Introduction
Esophageal cancer has a high morbidity rate and is
the sixth most common cause of cancer-related mortality worldwide
(1–3). A survey published in 2013 reported
that there were 450,000 new cases of esophageal cancer annually
(4), and China accounts for more
than half of all cases of this type of cancer (5). Each year, it is estimated that
150,000 individuals succumb to esophageal cancer in China, and the
5-year survival rate of patients is usually <30% (6–8).
Esophageal cancer has two main subtypes, namely esophageal squamous
cell carcinoma (ESCC) and esophageal adenocarcinoma (9,10).
Currently, the most commonly used treatments for ESCC are surgery,
chemotherapy, radiation therapy and comprehensive treatment
(11,12). The preferred treatment for ESCC is
surgical resection, but postoperative recurrence and distant
metastasis are clinical problems for which effective treatments
remain to be identified (13).
Cisplatin (DDP) is a first-line drug in the treatment for ESCC, and
the development of DDP resistance in ESCC cells is the main cause
of chemotherapy failure (14,15).
The effectiveness of chemotherapy depends on the sensitivity of the
tumor cells to chemotherapy drugs (16), and ESCC usually exhibits a high
resistance to chemotherapy (17,18).
Therefore, the identification of oncogenes that may be targeted to
combat resistance is likely to be a great benefit to clinical
practice.
C-terminal binding protein 2 (CtBP2) acts as a
transcriptional co-repressor, and modulates certain essential
cellular processes, such as proliferation, migration and (19). It has been reported that CtBP2 has
a critical function in tumorigenesis and tumor progression
(20,21). CtBP2 is overexpressed in a number
of different tumor types, including hepatocellular carcinoma
(22), prostate cancer (23), breast cancer (24,25)
and ovarian cancer (26).
Furthermore, preliminary studies conducted by the present research
team revealed that the expression of CtBP2 was upregulated in ESCC
tissues (27,28). In addition, CtBP2 predicts a poor
prognosis in human cancers, including ESCC (27). Therefore, it appears that CtBP2
serves an oncogene-like role in tumorigenesis and tumor
progression. It has been reported that CtBP2 represses the
sensitivity of breast cancer cells by p53-dependent and
-independent mechanisms (29).
However, the involvement of CtBP2 in the drug resistance of ESCC
remains unknown.
In order to further understand the DDP resistance
mechanisms of ESCC, the present study investigated the effect of
CtBP2 on DDP resistance in ECA109 cells. To the best of our
knowledge, the present study is the first to investigate this. The
effect of knocking down CtBP2 on the susceptibility of ESCC cells
to DDP was evaluated, and the underlying mechanism, such as the
regulation of the expression of apoptosis-related proteins, was
investigated. The results may indicate the potential of CtBP2 as a
therapeutic target for ESCC.
Materials and methods
Cell culture
The human ESCC cell line ECA109 was supplied by the
Cell Resource Center of Shanghai Institute for Biological Sciences,
Chinese Academy of Sciences (Shanghai, China). Cell culture was
performed in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
calf serum (FCS; Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA), and 10 kU/ml penicillin and 10 mg/ml streptomycin (Beyotime
Institute of Biotechnology, Haimen, China).
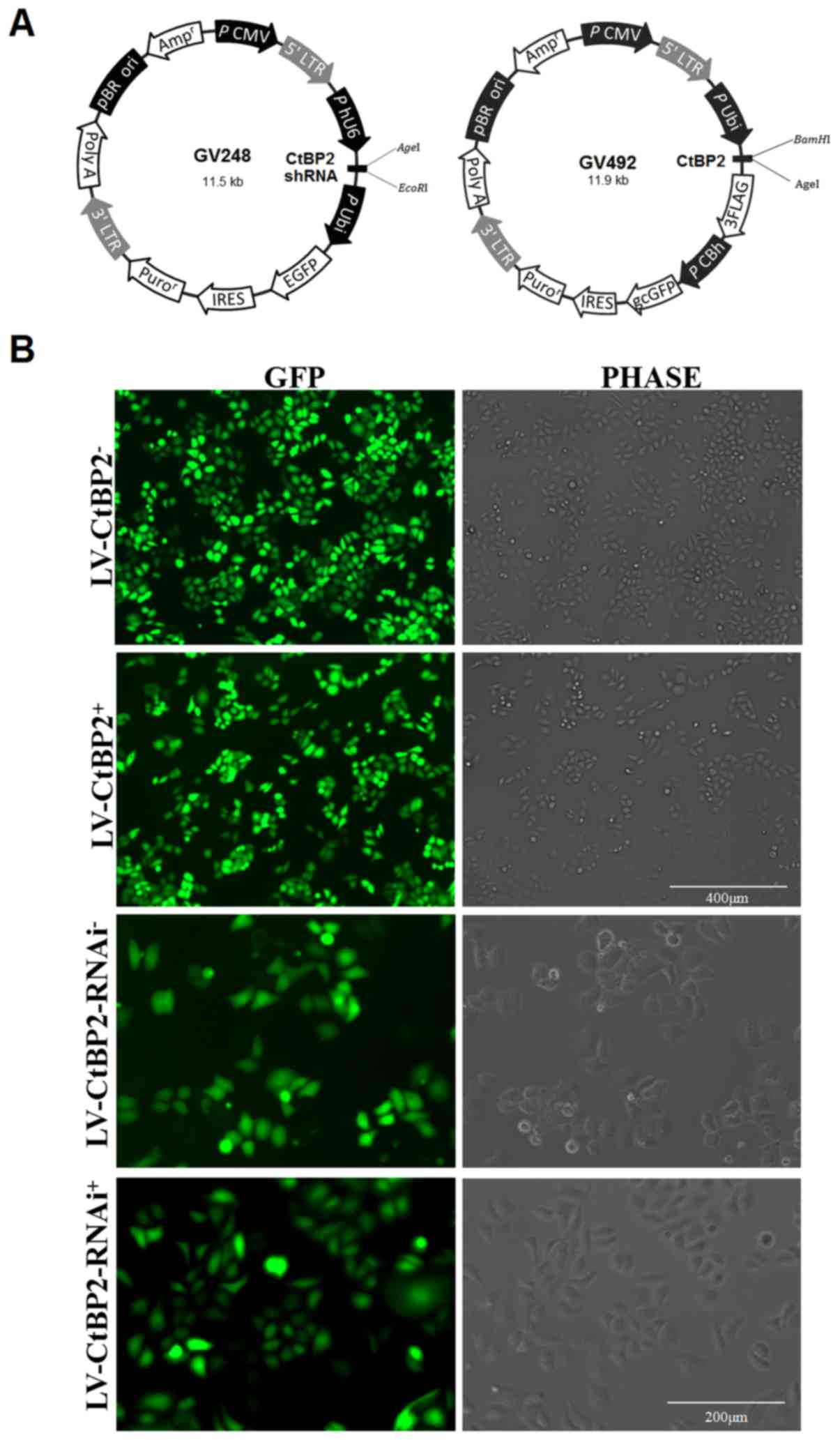
Knockdown or overexpression of CtBP2
Firstly, recombinant lentiviral vectors were
constructed to knockdown CtBP2 (LV-CtBP2-RNAi) or overexpress CtBP2
(LV-CtBP2). The sequences were designed using the software EPtiRNA
(http://optirna.unl.edu/) and synthesized by
GeneChem Co., Ltd. (Shanghai, China). The vector construction and
virus packaging were conducted by GeneChem Co., Ltd. For the
former, small hairpin RNA (shRNA) of CtBP2, whose target sequence
was 5′-GCGCCTTGGTCAGTAATAG-3′, was cloned into a GV248 vector
(GeneChem Co., Ltd.) via EcoRI and AgeI restriction
endonuclease sites. For the latter, the coding sequence of CtBP2
was cloned into a GV492 vector (GeneChem Co., Ltd.) via
BamHI and AgeI restriction endo-nuclease sites. The
primers used for overexpression were as follows: 5′-AGG TCG ACT CTA
GAG GAT CCC GCC ACC ATG GCC CTT GTG GAT AAG CAC-3′ (forward) and
5′-TCC TTG TAG TCC ATA CCT TGC TCG TTG GGG TGC TCT CGA TTG-3′
(reverse). Schematic diagrams of the recombinant lentiviral vector
constructs are presented in Fig.
