Introduction
The number of cancer patients worldwide has recently
been increasing. The failure of conventional chemotherapy requires
new approaches for successful cancer treatment. Considering that
patients with gastrointestinal cancer have represented one of the
largest segment of the population with cancer, diet is likely to be
intimately associated with cancer development. Therefore, diet and
nutrition have been considered to play an important role in the
pathogenesis of carcinogenesis (1).
Garlic (Allium sativum L.) is a species of
the onion family, Alliaceae that has been widely used as a food and
also as a folk medicine. A number of epidemiological studies have
suggested that garlic is effective in the prevention and treatment
of several human diseases with multiple pharmacological functions,
such as anticarcinogenic (2),
antithrombotic (3), hypolipidemic
(4) and hepatoprotective (5) activity. Over the past decades, this
herb has been reported to suppress carcinogenesis and to inhibit
the proliferation of cancer cells (e.g., esophageal, gastric,
colorectal, lung, skin and prostate cancer) in vivo and
in vitro (6). Several of
the beneficial effects of garlic have been demonstrated to be
attributed to several bioactive compounds isolated from garlic,
including the lipid-soluble allyl sulfur compounds (e.g., diallyl
sulfide, diallyl disulfide and diallyl trisulfide) and
water-soluble compounds, such as S-allyl cysteine (SAC) and
S-allylmercaptocysteine (SAMC) (7-14).
Aged garlic extract (AGE) has been shown to possess
various water-soluble organic sulfur compounds, such as SAC, SAMC
and S-1-propenylcysteine by unique manufacturing process (15). AGE possesses greater antioxidant
activity than fresh garlic by scavenging reactive oxygen species
(ROS), enhancing the activity of cellular antioxidant enzymes
(e.g., superoxide dismutase, catalase and glutathione peroxidase),
mainly observed following the administration of SAC in vivo
(16), and increasing glutathione
levels in cells (17-19). Several studies on both animals and
humans have shown that AGE contributes to a reduced risk of cancer
(20,21), cardiovascular disease (22-24),
Alzheimer's disease and other age-related degenerative conditions
(19,25). Moreover, the mode of action of AGE
in inhibiting the formation of atherosclerosis in apolipoprotein
E-knockout mice has been investigated by examining whether AGE
suppresses inflammation (26). An
interesting investigation was recently performed using SAC. As
reported above, SAC represents one of the active and main
constituents of AGE with anti-inflammatory and neuroprotective
properties. Therefore, SAC may be considered a potential candidate
in the therapy of neuroinflammatory conditions, such as multiple
sclerosis, a deleterious autoimmune and demyelinating disorder of
the central nervous system (CNS). That study aimed to evaluate
whether SAC can improve clinical and neuropathological
characteristics of experimental autoimmune encephalomyelitis in
C57BL/6 mice (27).
AGE exerts its cancer-inhibitory effects at both the
early and late stages by modulating carcinogen metabolism,
decreasing carcinogen binding to DNA and scavenging ROS (19). Studies have demonstrated that AGE
exerts chemopreventive effects on chemically-induced colon tumors
in rats (28,29). In vitro studies have also
demonstrated that AGE suppresses DLD-1 human colon cancer cell
proliferation, but does not inhibit that of MRC-5 normal
fibroblasts (29). The major
unique organosulfur compounds in AGE are SAC and SAMC, which are
produced during the long-term extraction of garlic (30,31).
A number of studies have demonstrated that SAC and SAMC exert
effective cell growth inhibitory effects on human colon and breast
cancer cells, and suppress cancer risk by altering the biological
behaviors of various human tumors, such as prostate, colon and
gastric cancers (7,8,10,13,14).
In patients that are diagnosed as suffering from
colorectal adenomas, following treatment with high doses of AGE,
both the size and the number of colon adenomas were suppressed
(29). Thus, these results suggest
that AGE may be a potential agent for use in the treatment of
cancer.
To further investigate the anti-proliferative
activity of AGE, in this study, we examined the effects of AGE on
the proliferation of both sensitive [wild-type (WT)] and
multi-drug-resistant (MDR) human cancer cell lines. This study was
performed at 37 and 42°C. Moreover, we attempted to elucidate its
intracellular mechanisms of action, by focusing on mitochondrial
activity in whole cancer cells and in isolated rat liver
mitochondria (RLM).
Mitochondria, the key bioenergetic intracellular
organelles, contain a high number of proteins having ion channel
functions. Increasing evidence points to the important contribution
of the channels to the regulation of mitochondrial functions. In
fact, ion homeostasis imbalance profoundly affects energy
transduction processes, ROS production and mitochondrial integrity.
Given the central role of the mitochondria in apoptosis, their ion
channels with the potential to compromise mitochondrial functions
become promising targets for the treatment of malignancies
(32).
Materials and methods
Reagents
Thiazolyl blue Tetrazolium bromide (MTT), verapamil,
fetal bovine serum (FBS) and
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All
cell culture flasks and dishes were obtained from Corning (Corning,
NY, USA). Nigericin, salinomycin, valinomycin, cyclosporine A
(CsA), bongkrekic acid (BKA), N-ethylmaleimide (NEM),
1,4-dithioerythritol (DTE) and spermine were purchased from
Sigma-Aldrich. Adenosine diphosphate (ADP) was purchased from
Boehringer (Mannheim, Germany). AGE provided by Wakunaga
Pharmaceutical Co. Ltd. (Hiroshima, Japan) was manufactured as
follows: Garlic cloves were sliced, immersed in a water-ethanol
mixture solution and naturally extracted for >10 months at room
temperature, as previously described (33). The AGE powder used in our
experiments was prepared by lyophilization. It contained
approximately 28.6% (w/v, 286 mg/ml) solid material, 0.63% (6.3
mg/ml) arginine and 0.1% SAC (calculated on a dry weight basis) as
a marker compound for standardization (34). The AGE powder was freshly dissolved
in Ham's F-12 or in RPMI-1640 medium prior to each experiment.
Cell cultures
A human colon adenocarcinoma cell line (LoVo WT)
isolated from a metastatic nodule, its MDR variant (LoVo DX), a
gastric adenocarcinoma cell line (AGS), a melanoma cell line (M14
WT), isolated from an epidermal melanoma, the corresponding MDR
variant M14 ADR2, a human cervical adenocarcinoma cell line (HeLa)
and a human ovarian carcinoma cell line (A2780) were used in this
study. The LoVo, M14 and HeLa cells were a kind gift from Professor
E. Dolfini (University of Milan, Milan, Italy), Dr A. Molinari
(National Institute of Health, Rome, Italy) and Professor M.T.
Conconi (University of Padova, Padova, Italy), respectively. The
A2780 cells were purchased from ECACC (Sigma-Aldrich) cat. no.
93112519. The MDR cell line, LoVo DX, was obtained by the prolonged
culture of drug-sensitive parental LoVo WT cells in medium
containing doxorubicin (Adriblastina, Pharmacia & Upjohn,
Milan, Italy) as previously described by Grandi et al
(35). The M14 ADR2 cell line was
obtained by us (36) by culturing
the M14 ADR or M14 DX cell line, previously selected for resistance
to adriamycin by Molinari et al (37), in medium containing 10 µM
doxorubicin constantly in each passage. Both resistant cell lines
are also resistant to other chemotherapeutic agents, such as
etoposide and vincristine (38,39).
The AGS cell line (homo sapiens gastric adenocarcinoma) was
obtained from the American Type Culture Collection
(ATCC® CRL-1739™; ATCC, Manassas, VA, USA). The LoVo and
AGS cell lines were grown in Ham's F-12 medium containing glutamine
supplemented with 10% FBS, 1% MEM vitamins, 1% MEM non-essential
amino acid, penicillin (100 U/ml) and streptomycin (100
µg/ml). The M14 cells were grown in RPMI-1640 with
glutamine, 10% FBS, 1% MEM non-essential amino acid, penicillin
(100 U/ml) and streptomycin (100 µg/ml). The HeLa and A2780
cells were grown in Nutrient Mixture F-12 (Ham's) and RPMI-1640,
respectively. In total, 1.5 g/l NaHCO3 (Sigma-Aldrich),
10% heat-inactivated fetal calf serum (Biowest, Nuaillé, France),
100 U/ml penicillin, 100 µg/ml streptomycin and 0.25
µg/ml amphotericin B (all from Sigma-Aldrich) were added to
both media.
All cell lines were incubated in a humidified
atmosphere of 5% CO2 in a water-jacketed incubator at
37°C. For each passage, exponentially growing M14 cells were
harvested with 10 mM EDTA. LoVo and AGS cells were harvested by
further addition of 0.25% trypsin solution. The trypsin activity
was quenched by the addition of complete F-12 medium.
AGE dose response assay
The LoVo WT, LoVo DX, M14 WT, M14 ADR2 and AGS cells
were seeded in a 96-well plate and incubated for 24 h to allow for
the complete reattachment of the cells to the plates. After
changing with fresh medium, the cells were incubated in the
presence of 0, 1, 5, 7 and 10 mg/ml AGE for 24, 48 and 72 h. The
anti-proliferative effects of AGE on human tumor cells were
examined by MTT assay. Briefly, MTT was added to each well.
Following 3 h of incubation at 37°C, dimethyl sulfoxide was added
to dissolve the crystals. The absorbance was determined at 577 and
660 nm using a spectrophotometer multi-mode plate reader [Synergy
HT BioTek, serial no. 270204; BioTek, Bernareggio (MB), Italy].
Anti-proliferative effects of
hyperthermia and AGE
The M14 WT cells were incubated in the presence of
0, 1, 3, 6 and 10 mg/ml AGE at 37 and 42°C for 1 h. After washing
the cells with PBS with 1% bovine serum albumin (BSA) twice, the
cells were seeded into 96-well plate and incubated in RPMI-1640
complete medium containing 0, 1, 3, 6 and 10 mg/ml of AGE at 37°C
for 48 h followed by MTT assay as described above.
Measurements 'in situ' of mitochondrial
membrane potential (Δψm)
The changes in Δψm in whole cells were assayed using
the lipophilic cationic probe, JC-1 dye. The LoVo WT, LoVo DX and
AGS cells were seeded in a 12-well plate and incubated for 24 h to
allow the cells to adhere to the plates. After changing with fresh
medium, the cells were incubated with 0, 1, 5 and 10 mg/ml of AGE
for 24 h at 37°C. Subsequently, the cells were stained with 2.5
µg/ml of JC-1 for 20 min at 37°C. In the LoVo cells, 100
µM verapamil was further added to the solution in order to
inhibit the P-glycoprotein-mediated efflux of JC-1 in the LoVo DX
cells. Exposure to verapamil significantly increased the
fluorescence intensity of JC-1 in the LoVo DX cells, while it did
not affect fluorescence in LoVo WT cells. The detached cells were
washed with PBS and then resuspended in PBS. The samples were then
analyzed using a BD Accuri C6 flow cytometer (BD Biosciences, San
Jose, CA, USA). JC-1 was excited using an argon laser at a
wavelength of 488 nm (using a BD Accuri C6 flow cytometer). The
emitted green (JC-1 monomer) and red (JC-1 aggregate) fluorescence
were detected at the FL-1 channel (533/30 nm) and FL-2 channel
(585/40 nm), respectively. At least 10,000 events/sample were
acquired in log mode. The ratio of red (FL2)/green (FL1)
fluorescence intensity was used to represent the Δψm.
The Δψm of the HeLa and A2780 cells was evaluated
using the BD™ MitoScreen kit (BD Pharmigen, San Diego, CA, USA)
containing JC-1. The cells (1.5×105) were seeded in a
24-well cell culture plate. following incubation for 24 h, AGE was
added to the complete medium at 5 and 10 mg/ml, and the cells were
incubated for a further 72 h. Following treatment, the cells were
centrifuged at 1,000 × g at room temperature, resuspended in JC-1
working solution and incubated for 30 min at 37°C in a
CO2 incubator. Following incubation, the cells were
washed twice, resuspended in assay buffer and analyzed using a
FACSCanto II flow cytometer (Becton-Dickinson, Mountain View, CA,
USA). The results are presented as dot plots and as the percentages
of cells with an energized or depolarized mitochondrial
membrane.
