Introduction
Adenocarcinoma is the predominant malignancy
affecting the colon and rectum (1). Colorectal cancer is the third most
common cancer diagnosed in the developed world (2,3). The
average 5-year survival rate of patients with colorectal cancer
remains poor at 55% (3), even
though the development of new drugs has improved the survival rate
of patients. The prognosis of patients with colorectal cancer
remains poor, in spite of the development of novel therapeutic
strategies (4–7). Human colorectal cancer represents a
heterogenous group of diseases, and its molecular classification is
increasingly important (4–7). The characterization of novel
biomarker targets may lead to the prolonged survival of patients
with colorectal cancer. Biomarkers may play a potential role in the
screening, diagnosis, prognosis and monitoring of the disease
(4–7). Mutations in the KRAS gene in ~40% of
tumors have been reported to be induced by genetic and epigenetic
alterations (8–11).
The expression of regucalcin, whose gene is
localized on the X chromosome (12–14),
has been shown to be suppressed in tumor tissues of mammalian and
human subjects in vivo (15,16),
suggesting that the suppressed expression of regucalcin plays an
important role in the promotion development of carcinogenesis
(15,16). Regucalcin has been demonstrated to
play a pivotal role as a repressor of manifold signaling pathways
and transcriptional activity in various types of cells (17,18);
this protein plays a regulatory role in the inhibition of various
signaling pathways implicated in calcium homeostasis, various
protein kinases and protein phosphatases, the suppression of
cytosolic protein synthesis, nuclear DNA and RNA synthesis, and the
regulation of nuclear gene expression (17–20).
Moreover, regucalcin has been found to suppress the proliferation
(15) and apoptotic cell death
mediated through diverse signaling molecules in the cells of
various types of tissues (20).
Thus, regucalcin may play a pivotal role in maintaining cell
homeostasis (17,18,21).
Importantly, regucalcin gene expression has been
shown to be decreased in various tissues of human cancer (15,16,22),
suggesting that a diminished regucalcin gene expression may induce
the promotion of carcinogenesis (15,16,22).
We have previously demonstrated that survival was prolonged in
patients with pancreatic cancer (23), breast cancer (24), hepatocellular carcinoma (25) and lung cancer (26) who had a higher regucalcin
expression in their tumor tissues as compared with those with a
lower regucalcin expression. In support of these findings, the
overexpression of regucalcin exerted repressive effects on the
growth of human pancreatic cancer MIA PaCa-2 cells (23), MDA-MB-231 breast cancer cells
(24), liver cancer HepG2 cells
(25) and lung adenocarcinoma A549
cells (26) in vitro.
Regucalcin may thus play a potential role as a suppressor of the
development of carcinogenesis in human subjects, demonstrating its
significance as a novel biomarker in the diagnosis of human
cancer.
The involvement of regucalcin in human colorectal
cancer has not yet been investigated, at least to best of our
knowledge. The current study was thus undertaken to determine
whether regucalcin is involved in the suppression of human
colrorectal cancer. Notably, we found that the prolonged survival
of patients with colorectal cancer was associated with a higher
regucalcin gene expression in the tumor tissues, as evaluated by
the analysis of gene expression using the Gene Expression Omnibus
(GEO) database (GSE12945). In addition, the overexpression of
regucalcin was shown to inhibit the growth of human colorectal
cancer RKO cells in vitro. Our findings thus support the
view that the diminished regucalcin gene expression predisposes
patients with colorectal cancer, suggesting a novel therapeutic
strategy involving regucalcin gene therapy in human colorectal
cancer.
Materials and methods
Materials and reagents
Dulbecco's modification of Eagle's medium (DMEM)
with 4.5 g/l glucose, L-glutamine and sodium pyruvate and
antibiotics (100 µg/ml penicillin and 100 µg/ml
streptomycin; P/S) were purchased from Corning (Mediatech, Inc.
Manassas, VA, USA). Fetal bovine serum (FBS) was from HyClone
(Logan, UT, USA). Lipofectamine reagent was obtained from Promega,
Madison, WI, USA). Tumor necrosis factor-α (TNF-α) was from R&D
Systems (Minneapolis, MN, USA). Sodium butyrate, roscovitine,
sulforaphane, dibucaine, PD98059, lipopolysaccharoide (LPS), Bay K
8644, wortmannin, 5, 6-dichloro-1-β-D-ribofuranosylbenzimidazole
(DRB), caspase-3 inhibitor, and all other reagents were purchased
from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise specified.
Gemcitabine was obtained from Hospira, Inc. (Lake Forest, IL, USA).
Gemcitabine and caspase-3 inhibitor were diluted in
phosphate-buffered saline (PBS) and thye other reagents were
dissolved in 100% ethanol for use.
Patient datasets
Regucalcin gene expression and the survival data of
62 patients with colorectal cancer were obtained though the GEO
database, GSE12945, for outcome analysis (27). These datasets contained gene
expression data derived from the Affymetrix U133A array. Microarray
analysis was performed as previously described (23–26).
Expression and raw expression data (CEL files) were summarized and
normalized using the Robust Multi-array Average algorithm and the
Bioconductor package affy (http://www.bioconductor.org/packages/2.0/bioc/html/affy.html).
Human colorectal cancer RKO cells
We used epithelial RKO cells originating from male
adult patients with colorectal carcinoma, which were obtained from
the American Type Culture Collection (ATCC; Rockville, MD, USA).
The RKO cells were suitable as a transfection host. The cells were
cultured using DMEM containing 10% FBS and 1% P/S.
Transfection of human regucalcin
cDNA
The RKO wild-type cells were transfected with pCXN2
vector expressing cDNA encoding human full-length (900 bp)
regucalcin (regucalcin cDNA/pCXN2) (28). To assay transient transfection, the
RKO cells were grown on 24-well plates to reach subconfluency.
Regucalcin cDNA/pCXN2 and empty pCXN2 vector alone were transfected
into the RKO cells using the synthetic cationic lipid components,
Lipofectamine reagent, according to manufacturer's instructions
(Promega) (28). Following
overnight incubation, Geneticin (600 µg/ml G418,
Sigma-Aldrich) was added to the culture wells, and the cells were
cultured to select the transfected cells for 3 weeks. Subsequently,
the cells were plated at limiting dilution to isolate
transfectants. Survival clones were isolated, transferred to 35-mm
dishes, and grown in medium without Geneticin. We obtained clones 1
and 2 of transfectants with the stable expression of regucalcin,
and the regucalcin levels in these clones were increased by 7.4- or
10.9-fold as compared with those of the wild-type cells,
respectively as shown in Fig. 2.
Clone 2 was used in the following experiments.
