Introduction
Malignant cells accumulating in the tissues of the
bladder form bladder cancer (BC) (1,2). BC
is one of several types of cancer developing from the epithelial
cells protecting the innermost tissue surface layer of the urinary
bladder, termed urothelial carcinoma. BC has become a serious
health problem and is the ninth most common type of cancer
worldwide (3). In 2012, ~430,000
new cases were diagnosed in patients, with an associated mortality
rate of ~165,000 worldwide (4).
Men are more often affected by BC than women, which represent 75%
of all BC cases (3). Those cases
of BC con fined to the mucosa and submucosa, designated as
non-muscle invasive BC (NMIBC), represent 75% of new BC cases
(5). However, in ~80% of patients
with NMIBC, disease recurs following initial treatment within 5
years (5). The high rates of
relapse and progression of BC have attracted global attention to
investigate the molecular mechanisms underlying its carcinogenesis
and progression.
The human trophoblast cell surface antigen 2
(Trop2), also termed GA733-1, EGP-1 or M1S1, is a cell-surface
glycoprotein (6). Trop2 is a
typical member of the tumor-associated calcium signal transducer
gene family and is identified as an epithelial adhesion molecule
(7). Trop2 was found to be a
biological marker of aggressive human trophoblast cells (8), originating from the outer layer of
the blastosphere and contributing to implantation. Trop2 also has
multifaceted roles in development and tumorigenesis by regulating
cell growth, migration and self-renewal (6). Trop2 was first identified as a
tumor-associated antigen when GA733 antibody immunoprecipitated
with Trop2 not only in gastrointestinal tumors, but also in
bladder, lung and cervical cancer (9). Cancer is a complex disorder during
development, which is always accompanied by the deregulation of
molecular events (10). Trop2 was
confirmed to trigger tumor proliferation, thus acting as a cancer
driver (11). The overexpression
of Trop2 has been found in the majority of types of human cancer,
including esophageal (12),
colorectal (13), oral (14), pancreatic (15), breast (16), glioma (17), uterine (18), ovarian (19) and prostate (20) cancer. The overexpression of Trop2
in human tumors promotes tumor aggressiveness and metastasis,
resulting in reduced survival rates (7). Although studies have investigated the
correlation between the expression of Trop2 and poor survival rates
in various cancer patients, the biological significance of Trop2 in
BC remains to be fully elucidated.
Curcumin is an active natural component of turmeric
derived from Curcuma longa. It has long been used across
Asia, particularly in India, as a food condiment and a traditional
herbal medicine. Curcumin exhibits anti-inflammatory,
anti-oxidative, antiproliferative, apoptosis-inducing, antitumor
and chemopreventive activities (21–23).
Accumulating data have shown that curcumin offers therapeutic
potential in a variety of cancer types (24,25).
Mechanistically, curcumin exhibits its therapeutic properties
through regulating multiple targets, including nuclear factor
(NF)-κB, Notch, S-phase kinase-associated protein 2 (Skp2), and
multiple microRNAs (22,25–29).
Gao et al found that curcumin promotes Krüppel-like factor 5
(KLF5) proteasome-dependent degradation by regulating
Yes-associated protein (YAP)/transcriptional coactivator with PDZ-
binding motif (TAZ) in BC cells (30). In T24 and 5637 cells, curcumin was
identified to decrease cell growth and migration, and to trigger
apoptosis via suppressing matrix metalloproteinase (MMP)-2 and
MMP-9 signaling pathways in vitro (31). However, whether curcumin affects
Trop2 in BC remains to be elucidated. Therefore, in the present
study, using a series of in vitro assays, the toxicity of
curcumin towards T24 and RT4 BC cell lines was examined, to reveal
whether Trop2 was a target of curcumin. In addition, whether Trop2
was associated with the antiproliferative property of curcumin
treatment was examined.
Materials and methods
Reagents and cell culture
The T24 and RT4 human BC cell lines were obtained
from the Chinese Academy of Science (Shanghai, China). The BC cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; cat. no.
MGC803; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% FBS and 100 U/ml penicillin/strep tomycin
(HyClone™; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in
a humidified atmosphere containing 5% CO2. Curcumin
(Sigma-Aldrich; EMD Millipore, Billerica, MA, USA) was dissolved in
dimethylsulfoxide (DMSO) and stored at −20°C. 3–4,5-dimethyl-2-
thiazolyl-2, 5-diphenyl-2-H-tetrazolium bromide (MTT; CAS no.
57360-69-7) was obtained from Sigma-Aldrich; EMD Millipore.
Lipofectamine 2000 was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. Primary antibodies targeting Trop2 (#90540), p27
(#2552), and cyclin E1 (#4129), and monoclonal anti-β-actin (#3700)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Secondary antibodies (#A-11031 and #A-11034) were obtained
from Thermo Fisher Scientific, Inc.
Cell proliferation assays
The T24 and RT4 cells were plated in 96-well plates
(5×103 cells/well) and cultured for ~24 h. The cells
were treated with curcumin (10, 15, 20 and 25 µM) at 37°C
for 48 and 72 h. The experimental control group (0.1% DMSO) was set
up as the zero group. Each group contained at least five wells.
Cell growth abilities were assessed using MTT assays following the
manufacturer's protocols. The MTT solution (10 µl; 0.5
mg/ml) was added to each well and incubated for 4 h. The
supernatant was then removed, and 100 µl DMSO was added to
dissolve the formazan product. Cell viability was determined by
detecting the absorbance at 590 nm.
Cell apoptosis analysis
The BC cells (2×105 cells/well) were
seeded in 6-well plates. A series of concentrations of curcumin
were added and the cells were cultured for 2 days. Cell apoptotic
death was assessed with an Annexin V/FITC Apoptosis Detection kit
(BD Biosciences, Franklin Lakes, NJ, USA). The curcumin-treated BC
cells were harvested and stained with propidium iodide (PI) and
annexin V-FITC, and were measured using a FACS calibur flow
cytometer (BD Biosciences).
Cell cycle assays
The T24 and RT4 BC cells were seeded in 6-well
plates and treated with curcumin for 468. The cells were collected
by trypsinization and washed with PBS and then suspended with
pre-cooling alcohol (70%). Following fixing overnight, the cells
were precipitated by centrifugation for 5 min at 200 × g and
pelleted cells were washed three times with 10 ml PBS. The cells
were then incubated with RNase and PI. Cell cycle distributions
were detected using a flow cytometer (BD Biosciences).
Wound-healing assay
The BC cells were plated into a 6-well plate and
incubated until the cells grew to ~90% confluence. The confluent
monolayer was injured with a sterile 100-µl tip and a
rectangular wound was created. Cell debris was carefully removed,
and the cells were cultured for ~16 h. The cells migrated into the
wound were visualized using an inverted microscope. The wound size
was scored by measuring the lesion border and comparing with the
size of the initial wound.
Cell invasion assay
Transwell chambers (8-µm pore size, Corning
Incorporated, Corning, NY, USA) were used to assess the invasive
ability of the BC cells. Following culture for 24 h at 37°C, the
cells were suspended in the FBS-free DMEM. The cells
(5×104 cells; 200 µl) were placed in the Matrigel
(BD Biosciences) pre-coated upper chamber, following which 600
µl of complete DMEM was added to the lower chamber.
Following incubation for 468, the remaining cells were removed with
cotton wool. Cells invading through the filters were stained with
Calcein-AM for 10 min, followed by rinsing the filters with water.
The stained cells were observed under a light microscope.
Transient transfection of plasmids and
small interfering RNAs (siRNAs)
The BC cells were transfected with pcDNA3.1-Trop2-
or Trop2-targeting siRNAs with Lipofectamine™ 2000 transfection
reagent, following the manufacturer's protocol. The siRNA sequences
(sense, 5′-ACA CTT GGA GGT TTT GGC CAC TGA CTG ACT CCA AGT GTC TGC
TGC TCAA-3′; antisense, 3′-CCT GTT GAG CAG CAG ACT TGG AGG TCA GTC
AGT CAG TGG CCA AAA CCT CCA AGT GTC TGC TGC TCA AC-5′) targeting
human Trop2 were purchased from GenePharma (Shanghai, China).
Western blot analysis
The BC cells were inoculated into the culture plate
at a concentration of 1×104 cells/cm2. The
cells were then transfected with pcDNA3.1-Trop2- or Trop2-targeting
siRNAs or treated with curcumin for a designated time course. The
pretreated BC cells were precipitated and lysed in lysis buffer
supplemented with protease inhibitor cocktail and PMSF (Roche
Diagnostics, Basel, Switzerland). A BCA protein assay was used to
quantify protein concentrations. Protein samples (30 µg)
were separated by 10% SDS-PAGE. The separated proteins were then
transferred onto a nitrocellulose membrane and were blocked with 5%
skimmed milk at room temperature for 1 h. The primary antibodies
(Trop2, 1:1,500; p27, 1:1,000; cyclin E1, 1:2,000; and
anti-β-actin, 1:3,000) were added and incubated at 4°C overnight.
After washing with TBS-Tween-20, a suitable secondary antibody
(anti-mouse, 1:2,500; anti-rabbit, 1:2,500) was added and incubated
at room temperature for ~1 h. The target proteins were developed
onto a film by an ECL imaging system (Pierce; Thermo Fisher
Scientific, Inc.). The protein expression levels were
semi-quantitated via densitometry using ImageJ software version
1.51 (National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/).
