, Zheng N, Xia J and Wang Z: The effects of curcumin on
proliferation, apoptosis, invasion, and NEDD4 expression in
pancreatic cancer. Biochem Pharmacol. 140:28–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F, et al: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar
|
|
42
|
Wang Q, Chen X and Hay N: Akt as a target
for cancer therapy: More is not always better (lessons from studies
in mice). Br J Cancer. 117:159–163. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Baer R, Cintas C, Therville N and
Guillermet-Guibert J: Implication of PI3K/Akt pathway in pancreatic
cancer: When PI3K isoforms matter? Adv Biol Regul. 59:19–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|