1A.
ECA109 cells were transfected with LV-CtBP2-RNAi or
LV-CtBP2 to knockdown or overexpress CtBP2, respectively. ECA109
cells transfected with empty vector GV248 and empty vector GV492
served as the negative controls, LV-CtBP2-RNAi− and
LV-CtBP2−, respectively. The following formula was used
to calculate to volume of virus to be added: Virus volume =
multiplicity of infection x cell number/virus titer. The cells
(1×106 cells/well) were seeded into 6-well plates prior
to transfection. In order to improve the transfection rate, 5
µg/ml polybrene and enhanced infection solution (without
FCS) were incubated overnight with the cells at 37°C with 5%
CO2. Puromycin (2 µg/ml; Gibco; Thermo Fisher
Scientific, Inc.) was added to the culture medium (DMEM containing
10% FCS) on the third day and refreshed every 2 days for 1 week to
select the transfected cells. The knockdown or overexpression of
CtBP2 in the ECA109 cells was confirmed using the observation of
green fluorescence, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blotting.
RNA extraction and RT-qPCR
Total RNA was extracted from the CtBP2 knockdown or
overexpressing cells using TRIzol reagent (Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
First-strand cDNA synthesis was conducted using a RevertAid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). The
temperature protocol was 60 min at 42°C, with termination of the
reaction by heating at 70°C for 5 min. qPCR was performed in
triplicate using SYBR Green Master mix (Roche Diagnostics, Basel,
Switzerland) in a 7500 Real-Time PCR instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.) to test the mRNA
expression level of CtBP2. The thermocycling conditions were as
follows: Firstly 95°C for 10 min, followed by 95°C for 10 sec, 60°C
for 15 sec and 72°C for 20 sec, for 40 cycles. The relative
expression of mRNA was calculated using the 2−ΔΔCq
method with GAPDH as an internal reference (30). The following primer sequences were
used: GAPDH, 5′-GAC CTG ACC TGC CGT CTA-3′ (sense) and 5′-AGG AGT
GGG TGT CGC TGT-3′ (antisense); CtBP2, 5′-CTG AGT TCC TGG CCT TTC
TG-3′ (sense) and 5′-GAC TTG ATA TCC GCG TCC TC-3′
(anti-sense).
Western blot analysis
Briefly, cells were homogenized in lysis buffer
containing 1 mM phenylmethane sulfonyl fluoride and complete
protein inhibitor mixture (Beyotime Institute of Biotechnology) for
15 min on ice, and then centrifuged at 13,400 × g for 10 min to
collect the supernatant. The supernatant was diluted in 5X sodium
dodecyl sulfate (SDS) loading buffer (Beyotime Institute of
Biotechnology), boiled for 5 min and then cooled on ice. The
protein concentration was measured at 280 nm using a One Drop
Spectrophotometer (Wuyi Technology Co., Ltd., Nanjing, China) prior
to loading the protein onto a gel (50 µg protein/lane). The
proteins were separated by 10% SDS-PAGE and then blotted to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with Tris-buffered saline and 0.1%
Tween-20 (TBST) supplemented with 5% non-fat milk for 2 h at room
temperature, and then reacted with the following primary antibodies
overnight at 4°C: Anti-CtBP2 (sc-17759; 1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), anti-activated caspase-3
(cleaved) (AB3623; 1:200; EMD Millipore), anti-B-cell lymphoma 2
(anti-Bcl-2; Ab-1; 1:1,000; EMD Millipore), anti-Bcl-2-associated X
protein (anti-Bax; ab53154; 1:500; Abcam, Cambridge, MA, USA) and
anti-β-actin (ab8227; 1:1,000; Abcam). After washing with TBST
three times, the membrane was then reacted with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (A8919; 1:1,000)
or HRP-conjugated rabbit anti-mouse IgG (A9044; 1,1000; both from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2 h at 37°C. The
protein bands were detected using ECL chemiluminescence reagent
(Thermo Fisher Scientific, Inc.) and imaged using a
chemiluminescence detection system (Tanon Science and Technology
Co., Ltd., Shanghai, China). ImageJ software (National Institutes
of Health, Bethesda, MD, USA) was used to analyze the density of
the bands and β-actin was used as a reference for normalization.
All experiments were repeated three times.
DDP treatment and cell viability
assay
ECA109 cells were seeded in triplicate in a 96-well
plate and cultured in 100 µl DMEM medium containing 10% FCS.
When the cells had become attached to the bottom of well, the cells
were treated with DDP (Sigma-Aldrich; Merck KGaA) in serial
dilutions (final concentration 1.5×10−3,
1.5×10−4, 1.5×10−5, 1.5×10−6,
1.5×10−7 or 1.5×10−8 M) in DMEM. Following
incubation for 48 h, methylthiazolyl tetrazolium solution (MTT;
Beyotime Institute of Biotechnology; 10 µl; 5 mg/ml) was
added to each well and the plate was incubated for a further 4 h.
The medium was eliminated and 100 µl dimethylsulfoxide
(Merck KGaA) was added to each well. The plate was shaken to
dissolve the MTT-formazan crystals and the absorbance at a
wavelength of 570 nm was read using an ELX800 microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
On the basis of the MTT results for the various
concentrations of DDP, the half maximal inhibitory concentration
(IC50) of DPP was determined. This concentration of DDP
was subjected to further testing of cell viability with different
treatment times (0, 12, 24 and 48 h) in order to determine the
appropriate treatment duration. For subsequent analysis of the role
of CtBP2 in the cytotoxicity of DDP, transfected and untransfected
ECA109 cells were exposed to the concentration of DPP closest to
the IC50 using the treatment duration identified to be
appropriate in this assay.
Hoechst 33342 staining
ECA109 cells on glass coverslips in 24-well plates
(5×104 cells/well) were fixed with 4% paraformaldehyde
for 30 min at room temperature and then washed with 0.01% PBS three
times, for 10 min each time at room temperature. The cells were
then stained with Hoechst 33342 (Shanghai Yeasen Biotechnology Co.,
Ltd., Shanghai, China) for 10 min at room temperature. The slides
were mounted with anti-fade solution (Beyotime Institute of
Biotechnology). The apoptotic cells were identified by detecting
the condensation and fragmentation of the cell nuclei under a
fluorescence microscope (Zeiss AG, Oberkochen, Germany). Apoptotic
cell numbers were counted in three randomly selected fields to
calculate the apoptosis rate in triplicate.
Flow cytometry (FCM) assay
Following the aforementioned treatments, the cells
were digested with trypsin and washed with 0.01% PBS twice. The
cells were then stained for 10–20 min with Annexin V-fluorescein
isothiocyanate and propidium iodide (Abcam) solution at room
temperature for 1 h in the dark, and then subjected to FCM (BD
Biosciences, San Jose, CA, USA). A total of 10,000 fluorescence
signals of each group were collected. The data were analyzed using
FACSuite software (BD Biosciences).
