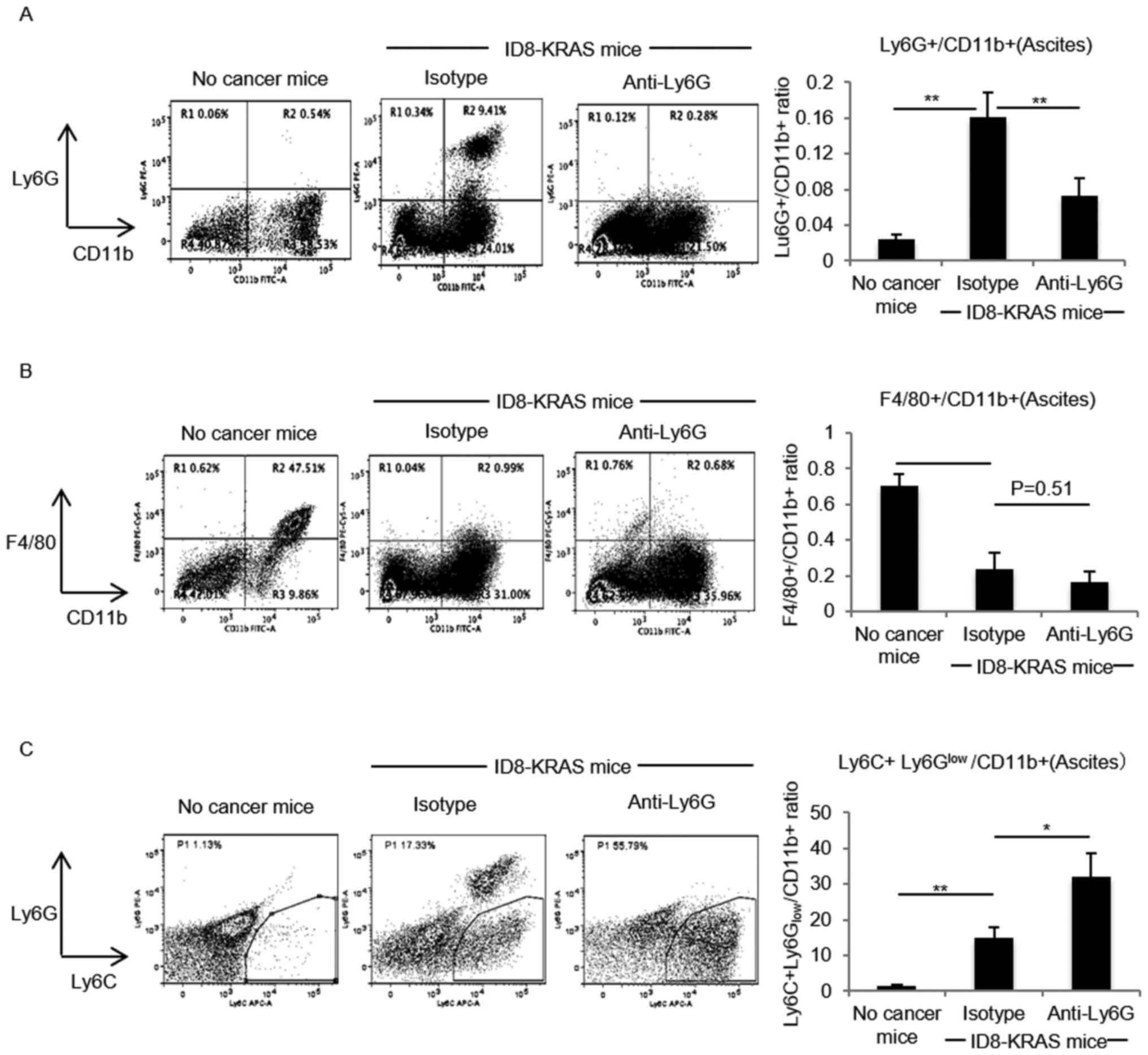
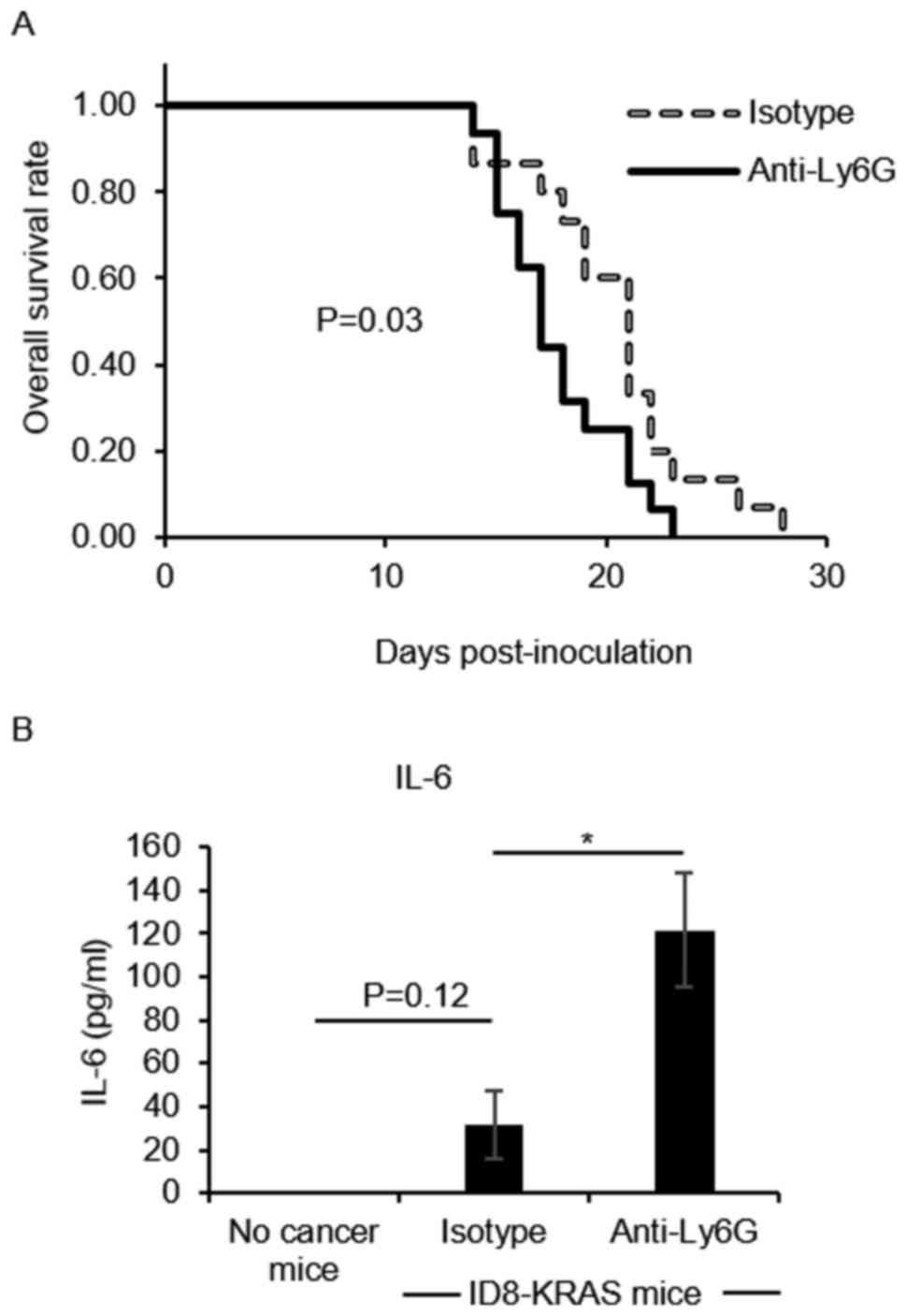
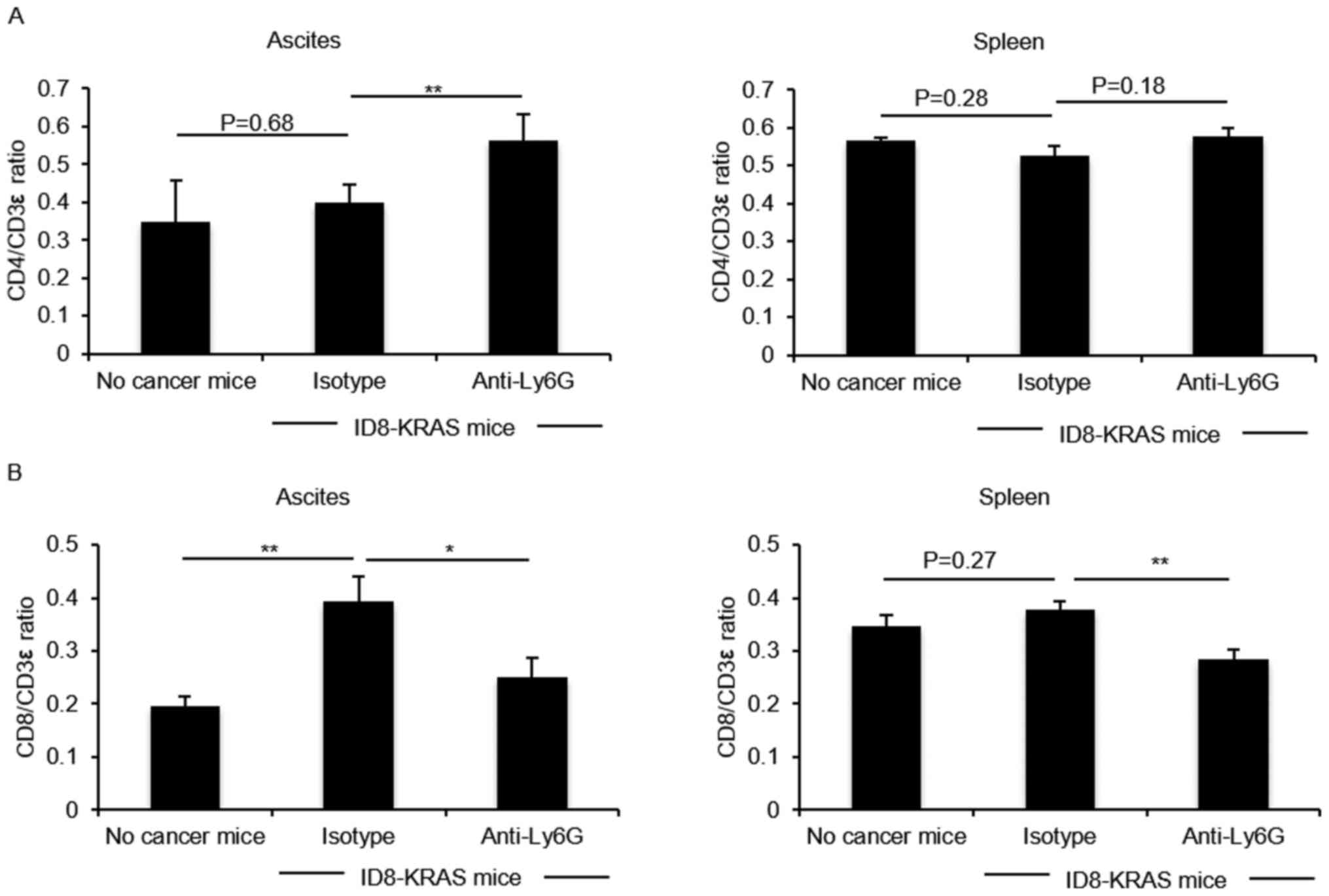
