Introduction
Breast cancer is one of the most common female
malignancies, as well as one of the leading causes of
cancer-associated morbidity and mortality in women (1,2). The
standard and classical treatments for breast cancer, including
surgical resection, radiotherapy and chemotherapy, are far from
satisfactory due to limited efficacy and high rates of recurrence
(3). Thus, the development of new
therapeutic strategies is urgently needed for the treatment of
breast cancer. Arsenic compounds, such as arsenic trioxide (ATO),
which has been used in standard treatment strategies for acute
promyelocytic leukemia (APL) (4,5), and
sodium arsenite, have been reported to show cytotoxic effects
against breast cancer cell lines (6–9).
However, such arsenic compounds are commonly regarded as toxic
compounds with adverse effects, including visceral organ damage,
serious acute toxicity, and potential carcinogenicity, which
consequently limit their clinical applications (10–12).
Arsenic disulfide (As2S2), as
the predominant active ingredient of realgar, also known as
‘Xiong-Huang’ in traditional Chinese medicine, is considered to be
a candidate for the treatment of several types of malignancies; it
exhibits relatively low toxicity, and has the advantage of being
orally administered (10,13–15).
A series of basic and clinical studies have reported the antitumor
effects of As2S2 in different types of
malignancies, particularly hematopoietic tumors such as APL
(13,16–19).
Recently, there is increasing evidence to support that
As2S2 has the ability to induce apoptosis and
inhibit the growth of hematopoietic and solid tumor cell lines,
such as human lymphoma cell lines (20), cervical cancer cell lines (21), human hepatocellular carcinoma cells
(22), and human osteosarcoma cell
lines (23). However, although the
cytocidal effect of realgar nanoparticles has been demonstrated
(24), the effects of
As2S2 on breast cancer cells, and the
underlying mechanisms, have yet to be fully investigated.
In recent decades, studies on the development of
three-dimensional (3D) cell culture systems to generate
multi-cellular tumor spheroids have attracted much attention. The
findings of such studies suggest that the natural manner in which
solid tumor cells grow in vivo is in 3D, indicating that
growing cancer cells in 3D culture mimics more closely the in
vivo environment compared with traditional two dimensional (2D)
cell culture systems (25–28). In this sense, 3D-cultured breast
cancer cells are able to provide more accurate evidence of the
drug’s activity in development of novel therapeutics (25). The actual tumor microenvironment in
the human body consists of cancer cells, fibroblasts, endothelial
cells and extracellular matrix (26). In comparison with 2D cell-culture
systems, the specific characteristics of 3D-culture systems include
tight cell-cell interactions, cell-microenvironment interactions,
and special internal and external cellular structures (27,28).
The aim of the present study was to investigate the
effects of As2S2 on MCF-7 cells (a human
breast cancer cell line), and to further disclose the possible
molecular mechanisms underlying the action of the drug. In the
present study, 2D- and 3D-cell culture systems were introduced in
order to observe and compare the cell formation, cellular
architecture, and drug responses between the two culture systems,
and to evaluate the effects of As2S2 on MCF-7
cells cultured in the two systems.
Materials and methods
Reagents
The cell counting kit-8 (CCK8) was obtained from
Dojindo Molecular Technologies, Inc. (Tokyo, Japan). The
fluorescein isothiocyanaste (FITC) Annexin V Apoptosis Detection
kit was from BD Biosciences (San Diego, CA, USA). The ECL™ Western
Blotting Analysis system and ECL™ Prime Western Blotting Detection
reagent were purchased from GE Healthcare (Buckinghamshire, UK).
As2S2 and mouse anti-human Bcl-2-associated X
protein (Bax) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The Pro-Survival Bcl-2 Family Antibody Sampler
kit, rabbit anti-human phosphoinositol 3-kinase (PI3K), rabbit
anti-human Akt, and rabbit anti-human mammalian target of rapamycin
(mTOR) were obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Mouse anti-human caspase-7 was purchased from BD
Pharmingen (BD Biosciences, San Jose, CA, USA). Mouse anti-human
p53 was obtained from BioLegend, Inc. (San Diego, CA, USA).
Cell lines and cell culture
The human breast cancer cell line, MCF-7, and the
human normal breast cell line, 184B5, were purchased from the
American Type Culture Collection (Manassas, VA, USA). MCF-7 cells
were cultured in Alpha-MEM medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with penicillin, streptomycin
and 10% fetal bovine serum (Merck KGaA, Darmstadt, Germany). 184B5
cells were cultured in DMEM/F12 medium supplemented with 5% FBS, 1x
Insulin, Transferrin and Selenium (ITS; Lonza Group Ltd., Anaheim,
CA, USA), 100 nM hydrocortisone (Merck KGaA), 2 mM sodium pyruvate
(Lonza Group, Ltd., Chiba, Japan), 20 ng/ml epidermal growth
factor, 0.3 nM trans-retinoic acid (both from Merck KGaA),
100 U/ml penicillin and 100 μg/ml streptomycin. Cells were
maintained as attached cells at 37°C in 5% carbon dioxide in a
humidified atmosphere.
2D cell-culture assay
MCF-7 cells were seeded at a density of 10,000
cells/well in 500 μl cell culture media into 48-well plates
(Iwaki Co., Ltd., Tokyo, Japan), and then incubated for 24 h.
Vehicle for controls (cell culture media) and different
concentrations of As2S2 were subsequently
added into the corresponding wells to adjust the final drug
concentrations of As2S2 to 0, 4, 8 and 16
μM. MCF-7 cells were allowed to grow for 72 h in the
presence of the drug. This was followed by a cytotoxicity assay and
other downstream applications, including apoptosis detection and
western blotting.
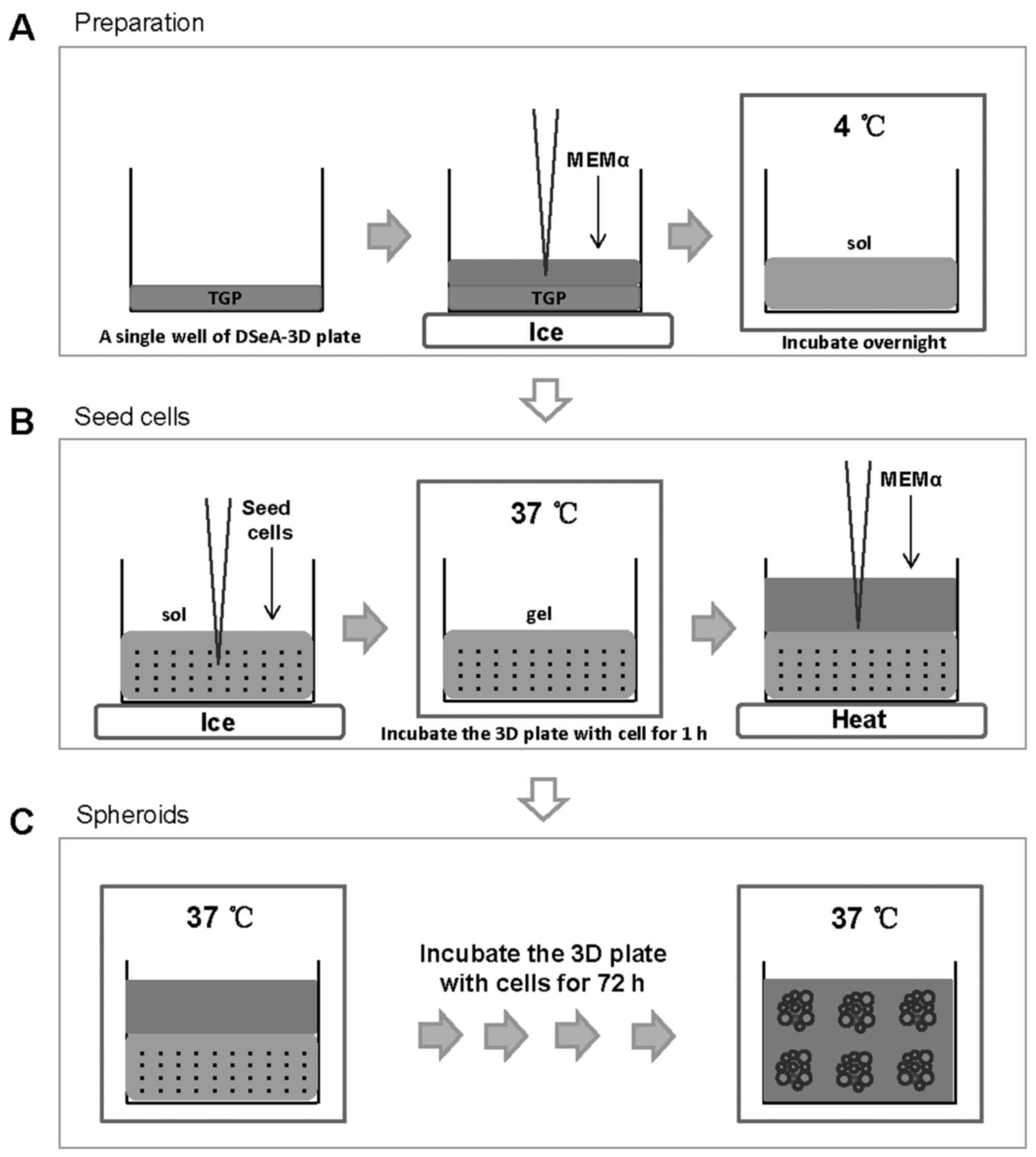
3D cell-culture assay
3D spheroids were formed using DSeA 3D micro-plates
(International Frontier Technology Laboratory, Inc., Tokyo, Japan)
with thermo-reversible gelatin polymer (TGP), as described
previously (29). Briefly, TGP
powder in each well was dissolved in Alpha-MEM medium (Thermo
Fisher Scientific, Inc.) and incubated at 4°C overnight to develop
the aqueous solution (the sol phase). MCF-7 cells were subsequently
seeded into cold Alpha-MEM medium with TGP on ice at a density of
10,000 cells/well, followed by incubation at 37°C to develop the
gel form. Vehicle and the drug were applied in the identical manner
as described above for the 2D cell culture. MCF-7 cells were
incubated in the gel at 37°C for 72 h to develop spheroids
(Fig. 1).