Statistical analysis
All data were analyzed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was
used to analyze the data and Tukey's post hoc test was used to
analyze the differences between specific groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Successful establishment of stable cell
lines
In order to detect the impact of CtBP2 on DDP
resistance in ESCC, the ECA109 cells were transfected with
lentivirus to knockdown or overexpress CtBP2. As the recombinant
lentiviral vector contained the enhanced green fluorescent protein
(EGFP) gene, the rate of transfection could be determined by direct
observation under a fluorescence microscope. The percentage of
cells positive for EGFP was >90% in the ECA109 cells transfected
with recombinant LV-CtBP2-RNAi or LV-CtBP2. Fluorescence images of
the transfected cells are presented in Fig. 1B.
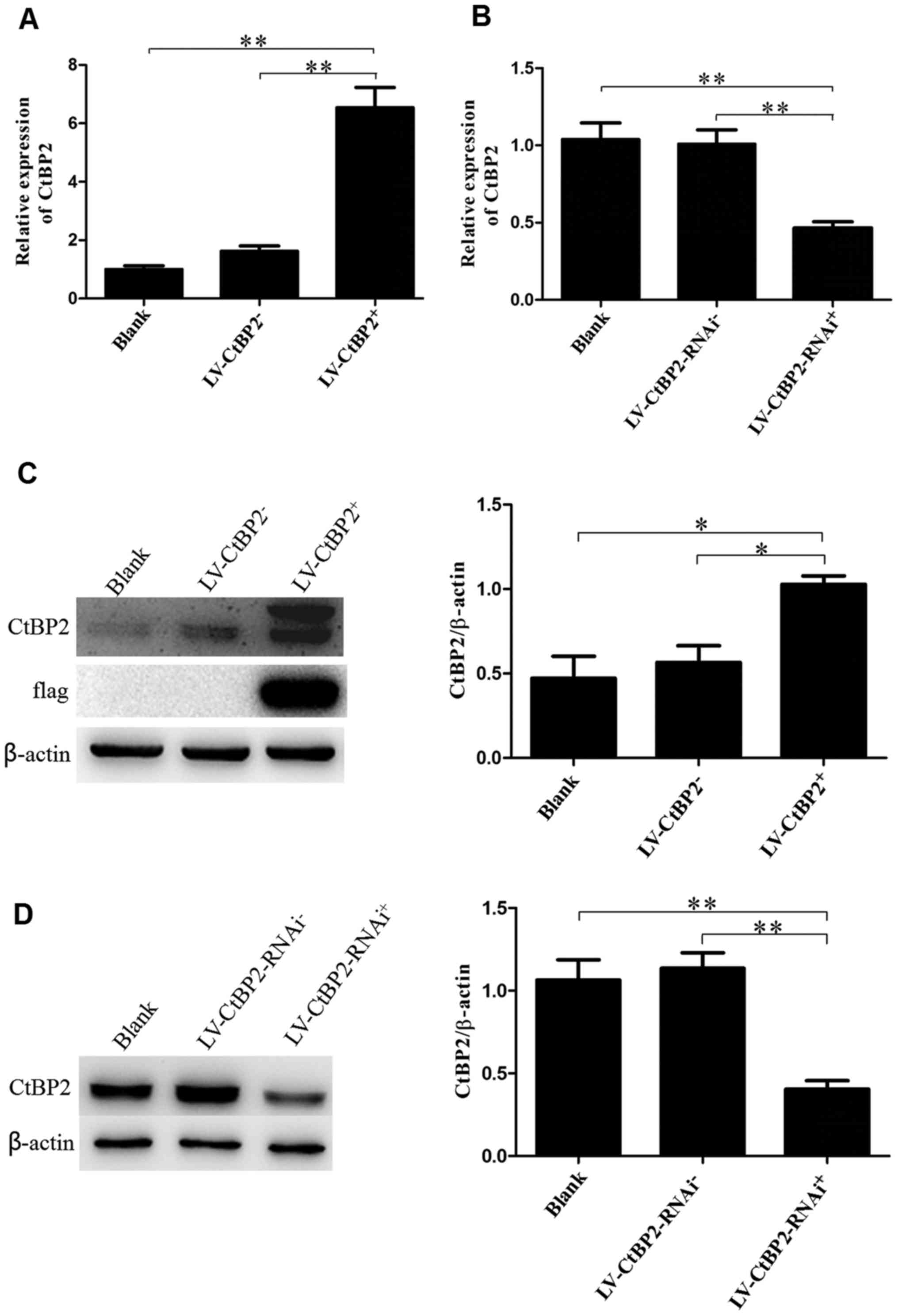
The expression levels of CtBP2 in the ECA109 cells
transfected with recombinant LV-CtBP2-RNAi or LV-CtBP2 (the
LV-CtBP2-RNAi+ and LV-CtBP2+ groups,
respectively) were further confirmed by RT-qPCR and western blot
analysis. The expression of CtBP2 mRNA was revealed to be
significantly changed by RT-qPCR analysis. Compared with the blank
and negative control (LV-CtBP2−) groups, the relative
expression of CtBP2 mRNA in the LV-CtBP2+ transfection
group was increased ~6-fold (P<0.01). By contrast, the relative
expression of CtBP2 mRNA in the LV-CtBP2-RNAi+
transfection group was decreased by more than half compared with
that in the blank and negative control (LV-CtBP2-RNAi−)
groups (P<0.01). No significant difference in CtBP2 mRNA levels
was detected between the blank group and the LV-CtBP2−
or LV-CtBP2-RNAi− group (P>0.05; Fig. 2A and B).
The protein levels of CtBP2 detected by western
blotting exhibited similar trends to the expression levels of CtBP2
mRNA in the ECA109 cells subjected to CtBP2 knockdown or
overexpression. Compared with the blank and respective negative
control groups, the expression of CtBP2 was increased ~2-fold in
the LV-CtBP2+ transfection group (P<0.05) and
decreased by two-thirds in the LV-CtBP2-RNAi+
transfection group (P<0.01; Fig. 2C
and D). Furthermore, no significant difference was detected
between the blank group and the LV-CtBP2− or
LV-CtBP2-RNAi− group (P>0.05).
These results suggest that stable cell lines with
the knockdown or overexpression of CtBP2 were successfully obtained
following recombinant lentiviral transfection for use in the
following experiments.
