Introduction
Osteosarcoma is a rare type of cancer (1,2).
However, its incidence has been reported to have increased yearly
in developed and developing countries, particularly in China
(3). Rapidly changing ecological
environments and living habits are thought to have contributed to
this increase (4). Unfortunately
there are no general solutions to address the increasing incidence.
Generally speaking, surgical resection is the primary treatment
mode of osteosarcoma, and hormone therapy, radiotherapy and
chemotherapy serve auxiliary therapeutic roles (5,6).
With the current treatment options, patient prognosis is relatively
poor (1,2,5,7).
Herpes simplex virus thymidine kinase/ganciclovir
(HSV-TK/GCV) systems have been widely applied in suicide cancer
gene therapy (8,9). Theoretically, HSV-TK phosphorylates
GCV to GCV-monophosphate, which is then converted to
GCV-triphosphate by endogenous cellular nucleoside kinases
(10). GCV-triphosphate acts as a
DNA chain terminator due to the lack of a functional 3′-OH group,
terminating DNA replication and causing apoptosis (11).
An important feature of the HSV-TK/GCV suicide gene
system is that its ability to kill tumor cells is largely dependent
on the integrity of gap junction intercellular communication (GJIC)
(12). Connexin 43 (Cx43), a
member of the connexin family, is a component of gap junctions.
These are intercellular channels that connect adjacent cells,
permitting the exchange of low molecular weight molecules,
including ions and secondary messengers to regulate cell death,
proliferation and differentiation (13-15).
Unfortunately, numerous types of cancer, including glioma, gastric
cancer, hepatocellular carcinoma, breast cancer, prostate cancer
and ovarian cancer, frequently lose Cx43 expression (16-19),
which leads to defects in GJIC and decreases the effectiveness of
HSV-TK/GCV systems (16-19).
Small ubiquitin-like modifier (SUMO) conjugation is
a post-translational regulatory process which functions in all
eukaryotes, mediated by SUMO activating enzyme, SUMO conjugating
enzyme and SUMO ligase, which attach SUMO to target proteins
(20-22). Ubc9, the only SUMO E2 conjugating
enzyme, is often overexpressed in tumors (23-25),
suggesting that it may be involved in molecular events required
during cancer development (21,24,25).
Recently, Kjenseth et al (26) reported that Cx43 is covalently
modified and regulated by SUMOylation in HeLa cells. However, the
role of this process in osteosarcoma remains poorly understood.
Therefore, the present study investigated Cx43 SUMOylation in
osteosar-coma, and assessed whether this process positively or
negatively influences the integrity of GJIC function, and whether
it may be used to enhance the efficacy of HSV-TK/GCV systems.
Materials and methods
Tissue specimens
Fresh surgical specimens were collected from 16
osteosarcoma patients diagnosed at the Department of Bone and Soft
Tissue Tumors (Tianjin Medical University Cancer Institute and
Hospital, Tianjin, China) between January 2016 and December 2016.
The diagnosis was made by a senior pathologist and confirmed by
another experienced pathologist (Department of Pathology, The Fifth
Central Hospital of Tianjin). The present study was approved by the
ethics committee of Tianjin Medical University Cancer Institute and
Hospital (Tianjin, China) and written informed consent was obtained
from all patients.
Immunohistochemistry
Paraffin-embedded tissues were cut into
5-μm-thick slices, which were then dewaxed in xylene,
hydrated in order of 100, 90, 70 and 50% ethanol and microwaved at
80 kPa, 117°C for 3 min for antigen retrieval. This was followed by
3% hydrogen peroxide treatment (OriGene Technologies, Inc.,
Beijing, China) to remove endogenous peroxidase, and blocking with
goat serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at room temperature for 30 min. Next, samples were
incubated with a rabbit polyclonal connexin 43/GJA1 primary
antibody (dilution, 1:2,000; cat. no. ab11370; Abcam, Cambridge,
UK) overnight at 4°C. A goat anti-rabbit IgG H&L hoseradish
peroxidase-conjugated secondary antibody (dilution, 1:5,000; cat.
no. ab205718; Abcam) was then applied at 37°C for 1 h. The sections
were stained with hematoxylin (cat. no. G1140; Soulebao Technology
Co., Ltd.; Beijing, China) at the stock concentration at room
temperature for 8 min, and mounted onto cover slips.
Cell lines and cell culture
The osteosarcoma cell lines, 143B, MG-63 and U-2OS,
and the osteoblast cell line, hFOB1.19 were purchased from the
American Type Culture Collection (Manassas, VA, USA). All cells
were maintained in Dulbecco's modified Eagle's medium (DMEM; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and 100
μg/ml streptomycin (Sigma-Aldrich; Merck KGaA), at 37°C in
5% CO2.
Plasmids and transfection
The lentiviral plasmids pWPXLD-His-siR-Ubc9,
pWPXLD-HA-Cx43 and pWPXLD-Flag-SUMO1 were synthesized by Biogot
Technology Co., Ltd., (Nanjing, China), and were packaged in 293
cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Then these viral plasmids were infected into
U-2OS cells at 70% confluence at a concentration of 20
μl/ml, according to the manufacturer's protocol. Fory-eight
hours after transfection, Ubc9 silencing was confirmed by western
blotting. Proliferation, colony formation ability, migration
capacity and apoptosis were detected by MTT assays (27,28),
soft agar colony formation assays (27,29),
wound healing assays (28),
Transwell assays (27-29) and flow cytometry (28), as previously described. Ubc9 and
Cx43 subcellular localizations were detected by immunocytochemistry
as previously described (30).
GJIC function was measured by the Lucifer Yellow dye transfer
assay, as previously described (31). Briefly, cells were plated in the
35-mm dishes and grown to confluency. Scrape loading was performed
using a sharp knife, and the monolayer cells were immersed in 0.05%
of Lucifer Yellow (MW 457.2, Sigma-Aldrich Inc., Shanghai, China)
for 3 min at room temperature, then the GJIC function was evaluated
through transfer of Lucifer Yellow to neighboring cells from the
border of scraped line. No dye transfer was evident in cells
incompetent in GJIC.
Immunoprecipitation
Total protein was extracted from cells, and
approximately 1 mg was diluted 10-fold with Triton X-100 lysis
buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Triton
X-100, 1 mM PMSF, 10 mM iodoacetamide and protease inhibitors),
pre-treated with protein-agarose beads for 1 h at 4°C, followed by
the addition of the anti-HA tag antibody (dilution, 1:500; cat. no.
ab18181; Abcam) or anti-Flag tag antibody (dilution, 1:50; cat. no.
ab1162; Abcam). Following an incubation at 4°C overnight,
immunoprecipitates were washed three times with 1 ml Triton X-100
lysis buffer, then diluted in 2X SDS sample buffer. After heating
for 10 min at 50°C, the samples were evaluated by western
blotting.