Statistical analysis
Data are presented as the mean ± standard deviation
of the mean following analysis with GraphPad Prism 4.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Statistical significance values
were evaluated through one-way analysis of variance with a
Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
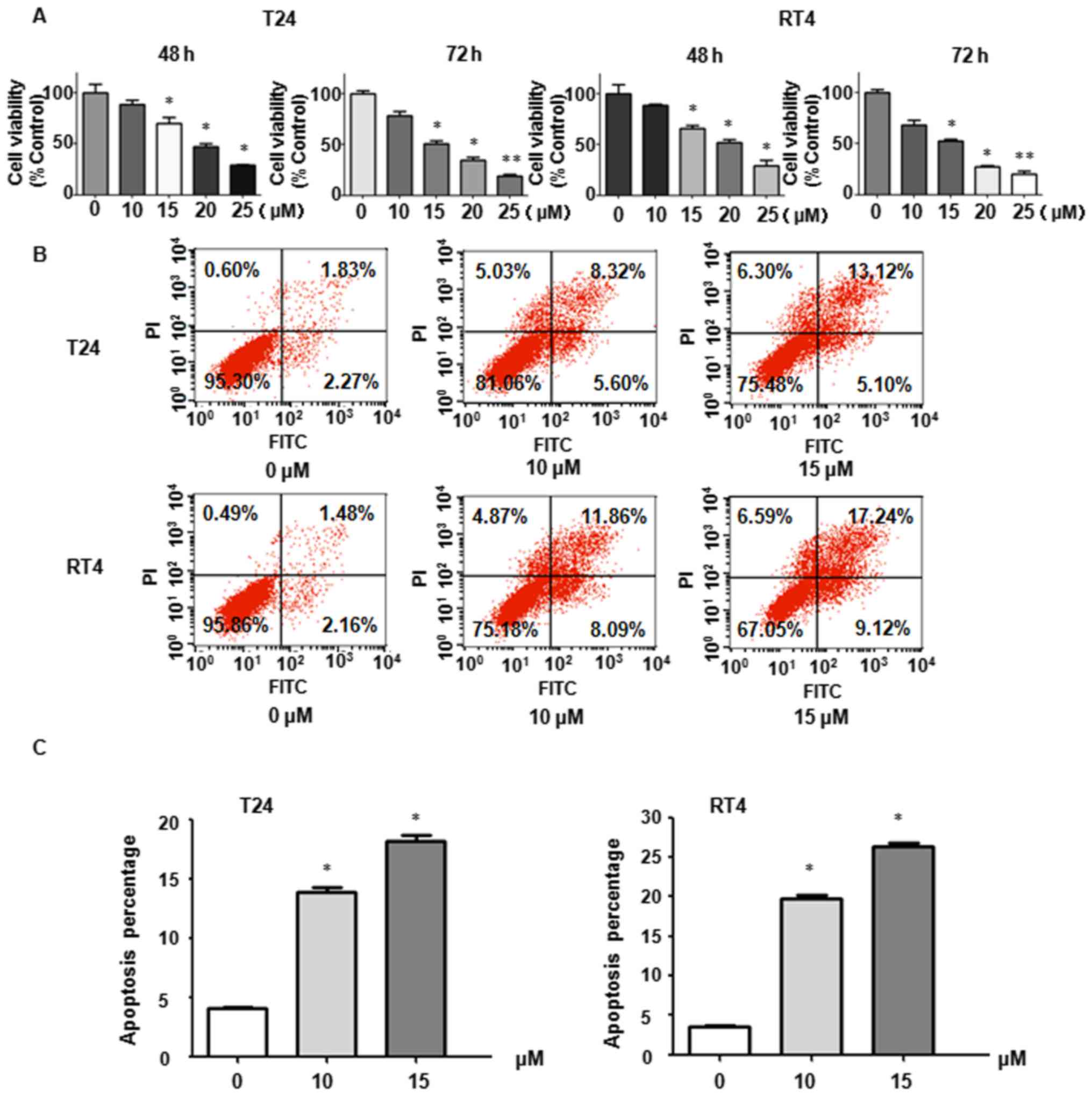
Curcumin inhibits BC cell growth
Curcumin has been reported to suppress proliferation
in various types of cancer. The present study aimed to determine
whether curcumin inhibits T24 and RT4 BC cell growth. The BC cells
were treated with curcumin for 48 and 72 h, respectively. An MTT
assay was performed and the resulting data showed that curcumin
suppressed BC cell proliferation in a time- and dose-dependent
manner (Fig. 1A). These data
supported the hypothesis that curcumin exerts its antitumor growth
property in BC cells.
Curcumin promotes apoptotic death of BC
cells
Subsequently, the present study detected whether
curcumin affects apoptotic death of BC cells. Annexin V-FITC/PI
apoptosis analysis was performed in T24 and RT4 BC cells following
curcumin treatment. As shown in Fig.
1B and C, curcumin induced apoptotic death of T24 and RT4 cells
in a dose-dependent manner. For example, treatment with 10 and 15
µM curcumin caused increased apoptotic death of RT4 cells
from 3.64% in the control group to 19.95 and 26.36% (Fig. 1B and C), respectively. It was found
that curcumin mainly induced late apoptosis, increasing from 1.83%
in the control group to 8.32 and 13.42% in T24 cells treated with
10 and 15 µM curcumin, respectively (Fig. 1B). A similar induction of late
apoptosis was induced by curcumin in RT4 cells (Fig. 1B). These results demonstrated that
curcumin promoted significant apoptotic death and may facilitate
inhibiting the proliferation of BC cells.
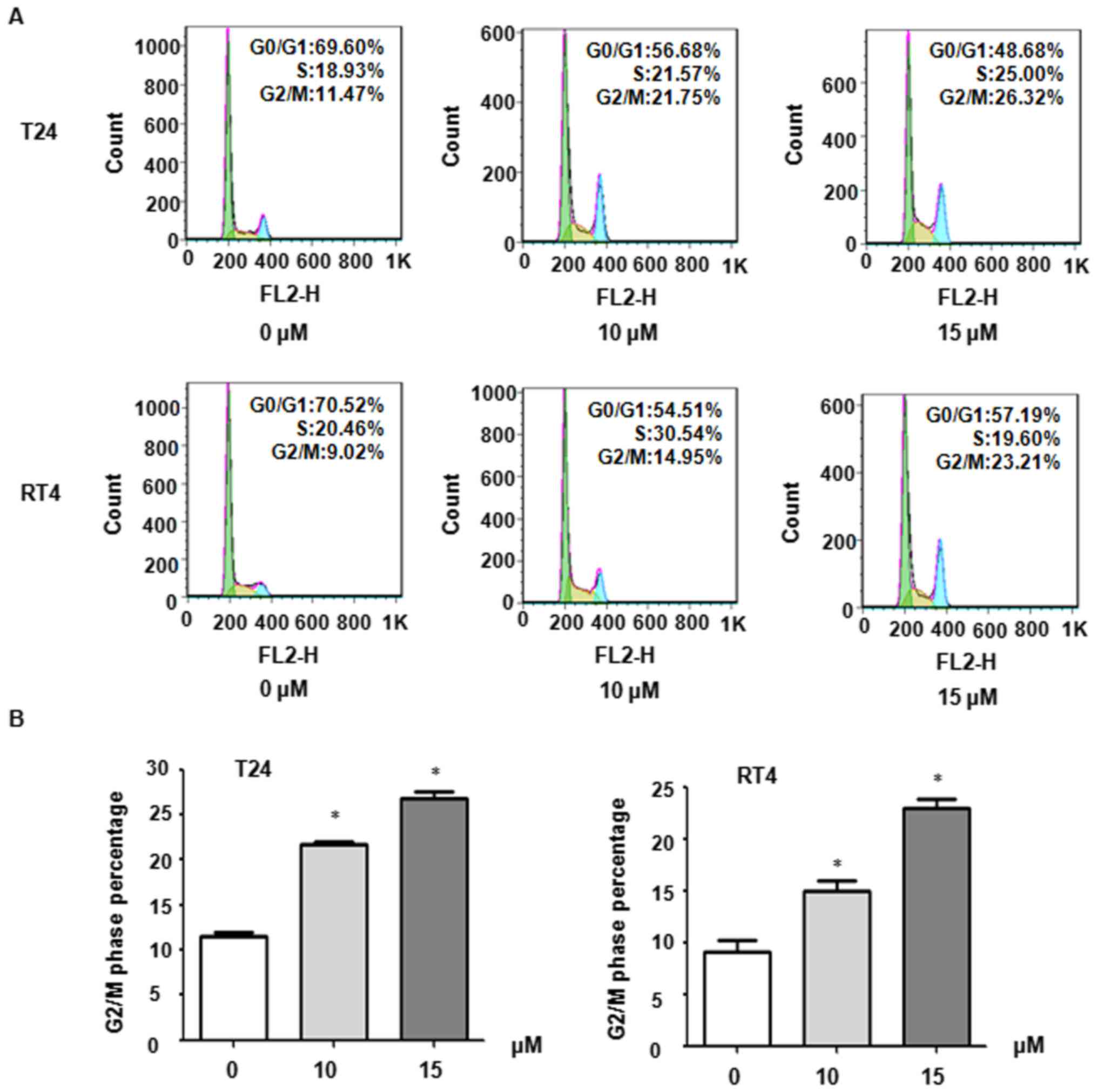
Curcumin induces cell cycle arrest of BC
cells
Further detection of the cell cycle distribution of
bladder cancer cells was performed following curcumin treatment.