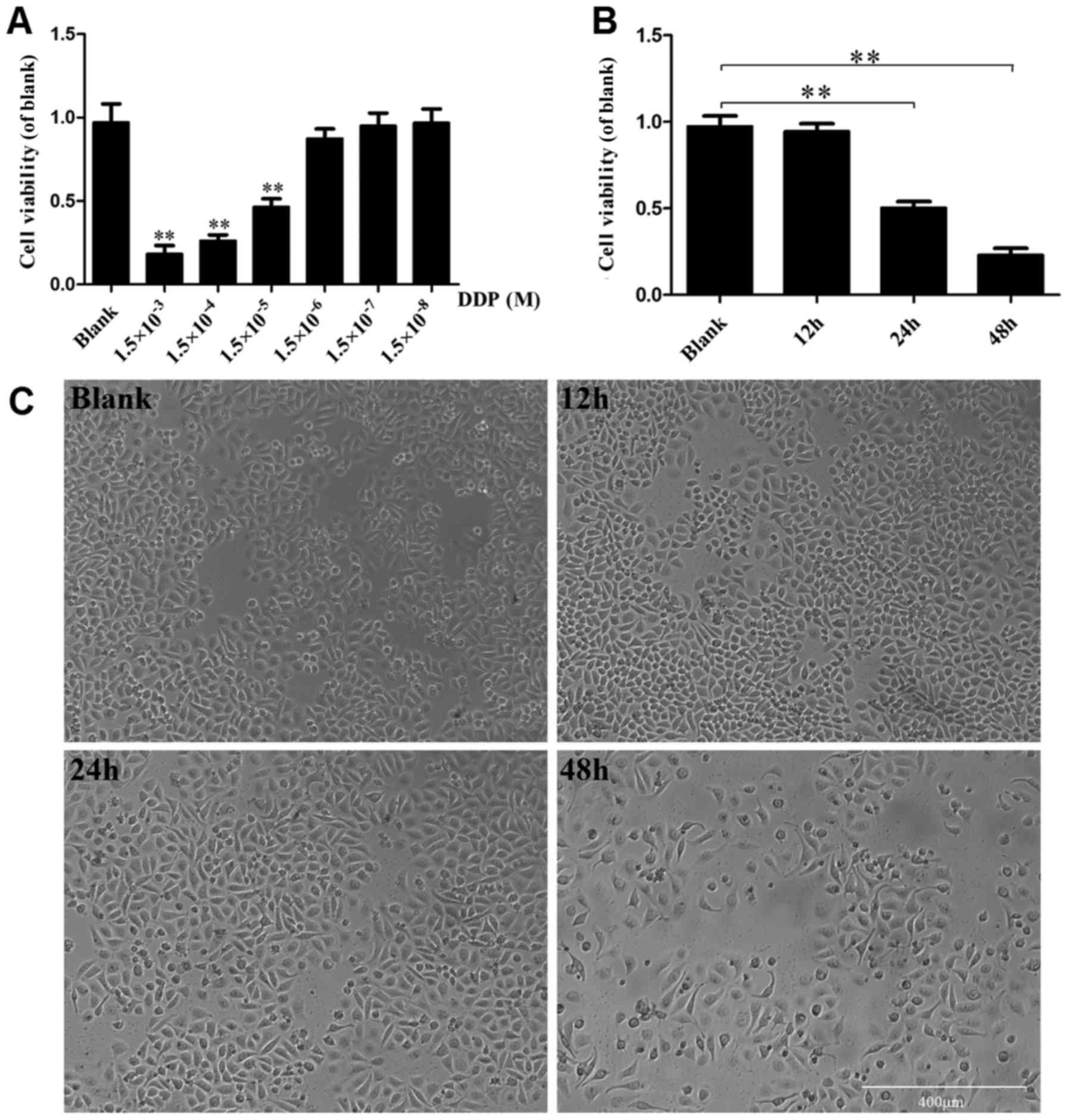
CtBP2 overexpression promotes ECA109 cell
viability following DDP treatment
In order to determine the optimized concentration
and treatment time for DDP, ECA109 cells were treated with DDP
solution in serial dilutions for different time periods. Firstly,
an MTT assay was conducted to test the changes in cell viability
following treatment with serial dilutions of DDP. The cell
viability was markedly decreased following treatment with
increasing concentrations of DDP for 24 h. Compared with the blank
group, the cell viability in the 1.5×10−3,
1.5×10−4 and 1.5×10−5 M DDP treated groups
was significantly reduced (P<0.01). Notably, the cell viability
of the 1.5×10−5 M DDP treatment group was reduced by
52.15% (Fig. 3A).
Secondly, the effects on cell viability of
incubation with 1.5×10−5 M DDP for different treatment
times were examined via MTT assay (Fig. 3B) and by observation under an
inverted phase contrast microscope (Fig. 3C). When observed under the
microscope, the number of dead cells appeared to increase gradually
as the treatment time with DDP was prolonged. The MTT assay
demonstrated that compared with the blank group, the viability of
the cells treated with DDP for 24 and 48 h was significantly
decreased (P<0.01), but the reduction in viability of the cells
treated with DDP for 12 h was not significant (P>0.05; Fig. 3B).
These results indicate that the effect of DDP on
cell viability was dependent on concentration and reaction time.
The optimized concentration and treatment time for DDP in ECA109
cells were 1.5×10−5 M and 24 h, respectively, and were
used in the following experiments.
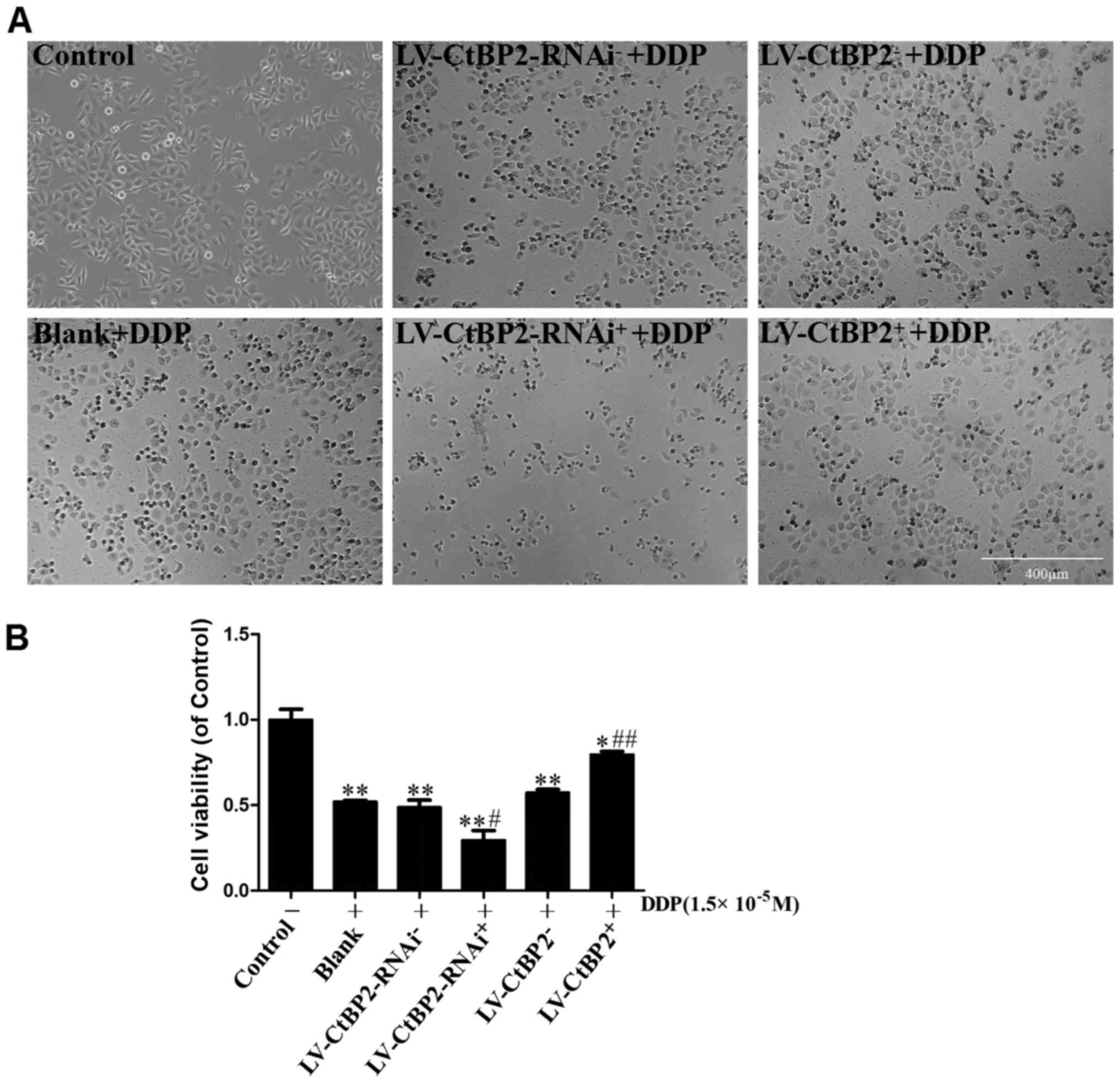
The impact of CtBP2 on the viability of the
DDP-treated ECA109 cells was examined by microscopy and MTT assay
(Fig. 4). The microscopy images
revealed that cell shrinkage occurred following treatment with DDP,
and the number of dead cells was increased. The viability of the
ECA109 cells treated with DDP for 24 h was significantly reduced
compared with that of the control cells (P<0.01). The cell
viability of the LV-CtBP2-RNAi+ + DDP group was
significantly reduced compared with that of the
LV-CtBP2-RNAi− + DDP group (P<0.05). Furthermore, the
cell viability of the LV-CtBP2+ + DDP group was
significantly increased compared with that of the
LV-CtBP2− + DDP group (P<0.01). No statistically
significant difference was detected between the
LV-CtBP2− + DDP and LV-CtBP2-RNAi− + DDP
groups and the blank + DDP group (P>0.05). These results
indicate that the overexpression of CtBP2 attenuated the reduction
of cell viability induced by DDP, and the knockdown of CtBP2
augmented the DDP-induced reduction of cell viability.
