Introduction
Neuroblastoma (NB) arising from neural crest cells
within the sympathetic nervous system is the most common
extracranial solid malignant tumor in childhood (1). This tumor generally occurs in young
children, with a median age of 17 months at diagnosis, and it
accounts for 15% of all pediatric oncological deaths (1,2). NB
exhibits marked heterogeneity as regards biological characteristics
and clinical features. For example, NBs occurring in patients
younger than 12 months of age usually regress or mature into a
benign ganglioneuroma spontaneously, while the majority of cases
are associated with an aggressive phenotype and a poor prognosis
when they occur in patients at 18 months or older. Although marked
improvements have been made for patients with lower-grade NBs, the
5-year survival rate of patients with high-risk NB remains <40%
(1).
A number of genetic aberrations in NBs, such as
aneuploidy, the amplification of oncogenes or allelic loss and
mutations, have been reported to be associated with clinical
outcome (3). Among these, the
amplification of the proto-oncogene MYCN is one of the few
predictive markers of a poor prognosis (4). NBs with MYCN amplification exhibit an
aggressive phenotype and resistance to chemotherapy, and patients
with NB harboring the amplification are classified as a high-risk
group. In addition to the MYCN aberration, the gain of chromosome
17q (5) and the deletion of
chromosome 1p or 11q (6) have also
been shown to be associated with a poor prognosis of patients with
NB. Gain-of-function mutations in the anaplastic lymphoma kinase
(ALK) gene have been observed in most cases of familial NB and in
some sporadic NB cases (7).
Nevertheless, the above-mentioned genomic abnormalities are lacking
in a significant number of malignant NBs.
Neuropilin 1 (NRP1) is a transmembrane glycoprotein
known to function as a co-receptor for many types of ligand,
including semaphorin 3A and 4A (SEMA3A, SEMA4A) (3,8) and
vascular endothelial growth factor (VEGF) (9). As NRP1 has the ability to modulate
the activity of a number of extracellular ligands, it is involved
in a wide range of physiological and pathological processes.
Numerous studies have demonstrated that NRP1 is
frequently overexpressed in a variety of tumors, such as leukemia
(10), gastric cancer (11), hepatocellular carcinoma (12) and osteosar-coma (13). In addition, an elevated NRP1
expression is generally associated with a poor prognosis in many
types of tumor (12–15). For example, a high NRP1 expression
level has been reported to be associated with an advanced stage and
lymph node invasion in pancreatic cancer (14).
The ability of NRP1 to enhance VEGF receptor 2
activity in response to VEGF-A suggests that one of the most
important roles of NRP1 in tumor development is its role in
angiogenesis (16). NRP1 has been
found to be expressed in blood vessels in >98% of carcinomas
derived from the breast, colon and lung (17). Based on these observations, NRP1
has been identified as a potential target for anti-angiogenic
therapies. In addition to tumor vessels, NRP1 is known to be
expressed in a variety of cancer tissues (17), and recent studies have indicated
that NRP1 regulates tumor cell functions in an
angiogenesis-independent manner. A previous study using an
esophageal cancer cell line indicated that NRP1 activates cell
proliferation by inducing p65 transcription via CREB activation
(15). It has also been reported
that the knockdown of NRP1 in gastric cancer cells results in cell
cycle arrest caused by p27 upregulation, and in a reduced cell
migratory ability via the inhibition of focal adhesion kinase
phosphorylation (11).
However, the role of NRP1 in NB has not yet been
elucidated. Although it has been reported that NRP1 is expressed at
higher levels in NB tissues compared to normal adrenal tissues, it
has also been reported that the expression levels of NRP1 are
higher in early-stage than in late-stage NB (18). Consistent with this observation, in
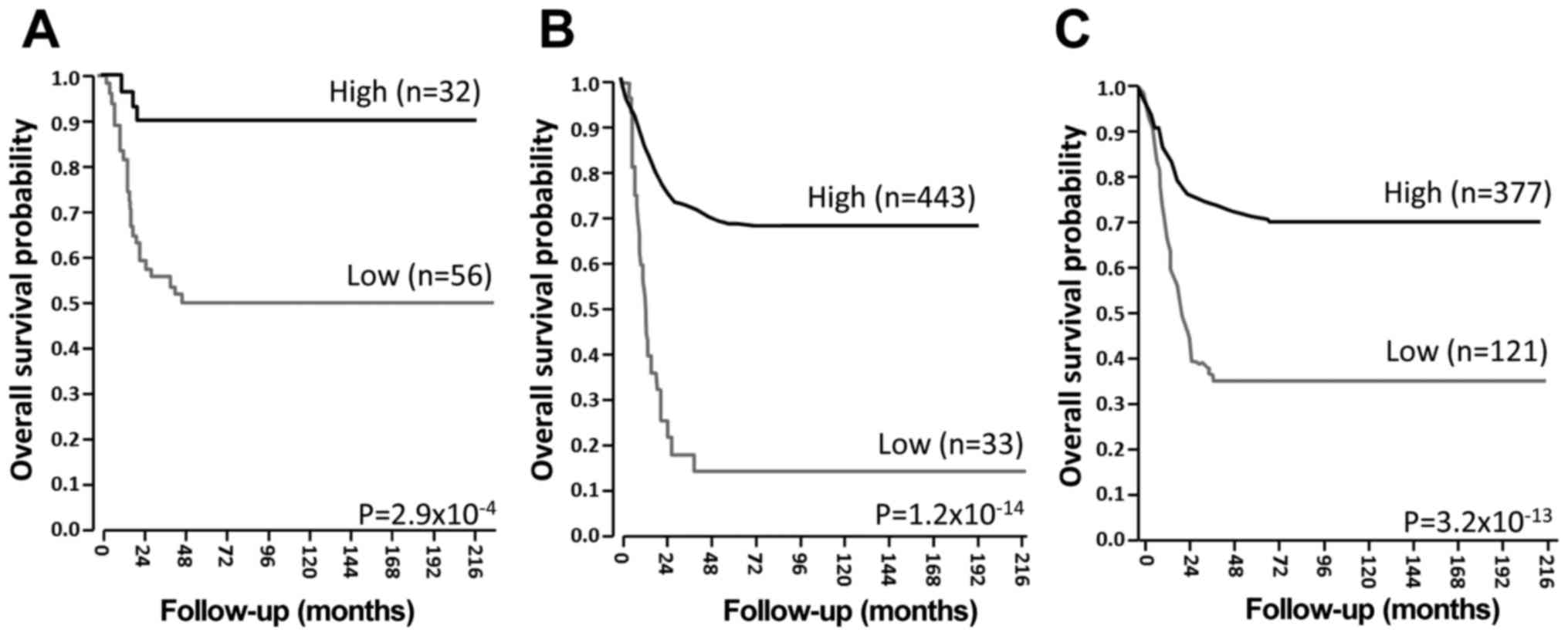
this study, the investigation of public datasets of global gene
expression analysis obtained from the R2 platform (http://r2.amc.nl), indicated that a higher level of
NRP1 expression was closely associated with a longer survival
period of patients with NB (Fig.