Western blotting
Total protein was extracted from fresh tissues or
cells with lysis buffer (50 mM β-glycerophosphate, 1 mM EDTA, 1 mM
EGTA, 0.5 mM Na3VO4 and 1% Triton X-100, pH
7.4) and protein concentration was analyzed by BCA assay (Thermo
Scientific Inc.). Then western blotting was performed by 4-15%
SDS-PAGE (Bio-Rad Laboratories, Hercules, CA, USA). After
electrophoresis, the proteins were transferred onto polyvinylidene
difluoride membranes (Bio-Rad Laboratories), and blocked with 0.1%
TBS-Tween and 5% skim milk powder for 1 h at room temperature.
Next, the membranes were incubated with anti-Ubc9 (dilution,
1:2000; cat. no. ab75854), anti-SUMO1 (dilution, 1:2,000; cat. no.
ab133352), anti-Cx43 (dilution, 1:2,000; cat. no. ab11370),
anti-His (dilution, 1:1,000; cat. no. ab9108), anti-HA (dilution,
1:5,000; cat. no. ab9110) or anti-β-actin (dilution, 1:1,000; cat.
no. ab8227) (all from Abcam) primary antibodies overnight at 4°C.
The membranes were then washed 5 times in 0.1% TBS-Tween and
incubated for 1 h at room temperature with a chicken anti-rabbit
IgG horseradish peroxidase-conjugated secondary antibody (dilution,
1:2,000; cat. no. sc-516087; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Labeled proteins were detected using a Super
Signal protein detection kit (Pierce; Thermo Fisher Scientific,
Inc.), and changes in protein levels were evaluated using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
In vitro HSV-TK/GCV treatment
The Ad-CMV-TK plasmid containing the HSV-TK gene was
provided by the Institute of Life Science, Nankai University
(Tianjin, China). HSV-TK mRNA expression was detected by reverse
transcription-polymerase chain reaction (RT-PCR) analysis. The
primer sequences used were as follows: HSV-TK, forward, 5′-CGAT
GACTTACTGGCAGGTG-3′ and reverse, 3′-TGGGAGTAGA AGCTGGCG-5′;
β-actin, forward, 5′-TCCCTGGAGAAGAGC TACGA-3′ and reverse,
3′-GATCCACACGGAGTACTTGC-5′. Stably transfected cells were selected
by G418 (1,000 mg/ml) and cultured in 24-well plates. When 50-80%
confluency was reached, various concentrations of GCV
(1×10−3, 1×10−2, 1×10−1, 1×100,
1×101 and 1×102 mg/ml) were added to each
well. After 48 h, Trypan blue (Sigma-Aldrich; Merck KGaA) staining
was performed and the percentage of dead cells was calculated using
a hemocytometer. In another group, a fixed concentration of GCV
(10−1 mg/ml) was added to the stably HSV-TK-transfected
cells, and 48 h later, lactate dehydroge-nase (LDH) activity was
measured using an LDH Activity Assay kit (BioVision, Inc.,
Milpitas, CA, USA), according to the manufacturer's
instructions.
In vivo HSV-TK/GCV treatment
A total of 60 4-week-old female nude mice were
purchased from the Animal Center of the Academy of Military Medical
Sciences (Beijing, China) and housed at the Experimental Animal
Center of The Fifth Central Hospital of Tianjin under controlled
temperature conditions (22-24°C), in a 12/12 h light/dark cycle.
All experimental procedures were carried out according to the
regulations and internal biosafety and bioethics guidelines of the
Animal ethics committee of The Fifth Central Hospital of Tianjin
(Tianjin, China). A tumor-bearing murine model was established as
previously described (32). The 60
mice were randomly divided into 5 groups: i) Control, untransfected
U-2OS cells were subcutaneously transplanted into the left
shoulder, followed by treatment with PBS for 25 days; ii) HSV-TK,
HSV-TK-transfected U-2OS cells were subcutaneously transplanted
into the left shoulder of mice, followed by treatment with PBS
every 2 days for 25 days; iii) HSV-TK/GCV, HSV-TK-transfected U-2OS
cells were subcutaneously transplanted into the left shoulder,
followed by treatment with 15 mg/kg GCV every 2 days for 25 days;
iv) siR-neg/HSV-TK/GCV, HSV-TK- and siR-neg-co-transfected U-2OS
cells were subcutaneously transplanted into the left shoulder,
followed by treatment with 15 mg/kg GCV every 2 days for 25 days,
and, v) siR-ubc9/HSV-TK/GCV, HSV-TK- and siR-ubc9-co-trans-fected
U-2OS cells were subcutaneously transplanted into the left
shoulder, followed by treatment with 15 mg/kg GCV every 2 days for
25 days. Tumor growth was measured using calipers every 5 days for
30 days. Tumor volume (V) was calculated as follows: V = L ×
W2 × 0.5 (L, length; W, width). The mice were
sacrificed, and paraffin-embedded tissue sections were prepared for
in situ apoptosis and immunohistochemical analyses. Apoptosis was
detected by TUNEL staining using an in situ cell death kit (Roche
Diagnostics, Basel, Switzerland), according to the manufacturer's
instructions. Ki67, Cx43 and Ubc9 protein expression was detected
by immunohistochemistry, as aforementioned, using the following
primary antibodies: Ki67 (dilution, 1:250; cat. no. ab16667), Cx43
(dilution, 1:2,000; cat. no. ab11370) and Ubc9 (dilution, 1:4,000;
cat. no. ab75854) (all from Abcam).
Statistical analysis
All experiments were repeated ≥3 times. All data are
expressed as the mean ± standard error of the mean. All tests were
two-tailed, and P<0.05 was considered to indicate a
statistically significant difference. GraphPad Prism 6 (GraphPad
Software, Inc., San Diego, CA, USA) was used for all statistical
tests.
Results
Ubc9 is highly expressed in osteosarcoma
tissues and cell lines
Recent studies have demonstrated that Ubc9 protein
levels are overexpressed in various types of tumor, including
colorectal, prostate, lung, breast and pancreatic cancer (33-35).
