Introduction
Controlled protein phosphorylation and
dephosphorylation are fundamental in all aspects of biology
(1), and the underlying mechanism
is regulated by protein kinases and protein phosphatases (PPs).
Protein phosphatase 2A (PP2A), a major serine/threonine phosphatase
and critical member of the PP family, is ubiquitously expressed in
eukaryotic cells. It serves a significant role in several essential
cellular functions, including cell cycle regulation, cell growth,
differentiation, metabolism and cell mobility (2–4).
Dysregulation of PP2A is associated with numerous diseases,
including human cancer.
PP2A is a large heterotrimeric holoenzyme consisting
of a wide variety of regulatory subunits B, a structural subunit A,
and a catalytic subunit C or PP2AC (5). The A subunits function as scaffold
proteins that are responsible for the formation of the holoenzyme
and for the stability of the complex. In addition, the regulatory B
subunits include four subfamilies, namely B (PR55), B′ (B56 or
PR61), B″ (PR72) and B′″ (PR93/PR110). Different families, isoforms
and splice variants of the regulatory subunits determine the broad
substrate specificity and allow the PP2A holoenzyme to selectively
regulate signaling pathways (6).
The B family of regulatory subunits are encoded by four genes known
as PPP2R2s, including PPP2R2A (B55α), PPP2R2B (B55β), PPP2R2C
(B55γ) and PPP2R2D (B55δ) (7),
which exhibit temporal and spatial expression patterns. PPP2R2A and
PPP2R2D are expressed ubiquitously in mammalian cells, while
PPP2R2B and PPP2R2C are highly enriched in the brain (7–9).
PP2A has been reported to function as a potential
tumor suppressor (10); however,
this predominant perception has been challenged over the past
decades. Certain studies indicated that the inactivation of PP2A
induces apoptosis in a number of tissues and cell types, including
the pancreas, testes, liver and leukemic cells (11–15).
This accumulating evidence suggested a different role for PP2A in
distinct cancer types. B subunits are key factors that determine
the substrate specificity of PP2A and, among them, PPP2R2D is
expressed ubiquitously in mammalian cells. Several investigators
have thus revealed the association between PPP2R2D and human
cancer. For instance, Zhuang et al (16) reported that PPP2R2D enhanced the
sensitivity of human hepatocellular carcinoma to chemotherapy. In
addition, Cunningham et al (17) observed that knockdown of PPP2R2D
increased cell death in pancreatic cancer cell lines. Although the
aforementioned studies indicated that PPP2R2D may be involved in
cancer progression, the precise function and clinical relevance of
PPP2R2D in human cancer remain unknown.
Gastric cancer (GC) is one of the most common
malignancies and the third leading cause of cancer-associated
mortality worldwide (18). Its
prognosis remains poor due to the advanced stage of cancer
progression at the time of diagnosis. Thus, the identification of
novel biomarkers for early stage diagnosis is an urgent requirement
for GC. In the present study, the expression pattern and function
of PPP2R2D in GC were investigated, and it was observed that it is
frequently overexpressed in tumor tissues and its expression may
indicate tumor progression and poor prognosis. Furthermore,
evidence was provided that PPP2R2D is involved in GC cell
proliferation and migration possibly through the mechanistic target
of rapamycin (mTOR) signaling pathway.
Materials and methods
Human tissue sample collection
Human GC and adjacent gastric mucosa specimens were
obtained from 28 patients following resection at the Shanghai East
Hospital of Tongji University School of Medicine (Shanghai, China)
between October 2013 and May 2014. Informed consent was obtained
from all patients prior to the sample collection. Primary GC in the
tissues of these patients was diagnosed according to the 7th
criteria of The American Joint Committee on Cancer (19) and confirmed by at least two
pathologists subsequent to the resection. All the samples were
snap-frozen in liquid nitrogen and preserved at −80°C prior to RNA
extraction. Kaplan Meier Plotter database (http://kmplot.com) was used to assess the effects of
PPP2R2D on the prognosis of GC. This study was approved by the
Ethics Committee of Shanghai East Hospital, Tongji University
School of Medicine.
Tissue microarray and
immunohistochemistry staining
A human GC tissue microarray (HStmA180Su09)
containing 90 patient tumor samples and adjacent gastric mucosa
specimens were purchased from Outdo Biotech Co., Ltd. (Shanghai,
China). The cohort comprised of 21 females and 69 males with an age
range between 17 and 84 years. The overall survival (OS) duration
of the patients was defined as the interval between initial surgery
and mortality. The sample sections (4 μm) were prepared and
processed for immunostaining using an antibody against PPP2R2D
(ab181071; Abcam, Cambridge, MA, USA) at a dilution of 1:500.
According to the percentage of positive cells, two pathologists
blinded to the clinical information evaluated the intensity of
PPP2R2D staining independently. The results were classified into
the following categories: Negative (−), <15%; weakly positive
(+), 15-40%; moderate positive (++), 40-75%; or strongly positive
(+++), >75%.
Cell culture and reagents
Human GC cell lines MGC803, SGC7901, BGC823, MKN28,
AGS and HGC27 were obtained from the Shanghai Cell Bank of the
Chinese Academy of Sciences (Shanghai, China). Among these cell
lines, MKN28 is a type of mixed gastric adenocarcinoma cell line
derived from MKN74 cells (20);
this does not affect the outcomes of the present study. Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Corning,
Inc., Corning, NY, USA) supplemented with 10% fetal bovine serum
(FBS) and 1% penicillin/streptomycin. All cell lines were
maintained at 37°C in a 5% CO2 humidified incubator
(Thermo Fisher Scientific, Inc., Waltham, MA, USA).
RNA extraction and quantitative
polymerase chain reaction (qPCR)
Total RNA was isolated from the cultured cells or
tissues using RNAiso Plus reagent and 1 μg total RNA was
reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit
(both from Takara Bio, Inc., Otsu, Japan) according to the
manufacturer’s protocol. The concentration of RNA was measured
using a Thermo Fisher Scientific NanoDrop™ spectrophotometer
(NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
qPCR was performed with SYBR-Green reagent (Takara Bio, Inc.) on an
ABI 7500 Real-Time PCR system (Thermo Fisher Scientific, Inc.). The
qPCR thermocycling conditions were: Initial hold at 95°C for 10
min, followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing/extension at 60°C for 60 sec. The primer sequences used
were as follows: PPP2R2D, 5′-CGAGTACCTGCGCAGCAAGCT-3′ (forward) and
5′-GACCCGGTCATGATGGCGCTATC-3′ (reverse); β-actin,
5′-CCTGGCACCCAGCACAATG-3′ (forward) and 5′-GGGCCGGACTCGTCATACT-3′
(reverse). β-actin served as an internal control, and the
2−ΔΔCt method (21) was
used for mRNA level quantification.