1). These results suggest that NRP1 may function to suppress
the malignant progression of NB.
In the present study, we performed a functional
analysis of NRP1 to determine its role in the development and/or
progression of NB, and to elucidate the molecular mechanisms
underlying its functions.
Materials and methods
Cell lines and culture conditions
The following human NB-derived cell lines were used
in this study: The SK-N-SH (HTB-11), SK-N-AS (CRL-2137) and SH-SY5Y
(CRL-2266) cells were obtained from the American Type Culture
Collection (ATCC, Manassas, VA, USA), NB69 cells (RCB0480) were
from Riken Cell Bank (Ibaraki, Japan) and Kelly cells
(EC92110411-F0) were from DS Pharma Biomedical (Osaka, Japan). The
Kelly and NB69 cells were cultured in RPMI-1640 medium (Nacalai
Tesque, Kyoto, Japan) supplemented with heat-inactivated fetal
bovine serum (FBS; Nichirei Bioscience, Tokyo, Japan) at a final
concentration of 10% (Kelly) or 15% (NB69). The SK-N-SH and SH-SY5Y
cells were cultured in MEM supplemented with 10% FBS, 0.1 mM
non-essential amino acids (Thermo Fisher Scientific, Waltham, MA,
USA) and 5 mM sodium pyruvate (Thermo Fisher Scientific). The
SK-N-AS cells were cultured in DMEM (Nacalai Tesque) supplemented
with 10% FBS. All the media contained 100 IU/ml of penicillin (Life
Technologies, Carlsbad, CA, USA) and 100 µl/ml of streptomycin
(Life Technologies). The cells were maintained at 37°C in a
CO2 incubator with a controlled humidified atmosphere
composed of 95% air and 5% CO2.
Analysis of cell viability
The SK-N-AS cells were seeded in 96-well plates at a
density of 1×104 cells per well, and immediately
transfected with control siRNA, NRP1 siRNA, or with β1 integrin
siRNA (Thermo Fisher Scientific) using Lipofectamine 3000 (Thermo
Fisher Scientific) according to the manufacturer's instructions.
The target sequence of the NRP1 siRNA was 5′-AGCAAAAGAAGGTTT-3′ and
that of the β1 integrin siRNA was 5′-CCGTAGCAAAGGAACAGCA-3′. As a
control siRNA, Silencer Select Negative Control #1, whose target
sequence information is not available, was used. Cell viability was
measured by WST8 assay using Cell Count Reagent CF (Nacalai Tesque)
immediately after the cells were attached to the plate bottom, or
at 24, 48 and 72 h following transfection.
Matrigel invasion assay
The SK-N-AS cells were seeded in dishes 6 cm in
diameter at a density of 5×105 cells per dish, and
immediately transfected with control siRNA, NRP1 siRNA, or β1
integrin siRNA, as described above. At 24 h after seeding, the
cells were removed from the plate using trypsin, and seeded into
cell culture inserts (Falcon, Durham, NC, USA) coated with human
fibronectin (Sigma-Aldrich, St. Louis, MO, USA) at a density of
7.5×104 cells/300 µl, and the inserts were placed in
24-well plates for cell culture inserts (Falcon) filled with 700 µl
of medium/well. After 48 h, the non-invading cells on the upper
surface of the membrane were removed, and the invading cells were
fixed with methanol and stained with Giemsa (Muto Pure Medicals,
Tokyo, Japan) for 1 min at room temperature, followed by washing
with PBS. The number of invading cells in 5 microscopic fields was
counted for each membrane under a light microscope at ×200
magnification. All of the analyses were performed in
triplicate.
Wound-healing cell migration assay
The SK-N-AS cells were seeded in 24-well plates at a
density of 2×105 cells per well, and immediately
transfected with control siRNA, NRP1 siRNA, or β1 integrin siRNA,
as described above. At 48 h following transfection, cell layers
were wounded using a Cell Scratcher Scratch stick (AGC Techno
Glass, Shizuoka, Japan) and the medium was replaced with fresh
medium. After 48 h, the cells were photographed by phase-contrast
microscope Leica DM IL (Leica, Wetzlar, Germany), and the widths of
the wounded areas were measured at 3 places in each sample. All of
the analyses were performed in triplicate.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells using RNeasy
mini kits (Qiagen, Valencia, CA, USA) according to the
manufacturer's instructions. For cDNA synthesis, 500 ng of total
RNA was reverse transcribed using an iScript cDNA synthesis system
(Bio-Rad Laboratories, Hercules, CA, USA). qPCR was performed using
a SYBR Premix Ex Taq™ system according to the manufacturer's
recommendations (Takara, Shiga, Japan). A mixture of cDNA derived
from total RNA of SK-N-AS cells was used as a reference.
Subsequently, a dilution series of the cDNA mixture was prepared
and used in qPCR as the templates to obtain a standard curve for
each gene, and then the expression levels of each genes were
estimated by extrapolation from a standard curves. Three
independent measurements were taken. The primer sets used for
qPCR-based amplification were as follows: NRP1 sense,
5′-ATGCGAATGGCTGATTCAGG-3′ and antisense,
5′-TCCATCGAAGACTTCCACGTAG-3′; β1 integrin sense,
5′-CATCCCTGAAAGTCCCAAGTG-3′ and antisense,
5′-TACCAACACGCCCTTCATTG-3′; and GAPDH sense,
5′-TCACCAGGGCTGCTTTTAAC-3′ and antisense,
5′-TGACGGTGCCATGGAATTTG-3′. The housekeeping gene GAPDH was used as
an internal reference. All of the PCR reactions were carried out
with an initial denaturation for 2 min at 94°C followed by 40
cycles of 94°C for 5 sec and 60°C for 30 sec using Thermal Cycler
Dice TP800 (Takara).