Furthermore, upregulation of Ubc9 expression has been suggested to
be accompanied by protein SUMOylation events (36). Therefore, in the present study, the
expression of Ubc9 protein was investigated in osteosarcoma and
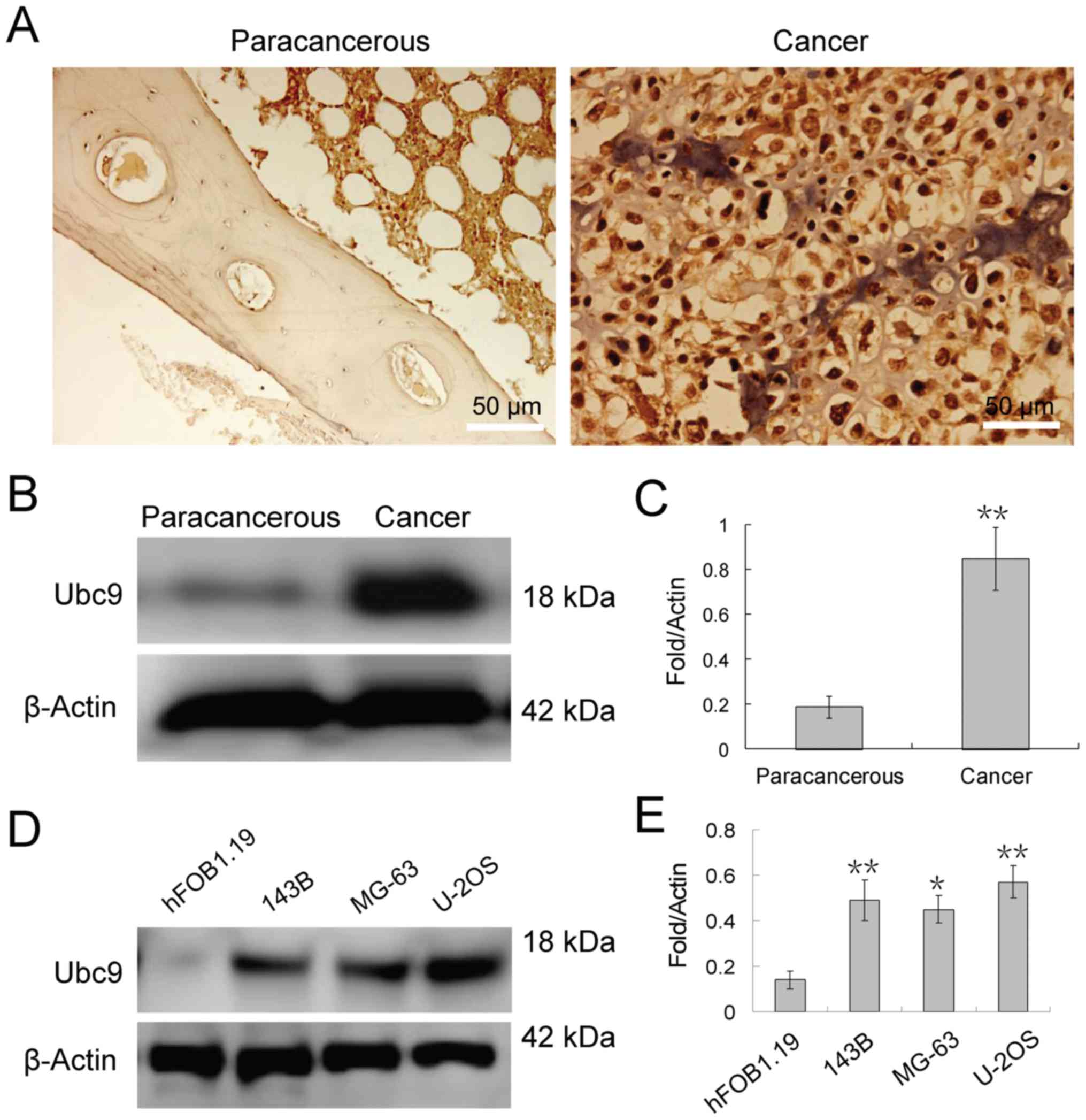
non-tumor tissues. Immunohistochemical staining revealed that Ubc9
protein was highly expressed in osteosarcoma tissue compared with
normal adjacent tissues and localized to the nucleus of
osteosarcoma cells (Fig. 1A).
Furthermore, western blotting analysis also demonstrated that Ubc9
expression in osteosarcoma tissue was approximately 4-fold of that
in adjacent tissues (Fig. 1B and
C). Similar results were achieved in the osteosarcoma cell
lines (Fig. 1D and E). Seeing as
the protein expression level of Ubc9 in U-2OS cells was the highest
of the 3 osteosarcoma cell lines tested, knockdown of Ubc9 may lead
to more pronounced effects in this cell line. Therefore, U-2OS
cells were selected for subsequent experiments.