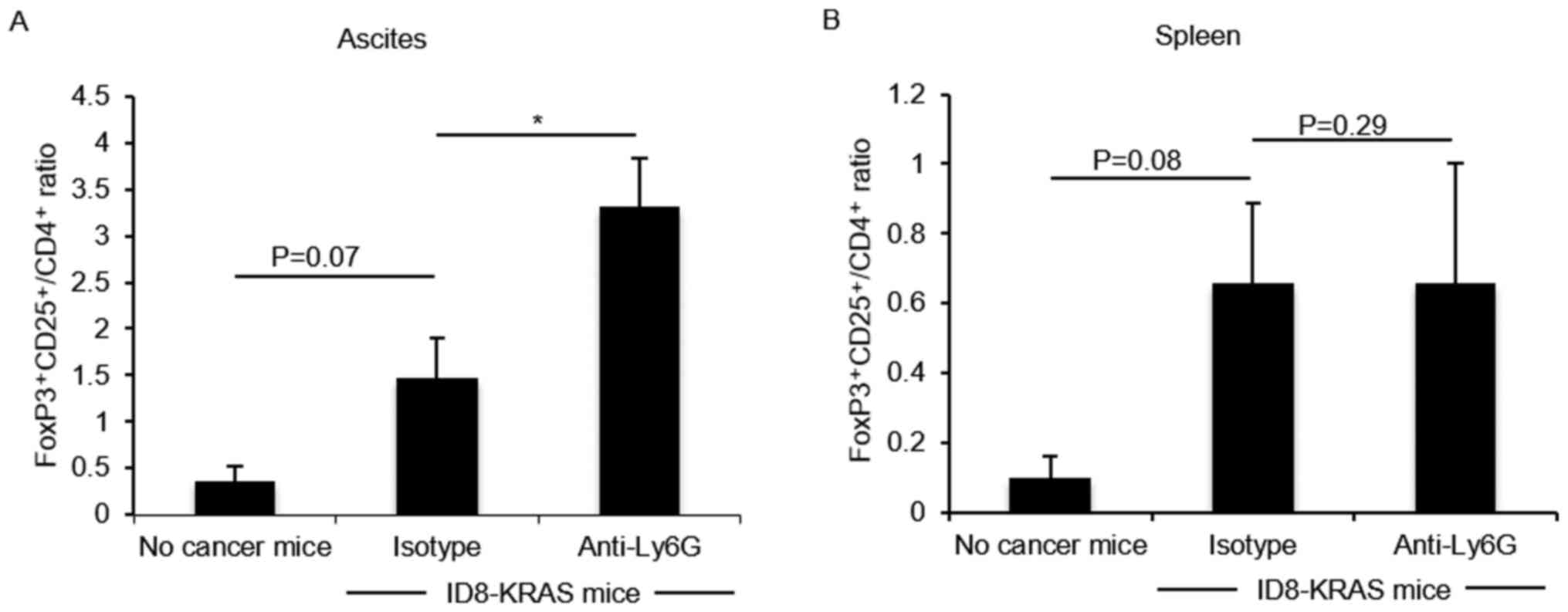
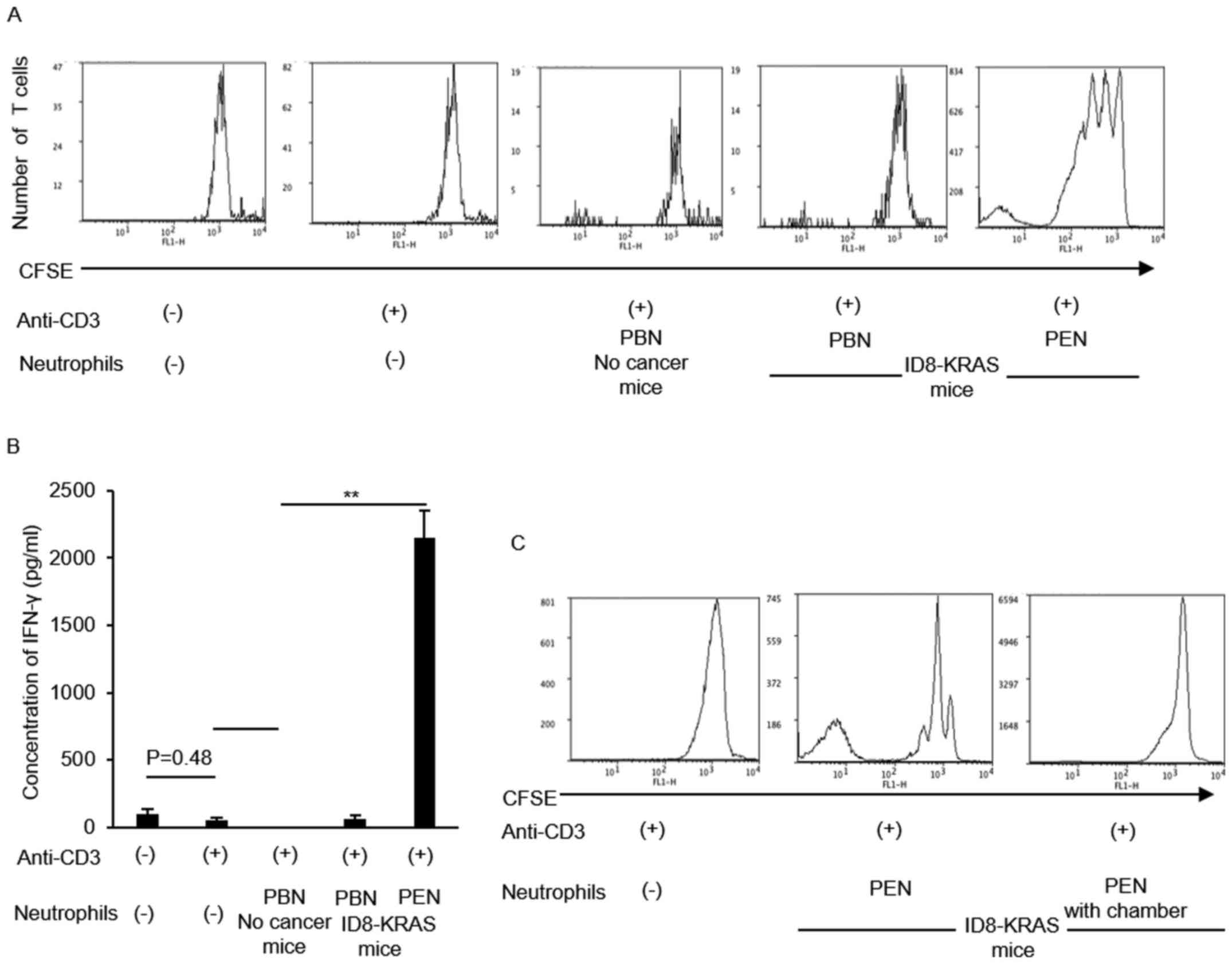