Immunoblotting
The cells were lysed in RIPA buffer containing
protease inhibitor cocktail (Nacalai Tesque) and phosphatase
inhibitor cocktail (Nacalai Tesque). Following the sonication of
the lysates, protein concentration was measured using Bio-Rad DC
kits (Bio-Rad Laboratories). Cell lysates (20 µg of protein) were
separated by 4–12% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and then electroblotted onto Immobilon-P membranes
(Millipore, Billerica, MA, USA) by the wet transfer method. The
membranes were then blocked with Blocking-one (Nacalai Tesque)
overnight at 4°C, and incubated with rabbit monoclonal antibodies
(Cell Signaling Technology, Beverly, MA, USA) to NRP1 (D62C6;
3725P), vimentin (D21H3; 5741P), N-cadherin (D4R1H; 13116P),
E-cadherin (24E10; 3195P), matrix metalloproteinase (MMP)2 (D8N9Y;
13132S), MMP9 (D603H; 13667S), β1 integrin (D2E5; 9699S), focal
adhesion kinase (FAK) (D2R2E; 13009S), FAK phosphorylated at Y397
(D20B1; 8556S), PI3K (p85) (19H8; 4257P), or with rabbit polyclonal
antibody to phosphorylated PI3K (p85; 4228P) (all from Cell
Signaling Technology), or with rabbit polyclonal antibody to GAPDH
(ab9485, Abcam, Cambridge, UK) at 4°C. All the antibodies were
diluted 500-fold for the reaction. After 24 h of incubation, the
membranes were washed with Tris-buffered saline containing 0.1%
Tween-20 (TBS-T), followed by incubation with 2,000-fold diluted
horseradish peroxidase-conjugated secondary antibody for rabbit IgG
(NA934-1ML, GE Healthcare Life Sciences, Buckinghamshire, UK), for
1 h at room temperature. The membranes were then washed extensively
with TBS-T, and treated with Chemi-Lumi-One Super (Nacalai Tesque)
to visualize immunoreactive signals using LAS4000 (Fujifilm, Tokyo,
Japan).
Analysis of the amount of filamentous
actin (F-actin) and globular actin (G-actin)
The SK-N-AS cells were seeded in dishes 10 cm in
diameter at a density of 1×106 cells per dish, and
immediately transfected with control siRNA or with NRP1 siRNA, as
described above. At 48 h after seeding, cell lysates were collected
by using the G-actin/F-actin In Vivo Assay kit (Cytoskeleton,
Denver, CO, USA), and G-actin and F-actin were separated by using
an ultracentrifuge according to the manufacturer's instructions. In
brief, cell lysates in lysis and F-actin stabilization buffer were
centrifuged at 100,000 × g, 37°C for 1 h, and precipitated F-actin
was dissolved in F-actin depolymerizaion buffer. As a positive
control, phalloidin, which can drive actin polymerization, was
added to the cell lysate. The amount of G-actin and F-actin was
analyzed by immunoblotting.
Statistical analysis
Statistical analyses were performed using the
Student's t-test. Data are presented as the means ± SD from at
least 3 independent experiments. In all analyses, a value of
P<0.05 was considered to indicate a statistically significant
difference. To generate survival curves for the overall survival of
patients with NB, 3 independent microarray datasets, GSE16476,
GSE45547 and GSE49710, were obtained from the R2 platform
(http://r2.amc.nl). Using this platform, survival
curves were calculated according to the Kaplan-Meier method, and
analyzed by the log-rank test followed by adjustment with
Bonferroni's test.
Results
A lower NRP1 expression level is closely
associated with the poor prognosis of patients with NB
To examine the clinical significance of NRP1 in the
development and/or progression of NB, Kaplan-Meier survival
analysis was performed utilizing public microarray datasets. As
shown in Fig. 1, a lower NRP1
expression level was significantly associated with a shorter
survival period of patients with NB. The results were confirmed in
all 3 independent datasets, suggesting that NRP1 may have a
suppressive effect on the malignant progression of NB.