Silencing Ubc9 inhibits proliferation and
migration, and promotes apoptosis of osteosarcoma cells
To analyze the role of Ubc9 in osteosarcoma and to
determine whether silencing of Ubc9 may inhibit carcinogenesis,
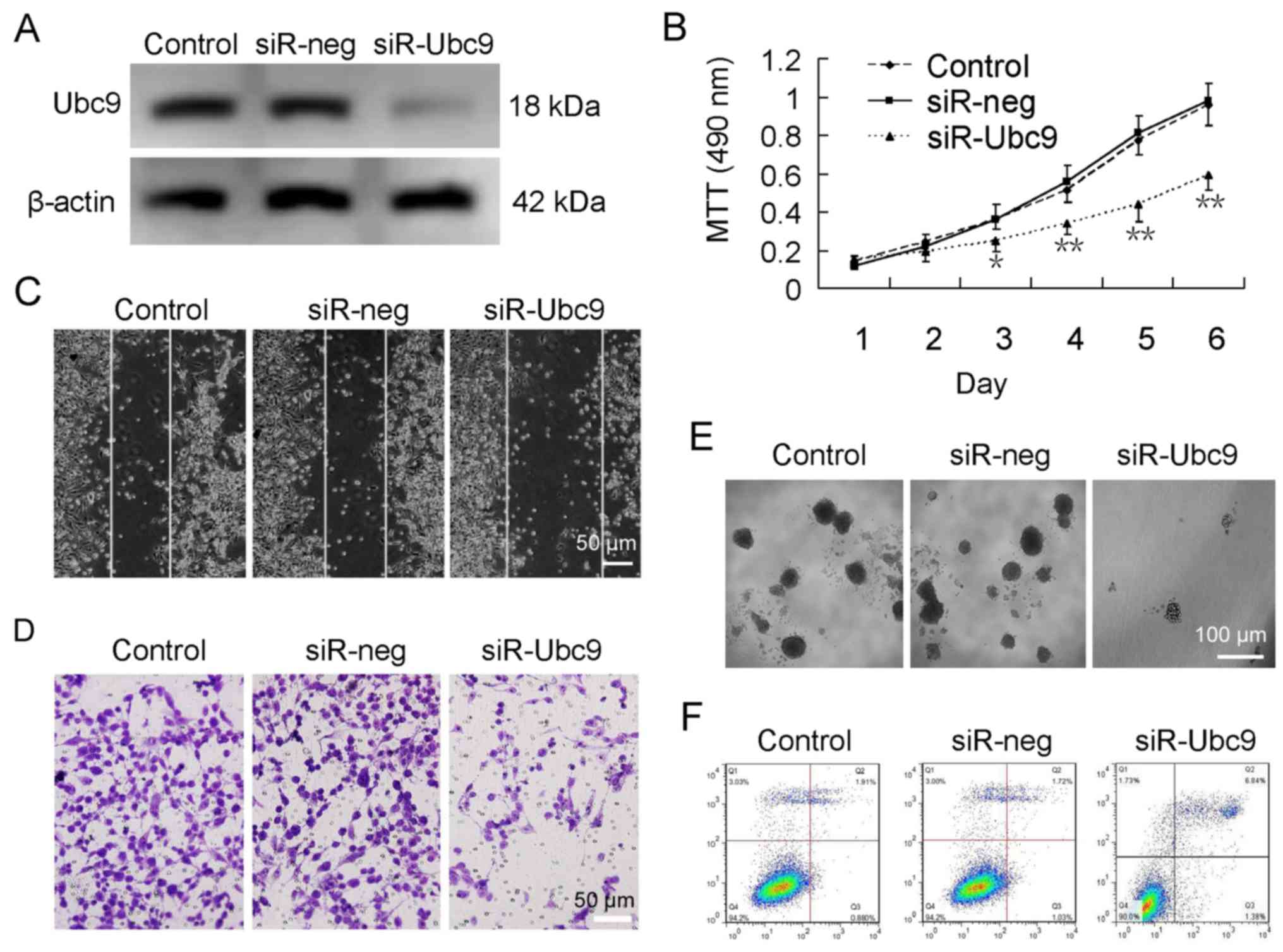
Ubc9 expression was silenced in U-2OS cells using siRNA (Fig. 2A). Further experimentation
demonstrated that the proliferation, migration and colony forming
abilities of U-2OS cells were significantly decreased following
Ubc9 silencing (Fig. 2B–E).
Furthermore, there was an increase in the apoptotic rate from ~2%
in untreated cells to ~7% in Ubc9-knockdown cells (Fig. 2F).
Silencing of Ubc9 partially restores GJIC
function in osteosarcoma and enhances sensitivity to
chemotherapy
Previous studies have reported that Cx43 is
covalently modified and regulated by SUMOylation (26); however, the specific role of Cx43
SUMOylation remains unknown. In the present study, the effect of
silencing Ubc9 on the function of GJIC was investigated in
osteosarcoma, as well as whether this mechanism may be used for
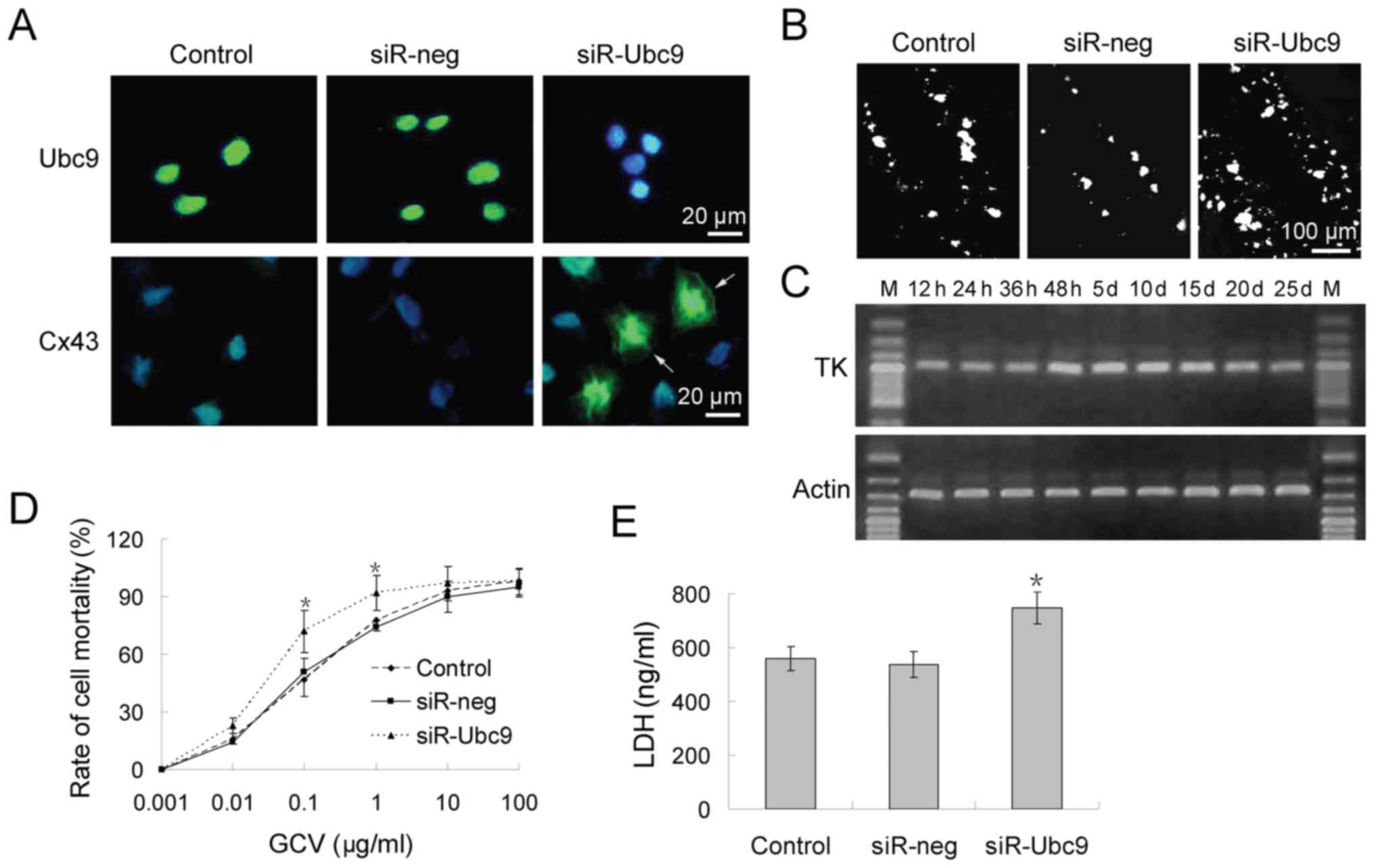
osteosarcoma treatment. Firstly, it was investigated whether Cx43
protein expression was restored by silencing Ubc9 in osteosarcoma
cells (Fig. 3A). Scrape loading
and dye transfer assays revealed that control the control group
exhibited poor dye-coupling. This was indicative of GJIC
inhibition. However, GJIC function was partially restored following
transfection with Ubc9 siRNA. Lucifer Yellow was transmitted to
neighboring cells from the loaded cells via the injured scraping
border (Fig. 3B).