CtBP2 overexpression reduces the
DDP-induced apoptosis of ECA109 cells
Hoechst 33342 staining and FCM were used to
investigate the effect of CtBP2 on the cell apoptosis induced by
DDP. The results of Hoechst 33342 staining demonstrated that the
numbers of apoptotic bodies were significantly increased
(P<0.01) in the DPP-treated cells compared with untreated
control group. The number of apoptotic bodies was increased
significantly in the LV-CtBP2-RNAi+ + DDP group compared
with the LV-CtBP2-RNAi− + DDP group, and decreased
significantly in the LV-CtBP2+ + DDP group compared with
the LV-CtBP2− + DDP group (P<0.05; Fig. 5A). On the basis of these results,
it appears that CtBP2 overexpression attenuates the increase of
apoptotic bodies induced by DDP.
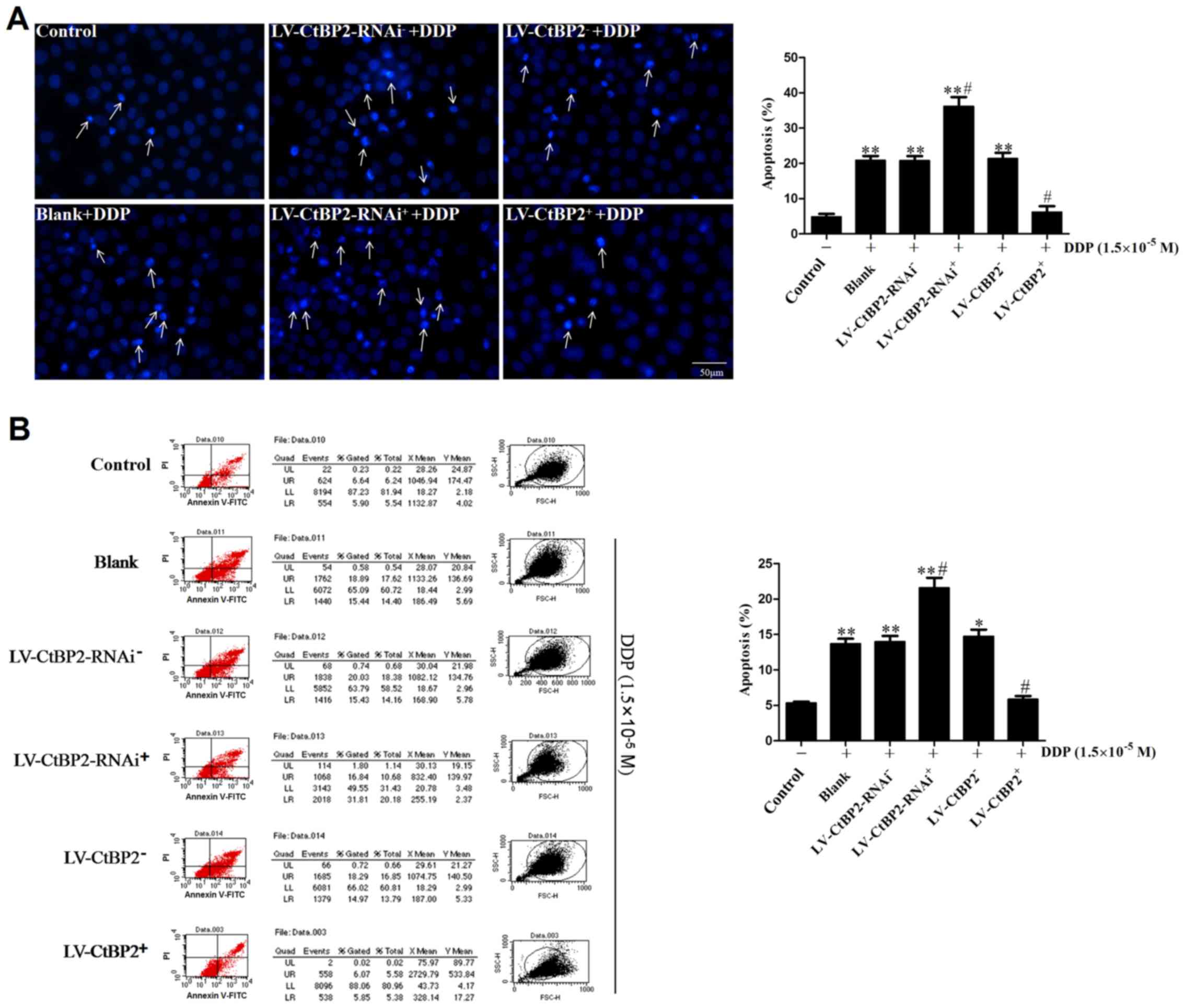 | Figure 5Effect of CtBP2 on the apoptosis of
ECA109 cells treated with DDP (1.5×10−5 M) was detected
by Hoechst 33342 staining and FCM. (A) Hoechst 33342 staining and
quantitative analysis of the results. In the fluorescence
microscopy images, apoptotic bodies are indicated by arrows (scale
bar, 50 µm). (B) The apoptosis of ECA109 cells was analyzed
by FCM assay. **P<0.01 vs. control;
#P<0.05 vs. the blank control group (blank + DPP) or
the respective negative control group (LV-CtBP2− + DPP
or LV-CtBP2-RNAi− + DPP). DDP, cisplatin; control,
untreated control group; blank, untransfected cells; LV-CtBP2-RNAi,
lentivirus for CtBP2 knockdown via RNA interference; LV-CtBP2,
lentivirus for CtBP2 overexpression; CtBP2, C-terminal binding
protein-2; FCM, flow cytometry; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
FCM was used to further verify the impact of CtBP2
on the DDP-induced apoptosis of ECA109 cells. The results are
consistent with those of Hoechst 33342 staining. The percentages of
apoptotic cells in the untreated control, blank + DDP,
LV-CtBP2-RNAi− + DDP and LV-CtBP2-RNAi+ + DDP
groups were 5.34, 13.7, 13.98 and 21.59% respectively. Compared
with the blank + DDP group and the LV-CtBP2-RNAi− + DDP
group, the percentage of apoptotic cells in the CtBP2 knockdown
(LV-CtBP2-RNAi+ + DDP) group was significantly increased
(P<0.05). The percentages of apoptotic cells in the blank + DDP,
LV-CtBP2− + DDP and LV-CtBP2+ + DDP groups
were 13.7, 14.75 and 5.86% respectively. The percentage of
apoptotic cells in the CtBP2 overexpression (LV-CtBP2+ +
DDP) group was significantly lower than those of the
LV-CtBP2− + DDP and blank + DDP groups (P<0.05;
Fig. 5B). The FCM results further
indicate that CtBP2 overexpression inhibited the DDP-induced
apoptosis of ECA109 cells.
