Introduction
Prostate cancer (PCa) is the most common non-skin
malignancy in males and the second leading cause of
cancer-associated mortality in men in the United States. It has
been estimated that ~161,360 new cases and ~26,730 cases of
PCa-associated mortality occurred in the United States in 2017
(1). However, the pathogenesis of
PCa has not yet been completely elucidated. PCa has a complex
etiology; numerous risk factors, including age, ethnicity, obesity
and family history, are most frequently reported to be associated
with the disease (2). Other
factors, including diet, inflammation and environment, may also
contribute to the development and progression of PCa (3). One important characteristic of PCa is
that it usually progresses from androgen-dependent to
androgen-independent phenotypes with highly metastatic properties
(3,4). Early-stage PCa, which is usually
confined to the prostate gland, may be cured by surgery, whereas
advanced-stage PCa that has metastasized to various organs is
typically treated by androgen ablation methods (5). It has been speculated that PCa may
develop into hormone-refractory tumors due to alternative cellular
processes that favor cancer cell proliferation and/or the
accumulation of mutations in the androgen receptor (6). Hormone-refractory PCa is usually
incurable and eventually fatal. Besides surgery and hormonal
treatment, radiotherapy and chemotherapy are also used to treat all
stages of PCa, although they frequently lead to unwanted side
effects and decreased quality of life for patients. Therefore, the
development of safe and effective novel anti-PCa medicines remains
a focus of research.
PC-SPES is an herbal mixture previously marketed by
Botanic Lab (Brea, CA, USA) that has been used as an alternative
treatment for patients with PCa (7). Numerous in vitro and in
vivo studies have reported that PC-SPES may exert promising
anticancer activities against PCa (8-10).
In addition, PC-SPES has been successfully tested, with promising
results in phase II clinical trials, as an effective agent in the
treatment of advanced PCa with very minimal side effects (11-16).
Ganoderma lucidum, also termed Lingzhi or Reshi, is one of
eight herbs used in PC-SPES. However, PC-SPES was withdrawn from
the market in 2002 following identification of contamination with
prescription drugs, e.g. warfarin (17,18).
Although PC-SPES was removed from the market, studies focusing on
novel formulas, and examining the anticancer activity and molecular
mechanisms underlying individual components of PC-SPES are of
critical importance. In particular, further research is required to
pinpoint the exact mechanism of action of each component against
PCa, including G. lucidum.
G. lucidum has been the most popular
medicinal mushroom used in Traditional Chinese Medicine (TCM) for
>2,000 years, and it has previously been used to promote
vitality and longevity in East Asia (19). Recently, it has been hypothesized
to possess anticancer activities against numerous types of cancer
(19). Previous studies have
suggested that G. lucidum may inhibit PCa cell
proliferation, angiogenesis and migration, induce apoptosis and
cell cycle arrest, and interfere with androgen receptor function
(6,20,21).
In the past few decades, several bioactive chemical substances,
including polysaccharides and triterpenoids extracted from the
fruiting bodies, cultured mycelia and spores of G. lucidum,
have been reported to be responsible for its anticancer activity.
Numerous studies have aimed to elucidate the molecular mechanism of
action against cancer of all the major bioactive compounds,
including polysaccharides and triterpenoids (6,20,21).
In particular, G. lucidum polysaccharides (GLP) have been
demonstrated to exert anticarcinogenic effects, which may be due to
their immunomodulatory and apoptotic activity (22). However, the exact molecular target
or signaling pathway of GLP against PCa is currently unclear.
Non-steroidal anti-inflammatory drug
(NSAID)-activated gene-1 (NAG-1), also termed growth
differentiation factor-15 (GDF15) or macrophage inhibitory cytokine
1, is a divergent member of the transforming growth factor-β
superfamily. NAG-1 serves a complex, although poorly understood,
role in normal physiology and in numerous human diseases, including
cancer (23). It has been
demonstrated that several tumor suppressor pathways, including p53,
glycogen synthase kinase-3β and early growth response-1 (EGR-1),
serve as upstream factors in NAG-1 transcriptional induction
(22,23); NAG-1 may also be induced by various
anticancer drugs or natural compounds. NAG-1 overexpression is able
to inhibit the development of prostate tumors in animal models
(24). Further laboratory and
clinical evidence suggested that NAG-1 may serve an
anticarcinogenic role in the early stage of carcinogenesis, and a
protumorigenic role in the late stage of carcinogenesis, as
reviewed by Wang et al (23). Previous studies have also suggested
that NAG-1 is proapoptotic, and thus inhibits cancer cell
proliferation (25-28). Recently, it was reported that water
extracts of G. lucidum (primarily containing GLP) inhibit
colorectal cancer carcinogenesis and induce NAG-1 (22). However, whether NAG-1 may be
induced in PCa cells by GLP, and its potential role in the anti-PCa
effects of GLP, remains unknown.
The present study assessed the effects and mechanism
of GLP extracted from sporoderm-broken spores of G. lucidum
on PCa, and examined the role of NAG-1 in androgen-independent and
highly metastatic PC-3 cells. These data suggested that GLP was
effective against PCa proliferation via NAG-1 mediated apoptosis.
To the best of our knowledge, the present study is the first to
examine the role of NAG-1 in GLP-induced anticancer effects in PCa.
The present results may aid in elucidating the anticancer
mechanisms of GLP.
Materials and methods
Materials
MTT was obtained from HXBIO (Hangzhou, China).
Hoechst 33342 was obtained from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Dulbecco's modified Eagle's
medium (DMEM) was purchased from Gibco (Thermo Fisher Scientific,
Inc.). The fluorescein isothiocyanate (FITC) Annexin V apoptosis
detection kit and propidium iodide (PI) staining kit were purchased
from BD Pharmingen; BD Biosciences (San Jose, CA, USA). Polyclonal
β-actin (cat. no. 4967S), poly(ADP-ribose) polymerase 1 (PARP)
(cat. no. 9542S), caspase-3 (cat. no. 9665), caspase-6 (cat. no.
9762), caspase-9 (cat. no. 9508), phos-phorylated (p)-extracellular
signal-regulated kinase (Erk)1/2 (cat. no. 9101S), Erk1/2 (cat. no.
9102S), p-protein kinase B (Akt) (cat. no. 9271S), Akt (cat. no.
9272S), EGR-1 (cat. no. 4153) and horseradish peroxidase-conjugated
anti-rabbit immunoglobulin G (IgG, cat. no. 7074) and anti-mouse
IgG (cat. no. 7076) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The NAG-1 polyclonal rabbit
antibody was generated in Dr Eling's laboratory (Laboratory of
Molecular Carcinogenesis, National Institute of Environmental
Health Sciences, Durham, NC, USA), as previously described
(29). The Quantikine Human
GDF15/NAG-1 ELISA kit (cat. no. DY957) was purchased from R&D
Systems, Inc. (Minneapolis, MN, USA). The RNA extraction kit was
obtained from Aidlab Biotechnologies, Co., Ltd. (Beijing, China).
NAG-1 small interfering (si) RNA (cat. no. M-019875-01) and control
siRNA (cat. no. D-001206-13) were obtained from Dharmacon, Inc.