Subsequently, a conventional HSV-TK/GCV system was
employed to detect whether Ubc9-silencing could increase
chemotherapy sensitivity. RT-qPCR analysis revealed that the
highest level of HSV-TK expression occurred 48 h after
transfection, and that HSV-TK expression was maintained for ≥25
days (Fig. 3C). Cells stably
expressing HSV-TK were incubated in medium containing
10−3-102 mg/ml GCV for 48 h. The cell
viability of U-2OS cells was 50% at 10−1 mg/ml GCV in
the control and siR-neg group. However, ≥70% cells died at this
concentration in the siR-Ubc9 group (Fig. 3D). LDH experiments confirmed these
results (Fig. 3E).
Ubc9-silencing reduces SUMOylated Cx43
and increases free Cx43 levels
To explore the association between Ubc9 silencing
and Cx43 SUMOylation, the protein levels of SUMO1 and Cx43 were
detected following Ubc9-silencing. It was revealed that
Ubc9-silencing significantly reduced the levels of conjugated
SUMO1, and increased the level of free SUMO1 protein. The level of
free Cx43 protein was also increased (Fig. 4A and B). Exogenous HA-Cx43 and
Flag-SUMO1 were co-transfected into U-2OS cells with or without
His-siR-Ubc9. The results confirmed that silencing of Ubc9
inhibited the conjugation of SUMO-1 to its substrate proteins, and
induced decoupling of SUMO1 from Cx43 (Fig. 4C).
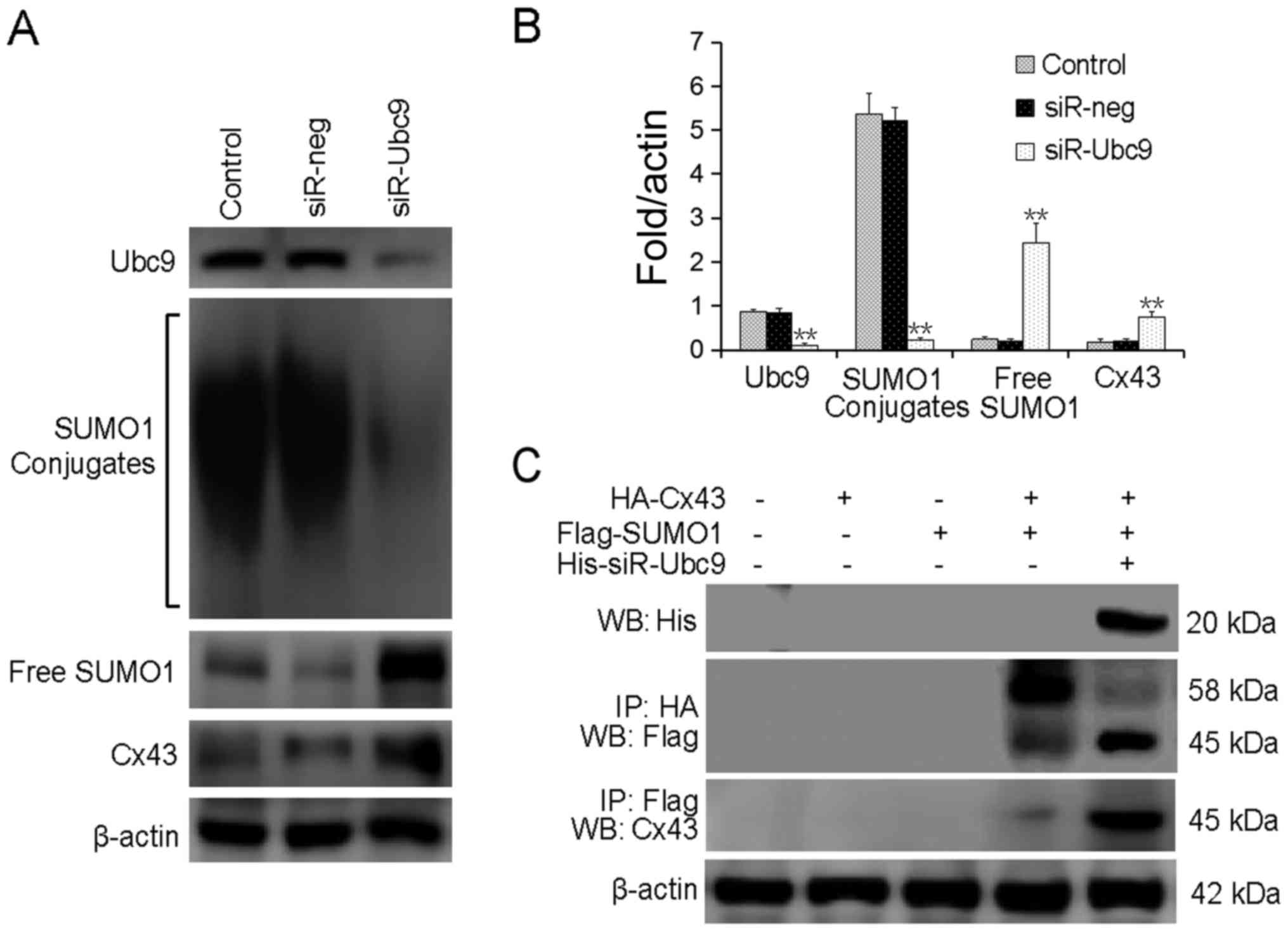 | Figure 4Ubc9-silencing reduces
Cx43-SUMOylation, increasing free Cx43 levels. (A) SUMOylated Cx43
levels were detected by western blotting. (B) The bar chart
presents the quantified SUMOylated Cx43 protein levels. (C)
Exogenous HA-Cx43 and Flag-SUMO1 were co-transfected into U-2OS
cells with or without His-siR-Ubc9. The cell lysates were subjected
to an immunoprecipitation assay. **P<0.01, compared
with control. Ubc9, SUMO-conjugating enzyme Ubc9; GJIC, gap
junction intercellular communication; LDH, lactate dehydrogenase;
siR, small interfering RNA; neg, negative control; Cx43, connexin