Western blot analysis
GC cells were lysed in lysis buffer (containing 25
mM Tris-Cl, pH 7.5, 1% SDS and 5 mM EDTA) supplemented with
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 30 min on ice. For phosphorylated protein, 1%
phosphatase inhibitor cocktail (Sigma-Aldrich; Merck KGaA) was
added to the lysis buffer. Total protein was assessed using
Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology, Haimen, China). Prior to the standard western blot
analysis, protein lysates were diluted in 5X SDS loading buffer and
boiled for 3 min. Total protein extracts (30-50 μg) were
electrophoresed by 10% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane, following which the membrane
was blocked with 5% non-fat dry milk in 0.1% Tween-20 in PBS (PBST)
for 2 h at room temperature. The membrane was subsequently
incubated with primary antibodies in PBST overnight at 4°C, and
then incubated with the corresponding secondary antibodies at room
temperature for 1 h. The antibodies used in this study included:
Anti-PPP2R2D (1:500; cat. no. 181071; Abcam), anti-FLAG (1:2,000;
cat. no. F1804; Sigma-Aldrich; Merck KGaA), anti-PPP2CA (1:500;
cat. no. 2038), mTOR (1:500; cat. no. 2983), phosphorylated
(p)-mTOR (Ser2448) (1:500; cat. no. 9864), protein kinase B (Akt;
1:1,000; cat. no. 9272), p-Akt (1:500; cat. no. 4051),
extracellular signal-regulated kinase (Erk; 1:500; cat. no. 4695),
p-Erk (1:500; cat. no. 4376) (all from Cell Signaling Technology,
Inc., Danvers, MA, USA), β-actin (1:500; cat. no. 81171; Santa Cruz
Biotechnology, Dallas, TX, USA) and IRDye 800DX-conjugated,
affinity-purified goat anti-rabbit or goat anti-mouse secondary
antibodies (#611-145-002 and #610-145-002, 1:1,000; Rockland
Immunochemicals, Inc., Pottstown, PA, USA). The Odyssey Infrared
imaging system (LI-COR Biosciences, Lincoln, NE, USA) was then used
for detection of the immunoreactive signal.
RNA interference and PPP2R2D
overexpression plasmid
In order to silence the expression levels of PPP2R2D
or PPP2CA in GC cell lines, small interfering RNA (siRNA)
oligonucleotides against PPP2R2D and PPP2CA were chemically
synthesized (GenePharma Co., Ltd., Shanghai, China). The siRNAs
sequences used were as follows: si-2R2D-1,
5′-GCACCUUUCAAAGUCAUGAdTdT-3′; si-2R2D-2,
5′-GCUCUCUCUAUGAGAACGAdTdT-3′; si-PPP2CA, 5′-CAUGGAACUUGACGA
UACUdTdT-3′; and a nonspecific control (si-NC,
5′-UUCUCCGAACGUGUCACGUdTdT-3′. Furthermore, in order to induce
overexpression of PPP2R2D, pEnter-PPP2R2D (GenBank accession no.
NM_018461) was purchased from Vigene Biosciences, Inc. (Jinan,
China). Cells were seeded at 30% confluence (for siRNA
transfection) or 80% confluence (for plasmid transfection) into
6-well plates prior to the transfection, and then
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to transfect cells at
37°C for 48 h with the plasmids or siRNAs according to the
manufacturer’s protocol. In order to obtain stably transfected
cells, a lentivirus knocking down PPP2R2D (LV-sh2R2D) or a
lentivirus control (LV-shNC) was generated and purchased from
GenePharma Co., Ltd., using the corresponding aforementioned
sequences (si-2R2D-1 and si-NC). The cells stably infected with the
lentivirus particles were enriched in the culture medium by
puromycin screening (Selleck Chemicals, Houston, TX, USA).
Cell proliferation assay
For the cell counting kit-8 (CCK-8) assay,
transfected GC cells were seeded into a 96-well culture plate at a
density of 3×103 cells per well. At 24, 48, 72, 96 and
120 h after transfection, cells were incubated with 10 μl
CCK-8 reagent (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) for 1 h at 37°C, and then the absorbance of each well was
measured spectrophotometrically at 450 nm in an automated plate
reader. The results were plotted as the mean ± standard deviation
of three independent experiments with five replicates per
experiment for each experimental condition.
Colony formation assay
Stably infected GC cells were seeded in a 6-well
plate at a density of 1×103 cells per well and cultured
for 2-3 weeks, following which the colonies formed were stained
with crystal violet for 10 min. Images of the colonies were
captured, and the number of cells was counted. All the experiments
were performed in triplicate and repeated at least for three
times.
5-Ethynyl-2′-deoxyuridine (EdU)
incorporation assay
GC cells were seeded at 1.5×105
cells/well in a 6-well plate and cultured for 24 h after
transfection with PPP2R2D-expressing plasmids. Subsequently, the
cells were incubated with 50 mM EdU for 2 h at room temperature.
Following staining with Apollo® 567 (both from RiboBio
Co., Ltd., Guangzhou, China) according to the manufacturer’s
protocol, the cells were observed with an inverted fluorescence
microscope and images were captured.
Cell migration assay
Cell migration assays were conducted using a 24-well
Transwell chamber (pore size, 8 μm; Costar; Corning, Inc.).
Briefly, at 24 h after transfection with plasmids or siRNAs, GC
cells were harvested and suspended in DMEM without FBS at a density
of 1×105 cells/ml. Next, 400 μl of the cell
suspension was loaded in the upper chamber, while 800 μl
DMEM containing 10% FBS was added to the bottom chamber. Subsequent
to incubation for 24 h at 37°C, the non-migrating cells in the
upper chamber were removed with a cotton swab, and the migrated
cells on the bottom surface of the filter were fixed in 4%
paraformaldehyde at room temperature for 5 min, stained with
crystal violet and counted under a phase contrast microscope in
five randomly selected fields at a magnification of ×200.
Wound healing assay
GC cells were harvested and re-suspended in DMEM
medium supplemented with 10% FBS in a 6-well plate at ~100%
confluence. A plastic tip was used to scratch a cell monolayer, and
then PBS was used to wash the samples and remove any cell debris,
followed by culturing of the cells in serum-free DMEM. After 0, 24
and 48 h, the cells were observed and images were captured using an
inverted microscope equipped with a camera.
Animal experiments
In order to establish a xenograft model of GC, 26
nude mice (BALA/c; age, 4-weeks-old) were purchased from Sippr-BK
Lab Animal Co., Ltd. (Shanghai, China). Mice were housed for 1 week
in a specific-pathogen-free environment prior to injection, under
the following standard conditions: 12-h light/dark cycle;
temperature, 25°C; humidity, 40-60%; free access to irradiated food
and autoclaved distilled water. A total of 2×106 stably
transfected cells in 100 μl PBS, namely MGC803-LV-shNC,
MGC803-LV-sh2R2D, MKN28-LV-shNC and MKN28-LV-sh2R2D, were
subcutaneously injected into the two flanks of each mouse,
respectively (n=8 per group). After 4 weeks, mice were euthanized,
and tumors were weighted. The weight of the mice was 18-20 g upon
purchase, and 25-28 g upon sacrifice, while the maximum diameter of
a single tumor was 14 mm. The tumor volumes were calculated using
the following equation: Volume = 0.5 × longitudinal diameter ×
(latitudinal diameter)2. The maximum volume of a single
tumor was 700 mm3.