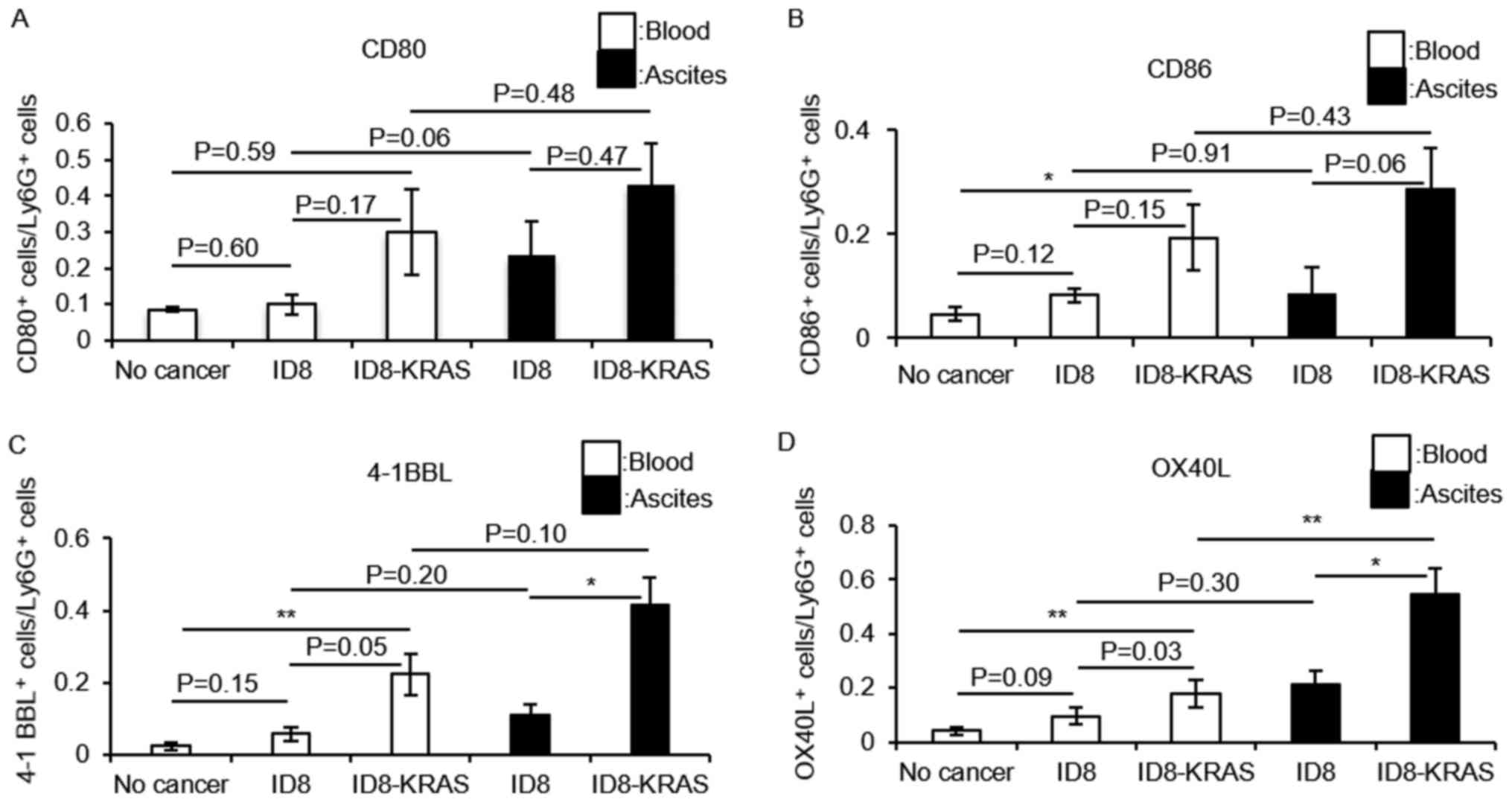
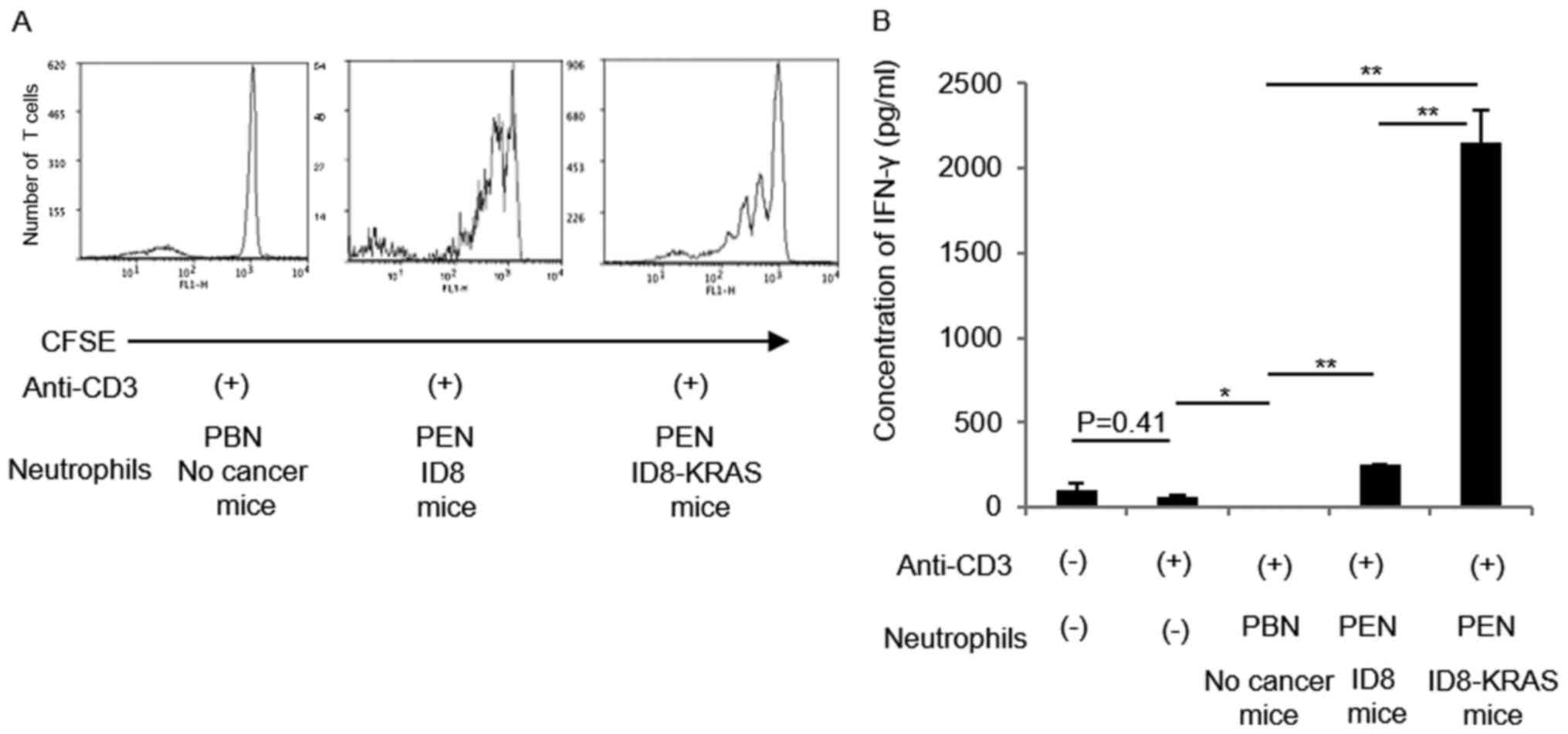
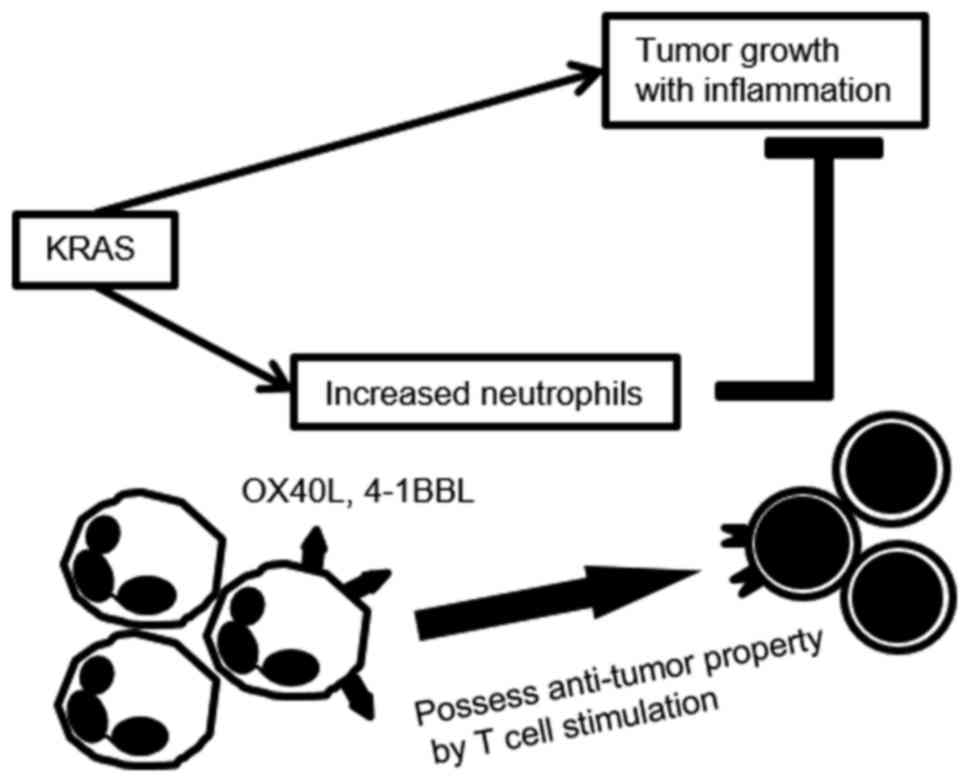
|
1
|
Trellakis S, Bruderek K, Dumitru CA,
Gholaman H, Gu X, Bankfalvi A, Scherag A, Hütte J, Dominas N,
Lehnerdt GF, et al: Polymorphonuclear granulocytes in human head
and neck cancer: Enhanced inflammatory activity, modulation by
cancer cells and expansion in advanced disease. Int J Cancer.