Animals
A total of 50 male Wistar rats, 2 months old,
weighing approximately 150 g, were used in our experiments. The
rats, housed in the animal facility of the Department of Biomedical
Sciences, University of Padova (Padova, Italy), were maintained
under controlled conditions (temperature 20-22°C, relative humidity
48-50%, water with antibacterial control and a 12:12 h light/dark
cycle) and provided with water and a standard diet (4RF25)
purchased by Mucedola s.r.l., Settimo Milanese (MI), Italy.
The experimental procedures were approved by the
local Ethics Committee for Animal Experimentation (CEASA) (protocol
no. 3619, 15.1.2014) and performed in agreement with the
international guidelines as well as European Communities Council
Directive and National Regulations (CEE Council 86/609 and DL
116/92).
RLM isolation and purification
RLM were isolated using the following method: The
rats were starved overnight and sacrificed by cervical dislocation.
The livers were rapidly explanted, immersed in ice-cold isolation
medium containing 250 mM sucrose, 5 mM HEPES (pH 7.4), 0.5 mM EGTA
and washed 4/5 times with the same medium. The livers were minced
into small sections and washed with ice-cold fresh medium without
EGTA. The suspension was transferred to a glass potter and
homogenized using a Teflon pestle operating at 1,600 rpm, by 3-4
time strokes.
The homogenate was centrifuged at 700 × g for 5 min
at 4°C and the obtained supernatant was centrifuged at 10,800 × g
for 10 min at 4°C. The pellet was washed with isolation medium,
resuspended and centrifuged at 15,900 × g for 10 min at 4°C.
Finally, the obtained pellet, containing the mitochondria, was
suspended in the standard medium (see the incubation procedure)
(40). Mitochondrial proteins were
measured by the biuret method with BSA, as a standard (41).
The mitochondria (1 mg protein/ml) were incubated in
a water-jacketed cell at 25°C under continuous stirring. The
standard medium contained 250 mM sucrose, 10 mM HEPES (pH 7.4), 5
mM Na-succinate, 1.25 µM rotenone and 1 mM Na-phosphate.
Variations and/or other additions are provided with each
experiment.
Determination of mitochondrial
functions
Δψm was calculated on the basis of the distribution
of the lipid-soluble cation tetraphenylphosphonium
(TPP+) (Sigma-Aldrich) measured across the inner
membrane using a TPP+-selective electrode (42). Mitochondrial swelling was
determined by measuring the apparent absorbance change of
mitochondrial suspensions at 540 nm on a Kontron Uvikon model 922
spectrophotometer equipped with thermostatic control. The protein
sulfydryl oxidation assay was performed as previously described by
Santos et al (43).
K+ was estimated in the supernatant by atomic
spectroscopy as previously described by Crompton and Costi
(44).
Nigericin, salinomycin, valinomycin are antibiotics
that behave as ionophores for K+ in the mitochondria.
They were all used at a 1 µM concentration, for
approximately 10 min, to compare their effects with that of AGE.
Mg2+ was used at a 1 mM concentration for approximately
10 min of incubation, as an inhibitor of
K+/H+ exchanger. CsA is an immunosuppressant,
while BKA is an inhibitor of AdNT, and both were used at a 1
µM concentration. ADP is a nucleotide, a substrate of AdNT,
and was used at a 1 mM concentration. All these compounds were
used, for approximately 30 min of incubation, as inhibitors of MPT.
NEM, used at 10 mM concentration, is a thiol reagent, DTE, used at
1 mM concentration, is a reducing agent, while spermine, at 50
µM, is a ROS scavenger. These compounds were incubated for
approximately 40 min, and used as MPT inhibitors.
Statistical analysis
The data were presented as the means ± SEM or means
± SD. Statistical analysis was performed using one-way ANOVA with
the Tukey-Kramer post hoc test was used. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Anti-proliferative effects of AGE on
sensitive and MDR human cancer cell lines
The results of MTT assays revealed that treatment
with AGE exerted anti-proliferative effects on all cell lines
examined in a dose-dependent manner. Treatment with 10 mg/ml of AGE
for 72 h decreased the viability of both the LoVo WT and LoVo DX
cells to 73 and 65%, respectively, when compared with the untreated
control cells (Fig. 1A and B). The
proliferation of the M14 WT and M14 ADR2 cells was markedly
decreased to approximately 43% of the control level following
treatment with 10 mg/ml AGE for only 24 h (Fig. 1C and D). A further extension of the
incubation time did not affect the viability of the cells.
Long-term treatment with >5 mg/ml of AGE induced a marked
inhibitory effect on growth of the AGS cells (Fig. 1E). An amount of AGE <5 mg/ml
exerted a modest effect on all cell lines.
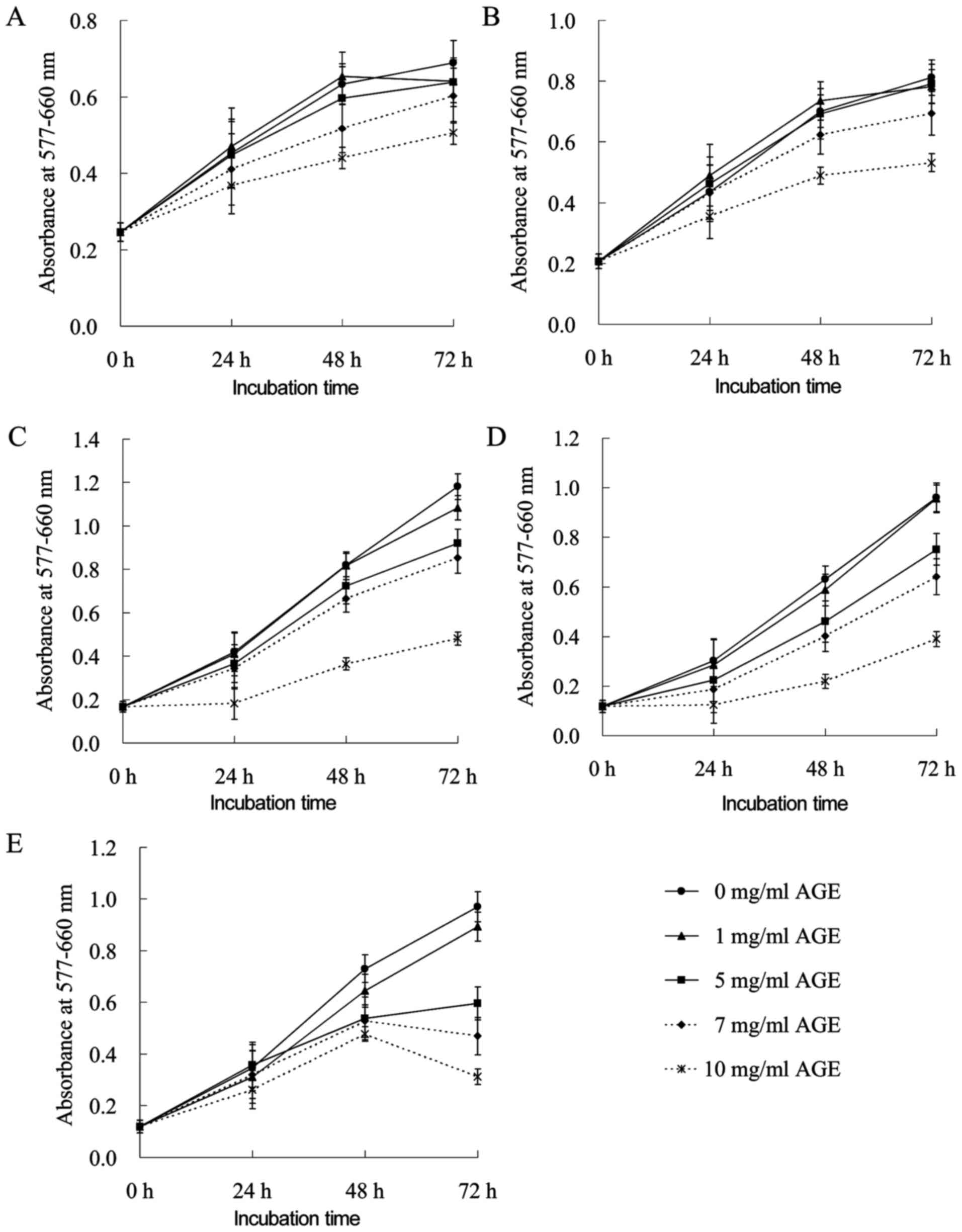 | Figure 1Anti-proliferative effects of AGE on
human tumor cells. (A) LoVo WT, (B) LoVo DX, (C) M14 WT, (D) M14
ADR2 and (E) AGS cells were treated with 0, 1, 5, 7 and 10 mg/ml of
AGE alone for 24, 48 and 72 h. The effects were determined by MTT
assay. Each point represents the mean ± SEM of 2 independent
experiments, with 3 wells per experiments. Where not shown, error
bars lie within symbols. AGE, aged garlic extract. |
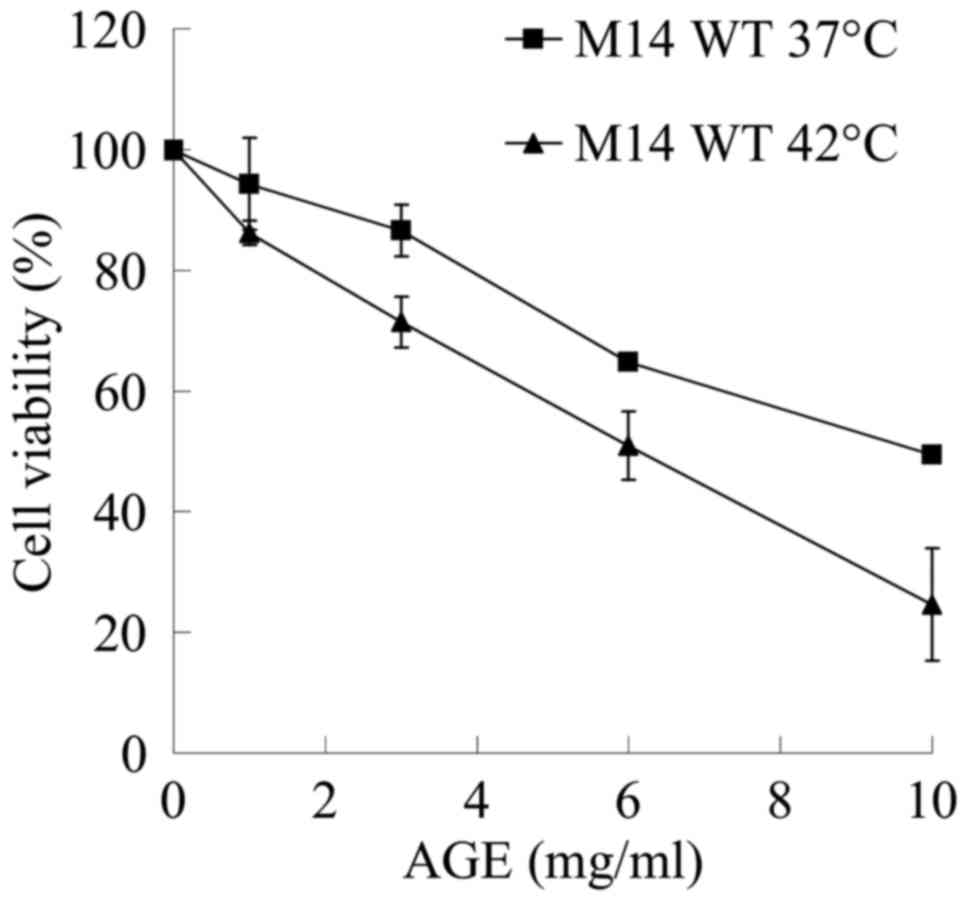
Anti-proliferative effects of
hyperthermia and AGE
The sensitivity of numerous cancer cell lines to an
increase in the temperature >37°C is the basis of clinical
hyperthermia. The potential of AGE to enhance the cytotoxic effects
of hyperthermia was investigated in the M14 cells as shown in
Fig. 2. Incubation at 42°C for 1 h
suppressed the viability of the M14 WT cells by approximately 29%
(data not shown). The M14 WT cells treated with 10 mg/ml of AGE at
42°C for 1 h exhibited a decrease in viability to 25% when compared
to the untreated cells at the same temperature, while 10 mg/ml AGE
at 37°C decreased cell viability only to 49% of the untreated
cells.