Flow cytometry was performed following PI staining. The findings
revealed that curcumin treatment caused a significant increase in
the numbers of cells in the G2/M phase in a dose-dependent manner,
compared with control cells (Fig. 2A
and B). For example, treatment with 10 and 15 µM
curcumin increased the G2/M cell populations to 14.95 and 23.21% in
RT4 cells, from 9.02% in the control (Fig. 2A and B). A similar cell cycle
arrest pattern was observed in T24 cells (Fig. 2A and B). These findings confirmed
that curcumin induced G2/M cell cycle arrest in the BC cells.
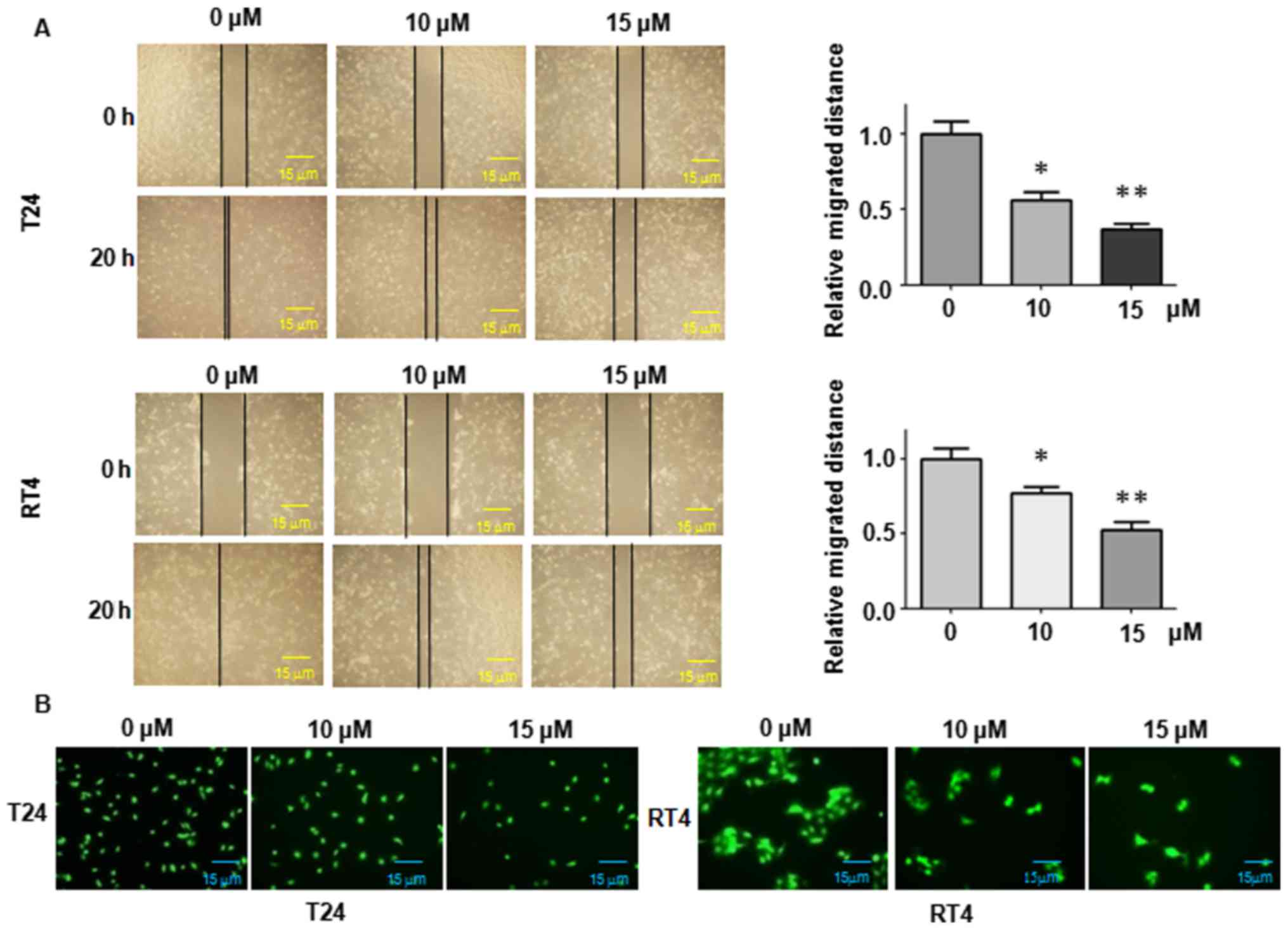
Curcumin suppresses cell migration of BC
cells
In order to detect the effects of curcumin on cell
motility, wound-healing assays were performed in T24 and RT4 cells,
respectively. As shown in Fig. 3A,
curcumin treatment significantly inhibited cell migration in the
two BC cell lines. These results revealed that curcumin treatment
notably inhibited cell migratory activity in the T24 and RT4 cancer
cells.
Curcumin suppresses cell invasion of BC
cells
The present study also measured whether curcumin
inhibits cell invasive ability using Transwell chambers. It was
found that the number of cells invaded through the pores of
Matrigel-coated filters was reduced in the two curcumin-treated BC
cell lines in a dose-dependent manner (Fig. 3B). It is important to note that 15
mM curcumin had a cytotoxic effect with ~20% growth inhibition in
the T24 cells and RT4 cells at 468. (Fig. 1A). However, treatment with 15 mM
curcumin for 24 h did not cause any cell growth inhibition in the
cell lines (data not shown). The invasion and migration were
measured in the two cell lines following treatment with 15 mM
curcumin for 20 h. Therefore, the effects on migration and invasion
by curcumin treatment were not due to cell viability inhibition.
The findings demonstrated that curcumin treatment significantly
inhibited the cell invasion potential of the T24 and RT4 BC
cells.
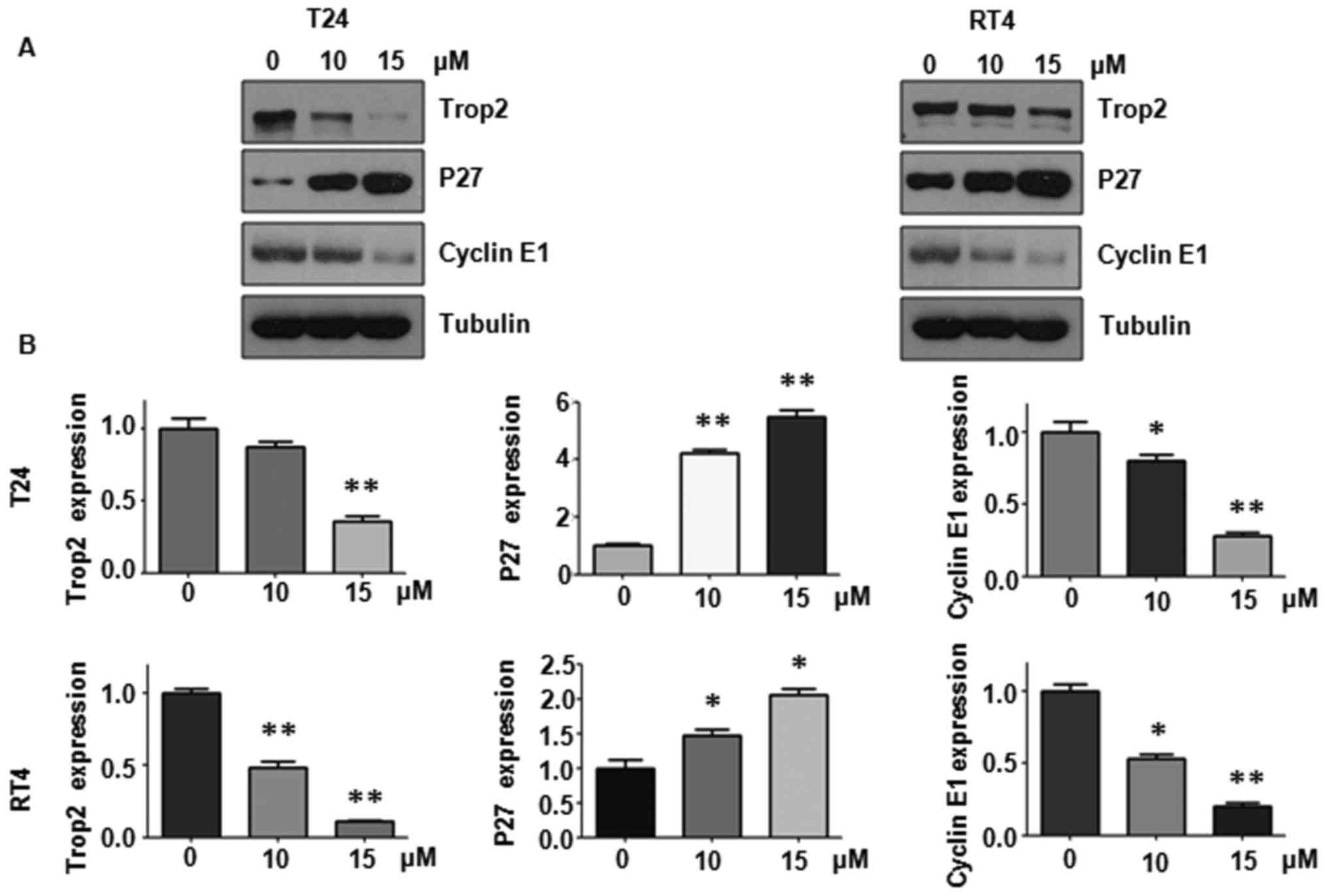
Curcumin suppresses the expression of
Trop2 in BC cells
Accumulating data have characterized Trop2 as a
tumor driver in various types of cancer, and pharmacological
inhibition of Trop2 may be a promising approach to treating BC.