(Lafayette, CO, USA). Lipofectamine® 2000 transfection reagent was
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). The
Dual-Luciferase assay kit was purchased from Promega Corp.
(Madison, WI, USA). The iScript cDNA synthesis kit and SYBR Master
Mix were purchased from Bio-Rad Laboratories, Inc. (Hercules, CA,
USA). The bicinchoninic acid (BCA) assay kit was purchased from
Pierce (Thermo Fisher Scientific, Inc.). The Western Lightening™
Plus-ECL, enhanced chemiluminescence substrate assay kit was
purchased from PerkinElmer, Inc. (Waltham, MA, USA).
GLP preparation
Powder from sporoderm-broken spores of G.
lucidum was obtained from Zhejiang Shouxiangu Pharmaceutical
Co., Ltd. (Wuyi, China). Polysaccharides were extracted from the
powder of sporoderm-broken spores of G. lucidum using the
hot water extraction method, as previously described (22). Following extraction, the GLP was
purified and dissolved in DMEM at 20 mg/ml as the stock
solution.
Cell culture
Human PCa cell lines PC-3, DU145 and LNCaP, and the
non-cancerous cell lines RWPE-1 and PrEC, were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). Cells
were maintained in an incubator containing 5% CO2 at
37°C in DMEM supplemented with 10% fetal bovine serum (FBS; Gemini
Bio Products, West Sacramento, CA, USA).
MTT assay
The MTT assay was used to assess cell viability.
Briefly, PC-3, DU145, LNCaP, RWPE-1 and PrEC cells were seeded in
96-well plates at 1×104 cells/well and incubated at 37°C
in DMEM until the cells reached 60% confluence. Subsequently, PC-3,
DU145 and LNCaP PCa cells were treated with various concentrations
of GLP (0, 1.25, 2.5, 5 and 10 mg/ml) for 24, 48 and 72 h, RWPE-1
and PrEC and nonmalignant prostate cells were treated for 48 h.
Subsequently, MTT solution (5 mg/ml) was added to each well and
incubated for 4 h at 37°C, after which, the supernatants were
discarded and 150 μl dimethyl sulfoxide (DMSO) was added to
dissolve the remaining water-insoluble formazan crystals. After a
10-min incubation, the absorbance was determined using a multi-well
plate reader (BioTek Instruments, Inc., Winooski, VT, USA) at 490
nm. All experiments were repeated at least three times.
Hoechst 33342 staining
Apoptosis was visualized according to nuclear
morphological alterations using the fluorescent dye Hoechst 33342.
When PC-3 cells had been treated with GLP (0, 1.25, 2.5, 5 and 10
mg/ml) for 48 h, the cells were rinsed with PBS and incubated with
10 μg/ml Hoechst 33342 for 10 min. A fluorescence microscope
was used to visualize the cells undergoing apoptosis, which display
morphological alterations in the nucleus at 350 nm.
Flow cytometric analysis of
apoptosis
Apoptosis was measured by flow cytometric analysis,
as previously described (22).
Briefly, 2×105 cells/well PC-3 cells were seeded in
6-well plates and treated with 0-10 mg/ml GLP for 24 and 48 h.
Subsequently, the cells were collected and stained with 50
μg/ml Annexin V-FITC/PI at room temperature in the dark for
15 min. Apoptosis was measured using a Guava® easyCyte flow
cytometer system (Merck KGaA, Darmstadt, Germany). The ratio of
Annexin V+/PI− (early) and Annexin
V+/PI+ (late) apoptotic cells was
calculated.
Western blotting
Total protein was extracted from PC-3 cells using
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA)
and protein concentrations were determined using the BCA method.
Proteins (35 μg) were separated by 10-12% SDS-PAGE, and
electrophoresed at 100 V for 2 h. Separated proteins were
transferred onto a polyvinylidene difluoride membrane at 100 V for
1 h on ice. After transfer, membranes were blocked with 5% non-fat
dry milk in 1X Tris-buffered saline with Tween-20 (50 mmol/l Tris,
pH 7.5; 150 mmol/l NaCl; 0.1% Tween-20) at room temperature for 1
h. The blots were subsequently incubated with primary antibodies
(1:1,000) overnight at 4°C. The blots were then incubated with the
secondary antibody (1:2,000) at room temperature for 1 h, according
to the manufacturer's protocol. The signals were detected using the
Western Lightning Plus-ECL Enhanced Chemiluminescence substrate
(PerkinElmer, Inc.), according to the manufacturer's protocol.
ImageJ 1.41 software (National Institutes of Health, Bethesda, MD,
USA) was used to calculate the optical density.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The quantity and quality of total RNA were assessed
using a NanoDrop 2000 (Nanodrop Technologies; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA). A total of 1 μg RNA
was reverse transcribed to cDNA, using iScript cDNA Synthesis kit
(Bio-Rad Laboratories, Inc.). The RT reaction was performed as
follows 5 min at 25°C, 30 min at 42°C, 5 min at 85°C. RT-qPCR
analysis was performed using SYBR Master Mix (Bio-Rad Laboratories,
Inc.) on a CFX96 Real-Time PCR system (Bio-Rad Laboratories, Inc.),
according to the manufacturer's protocol. The PCR conditions
consisted of 40 cycles, with 5 sec denaturation at 95°C, 30 sec
annealing at 60°C and 5 sec extension at 65°C. The fold change in
mRNA was calculated through relative quantification
(2−ΔΔCq) with β-actin used as the housekeeping gene
(30). The primers used were as
follows: β-actin, forward, 5′-CTGGAACGGTGAAGGTGACA-3′ and reverse,
5′-AAGGAACTTCCTTGAACAATGCA-3′; NAG-1, forward,
5′-CTCCAGATTCCGAGAGTTGC-3′ and reverse, 5′-AGAGATACGCAGGTGCAGGT-3′;
and EGR-1, forward, 5′-AGCCCTACGAGCACCTGAC-3′ and reverse,
5′-GGTTTGGCTGGGGTAACTG-3′.
RNA interference
NAG-1 RNA interference was conducted as previously
described (31). Briefly, PC-3
cells were grown to 60% confluence and were transfected with 100 nM
NAG-1 siRNA or negative control siRNA overnight at 37°C using
Lipofectamine® 2000 reagent, according to the manufacturer's
protocol. The effects of NAG-1siRNA were confirmed by western blot
analysis. To determine the role of NAG-1 in the anticancer effects
of GLP, following siRNA transfection overnight, the medium was
removed, and PC-3 cells were washed with phosphate-buffered saline
(PBS) and treated with 5 mg/ml GLP for up to 24 h. The cells were
subsequently harvested at 24 h and analyzed by western blotting and
flow cytometry for the analysis of apoptosis.
Luciferase reporter assay
The human NAG-1 promoter construct used in the
luciferase assay was previously described (32). Briefly, to generate pNAG-1
3′-untranslated region (UTR)-luciferase plasmid, NAG-1 3′-UTR was
amplified using the following primers: forward,
5′-GGCCGATCTAGA GTTAACGCAGTCCTGGTCCTTCCACTGT-3′ and reverse,
5′-GGCCGATCTAGAAAACAGTTCAGACAGCTTTATT-3′ (XbaI sites
are italicized). Subsequently, the amplified PCR product was
digested with XbaI and ligated to the XbaI site
positioned between luciferase and the SV polyadenylation site in
the pGL3 promoter vector (Promega Corp.). For the luciferase assay,
PC-3 cells were plated in 6-well plates at 2×105
cells/well in DMEM containing 10% FBS. When cells reached a
confluence of 50-60%, plasmid mixture containing 1 μg NAG-1
promoter linked to luciferase and 0.1 μg pRL-null (Promega
Corp.) were transfected using Lipofectamine® 2000 transfection
reagent at 37°C for 24 h, according to the manufacturer's protocol.