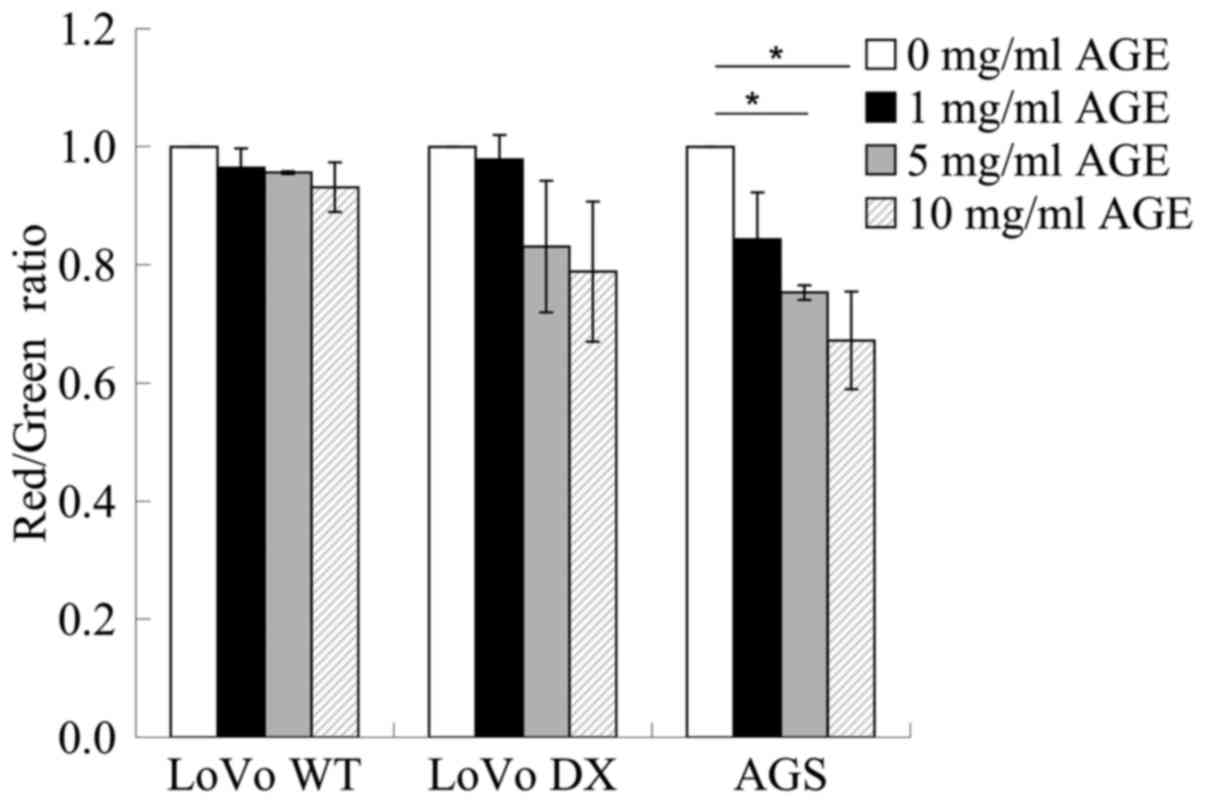
Depolarization of Δψm induced by AGE in
whole human cancer cells
To obtain information on mitochondrial
functionality, a flow cytometric analysis of the control and
treated cells loaded with the mitochondrial probe, JC-1, was
performed on several cancer cell lines. Flow cytometric analysis
revealed a tendency that AGE exerted toxic effects on the
mitochondria in a dose-response manner in the LoVo WT, LoVo DX and
AGS cells. Treatment with AGE induced an evident mitochondrial
membrane depolarization that was higher in the AGS cells than in
the LoVo WT and LoVo DX cells, as indicated by the ratio of red
(FL2 emission)/green (FL1 signal) fluorescence intensity (Fig. 3).
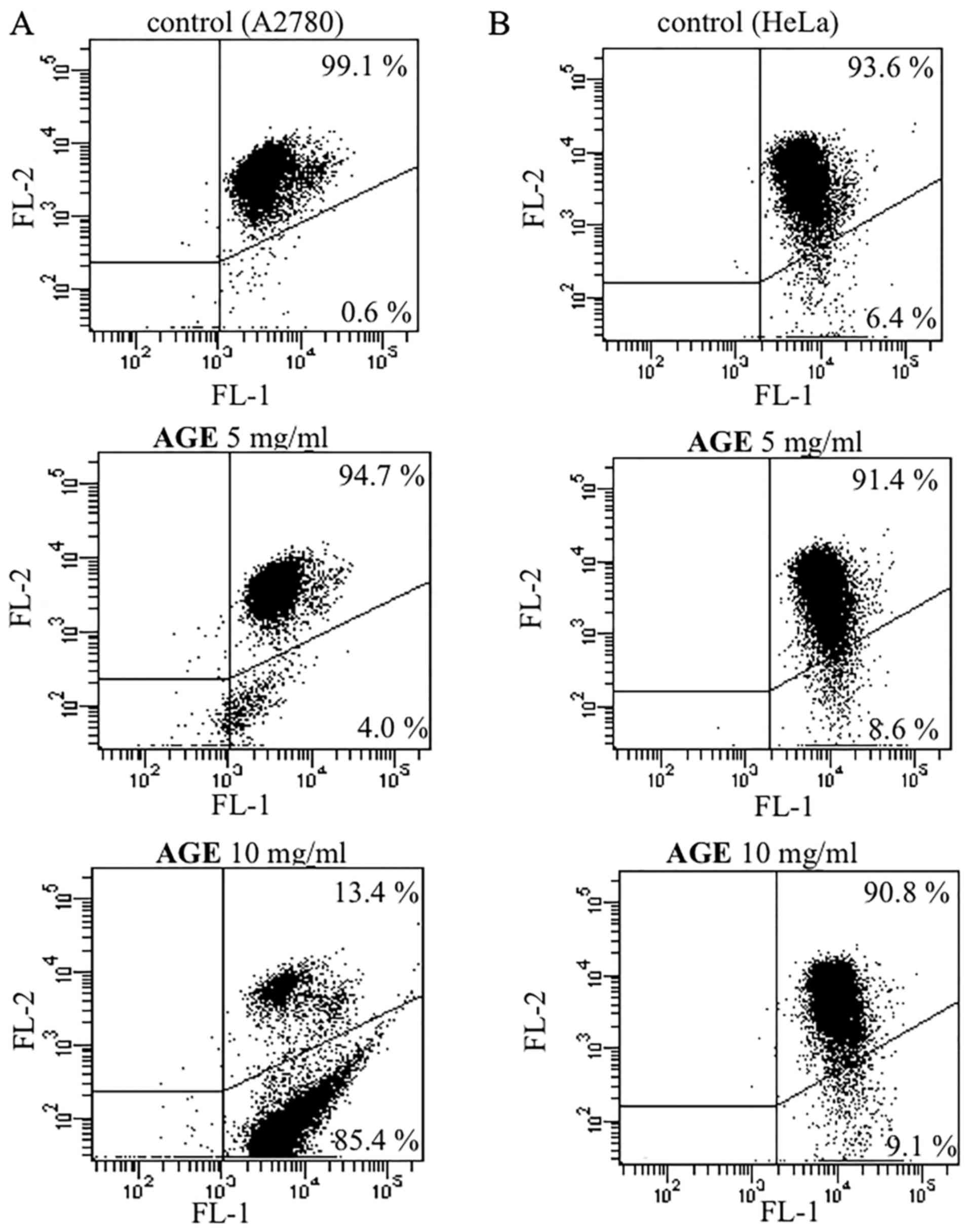
A similar effect was also obtained when incubating
the ovarian carcinoma A2780 (Fig.
4A) and cervical adenocarcinoma HeLa (Fig. 4B) cells in the presence of various
concentrations of AGE (5 and 10 mg/ml) for 72 h. The decrease in
red fluorescence in the dot plots of the treated cells indicated
mitochondrial membrane depolarization. Nevertheless, the extent of
the effect appeared to differ markedly between the two cell lines
taken into consideration. In particular, AGE induced a detectable
effect from the concentration of 5 mg/ml on the A2780 cells, with a
percentage of cells exhibiting depolarized mitochondria that
increased from 0.6% (untreated cells) to 4.0% (Fig. 4A). The ability of AGE to induce
mitochondrial membrane depolarization was clearly confirmed at the
maximum concentration considered, and indeed, at 10 mg/ml, the
above-mentioned percentage increased to 85.4%. In the HeLa cells,
the ability of AGE to provoke mitochondrial depolarization was
markedly less pronounced. Nevertheless, a dose-dependent effect was
also observed in this cell line, with the percentage of cells
exhibiting mitochondrial membrane depolarization increasing from
6.4% (untreated) to 8.6 and 9.1% in the presence of AGE at 5 and 10
mg/ml, respectively.