The siRNA-mediated knockdown of NRP1
enhances the invasive and migratory ability of NB cells
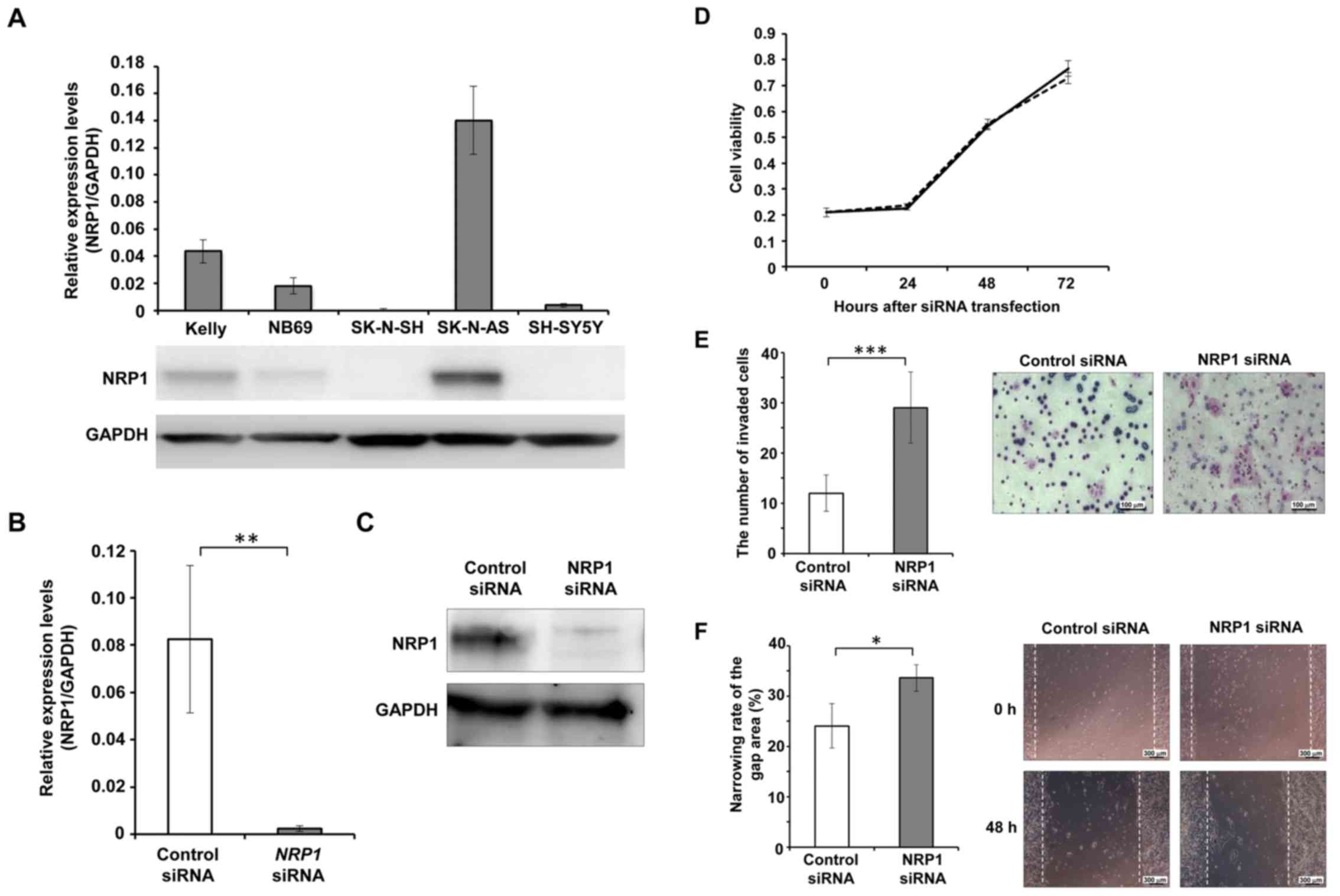
To clarify its function in NB cells, the expression
levels of NRP1 in NB-derived cell lines were analyzed. Among the 5
cell lines examined, the SK-N-AS cells exhibited he highest
expression of NRP1 at both the mRNA and protein level (Fig. 2A). Thus, we then performed the
siRNA-mediated knockdown of NRP1 in the SK-N-AS cells (Fig. 2B and C), and observed no
significant differences in cell viability between the cells in
which NRP1 was knocked down and the control cells at 24, 48 and 72
h following transfection (Fig.
2D).
Matrigel invasion assay was also performed to
evaluate the effects of NRP1 depletion on cell invasive ability.
The invasive ability of the cells in which NRP1 was knocked down
was significantly higher than that of the control cells. At 24 h
after cell seeding in the invasion chamber, the cells in which NRP1
was knocked down exhibited significantly greater numbers of
invading cells compared to the controls (Fig. 2E).
Wound-healing assay was performed to evaluate the
effects of NRP1 knockdown on cell migratory ability. At 48 h after
scratching the cell layer, the wound closure ratio of the cells in
which NRP1 was knocked down was significantly greater than that in
the control cells (Fig. 2F).
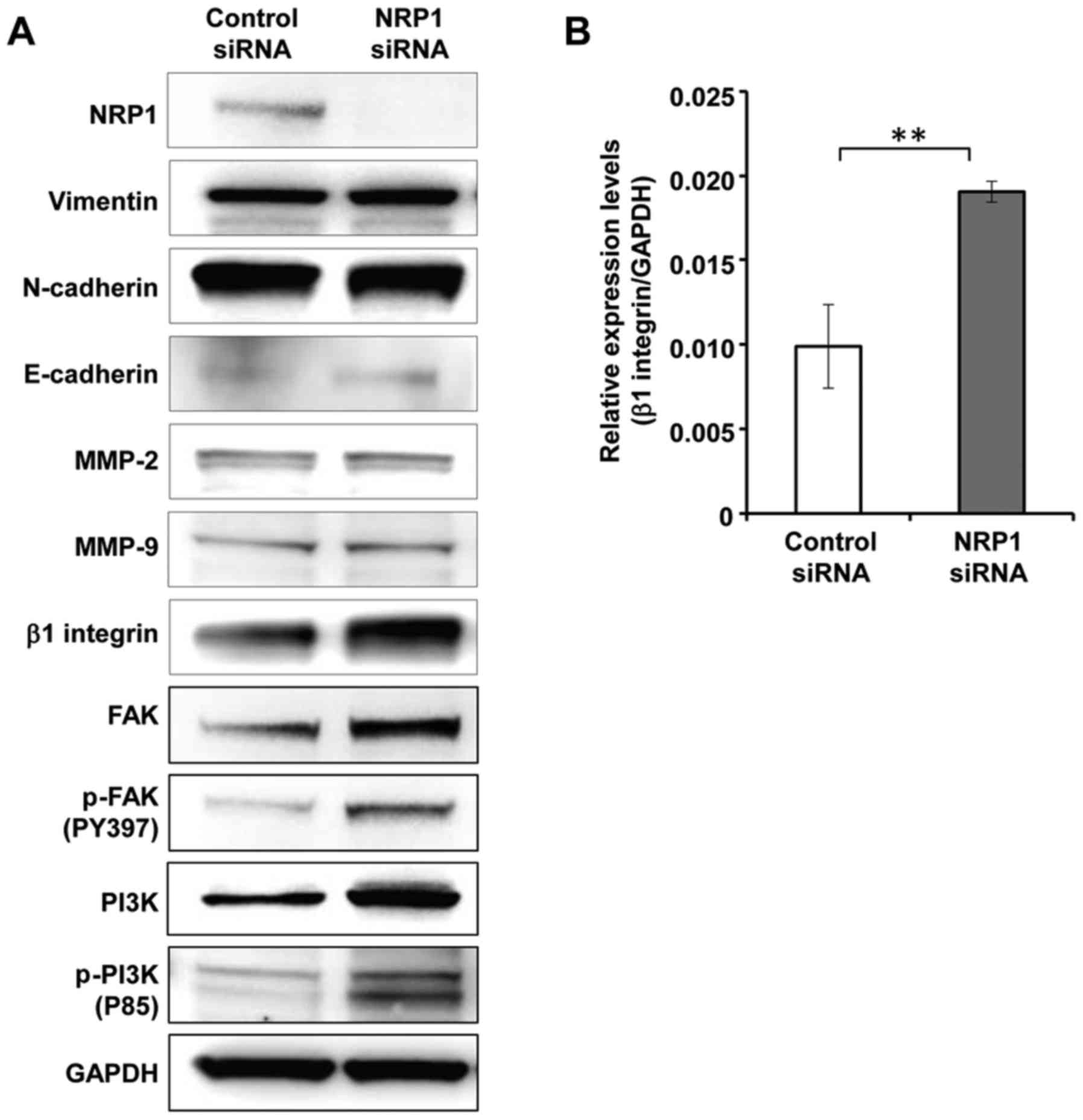
Expression of β1 integrin is upregulated
in cells in which NRP1 is knocked down
To elucidate the mechanisms underlying the
regulation of cell invasion and migration by NRP1, we examine the
expression levels of molecules that can affect the invasive