Therefore, the present study further examined whether curcumin
treatment modulates the expression of Trop2 in BC cells. Following
treatment with curcumin for 468, western blot analysis revealed
that Trop2 was markedly reduced in the T24 and RT4 BC cells
(Fig. 4A and B). It was also
detected that the protein levels of p27 and cyclin E1, two typical
cell cycle regulators, were modulated following curcumin treatment
(Fig. 4A and B). p27, the cell
cycle inhibitor, was significantly induced by curcumin treatment.
cyclin E1, which is crucial in promoting cell cycle progression and
contributes to tumorigenesis, was markedly suppressed in the
presence of curcumin in BC cells. Taken together, these results
suggested that curcumin exercised antitumor function in BC cells at
least partially by downregulating the expression of Trop2. In
addition, curcumin caused cell-cycle arrest, which may be
attributed to the modulated expression of p27 and cyclin E1.
Overexpression of Trop2 promotes cell
proliferation
In order to further identify whether Trop2 was
involved in the cytotoxic effects of curcumin, the present study
induced the overexpression of Trop2 in BC cells via Trop2 cDNA
transfection in the presence of curcumin. An empty vector was
transfected as a control. The subsequent MTT assays demonstrated
that the overexpression of Trop2 triggered BC cell proliferation
(Fig. 5A). In addition, the cell
growth inhibition induced by curcumin was partly abrogated under
Trop2 overexpression (Fig.
5A).
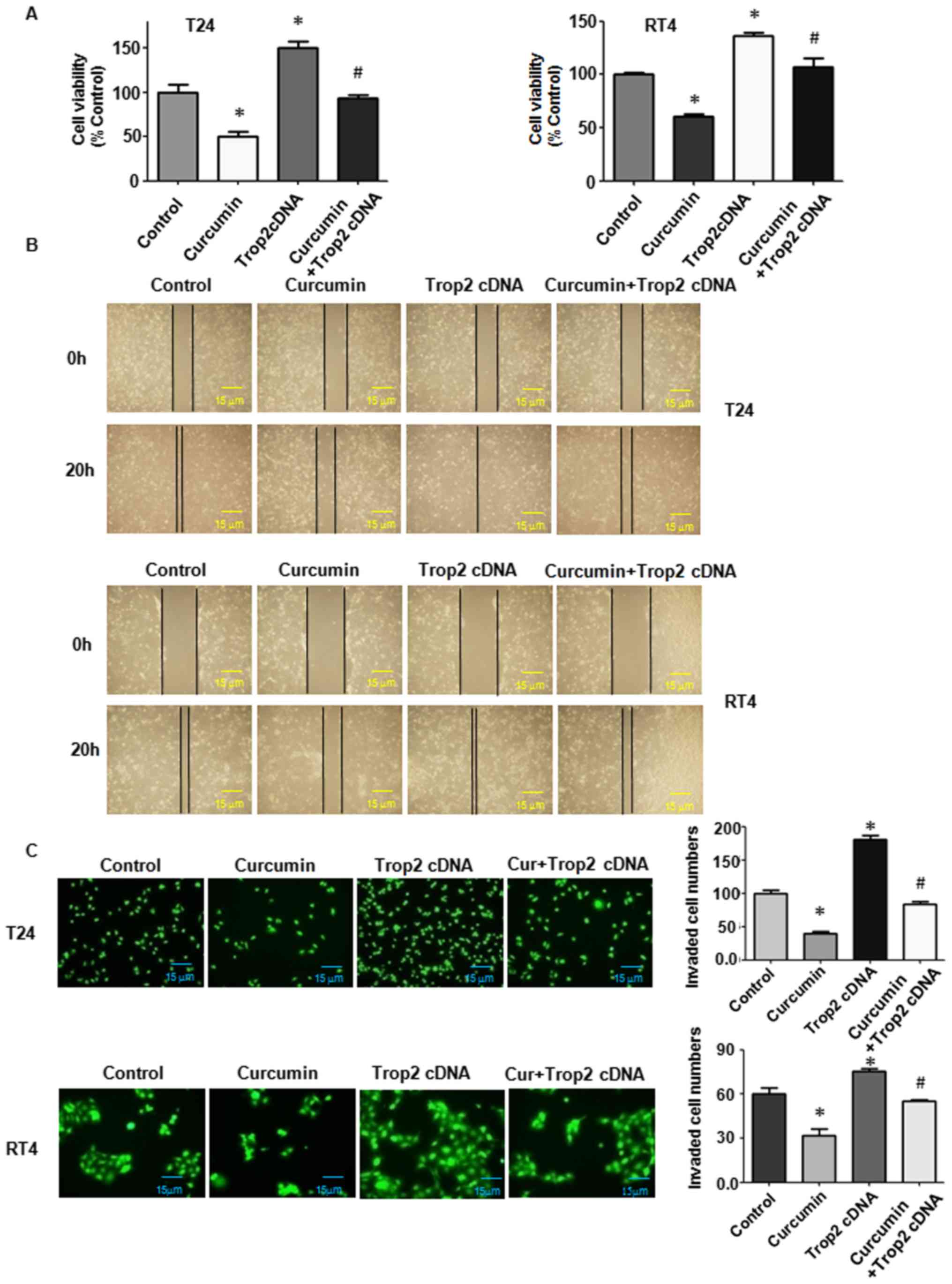 | Figure 5Overexpression of Trop2 enhances BC
cell proliferation, and increases cell migration and invasion. (A)
3–4,5-dimethyl-2- thiazolyl-2, 5-diphenyl-2-H-tetrazolium bromide
assay in BC cells to detect the growth changes following
overexpression of Trop2 alone or in combination with 15 µM
curcumin. (B) Wound-healing assay to determine cell migration
ability (magnification, ×100). (C) Invasion was detected using a
Transwell chamber assay. The experiments were repeated three times
(T24 cells, ×100 magnification; RT4 cells, ×200 magnification).
Quantitative results of the invasion assay are shown on the right.
*P<0.05, vs. control; #P<0.05, vs. 15
µM curcumin treatment or Trop2 cDNA transfection. BC,
bladder cancer; Trop2, trophoblast cell surface antigen 2; Trop2
cDNA, Trop2-expressing vector. |
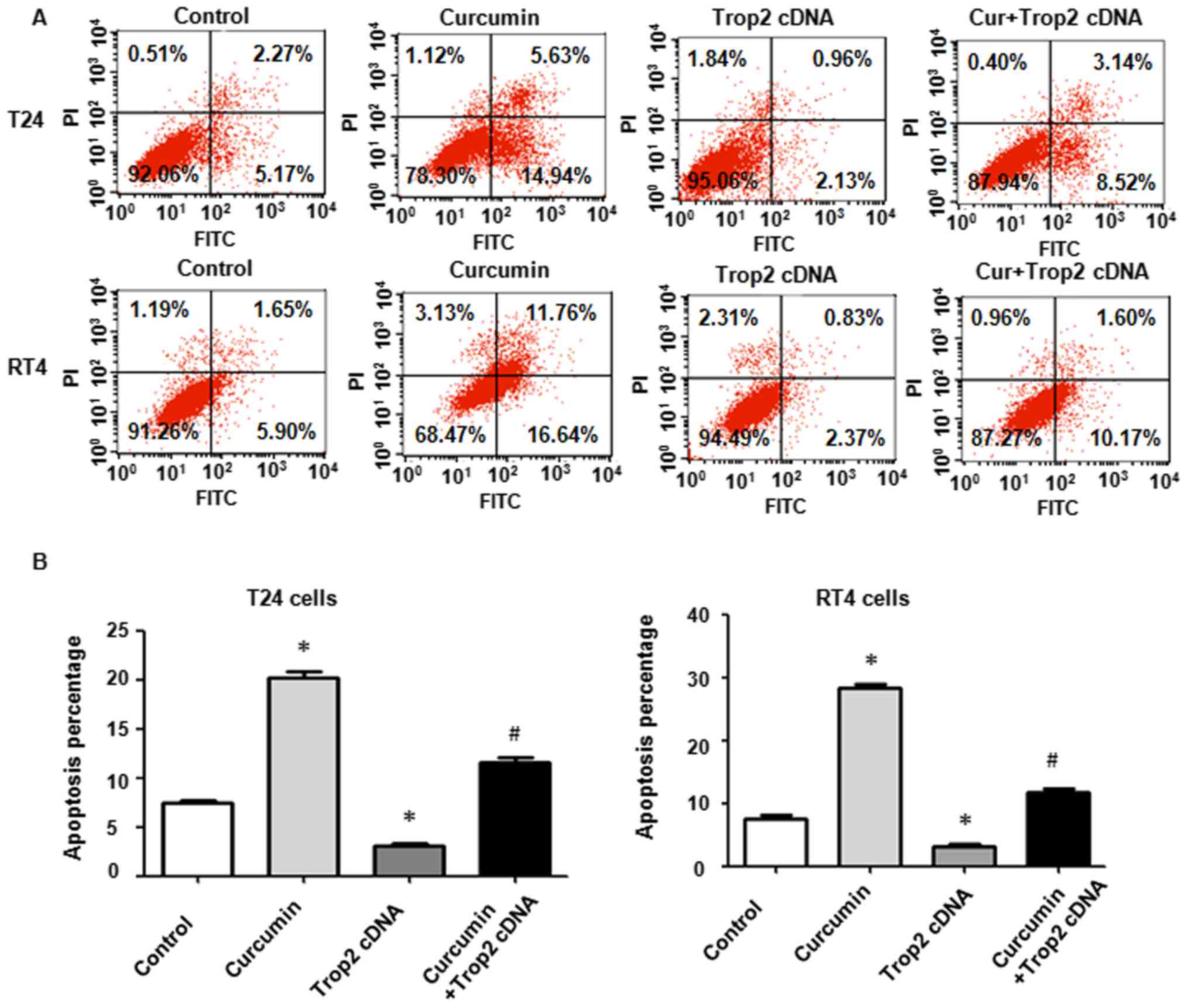
Overexpression of Trop2 promotes cell
migration and inhibits cell apoptosis
The cellular invasive and migratory properties were
measured to determine whether Trop2 regulates T24 and RT4 cell
mobility. Using a wound-healing assay, an increase in the migration
of BC cells was found under Trop2 overexpression (Fig. 5B). Consistently, the overexpression
of Trop2 eliminated curcumin-induced suppression of cell mobility
(Fig. 5B). The Transwell analysis
demonstrated that the overexpression of Trop2 markedly increased
the number of invasive BC cells (Fig.