Activation of the mitochondrial
K+/H+ exchanger by AGE
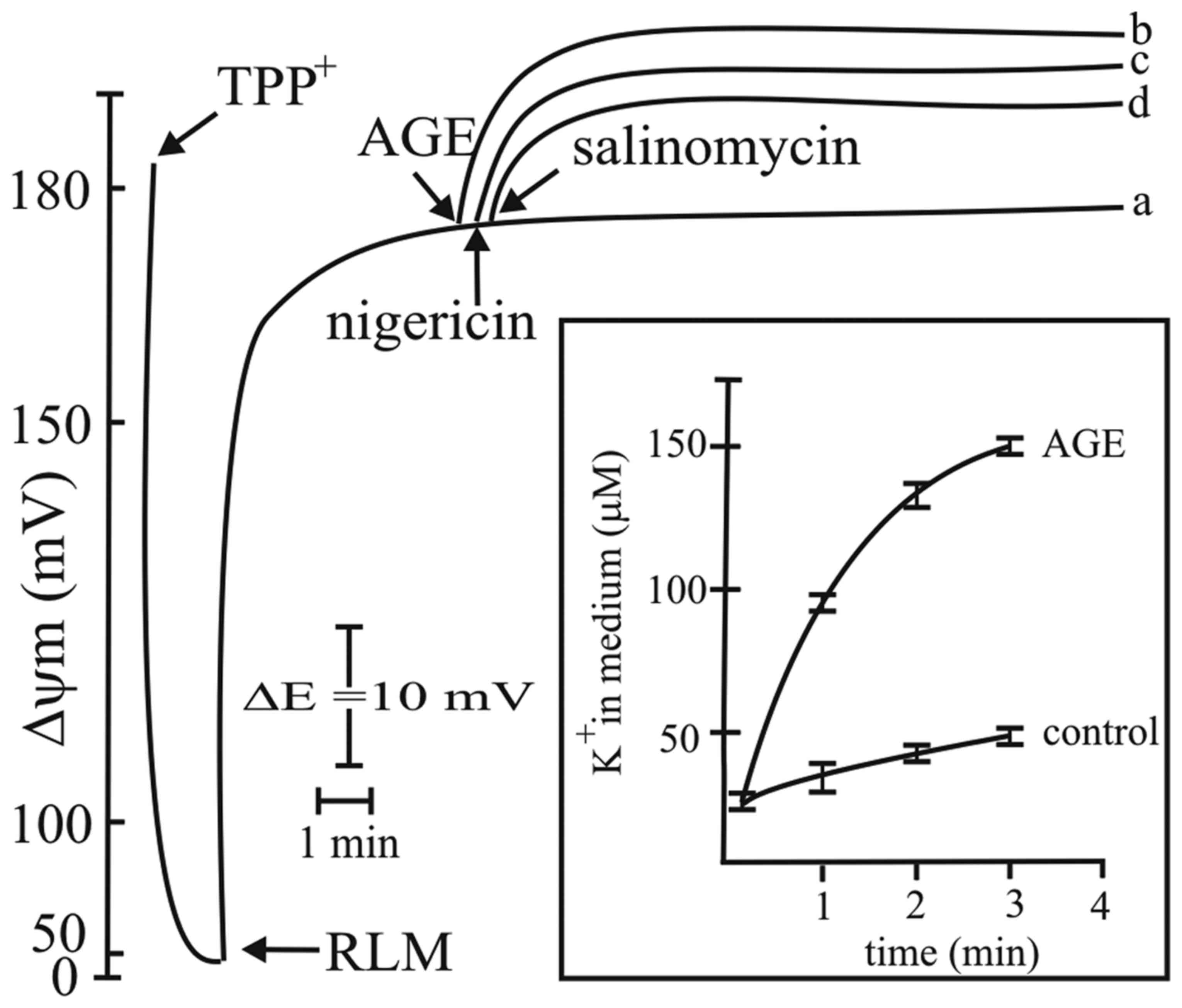
RLM incubated in standard medium at the
concentration of 1 mg protein/ml, as described in the Materials and
methods, and energized by the oxidation of succinate in the
presence of rotenone, exhibited a Δψm value of approximately 180 mV
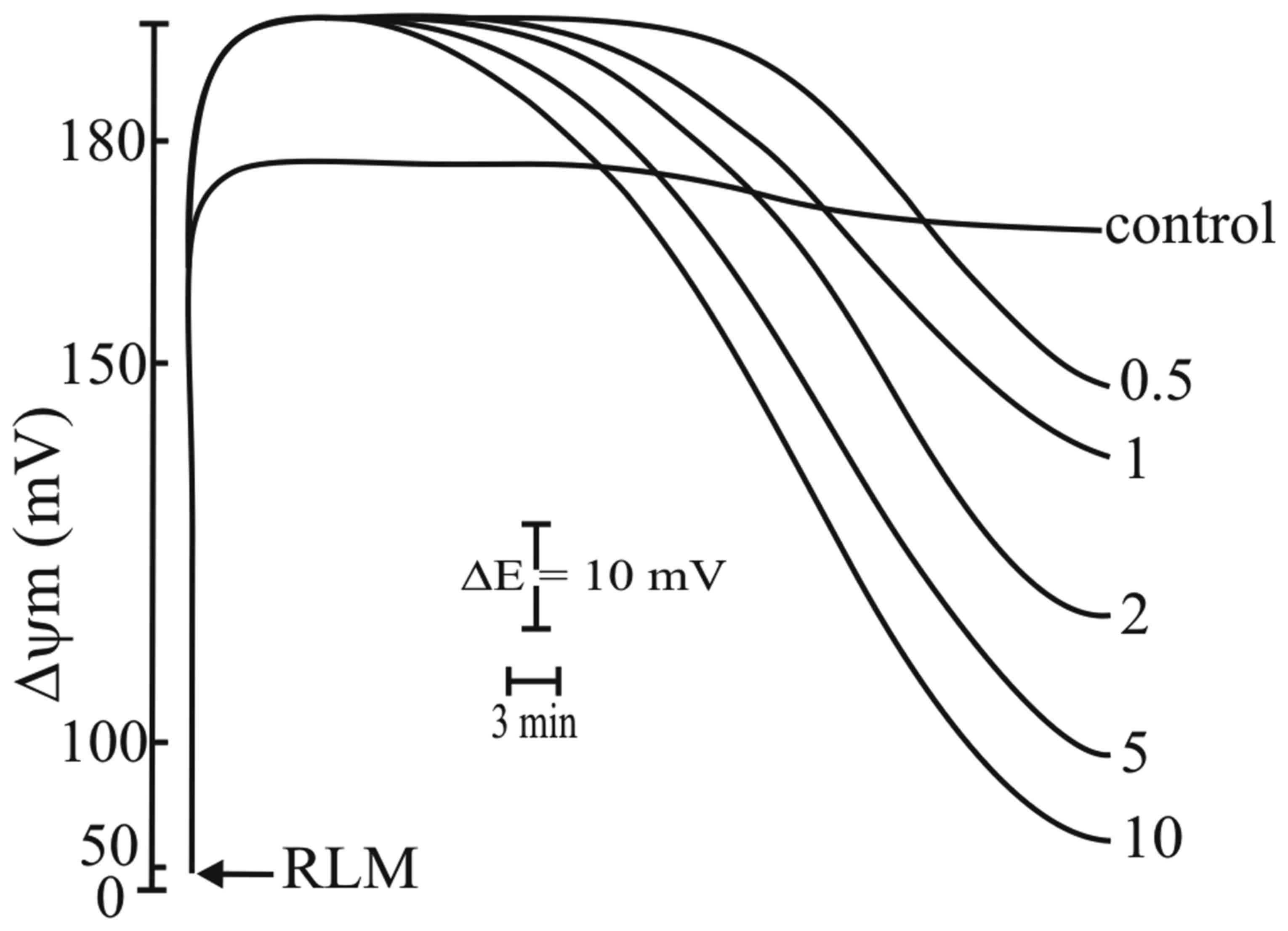
(Fig. 5, curve a). The addition of
AGE at 0.5 mg/ml induced a hyperpolarization of approximately 15 mV
that was maintained for several minutes (Fig. 5, curve b). This effect was similar
to that observed with the ionophore, nigericin (45) (Fig.
5, curve c) and with the anticancer drug salinomycin (Fig. 5, curve d) (46). The presence of AGE also induced the
release of K+ into the incubation medium (Fig. 5, insert). Higher AGE concentrations
did not significantly increase Δψm (Fig. 8, below). The addition of another
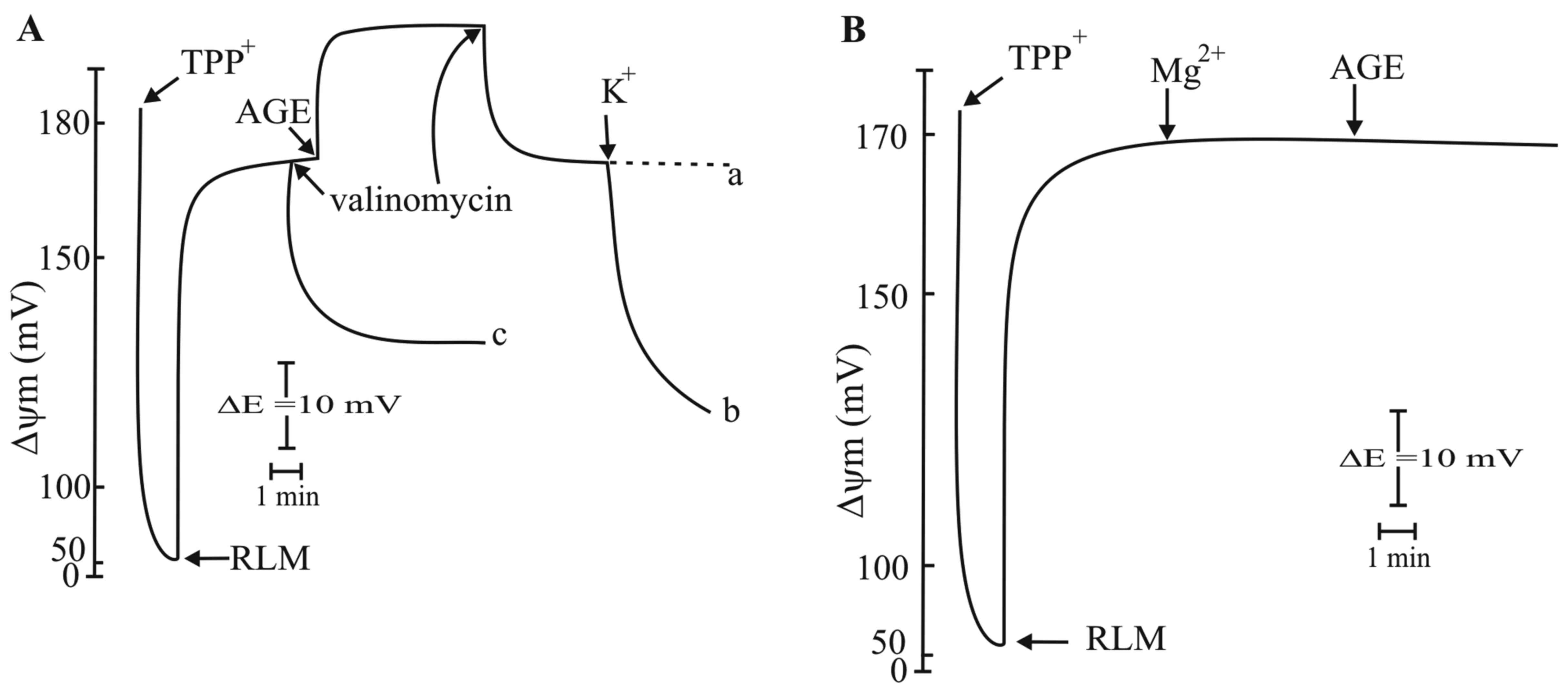
ionophore, valinomycin, after AGE, decreased the Δψm to the same
value obtained prior to the addition of AGE (Fig. 6A, curve a). Indeed, the addition of
K+ induced a further decrease in Δψm to a greater extent
(Fig. 6A, curve b) (47). These results indicated that the
addition of valinomycin provoked the electrophoretic uptake of
K+ previously released by AGE (Fig. 5, insert) and are in close agreement
with the mechanisms of the effect of valinomycin (Fig. 6A, curve c) that induces an
electrophoretic uptake of exogenous K+ driven by Δψm
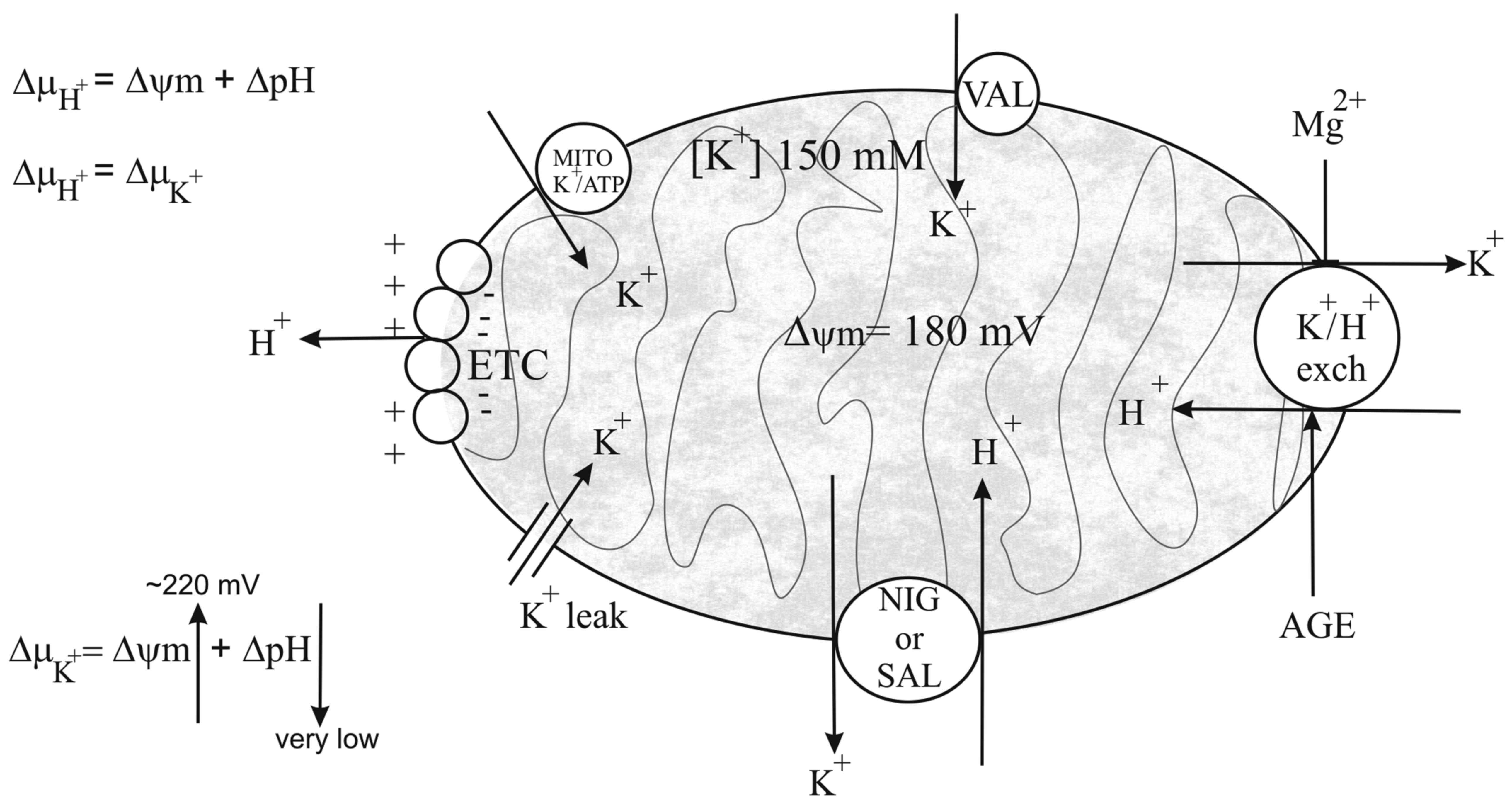
(Fig. 7). Most probably,
valinomycin was more efficient in inducing a decrease in Δψm with
respect to the increased hyperpolarization induced by AGE. The
observed effect of AGE may be explained by considering the
mechanisms of action of the above-mentioned antibiotics (nigericin
and salinomycin) on the mitochondrial membrane that activates an
exchange between endogenous K+ and exogenous
H+, as schematically depicted in Fig. 7. This exchange takes place to a
large extent, due to the mitochondrial matrix that contains high
concentrations of K+ (approximately 150 mM). Thus, the
electrochemical gradient (ΔμH+), that is the
sum of the electrical and chemical gradient
(ΔμH+ = Δψ + ΔpH), is completely transformed
in a K+ gradient, ΔμK+
(ΔμH + = ΔμK+). Under
this consideration, the large uptake of H+ completely
collapses ΔpH, with a strong acidification of the matrix, and
K+ gradient ΔμK+ (that is
ΔμH+) becomes equal to Δψm. This explains the
observed increase in Δψm following the addition of nigericin,
salinomycin, and likely also of AGE. Most probably, the effect of
AGE was attributable to an activation of the mitochondrial
K+/H+ exchanger, while nigericin and
salinomycin act as ionophores, forming a channel on the membrane
for K+/H+ exchange. The effects of AGE on the
K+/H+ exchanger were strongly supported by
the observations that after its addition to the RLM, a rapid