For in vivo metastasis experiments,
2.5×106 cells (MGC803-LV-shNC and MGC803-LV-sh2R2D) were
injected into the tail veins of the nude mice (n=5 per group).
After 10 weeks, mice were euthanized and the visible tumor nodules
on the lung surface were calculated. The maximum number of
metastatic nodules in a mouse was 4, and the maximum diameter of a
metastatic nodule was 3 mm. The lung tissues were immobilized in 4%
paraformaldehyde, and paraffin sections (8 μm) were made for
hematoxylin and eosin staining. All animal handling and
experimental procedures were approved by the Ethics Committee of
Shanghai East Hospital, Tongji University School of Medicine.
Statistical analysis
All the quantitative data are expressed as the mean
± standard deviation. The two-tailed χ2 test was used to
determine the significance of the difference among the covariates.
Survival durations were calculated using the Kaplan-Meier method
and statistical differences were determined by log-rank test. The
in vitro data were analyzed using Student’s t-test
(two-tailed) to determine any statistical significance. A P-value
of <0.05 was considered to indicate a statistically significant
difference.
Results
PPP2R2D is overexpressed in GC and is
associated with the progression and prognosis
To investigate the possible role of PPP2R2D in GC,
its protein expression level was first analyzed in a tissue
microarray consisting of 90 paired of GC tissues and adjacent
normal gastric mucosa specimens by an immunohistochemical approach.
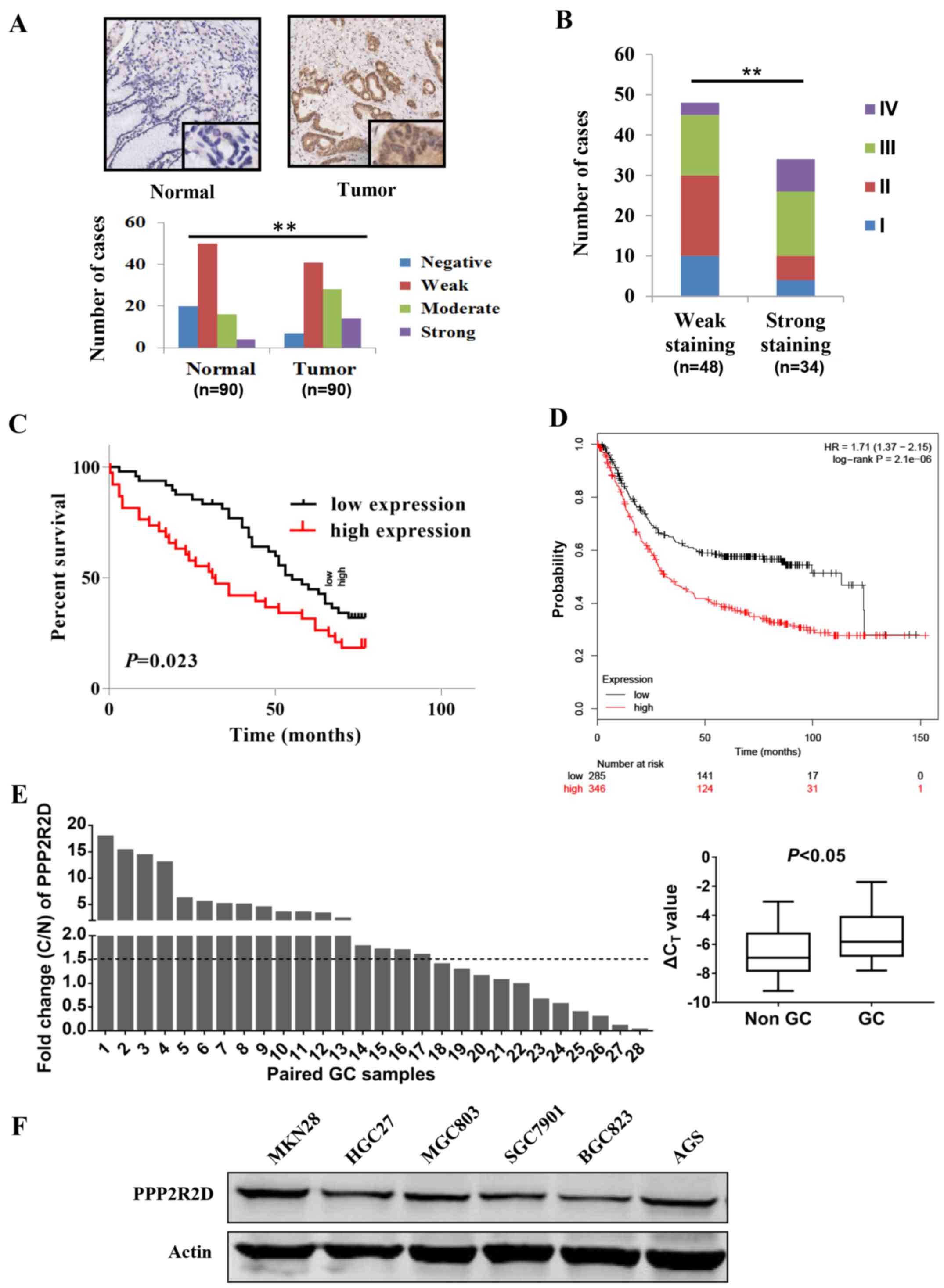
As shown in Fig. 1A, GC tissues
expressed more PPP2R2D compared with that in the paired normal
gastric mucosa tissues, as moderate and strong staining of PPP2R2D
was observed in a large number of tumor tissues (P<0.01). In
addition, following tissue staining, tumor samples were classified
into two groups: Weak staining group, which includes the negative
and weakly positive groups, and the strong staining group, which
includes the moderately and strongly positive groups. PPP2R2D
protein was highly expressed in TNM III and IV cancer tissues
[P<0.01 (χ2 test); Fig.
1B], thus indicating that the level of PPP2R2D may increase
gradually with GC progression.
The correlation between PPP2R2D expression and the
clinicopathological characteristics of 82 patients with available
follow-up data was analyzed and summarized in Table I. The data indicated that PPP2R2D
expression was positively correlated with disease stage (P=0.003),
T classification (P=0.009) and N classification (P=0.010). Notably,
PPP2R2D expression was negatively correlated with the OS rate of
patients (P<0.05; Fig. 1C),
which was in line with the results demonstrated in the Kaplan-Meier
survival curves (Fig. 1D).