Cells were then cultured in the absence or presence of 2.5 or 5
mg/ml GLP for an additional 24 h. At the end of the study, the
cells were collected and the Dual-Luciferase assay kit was used to
measure luciferase activity, which was then normalized to the
values of pRL-null luciferase activity.
ELISA
PC-3 cells were seeded in 6-well plates at
2×105 cells/well and incubated overnight. ELISA was
performed when the cells had been treated with culture medium
supplemented with GLP (0-10 mg/ml) for 48 h using cell culture
medium at 37°C. Cell lysates were also collected, and protein
concentrations were measured by BCA assay to correct for
variations. A Quantikine Human GDF15/NAG-1 ELISA kit was used to
determine the expression of NAG-1 protein in the medium, according
to the manufacturer's protocol, and the data were normalized to
total protein concentration determined from the cell lysates.
Statistical analysis
Data are presented as the means ± standard error of
three independent experiments. Statistical analysis was performed
using one-way analysis of variance (ANOVA) with Dunnett's
correction for pairwise comparisons, or two-way ANOVA with post hoc
Bonferroni's correction for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using GraphPad Prism 5.0 software (GraphPad
Software, Inc., La Jolla, CA, USA).
Results
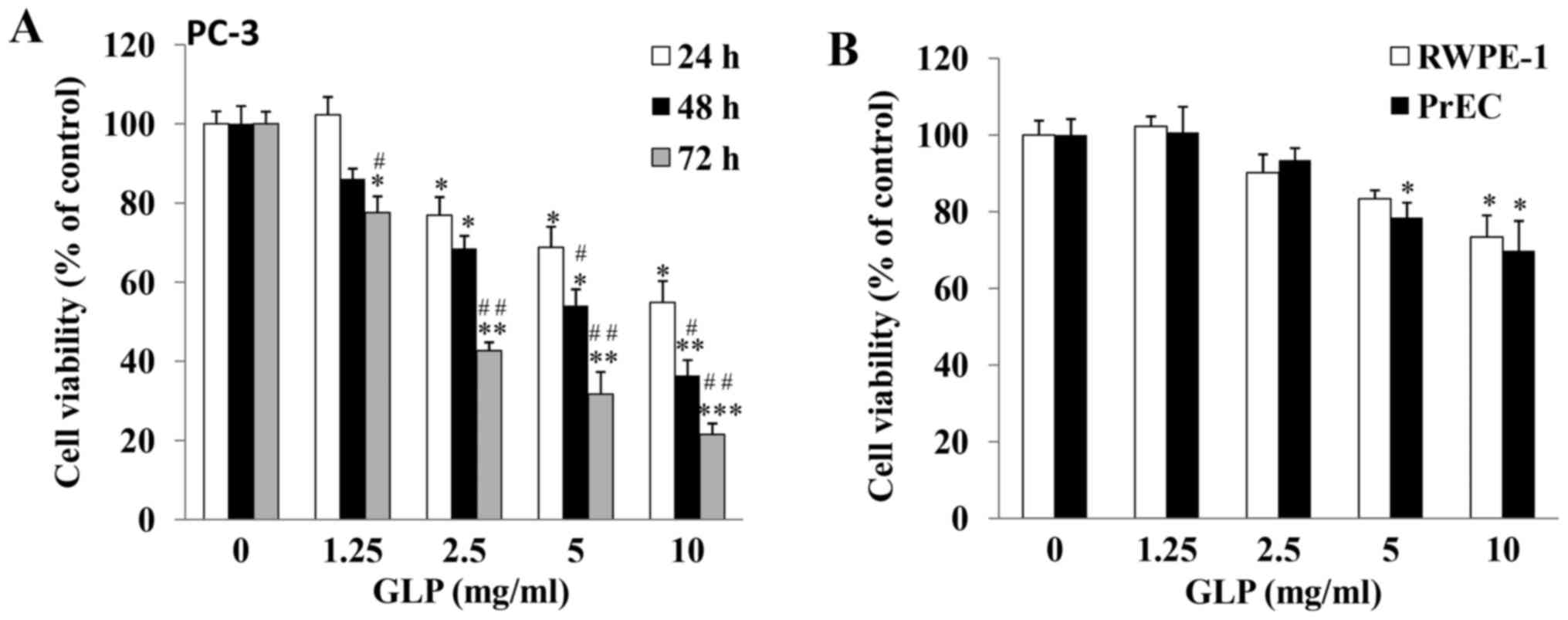
GLP inhibits PC-3 cell viability in a
dose- and time-dependent manner
The growth inhibitory effects of GLP against PCa
were examined. As determined by MTT assay, the viability of PC-3
cells was significantly reduced by GLP treatment in a dose- and
time-dependent manner (Fig. 1A).
PC-3 cells treated with 10 mg/ml GLP exhibited decreased cell
viability compared with the control group; viability was decreased
to 54.9±5.4, 36.4±3.9 and 21.5±2.8% at 24, 48 and 72 h,
respectively (P<0.01), thus suggesting that GLP may be a potent
anticancer agent against PCa. In addition, the inhibitory effects
of GLP on the viability of DU145 and LNCaP prostate cancer cells,
which are also commonly used to study PCa, were examined. The
results demonstrated that GLP exerted similar effects to those
observed in PC-3 cells (data not shown). Among these three cell
lines, PC-3 and DU145 cells are androgen-independent cell lines,
whereas LNCaP cells are androgen-dependent (33,34).
In addition, PC-3 cells demonstrate higher metastatic potential
compared with DU145 cells (33).
Due to the highly metastatic and androgen-independent properties of
PC-3 cells, subsequent experiments only focused on the PC-3 cell
line. It is well known that the majority of anticancer chemotherapy
drugs additionally cause cytotoxicity in normal cells. Therefore,
the present study investigated whether GLP exerted cytotoxic
effects on normal prostate cells. As presented in Fig. 1B, following treatment of prostate
non-malignant RWPE-1 and PrEC cells for 48 h, GLP at lower
concentrations (1.25 and 2.5 mg/ml) did not cause significant cell
death. However, GLP at 5 and 10 mg/ml did cause cytotoxicity in
RWPE-1 or PrEC cells. These results suggested that, similar to
other natural anticancer compounds, the effects of GLP on PCa
cancer cells are not cancer cell-specific.