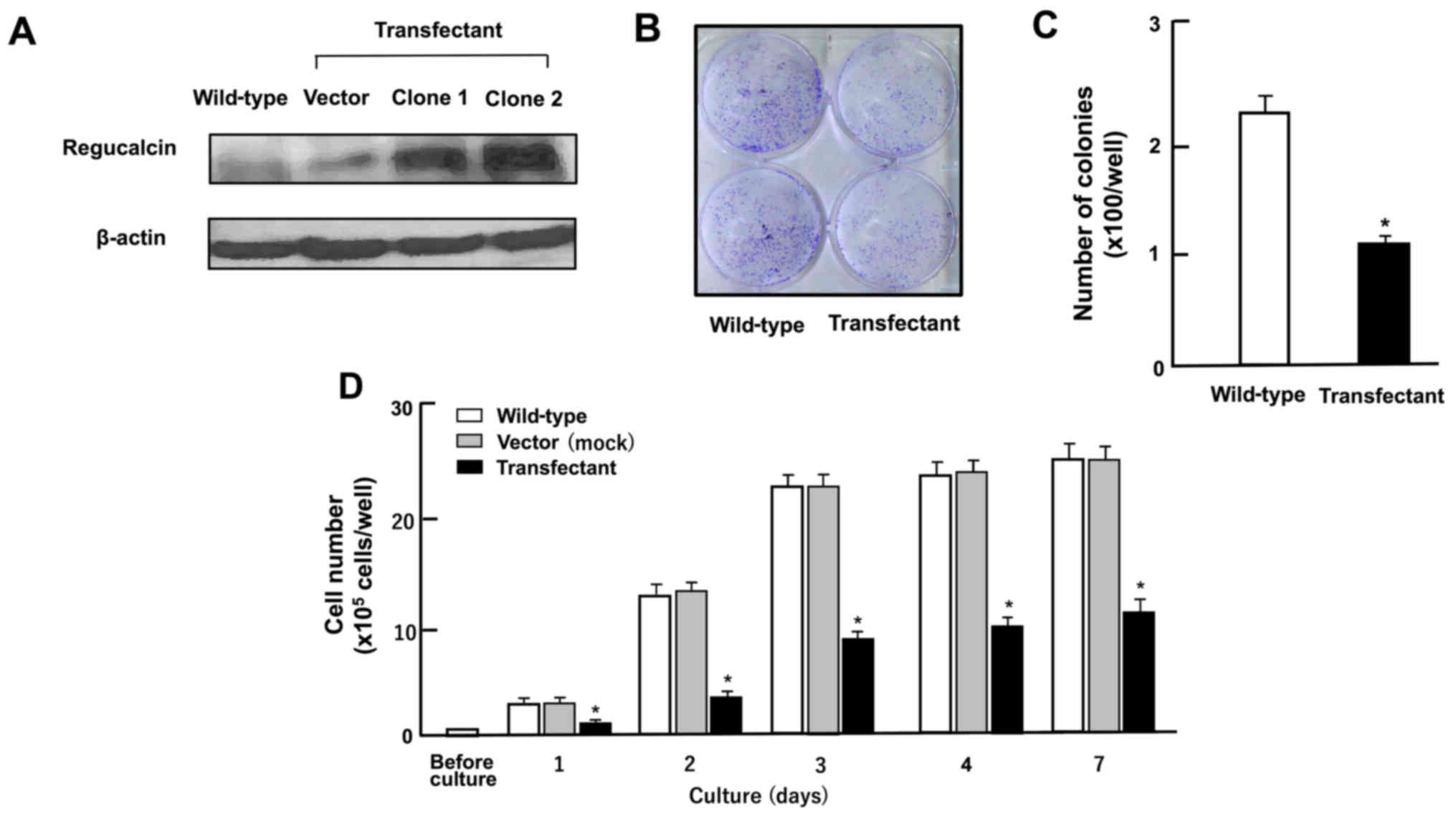 | Figure 2Overexpression of regucalcin
suppresses the colony formation and proliferation of human
colorectal cancer RKO cells in vitro. (A) Regucalcin
expression in the cells cultured for 3 days was analyzed by western
blot analysis with an anti-regucalcin antibody. The lanes from left
to right are as follows: Lane 1, wild-type cells; lane 2, cells
transfected with empty vector/pCXN2 (designated as vector); lanes 3
or 4; cells (clone 1 or 2) transfected with the human regucalcin
cDNA/pCXN2. (B and C) In colony formation, RKO wild-type cells and
transfectants were cultured for 7 days. After culture, colonies
were stained cells with 0.5% crystal violet, and stained colonies
was counted. (B) Image of colony formation. (C) Colonies containing
>50 cells was counted under a microscope. (D) In cell
proliferation assay, RKO wild-type cells, vector or clone 2 were
cultured in DMEM for 1, 2, 3, 4 or 7 days. After culture, the
number of cells attached on the dish was counted. Data are
presented as the means ± SD obtained from 8 wells of 2 replicate
plates per dataset using different dishes and cell preparation.
*P<0.001 vs. wild-type (white bar) or control vector
(grey bar), determined by one-way ANOVA with the Tukey-Kramer post
hoc test. |
Colony formation assay
The RKO wild-type cells or transfectants were seeded
into 6-well dishes at a density of 1×103/well and
cultured in medium containing 10% FBS and 1% P/S under the
condition of 5% CO2 and 37°C for 7 days, when visible
clonies were formed on the plates (29). The obtained colonies were washed
with PBS and fixed with methanol (0.5 ml per well) for 20 min at
room temperature, and then washed 3 times with PBS. Finally,
colonies were stained with 0.5% crystal violet for 30 min at room
temperature. Stained cells were washed 4 times with PBS. The plates
were air-dried for 2 h at room temperature. The colonies containing
>50 cells were counted under a microscope (Olympus MTV-3;
Olympus Corporation, Tokyo, Japan).
Cell proliferation assay
The RKO wild-type cells (1×105/ml per
well) or ROK cells (1×105/ml per well) transfected with
regucalcin cDNA were cultured in DMEM containing 10% FBS and 1% P/S
using a 24-well plate for 1, 2, 3, 4 or 7 days in a water-saturated
atmosphere containing 5% CO2 and 95% air at 37°C, as
previously described (23,26,30).
In separate experiments, RKO wild-type cells or transfectants were
cultured in DMEM containing 10% FBS and 1% P/S in the presence of
either sodium butyrate (10 and 100 µM), roscovitine (10 and
100 nM), sulphoraphan (1 and 10 nM), staurosporin (10 or 100 nM),
TNF-α (0.1 or 1 µg/ml), PD98059 (1 or 10 µM),
wortmannin (0.1 or 1 µM), DRB (0.1 or 1 µM), or
gemcitabine (50 or 100 nM) for 3 days. After culture, the cells
were detached from each culture dish by the addition of sterilized
solution (0.1 ml per well) of 0.05% trypsin plus EDTA in
Ca2+/Mg2+-free PBS (Thermo Fisher Scientific,
Waltham, MA, USA) with incubation for 2 min at 37°C, and then 0.9
ml of DMEM containing 10% FBS and 1% P/S were added to each well.
The cell number in the suspended medium was counted as described
below in the section 'Cell counting'.
Cell death assay
The RKO wild-type cells (1×105/ml per
well) cells or RKO cells (1×105/ml per well) transfected
with regucalcin cDNA were cultured in DMEM containing 10% FBS and
1% P/S using a 24-wells plate for 3 days until reaching
subcon-fluency, and they were then cultured for an additional 24 h
in the presence or absence of either Bay K 8644 (0.1 or 1
µM) or gemcitabine (10 or 100 nM) (31). In separate experiments, RKO
wild-type cells (1×105/ml per well) or transfectants
were cultured for 3 days until reaching subconfluency, and were
then cultured for an additional 24 h in the presence or absence of
either Bay K 8644 (1 µM) or gemcitabine (10 or 100 nM) with
or without caspase-3 inhibitor (10 µM) for 24 h, as
previously described (31). After
culture, the cells were detached by the addition of sterilized
solution (0.1 ml per well) of 0.05% trypsine plus EDTA in
Ca2+/Mg2+-free PBS into each well as
described above in the section 'Cell proliferation assay', and the
cell number was then counted as described below in the section
'Cell counting'.
Cell counting
To detach the cells on each well, the culture dishes
were incubated for 2 min at 37°C after the addition of the solution
(0.1 ml per well) of 0.05% trypsin plus EDTA in
Ca2+/Mg2+-free PBS, and the cells were then
detached through pipetting following the addition of DMEM (0.9 ml)
containing 10% FBS and 1% P/S (23,26,30,31).