release of K+ was induced (Fig. 5, insert).
These results demonstrate that AGE induces the
K+/H+ exchange, similar to nigericin or
salinomycin, but through a different mechanism. A further
confirmation of this effect is represented by the results reported
in Fig. 6B. In fact, a previous
addition of 1 mM Mg2+ to RLM completely prevented the
increase in Δψm caused by AGE. Mg2+ has been reported to
be a strong inhibitor of the K+/H+ exchanger
(48). The authors proposed
Mg2+ as a 'carrier brake' of the exchanger. Indeed the
inhibition on K+/H+ exchanger by
Mg2+ confirmed that AGE acts on the
K+/H+ exchanger.
Induction of mitochondrial permeability
transition following prolonged incubation of the RLM with AGE
As shown in Fig. 8,
the prolonged incubation of RLM with various concentrations of AGE,
until 40 min, a decrease in Δψm to a varying extent. This
observation appeared to be in contrast to the results reported in
Fig. 6A, showing that AGE
stimulated an increase in Δψm. In fact, the decrease in Δψm began
after several minutes of incubation and was dependent on the AGE
concentration. The decrease in Δψm could be ascribable to the
strong acidification of mitochondrial matrix following
H+ uptake in exchange with K+ that damages
RLM. However, the collapse of Δψm may be due also to another event.
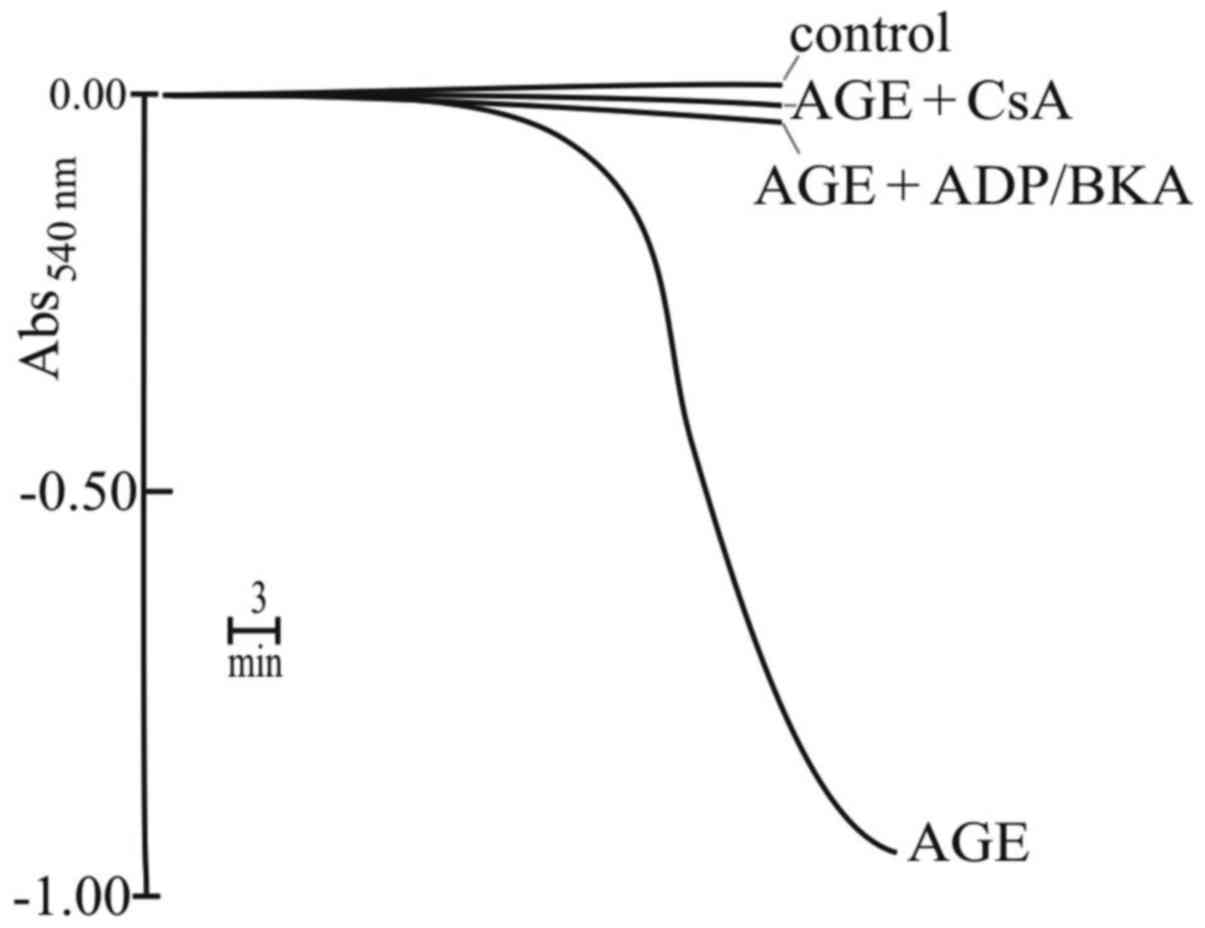
In this regard, it was also found that the incubation of RLM with
AGE, for approximately 15 min, caused mitochondrial swelling to a
large extent (Fig. 9). This
swelling was inhibited by CsA, BKA and ADP (Fig. 9), that are typical inhibitors of
the mitochondrial permeability transition (MPT), a phenomenon
closely related to cell death by intrinsic apoptosis. Generally,
this phenomenon is induced in the presence of supraphysiological
Ca2+ concentrations (primary inducer) and an oxidizing
agent (secondary inducer or amplifier) (for reviews see refs.
49,50). However, Ca2+ was not
added to the incubation medium; nevertheless, another experiment
revealed that ruthenium red (RR), an inhibitor of Ca2+
transport, completely prevented mitochondrial swelling (data not
shown). This indicates that in the presence of AGE, RR acts on MPT
by an unknown mechanism that, however, does not involve exogenous
Ca2+. A first conclusion of these results is that AGE,
following prolonged incubation, induced MPT of large amplitude, as
confirmed by the complete prevention due to the MPT inhibitors.
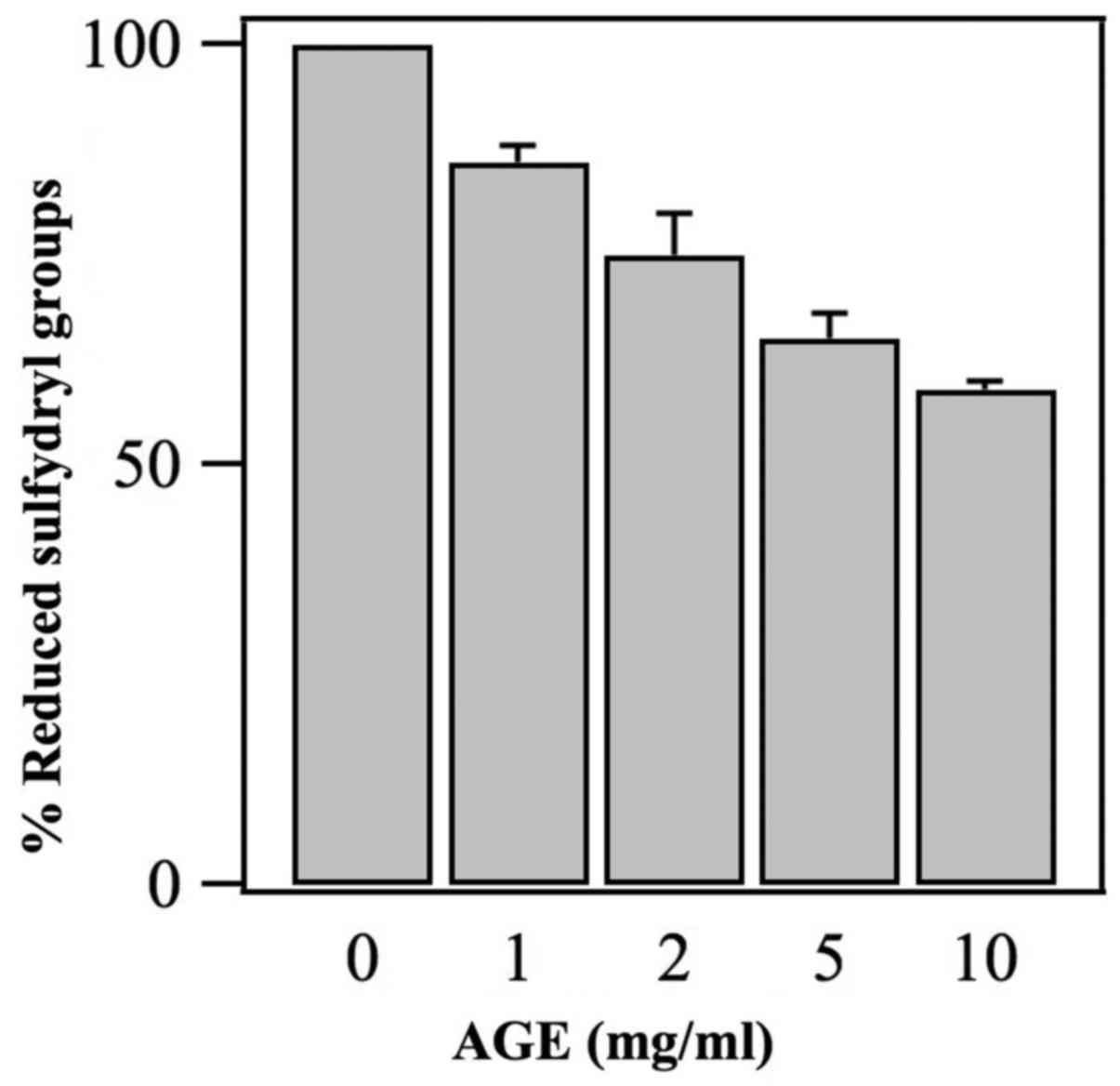
Oxidizing effects induced by AGE on the
mitochondria
The experiment shown in Fig. 10 was performed in order to
evaluate whether MPT was also related to oxidative stress. As shown
in the figure, AGE induced a decrease in the mitochondrial
sulfydryl groups with a dose response-dependent mechanism. Thus,
this observation may demonstrate that AGE acts as an oxidant with
the generation of disulfide bridge (see Discussion).