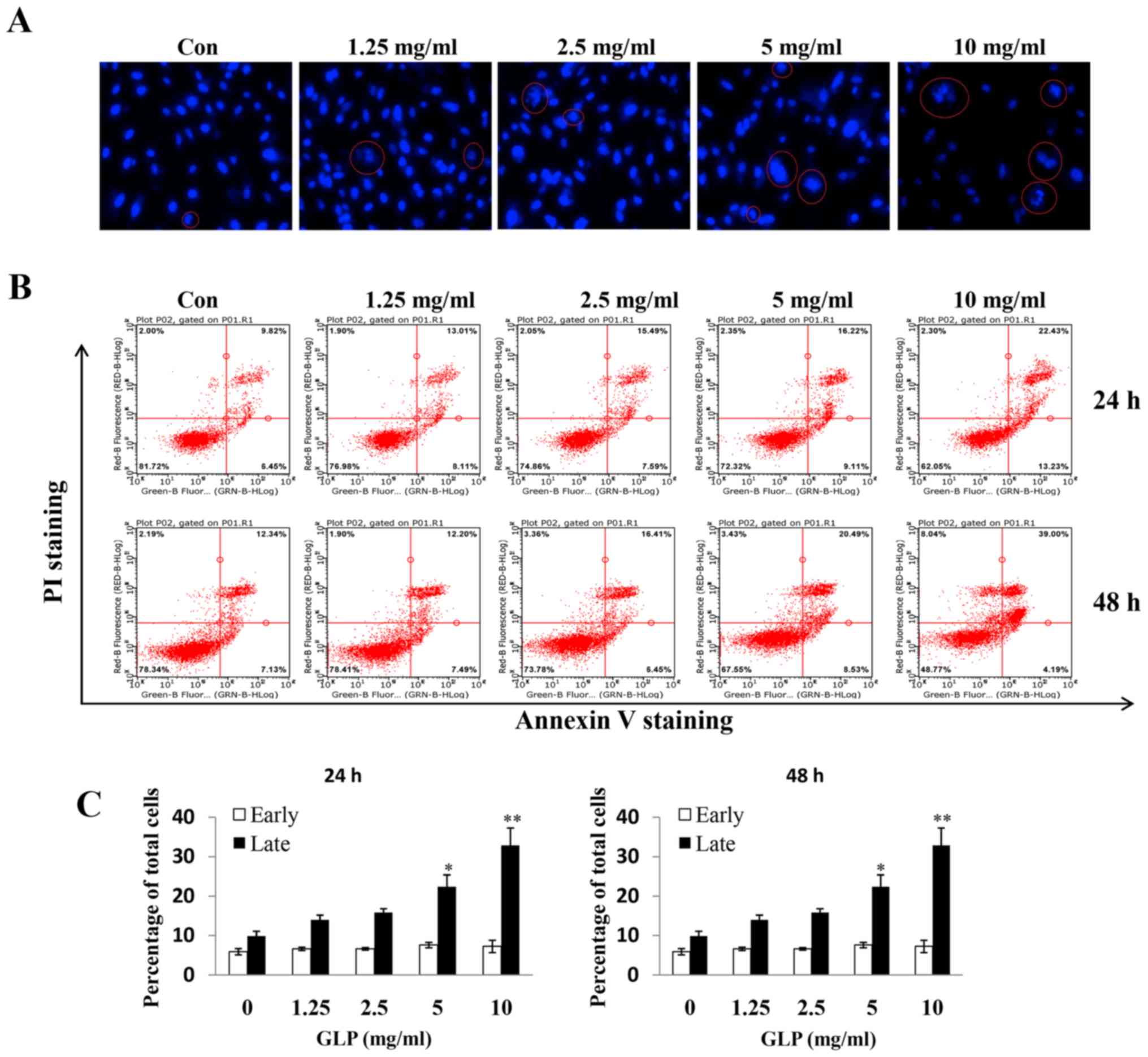
GLP induces apoptosis of PC-3 cells
Hoechst 33342 staining was conducted to examine
whether GLP induces apoptosis of PC-3 cells, which may be observed
microscopically. Nuclear morphological alterations were observed,
which indicated coagulation and fragmentation (indicated by a red
circle), upon treatment of PC-3 cells with increasing
concentrations of GLP (Fig. 2A).
Subsequently, flow cytometry was used to further study the effects
of GLP on apoptosis of PC-3 cells. It was observed that the amount
of Annexin V+/PI+ (late apoptosis) positive
cells was significantly increased upon GLP treatment (1.25-10
mg/ml) in a dose- and time-dependent manner (Fig. 2B). However, GLP seemed to have no
effect on early apoptosis, as there were no significant differences
in the percentage of Annexin V+/PI− (early
apoptosis) stained cells between treatment groups (Fig. 2B and C). The present data suggested
that GLP may inhibit cell proliferation through the stimulation of
late apoptosis in PC-3 cells. In addition, flow cytometric analysis
revealed that treatment with 10 mg/ml GLP slightly, but
significantly, induced apoptosis of PWPE-1 and PrEC cells (data not
shown), thus, suggesting a cytotoxic effect of GLP on normal
cells.
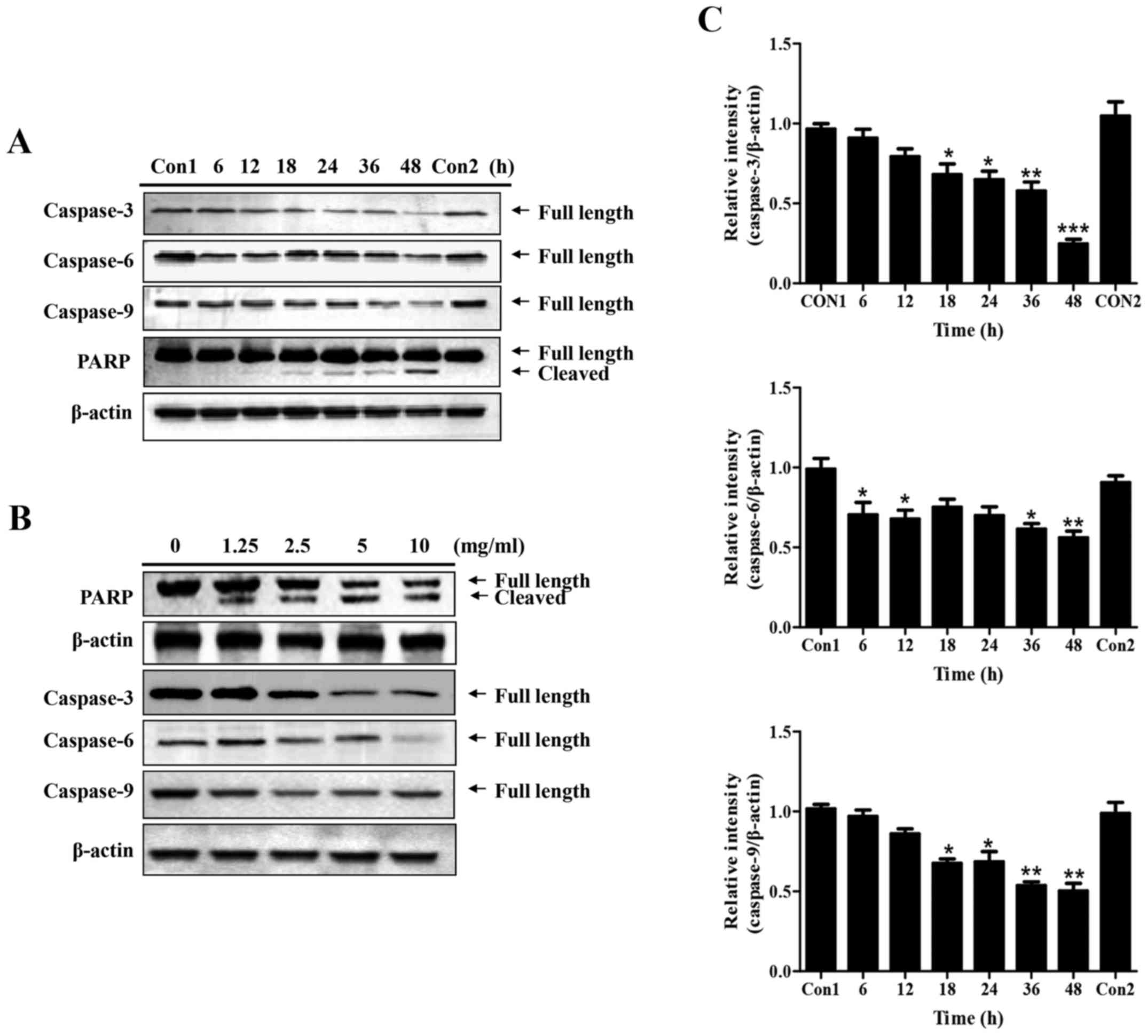
Western blotting was subsequently conducted to
examine the expression of key regulatory proteins associated with
apoptosis in PC-3 cells upon GLP treatment (Fig. 3). The effects of 5 mg/ml GLP on the
protein expression levels of pro-caspase-3, -6, -9 and PARP were
examined at various time-points. As presented in Fig. 3A and C, the expression levels of
the three pro-caspases were significantly downregulated between 6
and 48 h following GLP treatment. In addition, it was observed that
cleaved PARP expression was markedly increased with time; however,
the change in total PARP expression was not marked; this may have
been due to the strong signals in the blot. The first control group
(Con1) refers to when the protein sample was collected at 6 h,
whereas the second control group (Con2) refers to when the protein
sample was collected at 48 h. After 48 h of treatment, the protein
expression levels of all pro-caspases and PARP in untreated cells
(Con2) remained the same as at 6 h (Con1) (Fig. 3A). The dose-dependent effects of
GLP on the regulation of these proteins were subsequently examined.
Following treatment for 48 h, GLP (1.25-10 mg/ml) inhibited the
protein expression levels of pro-caspase-3, 6 and 9, although not