5C). The overexpression of Trop2 reversed the curcumin-induced
inhibition of cell invasion (Fig.
5C). Furthermore, the effect of the overexpression of Trop2 on
apoptosis was detected. The resulting data demonstrated that the
overexpression of Trop2 markedly reduced the apoptotic cell
percentage in BC cells and abrogated curcumin-induced apoptotic
cell death (Fig. 6A and B).
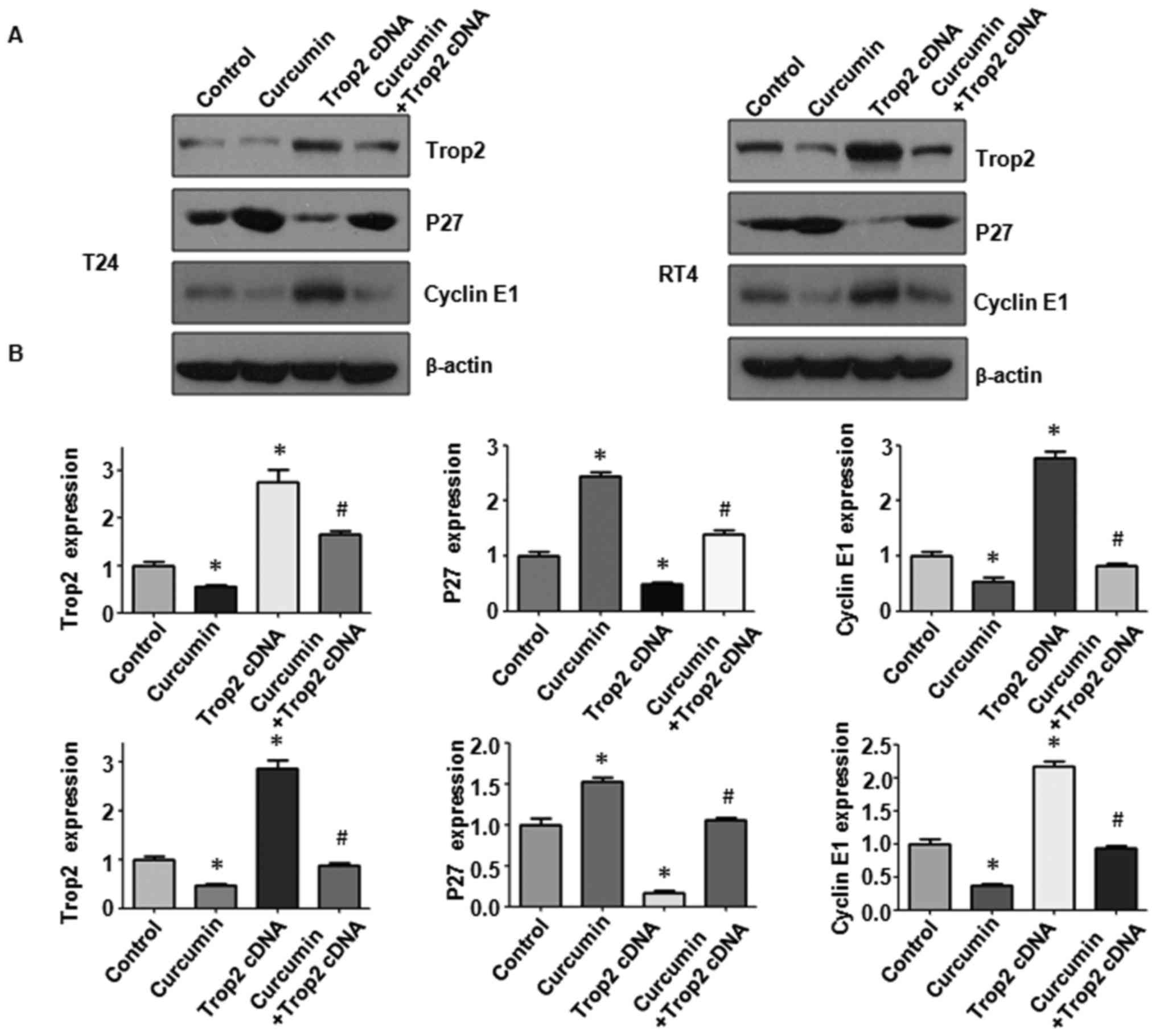
Overexpression of Trop2 modulates cell
cycle proteins
The protein levels of p27 and cyclin E1 were
measured in BC cells following Trop2 cDNA transfection. It was
observed that the overexpression of Trop2 significantly suppressed
protein levels of p27 in the T24 and RT4 cells (Fig. 7A and B; P<0.05). When the
overexpression of Trop2 was combined with curcumin treatment, the
induced expression of p27 was partially abrogated (Fig. 7A and B; P<0.05). Conversely, the
protein level of cyclin E1 was significantly induced in the two BC
cell lines with the overexpression of Trop2 (Fig. 7B; p<0.05). The curcumin-induced
suppression of cyclin E1 was partially abrogated when combined with
Trop2 cDNA transfection. Taken together, these findings supported
the hypothesis that curcumin exhibits anti-tumor activity in BC
cells partially via regulating the expression of Trop2 and its
downstream targets p27 and cyclin E1.