Furthermore, the qPCR results confirmed the upregulation of PPP2R2D
in an additional 28 GC tissues obtained at the Shanghai East
Hospital as compared with that in the paired adjacent normal
tissue. In total, 17/28 (60.7%) paired GC tissues demonstrated
>1.5-fold increase in PPP2R2D expression (P<0.05; Fig. 1E). Taken together, these findings
suggested that PPP2R2D is upregulated in GC and strongly associated
with GC progression and prognosis.
 | Table ICorrelation of PPP2R2D expression with
clinicopathological features of gastric cancer patients (n=82) from
GC tissue microarray. |
Table I
Correlation of PPP2R2D expression with
clinicopathological features of gastric cancer patients (n=82) from
GC tissue microarray.
| Feature | No. of cases | Weak Staining
(−,+) | Strong staining
(++,+++) | P-value |
|---|
| Age (years) | | | | |
| >60 | 46 | 25 | 21 | 0.384 |
| ≤60 | 36 | 23 | 13 | |
| Gender | | | | |
| Male | 64 | 38 | 26 | 0.771 |
| Female | 18 | 10 | 8 | |
|
Differentiation | | | | |
|
Well-differentiated | 16 | 10 | 6 | 0.934 |
| Moderately
differentiated | 24 | 14 | 10 | |
| Poorly
differentiated | 42 | 24 | 18 | |
| T
classificationa | | | | |
| T1 and T2 | 16 | 14 | 2 | 0.009 |
| T3 and T4 | 66 | 34 | 32 | |
| N
classificationa | | | | |
| N0 | 22 | 18 | 4 | 0.010 |
| N1-3 | 60 | 30 | 30 | |
| M
classificationa | | | | |
| M0 | 80 | 48 | 32 | 0.180 |
| M1 | 2 | 0 | 2 | |
| Stage | | | | |
| I and II | 40 | 30 | 10 | 0.003 |
| III and IV | 42 | 18 | 24 | |
PPP2R2D promotes GC cell proliferation
and colony formation in vitro
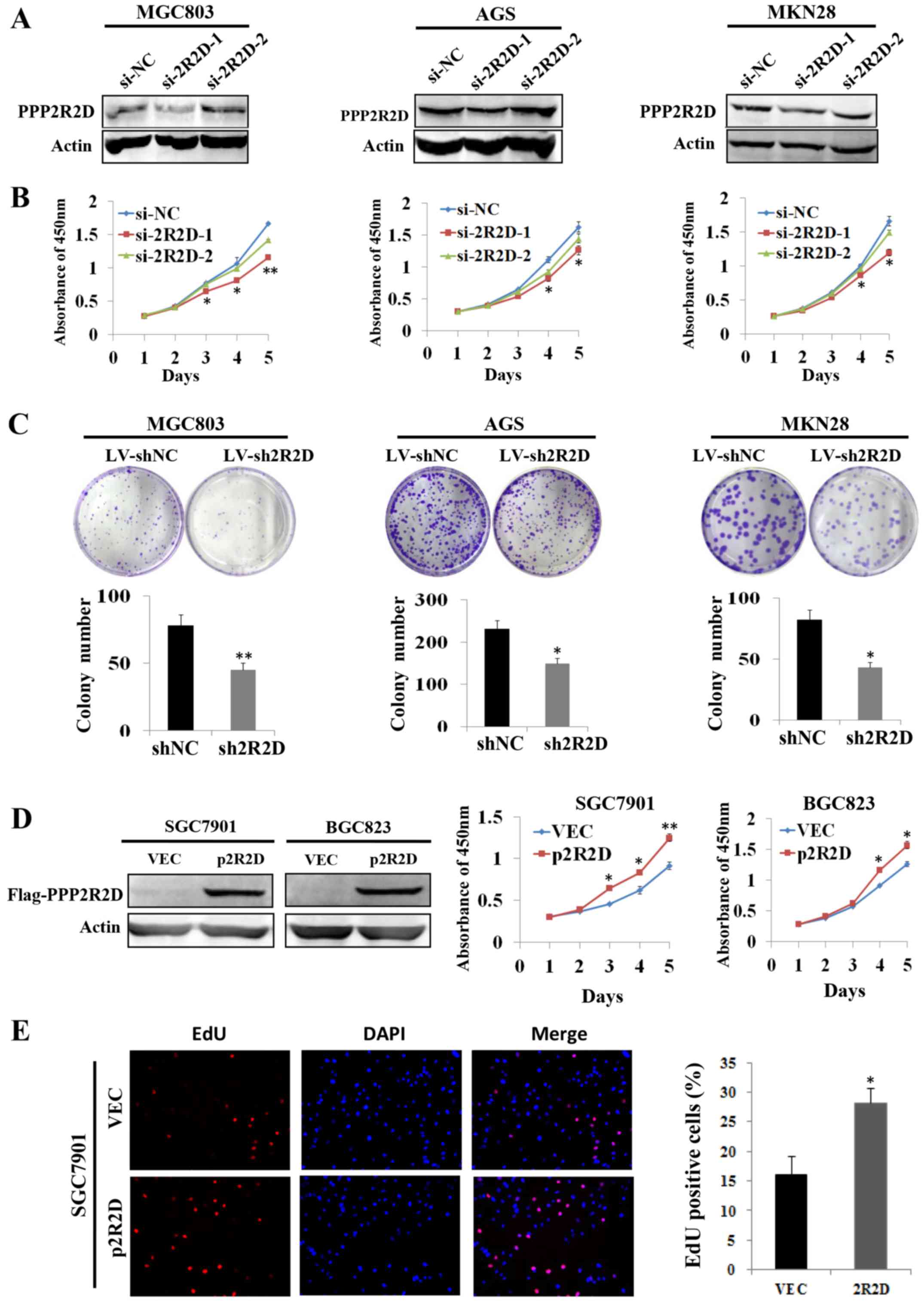
PPP2R2D protein expression level was examined in
several GC cell lines by western blot analysis in order to
determine which cell line was more appropriate for use in
overexpression or knockdown experiments. The results revealed that
PPP2R2D was highly expressed in MGC803, HGC27, AGS and MKN28 cells,
whereas it was expressed at relatively low levels in SGC7901and
BGC823cells (Fig. 1E).
PPP2R2D expression was knocked down in MGC803, AGS
and MKN28 cells by transient transfection with siRNAs against
PPP2R2D, and western blot analysis results indicated that si-2R2D-1
markedly decreased PPP2R2D expression compared with the si-NC group
(Fig. 2A). Subsequently, CCK-8
assays were conducted to determine the rate of cell growth. As
shown in Fig. 2B, knockdown of
PPP2R2D by si-2R2D-2 resulted in significantly reduced GC cell
growth compared with that in control cells. In addition, stable
cell lines were established by infecting with interference
lentivirus (LV-sh2R2D or control LV-shNC) in these cells. Colony
formation assays revealed that silencing PPP2R2D inhibited the
colony formation capacity of GC cells as indicated in Fig. 2C. Furthermore, PPP2R2D
overexpression was achieved by transiently transfecting a
FLAG-tagged PPP2R2D-expressing plasmid into SGC7901 and BGC823
cells (Fig. 2D). As expected, the
overexpression of PPP2R2D markedly promoted cell growth in the two
cell lines (Fig. 2D). Consistent
with these findings, the results of EdU incorporation assays
demonstrated that the percentage of EdU-positive cells increased
subsequent to the overexpression of PPP2R2D in SGC7901 cells
(Fig. 2E). The data presented in
these experiments suggested that PPP2R2D serves a critical role in
promoting cell proliferation and colony formation in GC.