43; Ip, immunoprecipitation; WB, western blot. |
Silencing of Ubc9 increases the
sensitivity of osteosarcoma to HSV-TK/GCV in vivo
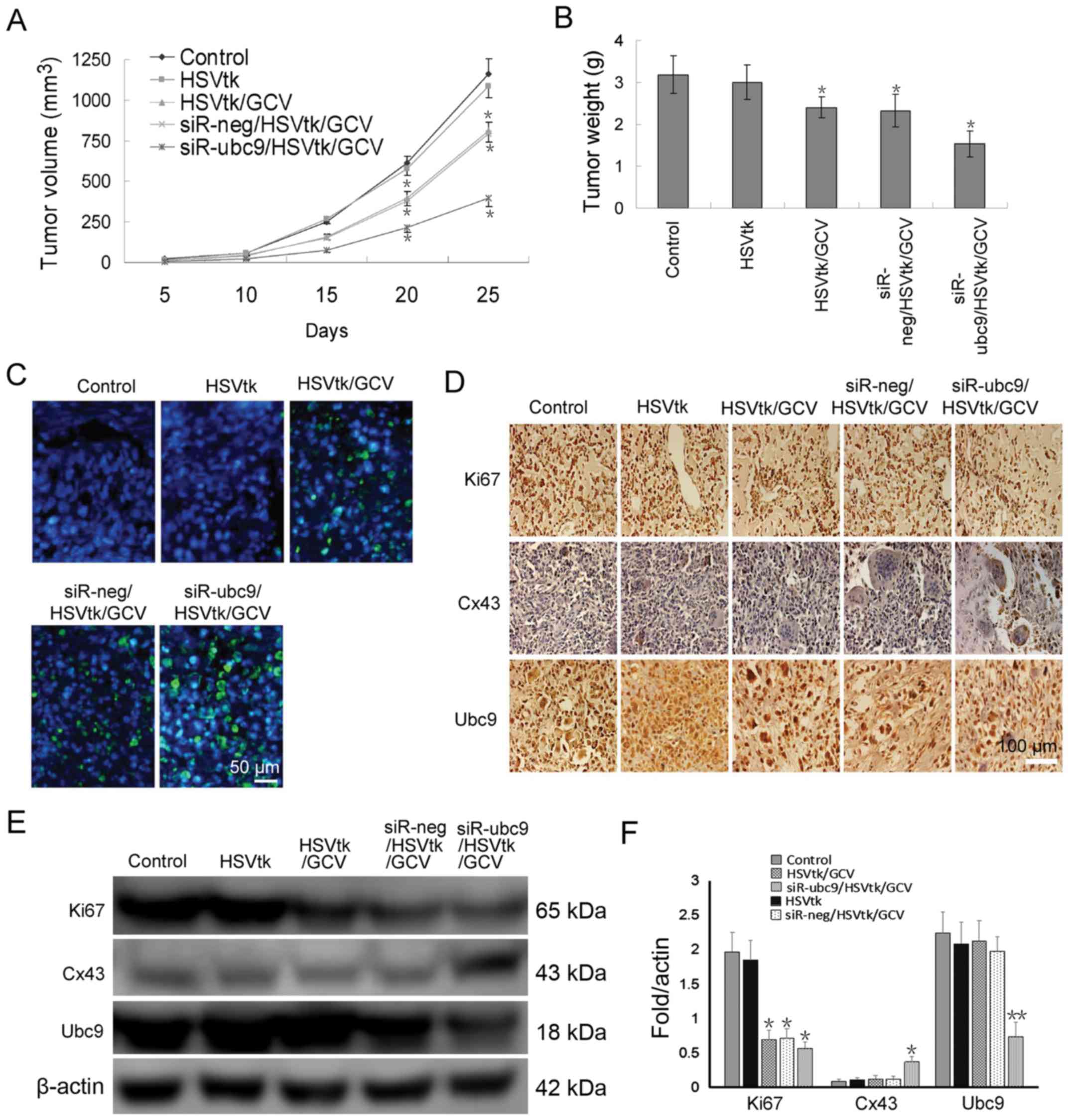
To verify whether Ubc9-silencing enhanced
chemosensitivity in vivo, xenografts tumors were established
in immunodeficient mice. The results demonstrated that transfection
of U-2OS cells with HSV-TK alone had an insignificant effect on
tumor growth. However, when GCV was intraperitoneally injected,
there was a significant decrease in tumor volume and weight
(Fig. 5A and B). Co-transfection
of the siR-Ubc9 plasmid and HSV-TK with GCV administration further
reduced tumor volume, and also induced apoptosis (Fig. 5A and C). In situ apoptosis
detection demonstrated that the HSV-TK/GCV system induced apoptosis
of a proportion of tumor cells, and that Ubc9-silencing further
enhanced the therapeutic effect (Fig.
5C). Finally, the protein expression levels of Ki67, Cx43 and
Ubc9 were detected in xenograft tumor tissues. The results
demonstrated that Ubc9 silencing significantly inhibited the rate
of proliferation, and restored GJIC function in vivo
(Fig. 5D–F).
Discussion
Recent studies have reported that SUMOylation is
frequently upregulated during malignant transformation in a range
of tumors, including lung cancer, prostate cancer, gastric cancer,
breast cancer and glioma (20-25,37).
Ubc9, the only SUMO-E2-conjugating enzyme, is has been demonstrated
to be overexpressed in various types of cancer cells (33-36).
Therefore, in the present study, Ubc9 expression was analyzed in
osteosarcoma tissues and in three osteosarcoma cell lines. The
results revealed that Ubc9 protein expression was significantly
increased in osteosarcoma tissues and cell lines. However, it was
not determined whether the level of Ubc9 protein was associated
with the malignancy of osteosarcoma due to the limited number of
tissue samples.