in a precise dose-dependent manner (Fig. 3B). Conversely, the expression
levels of full-length PARP were decreased, whereas cleaved PARP was
increased, upon GLP treatment (1.25-10 mg/ml) in a dose-dependent
manner (Fig. 3B). Taken together,
these data indicated that GLP was effective at inducing apoptosis
of PC-3 cells.
GLP induces EGR-1 and NAG-1 expression in
PC-3 cells
Numerous studies have demonstrated that the
expression of NAG-1 is induced by several natural compounds in
cancer (32,35-38).
A previous study suggested that EGR-1 may serve as an upstream
factor for the induction of NAG-1expression (31). Our previous study recently reported
that GLP is able to induce NAG-1 expression in colorectal cancer
HCT116 cells (22). However, to
the best of our knowledge, it remains unknown as to whether NAG-1
and its upstream gene EGR-1 may be induced in PC-3 cells by GLP. To
evaluate whether NAG-1 and EGR-1 were involved in the anticancer
effects of GLP in PC-3 cells, the mRNA and protein expression
levels of EGR-1 and NAG-1 were determined in PC-3 cells (Fig. 4A–D). Following exposure of the
cells to 5 mg/ml GLP, the mRNA and protein expression levels of
EGR-1 and NAG-1 were induced in a time-dependent manner (Fig. 4A and C). The mRNA expression levels
of EGR-1 and NAG-1 were significantly upregulated 6 h post-GLP
treatment, and this effect reached its maximum at 18 h and was
maintained at a high level until 24 h (Fig. 4A). The protein expression levels of
EGR-1 were markedly upreg-ulated at 12 h post-GLP treatment and the
upregulation was maintained until 24 h, whereas NAG-1 expression
occurred later, with the highest expression detected at 24 h
(Fig. 4C). The stimulation of
EGR-1 and NAG-1 expression by various doses of GLP (1.25-10 mg/ml),
following treatment for 24 h, was also determined. As illustrated
in Fig. 4B and D, the expression
levels of EGR-1 and NAG-1 were induced by GLP in a dose-dependent
manner at the mRNA and protein levels (Fig. 4B and 4D).
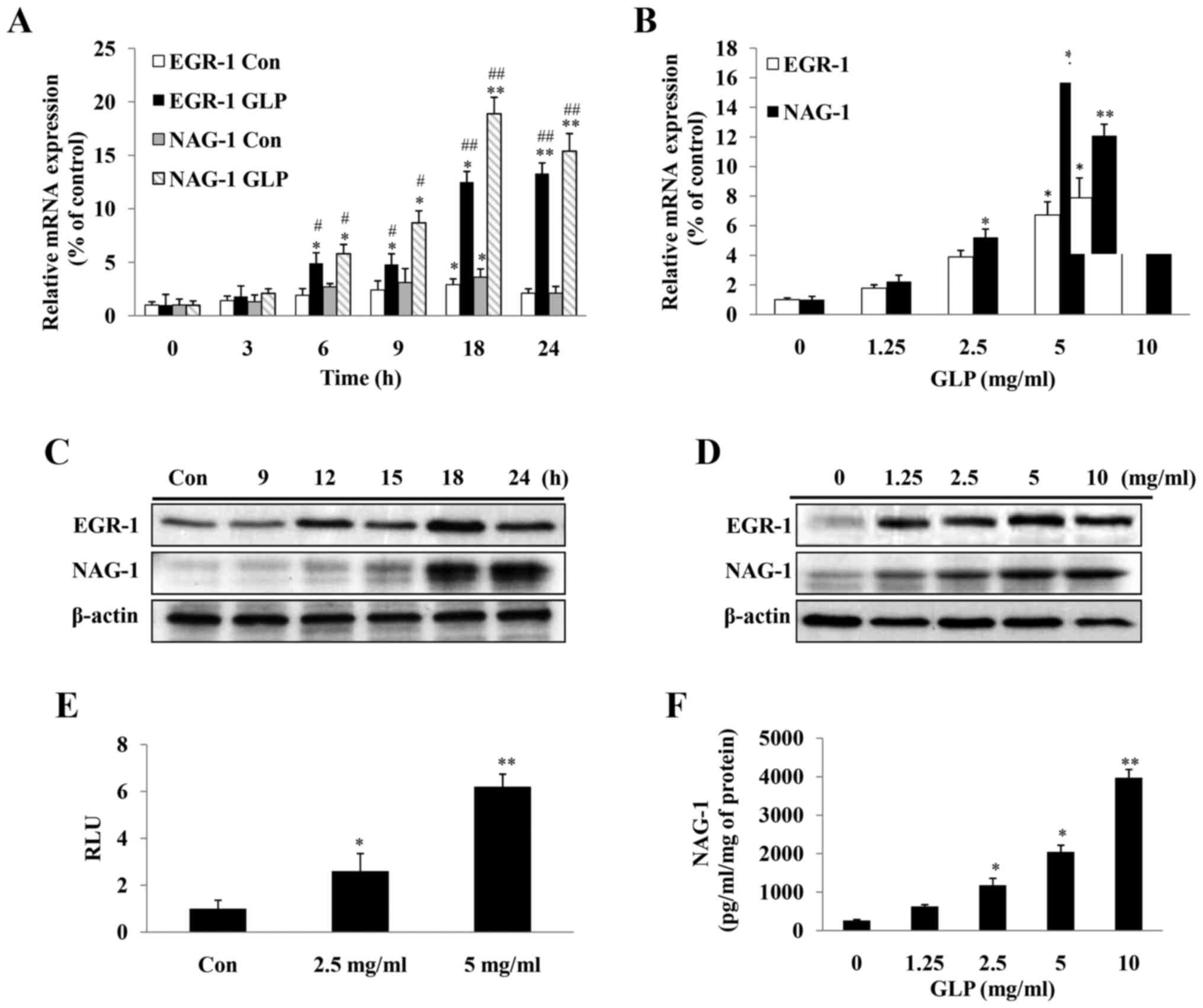 | Figure 4Expression levels of NAG-1, and its
transcription factor EGR-1, are induced in PC-3 cells upon GLP
treatment. (A) Quantification of the mRNA expression levels of
NAG-1 and EGR-1 in a time-dependent manner (0-24 h) following
treatment of PC-3 cells with 5 mg/ml GLP. Data are presented as the
means ± standard error from three independent experiments. Two-way
ANOVA with post hoc Bonferroni's correction for multiple comparison
was used to determine statistical significance. * indicates
time-dependent effects; # indicates effects of GLP treatment.