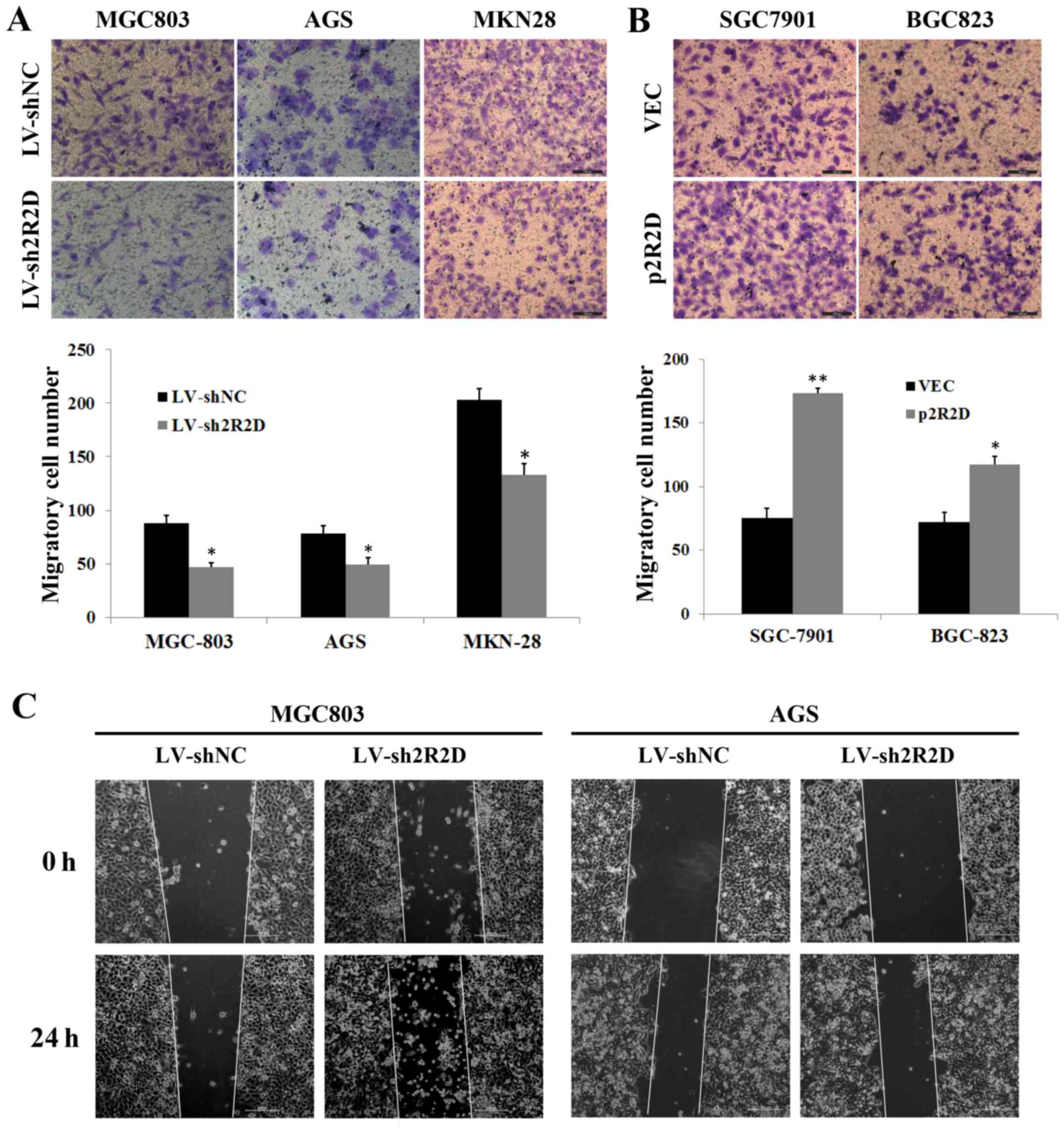
PPP2R2D enhances the migratory ability of
GC cells in vitro
Given that PPP2R2D expression was correlated with
the lymph node metastasis of GC cells (Table I), the study next determined the
impact of PPP2R2D knockdown or overexpression on GC cell migration
via Transwell chamber and wound healing assays. The data from the
Transwell assays indicated that silencing PPP2R2D significantly
inhibited the migratory ability of GC cells (Fig. 3A), whereas enforced expression of
PPP2R2D markedly facilitated the migratory capacity of GC cells
(Fig. 3B). Concurrently, PPP2R2D
knockdown strongly restricted the motility of MGC803 and AGS cells
towards the wound (Fig. 3C).
Therefore, these results indicated that PPP2R2D promotes GC cell
migration in vitro.