Medium containing the suspended cell (0.1 ml) was mixed by the
addition of 0.1 ml of 0.5% trypan blue staining solution. The
number of cells with viability were counted under a microscope
(Olympus MTV-3, Olympus) using a hemocytometer plate
(Sigma-Aldrich) with a cell counter (Line Seiki H-102P; Line Seiki
Co., Ltd., Tokyo, Japan). For each dish, we took the average of two
counting. The Cell number was shown as the number per well of
plate.
Western blot analysis
The RKO wild-type cells, control vector
cDNA-transfected cells, or regucalcin cDNA-transfected cells were
plated in 100-mm dishes at a density of 1×106 cells/well
in 10 ml of DMEM containing 10% FBS and 1% P/S, and were cultured
for 3 days. Following culture, the cells were washed 3 times with
cold PBS and removed from the dish by scraping using cell lysis
buffer (Cell Signaling Technology, Danvers, MA, USA) with the
addition of the inhibitors of protease and protein phosphatase
(Roche Diagnostics, Indianapolis, IN, USA). The lysates were then
centrifuged at 17,000 × g, at 4°C for 10 min. The protein
concentration of the supernatant obtained was determined for
western blotting using the Bio-Rad Protein Assay Dye (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with bovine serum albumin as
a standard. The supernatant was then stored at −80°C until use.
Samples of 40 µg of supernatant protein per lane were
separated by SDS polyacrylamide gel electrophoresis (12%, SDS-PAGE)
and transferred onto nylon membranes for immunoblotting using
specific antibodies against various proteins, which were obtained
from Cell Signaling Technology including Ras (#14429 rabbit), Akt
(#9272, rabbit), phospho-Akt (#9271, rabbit), mitogen-activated
protein kinase (MAPK; #4695, rabbit), phospho-MAPK (#4370, rabbit),
SAPK/JNK (#9252, rabbit), phospho-SAPK/JNK (#9251, rabbit), PI3
p110α (#4255, rabbit), Rb (#9309, mouse), p21 (#2947, rabbit),
c-jun (#9165, rabbit), β-catenin (#9581, rabbit), signal transducer
and activator of transcription 3 (Stat3; #12640, rabbit),
phospho-Stat3 (#9131, rabbit) and β-actin (#3700, mouse) and Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) including p53
(sc-126, mouse), c-fos (sc-52, rabbit), NF-κB p65 (sc-109, rabbit)
β-catenin (sc-39350, mouse) (28).
Rabbit anti-regucalcin antibody was obtained from Abcam (Cambridge,
MA, USA; diluted 1:1000, ab213459, rabbit), and it has been used
previously (22,28,32).
The target protein was incubated with one of the primary antibodies
(1:1,000) including phosphorylated types of various proteins (as
described above) overnight at 4°C, and followed by horseradish
peroxidase-conjugated secondary antibodies (mouse sc-2005 or rabbit
sc-2305; Santa Cruz Biotechnology, Inc.; diluted 1:2,000). The
immunoreactive blots were visualized with a SuperSignal West Pico
Chemiluminescent Substrate detection system (Thermo Scientific,
Rockford, IL, USA) according to the manufacturer's instructions.
β-actin (#3700, mouse; Cell Signaling Technology; diluted 1:2,000)
was used as a loading control. Three blots from independent
experiments were scanned on an Epson Perfection 1660 Photo scanner,
and bands quantified using ImageJ software.
Statistical analysis
Statistical significance was determined using
GraphPad InStat version 3 for Windows XP (GraphPad Software Inc. La
Jolla, CA, USA). Multiple comparisons were performed by one-way
analysis of variance (ANOVA) with the Tukey-Kramer multiple
comparisons post hoc test for parametric data as indicated.
Survival curves were constructed by Kaplan-Meier analysis and were
compared with the log-rank test as performed with IBM SPSS
software. Other data were analyzed with the paired or unpaired
Student's t-test as performed with IBM SPSS Statistics 18 software
(IBM, Chicago, IL, USA http://www.ibm.com). A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
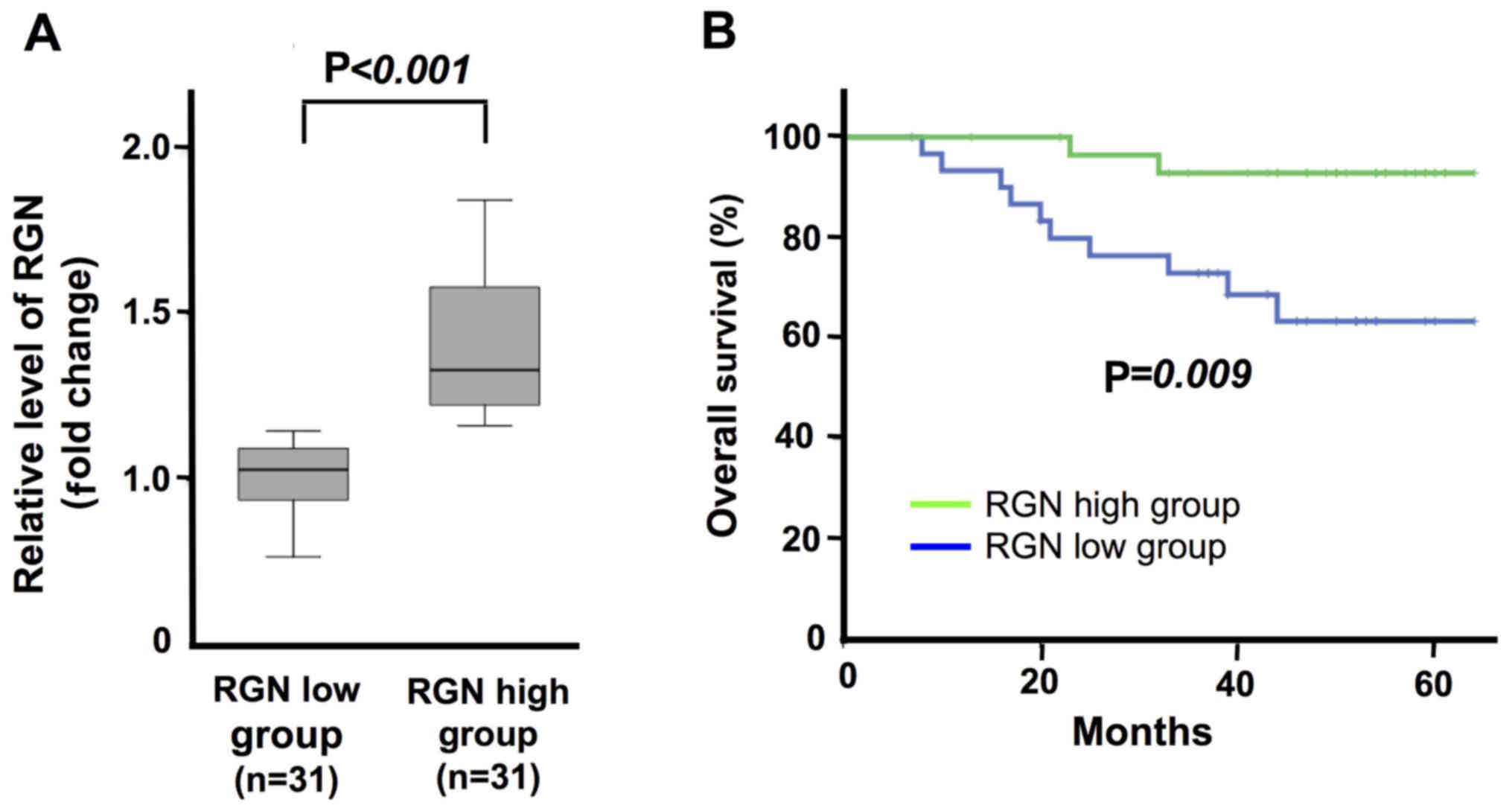
A higher regucalcin gene expression is
associated with the prolonged survival of patients with colorectal
cancer
To determine the involvement of regucalcin in
patients with colorectal cancer, we analyzed the expression levels
of regucalcin in colorectal tumor tissues of human subjects. We
compared regucalcin gene expression in the tumor tissues of
patients with colorectal cancer. The patients with colorectal
cancer were classified into 2 groups: One with a high (31 patients)
and another with a low (31 patients) mRNA expression of regucalcin
in the colorectal tumor tissues. As the group of patients with a
higher regucalcin mRNA expression was compared to the group of
patients with a lower regucalcin mRNA expression, a significant
difference was found between the 2 groups (Fig. 1A). The analysis of Kaplan-Meier
curve revealed that the survival of the group with a higher
regucalcin mRNA expression in the colorectal tumor tissues was
predominantly prolonged as compared with that of the group with a
lower regucalcin mRNA expression (Fig.