capacity and motility of the cells. First, we analyzed the proteins
involved in epithelial-mesenchymal transition (EMT), which is a
significant process for cancer cells to gain migratory and invasive
abilities. However, the expression levels of EMT-related proteins,
such as vimentin, N-cadherin and E-cadherin, were not altered by
NRP1 knockdown (Fig. 3A). We then
analyzed the expression levels of MMPs, which are required for
cells to digest extracellular matrix proteins. Again, no clear
differences were observed in the amounts of MMP2 or MMP9 between
the cells in which NRP1 was knocked down and the control cells
(Fig. 3A). In contrast, β1
integrin was strongly induced in NRP1 knockdown cells compared to
control cells (Fig. 3A). To
determine whether β1 integrin expression is regulated by NRP1 at
the transcriptional level, the mRNA expression level of β1 integrin
was measured by RT-qPCR. As shown in Fig. 3B, the mRNA expression level of β1
integrin was significantly higher in the cells in which NRP1 was
knocked down than in the control cells.
We also found that the levels of downstream
molecules of β1 integrin, such as FAK and PI3K, were increased and
these molecules were activated in the cells in which NRP1 was
knocked down (Fig. 3A). The
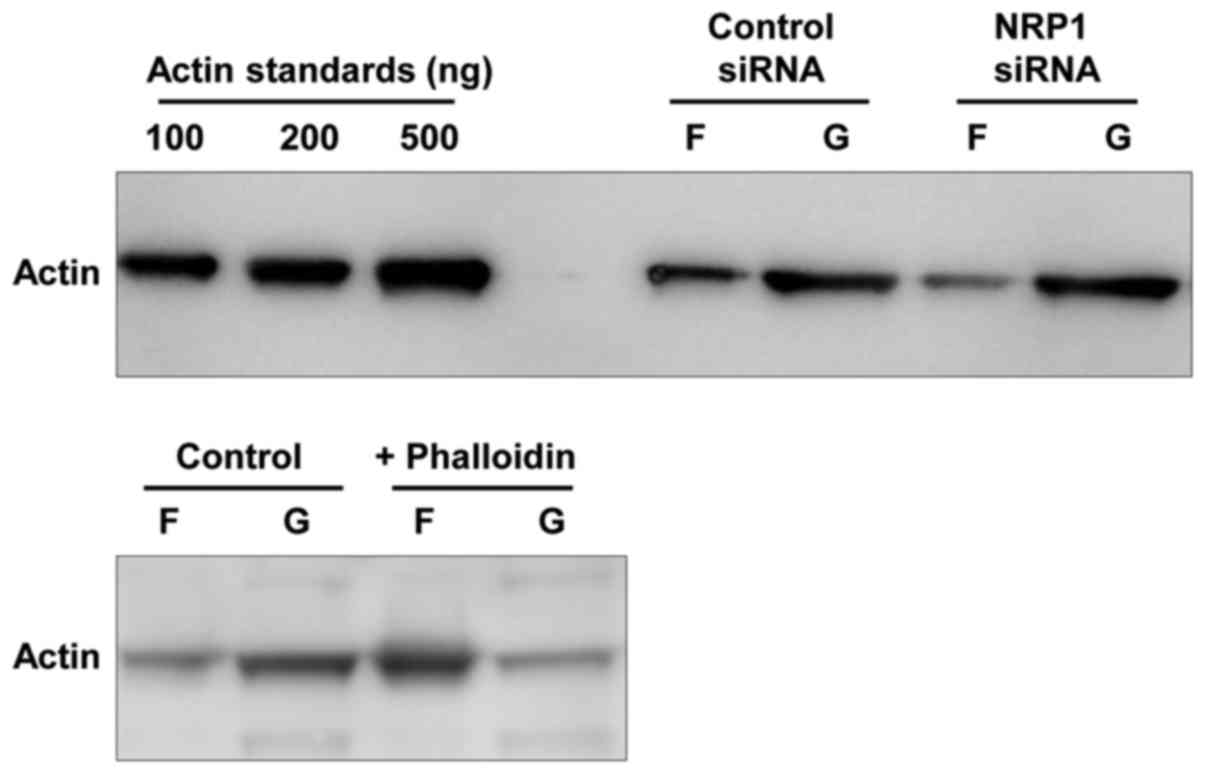
analysis of the amounts of F-actin and G-actin demonstrated that
the cells in which NRP1 was knocked down exhibited a markedly
reduced F-actin formation compared to the control cells (Fig. 4). These results indicated that the
induction of β1 integrin mediated by NRP1 knockdown resulted in the
alteration of actin fiber organization via the activation of its
downstream signal.