*P<0.05 and **P<0.01 compared with the
0 h group for each treatment. #P<0.05 and
##P<0.01 compared with the untreated control group at
each time-point. (B) Quantification of the mRNA expression levels
of NAG-1 and EGR-1 in a dose-dependent manner following treatment
of PC-3 cells with GLP (0-10 mg/ml) for 24 h. Induction of EGR-1
and NAG-1 protein expression upon GLP treatment in a (C)
time-dependent and (D) dose-dependent manner, as determined by
western blotting. β-actin was used as an internal control. (E) GLP
induced NAG-1 promoter activity, as determined by luciferase assay.
Fold-changes were normalized to pRL-null expressing Renilla
luciferase protein. (F) ELISA of the concentration of NAG-1 protein
in the cell culture medium following treatment with 0-10 mg/ml GLP
for 48 h. Data presented were normalized to the concentration of
protein in lysates from each sample. All data are presented as the
means ± standard error of three independent experiments.
*P<0.05, **P<0.01 compared with the
control group (one-way ANOVA with Dunnett's correction). ANOVA,
analysis of variance; EGR-1, early growth response-1; GLP,
Ganoderma lucidum polysaccharides; NAG-1, non-steroidal
anti-inflammatory drug-activated gene-1; RLU, relative light
units. |
To determine whether GLP was able to directly
activate the NAG-1 promoter, a luciferase reporter gene fused to
the NAG-1 promoter was transfected into PC-3 cells, which were
treated with GLP. GLP at 2.5 and 5 mg/ml significantly induced
NAG-1 promoter activity by 1.9 and 3.5-fold, respectively (Fig. 4E). In addition, the concentration
of secreted NAG-1in the medium was measured by ELISA. Considering
the cytotoxic effects caused by GLP treatment, the value determined
from the ELISA was normalized to the total protein concentration in
the cell lysates. It was observed that NAG-1 protein concentration
was significantly increased in response to GLP treatment in a
dose-dependent manner (Fig. 4F).
Taken together, the present data indicated that GLP significantly
induced expression of NAG-1 and its upstream factor EGR-1 in PC-3
cells.
GLP-induced apoptosis is mediated by
NAG-1 induction
NAG-1 is a proapoptotic gene, and numerous studies
have examined the role of NAG-1 in apoptosis (25-28).
To link the anticancer effects of GLP to NAG-1 activation and its
proapoptotic activity, PC-3 cells were transfected with scrambled
NAG-1 siRNA (vector) or NAG-1 siRNA, after which GLP-induced
apoptosis was detected. As presented in Fig. 5A, western blotting confirmed the
complete knockdown of NAG-1 expression in PC-3 cells, thus
suggesting that the NAG-1 siRNA used was effective. The present
study subsequently assessed whether GLP-induced NAG-1 expression
was also inhibited by siRNA transfection. As demonstrated
previously, NAG-1 expression was increased upon GLP treatment,
whereas NAG-1 siRNA-transfected cells exhibited reduced GLP-induced
NAG-1 expression (Fig. 5B).
However, NAG-1 expression was not completely blocked. To examine
whether NAG-1 serves a role in the induction of apoptosis by GLP,
NAG-1 expression was knocked down and apoptosis was measured by
flow cytometry. As displayed in Fig.
5C, GLP alone increased late apoptosis by 3.85-fold compared
with the control group (P<0.01). Conversely, apoptosis was
significantly reduced in cells transfected with NAG-1 siRNA and
treated with GLP, in which the percentage of late apoptosis was
increased only by 1.68-fold compared with NAG-1 siRNA-transfected
cells (Fig. 5C; P<0.01). There
was a significant reduction in the late apoptosis of GLP and NAG-1
siRNA-transfected cells compared with in cells treated with GLP
alone (Fig. 5C; P<0.01).
However, GLP-induced apoptosis was not completely blocked by NAG-1
siRNA transfection, thus suggesting that NAG-1 induction is only
partially responsible for GLP-induced apoptosis of PC-3 cells. The
expression levels of the apoptosis-associated proteins, PARP and
caspases, were detected following siRNA transfection by western
blotting. As presented in Fig. 5D,
the expression levels of pro-caspase-3, -6 and -9 (full length)
were reduced upon GLP treatment; however, cells transfected with
NAG-1 siRNA and treated with GLP exhibited a minimal reduction in
the expression of these proteins (Fig.
5D). Similarly, GLP alone induced a reduction in pro-PARP and
increased the induction of cleaved PARP, whereas NAG-1 siRNA
abolished the induction of cleaved PARP (Fig. 5D). Taken together, these data
supported the conclusion that GLP-induced apoptosis of PC-3 cells
may be mediated, at least in part, by NAG-1 induction.