Microscopy
The cellular morphological structures of MCF-7 cells
in both 2D- and 3D-cultures were observed on the fourth day
following seeding using an IX70® inverted microscope
(Olympus Corporation, Tokyo, Japan). A series of bright-field
images were recorded and examined with 10×, 20× and 40× objectives
(original magnifications: x100, x200 and x400, respectively).
Cytotoxicity assay
Cell cytotoxicity was analyzed using a CCK-8 assay
(Dojindo Molecular Technologies, Inc.). A total of 1×104
cells/well MCF-7 cells were seeded into 48-well plates (the cell
density was 1×104 cells/500 μl), and
As2S2 was added at final concentrations of 0,
4, 8 and 16 μM. The plate was subsequently incubated at 37°C
in a humidified atmosphere of 95% air and 5% CO2 for 72
h. Following incubation, 25 μl CCK-8 reagent was added into
each well, followed by an additional incubation for 3 h at 37°C in
a humidified atmosphere. The optical density (OD) value of each
well was measured using a Corona MT P-32 micro-plate reader (Corona
Electric Co., Ltd., Ibaraki, Japan) at 570 nm. The cell viability
rate was calculated according to the following equation: Cell
viability rate = (OD sample value − OD blank value)/(OD control
value − OD blank value) ×100%.
Assessment of apoptosis
Apoptotic rates of the MCF-7 cells were analyzed
using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences).
MCF-7 cells (2×105 cells/well) were seeded in 24-well
plates (cell density, 2×105 cells/ml; volume, 1 ml cell
suspension/well), and treated with serial concentrations of
As2S2 (final concentrations: 0, 4, 8 and 16
μM); this was followed by incubation for an additional 72 h.
Subsequently, the staining procedure was performed according to the
manufacturer’s protocol. A total of 1×104 cells was
analyzed using a flow cytom-eter (BD Biosciences). The cells were
subsequently assessed for viable (Annexin
V−/PI−), early apoptotic (Annexin
V+/PI−), late apoptotic (Annexin
V+/PI+) and necrotic (Annexin
V−/PI+) cells.
Western blotting
A western blotting protocol was utilized in order to
evaluate the protein expression levels of Bcl-2, Bax, p53,
caspase-7, PI3K, Akt and mTOR in both monolayers and spheroids.
Total protein was extracted from the MCF-7 cells treated with
various final concentrations of As2S2 (0, 4,
8 and 16 μM) in both 2D and 3D cell-culture systems.
Briefly, cell lysates were separated by SDS-PAGE and transferred
into a polyvinylidene difluoride (PVDF) transfer membrane
(Immobilon-P; Merck Millipore, Darmstadt, Germany). Membranes were
blocked with 5% skimmed milk for 1 h. The membranes were washed
with Tris-buffered saline/0.1% Tween-20 (TBST), and then incubated
overnight at 4°C with 1:500 anti-mouse p53 specific antibody (cat.
no. 628201; BioLegend, Inc.), 1:500 anti-mouse Bax-specific
antibody (cat. no. B8429; Merck KGaA), 1:1,000 anti-mouse
caspase-7-specific antibody (cat. no. 551238; BD Biosciences),
1:1,000 anti-rabbit Bcl-2-specific antibody (cat. no. 9941),
1:1,000 anti-rabbit PI3K p85 subunit-specific antibody (cat. no.
4228), 1:1,000 anti-rabbit Akt-specific antibody (cat. no. 4691),
and 1:1,000 anti-rabbit mTOR-specific antibody (cat. no. 2983) (all
from Cell Signaling Technology, Inc.). Membranes were also probed
with mouse anti-human β-actin (cat. no. ab49900; Abcam, Tokyo,
Japan) at 1:1,000 dilution as the internal control. The membranes
were incubated overnight with the primary antibodies listed above
at 4°C. The following day, the blots were incubated with 1:1,000
anti-mouse (cat. no. 7076) or 1:1,000 anti-rabbit (cat. no. 7074)
(both from Cell Signaling Technology, Inc.) specific polyclonal
secondary antibodies for 1 h at room temperature. The membranes
were washed three times with TBST. Signals were detected with an
ECL Western Blot detection kit in a luminescent image analyzer
(Fujifilm; LAS-3000; Fujifilm, Tokyo, Japan). The images obtained
were subsequently quantitatively analyzed using the ImageJ software
program (Rasband, W.S., ImageJ, U.S. National Institutes of Health,
Bethesda, MD, USA; http://rsb.info.nih.gov/ij/).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. One-way analysis of variance (ANOVA) followed by Tukey’s
post-hoc test was performed for multiple comparisons, whereas
Student’s t-test was used for the comparison of two groups.
P<0.05 was considered to indicate a statistically significant
difference. Each experiment was repeated independently at least
three times.
Results
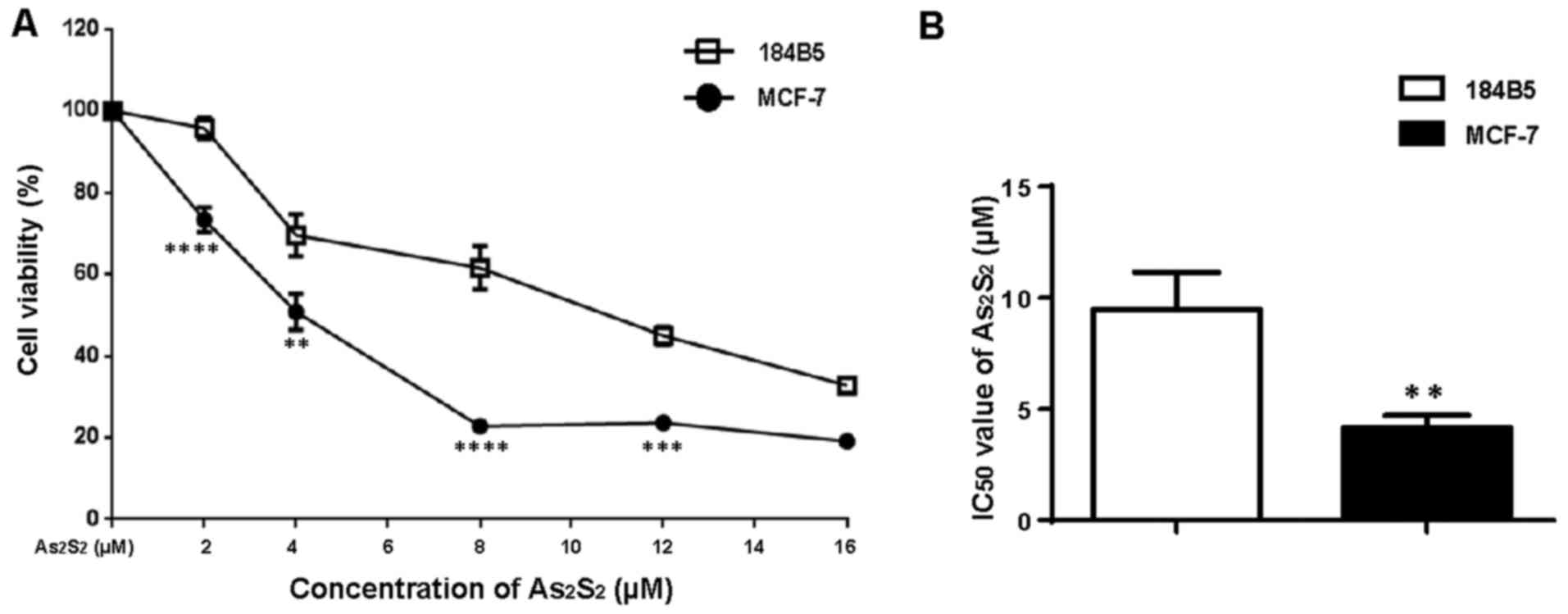
Human normal breast cells 184B5 are less
sensitive to As2S2 in comparison to MCF-7
cells
In order to determine the cytotoxic selectivity of
As2S2, a normal human breast epithelial cell
line (184B5) was used as a normal control (30,31),
and the cytotoxic effect of As2S2 on both the
breast cancer cell line (MCF-7) and 184B5 cells was examined using
a CCK-8 assay. As shown in Fig. 2,
dose-dependent inhibition was observed in the two cell lines
following exposure to different concentrations of
As2S2 (2-16 μM) for 72 h. In
comparison with 184B5 cells, however, the MCF-7 cells exhibited
significantly higher sensitivity to As2S2,
with increased inhibitory ratios in cell viability (Fig. 2A) and decreased IC50
values of As2S2 (Fig. 2B). These results suggested that
As2S2 is less cytotoxic to normal breast
epithelial cells than it is to breast cancer cells.
Microscopic imaging of 2D cultured and 3D
cultured MCF-7 cells
Following treatment with various concentrations (0,
4, 8 and 16 μM) of As2S2 for 72 h, the
bright-field images of MCF-7 cells in both 2D and 3D cultures were
recorded using an inverted microscope (Olympus Corporation) with
original magnifications of ×100, ×200 and ×400 (objectives: 10×,
20× and 40×, respectively). As shown in Fig. 3, MCF-7 cells attached simply to the
bottom of the wells in a monolayer manner in the 2D culture plates,
whereas they formed multicellular spheroids (MCSs) in the 3D
culture plates. The morphological changes of MCF-7 cells cultured
as 2D monolayers and 3D spheroids following exposure to 0, 4, 8 and
16 μM As2S2 were examined. Exposure to
a relatively high concentration (i.e., 8 or 16 μM) of
As2S2 caused substantial morphological
defects in both the 2D and 3D culture systems. Specifically,
following treatment with As2S2 for 72 h, the
MCF-7 monolayers in the 2D plates clearly decreased in a
dose-dependent manner, whereas the structural integrity of the
MCF-7 spheroids in the 3D plates deteriorated and fragmented, with
a destructive configuration (Fig.