Mechanisms underlying the effect of CtBP2
on DDP chemoresistance
Caspase serves essential roles in cell apoptosis,
which is a cellular event considered as programmed cell death
(31,32). Caspase-3 is one of the crucial
downstream effectors of apoptosis (33). The effects of CtBP2 on the protein
levels of p53, Bcl-2, Bax and activated caspase-3 were analyzed by
western blotting (Fig. 6). The
results shown in Fig. 6A and B
demonstrate that the expression of p53 was significantly increased
(P<0.01) in the ECA109 cells treated with 1.5×10−5 M
DDP for 24 h compared with the untreated control cells. Notably,
the expression of p53 in the CtBP2 overexpression
(LV-CtBP2+ + DDP) group was significantly decreased
compared with that of the LV-CtBP2− + DDP group
(P<0.05). The changes in cleaved caspase-3 levels (Fig. 6D) exhibited a similar pattern to
those of p53. These results demonstrate that the overexpression of
CtBP2 inhibited the DDP-induced increase in the protein levels of
p53 and activated caspase-3 in ECA109 cells.
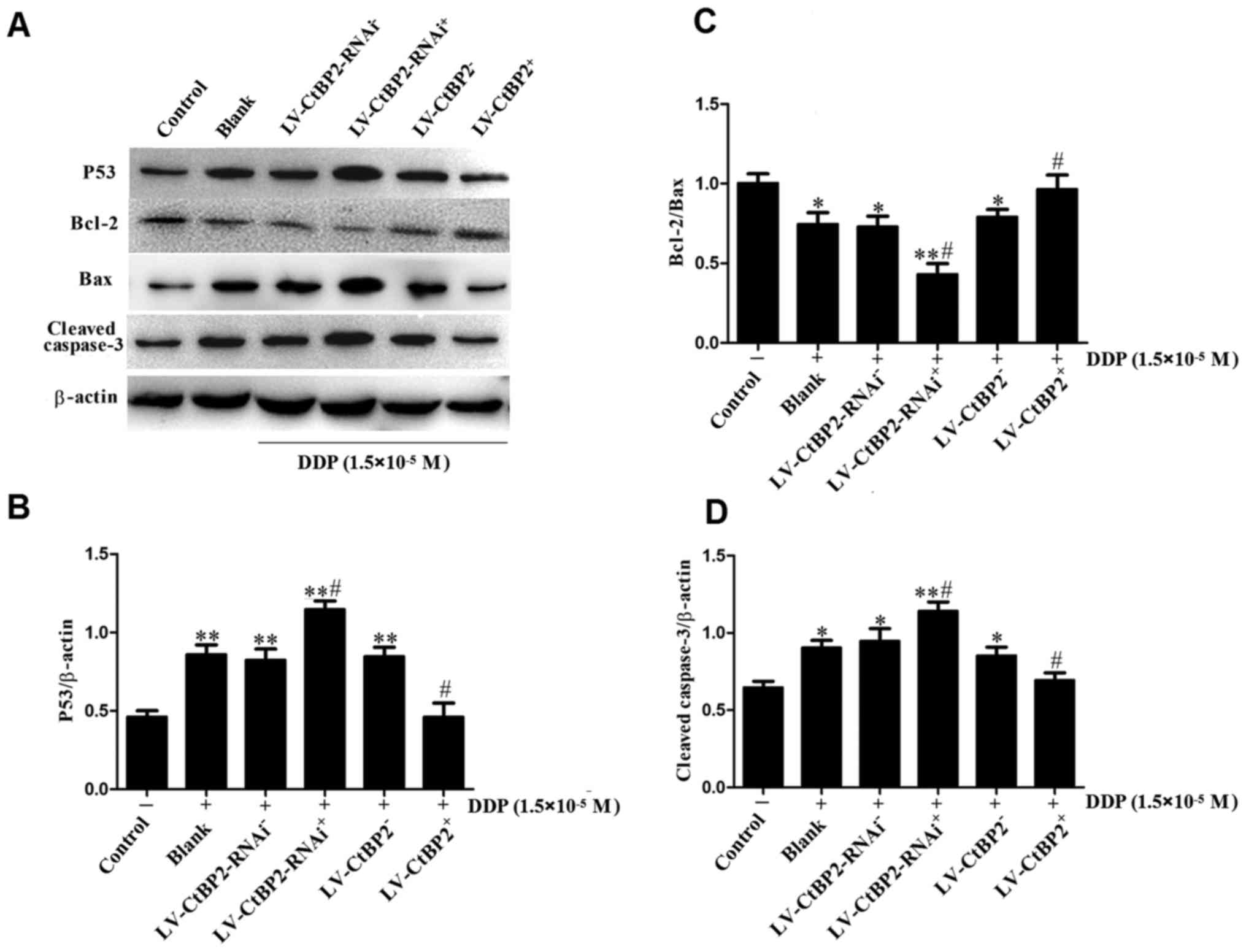 | Figure 6Levels of apoptosis-associated
proteins, namely p53, Bcl-2, Bax and cleaved caspase-3, were
measured by western blotting. (A) Representative western blots for
p53, Bcl-2, Bax and cleaved caspase-3. Quantified data for (B) p53,
(C) the ratio of Bcl-2/Bax and (D) cleaved caspase-3.
*P<0.05 and **P<0.01 vs. control;
#P<0.05 vs. blank control group (blank + DPP) or the
respective negative control group (LV-CtBP2− + DPP or
LV-CtBP2-RNAi− + DPP). DDP, cisplatin; control,
untreated control group; blank, untransfected cells; LV-CtBP2-RNAi,
cells transfected with a lentivirus for CtBP2 knockdown; LV-CtBP2,
cells transfected with a lentivirus for CtBP2 overexpression;
CtBP2, C-terminal binding protein-2; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein. |
The protein levels of Bax and Bcl-2 were also
detected using western blotting. The results clearly demonstrate
that Bcl-2 expression was downregulated while Bax expression was
upregulated in the CtBP2 knockdown (LV-CtBP2-RNAi+ +
DDP) group. Therefore, the Bcl-2/Bax ratio in the
LV-CtBP2-RNAi+ + DDP group was significantly decreased
compared with those in the untreated control, blank + DDP and
LV-CtBP2-RNAi− + DDP groups (P<0.05). By contrast,
the ratio of Bcl-2/Bax in the CtBP2 overexpression
(LV-CtBP2+ + DDP) group was significantly increased
compared with those in the blank + DDP and LV-CtBP2+ +
DDP groups (P<0.05; Fig. 6C).
These results indicate that CtBP2 overexpression alleviated the
DDP-induced apoptosis of ECA109 cells by reducing the protein
levels of p53, cleaved caspase-3 and Bax, and increasing Bcl-2
expression.
Discussion
Understanding of the biology and molecular
mechanisms underlying ESCC development and progression is required
for advances in the treatment of ESCC (34). Chemotherapy is one of the main
therapeutic strategies for the treatment of ESCC (35,36).
However, chemoresistance to anticancer drugs greatly reduces the
effectiveness of these drugs, and is a huge obstacle to the
discovery of a successful therapy for ESCC (37). Therefore, it is urgently necessary
to identify an effective potential therapeutic target for
combatting drug resistance in ESCC. DDP is a first-line drug in the
treatment for ESCC, and DDP resistance remains a serious
challenge.