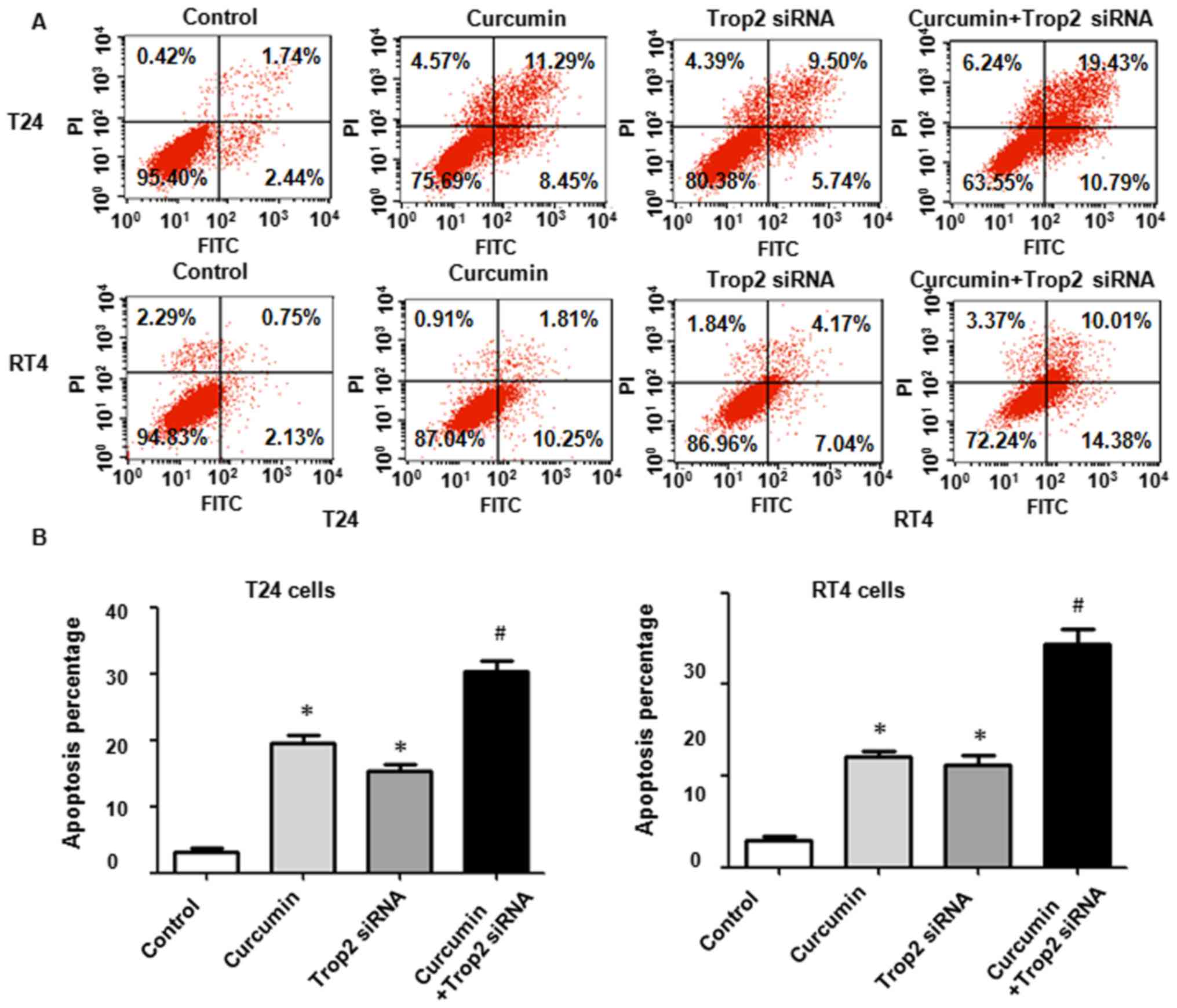
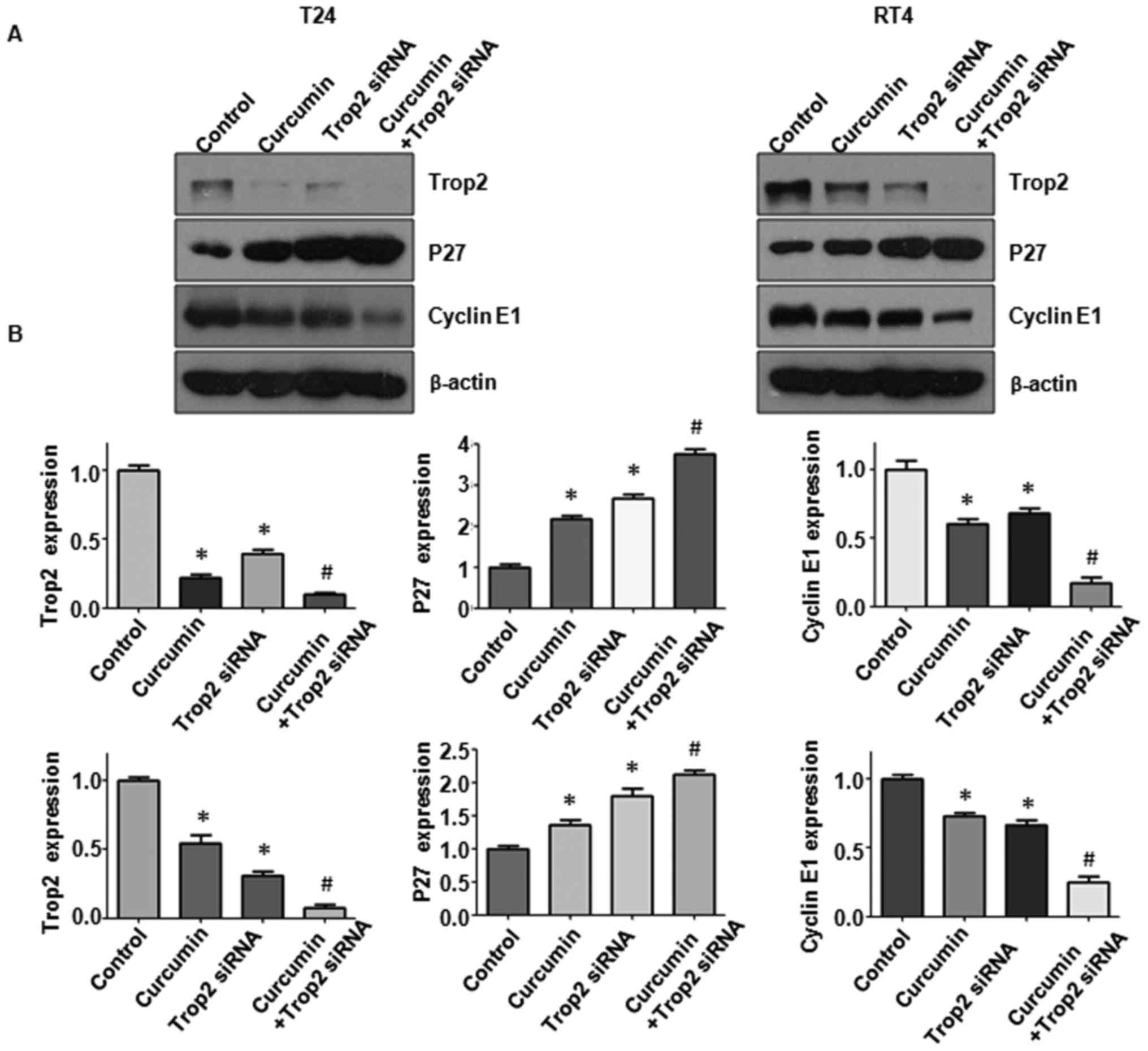
Downregulation of Trop2 by siRNA
transfection sensitizes BC cells to curcumin treatment
In addition, Trop2 siRNA was used to knock down
Trop2 in the BC cells and its effects on cell proliferation,
mobility, apoptosis, and the expression of p27 and cyclin E1 were
examined. Following Trop2 siRNA transfection, the MTT assay results
showed that T24 and RT4 cell proliferation was inhibited (Fig. 8A). When Trop2 was downregulated in
the presence of curcumin, the suppression of cell proliferation was
significantly enhanced compared with that in the cells treated with
either curcumin or siRNA transfection alone (Fig. 8A; P<0.05). The downregulation of
Trop2 combined with curcumin treatment also suppressed cell
invasion and migration (Fig. 8B and
C; P<0.05). The sensitivity of BC cells to apoptosis from
curcumin was markedly enhanced in the Trop2-silenced cells
(Fig. 9A and B). Furthermore, the
downregulation of Trop2 altered the levels of p27 and cyclin E1 in
the T24 and RT4 cells (Fig. 10A and
B). Taken together, these findings demonstrated that Trop2 was
associated with the curcumin-induced suppression of cell
proliferation, increase of apoptosis, and inhibition of invasive
and migration abilities in BC cells. Curcumin treatment together
with the downregulation of Trop2 enhanced the antineoplastic
property of curcumin in BC cells.
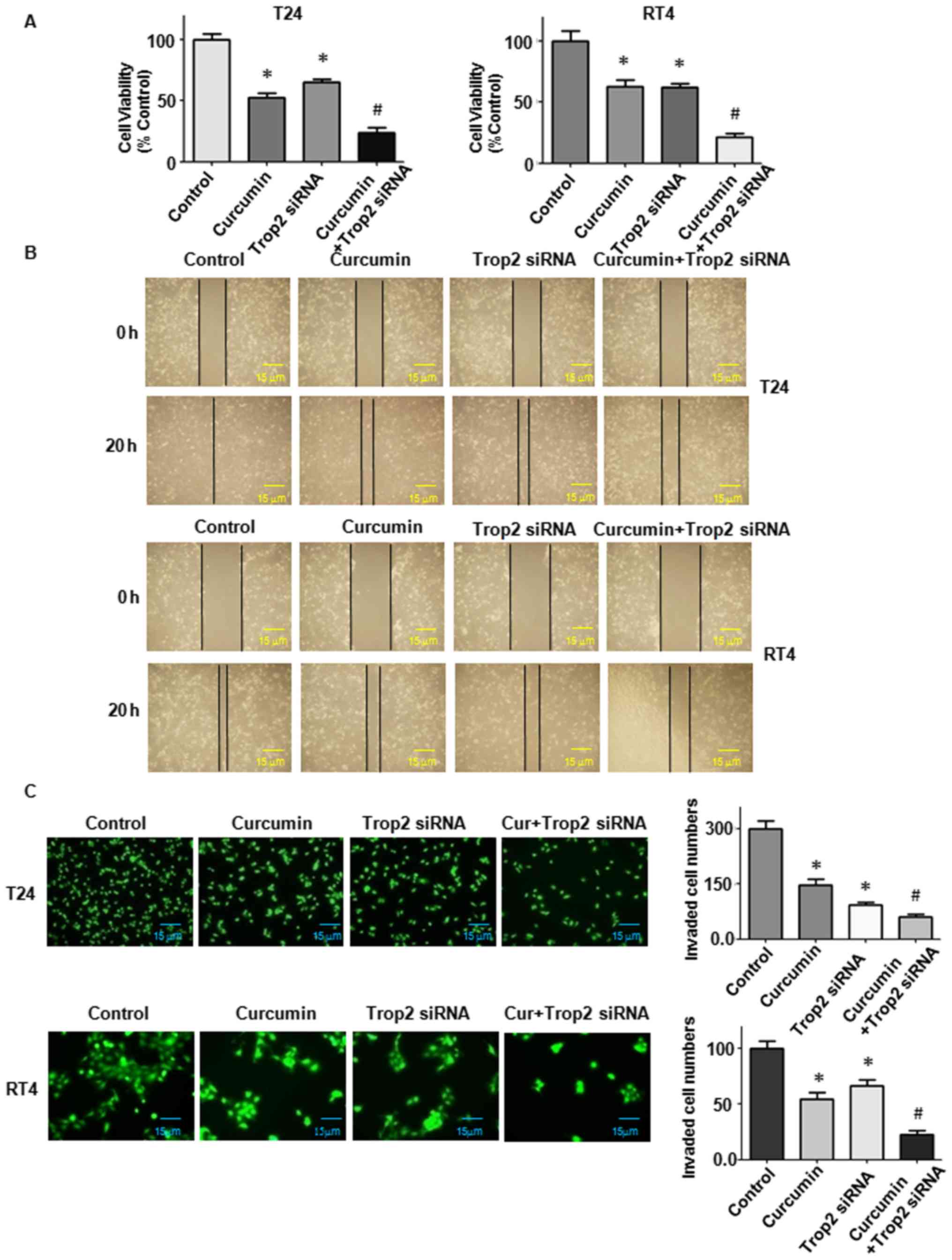 | Figure 8Downregulation of Trop2 suppresses BC
cell proliferation, and inhibits cell migration and invasion. (A)
3–4,5-dimethyl-2- thiazolyl-2, 5-diphenyl-2-H-tetrazolium bromide
assay to detect BC cell growth following downregulation of Trop2
alone or in combination with 15 µM curcumin. (B)
Wound-healing assay to determine cell migration ability
(magnification, ×100). (C) Invasion was detected using a Transwell
chamber assay (T24 cells, ×100 magnification; RT4 cells, ×200
magnification). Quantitative results are shown on the right.