3). In contrast, treatment with 4 μM
As2S2 (a relatively low concentration) led to
a modest decrease in 2D-cultured MCF-7 cells, whereas it failed to
cause any morphological changes in the 3D spheroids.
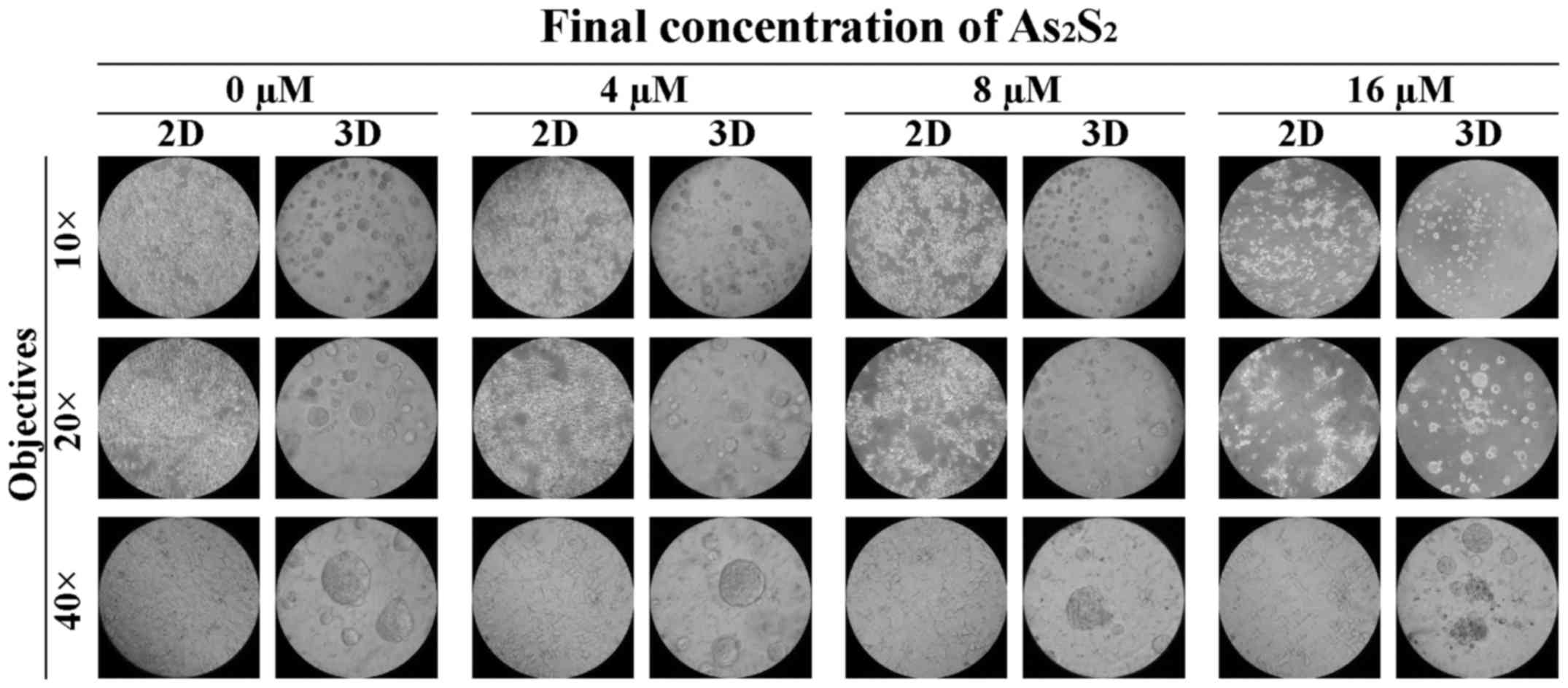 | Figure 3Different morphology of the MCF-7
cells in 2D and 3D cultures and the responses of the monolayers and
the spheroids following exposure to As2S2.
MCF-7 cells were seeded at a density of 10,000 cells/well in the 2D
and 3D cultured systems. Subsequently, the cells were treated with
a range of concentrations of As2S2 (0, 4, 8
and 16 μM) for 72 h. Bright-field images of MCF-7 monolayers
and spheroids were acquired using an IX70® inverted
microscope (Olympus Corporation, Tokyo, Japan), with ×10, ×20 and
×40 objectives (original magnifications, ×100, ×200 and ×400,
respectively). As2S2, arsenic disulfide. |
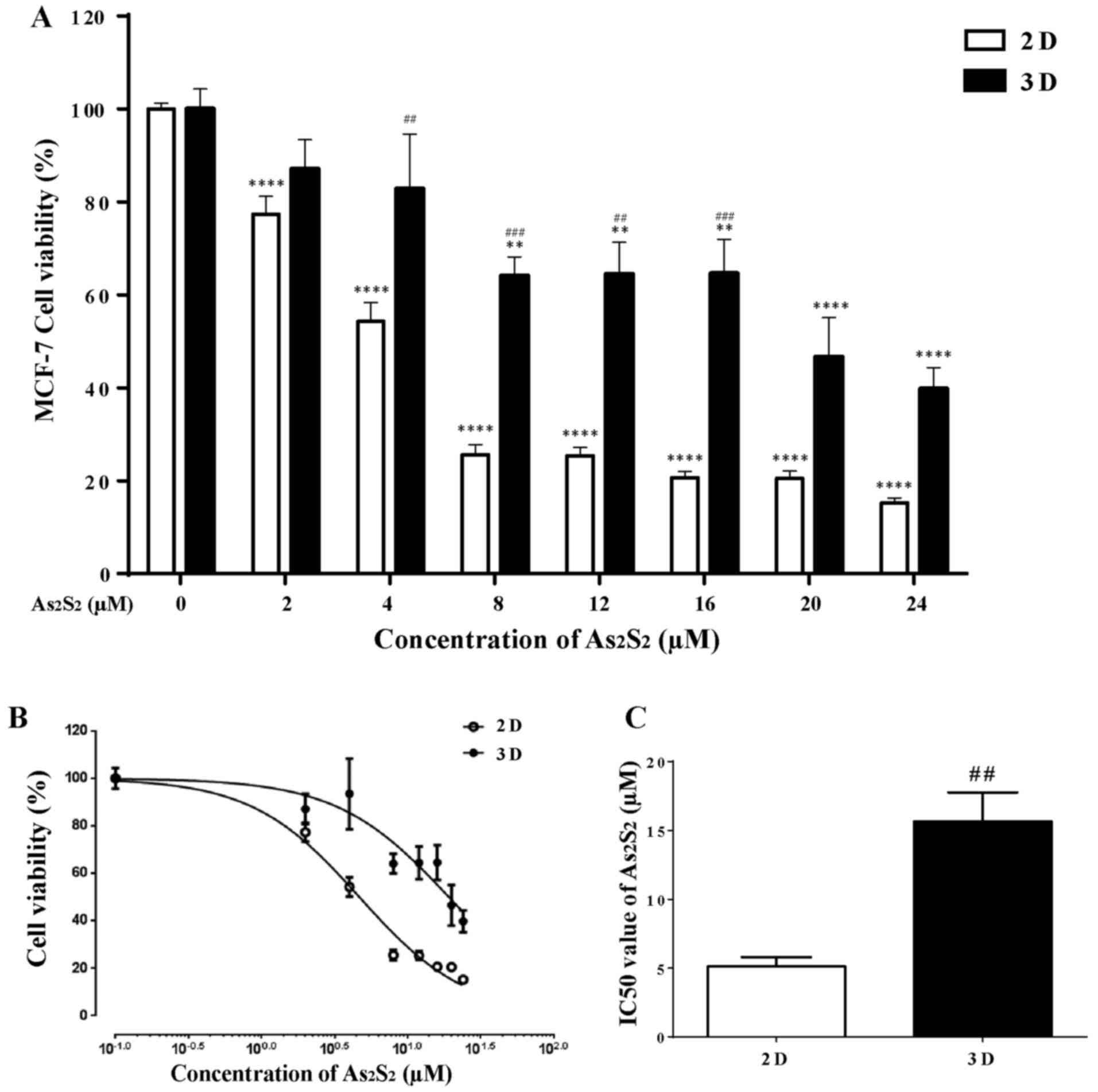
Cytotoxic effects of
As2S2 on 2D- and 3D-cultured MCF-7 cells
CCK-8 assays were performed to assess the viability
of cells (in both the 2D- and 3D-cell culture systems) exposed to
0–24 μM of As2S2 for 72 h. As shown in
Fig. 4A, a significant decrease in
cell viability was observed in 2D-cultured MCF-7 cells following
treatment with various concentrations of
As2S2; this occurred in a dose-dependent
manner. Specifically, in comparison with the control group (0
μM As2S2), cell viability was markedly
reduced to 77.32±3.92 and 15.24±1.04% after exposure to 2 and 24
μM As2S2, respectively. In contrast,
relatively higher concentrations (≥8 μM) of
As2S2 were observed to have a significant
dose-dependent inhibitory effect on 3D-cultured MCF-7 cells. There
were significant differences between the 2D- and 3D-cultured
systems in cell viability following exposure to
As2S2 with the final drug concentrations of
4, 8, 12 and 16 μM. Although similar dose-dependent growth
inhibition was observed in 3D-cultured MCF-7 cells, the growth
inhibition rates were much lower compared with those observed in
the 2D culture system. In particular, the growth inhibition rates
induced by a relatively high concentration (≥8 μM) of
As2S2 in the 2D-culure system were more than
two times higher compared with those observed in the 3D culture
system, indicating that the MCF-7 spheroids in the 3D culture
system were more resistant to the cytotoxicity of
As2S2 in comparison to the monolayers in the
2D culture system.