1B). This result supports the view that a decreased regucalcin
gene expression leads to the development of carcinogenesis in human
colorectal cells, and that this causes a worse clinical outcome.
However, further studies using multiple datasets are warranted to
confirm the results of this study.
Overexpression of regucalcin suppresses
the growth of RKO cells
To generate regucalcin-overexpressing cells, RKO
cells were transiently transfected with the empty pCXN2 vector or
human regucalcin cDNA (coding full-length 33 kDa protein)/pCXN2
construct using Lipofectamine. We isolated clone 1 and 2 of
transfectants with a stable expression of regucalcin, and the
regucalcin levels were higher in clone 2 as compared with those of
clone 1 (Fig. 2A). Thus, clone 2
was used to determine the effects of the overexpression of
regucalcin on the growth of RKO cells in vitro. Firstly, to
determine the effects of the overexpression of regucalcin on colony
formation, RKO wild-type cells and transfectants were cultured for
7 days (Fig. 2B and C). The number
of colonies formed was found to decrease in the
regucalcin-overexpressing transfectants as compared with the
wild-type cells (Fig. 2B and C).
The proliferation of the wild-type RKO cells was markedly enhanced
with increasing days of culture (Fig.
2D). This enhancement of proliferation was clearly suppressed
in the transfectants (Fig. 2D).
Thus, the overexpression of regucalcin suppressed the colony
formation and proliferation of human colorectal cancer RKO
cells.
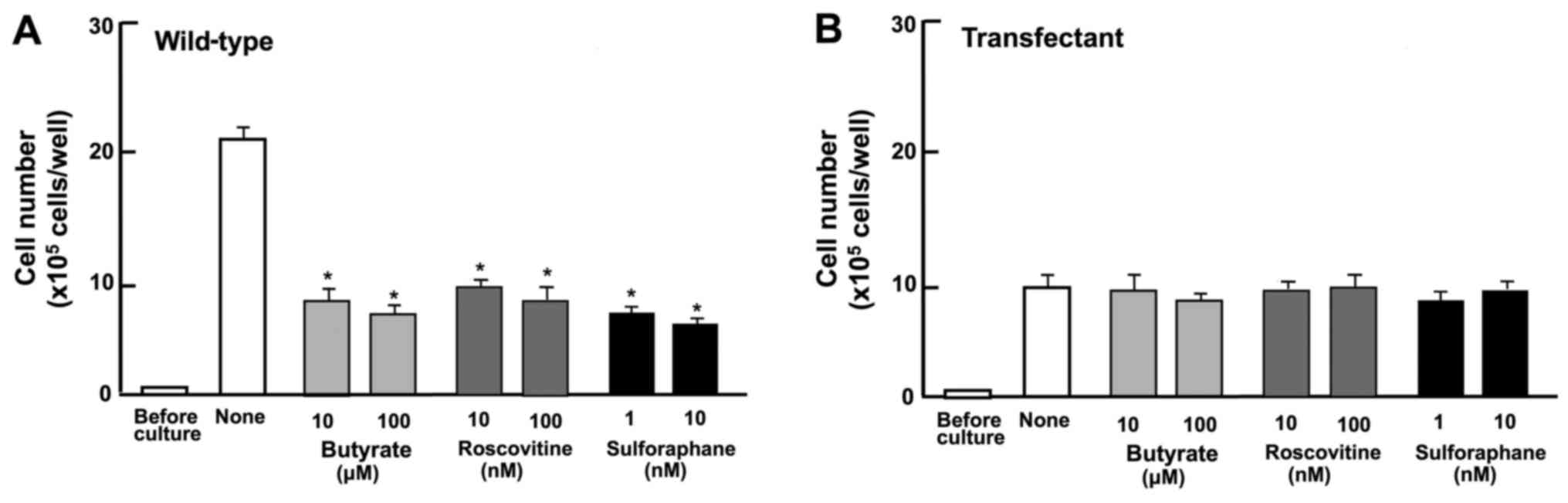
Suppressive effects of the overexpression
of regucalcin on the proliferation of RKO cells involve various
signaling pathways
To determine the mechanisms through which the
overexpression of regucalcin suppresses the proliferation of RKO
cells, it was investigated whether the revelation of suppressive
effects of the overexpression of regucalcin are attenuated in the
presence of various inhibitors that induce cell cycle arrest in
vitro (Fig. 3). Wild-type
cells were cultured for 3 days in the presence of butyrate (10 and
100 µM) (33), roscovitine
(10 and 100 nM) (34) or
sulforaphane (1 and 10 nM) (35).
The proliferation of the wild-type cells was suppressed in the
presence of these inhibitors (Fig.
3A). The effects of these inhibitors were not potentiated in
the transfectants (Fig. 3B). Thus,
this result suggested that the overexpression of regucalcin induces
G1 and G2/M phase cell cycle arrest in the RKO cells, although
further experiments using immunofluorescence assay are required to
confirm our findings.