A previous study conducted by the present research
team indicated that the expression of CtBP2 was upregulated in ESCC
tissues compared with adjacent non-tumorous tissues (17). However, the effect of CtBP2 on the
susceptibility of ESCC cells to DDP was unclear. Therefore, the
present study established stable ECA109 cells with the
overexpression or knockdown of CtBP2 via recombinant lentiviral
transfection in order to investigate the effect of CtBP2 on these
cells when treated with DDP.
In the present study, the optimized concentration
and treatment time of DDP for use in vitro were determined
by MTT assay; 1.5×10−5 M DDP treatment for 24 h was
selected for further investigation. The optimized concentration
(1.5×10−5 M) in the study is similar to the clinically
used dose of DDP (20 mg/m2/day) (38,39).
The effect of CtBP2 on the cell apoptosis induced by DDP was
investigated by Hoechst 33342 staining and FCM. The overexpression
of CtBP2 attenuated the reduction of cell viability and inhibited
the cell apoptosis induced by DDP. By comparison, the knockdown of
CtBP2 increased the susceptibility of ECA109 cells to DDP, as it
increased the number of apoptotic cells following treatment with
DDP. These findings indicate that CtBP2 is a potential target for
the chemotherapy of ESCC, via which drug resistance may be reversed
in patients with ESCC.
There are various mechanisms by which tumor cells
develop resistance to chemotherapy. CtBP2 has been demonstrated to
reduce the chemosensitivity of breast cancer cells to various
chemotherapeutic drugs via p53-dependent and p53-independent
effects (29). The present study
attempted to further investigate the effect of CtBP2 on the
chemoresistance of ECA109 cells. The protein levels of the
apoptosis-related proteins p53, Bcl-2, Bax and cleaved caspase-3 in
the DDP-treated ECA109 cells with CtBP2 overexpression or knockdown
were determined using western blotting. The overexpression of CtBP2
reduced the protein levels of p53, cleaved caspase-3 and Bax, and
increased the protein levels of Bcl-2 in DDP-treated ECA109 cells,
and the knockdown of CtBP2 exhibited opposing effects. These
results indicated that CtBP2 reduced the chemosensitivity of ECA109
cells to DPP via the inhibition of p53, caspase-3 and Bax. This
information supplements the findings of a previous study by the
present research team, which demonstrated that CtBP2 promotes the
progression of ESCC via the negative transcriptional regulation of
p16INK4A (27). Nuclear
p16 has been reported to be important to the chemosensitivity of
multiple cancers (40,41), and in ESCC, CtBP2 promotes
chemoresistance through the negative transcriptional regulation of
p16INK4A. However, a limitation of the present study is
that only a single cell line was investigated, and the further
investigation of other cell types of esophageal cancer is required
to confirm the findings of the study.
In conclusion, the results of the present study
indicate that CtBP2 attenuated the susceptibility of ESCC cells to
DDP by regulating the expression of apoptosis-related proteins and
thereby inhibiting cell apoptosis. This knowledge may provide a new
strategy for decreasing the chemoresistance to DDP in the treatment
of ESCC.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81101159
and 81502055), and the Natural Science Foundation of Jiangsu
Province, China (grant no. BK20151268).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
MJ, YZ, HS, YM and QJ designed and did the research.
QJ, YW, WB and PW analyzed the data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests with respect to the data appearing in the manuscript.
References
|
1
|
Wakatsuki K, Matsumoto S, Migita K, Ito M,
Kunishige T, Nakade H, Nakatani M, Kitano M, Takano M, Obayashi C,
et al: Usefulness of computed tomography density of a tumor in
predicting the response of advanced esophageal cancer to
preoperative chemotherapy. Surgery. 162:823–835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Servagi-Vernat S, Créhange G, Bonnetain F,
Mertens C, Brain E and Bosset JF: Chemoradiation in elderly
esophageal cancer patients: Rationale and design of a phase I/II
multicenter study (OSAGE). BMC Cancer. 17:4832017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Canto MI, Abrams J, Kunzli H, Weusten B,
Komatsu Y, Jobe BA and Lightdale CJ: Nitrous oxide cryotherapy for
treatment of esophageal squamous cell neoplasia: Initial
multicenter international experience with a novel portable
cryoballoon ablation system (with video). Gastrointest Endosc.
87:574–581. 2018. View Article : Google Scholar
|
|
4
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Wu X, Li L, Liu Y, Xu C, Su D and
Liu Z: The E3 ligase HECTD3 promotes esophageal squamous cell
carcinoma (ESCC) growth and cell survival through targeting and
inhibiting caspase-9 activation. Cancer Lett. 404:44–52. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kjaer DW, Larsson H, Svendsen LB and
Jensen LS: Changes in treatment and outcome of oesophageal cancer
in Denmark between 2004 and 2013. Br J Surg. 104:1338–1345. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin-Richard M, Díaz Beveridge R,
Arrazubi V, Alsina M, Galan Guzmán M, Custodio AB, Gómez C, Muñoz
FL, Pazo R and Rivera F: SEOM Clinical Guideline for the diagnosis
and treatment of esophageal cancer (2016). Clin Transl Oncol.
18:1179–1186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sohda M and Kuwano H: Current status and
future prospects for esophageal cancer treatment. Ann Thorac
Cardiovasc Surg. 23:1–11. 2017. View Article : Google Scholar :
|
|
9
|
Arnold M, Laversanne M, Brown LM, Devesa
SS and Bray F: Predicting the future burden of esophageal cancer by
histological subtype: International trends in incidence up to 2030.
Am J Gastroenterol. 112:1247–1255. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palumbo Júnior A, Da Costa NM, Esposito F,
Fusco A and Pinto LF: High Mobility Group A proteins in esophageal
carcinomas. Cell Cycle. 15:2410–2413. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan F, Mao H, Bu F, Tong X, Li J, Zhang S,
Liu X, Wang L, Wu L, Chen R, et al: Sp1-mediated transcriptional
activation of miR-205 promotes radioresistance in esophageal
squamous cell carcinoma. Oncotarget. 8:5735–5752. 2017.
|
|
12
|
Sugihara H, Ishimoto T, Miyake K, Izumi D,
Baba Y, Yoshida N, Watanabe M and Baba H: Noncoding RNA expression
aberration is associated with cancer progression and is a potential
biomarker in esophageal aquamous cell carcinoma. Int J Mol Sci.
16:27824–27834. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goense L, van Rossum PS, Kandioler D,
Ruurda JP, Goh KL, Luyer MD, Krasna MJ and van Hillegersberg R:
Stage-directed individualized therapy in esophageal cancer. Ann N Y
Acad Sci. 1381:50–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cleary JM, Mamon HJ, Szymonifka J, Bueno
R, Choi N, Donahue DM, Fidias PM, Gaissert HA, Jaklitsch MT, Kulke
MH, et al: Neoadjuvant irinotecan, cisplatin, and concurrent
radiation therapy with celecoxib for patients with locally advanced
esophageal cancer. BMC Cancer. 16:4682016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Phatak P, Byrnes KA, Mansour D, Liu L, Cao
S, Li R, Rao JN, Turner DJ, Wang JY and Donahue JM: Overexpression
of miR-214-3p in esophageal squamous cancer cells enhances
sensitivity to cisplatin by targeting survivin directly and
indirectly through CUG-BP1. Oncogene. 35:2087–2097. 2016.
View Article : Google Scholar :
|
|
16
|
Wu Y and Jiang M: The revolution of lung
cancer treatment: from vaccines, to immune checkpoint inhibitors,
to chimeric antigen receptor T therapy. Biotarget. 1:72017.