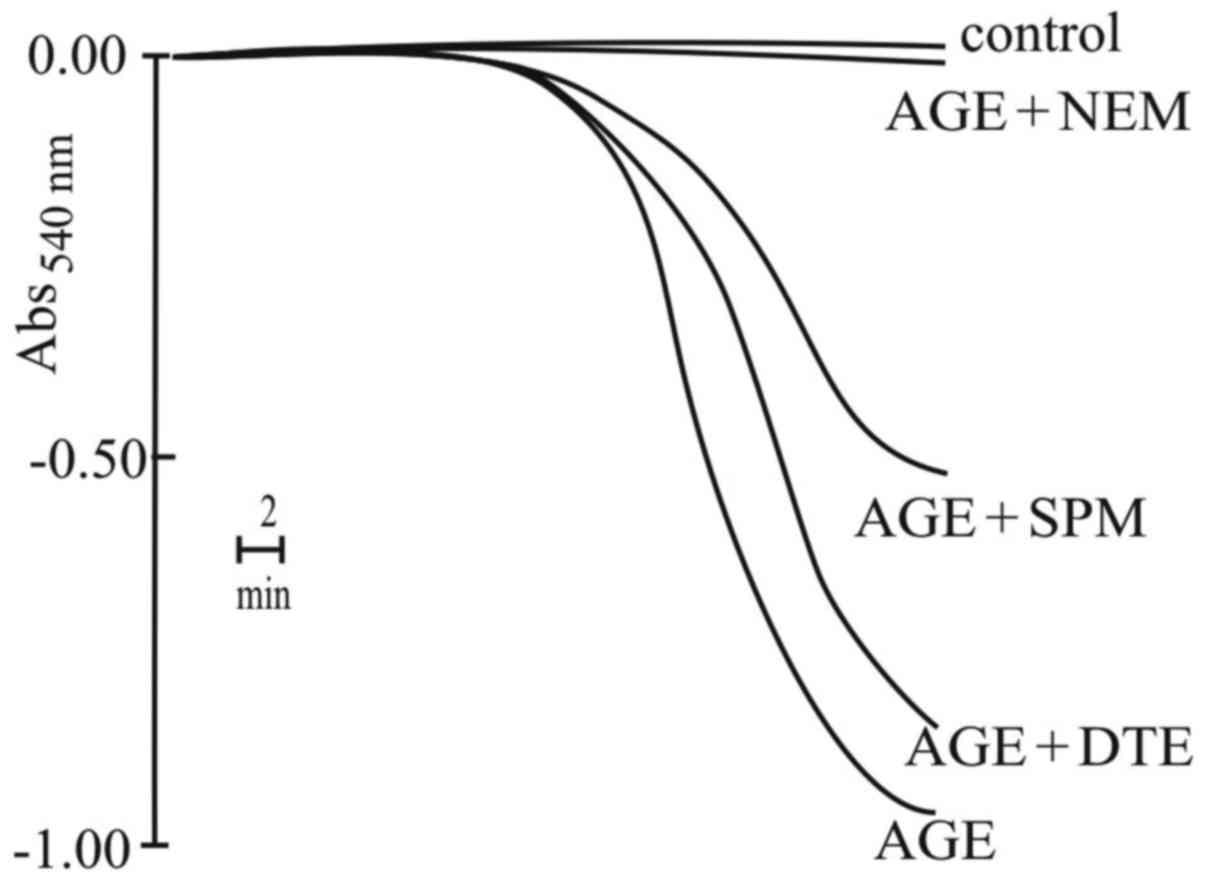
To support this result, another experiment on
mitochondrial swelling induced by AGE, in the presence of
antioxidant agents was performed. The results reported in Fig. 11 indicated that the alkylating
reagent, NEM, completely prevented the swelling, while the reducing
agent, DTE, or the scavenger of ROS, spermine, exerted negligible
or only partial inhibitory effects, respectively. Very
surprisingly, AGE was able to induce oxidative stress; however, MPT
induction was not closely related to this event. Most probably, the
oxidation of the SH groups by AGE was only partially involved in
the induction of MPT, as demonstrated by the ineffectiveness of DTE
and spermine. Of note, the inhibitory effects induced by NEM
warrant further investigation.
Discussion
In conventional cancer chemotherapy, numerous issues
hamper successful treatment. Among these, the lack of tumor
specificity of the cytotoxic drugs, and the development of MDR
cancer cells are the most difficult issues which need to be
resolved. Hence, there is a demand for alternative therapeutic
strategies. Treatment of the AGS, HeLa, A2780, LoVo WT and LoVo DX
cells with AGE was accompanied by characteristic mitochondrial
alterations. JC-1 exposure on the outer surface of the
mitochondrial membrane clearly revealed the onset of the
mitochondrial membrane depolarization process (Figs. 3 and 4). In general, the mitochondrial changes
of the AGS, LoVo WT cells and its resistant phenotype mirrored the
results of the cell survival experiments (Fig. 1).
The presence of AGE near the tumor mass may enhance
the effects of currently used antineoplastic therapies, such as
hyperthermia therapy. Therefore, from a therapeutic point of view,
the improvement of the efficacy of the in situ presence of
cytotoxic drugs is essential. As reported above, AGE induced a
decrease in the viability of the melanoma M14 WT cells (Fig. 1C). Of note, a considerable
enhancement of cytotoxicity at 42°C, compared to 37°C, was also
observed (Fig. 2). It has been
reported that cancer cells are selectively killed by hyperthermia
alone (51). Moreover, numerous
studies have demonstrated a beneficial antineoplastic effect of
hyperthermia, particularly when used in combination with anticancer
agents or associated with other therapeutic modalities, such as
irradiation or chemotherapy, in the treatment of human cancers
(39,52,53).
This has led researchers to evaluate the clinical potential of
hyperthermia using several temperatures (ranging from 40 to 43°C)
(51). Localized hyperthermia
enhances the cytotoxic process of several antitumor drugs and has
considerable potential in cancer therapy (54,55).
This has been explained by a favourable influence on blood flow,
cell membrane permeability and drug uptake (56). Hyperthermia can act at the initial
stage of treatment, probably by accelerating the kinetics of the
membrane molecular interactions and by favouring drug delivery into
the cancer cells (51,57). In view of these results, the use of
AGE in cancer therapy deserves to be taken into consideration. By
delivering AGE into cancer cells, a cytotoxic effect can be induced
in situ. The main challenge is the mechanism through which
AGE can be delivered in vivo to cancer cells for possible
clinical application. Molecular anticancer drugs can be conjugated
with biocompatible polymers which function as carriers and
stabilizers, resulting in decreased drug toxicity and an enhanced
therapeutic efficacy (58). It has
been shown that by conjugating macromolecules with polyethylene
glycol (PEG) hydrogels, with the aim of increasing its plasmatic
half-life or its targetability under administrable form, the yield
of immobilization is very high (59). Currently, nanotechnology concerning
particles and devices in the range of a 1-100 nm dimension,
provides novel opportunities in cancer therapy. Nanoparticle-based
therapies have been shown to reduce systemic toxicities and to
enhance therapeutic efficacy of drugs (60,61).
Our research group has previously performed several studies into
the research of novel nanoparticles in inducing polyamine and
bovine serum amine oxidase (BSAO)-based nanoparticles to overcome
some of the issues associated with conventional anticancer therapy,
including the limitations of treating drug resistant tumors
(62). To increase the stability
of the enzyme and the release of cytotoxic products, core-shell
gold nanoparticles have been employed for the immobilization of
BSAO (63). BSAO was also
conjugated on a new injectable nanohydrogel, obtained derivatizing
hyaluronic acid with cholesterol (64). The results indicate that the
above-mentioned nanosystems and the superparamagnetic and iron
oxide nanoparticles (65) are
useful controlled delivery systems, promising for future biomedical
enzyme applications. Moreover, in our opinion, the systematic
exploration of AGE in combination with conventional anticancer
drugs promises new and efficient anticancer therapies within a
short period of time.
As regards the mechanisms of action, the results
obtained by this study first of all strongly support the hypothesis
that AGE functions in a similar manner as K+ ionophores,
such as nigericin or salinomycin (46) (Fig.
5), and opposite to that of another K+ ionophore
valinomycin (47) (Fig. 6A). In particular, AGE acts in the
mitochondria as a K+/H+ antiporter that
directly affects some mitochondrial bioenergetic functions, not
only a few minutes later upon its addition, but also after longer
incubation times.
Thus, these results clearly demonstrate that AGE is
able to interact at the mitochondrial K+ cycle level.
The K+ cycle consists of influx and efflux pathways for
K+, H+ and anions (Fig. 7). It is well known that
electrogenic proton ejection, by the electron transport chain,
generates an electrical membrane potential
(ΔμH+) which drives K+ influx by
diffusion ('K+ leak') and via the mitochondrial
ATP-sensitive K+ channel (mito KATP)
(Fig. 7). This
K+/H+ exchange will alkalinize the matrix
causing the uptake of phosphate via the electroneutral
Pi-H+ symporter. Net uptake of K+ will be
accompanied by osmotically obligated water that would result in
matrix swelling. However, excess matrix K+ is ejected by
the K+/H+ antiporter that prevents
mitochondrial swelling, thus maintaining matrix volume homeostasis
(66). Therefore, the
mitochondrial K+ cycle plays two distinct roles in
mitochondrial and cell physiology: i) Volume homeostasis to prevent
excessive matrix swelling; ii) volume regulation to prevent
excessive matrix contraction (67). Changes in the regulated activity of
mito KATP, K+ leak or
K+/H+ antiporter can lead to matrix swelling
or contraction with catastrophic effect on inner membrane, and to
vesicular system assembly. This can lead to dysfunctions of
mitochondrial complexes and can alter electron flux with the
generation of ROS (68,69) and consequent bioenergetic collapse.