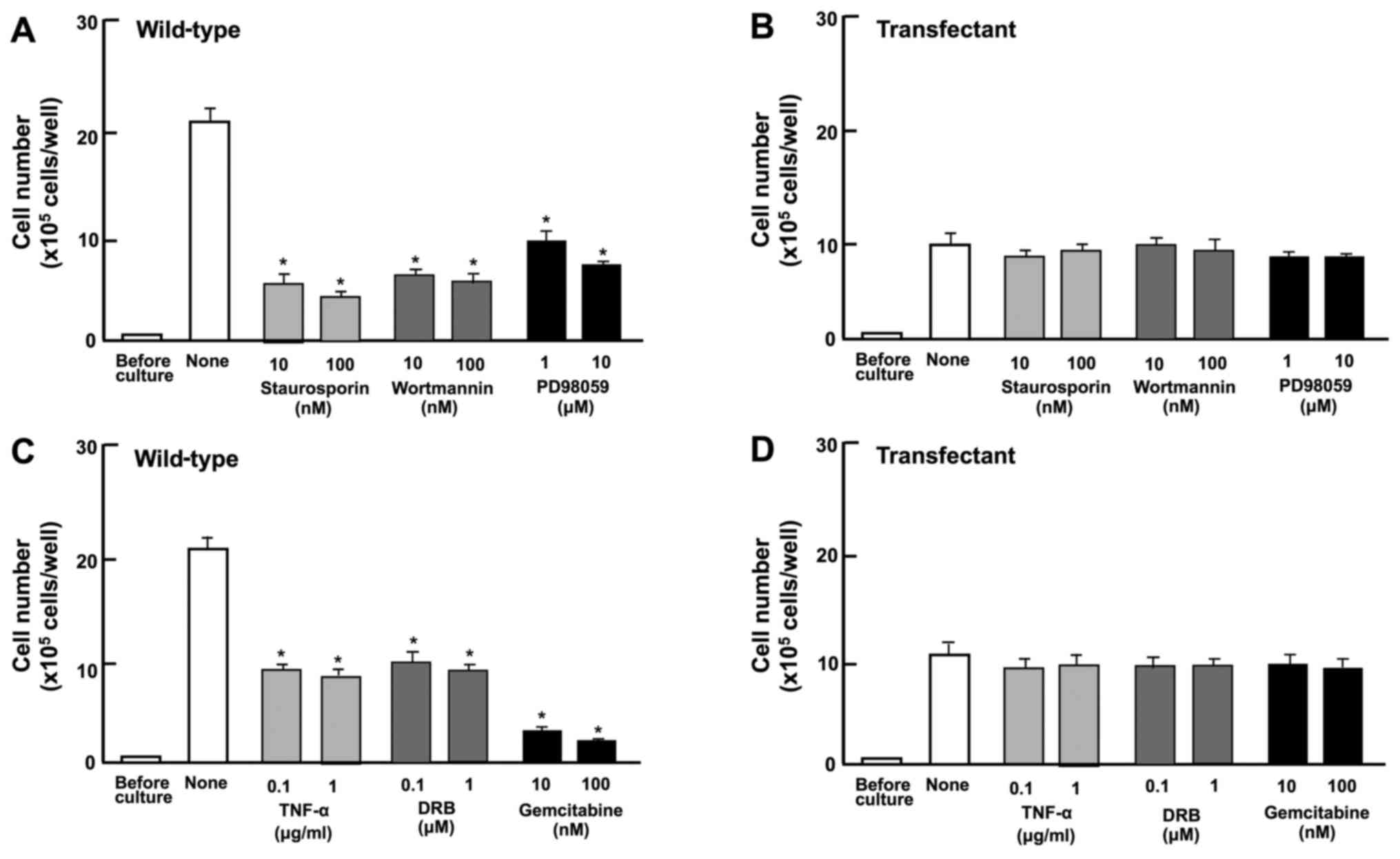
Next, we determined the involvement of signaling
factors in the suppressive effects induced by the overexpression of
regucalcin on cell proliferation. The proliferation of RKO
wild-type cells was suppressed by staurosporine (10 or 100 nM), a
calcium signaling protein kinase C-related inhibitor (36,37),
and PD98059 (1 or 10 µM), an inhibitor of extracellular
signal-regulated kinase (ERK) and mitogen-activated protein kinase
(MAPK) (38), and wortmannin (10
or 100 nM), an inhibitor of phosphatidylinositol 3-kinase (PI3K)
(39) (Fig. 4A). The blocking of these pathways
did not potentiate the suppressive effects of the overexpression of
regucalcin on cell proliferation (Fig.
4B).
DRB is an inhibitor of the transcriptional activity
with RNA polymerase II inhibition (40). Gemcitabine is a potent antitumor
agent that induces nuclear DNA damage (41). In this study, these inhibitors
suppressed the proliferation of wild-type cells (Fig. 4C). However, such effects were not
potentiated in the transfectants (Fig.
4D). These results suggest that the overexpression of
regucalcin suppresses various signaling processes linked to cell
proliferation, and that regucalcin-overexpressing cells exhibit a
lack of responses to the above-mentioned inhibitors of these
pathways.
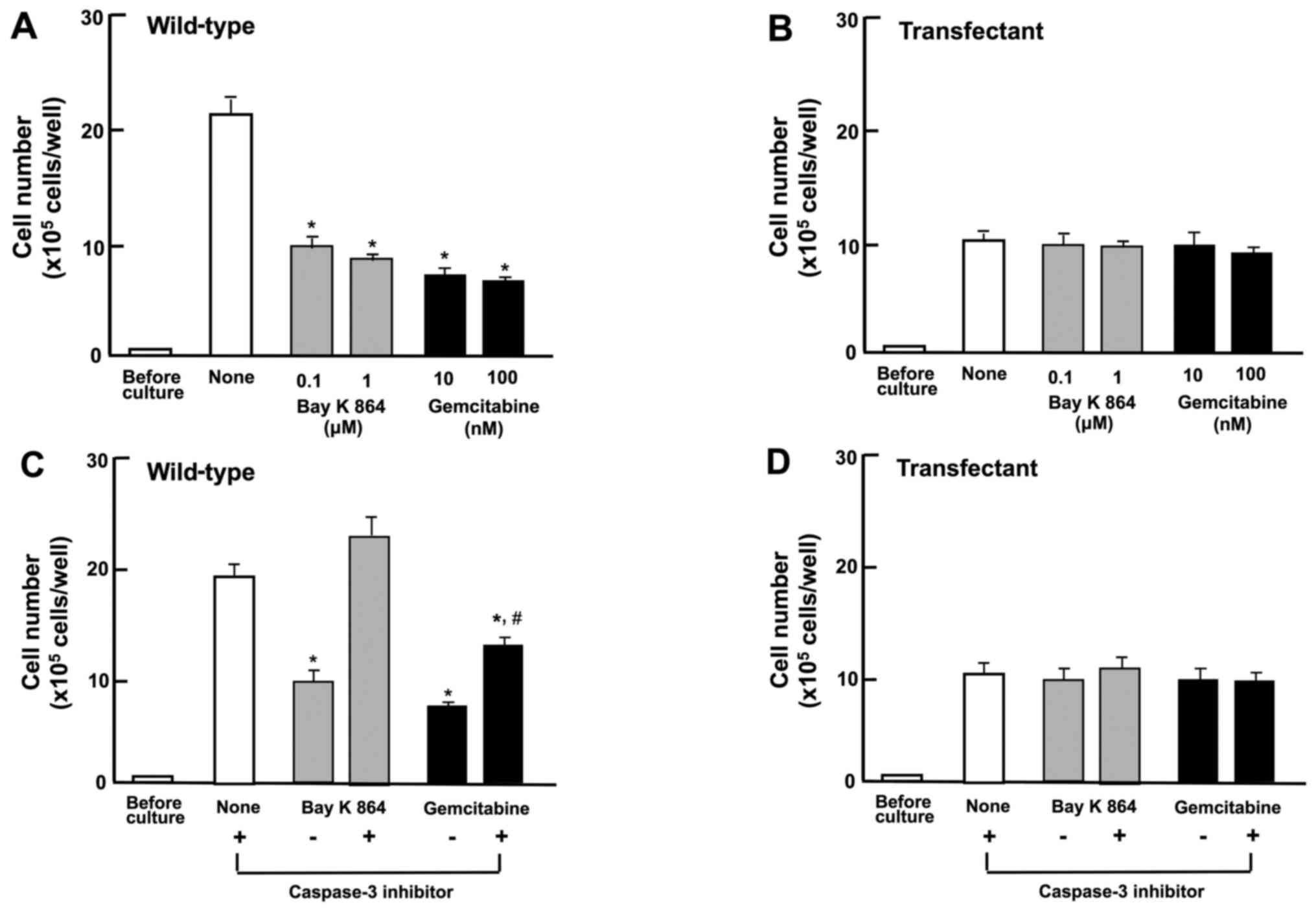
Suppressive effects of the overexpression
of regucalcin on colorectal carcinoma cell proliferation are
independent of cell death
The effects of the overexpression of regucalcin on
the death of RKO cells were then investigated. The wild-type cells
or transfectants were cultured for 3 days until reaching
subconfluency, and they were then cultured for 24 h after the
addition of various stimulatory factors that induce apoptotic cell
death. The number of wild-type cells was decreased by culture with
Bay K 8644 (0.1 and 1 µM) or gemcitabine (10 or 100 nM),
which are known to induce apoptotic cell death (20,31)
(Fig. 5A). The overexpression of
regucalcin did not lead to the death of the wild-type cells, and
the apoptotic cell death-inducing factors did not cause the cell
death of the transfectants (Fig.