The growth inhibition curves were obtained using the
probit regression analysis method (Fig. 4B), and the half-maximal inhibitory
concentration (IC50 value) of
As2S2 in the 2D and 3D culture systems was
further calculated from their respective inhibition curves. As
shown in Fig. 4C, the mean
IC50 value of As2S2 in the 3D
culture system was approximately three times higher compared with
that in the 2D culture system (5.11±0.69 μM in the 2D
culture system; 15.65±2.12 μM in the 3D culture system;
P=0.0091). These results suggested that the monolayer cells were
more sensitive to As2S2 in comparison with
the spheroid cells.
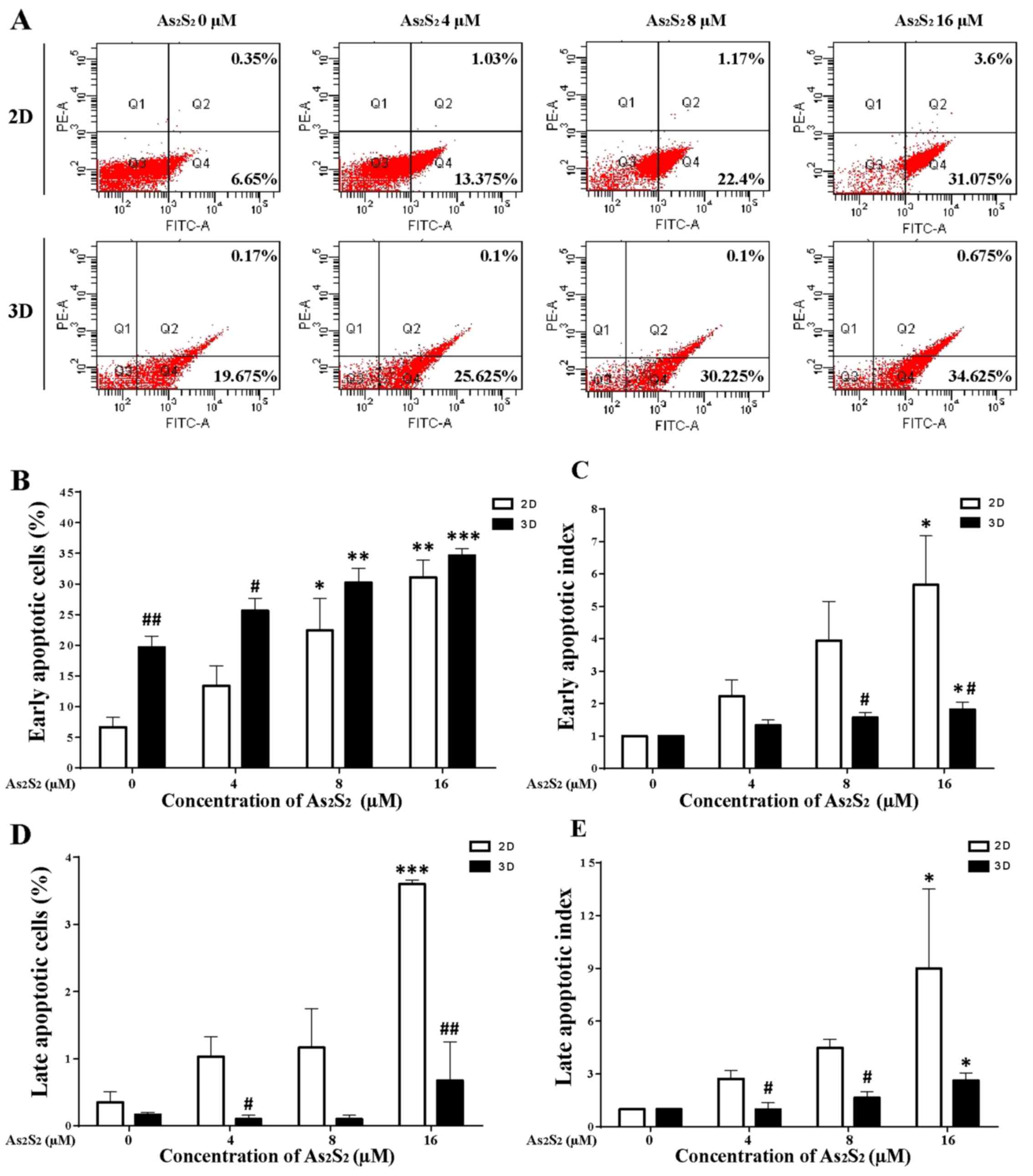
As2S2 treatment
induced apoptosis of 2D- and 3D-cultured MCF-7 cells
To explore whether the induction of apoptosis was
involved in the cytotoxicity of As2S2 in
MCF-7 cells cultured in the 2D and 3D cultured systems, an Annexin
V-FITC/PI dual staining assay was performed. The MCF-7 cells of
monolayers and spheroids were exposed to 0, 4, 8 and 16 μM
As2S2 for 72 h. The number of apoptotic MCF-7
cells was measured by an Annexin V-FITC/PI dual staining assay,
followed by flow cytometry, which was based on the probe of the
early (Q4) and late apoptosis (Q2) of MCF-7 cells (Fig. 5A).
As shown in Fig.
5B, the early apoptosis of MCF-7 cells was induced by
As2S2 in a dose-dependent manner in the 2D-
and the 3D-culture system. Without any drug exposure, MCF-7 cell
spheroids seemed to be more susceptible to early apoptosis than the
2D monolayers, and the median values of the Annexin
V+/PI− (early apoptosis) cells in the 2D
monolayers and the 3D spheroids were 6.65±1.60 and 19.68±1.81%,
respectively. The number of early apoptotic cells in the drug-free
3D culture was signifi-cantly increased in comparison with the 2D
culture (P=0.0017). Following treatment with 4, 8 and 16 μM
of As2S2 for 72 h in the 2D culture
monolayers, the percentages of cells in early apop-tosis were
13.38±3.25% (P=0.5439), 22.40±5.24% (P=0.0340), and 31.08±2.86%
(P=0.0017), respectively, in comparison with control (0 μM
As2S2). By contrast, following treatment with
4, 8 and 16 μM of As2S2 for 72 h in 3D
culture spheroids, the percentages of cells in early apoptosis were
25.63±2.03% (P=0.1628), 30.23±2.29% (P=0.0082), and 34.63±1.10%
(P=0.0005), respectively, in comparison with the control.
In the absence of As2S2, the
3D spheroids were more susceptible to early apoptosis in comparison
with the 2D monolayers. The pro-early apoptotic effects of
As2S2 between the 2D and 3D systems were
therefore compared by evaluating the apoptotic index, i.e., the
ratio of apoptotic cell percentages between the drug treatment
groups and the control. As shown in Fig. 5C, the early apoptotic indices in
the 2D-cultured MCF-7 cells treated with 4, 8 and 16 μM of
As2S2 for 72 h increased 2.22±0.50-fold
(P=0.8209), 3.95±1.20-fold (P=0.2107), and 5.67±1.51-fold
(P=0.0275), respectively, in comparison with the control (1.00). In
3D-cultured spheroids, the early cell-apoptotic indices following
treatment with 4, 8 and 16 μM of As2S2
increased 1.33±0.16-fold (P=0.4645), 1.57±0.16-fold (P= 0.0988),
and 1.82±0.22-fold (P= 0.0140), respectively, in comparison with
the control (1.00). These data demonstrated that MCF-7 cells
cultured as spheroids were more resistant to
As2S2 in terms of the early induction of
apoptosis, in comparison with cells cultured as monolayers, with
statistically significant differences in the presence of 8
(P=0.0457) and 16 μM (P=0.0448)
As2S2.
As shown in Fig.
5D, late apoptosis of the MCF-7 cells was induced by
As2S2 in a dose-dependent manner,
particularly in 2D monolayers. In 2D culture monolayers, following
treatment with 4, 8 and 16 μM of As2S2
for 72 h, the percentages of cells in late apoptosis (Annexin
V+/PI+) were 0.53±0.23% (P=0.9568),
1.17±0.58% (P=0.3166), and 3.60±0.06% (P=0.0005), respectively, in
comparison with the control. By contrast, the MCF-7 spheroids were
more resistant to As2S2-induced late
apoptosis, without any significant increases in those cells in the
presence of any concentration of As2S2.
As shown in Fig.
5E, the late apoptotic index of MCF-7 cells was induced by
As2S2 in a dose-dependent manner in both 2D-
and 3D-culture systems. In 2D culture monolayers, following
treatment with 4, 8 and 16 μM As2S2
for 72 h the late cell-apoptotic index increased 2.72±0.49-fold
(P=0.8894), 4.48±0.49-fold (P=0.5016), and 8.99±4.52-fold
(P=0.0458), respectively, in comparison with the control (1.00). In
the 3D-cultured spheroids, following As2S2
treatment at concentrations of 4, 8 and 16 μM for 72 h, the
late cell-apop-totic index increased 0.98±0.38-fold (P>0.99),
1.65±0.35-fold (P=0.5419), and 2.63±0.42-fold (P=0.0352),
respectively, in comparison with the control (1.00). Therefore, the
data in the present study demonstrated that MCF-7 cells cultured as
spheroids were more resistant to As2S2 in
terms of the late induction of apoptosis, in comparison with cells
cultured as monolayers, with statistically significant differences
in the presence of 4 (P=0.0206) and 8 (P=0.0231) μM
As2S2, respectively.