To further analyze the role of Cx43 SUMOylation in
maintaining the integrity and function of the GJIC between cancer
cells, a lentiviral plasmid that induced Ubc9 silencing was
constructed. The majority of substrate proteins, which were
originally bound to SUMO1, underwent deSUMOylation following
Ubc9-silencing. The levels of free Cx43 were also significantly
increased. Immunocytochemistry and Lucifer Yellow dye transfer
experiments confirmed that Ubc9-silencing partially restored the
structure and function of GJIC, which was likely mediated by free
Cx43.
SUMO1 competes with ubiquitin for the same lysine
binding sites on a substrate protein, preventing the target protein
from being hydrolyzed (38). This
may explain the increased free Cx43 protein levels. However,
contrary to expectation, Cx43 deSUMOylation increased Cx43 levels
via silencing Ubc9, which improved the GJIC function between cells.
Proteins that perform different functions in different stress
conditions are often modified by a variety of post-translational
modifications, including phosphorylation, acetylation, methylation
and ubiquitination (39).
Unfortunately, the specific regulatory mechanism that underlies the
relationship between Cx43 levels and decreased SUMOylation remains
unclear.
Whether the recovery of GJIC triggered by
Ubc9-silencing could be transformed and utilized to improve the
sensitivity of chemotherapeutic drugs was a major focus of the
present study. Silencing of Ubc9 improved the sensitivity of
osteosarcoma cells to HSV-TK/GCV chemotherapy both in vitro
and in vivo.
In addition to the above findings, the present study
also examined the effect of Ubc9-silencing on proliferation,
migration and apoptosis of osteosarcoma cells. It was demonstrated
that inhibition of Ubc9 expression directly suppressed the
proliferation and migration of osteosarcoma cells, and induced
apoptosis. However, the apoptotic rate only increased from 2~7%
following Ubc9 silencing.
Recent studies have demonstrated that osteosarcoma
cells maintain their proliferation and migration capabilities via
the PI3K/Akt pathway (40-42). Other studies confirmed that Akt
SUMOylation regulates proliferation, tumorigenesis and the cell
cycle (43,44). In addition, Akt-SUMOylation
regulates global SUMOylation, including that of Akt and Ubc9, STAT1
and CREB (45). Due to the
important role of Akt-SUMOylation in tumorigenesis, this mechanism
may also be involved in osteosarcoma formation.
In conclusion, the present study indicates that
Cx43-SUMOylation occurs in osteosarcoma tissues and is involved in
regulating Cx43 gap junctions. However, the underlying molecular
mechanism remains unclear. Importantly, the present study provides
a novel strategy to improve the chemotherapy sensitivity of
osteosarcoma by inducing deSU-MOylation of Cx43. This gives us an
important indication that there will be a broad space for
development in this field in the future.
Acknowledgments
The authors would like to thank Tianjin Medical
University Cancer Institute and Hospital for providing samples of
osteo-sarcoma tissue, and Dr Baojiang Li and Dr Zhongmin Jiang
(Department of Pathology, Tianjin Fifth Central Hospital, Tianjin,
China) for their help with pathological diagnosis.
References
|
1
|
Akoluk A, Barazani Y, Slova D, Shah S and
Tareen B: Carcinosarcoma of the bladder: Case report and review of
the literature. Can Urol Assoc J. 5:E69–E73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar
|
|
3
|
Gu X, Ding J, Zhang Z, Li Q, Zhuang X and
Chen X: Polymeric nanocarriers for drug delivery in osteosarcoma
treatment. Curr Pharm Des. 21:5187–5197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grote HJ, Braun M, Kalinski T, Pomjanski
N, Back W, Bleyl U, Böcking A and Roessner A: Spontaneous malignant
transformation of conventional giant cell tumor. Skeletal Radiol.
33:169–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bennani S, Louahlia S, Aboutaieb R, el
Mrini M and S: Carcinosarcoma of the bladder. Apropos of two cases
J Urol (Paris). 100:210–216. 1994.In French.
|
|
6
|
Chiu KC, Lin MC, Liang YC and Chen CY:
Renal carcinosarcoma: Case report and review of literature. Ren
Fail. 30:1034–1039. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishisho T, Sakai T, Tezuka F, Higashino
K, Takao S, Takata Y, Miyagi R, Toki S, Abe M, Yamashita K, et al:
Delayed diagnosis of primary bone and soft tissue tumors initially
treated as degenerative spinal disorders. J Med Invest. 63:274–277.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yi BR, Choi KJ, Kim SU and Choi KC:
Therapeutic potential of stem cells expressing suicide genes that
selectively target human breast cancer cells: Evidence that they
exert tumoricidal effects via tumor tropism (review). Int J Oncol.
41:798–804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wildner O: In situ use of suicide genes
for therapy of brain tumours. Ann Med. 31:421–429. 1999. View Article : Google Scholar
|
|
10
|
van Dillen IJ, Mulder NH, Vaalburg W, de
Vries EF and Hospers GA: Influence of the bystander effect on
HSV-tk/GCV gene therapy. A review Curr Gene Ther. 2:307–322. 2002.
View Article : Google Scholar
|
|
11
|
Määttä AM, Samaranayake H, Pikkarainen J,
Wirth T and Ylä-Herttuala S: Adenovirus mediated herpes simplex
virus-thymidine kinase/ganciclovir gene therapy for resectable
malignant glioma. Curr Gene Ther. 9:356–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Czyż J, Szpak K and Madeja Z: The role of
connexins in prostate cancer promotion and progression. Nat Rev
Urol. 9:274–282. 2012. View Article : Google Scholar
|
|
13
|
El-Sabban ME, Abi-Mosleh LF and Talhouk
RS: Developmental regulation of gap junctions and their role in
mammary epithelial cell differentiation. J Mammary Gland Biol
Neoplasia. 8:463–473. 2003. View Article : Google Scholar
|
|
14
|
Abbaci M, Barberi-Heyob M, Blondel W,
Guillemin F and Didelon J: Advantages and limitations of commonly
used methods to assay the molecular permeability of gap junctional
intercellular communication. Biotechniques. 45:33–52. 56–62. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aasen T: Connexins: Junctional and
non-junctional modulators of proliferation. Cell Tissue Res.