5B). This result suggests that the suppressive effects of the
overexpression of regucalcin on the proliferation of RKO cells are
not a result of cell death.
Moreover, it was investigated whether the effects of
overexpressed regucalcin on cell death are mediated through
caspase-3. The RKO wild-type cells and transfectants, upon reaching
subconfluency, were cultured in the presence of Bay K 8644 (1
µM) or gemcitabine (100 nM) with or without caspase-3
inhibitors (10 µM) for 24 h (Fig. 5C and D). The effects of Bay K 8644
or gemcitabine on cell death were not observed in the presence of
the caspase-3 inhibitor (Fig. 5C).
The stimulatory effects of Bay K 8644 or gemcitabine on cell death
were not observed in transfectants cultured with or without
caspase-3 inhibitor (Fig. 5D).
These findings suggest that regucalcin prevents cell death by
decreasing the activity of caspase-3 that activates DNA
fragmentation in the nucleus, leading to apoptotic cell death.
Thus, the suppressive effects of the overexpression of regucalcin
on the proliferation of RKO cells are not a result of cell
death.
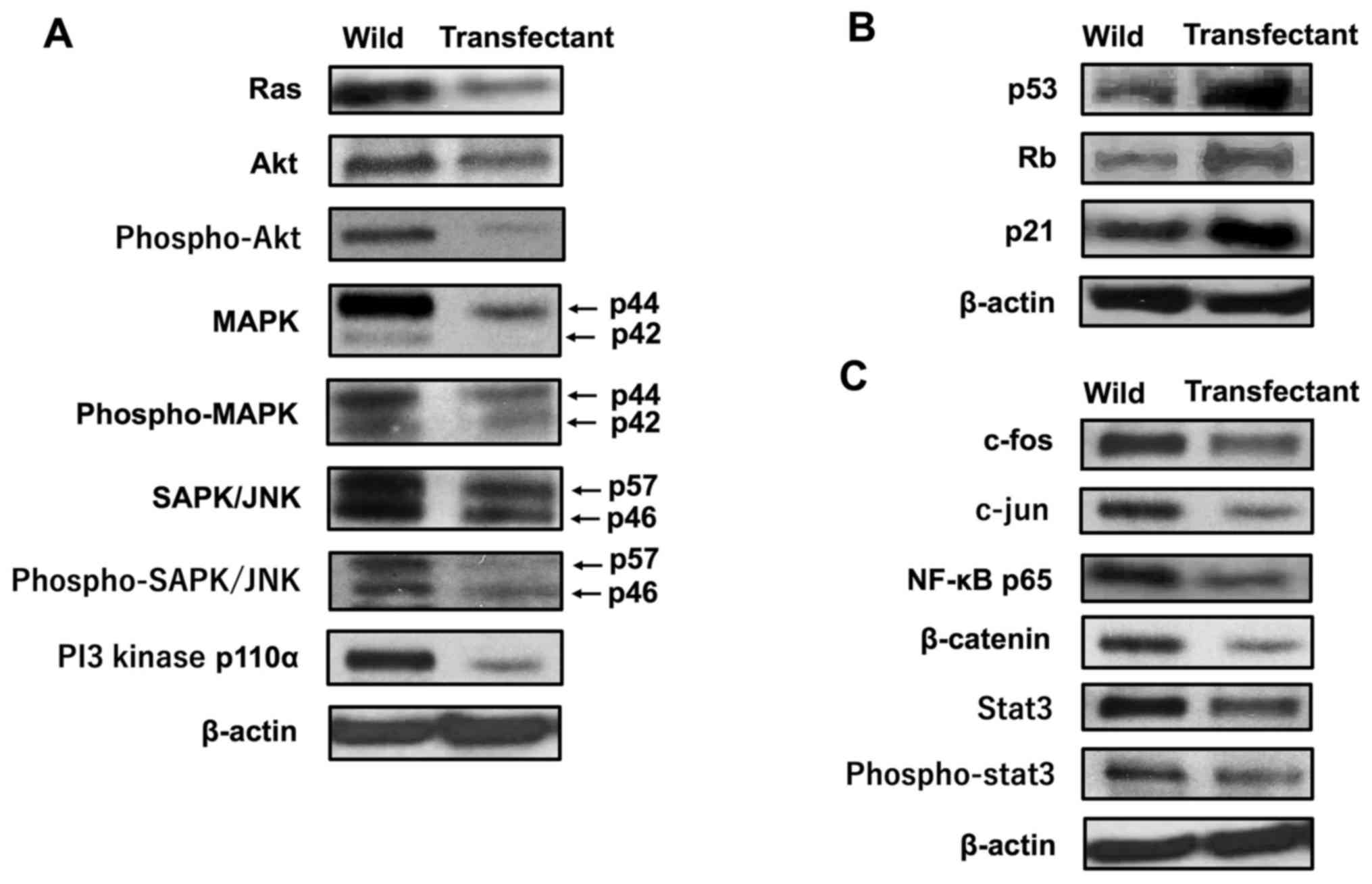
Overexpression of regucalcin regulates
the expression of various proteins related to cell signaling and
transcription activity
Mechanistically, it was investigated whether the
overexpression of regucalcin regulates the expression of key
proteins, which are involved in signaling pathways and
transcriptional activity, by western blot analysis. The results
revealed that the levels of Ras, Akt, phospho-Akt, MAPK,
phospho-MAPK, SAPK/JNK, phospho-SAPK/JNK and PI3 kinase 110α were
diminished by the overexpression of regucalcin (Fig. 6A). These results suggest that the
overexpression of regucalcin suppresses the activation of
Ras-linked signaling pathways in RKO cells. Of note, the
overexpression of regucalcin elevated the protein levels of p53 and
Rb, tumor suppressors, and that of p21, an inhibitor of the cell
cycle (Fig. 6B). In addition, the
overexpression of regucalcin diminished the levels of c-fos, c-jun,
NF-κB p65, β-catenin, Stat3 and phospho-Stat3 which are
transcription factors linked to the proliferation of RKO cells
(Fig. 6C). Thus, we determined the
changes in the levels of proteins (14 molecules), which may be
major signaling proteins related to the proliferation of cancer
cells. However, various other proteins are implicated in the
proliferation of cancer cells. Thus, further investigations are
required to determine involvement of other proteins.
Discussion
In this study, we performed the profiling of gene
expression and analysis of the survival of 62 patients with
colorectal cancer using the GEO database (GSE12945) for outcome
analysis. The prolonged survival of the patients with colorectal
cancer was found to be associated with a higher regucalcin gene
expression in the tumor tissues. The diminished gene expression of
regucalcin was accompanied by a poor prognosis of patients with
colorectal cancer. This finding supports the view that the
suppression of regucalcin gene expression may contribute to the
promotion or aggressiveness of the development of human colorectal
cancer. A downregulated regucalcin gene expression may lead to a
worse clinical outcome of cancer patients. Moreover, to determine a
translational mechanism for this clinical finding, it was
investigated whether the overexpression of regucalcin suppresses
the proliferation of human colorectal cancer RKO cells in
vitro. The overexpression of regucalcin was shown to suppress
colony formation and the proliferation of RKO cells without
inducing direct cell toxicity with necrotic or apoptotic cell death
in vitro. Thus, this study demonstrated a crucial role of
regucalcin as a suppressor in the growth of human colorectal cancer
cells. Endogenous regucalcin may play a suppressive role in the
development of human colorectal cancer. However, further studies
using multiple datasets are warranted to confirm the results of
this study.