129:2183–2193. 2011. View Article : Google Scholar
|
|
2
|
Jensen HK, Donskov F, Marcussen N,
Nordsmark M, Lundbeck F and von der Maase H: Presence of
intratumoral neutrophils is an independent prognostic factor in
localized renal cell carcinoma. J Clin Oncol. 27:4709–4717. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jensen TO, Schmidt H, Møller HJ, Donskov
F, Høyer M, Sjoegren P, Christensen IJ and Steiniche T:
Intratumoral neutrophils and plasmacytoid dendritic cells indicate
poor prognosis and are associated with pSTAT3 expression in AJCC
stage I/II melanoma. Cancer. 118:2476–2485. 2012. View Article : Google Scholar
|
|
4
|
Templeton AJ, McNamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: A systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taguchi S, Nakagawa T, Matsumoto A, Nagase
Y, Kawai T, Tanaka Y, Yoshida K, Yamamoto S, Enomoto Y, Nose Y, et
al: Pretreatment neutrophil-to-lymphocyte ratio as an independent
predictor of survival in patients with metastatic urothelial
carcinoma: A multi-institutional study. Int J Urol. 22:638–643.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakamura K, Nagasaka T, Nishida T, Haruma
T, Ogawa C, Kusumoto T, Seki N and Hiramatsu Y: Neutrophil to
lymphocyte ratio in the pre-treatment phase of final-line
chemotherapy predicts the outcome of patients with recurrent
ovarian cancer. Oncol Lett. 11:3975–3981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng Z, Wen H, Bi R, Ju X, Chen X, Yang W
and Wu X: Preoperative neutrophil-to-lymphocyte ratio as a
predictive and prognostic factor for high-grade serous ovarian
cancer. PLoS One. 11:e01561012016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida M, Taguchi A, Kawana K, Adachi K,
Kawata A, Ogishima J, Nakamura H, Fujimoto A, Sato M, Inoue T, et
al: Modification of the tumor microenvironment in KRAS or
c-MYC-induced ovarian cancer-associated peritonitis. PLoS One.
11:e01603302016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chandler RL, Damrauer JS, Raab JR,
Schisler JC, Wilkerson MD, Didion JP, Starmer J, Serber D, Yee D,
Xiong J, et al: Coexistent ARID1A-PIK3CA mutations promote ovarian
clear-cell tumorigenesis through pro-tumorigenic inflammatory
cytokine signalling. Nat Commun. 6:61182015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eser S, Schnieke A, Schneider G and Saur
D: Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer.
111:817–822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pylayeva-Gupta Y, Grabocka E and Bar-Sagi
D: RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer.
11:761–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daniluk J, Liu Y, Deng D, Chu J, Huang H,
Gaiser S, Cruz-Monserrate Z, Wang H, Ji B and Logsdon CD: An NF-κB
pathway-mediated positive feedback loop amplifies Ras activity to
pathological levels in mice. J Clin Invest. 122:1519–1528. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caruso RA, Bellocco R, Pagano M, Bertoli
G, Rigoli L and Inferrera C: Prognostic value of intratumoral
neutrophils in advanced gastric carcinoma in a high-risk area in
northern Italy. Mod Pathol. 15:831–837. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jablonska J, Leschner S, Westphal K,
Lienenklaus S and Weiss S: Neutrophils responsive to endogenous
IFN-beta regulate tumor angiogenesis and growth in a mouse tumor
model. J Clin Invest. 120:1151–1164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: 'N1' versus "N2"
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao
YS and Xu YF: Intratumoral neutrophils: A poor prognostic factor
for hepatocellular carcinoma following resection. J Hepatol.
54:497–505. 2011. View Article : Google Scholar
|
|
17
|
Houghton AM: The paradox of
tumor-associated neutrophils: Fueling tumor growth with cytotoxic
substances. Cell Cycle. 9:1732–1737. 2010. View Article : Google Scholar
|
|
18
|
Eruslanov EB, Bhojnagarwala PS, Quatromoni
JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A,
Litzky L, Hancock WW, et al: Tumor-associated neutrophils stimulate
T cell responses in early-stage human lung cancer. J Clin Invest.
124:5466–5480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasaki R, Narisawa-Saito M, Yugawa T,
Fujita M, Tashiro H, Katabuchi H and Kiyono T: Oncogenic
transformation of human ovarian surface epithelial cells with
defined cellular oncogenes. Carcinogenesis. 30:423–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Overmyer KA, Thonusin C, Qi NR, Burant CF
and Evans CR: Impact of anesthesia and euthanasia on metabolomics
of mammalian tissues: Studies in a C57BL/6J mouse model. PLoS One.
10:e01172322015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arnold L, Tyagi RK, Mejia P, Van Rooijen
N, Pérignon JL and Druilhe P: Analysis of innate defences against
Plasmodium falciparum in immunodeficient mice. Malar J. 9:1972010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao H, Heeringa P, Liu Z, Huugen D, Hu P,
Maeda N, Falk RJ and Jennette JC: The role of neutrophils in the
induction of glomerulonephritis by anti-myeloperoxidase antibodies.
Am J Pathol. 167:39–45. 2005. View Article : Google Scholar
|
|
23
|
Tempfer C, Zeisler H, Sliutz G, Haeusler
G, Hanzal E and Kainz C: Serum evaluation of interleukin 6 in
ovarian cancer patients. Gynecol Oncol. 66:27–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scambia G, Testa U, Benedetti Panici P,
Foti E, Martucci R, Gadducci A, Perillo A, Facchini V, Peschle C
and Mancuso S: Prognostic significance of interleukin 6 serum
levels in patients with ovarian cancer. Br J Cancer. 71:354–356.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang J, Yang L, Yu L, Wang YY, Chen R,
Qian J, Hong ZP and Su XS: Surgery-induced monocytic
myeloid-derived suppressor cells expand regulatory T cells in lung
cancer. Oncotarget. 8:17050–17058. 2017.PubMed/NCBI
|
|
26
|
O'Connor MA, Vella JL and Green WR:
Reciprocal relationship of T regulatory cells and monocytic
myeloid-derived suppressor cells in LP-BM5 murine
retrovirus-induced immunodeficiency. J Gen Virol. 97:509–522. 2016.