Effects of As2S2 on
the expression of apoptosis-associated proteins in 2D- and 3D-
cultured MCF-7 cells
To further investigate the mechanism underlying
As2S2-induced apoptosis in MCF-7 monolayers
and spheroids, the expression levels of apoptosis-associated
proteins were evaluated. To accomplish this, the levels of
pro-apoptotic proteins, such as Bax, p53 and caspase-7, as well as
of the anti-apoptotic protein, Bcl-2, were assessed by western
blotting. As shown in Fig. 6A, C and
D, the expression levels of the pro-apoptotic proteins, Bax,
p53 and caspase-7, were significantly upregulated by
As2S2 in MCF-7 monolayers. The protein levels
of Bax were markedly increased following treatment with 4
(P=0.004), 8 (P=0.0032) and 16 (P=0.0258) μM
As2S2, in comparison with the control (0
μM As2S2) (Fig. 6A). For p53, the protein levels
increased in a dose-dependent manner, following exposure to 4, 8
and 16 μM As2S2 (in comparison with
the control). A statistically significant increase was observed
following treatment with 16 μM As2S2
(P=0.0045) (Fig. 6C). The protein
expression of caspase-7 was significantly upregulated after
incubation with 8 (P=0.0003) and 16 (P=0.0009) μM
As2S2, in comparison with the control
(Fig. 6D). By contrast, a similar
increase in the expression level of Bax was confirmed in treated 3D
spheroids after treatment with 4, 8 and 16 μM
As2S2 (P= 0.0026, P=0.0265, P=0.0226 vs.
control, respectively), and As2S2 treatment
tended to induce the increased expression of p53; however, the
expression of caspase-7 was not significantly increased in the 3D
culture system (Fig. 6A, C and
D).
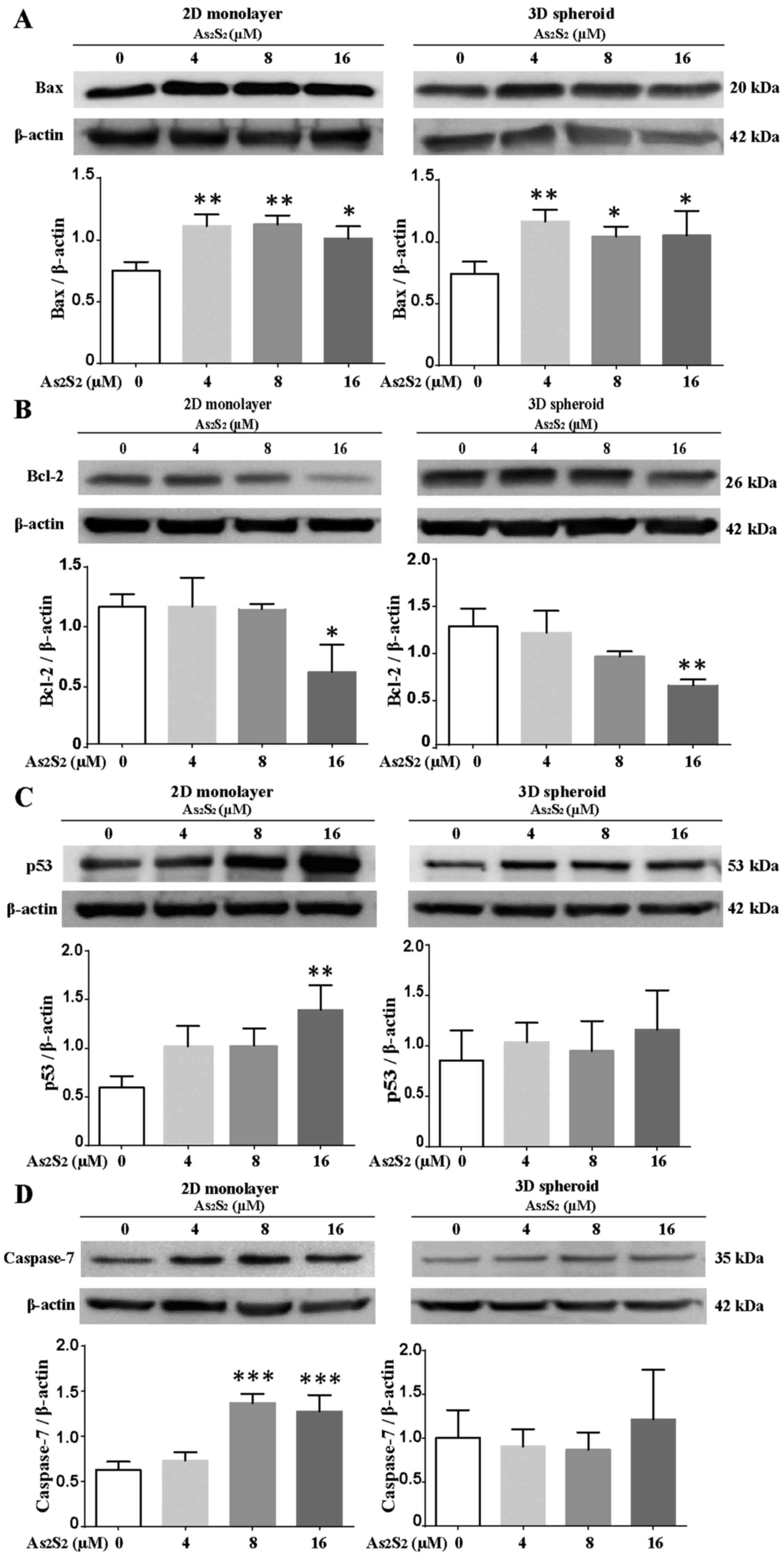 | Figure 6Effects of
As2S2 on the expression levels of
apoptosis-associated proteins in 2D- and 3D-cultured MCF-7 cells.
The expression levels of apoptosis-associated proteins were
analyzed by western blotting. (A) As2S2
significantly increased the levels of the pro-apoptotic protein,
Bax, in MCF-7 2D monolayers and 3D spheroids. (B)
As2S2 significantly reduced the levels of the
anti-apoptotic protein, Bcl-2, in MCF-7 2D monolayers and 3D
spheroids in a dose-dependent manner. (C)
As2S2 significantly increased the levels of
the pro-apoptotic protein, p53, in MCF-7 2D monolayers in a
dose-dependent manner, whereas the agent slightly increased the
expression of p53 in MCF-7 3D spheroids. (D)
As2S2 significantly increased the levels of
the pro-apoptotic protein, caspase-7, in MCF-7 2D monolayers in a
dose-dependent manner, whereas the agent exerted only a small
effect on caspase-7 in MCF-7 3D spheroids. All data are expressed
as the mean ± standard error of the mean (n≥3). Asterisks indicate
significant differences between the control and drug treatment
groups (*P<0.05; **P<0.01;
***P<0.001). As2S2, arsenic
disulfide; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
As shown in Fig.
6B, following treatment with As2S2 for 72
h, the expression of the anti-apoptotic protein, Bcl-2, was
down-regulated in both MCF-7 monolayers and spheroids. Following
treatment with 16 μM As2S2,
statistically significant decreases were observed in MCF-7
monolayers (P=0.0221 vs. control) and spheroids (P=0.0051 vs.
control) (Fig. 6B).
Effects of As2S2 on
the expression of pro-survival proteins in 2D- and 3D- cultured
MCF-7 cells
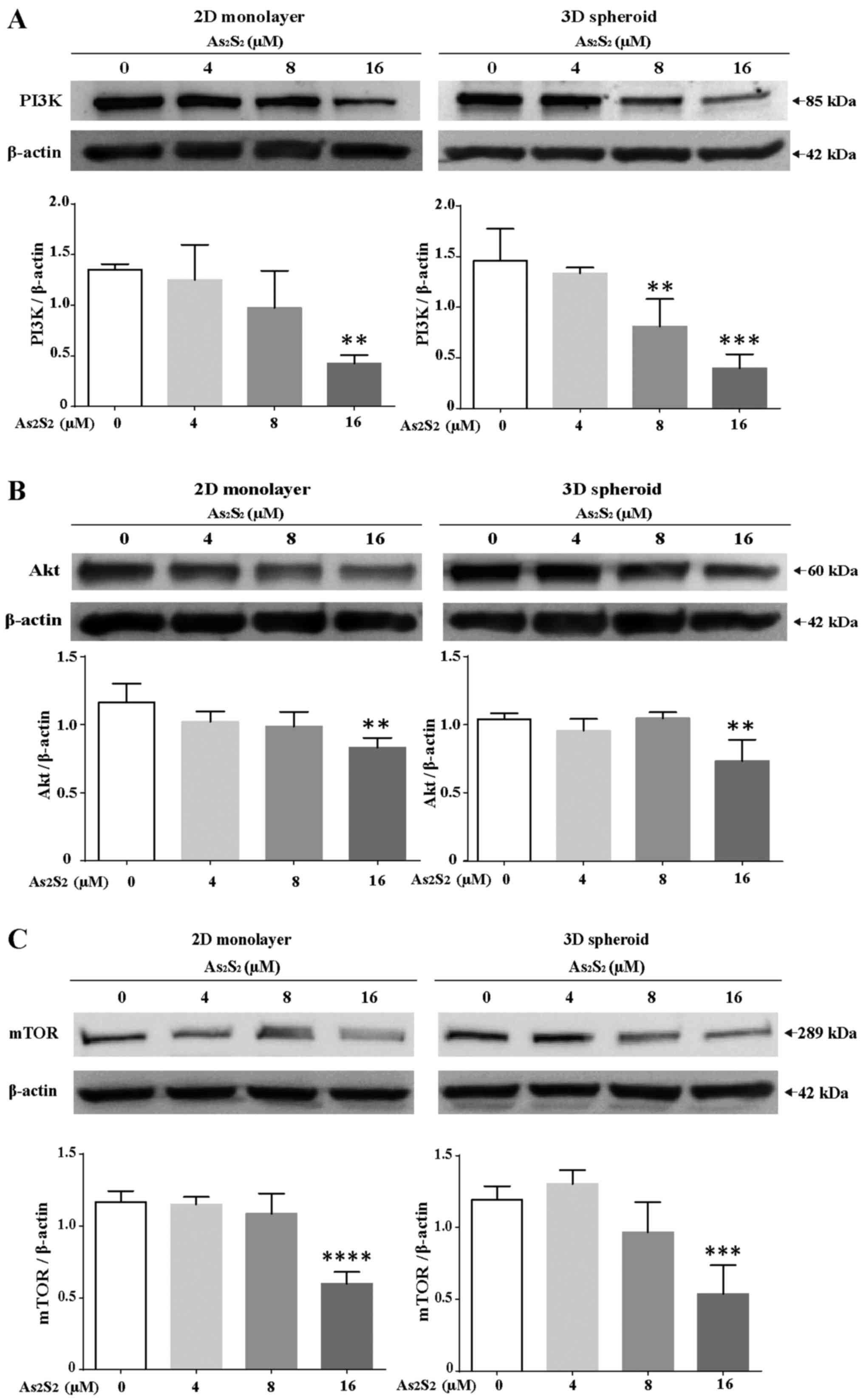
Since the PI3K/Akt/mTOR pathway serves an essential
role in cell growth, proliferation, and survival (32,33),
the protein expression of these pro-survival mediators were
detected in both 2D monolayers and 3D spheroids of MCF-7 cells.