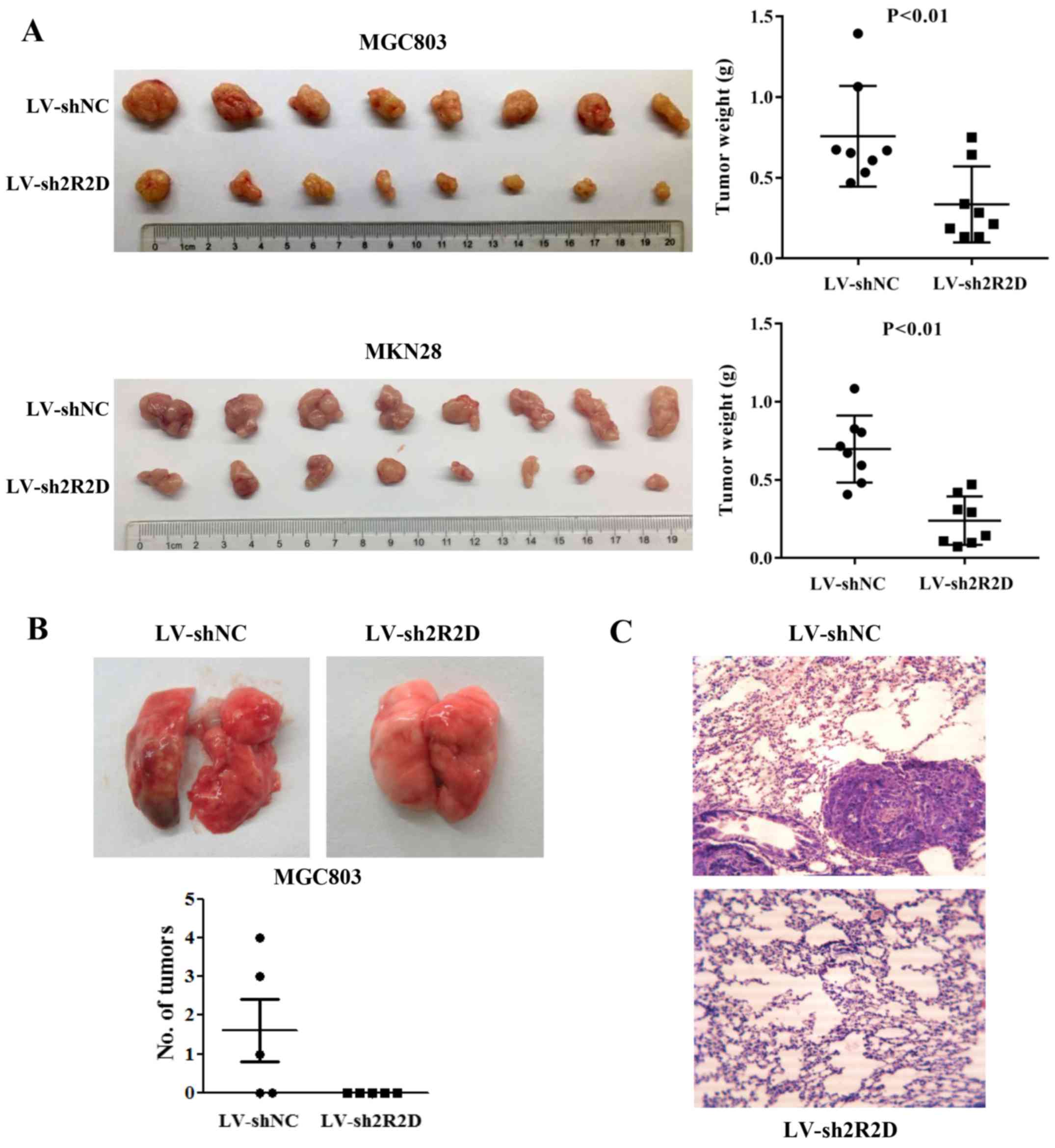
Silencing of PPP2R2D attenuates
tumorigenicity and metastasis of GC cells in vivo
The effect of PPP2R2D expression on GC cell
tumorigenicity was also evaluated in a nude mouse model. The stable
knockdown cells (MGC803/LV-sh2R2D and MKN28/LV-sh2R2D) and their
corresponding control cells were subcutaneously inoculated into
4-week-old male nude mice (n=8 per group). After 4 weeks, all mice
were euthanized and tumors were excised. The results demonstrated
that PPP2R2D knockdown significantly suppressed the tumorigenicity
of the two GC cells in vivo, and the size and weight of
tumors were lower compared with those of xenografts formed from
control-transfected cells (Fig.
4A). Furthermore, the pulmonary metastasis potential of cells
with PPP2R2D knockdown was elucidated by tail vein injection. As
expected, no visible metastatic foci were observed on lung surfaces
in all 5 mice injected with MGC803/LV-sh2R2D cells. By contrast, 3
out of 5 mice injected with control-transfected cells developed
metastatic tumors on the lung surface (Fig. 4B and C). These data further
confirmed the oncogenic role of PPP2R2D in animal models.
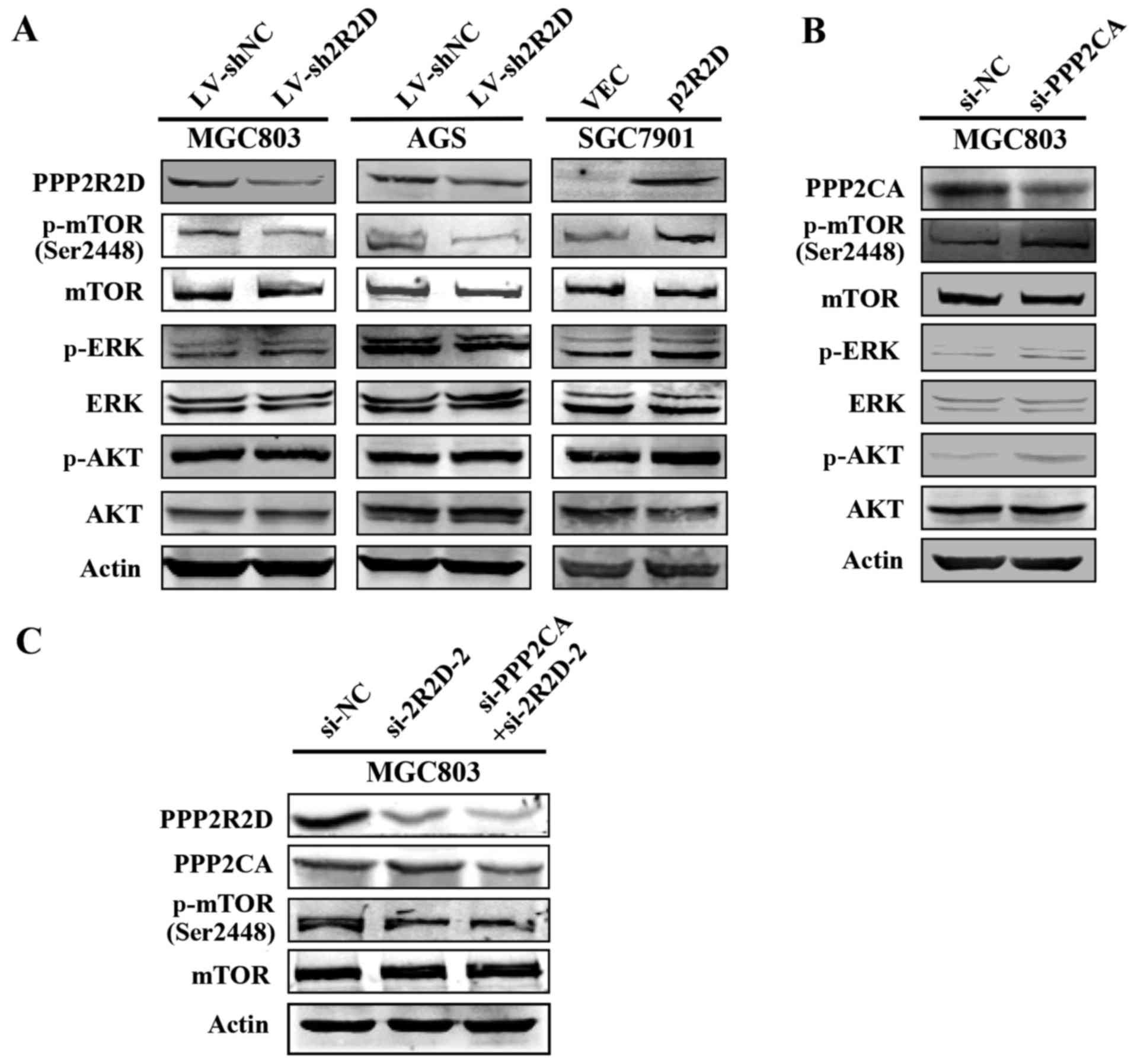
PPP2R2D influences mTOR phosphorylation
level
PP2A holoenzyme is known to serve an essential role
in the regulation of critical signaling pathways during
tumorigenesis (22,23). Since PPP2R2D is a regulatory
subunit of PP2A, the current study attempted to explore whether it
is involved in the PP2A-associated signaling pathways. Western blot
analysis results revealed that the phosphorylation level of mTOR
(p-mTOR) was evidently reduced following knockdown of PPP2R2D in
MGC803 and AGS cells, whereas no visible change occurred on the
level of p-Akt and p-Erk proteins (Fig. 5A). By contrast, overexpression of
PPP2R2D markedly increased the level of p-mTOR without altering the
abundance of total mTOR proteins in SGC7901 cells (Fig. 5A). Furthermore, the phosphorylation
levels of these three kinases were elevated when PPP2CA, the
catalytic subunit of PP2A, was knocked down in GC cells (Fig. 5B), which is in accordance with
previous findings (24). Notably,
knockdown of PPP2CA did not affect p-mTOR level in the PPP2R2D
silenced cells (Fig. 5C),
suggesting that the effect of PPP2R2D on p-mTOR level was
independent of PPP2CA. Taken together, these results suggested that
PPP2R2D contributes to GC progression putatively via mTOR
activation.