The mechanistic characterization of the suppressive
effects of the overexpression of regucalcin on the proliferation of
RKO cells was investigated using various inhibitors that regulate
cell signaling pathways. The suppressive effects of the
overexpression of regucalcin on the proliferation of RKO cells were
not potentiated in the presence of butyrate, roscovitine or
sulphoraphan, that induce cell cycle arrest. Butyrate induces an
inhibition of G1 progression (33). Roscovitine is a potent and
selective inhibitor of the cyclin-dependent kinase cdc2, cdk2m and
cdk5 (34). Sulforaphane induces
G2/M phase cell cycle arrest (35). The overexpression of regucalcin was
suggested to cause G1 and G2/M phase cell cycle arrest in RKO
cells. Such findings have been shown in various types of cells,
including normal rat kidney proximal tubular epithelial NRK52E
cells (36), rat hepatoma H4-II-E
cells (28) and human cancer cells
of various types (23–26) in vitro. Importantly, the
overexpression of regucalcin has been shown to increase the
expression of p21, a cell cycle inhibitor, supporting the view that
regucalcin plays a role in cell cycle arrest (23–26).
It was then determined whether regucalcin regulates
cell signaling pathways using various inhibitors. The suppressive
effects of the overexpression of regucalcin on the proliferation of
RKO cells were not potentiated in the presence of dibucaine, an
inhibitor of calcium/calmodulin-dependent protein kinases (30), staurosporine, an inhibitor of
protein kinase C (37),
wortmannin, an inhibitor of the PI3K/Akt signaling pathway
(38) and PD98059, an inhibitor of
the ERK/MAP kinase-related signaling pathway (39). The overexpression of regucalcin was
shown to exert suppressive effects on cell proliferation due to the
inhibition of various signaling pathways, namely
Ca2+-dependent kinases, PI3K/Akt, and ERK/MAPK in RKO
cells. Thus, regucalcin has potential as a suppressor of diverse
signaling pathways in human cancer cells. Furthermore, results of
western blot analysis revealed that the overexpression of
regucalcin induced a decrease in the levels of various proteins
that are involved in signaling pathways linked to Ras, Akt, MAPK,
SAPK/JNK and PI3 kinase in RKO cells. The suppressive effects of
regucalcin, which are mediated through the regulation of various
signaling pathways, have also been observed in various types of
human cancer cells, including pancreatic MIA-PaCa2 cells (23), MDA-MB-231 human breast cancer cells
(24), human liver cancer HepG2
cells (25) and human lung cancer
A549 cells (26) in
vitro.
The suppressive effects of the overexpression of
regucalcin on the proliferation of RKO cells were not altered by
culture with DRB, an inhibitor of transcriptional activity with RNA
polymerase II inhibition (40).
The suppressive effects of the overexpression of regucalcin on the
proliferation of RKO cells were not potentiated by culture with
gemcitabine, which is used in the therapy of human cancer as an
antitumor agent that induces DNA damage in the nuclei (41). This drug inhibits the proliferation
and stimulates apoptotic cell death in various types of cancer
cells (41). Our results suggest
that regucalcin partly regulates pathways implicated in the mode of
action of gemcitabine. Regucalcin has been demonstrated to directly
suppress DNA and RNA synthesis using isolated rat liver nuclei
(17–19).
Regucalcin has been shown to play a role in the
regulation of cell nuclear function (19). Importantly, the overexpression of
regucalcin has been demonstrated to enhance the gene expression
levels of p53 and Rb, tumor suppressors, and those of p21, an
inhibitor of the cell cycle, and to suppress the gene expression
levels of ras, c-jun and c-myc, oncogenes, due to binding to
nuclear DNA in cloned rat hepatoma H4-II-E cells in vitro
(19,42). Moreover, in this study, the
overexpression of regucalcin was found to elevate the protein
levels of p53, Rb and p21 and to diminish those of ras, c-fos,
c-fos, NF-κB p65, β-catenin and Stat3, which are transcription
factors linked to cancer cell proliferation, in RKO cells in
vitro. These findings may support the view that endogenous
regucalcin plays a pivotal role in mediating suppressive effects on
the growth of cancer cells due to the regulation of the expression
of various proteins linked to transcription factors, tumor
suppressors and oncogenes linked to tumor development. Regucalcin
binds to DNA (42) and regulates
the gene expressions of various proteins in the nucleus of normal
and cancer cells (19,42).
The overexpression of regucalcin was shown to
prevent the colony formation of RKO cells in vitro. Such
effects of the overexpression of regucalcin on the colony formation
of RKO cells may be a result of the suppression of proliferation
induced by the overexpression of regucalcin. Colorectal cancer
cells may express a particularly aggressive metastatic phenotype of
primary neoplastic cells to regional lymph nodes, liver, adrenal
glands, contralateral lung, brain and bone marrow (1,2). The
overexpression of regucalcin may lead to the suppression of the
colony formation and metastasis of colorectal cancer. In addition,
we hypothesize that the overexpression of regucalcin may suppress
tumor growth in animal models following the transplantation of
cancer cells in vivo. This remains to be elucidated.
In conclusion, the current study demonstrates that
the prolonged survival of patients with colorectal cancer is
associated with a higher regucalcin gene expression in the tumor
tissues, and that the overexpression of regucalcin suppresses the
proliferation of human colorectal cancer RKO cells in vitro.
A higher expression of endogenous regucalcin plays a potential role
as a suppressor of the development of human colorectal cancer, and
the downregulation of regucalcin leads to the progression of
carcinogenesis. Previously, we demonstrated that a higher
regucalcin expression in tumor tissue prolonged the survival of
patients with pancreatic cancer (23), breast cancer (24), hepatocellular carcinoma (25) and lung adenocarcinoma (26), and that the overexpression of
regucalcin suppressed the growth of their related human cancer
cells in vitro (23–26).
Thus, regucalcin may play a crucial role as a suppressor of
carcinogenesis in various types of human cancer. The downregulation
of regucalcin gene expression may predispose patients to the
promotion of cancer. The delivery of the regucalcin gene, which is
overexpressed in tumor tissues, may provide a novel therapeutic
strategy for human cancer.
Acknowledgments
The authors would like to thank Dr Oliver Hankinson
(David Geffen School of Medicine, University of California, Los
Angeles, USA) for his encouragement.
References
|
1
|
Porter MG and Stoeger SM: Atypical
colorectal neoplasms. Surg Clin North Am. 97:641–656. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Society Cancer: Cancer facts
& figures. 2016, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html.
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar
|
|
5
|
Alnabulsi A and Murray GI: Integrative
analysis of the colorectal cancer proteome: Potential clinical
impact. Expert Rev Proteomics. 13:1–11. 2016. View Article : Google Scholar
|
|
6
|
Alnabulsi A, Swan R, Cash B, Alnabulsi A
and Murray GI: The differential expression of omega-3 and omega-6
fatty acid metabolising enzymes in colorectal cancer and its
prognostic significance. Br J Cancer. 116:1612–1620. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carini F, Mazzola M, Rappa F, Jurjus A,
Geagea AG, Al Kattar S, Bou-Assi T, Jurjus R, Damiani P, Leone A,
et al: Colorectal carcinogenesis: Role of oxidative stress and
antioxidants. Anticancer Res. 37:4759–4766. 2017.PubMed/NCBI
|
|
8
|
Colussi D, Brandi G, Bazzoli F and
Ricciardiello L: Molecular pathways involved in colorectal cancer:
Implications for disease behavior and prevention. Int J Mol Sci.