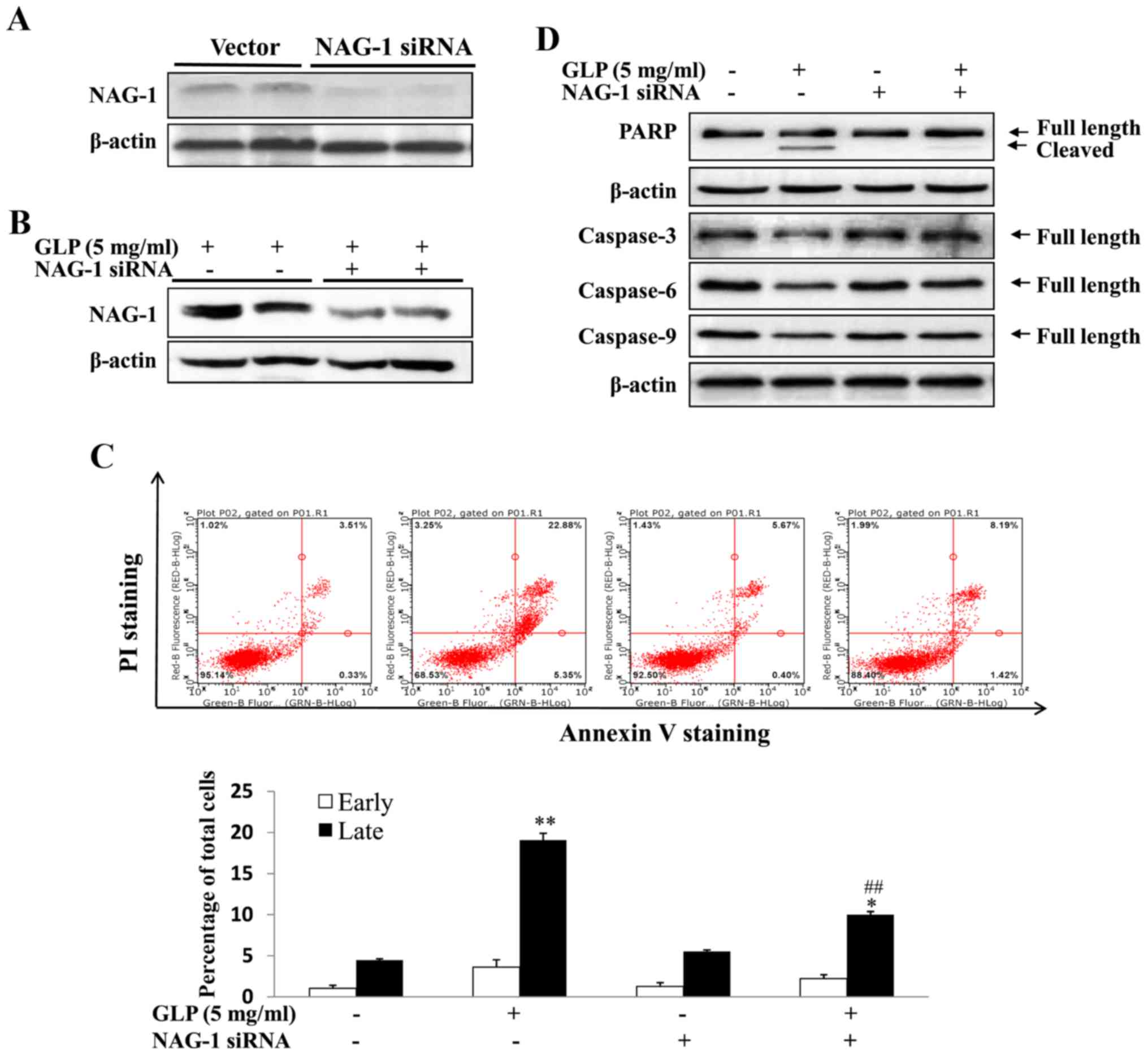 | Figure 5GLP-induced apoptosis of PC-3 cells
is mediated through NAG-1 induction. (A) NAG-1 siRNA successfully
knocked down NAG-1 expression in PC-3 cells, as determined by
western blotting. (B) NAG-1 siRNA inhibited GLP-induced NAG-1
expression, as determined by western blotting. (C) NAG-1 siRNA
inhibited GLP-induced apoptosis, as determined by flow cytometry.
Percentage of early and late apoptotic of PC-3 cells induced by GLP
is presented in the lower panel. (D) NAG-1 siRNA inhibited
GLP-induced PARP cleavage and the suppression of pro-caspase-3, -6
and -9 protein expression. β-actin was used as an internal control.
Data are presented as the means ± standard error.
*P<0.05, **P<0.01 compared with the
control group (one-way analysis of variance with Dunnett's
correction). ##P<0.01, compared with GLP-treated
cells. GLP, Ganoderma lucidum polysaccharides; NAG-1,
non-steroidal anti-inflammatory drug-activated gene-1; PARP,
poly(ADP-ribose) polymerase; PI, propidium iodide; siRNA, small
interfering RNA. |
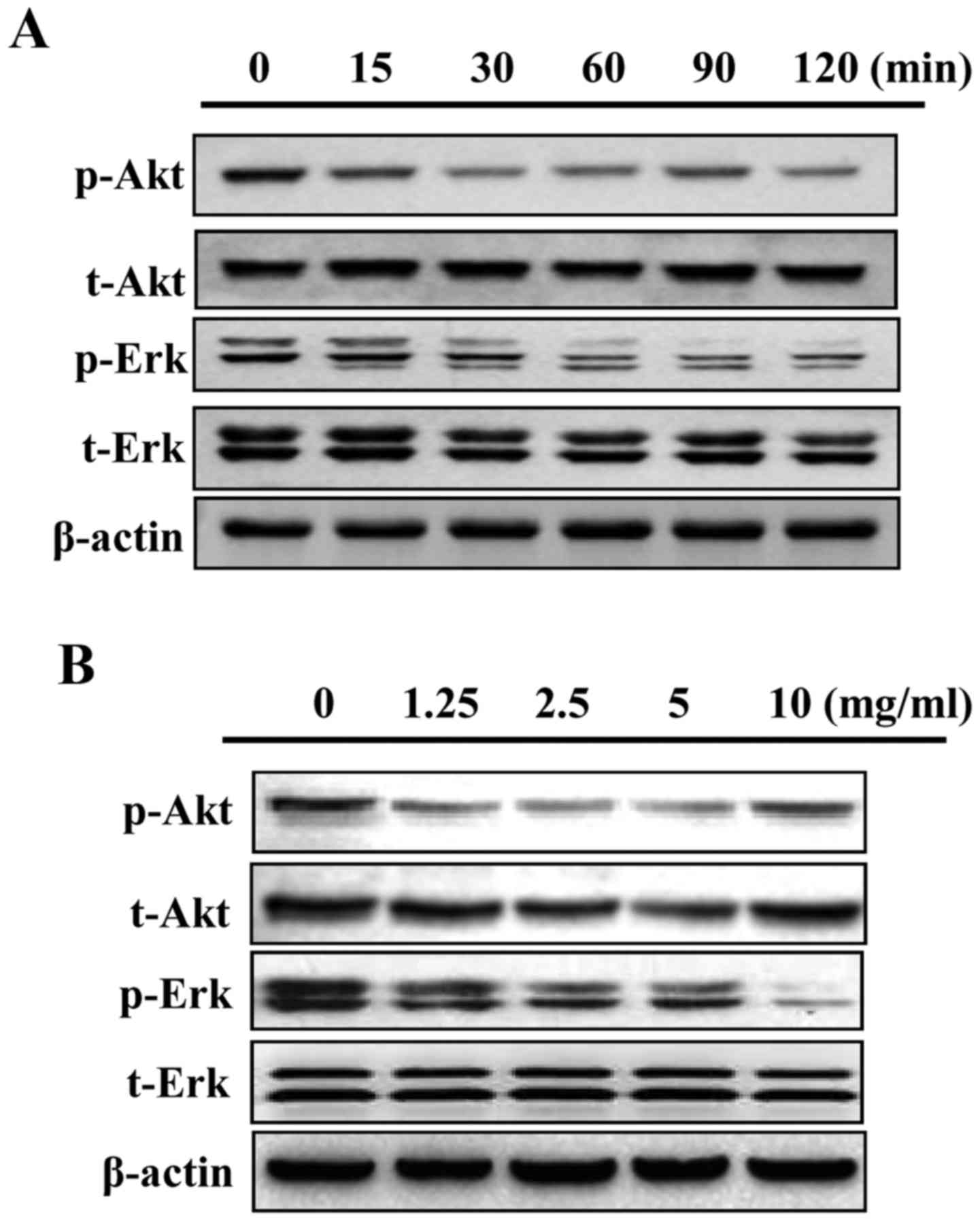
GLP inhibits mitogen-activated protein
kinase(MAPK)/Erk and Akt phosphorylation in PC-3 cells
The present study examined whether the MAPK/Erk and
Akt signaling pathways may be regulated by GLP in PC-3 cells. As
presented in Fig. 6A, 5 mg/ml GLP
markedly reduced the phosphorylation of Akt and MAPK/Erk signaling
from as early as 15 min. In addition, GLP reduced the
phosphorylation of Akt and MAPK/Erk proteins in a dose-dependent
manner between 1.25 and 10 mg/ml (Fig.