Discussion
PPP2R2D, also known as B55δ, is a crucial regulatory
subunit of PP2A. Previous studies have reported that PPP2R2D is
involved in the regulation of DNA repair (25), while PPP2R2D knockdown inhibited T
cell apoptosis and enhanced T cell proliferation, as well as
cytokine production in tumors (26). In addition, PPP2R2D has been
demonstrated to be associated with specific types of human cancer
(16,17). However, the expression and precise
function of PPP2R2D in GC have not yet been elucidated.
In the present study, compelling evidence was
provided demonstrating an oncogenic role of PPP2R2D in GC
progression and development for the first time, to the best of our
knowledge. At the cellular level, PPP2R2D promotes GC cell
proliferation, colony formation and cell migration. Furthermore,
PPP2R2D significantly facilitated the tumorigenicity and metastasis
in nude mice. Clinically, PPP2R2D expression was significantly
upregulated at the mRNA and protein levels in GC specimens.
Immunostaining results also indicated that PPP2R2D expression was
positively associated with GC disease stage, T classification and N
classification. Notably, a high expression of PPP2R2D was
correlated with poor prognosis of patients with GC. Thus, the
current study may provide a good indicator for GC progression and
prognosis. Furthermore, the clinical data obtained in the present
study were in agreement with the findings of the cellular and
animal experiments on the physiological function of PPP2R2D.
A number of studies have reported that B subunits
direct PP2A towards the regulation of signaling pathways that
control the cell cycle progression, cell proliferation and
apoptosis in eukaryotes (27–29).
mTOR is one of the critical kinases involved in the regulation of
numerous cellular events, including cell proliferation, protein
synthesis, migration, angiogenesis and metastasis in normal and
cancer cells (30–33). In addition, the PI3K/Akt/mTOR
signaling pathway is known to be regulated by phosphatases such as
PP2A (24,34). Nevertheless, a direct link between
PPP2R2D and mTOR in tumor development has yet to be established. In
the current study, the PPP2R2D-mediated phosphorylation of mTOR
protein at serine 2448 was described, which may be independent of
the PP2A catalytic subunit PPP2CA as determined by western blot
analysis. The current results also indicated that PPP2R2D is
endowed with tumor-promoting capacity via an increased level of
active mTOR. Notably, unlike PPP2CA, overexpression and knockdown
of PPP2R2D did not exert any significant effects on Akt and Erk
phosphorylation, implying that the involvement of PPP2R2D in mTOR
activation varies in the case of Akt or Erk activation. However,
how PPP2R2D regulates the mTOR phosphorylation remains unclear, and
its association with PPP2CA would also be worth exploring in future
studies.
In conclusion, the results of the cell biological
experiments and analysis of clinical data in the present study
consistently indicated that the expression of PPP2R2D serves
pivotal roles in GC progression and metastasis. In addition, mTOR
signaling may mediate the oncogenic roles of PPP2R2D. The current
observations also provided a new insight into the link between
regulatory subunits of PP2A and tumor development. Taken together,
PPP2R2D may serve as a new independent prognostic indicator and
therapeutic target for GC treatment.
Acknowledgments
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81772567, 81272917 and
81302064), and the Outstanding Leaders Training Program of Pudong
Health Bureau of Shanghai (no. PWRl2013-02).
Availability of data and materials
The data and materials used and/or analyzed during
the current study are available from the corresponding author on
reasonable request.
Authors’ contributions
YL and YG conceived and designed the study. SY, LL
and QW acquired, analyzed and interpreted the data. SY and YL wrote
and reviewed the manuscript. ND cultured all of the cell lines, and
performed cell transfection and lentiviral infection experiments.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Ethics
Committee of Shanghai East Hospital, Tongji University School of
Medicine (Shanghai, China). Informed consent was obtained from all
patients prior to the sample collection.
Consent for publication
The patient, or parent, guardian or next of kin
provided written informed consent for the publication of any
associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hunter T: Protein kinases and
phosphatases: The yin and yang of protein phosphorylation and
signaling. Cell. 80:225–236. 1995. View Article : Google Scholar
|
|
2
|
Janssens V and Goris J: Protein
phosphatase 2A: A highly regulated family of serine/threonine
phosphatases implicated in cell growth and signalling. Biochem J.
353:417–439. 2001. View Article : Google Scholar
|
|
3
|
Lechward K, Awotunde OS, Swiatek W and
Muszyńska G: Protein phosphatase 2A: Variety of forms and diversity
of functions. Acta Biochim Pol. 48:921–933. 2001.
|
|
4
|
Virshup DM: Protein phosphatase 2A: A
panoply of enzymes. Curr Opin Cell Biol. 12:180–185. 2000.
View Article : Google Scholar
|
|
5
|
Sontag E: Protein phosphatase 2A: The
Trojan Horse of cellular signaling. Cell Signal. 13:7–16. 2001.
View Article : Google Scholar
|
|
6
|
Seshacharyulu P, Pandey P, Datta K and
Batra SK: Phosphatase: PP2A structural importance, regulation and
its aberrant expression in cancer. Cancer Lett. 335:9–18. 2013.
View Article : Google Scholar
|
|
7
|
Strack S, Chang D, Zaucha JA, Colbran RJ
and Wadzinski BE: Cloning and characterization of B delta, a novel
regulatory subunit of protein phosphatase 2A. FEBS Lett.
460:462–466. 1999. View Article : Google Scholar
|
|
8
|
Mayer RE, Hendrix P, Cron P, Matthies R,
Stone SR, Goris J, Merlevede W, Hofsteenge J and Hemmings BA:
Structure of the 55-kDa regulatory subunit of protein phosphatase
2A: Evidence for a neuronal-specific isoform. Biochemistry.