Following 72 h of exposure to 4, 8 and 16 μM
As2S2, the protein levels of PI3K in 2D- and
3D-cultured MCF-7 cells were decreased in a dose-dependent manner
in comparison with their untreated counterparts (Fig. 7A). At a concentration of 16
μM, As2S2 had a significant inhibitory
effect on 2D monolayers (P=0.01). As2S2 also
induced a significant downregulation of PI3K in 3D spheroids at
final concentrations of 8 (P=0.0066) and 16 (P=0.0001) μM,
respectively. As shown in Fig. 7B,
subsequently to treatment with As2S2 for 72
h, the Akt protein expression levels were downregulated in MCF-7
monolayers and spheroids. After treatment with 16 μM
As2S2, statistically significant decreases
were observed in MCF-7 monolayers (P=0.0031) and spheroids
(P=0.0028) (Fig. 7B). Following
exposure to 4, 8 and 16 μM As2S2, the
protein levels of mTOR decreased in a dose-dependent manner in both
2D and 3D MCF-7 cells, in comparison with their untreated
counterparts; after treatment with 16 μM
As2S2, statistically significant differences
were observed in both 2D monolayers (P<0.0001) and 3D spheroids
(P=0.0004) (Fig. 7C).
Discussion
Several arsenic compounds have been investigated
for their antitumor potency and efficacy against different breast
cancer cell lines (7–9). For instance, ATO, as a Food and Drug
Administration (FDA)-approved drug, was reported to have the
potential to suppress cell growth, inhibit cell migration, induce
apoptosis and cell cycle arrest, and downregulate cancer
pro-coagulant activity in breast cancer cell lines (34–37).
However, ATO is regarded as a toxic agent that must be carefully
monitored after its intravenous administration. ATO is known to
cause severe adverse effects, including liver damage and QT
prolongation (10–12). As2S2, which
shows similar efficacy and fewer adverse effects (14,15),
is much less toxic than arsenic trioxide and other common arsenic
compounds, including sodium arsenite and arsenate (14,38),
and has been considered as an alternative oral arsenic agent for
cancer treatment. In China, As2S2 has already
been approved for clinical use in the treatment of leukemia
(16–18,39).
A series of clinical studies and trials proved that treatment with
As2S2 was well tolerated, with only moderate
side-effects in patients suffering from APL (13,39,40).
Recent studies have also reported the potent anti-tumor properties
of As2S2 in various solid cancer cell lines,
along with a marked reduction in cytotoxic effects in human normal
cell lines (41–43). However, there have been very few
studies on the anti-tumor effects of As2S2 in
breast cancer cells (24), and
fewer studies on the underlying mechanisms. Therefore, the present
study was performed to investigate the anticancer effects of
As2S2, and the underlying mechanisms in 2D-
and 3D-cultured MCF-7 cells. To the best of our knowledge, this is
the first report to show the mechanism underlying the cytotoxicity
induced by As2S2 in MCF-7 cells cultured in
2D and 3D culture systems.
In the present study, an in vitro cell
culture method was adapted to create 3D spheroids of MCF-7 cells.
The 3D cell-culture system established in the present research was
based on the adoption of TGP, in which the sol-gel transiting phase
is reversibly regulated by temperature (44). By way of comparison, the effects of
As2S2 on MCF-7 cells cultured as a 2D
monolayer were also investigated. The results demonstrated that
As2S2 decreased the cellular viability of
MCF-7 cells by inducing cellular apoptosis in both 2D and 3D cell
cultures. These effects were also associated with the activation of
pro-apoptotic signals, such as executioner caspase-7, p53 and Bax,
and the inhibition of Bcl-2 (associated with anti-apoptotic
signaling), as well as the pro-survival PI3K/Akt/mTOR pathway. In
addition, the microscopic observations revealed that 2D cells
simply attach to the bottom of the wells in a traditional monolayer
cell distribution, whereas the 3D cells form multicellular
spheroids via cell-cell interactions. The data from the cell
viability assays and apoptosis detection experiments revealed that
3D spheroids exhibited a certain degree of drug resistance, in
comparison with the 2D monolayer cell culture. This could be
attributed to the specific morphological structure of 3D spheroids
(Fig. 3), which is similar to the
features that studies have previously reported (45,46).
Unregulated cell survival and uncontrolled cell
growth may result in excessive cellular proliferation and the loss
of controlled cell death, which are two typical phenotypes of
cancer cells (47,48), including breast cancer cells. The
results obtained from the WST cell proliferation assays
demonstrated that As2S2 strongly inhibited
the cellular viability of MCF-7 cells in a dose-dependent manner,
in both 2D monolayers and in 3D spheroids. Consistently with these
findings, the data revealed that As2S2 is
able to induce apoptosis in 2D- and 3D-cultured MCF-7 cells in a
concentration-dependent manner. Since 3D cell-culture models enable
cells to form structures that mimic the in vivo architecture
in a more physiologically relevant condition compared with
traditional 2D monolayers (49–51),
the present study provided a more reliable method for investigating
the actual function of As2S2 in MCF-7 cells.
In addition, the data of the present study demonstrated the
increased drug resistance of MCF-7 cells cultured in a 3D system in
comparison with those cultured in a 2D system, either through the
inhibition of cell viability or the induction of apoptosis by
As2S2. Notably, it was revealed that, in the
absence of any drug treatment, MCF-7 cell spheroids were more
susceptible to apoptosis than the 2D monolayers, which is
consistent with observations made by other researchers (52–54).
This might be associated with the specific hypoxic and endocrinal
changes in the 3D spheroid microenvironment. This phenomenon was
different from anoikis (55,56),
since the MCF-7 spheroids developed in the present study
demonstrated cell-cell interactions and interacted with each other,
with the extracellular matrix, and with their microenvironment.
Emerging evidence has demonstrated that arsenic
compounds such as arsenic trioxide exert anti-tumor effects by
inducing apoptosis in hematological cancer and in solid tumors
(6,57,58).
The molecular mechanisms underlying arsenic-mediated apoptosis
include the activation of intrinsic and extrinsic apoptosis
pathways, as well as the suppression of pro-survival pathways in
various cancer cell lines. In the current study, the results
demonstrated that As2S2 induced the
expression of pro-apoptotic proteins and inhibited the levels of
pro-survival proteins, suggesting the possible pathways responsible
for the cytotoxicity of As2S2 in MCF-7 cells.
Caspases-3 and -7 play critical roles as effectors in the execution
phase, namely, the final phase of apoptosis (59,60).
Although the expression of caspase-3 was not detected in either 2D
monolayers or 3D spheroids (data not shown), the results of the
present study indicated that As2S2
significantly increased the protein expression of caspase-7 in
2D-cultured MCF-7 cells in a dose-dependent manner. In that
respect, the MCF-7 cell apoptosis that was induced by
As2S2 in the present study might be mediated
via the activation of caspase-7 instead of caspase-3, which is
congruent with the findings of previous studies showing that the
MCF-7 cell line does not express caspase-3, but that it may undergo
apoptosis through the activation of caspases-7 and -6 (61,62).
The tumor suppressor p53 is capable of inducing cell apoptosis via
activation of the mitochondrial and death receptor-induced
apoptotic pathways (63,64). The results of the present study
revealed that As2S2 increased the protein
level of p53 in a dose-dependent manner in 2D- and 3D-cultured
MCF-7 cells, which is consistent with the results showing that
As2S2 induced the expression of activated
caspase-7 expression in 2D culture. Furthermore,
As2S2 activated Bax (a pro-apoptotic
protein), but inhibited Bcl-2 (an anti-apoptotic protein from the
Bcl-2 family), in MCF-7 monolayers as well as in spheroids,
indicating the significant role that As2S2
exerted in stimulating the intrinsic pathway of apoptosis. The
PI3K/Akt/mTOR pathway, which is thought to promote cellular
proliferation and survival and which has been shown to be
associated with inactivation of the apoptotic signals, is the most
frequently activated signaling pathway in breast cancer (32,33,65,66).
Blocking the expression of PI3K/Akt/mTOR simultaneously inhibits
cell survival and promotes pro-apoptotic activity. In the present
study, the decrease in PI3K/Akt/mTOR expression that occurred in
MCF-7 monolayers and spheroids following treatment with
As2S2 revealed the possible inhibitory role
of the arsenic compound in the downregulation of cancer
cell-survival signals, which suggests the activation of
pro-apoptotic signaling.
In conclusion, the present study has provided
evidence that As2S2 may be a potential
therapeutic agent to be applied in the treatment of breast cancer.
The in vitro results in the present study suggested that
As2S2 inhibited the viability of MCF-7 cells
and induced cell apoptosis in both 2D monolayers and 3D spheroids
in a concentration-dependent manner. Activation of the
mitochondrial apoptosis pathway may be involved in this mechanism,
since the upregulation of the pro-apoptotic mediators, Bax, p53,
caspase-7, as well as the downregulation of the anti-apoptotic
mediator, Bcl-2, were observed following exposure to
As2S2. In addition, the inhibition of the
pro-survival PI3K/Akt/mTOR pathway by As2S2
treatment contributed to the suppression of cell viability and the
induction of apoptosis in 2D- and 3D-cultured MCF-7 cells.