On the basis of the reported results, the effects of AGE on the
mitochondria exhibited a close correlation with nigericin and
salinomycin and are comparable to the above-mentioned changes in
the activity of the K+ cycle.
Considering the above-mentioned correlation, in
particular, the acidification of mitochondrial matrix due to the
H+ influx, it is possible to propose, besides the
above-mentioned alterations of inner membrane, other
pathophysiological effect for AGE. Matrix pH is a factor that
controls oxidative phosphorylation in the mitochondria of normal
cells. Its value ranges from 7.7 to 8.2 in different cell types,
while the pH of cytosol is approximately 7.0. This ΔpH contributes
in significant way to the driving force for ATP synthesis, but also
for several transport processes responsible for exchanging
metabolites (e.g., phosphate, pyruvate and glutamate) (70). Hence, matrix acidification induced
by AGE addition attenuates mitochondrial ATP synthesis, despite
hyperpolarization of the membrane observed in the first 10-20 min.
In fact, the prolonged acidification alters the activity of the
enzymes of Krebs cycle and respiratory chain causing a sustained
decrease in oxygen consumption and a decrease in Δψm, the extent of
which depends on AGE concentration (Fig. 8).
Apart from considering the effect of AGE as a
K+/H+ exchanger, its action following
prolonged incubation should also be taken into account, leading to
mitochondrial swelling and Δψm collapse (Figs. 8 and 9). As emphasized in the Results, AGE
induces MPT that, however, seems to exhibit a different mechanism
with respect to the well-known one (48). In fact, on the basis of the
reported results, this mechanism, as mentioned above, seems to not
be dependent on exogenous supraphysiological Ca2+
concentrations, while the involvement of an oxidative stress is
strongly in doubt, due to the apparent scarce effect of DTE and
spermine (Fig. 11). To provide a
possible explanation for these observations, a previous theory on
MPT proposed by Halestrap and Davidson (71) should be considered. These authors
suggested that, in particular conditions, the adenine nucleotide
translocase (AdNT), generally considered as the main protein on
which the transition pore is open, can be transformed in a
K+ channel that, in turn, forms the aspecific transition
pore leading to MPT. Thus, the effect of AGE, besides activating
the K+/H+ exchanger, could be that of
transforming AdNT in the transition pore. The Halestrap-Davidson
theory predicted that the transformation of AdNT in the
K+ channel and, subsequently, in the transition pore,
needed the presence of phosphate and Ca2+. In this
regard, it is worth noting that phosphate is present in the
incubation medium, while Ca2+ is absent. Thus, the
effect of AGE in altering the architecture of the membrane, by
acting on the K+/H+ exchanger, should be
responsible for transforming AdNT in a K+ channel. Then,
AGE should be able to induce the binding of endogenous
Ca2+ to the critical site(s) located on AdNT and
responsible of transforming K+ channel in the aspecific
pore without involving an oxidative stress (70). Thus, it can be emphasized that AGE
induces MPT by involving only endogenous Ca2+. In this
regard, it should be considered that endogenous Ca2+, in
the presence of phosphate, cycles across the mitochondrial
membranes and, most probably, AGE causes the interaction of the
cation with the critical site(s) responsible of MPT induction
(71). The presence of RR, that
blocks the uptake of Ca2+ during its cycling, prevents
the cation binding to the above mentioned site(s) and consequently
prevents MPT induction.
The oxidizing effect exhibited by AGE in the
mitochondria seems to be very surprising, as the compound generally
exerts antioxidant and ROS-scavenging effects (17,19).
However, an explanation for this may be that AGE functions as a
redox cycler in the mitochondria, similarly to several polyphenols
and triterpene compounds, exhibiting both antioxidizing and
oxidizing properties (72,73 and refs. therein). These opposite
effects are generally observed in the mitochondria, since the
respiratory chain contains iron-sulfur proteins that are involved
in the above-mentioned mechanism coupled to the redox cyclers. Of
note, it should be emphasized that the oxidative processes of AGE
should not be considered as damage, but rather that they can
contribute to the balance of both SH/SS and GSH/GSSG pools that are
essential for mitochondrial physiology.
In conclusion, this study demonstrates that AGE is
able to induce the phenomenon of MPT without the strong involvement
of oxidative stress, even though the compound is able to cause it.
Most probably, the contribution of the oxidizing effect of AGE in
inducing MPT is negligible. Consequently, as it is well known, MPT
is related to the release of pro-apoptotic factors, such as
cytochrome c, apoptosis-inducing factor and Smac/Diablo,
inducing cell death by intrinsic apoptosis (50).
Abbreviations:
|
AGE
|
aged garlic extract
|
|
SAC
|
S-allylcysteine
|
|
SAMC
|
S-allylmercaptocysteine
|
|
ROS
|
reactive oxygen species
|
|
CNS
|
central nervous system
|
|
WT
|
wild-type
|
|
MDR
|
multidrug-resistant
|
|
RLM
|
rat liver mitochondria
|
|
MTT
|
thiazolyl blue tetrazolium
bromide
|
|
FBS
|
fetal bovine serum
|
|
JC-1
|
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine
iodide
|
|
BSA
|
bovine serum albumin
|
|
Δψm
|
mitochondrial membrane potential
|
|
TPP+
|
tetraphenylphosphonium
|
|
ΔμH+
|
electrochemical gradient
|
|
CsA
|
cyclosporin A
|
|
BKA
|
bongkrekic acid
|
|
ADP
|
adenosine diphosphate
|
|
MPT
|
mitochondrial permeability
transition
|
|
RR
|
ruthenium red
|
|
NEM
|
N-ethylmaleimide
|
|
DTE
|
1,4-dithioerythritol
|
|
PEG
|
polyethylene glycol
|
|
BSAO
|
bovine serum amine oxidase
|
|
AdNT
|
adenine nucleotide translocase
|
Acknowledgments
The authors would like to thank Dr Naoaki Morihara
for reading the manuscript.
References
|
1
|
Gallo M, Altieri F, Di Stadio CS, Miselli
G, Villano V, Arcari P and Rippa E: An overview on factors
underlying gastric cancer; strategies for its management with
particular reference to diet. J Gastrointest Dig Syst. 6:399–408.
2016. View Article : Google Scholar
|
|
2
|
Fleischauer AT, Poole C and Arab L: Garlic
consumption and cancer prevention: Meta-analyses of colorectal and
stomach cancers. Am J Clin Nutr. 72:1047–1052. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rahman K and Lowe GM: Garlic and
cardiovascular disease: A critical review. J Nutr. 136(Suppl 3):
736S–740S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banerjee SK and Maulik SK: Effect of
garlic on cardiovascular disorders: A review. Nutr J. 1:42002.
View Article : Google Scholar
|
|
5
|
Chu Q, Ling MT, Feng H, Cheung HW, Tsao
SW, Wang X and Wong YC: A novel anticancer effect of garlic
derivatives: Inhibition of cancer cell invasion through restoration
of E-cadherin expression. Carcinogenesis. 27:2180–2189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomson M and Ali M: Garlic [Allium
sativum]: A review of its potential use as an anti-cancer agent.
Curr Cancer Drug Targets. 3:67–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li G, Qiao C, Lin R, Pinto J, Osborne M
and Tiwari R: Antiproliferative effects of garlic constituents in
cultured human breast-cancer cells. Oncol Rep. 2:787–791.
1995.PubMed/NCBI
|
|
8
|
Shirin H, Pinto JT, Kawabata Y, Soh JW,
Delohery T, Moss SF, Murty V, Rivlin RS, Holt PR and Weinstein IB:
Antiproliferative effects of S-allylmercaptocysteine on colon
cancer cells when tested alone or in combination with sulindac
sulfide. Cancer Res. 61:725–731. 2001.PubMed/NCBI
|
|
9
|
Hosono T, Fukao T, Ogihara J, Ito Y, Shiba
H, Seki T and Ariga T: Diallyl trisulfide suppresses the
proliferation and induces apoptosis of human colon cancer cells
through oxidative modification of beta-tubulin. J Biol Chem.
280:41487–41493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Howard EW, Ling MT, Chua CW, Cheung HW,
Wang X and Wong YC: Garlic-derived S-allylmercaptocysteine is a
novel in vivo antimetastatic agent for androgen-independent
prostate cancer. Clin Cancer Res. 13:1847–1856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sriram N, Kalayarasan S, Ashokkumar P,
Sureshkumar A and Sudhandiran G: Diallyl sulfide induces apoptosis
in Colo 320 DM human colon cancer cells: Involvement of caspase-3,
NF-kappaB, and ERK-2. Mol Cell Biochem. 311:157–165. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lai KC, Kuo CL, Ho HC, Yang JS, Ma CY, Lu
HF, Huang HY, Chueh FS, Yu CC and Chung JG: Diallyl sulfide,
diallyl disulfide and diallyl trisulfide affect drug resistant gene
expression in colo 205 human colon cancer cells in vitro and in
vivo. Phytomedicine. 19:625–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yan JY, Tian FM, Hu WN, Zhang JH, Cai HF
and Li N: Apoptosis of human gastric cancer cells line SGC 7901
induced by garlic-derived compound S-allylmercaptocysteine (SAMC).
Eur Rev Med Pharmacol Sci. 17:745–751. 2013.PubMed/NCBI
|
|
14
|
Zhang H, Wang K, Lin G and Zhao Z:
Antitumor mechanisms of S-allyl mercaptocysteine for breast cancer
therapy. BMC Complement Altern Med. 14:2702014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kodera Y, Ushijima M, Amano H, Suzuki JI
and Matsutomo T: Chemical and biological properties of
S-1-propenyl-l-cysteine in aged garlic extract. Molecules.
22:E5702017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Franco-Enzástiga Ú, Santana-Martínez RA,
Silva-Islas CA, Barrera-Oviedo D, Chánez-Cárdenas ME and Maldonado
PD: Chronic administration of S-allylcysteine activates Nrf2 factor
and enhances the activity of antioxidant enzymes in the striatum,
frontal cortex and hippocampus. Neurochem Res. 42:3041–3051. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imai J, Ide N, Nagae S, Moriguchi T,
Matsuura H and Itakura Y: Antioxidant and radical scavenging
effects of aged garlic extract and its constituents. Planta Med.
60:417–420. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei Z and Lau BHS: Garlic inhibits free
radical generation and augments antioxidant enzyme activity in
vascular endothelial cells. Nutr Res. 18:61–70. 1998. View Article : Google Scholar
|
|
19
|
Borek C: Antioxidant health effects of
aged garlic extract. J Nutr. 131:1010S–1015S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka S, Haruma K, Kunihiro M, Nagata S,
Kitadai Y, Manabe N, Sumii M, Yoshihara M, Kajiyama G and Chayama
K: Effects of aged garlic extract (AGE) on colorectal adenomas: A
double-blinded study. Hiroshima J Med Sci. 53:39–45. 2004.
|
|
21
|
Matsuura N, Miyamae Y, Yamane K, Nagao Y,
Hamada Y, Kawaguchi N, Katsuki T, Hirata K, Sumi S and Ishikawa H:
Aged garlic extract inhibits angiogenesis and proliferation of
colorectal carcinoma cells. J Nutr. 136(Suppl 3): 842S–846S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Budoff MJ, Takasu J, Flores FR, Niihara Y,
Lu B, Lau BH, Rosen RT and Amagase H: Inhibiting progression of
coronary calcification using Aged Garlic Extract in patients
receiving statin therapy: A preliminary study. Prev Med.
39:985–991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morihara N, Hino A, Yamaguchi T and Suzuki
J: Aged garlic extract suppresses the development of
atherosclerosis in apolipoprotein E-knockout mice. J Nutr.
146:460S–463S. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ried K, Travica N and Sali A: The effect
of aged garlic extract on blood pressure and other cardiovascular
risk factors in uncontrolled hypertensives: The AGE at Heart trial.