View Article : Google Scholar
|
|
17
|
Liu T, Li R, Zhao H, Deng J, Long Y, Shuai
MT, Li Q, Gu H, Chen YQ and Leng AM: eIF4E promotes tumorigenesis
and modulates chemosensitivity to cisplatin in esophageal squamous
cell carcinoma. Oncotarget. 7:66851–66864. 2016.PubMed/NCBI
|
|
18
|
Komatsu S, Ichikawa D, Kawaguchi T,
Miyamae M, Okajima W, Ohashi T, Imamura T, Kiuchi J, Konishi H,
Shiozaki A, et al: Circulating miR-21 as an independent predictive
biomarker for chemoresistance in esophageal squamous cell
carcinoma. Am J Cancer Res. 6:1511–1523. 2016.PubMed/NCBI
|
|
19
|
Sumner ET, Chawla AT, Cororaton AD,
Koblinski JE, Kovi RC, Love IM, Szomju BB, Korwar S, Ellis KC and
Grossman SR: Transforming activity and therapeutic targeting of
C-terminal-binding protein 2 in Apc-mutated neoplasia. Oncogene.
36:4810–4816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai F, Xuan Y, Jin JJ, Yu S, Long ZW, Cai
H, Liu XW, Zhou Y, Wang YN, Chen Z, et al: CtBP2 overexpression
promotes tumor cell proliferation and invasion in gastric cancer
and is associated with poor prognosis. Oncotarget. 8:28736–28749.
2017.PubMed/NCBI
|
|
21
|
Riku M, Inaguma S, Ito H, Tsunoda T, Ikeda
H and Kasai K: Down-regulation of the zinc-finger homeobox protein
TSHZ2 releases GLI1 from the nuclear repressor complex to restore
its transcriptional activity during mammary tumorigenesis.
Oncotarget. 7:5690–5701. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng X, Song T, Dou C, Jia Y and Liu Q:
CtBP2 is an independent prognostic marker that promotes GLI1
induced epithelial-mesenchymal transition in hepatocellular
carcinoma. Oncotarget. 6:3752–3769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang C, Li S, Qiao B, Yang K, Liu R, Ma
B, Liu Y, Zhang Z and Xu Y: CtBP2 overexpression is associated with
tumorigenesis and poor clinical outcome of prostate cancer. Arch
Med Sci. 11:1318–1323. 2015. View Article : Google Scholar
|
|
24
|
Yang X, Sun Y, Li H, Shao Y, Zhao D, Yu W
and Fu J: C-terminal binding protein-2 promotes cell proliferation
and migration in breast cancer via suppression of p16INK4A.
Oncotarget. 8:26154–26168. 2017.PubMed/NCBI
|
|
25
|
Frietze S, O'Geen H, Littlepage LE, Simion
C, Sweeney CA, Farnham PJ and Krig SR: Global analysis of ZNF217
chromatin occupancy in the breast cancer cell genome reveals an
association with ERalpha. BMC Genomics. 15:5202014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
May T, Yang J, Shoni M, Liu S, He H, Gali
R, Ng SK, Crum C, Berkowitz RS and Ng SW: BRCA1 expression is
epigenetically repressed in sporadic ovarian cancer cells by
overexpression of C-terminal binding protein 2. Neoplasia.
15:600–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan C, Shi H, Wang H, Zhang J, Ni W, Chen
B, Hou S, Yang X, Shen A and Ni R: CtBP2 contributes to malignant
development of human esophageal squamous cell carcinoma by
regulation of p16INK4A. J Cell Biochem. 114:1343–1354. 2013.
View Article : Google Scholar
|
|
28
|
Zhang J, Zhu J, Yang L, Guan C, Ni R, Wang
Y, Ji L and Tian Y: Interaction with CCNH/CDK7 facilitates CtBP2
promoting esophageal squamous cell carcinoma (ESCC) metastasis via
upregulating epithelial-mesenchymal transition (EMT) progression.
Tumour Biol. 36:6701–6714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Birts CN, Harding R, Soosaipillai G,
Halder T, Azim-Araghi A, Darley M, Cutress RI, Bateman AC and
Blaydes JP: Expression of CtBP family protein isoforms in breast
cancer and their role in chemoresistance. Biol Cell. 103:1–19.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Ansari S, Chen C, Hasani-Sadrabadi MM, Yu
B, Zadeh HH, Wu BM and Moshaverinia A: Hydrogel elasticity and
microarchitecture regulate dental-derived mesenchymal stem
cell-host immune system cross-talk. Acta Biomater. 60:181–189.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mihaly SR, Sakamachi Y, Ninomiya-Tsuji J
and Morioka S: Noncanocial cell death program independent of
caspase activation cascade and necroptotic modules is elicited by
loss of TGFβ-activated kinase 1. Sci Rep. 7:29182017. View Article : Google Scholar
|
|
33
|
Luna C, Mendoza N, Casao A, Pérez-Pé R,
Cebrián-Pérez JA and Muiño-Blanco T: c-Jun N-terminal kinase and
p38 mitogen-activated protein kinase pathways link capacitation
with apoptosis and seminal plasma proteins protect sperm by
interfering with both routes. Biol Reprod. 96:800–815. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Whiteside TL: Stimulatory role of exosomes
in the context of therapeutic anti-cancer vaccines. Biotarget.
1:52017. View Article : Google Scholar
|
|
35
|
Kwon D, Yun JY, Keam B, Kim YT and Jeon
YK: Prognostic implications ofFGFR1andMYCstatus in esophageal
squamous cell carcinoma. World J Gastroenterol. 22:9803–9812. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen P, Zhang JY, Sha BB, Ma YE, Hu T, Ma
YC, Sun H, Shi JX, Dong ZM and Li P: Luteolin inhibits cell
proliferation and induces cell apoptosis via down-regulation of
mitochondrial membrane potential in esophageal carcinoma cells EC1
and KYSE450. Oncotarget. 8:27471–27480. 2017.PubMed/NCBI
|
|
37
|
Zhang HF, Wu C, Alshareef A, Gupta N, Zhao
Q, Xu XE, Jiao JW, Li EM, Xu LY and Lai R: The PI3K/AKT/c-MYC axis
promotes the acquisition of cancer stem-like features in esophageal
squamous cell carcinoma. Stem Cells. 34:2040–2051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Konishi H, Fujiwara H, Shiozaki A, Shoda
K, Kosuga T, Kubota T, Okamoto K and Otsuji E: Effects of
neoadjuvant 5-fluorouracil and cisplatin therapy in patients with
clinical stage II/III esophageal squamous cell carcinoma.
Anticancer Res. 38:1017–1023. 2018.PubMed/NCBI
|
|
39
|
Liu B, Wang C, Chen P, Cheng B and Cheng
Y: RACKI induces chemotherapy resistance in esophageal carcinoma by
upregulating the PI3K/AKT pathway and Bcl-2 expression. Onco
Targets Ther. 11:211–220. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Veena MS, Wilken R, Zheng JY, Gholkar A,
Venkatesan N, Vira D, Ahmed S, Basak SK, Dalgard CL, Ravichandran
S, et al: p16 protein and gigaxonin are associated with the
ubiquitination of NFκB in cisplatin-induced senescence of cancer
cells. J Biol Chem. 289:34921–34937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Geiger JL, Lazim AF, Walsh FJ, Foote RL,
Moore EJ, Okuno SH, Olsen KD, Kasperbauer JL, Price DL, Garces YI,
et al: Adjuvant chemoradiation therapy with high-dose versus weekly
cisplatin for resected, locally-advanced HPV/p16-positive and
negative head and neck squamous cell carcinoma. Oral Oncol.
50:311–318. 2014. View Article : Google Scholar : PubMed/NCBI
|