Furthermore, the present study has revealed that 3D spheroids of
MCF-7 cells had a higher rate of cell viability and reduced cell
apoptosis in comparison with 2D monolayer cells following treatment
with As2S2, indicating the drug resistance of
3D spheroids. However, the present study focused on delineating the
pro-apoptotic effect of As2S2 in breast
cancer cells, and therefore it was confined to one of the cell
death mechanisms. Further studies will be performed to elucidate
other potential mechanisms involved in the anti-tumor function of
As2S2, particularly with respect to the
induction of cell cycle and autophagy. Furthermore, the mechanism
of drug resistance in 3D spheroids also requires further
investigation.
Glossary
Abbreviations
Abbreviations:
|
As2S2
|
arsenic disulfide
|
|
2D
|
two-dimensional
|
|
3D
|
three-dimensional
|
|
ATO
|
arsenic trioxide
|
|
APL
|
acute promyelocytic leukemia
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bax
|
Bcl-2-associated X protein
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
MCS
|
multicellular spheroid
|
|
TGP
|
thermo-reversible gelatin polymer
|
|
OD
|
optical density
|
Acknowledgments
Not applicable.
Funding
This study was supported in part by grants from
China Scholarship Council (file no. 201709110064).
Availability of data and materials
The analyzed data sets generated during the current
study are available from the corresponding author on reasonable
request for non-commercial purposes, while preserving necessary
confidentiality and anonymity.
Authors’ contributions
TH conceived the study and was in charge of overall
direction and planning and critically revised the manuscript. YXZ
designed and performed the experiments, analyzed data and was major
contributor in writing the manuscript. KO and BY gave advice on the
experiments and writing of the manuscript and critically revised
the manuscript. KO, BY, KS, AK and ST contributed to technical
assistance. TH, MS and NT supervised the study. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
3
|
Recht A, Come SE, Henderson IC, Gelman RS,
Silver B, Hayes DF, Shulman LN and Harris JR: The sequencing of
chemotherapy and radiation therapy after conservative surgery for
early-stage breast cancer. N Engl J Med. 334:1356–1361. 1996.
View Article : Google Scholar
|
|
4
|
Rojewski MT, Körper S and Schrezenmeier H:
Arsenic trioxide therapy in acute promyelocytic leukemia and
beyond: From bench to bedside. Leuk Lymphoma. 45:2387–2401. 2004.
View Article : Google Scholar
|
|
5
|
Cicconi L and Lo-Coco F: Current
management of newly diagnosed acute promyelocytic leukemia. Ann
Oncol. 27:1474–1481. 2016. View Article : Google Scholar
|
|
6
|
Miller WH Jr, Schipper HM, Lee JS, Singer
J and Waxman S: Mechanisms of action of arsenic trioxide. Cancer
Res. 62:3893–3903. 2002.
|
|
7
|
Chow SK, Chan JY and Fung KP: Suppression
of cell proliferation and regulation of estrogen receptor alpha
signaling pathway by arsenic trioxide on human breast cancer MCF-7
cells. J Endocrinol. 182:325–337. 2004. View Article : Google Scholar
|
|
8
|
Yao M, Yuan B, Wang X, Sato A, Sakuma K,
Kaneko K, Komuro H, Okazaki A, Hayashi H, Toyoda H, et al:
Synergistic cytotoxic effects of arsenite and tetrandrine in human
breast cancer cell line MCF-7. Int J Oncol. 51:587–598. 2017.
View Article : Google Scholar
|
|
9
|
Soria EA, Bongiovanni GA, Luján CD and
Eynard AR: Effect of arsenite on nitrosative stress in human breast
cancer cells and its modulation by flavonoids. Nutr Cancer.
67:659–663. 2015. View Article : Google Scholar
|
|
10
|
Wu JZ and Ho PC: Comparing the relative
oxidative DNA damage caused by various arsenic species by
quantifying urinary levels of 8-hydroxy-2′-deoxyguanosine with
isotope-dilution liquid chromatography/mass spectrometry. Pharm
Res. 26:1525–1533. 2009. View Article : Google Scholar
|
|
11
|
Horibe Y, Adachi S, Yasuda I, Yamauchi T,
Kawaguchi J, Kozawa O, Shimizu M and Moriwaki H: Anticancer effect
of arsenite on cell migration, cell cycle and apoptosis in human
pancreatic cancer cells. Oncol Lett. 12:177–182. 2016. View Article : Google Scholar
|
|
12
|
Dangleben NL, Skibola CF and Smith MT:
Arsenic immunotoxicity: A review. Environ Health. 12:732013.
View Article : Google Scholar
|
|
13
|
Lu DP, Qiu JY, Jiang B, Wang Q, Liu KY,
Liu YR and Chen SS: Tetra-arsenic tetra-sulfide for the treatment
of acute promyelocytic leukemia: A pilot report. Blood.
99:3136–3143. 2002. View Article : Google Scholar
|
|
14
|
Lu YF, Yan JW, Wu Q, Shi JZ, Liu J and Shi
JS: Realgar- and cinnabar-containing an-gong-niu-huang wan (AGNH)
is much less acutely toxic than sodium arsenite and mercuric
chloride. Chem Biol Interact. 189:134–140. 2011. View Article : Google Scholar
|
|
15
|
Liu J, Liang SX, Lu YF, Miao JW, Wu Q and
Shi JS: Realgar and realgar-containing Liu-Shen-Wan are less
acutely toxic than arsenite and arsenate. J Ethnopharmacol.
134:26–31. 2011. View Article : Google Scholar
|
|
16
|
Wang L, Zhou GB, Liu P, Song JH, Liang Y,
Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al: Dissection of
mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as
an effective treatment for promyelocytic leukemia. Proc Natl Acad
Sci USA. 105:4826–4831. 2008. View Article : Google Scholar
|
|
17
|
Zhang QY, Mao JH, Liu P, Huang QH, Lu J,
Xie YY, Weng L, Zhang Y, Chen Q, Chen SJ, et al: A systems biology
understanding of the synergistic effects of arsenic sulfide and
Imatinib in BCR/ABL-associated leukemia. Proc Natl Acad Sci USA.
106:3378–3383. 2009. View Article : Google Scholar
|
|
18
|
Liu Y, He P, Cheng X and Zhang M:
Long-term outcome of 31 cases of refractory acute promyelocytic
leukemia treated with compound realgar natural indigo tablets
administered alternately with chemotherapy. Oncol Lett.
10:1184–1190. 2015. View Article : Google Scholar
|
|
19
|
Wu F, Wu D, Ren Y, Duan C, Chen S and Xu
A: Bayesian network meta-analysis comparing five contemporary
treatment strategies for newly diagnosed acute promyelocytic
leukaemia. Oncotarget. 7:47319–47331. 2016.
|
|
20
|
Wang L, Liu X, Li X, Lv X, Lu K, Chen N,
Li P and Wang X: Arsenic disulfide induces apoptosis of human
diffuse large B cell lymphoma cells involving Bax cleavage. Oncol
Rep. 30:2427–2434. 2013. View Article : Google Scholar
|
|
21
|
Cheng YX, Liu R, Wang Q, Li BS, Xu XX, Hu
M, Chen L, Fu Q, Pu DM and Hong L: Realgar-induced apoptosis of
cervical cancer cell line Siha via cytochrome c release and
caspase-3 and caspase-9 activation. Chin J Integr Med. 18:359–365.
2012. View Article : Google Scholar
|
|
22
|
Song P, Chen P, Wang D, Wu Z, Gao Q, Wang
A, Zhu R, Wang Y, Wang X, Zhao L, et al: Realgar transforming
solution displays anticancer potential against human hepatocellular
carcinoma HepG2 cells by inducing ROS. Int J Oncol. 50:660–670.
2017. View Article : Google Scholar
|
|
23
|
Wang G, Zhang T, Sun W, Wang H, Yin F,
Wang Z, Zuo D, Sun M, Zhou Z, Lin B, et al: Arsenic sulfide induces
apoptosis and autophagy through the activation of ROS/JNK and
suppression of Akt/mTOR signaling pathways in osteosarcoma. Free
Radic Biol Med. 106:24–37. 2017. View Article : Google Scholar
|
|
24
|
Tian Y, Wang X, Xi R, Pan W, Jiang S, Li
Z, Zhao Y, Gao G and Liu D: Enhanced antitumor activity of realgar
mediated by milling it to nanosize. Int J Nanomedicine. 9:745–757.
2014.
|
|
25
|
Breslin S and O’Driscoll L:
Three-dimensional cell culture: The missing link in drug discovery.
Drug Discov Today. 18:240–249. 2013. View Article : Google Scholar
|
|
26
|
Yamada KM and Cukierman E: Modeling tissue
morphogenesis and cancer in 3D. Cell. 130:601–610. 2007. View Article : Google Scholar
|
|
27
|
Weigelt B, Ghajar CM and Bissell MJ: The
need for complex 3D culture models to unravel novel pathways and
identify accurate biomarkers in breast cancer. Adv Drug Deliv Rev.
69–70:42–51. 2014. View Article : Google Scholar
|
|
28
|
Rimann M and Graf-Hausner U: Synthetic 3D
multicellular systems for drug development. Curr Opin Biotechnol.
23:803–809. 2012. View Article : Google Scholar
|
|
29
|
Kiyomi A, Makita M, Ozeki T, Li N,
Satomura A, Tanaka S, Onda K, Sugiyama K, Iwase T and Hirano T:
Characterization and clinical implication of Th1/Th2/Th17 cytokines
produced from three-dimensionally cultured tumor tissues resected
from breast cancer patients. Transl Oncol. 8:318–326. 2015.
View Article : Google Scholar
|
|
30
|
Dubois V, Jardé T, Delort L, Billard H,
Bernard-Gallon D, Berger E, Geloen A, Vasson MP and Caldefie-Chezet
F: Leptin induces a proliferative response in breast cancer cells
but not in normal breast cells. Nutr Cancer. 66:645–655. 2014.