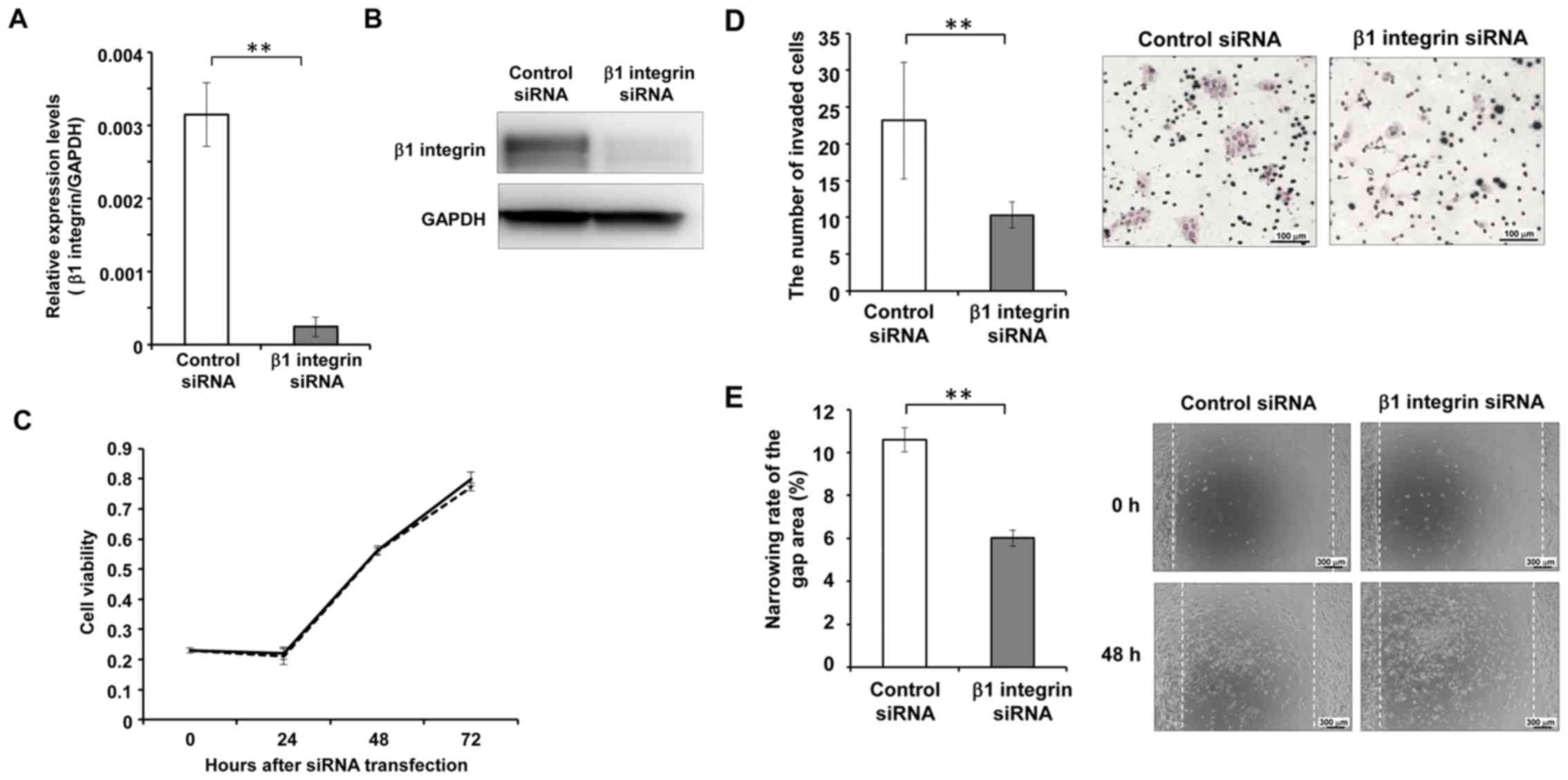
The siRNA-mediated knockdown of β1
integrin suppresses the invasive and migratory abilities of NB
cells
To confirm that the integrin signal mediates the
effects of NRP1 knockdown, we examined the phenotype of SK-N-AS
cells after the silencing of β1 integrin (Fig. 5A and B). No obvious differences
were observed in cell viability between the cells in which β1
integrin was knocked down and the control cells at any time-point
after siRNA transfection (Fig.
5C). On the other hand, the cells in which β1 integrin was
knocked down exhibited a significantly decreased invasive ability
compared to the control cells 24 h after cell seeding in the
invasion chamber (Fig. 5D). The
cell migratory ability was also shown to be suppressed in the cells
in which β1 integrin was knocked down. The wound closure ratio of
the cells in which β1 integrin was knocked down was significantly
lower than that of the control cells at 48 h after wounding the
cell layer (Fig. 5E).
Discussion
In the present study, we analyzed public datasets
and demonstrated that a decreased expression of NRP1 was associated
with the shorter survival length of NB. The NB cells in which NRP1
was knocked down exhibited markedly enhanced invasive and migratory
abilities, suggesting that NRP1 may have a tumor suppressive
function in NB.
Neuropilin, consisting of NRP1 and its homolog NRP2,
is a transmembrane protein that functions as a co-receptor for
several ligands, such as VEGF, semaphorin and transforming growth
factor (TGF)-β1, and enhances their signals (19). Under physiological conditions, NRPs
are known to play significant roles in angiogenesis, the
development of the nervous system and immunity. Almost all previous
reports on malignancies have demonstrated that NRP1 is highly
expressed in cancer tissues compared to normal tissues, and/or
patients with a higher expression of NRP1 in cancer tissues exhibit
a shorter survival period than those with lower NRP1 expression
levels (10–15). As many of the ligands for NRPs are
relevant to angiogenesis, the acceleration of tumor vessel
formation is one of the most likely mechanisms for the
tumor-promoting effect of NRPs (16). Indeed, it has been reported that
the co-expression of NRP1 and VEGF2 in endothelial cells and
melanoma cells promotes vascular formation (20). In addition, NRPs are known to
affect tumor cells directly, activating cell growth and migration
(6,15). In breast cancer, NRP1 plays a key
role in mammosphere formation, which is one of the typical features
of breast cancer stem cells, via activating the NF-κB signal
(21). The ability of NRPs to bind
to TGF-β1 and its receptors suggests that NRPs can promote tumor
metastasis (22). Based on these
observations, NRPs have been proposed to be candidate therapeutic
target for malignancies.
The findings of the present study indicate that NRP1
has a tumor suppressive function in NB, which contradicts the
findings of previous reports on NRP1 in other types of cancer.