30:3589–3597. 1991. View Article : Google Scholar
|
|
9
|
Zolnierowicz S, Csortos C, Bondor J, Verin
A, Mumby MC and DePaoli-Roach AA: Diversity in the regulatory
B-subunits of protein phosphatase 2A: Identification of a novel
isoform highly expressed in brain. Biochemistry. 33:11858–11867.
1994. View Article : Google Scholar
|
|
10
|
Janssens V, Goris J and Van Hoof C: PP2A:
The expected tumor suppressor. Curr Opin Genet Dev. 15:34–41. 2005.
View Article : Google Scholar
|
|
11
|
Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y,
Xu Z and Han X: Cantharidin, a potent and selective PP2A inhibitor,
induces an oxidative stress-independent growth inhibition of
pancreatic cancer cells through G2/M cell-cycle arrest and
apoptosis. Cancer Sci. 101:1226–1233. 2010. View Article : Google Scholar
|
|
12
|
Schweyer S, Bachem A, Bremmer F,
Steinfelder HJ, Soruri A, Wagner W, Pottek T, Thelen P, Hopker WW,
Radzun HJ, et al: Expression and function of protein phosphatase
PP2A in malignant testicular germ cell tumours. J Pathol.
213:72–81. 2007. View Article : Google Scholar
|
|
13
|
Duong FH, Dill MT, Matter MS, Makowska Z,
Calabrese D, Dietsche T, Ketterer S, Terracciano L and Heim MH:
Protein phosphatase 2A promotes hepatocellular carcinogenesis in
the diethylnitrosamine mouse model through inhibition of p53.
Carcinogenesis. 35:114–122. 2014. View Article : Google Scholar
|
|
14
|
Lu J, Kovach JS, Johnson F, Chiang J,
Hodes R, Lonser R and Zhuang Z: Inhibition of serine/threonine
phosphatase PP2A enhances cancer chemotherapy by blocking DNA
damage induced defense mechanisms. Proc Natl Acad Sci USA.
106:11697–11702. 2009. View Article : Google Scholar
|
|
15
|
Boudreau RT, Conrad DM and Hoskin DW:
Apoptosis induced by protein phosphatase 2A (PP2A) inhibition in T
leukemia cells is negatively regulated by PP2A-associated p38
mitogen-activated protein kinase. Cell Signal. 19:139–151. 2007.
View Article : Google Scholar
|
|
16
|
Zhuang Q, Zhou T, He C, Zhang S, Qiu Y,
Luo B, Zhao R, Liu H, Lin Y and Lin Z: Protein phosphatase 2A-B55δ
enhances chemotherapy sensitivity of human hepatocellular carcinoma
under the regulation of microRNA-133b. J Exp Clin Cancer Res.
35:672016. View Article : Google Scholar
|
|
17
|
Cunningham CE, Li S, Vizeacoumar FS,
Bhanumathy KK, Lee JS, Parameswaran S, Furber L, Abuhussein O, Paul
JM, McDonald M, et al: Therapeutic relevance of the protein
phosphatase 2A in cancer. Oncotarget. 7:61544–61561. 2016.
View Article : Google Scholar
|
|
18
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
19
|
Edge SB; American Joint Committee on
Cancer: AJCC Cancer Staging Manual. Springer; New York: 2010
|
|
20
|
Capes-Davis A, Theodosopoulos G, Atkin I,
Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA,
Reddel RR, et al: Check your cultures! A list of cross-contaminated
or misidentified cell lines. Int J Cancer. 127:1–8. 2010.
View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Rodgers JT, Vogel RO and Puigserver P:
Clk2 and B56β mediate insulin-regulated assembly of the PP2A
phosphatase holoenzyme complex on Akt. Mol Cell. 41:471–479. 2011.
View Article : Google Scholar
|
|
23
|
Zeng Q, Zhang H, Qin J, Xu Z, Gui L, Liu
B, Liu C, Xu C, Liu W, Zhang S, et al: Rapamycin inhibits
BAFF-stimulated cell proliferation and survival by suppressing
mTOR-mediated PP2A-Erk1/2 signaling pathway in normal and
neoplastic B-lymphoid cells. Cell Mol Life Sci. 72:4867–4884. 2015.
View Article : Google Scholar
|
|
24
|
Sablina AA, Hector M, Colpaert N and Hahn
WC: Identification of PP2A complexes and pathways involved in cell
transformation. Cancer Res. 70:10474–10484. 2010. View Article : Google Scholar
|
|
25
|
Kalev P, Simicek M, Vazquez I, Munck S,
Chen L, Soin T, Danda N, Chen W and Sablina A: Loss of PPP2R2A
inhibits homologous recombination DNA repair and predicts tumor
sensitivity to PARP inhibition. Cancer Res. 72:6414–6424. 2012.
View Article : Google Scholar
|
|
26
|
Zhou P, Shaffer DR, Alvarez Arias DA,
Nakazaki Y, Pos W, Torres AJ, Cremasco V, Dougan SK, Cowley GS,
Elpek K, et al: In vivo discovery of immunotherapy targets in the
tumour microenvironment. Nature. 506:52–57. 2014. View Article : Google Scholar
|
|
27
|
Eichhorn PJ, Creyghton MP and Bernards R:
Protein phosphatase 2A regulatory subunits and cancer. Biochim
Biophys Acta. 1795:1–15. 2009.
|
|
28
|
Letourneux C, Rocher G and Porteu F:
B56-containing PP2A dephosphorylate ERK and their activity is
controlled by the early gene IEX-1 and ERK. EMBO J. 25:727–738.
2006. View Article : Google Scholar
|
|
29
|
Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY
and Chiang CW: Regulation of phosphorylation of Thr-308 of Akt,
cell proliferation, and survival by the B55alpha regulatory subunit
targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol
Chem. 283:1882–1892. 2008. View Article : Google Scholar
|
|
30
|
Wullschleger S, Loewith R and Hall MN: TOR
signaling in growth and metabolism. Cell. 124:471–484. 2006.
View Article : Google Scholar
|
|
31
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar
|
|
32
|
Chen L, Xu B, Liu L, Liu C, Luo Y, Chen X,
Barzegar M, Chung J and Huang S: Both mTORC1 and mTORC2 are
involved in the regulation of cell adhesion. Oncotarget.
6:7136–7150. 2015.
|
|
33
|
Bai H, Li H, Li W, Gui T, Yang J, Cao D
and Shen K: The PI3K/AKT/mTOR pathway is a potential predictor of
distinct invasive and migratory capacities in human ovarian cancer
cell lines. Oncotarget. 6:25520–25532. 2015. View Article : Google Scholar
|
|
34
|
Zhang Q and Claret FX: Phosphatases: The
new brakes for cancer development? Enzyme Res. 2012:6596492012.
View Article : Google Scholar
|