View Article : Google Scholar
|
|
31
|
Alcolea V, Plano D, Encío I, Palop JA,
Sharma AK and Sanmartín C: Chalcogen containing heterocyclic
scaffolds: New hybrids with antitumoral activity. Eur J Med Chem.
123:407–418. 2016. View Article : Google Scholar
|
|
32
|
Ciruelos Gil EM: Targeting the
PI3K/AKT/mTOR pathway in estrogen receptor-positive breast cancer.
Cancer Treat Rev. 40:862–871. 2014. View Article : Google Scholar
|
|
33
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar
|
|
34
|
Yun SM, Woo SH, Oh ST, Hong SE, Choe TB,
Ye SK, Kim EK, Seong MK, Kim HA, Noh WC, et al: Melatonin enhances
arsenic trioxide-induced cell death via sustained upregulation of
Redd1 expression in breast cancer cells. Mol Cell Endocrinol.
422:64–73. 2016. View Article : Google Scholar
|
|
35
|
Zhang S, Ma C, Pang H, Zeng F, Cheng L,
Fang B, Ma J, Shi Y, Hong H, Chen J, et al: Arsenic trioxide
suppresses cell growth and migration via inhibition of miR-27a in
breast cancer cells. Biochem Biophys Res Commun. 469:55–61. 2016.
View Article : Google Scholar
|
|
36
|
Zhang YF, Zhang M, Huang XL, Fu YJ, Jiang
YH, Bao LL, Maimaitiyiming Y, Zhang GJ, Wang QQ and Naranmandura H:
The combination of arsenic and cryptotanshinone induces apoptosis
through induction of endoplasmic reticulum stress-reactive oxygen
species in breast cancer cells. Metallomics. 7:165–173. 2015.
View Article : Google Scholar
|
|
37
|
Kasukabe T, Okabe-Kado J, Kato N, Honma Y
and Kumakura S: Cotylenin A and arsenic trioxide cooperatively
suppress cell proliferation and cell invasion activity in human
breast cancer cells. Int J Oncol. 46:841–848. 2015. View Article : Google Scholar
|
|
38
|
Luo XQ, Ke ZY, Huang LB, Guan XQ, Zhang YC
and Zhang XL: Improved outcome for Chinese children with acute
promyelocytic leukemia: A comparison of two protocols. Pediatr
Blood Cancer. 53:325–328. 2009. View Article : Google Scholar
|
|
39
|
Zhu HH, Wu DP, Jin J, Li JY, Ma J, Wang
JX, Jiang H, Chen SJ and Huang XJ: Oral tetra-arsenic tetra-sulfide
formula versus intravenous arsenic trioxide as first-line treatment
of acute promyelocytic leukemia: A multicenter randomized
controlled trial. J Clin Oncol. 31:4215–4221. 2013. View Article : Google Scholar
|
|
40
|
Xiang-Xin L, Lu-Qun W, Hao L, Xiao-Peng H,
Fang-Lin L, Ling-Ling W, Xue-Liang C and Ming H: Clinical study on
prospective efficacy of all-trans Acid, realgar-indigo naturalis
formula combined with chemotherapy as maintenance treatment of
acute promyelocytic leukemia. Evid Based Complement Alternat Med.
2014:9875602014. View Article : Google Scholar
|
|
41
|
Wu JZ and Ho PC: Evaluation of the in
vitro activity and in vivo bioavailability of realgar nanoparticles
prepared by cryogrinding. Eur J Pharm Sci. 29:35–44. 2006.
View Article : Google Scholar
|
|
42
|
Wang H, Liu Z, Gou Y, Qin Y, Xu Y, Liu J
and Wu JZ: Apoptosis and necrosis induced by novel realgar quantum
dots in human endometrial cancer cells via endoplasmic reticulum
stress signaling pathway. Int J Nanomedicine. 10:5505–5512. 2015.
View Article : Google Scholar
|
|
43
|
Ding W, Zhang L, Kim S, Tian W, Tong Y,
Liu J, Ma Y and Chen S: Arsenic sulfide as a potential anti cancer
drug. Mol Med Rep. 11:968–974. 2015. View Article : Google Scholar
|
|
44
|
Tsukikawa S, Matsuoka H, Kurahashi Y,
Konno Y, Satoh K, Satoh R, Isogai A, Kimura K, Watanabe Y, Nakano
S, et al: A new method to prepare multicellular spheroids in cancer
cell lines using a thermo-reversible gelation polymer. Artif
Organs. 27:598–604. 2003. View Article : Google Scholar
|
|
45
|
Khaitan D, Chandna S, Arya MB and
Dwarakanath BS: Establishment and characterization of multicellular
spheroids from a human glioma cell line; Implications for tumor
therapy. J Transl Med. 4:122006. View Article : Google Scholar
|
|
46
|
Edmondson R, Broglie JJ, Adcock AF and
Yang L: Three-dimensional cell culture systems and their
applications in drug discovery and cell-based biosensors. Assay
Drug Dev Technol. 12:207–218. 2014. View Article : Google Scholar
|
|
47
|
Biswas DK, Shi Q, Baily S, Strickland I,
Ghosh S, Pardee AB and Iglehart JD: NF-kappa B activation in human
breast cancer specimens and its role in cell proliferation and
apoptosis. Proc Natl Acad Sci USA. 101:10137–10142. 2004.
View Article : Google Scholar
|
|
48
|
Vermeulen K, Berneman ZN and Van
Bockstaele DR: Cell cycle and apoptosis. Cell Prolif. 36:165–175.
2003. View Article : Google Scholar
|
|
49
|
Fischbach C, Chen R, Matsumoto T,
Schmelzle T, Brugge JS, Polverini PJ and Mooney DJ: Engineering
tumors with 3D scaffolds. Nat Methods. 4:855–860. 2007. View Article : Google Scholar
|
|
50
|
Debnath J and Brugge JS: Modelling
glandular epithelial cancers in three-dimensional cultures. Nat Rev
Cancer. 5:675–688. 2005. View Article : Google Scholar
|
|
51
|
Breslin S and O’Driscoll L: The relevance
of using 3D cell cultures, in addition to 2D monolayer cultures,
when evaluating breast cancer drug sensitivity and resistance.
Oncotarget. 7:45745–45756. 2016. View Article : Google Scholar
|
|
52
|
Zeng H, Sun M, Zhou C, Yin F, Wang Z, Hua
Y and Cai Z: Hematoporphyrin monomethyl ether-mediated photodynamic
therapy selectively kills sarcomas by inducing apoptosis. PLoS One.
8:e777272013. View Article : Google Scholar
|
|
53
|
Akeda K, Nishimura A, Satonaka H, Shintani
K, Kusuzaki K, Matsumine A, Kasai Y, Masuda K and Uchida A:
Three-dimensional alginate spheroid culture system of murine
osteosarcoma. Oncol Rep. 22:997–1003. 2009. View Article : Google Scholar
|
|
54
|
Chandrasekaran S, Marshall JR, Messing JA,
Hsu JW and King MR: TRAIL-mediated apoptosis in breast cancer cells
cultured as 3D spheroids. PLoS One. 9:e1114872014. View Article : Google Scholar
|
|
55
|
Sodek KL, Murphy KJ, Brown TJ and
Ringuette MJ: Cell-cell and cell-matrix dynamics in intraperitoneal
cancer metastasis. Cancer Metastasis Rev. 31:397–414. 2012.
View Article : Google Scholar
|
|
56
|
Daubriac J, Fleury-Feith J, Kheuang L,
Galipon J, Saint-Albin A, Renier A, Giovannini M, Galateau-Sallé F
and Jaurand MC: Malignant pleural mesothelioma cells resist anoikis
as quiescent pluricellular aggregates. Cell Death Differ.
16:1146–1155. 2009. View Article : Google Scholar
|
|
57
|
Khairul I, Wang QQ, Jiang YH, Wang C and
Naranmandura H: Metabolism, toxicity and anticancer activities of
arsenic compounds. Oncotarget. 8:23905–23926. 2017. View Article : Google Scholar
|
|
58
|
Boehme KA, Nitsch J, Riester R,
Handgretinger R, Schleicher SB, Kluba T and Traub F: Arsenic
trioxide potentiates the effectiveness of etoposide in Ewing
sarcomas. Int J Oncol. 49:2135–2146. 2016. View Article : Google Scholar
|
|
59
|
Kadam CY and Abhang SA: Apoptosis markers
in breast cancer therapy. Adv Clin Chem. 74:143–193. 2016.
View Article : Google Scholar
|
|
60
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: Structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar
|
|
61
|
Kurokawa H, Nishio K, Fukumoto H, Tomonari
A, Suzuki T and Saijo N: Alteration of caspase-3
(CPP32/Yama/apopain) in wild-type MCF-7, breast cancer cells. Oncol
Rep. 6:33–37. 1999.
|
|
62
|
Simstein R, Burow M, Parker A, Weldon C
and Beckman B: Apoptosis, chemoresistance, and breast cancer:
Insights from the MCF-7 cell model system. Exp Biol Med (Maywood).
228:995–1003. 2003. View Article : Google Scholar
|
|
63
|
Wang X, Simpson ER and Brown KA: p53:
Protection against tumor growth beyond effects on cell cycle and
apoptosis. Cancer Res. 75:5001–5007. 2015. View Article : Google Scholar
|
|
64
|
Pietsch EC, Sykes SM, McMahon SB and
Murphy ME: The p53 family and programmed cell death. Oncogene.
27:6507–6521. 2008. View Article : Google Scholar
|
|
65
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar
|
|
66
|
Miller TW, Rexer BN, Garrett JT and
Arteaga CL: Mutations in the phosphatidylinositol 3-kinase pathway:
Role in tumor progression and therapeutic implications in breast
cancer. Breast Cancer Res. 13:2242011. View Article : Google Scholar
|