Among the molecules relevant to cell invasion and/or motility, we
found that β1 integrin was significantly upregulated following NRP1
knockdown. The integrins are a family of transmembrane receptors
through which cells adhere to the extracellular matrix, regulating
cell proliferation and migration. The integrin signal has been
known to induce the autophosphorylation of FAK followed via the
activation of downstream signals, such as PI3K and F-actin
formation (23,24). On the other hand, it has been
reported that reactive oxygen species produced upon integrin
receptor activation oxidize actin and this modification results in
disassembly of the actin-myosin complex (25). It has been also shown that the
oxidation of actin causes the prevention of actin polymerization
(26). These cytoskeleton dynamics
induced by integrin signal is necessary for cell ability of
migration and invasion. Our results indicated that FAK and PI3K
were upregulated and activated in NRP1-depleted cells (Fig. 3A). In addition, NRP1-depleted cells
demonstrated an obviously reduced amount of F-actin compared to
control cells (Fig. 4). These
observations suggested that the decreased expression of NRP1
resulted in an enhanced cell invasion and motility via the
induction of β1 integrin expression. The observation that β1
integrin silencing suppressed cell invasion and migration supports
this suggestion.
It is worth noting that β1 integrin expression was
upregulated at the transcriptional level following the knockdown of
NRP1. Although several studies have demonstrated that the activity
of integrins can be regulated by semaphorins via its receptors,
including NRPs and plexins (27–29),
there are few reports available regarding the regulation of
integrin expression by semaphorins/NRPs. In a previous study using
a breast cancer cell line, cells treated with SEMA3A exhibited an
increased expression of α2 and β1 integrin at the transcriptional
level (30). As NRP1 should be
activated by SEMA3A, this report was in contrast with the findings
of the present study. It has been reported that the expression of
β1 integrin is regulated by the homeobox family gene, HOXD1, in
endothelial cells (31),
hypoxia-inducible factor in fibroblasts (32), or fork head box M1 in breast cancer
cells (33). A detailed analysis
indicated that the β1 integrin promoter contains binding sites for
these transcription factors (31–33).
To elucidate the mechanisms through which NRPs regulate the
expression of integrins, we are currently planning a study to
evaluate whether there is a crosstalk between NRP signals and the
above transcription factors.
Intriguingly, the present findings suggested that
therapeutics to inhibit the function of NRP1 can promote the
malignant alteration of NB, even though NRP1 is assumed to be a
promising therapeutic target for many other tumors. The reason why
NRP1 exerts tumor suppressive effects against NB cells in contrast
to the findings in other types of cancers remains unclear. NRP1 and
its ligand, SEMA3A, are involved in axonal guidance during nervous
system development (27,34,35).
As NB is derived from neural crest progenitor cells in the
sympathetic nervous system and differentiated NB cells exhibit a
neuron-like morphology with elongated dendrites, we speculated that
the knockdown of NRP1 may inhibit the neuron-like phenotype of NB
cells and result in undifferentiated, malignant properties. Further
analyses are warranted to evaluate this possibility.
In conclusion, the present study indicated that a
decreased NRP1 expression level was associated with a poor
prognosis of patients with NB, and that the silencing of NRP1
results in the promotion of the migratory and invasive activities
of NB-derived SK-N-AS cells, along with the upregulated expression
of β1 integrin. These results indicate that NRP1 exerts its tumor
suppressive effects by reducing β1 integrin expression in NB.
Acknowledgments
The authors would like to thank Ms. A. Oguni for her
excellent technical assistance and Ms. K. Tagata and Ms. E.
Fukushima for her secretarial assistance.
Funding
The present study was supported in part by JSPS
KAKENHI (grant no. 17K17006 to RH), and by the MEXT-Supported
Program for the Strategic Research Foundation at Private
Universities (2011–2015) to NF, MS, TK and KF.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YI, KS and KF planned the experiments. YI and KF
performed the experiments. YI, TK and KF interpret experimental
data. SY designed and tested the primers for real-time PCR. TH,
ENM, YW, RH, HK and SU have maintained the NB cells and confirm the
identity of these cells. YW, NF and MS performed statistical
analysis of the data. KF and YI wrote the paper. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner JH and Reh DD: Incidence and
survival in patients with sinonasal cancer: A historical analysis
of population-based data. Head Neck. 34:877–885. 2012. View Article : Google Scholar
|
|
3
|
Brodeur GM: Neuroblastoma: Biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwab M, Alitalo K, Klempnauer KH, Varmus
HE, Bishop JM, Gilbert F, Brodeur G, Goldstein M and Trent J:
Amplified DNA with limited homology to myc cellular oncogene is
shared by human neuroblastoma cell lines and a neuroblastoma
tumour. Nature. 305:245–248. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caron H: Allelic loss of chromosome 1 and
additional chromosome 17 material are both unfavourable prognostic
markers in neuroblastoma. Med Pediatr Oncol. 24:215–221. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Attiyeh EF, London WB, Mosse YP, Wang Q,
Winter C, Khazi D, McGrady PW, Seeger RC, Look AT, Shimada H, et
al: Chromosome 1p and 11q deletions and outcome in neuroblastoma. N
Engl J Med. 353:2243–2253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carpenter EL and Mosse YP: Targeting ALK
in neuroblastoma - preclinical and clinical advancements. Nat Rev
Clin Oncol. 9:391–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oberthuer A, Hero B, Berthold F, Juraeva
D, Faldum A, Kahlert Y, Asgharzadeh S, Seeger R, Scaruffi P, Tonini
GP, et al: Prognostic impact of gene expression-based
classification for neuroblastoma. J Clin Oncol. 28:3506–3515. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baker DL, Schmidt ML, Cohn SL, Maris JM,
London WB, Buxton A, Stram D, Castleberry RP, Shimada H, Sandler A,
et al: Outcome after reduced chemotherapy for intermediate-risk
neuroblastoma. N Engl J Med. 363:1313–1323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Younan S, Elhoseiny S, Hammam A, Gawdat R,
El-Wakil M and Fawzy M: Role of neuropilin-1 and its expression in
Egyptian acute myeloid and acute lymphoid leukemia patients. Leuk
Res. 36:169–173. 2012. View Article : Google Scholar
|
|
11
|
Li L, Jiang X, Zhang Q, Dong X, Gao Y, He
Y, Qiao H, Xie F, Xie X and Sun X: Neuropilin-1 is associated with
clinicopathology of gastric cancer and contributes to cell
proliferation and migration as multifunctional co-receptors. J Exp
Clin Cancer Res. 35:162016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Liu P, Jiang Y, Dou X, Yan J, Ma
C, Fan Q, Wang W, Su F, Tang H, et al: High expression of
neuropilin-1 associates with unfavorable clinicopathological
features in hepatocellular carcinoma. Pathol Oncol Res. 22:367–375.