Integr Blood Press Control. 9:9–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thorajak P, Pannangrong W, Welbat JU,
Chaijaroonkhanarak W, Sripanidkulchai K and Sripanidkulchai B:
Effects of Aged Garlic Extract on cholinergic, glutamatergic and
GABAergic systems with regard to cognitive impairment in Aβ-induced
rats. Nutrients. 9:686–698. 2017. View Article : Google Scholar
|
|
26
|
Morihara N, Hino A, Miki S, Takashima M
and Suzuki JI: Aged garlic extract suppresses inflammation in
apolipoprotein E-knockout mice. Mol Nutr Food Res. 61:10–16. 2017.
View Article : Google Scholar
|
|
27
|
Zeinali H, Baluchnejadmojarad T, Fallah S,
Sedighi M, Moradi N and Roghani M: S-allyl cysteine improves
clinical and neuropathological features of experimental autoimmune
encephalomyelitis in C57BL/6 mice. Biomed Pharmacother. 97:557–563.
2018. View Article : Google Scholar
|
|
28
|
Katsuki T, Hirata K, Ishikawa H, Matsuura
N, Sumi S and Itoh H: Aged garlic extract has chemopreventative
effects on 1,2-dimethylhydrazine-induced colon tumors in rats. J
Nutr. 136(Suppl 3): 847S–851S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jikihara H, Qi G, Nozoe K, Hirokawa M,
Sato H, Sugihara Y and Shimamoto F: Aged garlic extract inhibits
1,2-dimethylhydrazine-induced colon tumor development by
suppressing cell proliferation. Oncol Rep. 33:1131–1140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amagase H: Clarifying the real bioactive
constituents of garlic. J Nutr. 136(Suppl 3): 716S–725S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsutomo T and Kodera Y: Development of
an analytic method for sulfur compounds in aged garlic extract with
the use of a postcolumn high performance liquid chromatography
method with sulfur-specific detection. J Nutr. 146:450S–455S. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leanza L, Zoratti M, Gulbins E and Szabo
I: Mitochondrial ion channels as oncological targets. Oncogene.
33:5569–5581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr.
131:1075S–1079S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morihara N, Sumioka I, Moriguchi T, Uda N
and Kyo E: Aged garlic extract enhances production of nitric oxide.
Life Sci. 71:509–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Grandi M, Geroni C and Giuliani FC:
Isolation and characterization of a human colon adenocarcinoma cell
line resistant to doxorubicin. Br J Cancer. 54:515–518. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agostinelli E, Belli F, Molinari A,
Condello M, Palmigiani P, Vedova LD, Marra M, Seiler N and Arancia
G: Toxicity of enzymatic oxidation products of spermine to human
melanoma cells (M14): Sensitization by heat and MDL 72527. Biochim
Biophys Acta. 1763:1040–1050. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Molinari A, Toccacieli L, Calcabrini A,
Diociaiuti M, Cianfriglia M and Arancia G: Induction of
P-glycoprotein expression on the plasma membrane of human melanoma
cells. Anticancer Res. 20:2691–2696. 2000.PubMed/NCBI
|
|
38
|
Dolfini E, Dasdia T, Arancia G, Molinari
A, Calcabrini A, Scheper RJ, Flens MJ, Gariboldi MB and Monti E:
Characterization of a clonal human colon adenocarcinoma line
intrinsically resistant to doxorubicin. Br J Cancer. 76:67–76.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Agostinelli E, Condello M, Molinari A,
Tempera G, Viceconte N and Arancia G: Cytotoxicity of spermine
oxidation products to multidrug resistant melanoma M14 ADR2 cells:
Sensitization by the MDL 72527 lysosomotropic compound. Int J
Oncol. 35:485–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Frezza C, Cipolat S and Scorrano L:
Organelle isolation: Functional mitochondria from mouse liver,
muscle and cultured fibroblasts. Nat Protoc. 2:287–295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gornall AG, Bardawill CJ and David MM:
Determination of serum proteins by means of the biuret reaction. J
Biol Chem. 177:751–766. 1949.PubMed/NCBI
|
|
42
|
Kamo N, Muratsugu M, Hongoh R and Kobatake
Y: Membrane potential of mitochondria measured with an electrode
sensitive to tetraphenyl phosphonium and relationship between
proton electrochemical potential and phosphorylation potential in
steady state. J Membr Biol. 49:105–121. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Santos AC, Uyemura SA, Lopes JLC, Bazon
JN, Mingatto FE and Curti C: Effect of naturally occurring
flavonoids on lipid peroxidation and membrane permeability
transition in mitochondria. Free Radic Biol Med. 24:1455–1461.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Crompton M and Costi A: Kinetic evidence
for a heart mitochondrial pore activated by Ca2+,
inorganic phosphate and oxidative stress. A potential mechanism for
mitochondrial dysfunction during cellular Ca2+ overload.
Eur J Biochem. 178:489–501. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Toninello A, Miotto G, Siliprandi D,
Siliprandi N and Garlid KD: On the mechanism of spermine transport
in liver mitochondria. J Biol Chem. 263:19407–19411.
1988.PubMed/NCBI
|
|
46
|
Managò A, Leanza L, Carraretto L, Sassi N,
Grancara S, Quintana-Cabrera R, Trimarco V, Toninello A, Scorrano
L, Trentin L, et al: Early effects of the antineoplastic agent
salinomycin on mitochondrial function. Cell Death Dis. 6:e19302015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mitchell P and Moyle J: Estimation of
membrane potential and pH difference across the cristae membrane of
rat liver mitochondria. Eur J Biochem. 7:471–484. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nakashima RA and Garlid KD: Quinine
inhibition of Na+ and K+ transport provides
evidence for two cation/H+ exchangers in rat liver
mitochondria. J Biol Chem. 257:9252–9254. 1982.PubMed/NCBI
|
|
49
|
Zoratti M and Szabò I: The mitochondrial
permeability transition. Biochim Biophys Acta. 1241:139–176. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Susin SA, Zamzami N and Kroemer G:
Mitochondria as regulators of apoptosis: Doubt no more. Biochim
Biophys Acta. 1366:151–165. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Agostinelli E, Belli F, Dalla Vedova L,
Marra M, Crateri P and Arancia G: Hyperthermia enhances
cytotoxicity of amine oxidase and spermine on drug-resistant LoVo
colon adenocarcinoma cells. Int J Oncol. 28:1543–1553.
2006.PubMed/NCBI
|
|
52
|
Takahashi T, Horie H, Kojima O and Itoh M:
Preoperative combined treatment with radiation, intraluminal
hyperthermia, and 5-fluorouracil suppositories for patients with
rectal cancer. Surg Today. 23:1043–1048. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vernon CC, Hand JW, Field SB, Machin D,
Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD,
González González D, et al International Collaborative Hyperthermia
Group: Radiotherapy with or without hyperthermia in the treatment
of superficial localized breast cancer: Results from five
randomized controlled trials. Int J Radiat Oncol Biol Phys.
35:731–744. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bates DA and Mackillop WJ: The effect of
hyperthermia in combination with melphalan on drug-sensitive and
drug-resistant CHO cells in vitro. Br J Cancer. 62:183–188. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dahl O: Mechanisms of thermal enhancement
of chemotherapeutic cytotoxicity. Hyperthermia and Oncology. Urano
M and Douple E: 4. Utrecht: VSP; pp. 9–28. 1994
|
|
56
|
Reinhold HS and Endrich B: Tumour
microcirculation as a target for hyperthermia. Int J Hyperthermia.
2:111–137. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Agostinelli E, Arancia G, Calcabrini A,
Matarrese P, Mondovì B and Pietrangeli P: Hyperthermia-induced
biochemical and ultra-structural modifications in cultured cells.
Exp Oncol. 17:269–276. 1995.
|
|
58
|
Maeda H, Seymour LW and Miyamoto Y:
Conjugates of anticancer agents and polymers: Advantages of
macromolecular therapeutics in vivo. Bioconjug Chem. 3:351–362.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Demers N, Agostinelli E, Averill-Bates DA
and Fortier G: Immobilization of native and poly(ethylene
glycol)-treated ('PEGylated') bovine serum amine oxidase into a
biocompatible hydrogel. Biotechnol Appl Biochem. 33:201–207. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR
and Tsourkas A: Multifunctional nanoparticles: Cost versus benefit
of adding targeting and imaging capabilities. Science. 338:903–910.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bertrand N, Wu J, Xu X, Kamaly N and
Farokhzad OC: Cancer nanotechnology: The impact of passive and
active targeting in the era of modern cancer biology. Adv Drug
Deliv Rev. 66:2–25. 2014. View Article : Google Scholar :
|
|
62
|
Agostinelli E, Vianello F, Magliulo G,
Thomas T and Thomas TJ: Nanoparticle strategies for cancer
therapeutics: Nucleic acids, polyamines, bovine serum amine oxidase
and iron oxide nanoparticles (Review). Int J Oncol. 46:5–16. 2015.
View Article : Google Scholar
|
|
63
|
Venditti I, Hassanein TF, Fratoddi I,
Fontana L, Battocchio C, Rinaldi F, Carafa M, Marianecci C,
Diociaiuti M, Agostinelli E, et al: Bioconjugation of gold-polymer
core-shell nanoparticles with bovine serum amine oxidase for
biomedical applications. Colloids Surf B Biointerfaces.
134:314–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Montanari E, Capece S, Di Meo C, Meringolo
M, Coviello T, Agostinelli E and Matricardi P: Hyaluronic acid
nanohydrogels as a useful tool for BSAO immobilization in the
treatment of melanoma cancer cells. Macromol Biosci. 13:1185–1194.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sinigaglia G, Magro M, Miotto G, Cardillo
S, Agostinelli E, Zboril R, Bidollari E and Vianello F:
Catalytically active bovine serum amine oxidase bound to
fluorescent and magnetically drivable nanoparticles. Int J
Nanomedicine. 7:2249–2259. 2012.PubMed/NCBI
|
|
66
|
Garlid KD, Dos Santos P, Xie ZJ, Costa AD
and Paucek P: Mitochondrial potassium transport: The role of the
mitochondrial ATP-sensitive K(+) channel in cardiac function and
cardioprotection. Biochim Biophys Acta. 1606:1–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Garlid KD and Paucek P: Mitochondrial
potassium transport: The K(+) cycle. Biochim Biophys Acta.
1606:23–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Garlid KD: Opening mitochondrial K(ATP) in
the heart - what happens, and what does not happen. Basic Res
Cardiol. 95:275–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tian J, Liu J, Garlid KD, Shapiro JI and
Xie Z: Involvement of mitogen-activated protein kinases and
reactive oxygen species in the inotropic action of ouabain on
cardiac myocytes. A potential role for mitochondrial K(ATP)
channels. Mol Cell Biochem. 242:181–187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Greenbaum NL and Wilson DF: The
distribution of inorganic phosphate and malate between intra- and
extramitochondrial spaces. Relationship with the transmembrane pH
difference. J Biol Chem. 260:873–879. 1985.PubMed/NCBI
|
|
71
|
Halestrap AP and Davidson AM: Inhibition
of Ca2(+)-induced large-amplitude swelling of liver and heart
mitochondria by cyclosporin is probably caused by the inhibitor
binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase
and preventing it interacting with the adenine nucleotide
translocase. Biochem J. 268:153–160. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Battaglia V, Brunati AM, Fiore C, Rossi
CA, Salvi M, Tibaldi E, Palermo M, Armanini D and Toninello A:
Glycyrrhetinic acid as inhibitor or amplifier of permeability
transition in rat heart mitochondria. Biochim Biophys Acta.
1778:313–323. 2008. View Article : Google Scholar
|
|
73
|
Salvi M, Brunati AM, Clari G and Toninello
A: Interaction of genistein with the mitochondrial electron
transport chain results in opening of the membrane transition pore.
Biochim Biophys Acta. 1556:187–196. 2002. View Article : Google Scholar : PubMed/NCBI
|