*P<0.05, vs. control; #P<0.05, vs. 15
µM curcumin treatment or Trop2 siRNA transfection. BC,
bladder cancer; Trop2, trophoblast cell surface antigen 2; siRNA,
small interfering RNA. |
Discussion
BC is one of the most commonly diagnosed urological
tumors and causes severe tumor-associated mortality worldwide. In
China, BC has become the most frequent urological malignancy
(32). At initial diagnosis, ~75%
of cases are NMIBC. However, disease in ~80% of patients with NMIBC
recurs following initial treatment within 5 years (5). Despite surgical techniques and
adjuvant chemotherapy having progressed extensively, BC represents
a prevalent and life-threatening form of tumor (32,33).
In order to improve the poor prognosis of BC, the development of
novel treatment methods based on novel molecular networks is
urgently required.
Curcumin has been described to exhibit
antineoplastic properties in various types of cancer, including the
inhibition of cell growth and metastasis, and promotion of
apoptosis, via interacting with numerous cell signaling molecules
(28,34). Kamat et al found that
curcumin enhanced the antitumor effects of Bacillus Calmette-Guerin
on BC by reducing NF-κB and inducing tumor necrosis factor-related
apoptosis-inducing ligand receptors (35). Curcumin has been found to inhibit
cell proliferation and invasive ability and trigger apoptosis by
the suppression of Skp2 and induction of p21 in pancreatic cancer
cells (27). Curcumin enhances the
effect of 5-fluorouracil by disrupting AMP-activated protein
kinase/Unc-51 like autophagy activating kinase-dependent autophagy
and inducing apoptotic death in colon cancer cells (36). Curcumin inhibits cell growth
through increasing p21 and p27 cyclin-dependent kinase inhibitors
and inhibiting cyclin D1 and phosphatidylinositol-3 kinase
(PI3K)/Akt signaling (37).
YAP/TAZ are markedly suppressed by curcumin treatment, and the
expression of Notch-1 is also suppressed (38). Curcumin triggers the degradation of
KLF5 by the suppression of YAP/TAZ in BC cells (30). It has been reported that curcumin
inhibits the mobility of BC cells through modulating the level of
β-catenin and abrogating epithelial-mesenchymal transition (EMT)
(39). In the present study,
curcumin notably inhibited BC cell growth, invasion and migration,
and triggered apoptotic cell death and G2/M phase arrest (Figs. 1 and 2A and B). These findings suggested the
therapeutic possibility of curcumin for treating BC. Furthermore,
it was found that Trop2 was a target of curcumin in the BC cell
lines.
A number of studies have implicated the oncogenic
role of Trop2 in tumorigenesis, most likely through triggering cell
proliferation. A high expression level of Trop2 has been observed
in the majority of types of epithelial cancer. The overexpression
of Trop2 was shown to promote cancer cell growth and enhance the
tumorigenic potential of cells when injected into mice (11,40,41),
supporting Trop2 as a key cancer driver. It was found that murine
Trop2 activated the extracellular signal-regulated kinase
(ERK)/mitogen-activated protein kinase pathway through inducing
cyclin D1 and cyclin E, and reducing p27. ERK was also activated
upon overexpressing Trop2 in human pancreatic cancer and colorectal
cancer cells (40). Trop2 has been
revealed to enhance invasion of thyroid cancer by increasing MMP-2
(42). In the present study, the
overexpression of Trop2 by cDNA transfection increased BC cell
growth (Fig. 3A). The invasion and
migration abilities were also enhanced (Fig. 3B and C). Noteworthy, the
overexpression of Trop2 significantly decreased the apoptotic cell
percentage in the two BC cell lines and inhibited curcumin-induced
apoptosis (Fig. 4A). Consistent
with a previous study, the overexpression of Trop2 significantly
suppressed the protein levels of p27 in the T24 and RT4 cells
(Fig. 4B and C). When combined
with curcumin treatment, the induced expression of p27 was
partially abrogated (Fig. 4B and
C). The expression of cyclin E1 was upregulated by the
overexpression of Trop2 (Fig. 4B and
C). By contrast, the depletion of Trop2 through siRNA
transfection in colon and breast cancer cells suppresses growth and
colony forming abilities (11,41).
The depletion of endogenous Trop2 by Trop2-siRNA retroviral
infection also inhibits the invasion and migration of thyroid
cancer cells (42), and Trop2
deletion in gallbladder cancer cells notably suppressed cell
growth, colonies formation, and invasive and migration abilities
via modulating PI3K/AKT signaling and EMT characteristics (43). Similarly, the silencing of Trop2 by
siRNA transfection in the present study led to a significant
suppression of cell proliferation, invasion and migration of BC
cells (Fig. 5), whereas apoptotic
cell death was markedly increased (Fig. 6A). The silencing of Trop2
sensitized the BC cells to curcumin treatment (Figs. 5 and 6). The expression of p27 and cyclin E1
were also modulated by silencing Trop2 (Fig. 6B and C). Taken together, Trop2 may
serve as an attractive therapeutic target for the clinical
treatment of patients with BC.
Acknowledgments
Not applicable.
Funding
This study was supported by a grant from the New
Leading Technology Project for Municipal Hospitals supported by
Shanghai Shen Kang Hospital Development Center (grant no.
SHDC12015125).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LZ, JB, WX and YH were involved in conception and
design of the study; LZ, GY and RZ were involved in data
collection; LD, JB, WX and HC were involved in data analysis; LZ,
GY, RZ, LD, JB, WX and HC conducted investigative experiments; JB,
WX and YH were involved in project administration; JB and YH
supervised the study; LZ, JB and YH wrote and edited the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
PDQ Adult Treatment Editorial Board:
Bladder cancer treatment (PDQ®): Patient version. PDQ
Cancer Information Summaries. National Cancer Institute; Bethesda,
MD: 2002
|
|
2
|
Disease GBD, Injury I and Prevalence C;
GBD 2015 disease and injury incidence and prevalence collaborators:
Global, regional, and national incidence, prevalence, and years
lived with disability for 310 diseases and injuries, 1990–2015: A
systematic analysis for the Global Burden of Disease Study 2015.
Lancet. 388:1545–1602. 2016. View Article : Google Scholar
|
|
3
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
5
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar
|
|
6
|
McDougall AR, Tolcos M, Hooper SB, Cole TJ
and Wallace MJ: Trop2: From development to disease. Dev Dyn.
244:99–109. 2015. View Article : Google Scholar
|
|
7
|
Cubas R, Li M, Chen C and Yao Q: Trop2: A
possible therapeutic target for late stage epithelial carcinomas.
Biochim Biophys Acta. 1796:309–314. 2009.
|
|
8
|
Lipinski M, Parks DR, Rouse RV and
Herzenberg LA: Human trophoblast cell-surface antigens defined by
monoclonal antibodies. Proc Natl Acad Sci USA. 78:5147–5150. 1981.
View Article : Google Scholar
|
|
9
|
Linnenbach AJ, Wojcierowski J, Wu SA, Pyrc
JJ, Ross AH, Dietzschold B, Speicher D and Koprowski H: Sequence
investigation of the major gastrointestinal tumor-associated
antigen gene family, GA733. Proc Natl Acad Sci USA. 86:27–31. 1989.
View Article : Google Scholar
|
|
10
|
Dean M: Cancer as a complex developmental
disorder–nineteenth Cornelius P. Rhoads Memorial Award Lecture.
Cancer Res. 58:5633–5636. 1998.
|
|
11
|
Trerotola M, Cantanelli P, Guerra E,
Tripaldi R, Aloisi AL, Bonasera V, Lattanzio R, de Lange R, Weidle
UH, Piantelli M, et al: Upregulation of Trop-2 quantitatively
stimulates human cancer growth. Oncogene. 32:222–233. 2013.