View Article : Google Scholar
|
|
27
|
Idorn M, Køllgaard T, Kongsted P, Sengeløv
L and Thor Straten P: Correlation between frequencies of blood
monocytic myeloid-derived suppressor cells, regulatory T cells and
negative prognostic markers in patients with castration-resistant
metastatic prostate cancer. Cancer Immunol Immunother.
63:1177–1187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng
YX, Cai MY and Xie D: Increased intratumoral neutrophil in
colorectal carcinomas correlates closely with malignant phenotype
and predicts patients' adverse prognosis. PLoS One. 7:e308062012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamanaka T, Matsumoto S, Teramukai S,
Ishiwata R, Nagai Y and Fukushima M: The baseline ratio of
neutrophils to lymphocytes is associated with patient prognosis in
advanced gastric cancer. Oncology. 73:215–220. 2007. View Article : Google Scholar
|
|
30
|
Nuhn P, Vaghasia AM, Goyal J, Zhou XC,
Carducci MA, Eisenberger MA and Antonarakis ES: Association of
pretreatment neutrophil-to-lymphocyte ratio (NLR) and overall
survival (OS) in patients with metastatic castration-resistant
prostate cancer (mCRPC) treated with first-line docetaxel. BJU Int.
114(6b): E11–E17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azab B, Bhatt VR, Phookan J, Murukutla S,
Kohn N, Terjanian T and Widmann WD: Usefulness of the
neutrophil-to-lymphocyte ratio in predicting short- and long-term
mortality in breast cancer patients. Ann Surg Oncol. 19:217–224.
2012. View Article : Google Scholar
|
|
32
|
Movahedi K, Guilliams M, Van den Bossche
J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P and Van
Ginderachter JA: Identification of discrete tumor-induced
myeloid-derived suppressor cell subpopulations with distinct T
cell-suppressive activity. Blood. 111:4233–4244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Youn JI, Nagaraj S, Collazo M and
Gabrilovich DI: Subsets of myeloid-derived suppressor cells in
tumor-bearing mice. J Immunol. 181:5791–5802. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Solito S, Marigo I, Pinton L, Damuzzo V,
Mandruzzato S and Bronte V: Myeloid-derived suppressor cell
heterogeneity in human cancers. Ann N Y Acad Sci. 1319:47–65. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY,
Chung YH, Chen WC and Lee KD: IL-6-stimulated CD11b+
CD14+ HLA-DR− myeloid-derived suppressor
cells, are associated with progression and poor prognosis in
squamous cell carcinoma of the esophagus. Oncotarget. 5:8716–8728.
2014.PubMed/NCBI
|
|
36
|
Cuenca AG, Delano MJ, Kelly-Scumpia KM,
Moreno C, Scumpia PO, Laface DM, Heyworth PG, Efron PA and Moldawer
LL: A paradoxical role for myeloid-derived suppressor cells in
sepsis and trauma. Mol Med. 17:281–292. 2011. View Article : Google Scholar :
|
|
37
|
Dolcetti L, Peranzoni E, Ugel S, Marigo I,
Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati
A, et al: Hierarchy of immunosuppressive strength among
myeloid-derived suppressor cell subsets is determined by GM-CSF.
Eur J Immunol. 40:22–35. 2010. View Article : Google Scholar
|
|
38
|
Haverkamp JM, Smith AM, Weinlich R, Dillon
CP, Qualls JE, Neale G, Koss B, Kim Y, Bronte V, Herold MJ, et al:
Myeloid-derived suppressor activity is mediated by monocytic
lineages maintained by continuous inhibition of extrinsic and
intrinsic death pathways. Immunity. 41:947–959. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Umemura N, Saio M, Suwa T, Kitoh Y, Bai J,
Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, et al:
Tumor-infiltrating myeloid-derived suppressor cells are
pleiotropic-inflamed monocytes/macrophages that bear M1- and
M2-type characteristics. J Leukoc Biol. 83:1136–1144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sumida K, Wakita D, Narita Y, Masuko K,
Terada S, Watanabe K, Satoh T, Kitamura H and Nishimura T:
Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells
and inhibits tumor growth by enhancing T-cell responses. Eur J
Immunol. 42:2060–2072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen W, Jin W, Hardegen N, Lei KJ, Li L,
Marinos N, McGrady G and Wahl SM: Conversion of peripheral
CD4+CD25− naive T cells to
CD4+CD25+ regulatory T cells by TGF-beta
induction of transcription factor Foxp3. J Exp Med. 198:1875–1886.
2003. View Article : Google Scholar : PubMed/NCBI
|