360:685–699. 2015. View Article : Google Scholar
|
|
16
|
Ehrlich HP: A snapshot of direct cell-cell
communications in wound healing and scarring. Adv Wound Care (New
Rochelle). 2:113–121. 2013. View Article : Google Scholar
|
|
17
|
Falk MM, Fong JT, Kells RM, O'Laughlin MC,
Kowal TJ and Thévenin AF: Degradation of endocytosed gap junctions
by autophagosomal and endo-/lysosomal pathways: A perspective. J
Membr Biol. 245:465–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang JX, Siller-Jackson AJ and Burra S:
Roles of gap junctions and hemichannels in bone cell functions and
in signal transmission of mechanical stress. Front Biosci.
12:1450–1462. 2007. View
Article : Google Scholar :
|
|
19
|
Klaunig JE: Alterations in intercellular
communication during the stage of promotion. Proc Soc Exp Biol Med.
198:688–692. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barry J and Lock RB: Small
ubiquitin-related modifier-1: Wrestling with protein regulation.
Int J Biochem Cell Biol. 43:37–40. 2011. View Article : Google Scholar
|
|
21
|
Morris JR: SUMO in the mammalian response
to DNA damage. Biochem Soc Trans. 38:92–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoeller D and Dikic I: Targeting the
ubiquitin system in cancer therapy. Nature. 458:438–444. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moschos SJ and Mo YY: Role of SUMO/Ubc9 in
DNA damage repair and tumorigenesis. J Mol Histol. 37:309–319.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Praefcke GJ, Hofmann K and Dohmen RJ: SUMO
playing tag with ubiquitin. Trends Biochem Sci. 37:23–31. 2012.
View Article : Google Scholar
|
|
25
|
Princz A and Tavernarakis N: The role of
SUMOylation in ageing and senescent decline. Mech Ageing Dev.
162:85–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kjenseth A, Fykerud TA, Sirnes S, Bruun J,
Yohannes Z, Kolberg M, Omori Y, Rivedal E and Leithe E: The gap
junction channel protein connexin 43 is covalently modified and
regulated by SUMOylation. J Biol Chem. 287:15851–15861. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Jiang Z, Huang J, Huang S, Li Y, Yu
S, Yu S and Liu X: miR-7 inhibits glioblastoma growth by
simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK
pathways. Int J Oncol. 44:1571–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chai L, Kang X-J, Sun Z-Z, Zeng MF, Yu SR,
Ding Y, Liang JQ, Li TT and Zhao J: MiR-497-5p, miR-195-5p and
miR-455-3p function as tumor suppressors by targeting hTERT in
melanoma A375 cells. Cancer Manag Res. 10:989–1003. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Li G, Su Z, Jiang Z, Chen L, Wang
J, Yu S and Liu Z: Poly(amido amine) is an ideal carrier of miR-7
for enhancing gene silencing effects on the EGFR pathway in U251
glioma cells. Oncol Rep. 29:1387–1394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Z, Zhang L, Zhang L, Wang S, Zheng
M, Li Y and Liu X: Enhancement of B-cell translocation gene-2
inhibits proliferation and metastasis of colon cancer cells].
Zhonghua Zhong Liu Za Zhi. 37:330–335. 2015.In Chinese. PubMed/NCBI
|
|
31
|
Li XD, Chang B, Chen B, Liu ZY, Liu DX,
Wang JS, Hou GQ, Huang DY and Du SX: Panax notoginseng saponins
potentiate osteogenesis of bone marrow stromal cells by modulating
gap junction intercellular communication activities. Cell Physiol
Biochem. 26:1081–1092. 2010. View Article : Google Scholar
|
|
32
|
Liu D and Liu A: Administration of vitamin
E prevents thymocyte apoptosis in murine sarcoma S180 tumor bearing
mice. Cell Mol Biol (Noisy-le-grand). 58(Suppl): OL1671–OL1679.
2012.
|
|
33
|
Wasik U and Filipek A: The CacyBP/SIP
protein is sumoylated in neuroblastoma NB2a cells. Neurochem Res.
38:2427–2432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu S, Sachdeva M, Wu F, Lu Z and Mo YY:
Ubc9 promotes breast cell invasion and metastasis in a
sumoylation-independent manner. Oncogene. 29:1763–1772. 2010.
View Article : Google Scholar :
|
|
35
|
Galanty Y, Belotserkovskaya R, Coates J,
Polo S, Miller KM and Jackson SP: Mammalian SUMO E3-ligases PIAS1
and PIAS4 promote responses to DNA double-strand breaks. Nature.
462:935–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matt S and Hofmann TG: The DNA
damage-induced cell death response: A roadmap to kill cancer cells.
Cell Mol Life Sci. 73:2829–2850. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hong SS, Lee H and Kim KW: HIF-1alpha: A
valid therapeutic target for tumor therapy. Cancer Res Treat.
36:343–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garbuz DG: Regulation of heat shock gene
expression in response to stress. Mol Biol (Mosk). 51:400–417.
2017.In Russian. View Article : Google Scholar
|
|
39
|
Rape M: Ubiquitylation at the crossroads
of development and disease. Nat Rev Mol Cell Biol. 19:59–70. 2018.
View Article : Google Scholar
|
|
40
|
Perry JA, Kiezun A, Tonzi P, Van Allen EM,
Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS,
et al: Complementary genomic approaches highlight the PI3K/mTOR
pathway as a common vulnerability in osteosarcoma. Proc Natl Acad
Sci USA. 111:E5564–E5573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Graziano AC, Cardile V, Avola R, Vicario
N, Parenti C, Salvatorelli L, Magro G and Parenti R: Wilms' tumor
gene 1 silencing inhibits proliferation of human osteosarcoma MG-63
cell line by cell cycle arrest and apoptosis activation.
Oncotarget. 8:13917–13931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xia W, Tian H, Cai X, Kong H, Fu W, Xing
W, Wang Y, Zou M, Hu Y and Xu D: Inhibition of SUMO-specific
protease 1 induces apoptosis of astroglioma cells by regulating
NF-κB/Akt pathways. Gene. 595:175–179. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de la Cruz-Herrera CF, Campagna M, Lang V,
del Carmen González-Santamaría J, Marcos-Villar L, Rodríguez MS,
Vidal A, Collado M and Rivas C: SUMOylation regulates AKT1
activity. Oncogene. 34:1442–1450. 2015. View Article : Google Scholar
|
|
45
|
Lin CH, Liu SY and Lee EH: SUMO
modification of Akt regulates global SUMOylation and substrate
SUMOylation specificity through Akt phosphorylation of Ubc9 and
SUMO1. Oncogene. 35:595–607. 2016. View Article : Google Scholar
|