View Article : Google Scholar
|
|
12
|
Nakashima K, Shimada H, Ochiai T,
Kuboshima M, Kuroiwa N, Okazumi S, Matsubara H, Nomura F, Takiguchi
M and Hiwasa T: Serological identification of TROP2 by recombinant
cDNA expression cloning using sera of patients with esophageal
squamous cell carcinoma. Int J Cancer. 112:1029–1035. 2004.
View Article : Google Scholar
|
|
13
|
Ohmachi T, Tanaka F, Mimori K, Inoue H,
Yanaga K and Mori M: Clinical significance of TROP2 expression in
colorectal cancer. Clin Cancer Res. 12:3057–3063. 2006. View Article : Google Scholar
|
|
14
|
Fong D, Spizzo G, Gostner JM, Gastl G,
Moser P, Krammel C, Gerhard S, Rasse M and Laimer K: TROP2: A novel
prognostic marker in squamous cell carcinoma of the oral cavity.
Mod Pathol. 21:186–191. 2008. View Article : Google Scholar
|
|
15
|
Fong D, Moser P, Krammel C, Gostner JM,
Margreiter R, Mitterer M, Gastl G and Spizzo G: High expression of
TROP2 correlates with poor prognosis in pancreatic cancer. Br J
Cancer. 99:1290–1295. 2008. View Article : Google Scholar
|
|
16
|
Pau Ni IB, Zakaria Z, Muhammad R, Abdullah
N, Ibrahim N, Aina Emran N, Hisham Abdullah N and Syed Hussain SN:
Gene expression patterns distinguish breast carcinomas from normal
breast tissues: The Malaysian context. Pathol Res Pract.
206:223–228. 2010. View Article : Google Scholar
|
|
17
|
Ning S, Liang N, Liu B, Chen X, Pang Q and
Xin T: TROP2 expression and its correlation with tumor
proliferation and angiogenesis in human gliomas. Neurol Sci.
34:1745–1750. 2013. View Article : Google Scholar
|
|
18
|
Varughese J, Cocco E, Bellone S, de Leon
M, Bellone M, Todeschini P, Schwartz PE, Rutherford TJ, Pecorelli S
and Santin AD: Uterine serous papillary carcinomas overexpress
human trophoblast-cell-surface marker (Trop-2) and are highly
sensitive to immunotherapy with hRS7, a humanized anti-Trop-2
monoclonal antibody. Cancer. 117:3163–3172. 2011. View Article : Google Scholar
|
|
19
|
Varughese J, Cocco E, Bellone S, Bellone
M, Todeschini P, Carrara L, Schwartz PE, Rutherford TJ, Pecorelli S
and Santin AD: High-grade, chemotherapy-resistant primary ovarian
carcinoma cell lines overexpress human trophoblast cell-surface
marker (Trop-2) and are highly sensitive to immunotherapy with
hRS7, a humanized monoclonal anti-Trop-2 antibody. Gynecol Oncol.
122:171–177. 2011. View Article : Google Scholar
|
|
20
|
Trerotola M, Jernigan DL, Liu Q, Siddiqui
J, Fatatis A and Languino LR: Trop-2 promotes prostate cancer
metastasis by modulating β(1) integrin functions. Cancer Res.
73:3155–3167. 2013. View Article : Google Scholar
|
|
21
|
Okudan N, Belviranlı M, Gökbel H, Oz M and
Kumak A: Protective effects of curcumin supplementation on
intestinal ischemia reperfusion injury. Phytomedicine. 20:844–848.
2013. View Article : Google Scholar
|
|
22
|
Kanai M: Therapeutic applications of
curcumin for patients with pancreatic cancer. World J
Gastroenterol. 20:9384–9391. 2014.
|
|
23
|
Aggarwal BB and Harikumar KB: Potential
therapeutic effects of curcumin, the anti-inflammatory agent,
against neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and neoplastic diseases. Int J Biochem Cell Biol.
41:40–59. 2009. View Article : Google Scholar
|
|
24
|
Zong H, Wang F, Fan QX and Wang LX:
Curcumin inhibits metastatic progression of breast cancer cell
through suppression of urokinase-type plasminogen activator by
NF-kappa B signaling pathways. Mol Biol Rep. 39:4803–4808. 2012.
View Article : Google Scholar
|
|
25
|
Gonçalves VP, Ortega AA, Guimarães MR,
Curylofo FA, Rossa Junior C, Ribeiro DA and Spolidorio LC:
Chemopreventive activity of systemically administered curcumin on
oral cancer in the 4-nitroquinoline 1-oxide model. J Cell Biochem.
116:787–796. 2015. View Article : Google Scholar
|
|
26
|
Beevers CS, Zhou H and Huang S: Hitting
the golden TORget: Curcumin's effects on mTOR signaling. Anticancer
Agents Med Chem. 13:988–994. 2013. View Article : Google Scholar
|
|
27
|
Su J, Zhou X, Wang L, Yin X and Wang Z:
Curcumin inhibits cell growth and invasion and induces apoptosis
through downregulation of Skp2 in pancreatic cancer cells. Am J
Cancer Res. 6:1949–1962. 2016.
|
|
28
|
Shehzad A and Lee YS: Molecular mechanisms
of curcumin action: Signal transduction. Biofactors. 39:27–36.
2013. View Article : Google Scholar
|
|
29
|
Momtazi AA, Shahabipour F, Khatibi S,
Johnston TP, Pirro M and Sahebkar A: Curcumin as a microRNA
regulator in cancer: A review. Rev Physiol Biochem Pharmacol.
171:1–38. 2016. View Article : Google Scholar
|
|
30
|
Gao Y, Shi Q, Xu S, Du C, Liang L, Wu K,
Wang K, Wang X, Chang LS, He D, et al: Curcumin promotes KLF5
proteasome degradation through downregulating YAP/TAZ in bladder
cancer cells. Int J Mol Sci. 15:15173–15187. 2014. View Article : Google Scholar
|
|
31
|
Shi J, Zhang X, Shi T and Li H: Antitumor
effects of curcumin in human bladder cance in vitro. Oncol Lett.
14:1157–1161. 2017. View Article : Google Scholar
|
|
32
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A;
European Association of Urology: EAU guidelines on muscle-invasive
and metastatic bladder cancer: Summary of the 2013 guidelines. Eur
Urol. 65:778–792. 2014. View Article : Google Scholar
|
|
33
|
Shirodkar SP and Lokeshwar VB: Potential
new urinary markers in the early detection of bladder cancer. Curr
Opin Urol. 19:488–493. 2009. View Article : Google Scholar
|
|
34
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
35
|
Kamat AM, Tharakan ST, Sung B and Aggarwal
BB: Curcumin potentiates the antitumor effects of Bacillus
Calmette-Guerin against bladder cancer through the downregulation
of NF-kappaB and upregulation of TRAIL receptors. Cancer Res.
69:8958–8966. 2009. View Article : Google Scholar
|
|
36
|
Zhang P, Lai ZL, Chen HF, Zhang M, Wang A,
Jia T, Sun WQ, Zhu XM, Chen XF, Zhao Z, et al: Curcumin synergizes
with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT
activity and enhancing apoptosis in colon cancer cells with tumor
growth inhibition in xenograft mice. J Exp Clin Cancer Res.
36:1902017. View Article : Google Scholar
|
|
37
|
Zhao Z, Li C, Xi H, Gao Y and Xu D:
Curcumin induces apoptosis in pancreatic cancer cells through the
induction of forkhead box O1 and inhibition of the PI3K/Akt
pathway. Mol Med Rep. 12:5415–5422. 2015. View Article : Google Scholar
|
|
38
|
Zhou X, Su J, Feng S, Wang L, Yin X, Yan J
and Wang Z: Antitumor activity of curcumin is involved in
downregulation of YAP/TAZ expression in pancreatic cancer cells.
Oncotarget. 7:79076–79088. 2016. View Article : Google Scholar
|
|
39
|
Shi J, Wang Y, Jia Z, Gao Y, Zhao C and
Yao Y: Curcumin inhibits bladder cancer progression via regulation
of β-catenin expression. Tumour Biol. 39:1010428317702548. 2017.
View Article : Google Scholar
|
|
40
|
Cubas R, Zhang S, Li M, Chen C and Yao Q:
Trop2 expression contributes to tumor pathogenesis by activating
the ERK MAPK pathway. Mol Cancer. 9:2532010. View Article : Google Scholar
|
|
41
|
Wang J, Day R, Dong Y, Weintraub SJ and
Michel L: Identification of Trop-2 as an oncogene and an attractive
therapeutic target in colon cancers. Mol Cancer Ther. 7:280–285.
2008. View Article : Google Scholar
|
|
42
|
Guan H, Guo Z, Liang W, Li H, Wei G, Xu L,
Xiao H and Li Y: Trop2 enhances invasion of thyroid cancer by
inducing MMP2 through ERK and JNK pathways. BMC Cancer. 17:4862017.
View Article : Google Scholar
|
|
43
|
Li X, Teng S, Zhang Y, Zhang W, Zhang X,
Xu K, Yao H, Yao J, Wang H, Liang X, et al: TROP2 promotes
proliferation, migration and metastasis of gallbladder cancer cells
by regulating PI3K/AKT pathway and inducing EMT. Oncotarget.
8:47052–47063. 2017.
|