2016. View Article : Google Scholar
|
|
13
|
Zhu H, Cai H, Tang M and Tang J:
Neuropilin-1 is overexpressed in osteosarcoma and contributes to
tumor progression and poor prognosis. Clin Transl Oncol.
16:732–738. 2014. View Article : Google Scholar
|
|
14
|
Ben Q, Zheng J, Fei J, An W, Li P, Li Z
and Yuan Y: High neuropilin 1 expression was associated with
angiogenesis and poor overall survival in resected pancreatic
ductal adenocarcinoma. Pancreas. 43:744–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi F, Shang L, Yang LY, Jiang YY, Wang
XM, Hao JJ, Zhang Y, Huang DK, Cai Y, Xu X, et al: Neuropilin-1
contributes to esophageal squamous cancer progression via promoting
P65-dependent cell proliferation. Oncogene. 37:935–943. 2018.
View Article : Google Scholar
|
|
16
|
Fuh G, Garcia KC and de Vos AM: The
interaction of neuropilin-1 with vascular endothelial growth factor
and its receptor flt-1. J Biol Chem. 275:26690–26695.
2000.PubMed/NCBI
|
|
17
|
Jubb AM, Strickland LA, Liu SD, Mak J,
Schmidt M and Koeppen H: Neuropilin-1 expression in cancer and
development. J Pathol. 226:50–60. 2012. View Article : Google Scholar
|
|
18
|
Fakhari M, Pullirsch D, Abraham D, Paya K,
Hofbauer R, Holzfeind P, Hofmann M and Aharinejad S: Selective
upregulation of vascular endothelial growth factor receptors
neuropilin-1 and -2 in human neuroblastoma. Cancer. 94:258–263.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prud'homme GJ and Glinka Y: Neuropilins
are multifunctional coreceptors involved in tumor initiation,
growth, metastasis and immunity. Oncotarget. 3:921–939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ruffini F, D'Atri S and Lacal PM:
Neuropilin-1 expression promotes invasiveness of melanoma cells
through vascular endothelial growth factor receptor-2-dependent and
-independent mechanisms. Int J Oncol. 43:297–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Glinka Y, Mohammed N, Subramaniam V, Jothy
S and Prud'homme GJ: Neuropilin-1 is expressed by breast cancer
stem-like cells and is linked to NF-κB activation and tumor sphere
formation. Biochem Biophys Res Commun. 425:775–780. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glinka Y, Stoilova S, Mohammed N and
Prud'homme GJ: Neuropilin-1 exerts co-receptor function for
TGF-beta-1 on the membrane of cancer cells and enhances responses
to both latent and active TGF-beta. Carcinogenesis. 32:613–621.
2011. View Article : Google Scholar
|
|
23
|
Chen HC and Guan JL: Association of focal
adhesion kinase with its potential substrate phosphatidylinositol
3-kinase. Proc Natl Acad Sci USA. 91:10148–10152. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westhoff MA, Serrels B, Fincham VJ, Frame
MC and Carragher NO: SRC-mediated phosphorylation of focal adhesion
kinase couples actin and adhesion dynamics to survival signaling.
Mol Cell Biol. 24:8113–8133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fiaschi T, Cozzi G, Raugei G, Formigli L,
Ramponi G and Chiarugi P: Redox regulation of beta-actin during
integrin-mediated cell adhesion. J Biol Chem. 281:22983–22991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lassing I, Schmitzberger F, Bjornstedt M,
Holmgren A, Nordlund P, Schutt CE and Lindberg U: Molecular and
structural basis for redox regulation of beta-actin. J Mol Biol.
370:331–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neufeld G and Kessler O: The semaphorins:
Versatile regulators of tumour progression and tumour angiogenesis.
Nat Rev Cancer. 8:632–645. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Serini G, Valdembri D, Zanivan S, Morterra
G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L,
Logan M, et al: Class 3 semaphorins control vascular morphogenesis
by inhibiting integrin function. Nature. 424:391–397. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Herman JG and Meadows GG: Increased class
3 semaphorin expression modulates the invasive and adhesive
properties of prostate cancer cells. Int J Oncol. 30:1231–1238.
2007.PubMed/NCBI
|
|
30
|
Pan H, Wanami LS, Dissanayake TR and
Bachelder RE: Autocrine semaphorin3A stimulates alpha2 beta1
integrin expression/function in breast tumor cells. Breast Cancer
Res Treat. 118:197–205. 2009. View Article : Google Scholar
|
|
31
|
Park H, Choi HJ, Kim J, Kim M, Rho SS,
Hwang D, Kim YM and Kwon YG: Homeobox D1 regulates angiogenic
functions of endothelial cells via integrin β1 expression. Biochem
Biophys Res Commun. 408:186–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Keely S, Glover LE, MacManus CF, Campbell
EL, Scully MM, Furuta GT and Colgan SP: Selective induction of
integrin beta1 by hypoxia-inducible factor: Implications for wound
healing. FASEB J. 23:1338–1346. 2009. View Article : Google Scholar :
|
|
33
|
Hamurcu Z, Kahraman N, Ashour A and
Ozpolat B: FOXM1 transcriptionally regulates expression of integrin
β1 in triple-negative breast cancer. Breast Cancer Res Treat.
163:485–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kolodkin AL, Levengood DV, Rowe EG, Tai
YT, Giger RJ and Ginty DD: Neuropilin is a semaphorin III receptor.
Cell. 90:753–762. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He Z and Tessier-Lavigne M: Neuropilin is
a receptor for the axonal chemorepellent Semaphorin III. Cell.
90:739–751. 1997. View Article : Google Scholar : PubMed/NCBI
|