14:16365–16385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kudryavtseva AV, Lipatova AV, Zaretsky AR,
Moskalev AA, Fedorova MS, Rasskazova AS, Shibukhova GA, Snezhkina
AV, Kaprin AD, Alekseev BY, et al: Important molecular genetic
markers of colorectal cancer. Oncotarget. 7:53959–53983. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones RP, Sutton PA, Evans JP, Clifford R,
McAvoy A, Lewis J, Rousseau A, Mountford R, McWhirter D and Malik
HZ: Specific mutations in KRAS codon 12 are associated with worse
overall survival in patients with advanced and recurrent colorectal
cancer. Br J Cancer. 116:923–929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimokawa N and Yamaguchi M: Molecular
cloning and sequencing of the cDNA coding for a calcium-binding
protein regucalcin from rat liver. FEBS Lett. 327:251–255. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimokawa N, Matsuda Y and Yamaguchi M:
Genomic cloning and chromosomal assignment of rat regucalcin gene.
Mol Cell Biochem. 151:157–163. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thiselton DL, McDowall J, Brandau O,
Ramser J, d'Esposito F, Bhattacharya SS, Ross MT, Hardcastle AJ and
Meindl A: An integrated, functionally annotated gene map of the
DXS8026-ELK1 interval on human Xp11.3-Xp11.23: Potential hotspot
for neuro-genetic disorders. Genomics. 79:560–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamaguchi M: Suppressive role of
regucalcin in liver cell proliferation: Involvement in
carcinogenesis. Cell Prolif. 46:243–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi M: Involvement of regucalcin as
a suppressor protein in human carcinogenesis: Insight into the gene
therapy. J Cancer Res Clin Oncol. 141:1333–1341. 2015. View Article : Google Scholar
|
|
17
|
Yamaguchi M: Role of regucalcin in
maintaining cell homeostasis and function (review). Int J Mol Med.
15:371–389. 2005.PubMed/NCBI
|
|
18
|
Yamaguchi M: Regucalcin and cell
regulation: Role as a suppressor protein in signal transduction.
Mol Cell Biochem. 353:101–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamaguchi M: Role of regucalcin in cell
nuclear regulation: Involvement as a transcription factor. Cell
Tissue Res. 354:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi M: The anti-apoptotic effect of
regucalcin is mediated through multisignaling pathways. Apoptosis.
18:1145–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaguchi M: The Role of Regucalcin in
Cell Homeostasis and Disorder. Nova Science Publishers, Inc; New
York, NY: 2017
|
|
22
|
Murata T and Yamaguchi M: Alternatively
spliced variants of the regucalcin gene in various human normal and
tumor tissues. Int J Mol Med. 34:1141–1146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamaguchi M, Osuka S, Weitzmann MN,
El-Rayes BF, Shoji M and Murata T: Prolonged survival in pancreatic
cancer patients with increased regucalcin gene expression:
Overexpression of regucalcin suppresses the proliferation in human
pancreatic cancer MIA PaCa-2 cells in vitro. Int J Oncol.
48:1955–1964. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaguchi M, Osuka S, Weitzmann MN, Shoji
M and Murata T: Increased regucalcin gene expression extends
survival in breast cancer patients: Overexpression of regucalcin
suppresses the proliferation and metastatic bone activity in
MDA-MB-231 human breast cancer cells in vitro. Int J Oncol.
49:812–822. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi M, Osuka S, Weitzmann MN,
El-Rayes BF, Shoji M and Murata T: Prolonged survival in
hepatocarcinoma patients with increased regucalcin gene expression:
HepG2 cell proliferation is suppressed by overexpression of
regucalcin in vitro. Int J Oncol. 49:1686–1694. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaguchi M, Osuka S, Shoji M, Weitzmann
MN and Murata T: Survival of lung cancer patients is prolonged with
higher regucalcin gene expression: Suppressed proliferation of lung
adenocarcinoma A549 cells in vitro. Mol Cell Biochem. 430:37–46.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Staub E, Groene J, Heinze M, Mennerich D,
Roepcke S, Klaman I, Hinzmann B, Castanos-Velez E, Pilarsky C, Mann
B, et al: An expression module of WIPF1-coexpressed genes
identifies patients with favorable prognosis in three tumor types.
J Mol Med (Berl). 87:633–644. 2009. View Article : Google Scholar
|
|
28
|
Misawa H, Inagaki S and Yamaguchi M:
Suppression of cell proliferation and deoxyribonucleic acid
synthesis in the cloned rat hepatoma H4-II-E cells overexpressing
regucalcin. J Cell Biochem. 84:143–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fang Z, Tang Y, Fang J, Zhou Z, Xing Z,
Guo Z, Guo X, Wang W, Jiao W, Xu Z, et al: Simvastatin inhibits
renal cancer cell growth and metastasis via AKT/mTOR, ERK and
JAK2/STAT3 pathway. PLoS One. 17:e628232013. View Article : Google Scholar
|
|
30
|
Yamaguchi M and Daimon Y: Overexpression
of regucalcin suppresses cell proliferation in cloned rat hepatoma
H4-II-E cells: Involvement of intracellular signaling factors and
cell cycle-related genes. J Cell Biochem. 95:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Izumi T and Yamaguchi M: Overexpression of
regucalcin suppresses cell death in cloned rat hepatoma H4-II-E
cells induced by tumor necrosis factor-alpha or thapsigargin. J
Cell Biochem. 92:296–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamaguchi M and Isogai M: Tissue
concentration of calcium-binding protein regucalcin in rats by
enzyme-linked immunoadsorbent assay. Mol Cell Biochem. 122:65–68.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Charollais RH, Buquet C and Mester J:
Butyrate blocks the accumulation of CDC2 mRNA in late G1 phase but
inhibits both the early and late G1 progression in chemically
transformed mouse fibroblasts BP-A31. J Cell Physiol. 145:46–52.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meijer L, Borgne A, Mulner O, Chong JP,
Blow JJ, Inagaki N, Inagaki M, Delcros JG and Moulinoux JP:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh SV, Herman-Antosiewicz A, Singh AV,
Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L and Baskaran R:
Sulforaphane-induced G2/M phase cell cycle arrest involves
checkpoint kinase 2-mediated phosphorylation of cell division cycle
25C. J Biol Chem. 279:25813–25822. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakagawa T, Sawada N and Yamaguchi M:
Overexpression of regucalcin suppresses cell proliferation of
cloned normal rat kidney proximal tubular epithelial NRK52E cells.
Int J Mol Med. 16:637–643. 2005.PubMed/NCBI
|
|
37
|
Tamaoki T, Nomoto H, Takahashi I, Kato Y,
Morimoto M and Tomita F: Staurosporine, a potent inhibitor of
phospholipid/Ca++dependent protein kinase. Biochem
Biophys Res Commun. 135:397–402. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Serrano-Nascimento C, da Silva Teixeira S,
Nicola JP, Nachbar RT, Masini-Repiso AM and Nunes MT: The acute
inhibitory effect of iodide excess on sodium/iodide symporter
expression and activity involves the PI3K/Akt signaling pathway.
Endocrinology. 155:1145–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pelech SL, Charest DL, Mordret GP, Siow
YL, Palaty C, Campbell D, Charlton L, Samiei M and Sanghera JS:
Networking with mitogen-activated protein kinases. Mol Cell
Biochem. 127–128:157–169. 1993. View Article : Google Scholar
|
|
40
|
Palangat M, Grass JA, Langelier MF,
Coulombe B and Landick R: The RPB2 flap loop of human RNA
polymerase II is dispensable for transcription initiation and
elongation. Mol Cell Biol. 31:3312–3325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tang SC and Chen YC: Novel therapeutic
targets for pancreatic cancer. World J Gastroenterol.
20:10825–10844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsurusaki Y and Yamaguchi M: Role of
regucalcin in liver nuclear function: Binding of regucalcin to
nuclear protein or DNA and modulation of tumor-related gene
expression. Int J Mol Med. 14:277–281. 2004.PubMed/NCBI
|