6B). These data suggested that inhibition of MAPK/Erk and Akt
activity may serve as a downstream signaling target of GLP in PC-3
cells; however, more definitive studies are required in the future
to elucidate this association.
Discussion
During the late stages of the disease, PCa is
characterized by the development of an androgen-refractory
phenotype with a highly metastatic nature. Much effort has been
made to identify novel therapies for the treatment of highly
metastatic PCa. The use of TCM has received recognition in Western
countries as an adjunct/alternative to conventional strategies for
cancer therapy and prevention (39). Gaining an understanding of the
molecular basis of TCM compounds and highlighting their potential
applications for cancer treatment is crucial. Among the numerous
TCM compounds, G. lucidum is one of the most widely studied
for cancer prevention. The present study demonstrated that GLP from
the broken spores of G. lucidum may be a potent inhibitor of
PCa cell proliferation by inducing apoptosis of PC-3 cells, which
was partially mediated by NAG-1 induction.
G. lucidum exerts numerous biological
functions, including immunoregulatory, antiviral, antibacterial,
antioxidant and hepatoprotective effects; in particular, its
promising anticancer activity has been reported (19,40).
The anticancer activities of GLP have remained a focus of interest
for cancer prevention. Numerous studies have demonstrated that GLP
is able to inhibit carcinogenesis in various types of cancer,
including ovarian (41), lung
(42,43), breast (44), liver cancer (45), leukemia (46), colon cancer (22) and PCa (47). Amongst these cancer types, the
anticancer effects of G. lucidum on PCa have been the most
intensely investigated; this is due to earlier reports on PC-SPES
and its anticancer activity in PCa. The first study was published
in 1997, wherein Hsieh et al (48) reported that ethanol extracts of
PC-SPES inhibit cell proliferation and reduce prostate specific
antigen levels in LNCaP cells. Subsequently, clinical and
laboratory studies have reported the potential activity of PC-SPES
against hormone-independent PCa (11,49).
Although PC-SPES was criticized for its adulteration
of the Food and Drug Administration-controlled prescription drug
synthetic estrogen diethylstilbestrol and warfarin, and was thus
withdrawn from the market, the interest in G. lucidum as an
individual TCM continues. Silva et al (21) reported that the spores and
unpurified fruiting body of G. lucidum inhibit the
constitutively active transcription factors activator protein-1 and
nuclear factor-κB in PC-3 cells, and thus suppress cell migration.
Ethanol extracts from G. lucidum, containing ganoderic acid
subtypes F, have also been reported to inhibit cell proliferation
of LNCaP cells and block hormone-induced prostate growth in
castrated rats (50). Jiang et
al (20) reported that G.
lucidum extracts containing GLP and triterpenoids inhibit PC-3
cell proliferation in vitro. Further examination
demonstrated that the growth inhibition of PC-3 cells mediated by
G. lucidum is caused by dysregulation of the expression of
cell cycle arrest-associated proteins (20). However, very few of these studies
have examined the effects of GLP, particularly those extracted from
the broken spores of G. lucidum, against PCa. GLP is usually
isolated from the fruiting bodies, mycelia and spores of G.
lucidum (51). GLP isolated
from the sporoderm-broken spores of G. lucidum may be more
bioactive, exerting more potent cancer cell growth inhibitory
effects compared with GLP isolated from other parts of G.
lucidum (22,52). The present study demonstrated that
GLP from the sporoderm-broken spores of G. lucidum was a
potent inhibitor of PCa PC-3 cell growth in a time- and
dose-dependent manner. In the present study, the three most
commonly used PCa cell lines, DU145, PC-3 and LNCaP, were assessed,
and it was demonstrated that GLP was effective at inhibiting cell
proliferation in all three cell lines. However, due to the highly
metastatic and androgen-independent properties of PC-3 cells
(33,34), subsequent experiments focused on
PC-3 cells.
Cancer is driven by an imbalance between cell
proliferation and cell death (53). Apoptosis is a type of programmed
cell death, which is regulated by a strict and complex signaling
network that comprises a series of associated genes that must be
coordinated (53,54). In the present study, it was
observed that GLP was effective at inducing apoptosis of PC-3 cells
in a time- and dose-dependent manner. Notably, treatment with GLP
at various concentrations did not induce early-stage apoptosis,
although it significantly induced late-stage apop-tosis of PC-3
cells. Numerous proteins regulate apoptosis; therefore, the
expression of key regulatory proteins associated with apoptosis was
detected. Caspases are important proteins for maintaining
homeostasis by regulating cell death, and are key markers during
apoptosis (55). The cleavage of
caspases from their pro-form serves an important role in inducing
apoptosis. The present study revealed that GLP downregulated the
protein expression levels of pro-caspase 3, -6 and -9, and
significantly cleaved PARP in PC-3 cells, as determined by western
blotting.
Consistent with the present study, GLP has also been
reported to induce apoptosis of other cancer cells, including
breast (56) and colorectal cancer
(57). Liang et al
(57) reported that GLP-induced
apoptosis of human colorectal cancer HCT116 cells is mediated by
the activation of caspase-3 and -9, and cleavage of PARP. In
addition, a recent study demonstrated that GLP induces apoptosis of
HCT116 cells by reducing total PARP, and pro-caspase-3 and -9
expression at the protein level (22). However, to the best of our
knowledge, few studies have examined the proapoptotic activity of
GLP in PCa cells. Zaidman et al (2) reported that G. lucidum ethanol
extract, which primarily contains terpenoids, inhibits LNCaP cell
proliferation by activating caspase-3 and -8, thus inducing cell
apoptosis. In a previous study, it was demonstrated that GLP is
also able to induce death receptor-mediated apoptosis through the
induction of Fas-associated protein with death domain, tumor
necrosis factor receptor-associated factor 2 and caspase-8
expression in colorectal cancer xenograft tumors (22). It is well established that
apoptosis is composed of two pathways: The extrinsic pathway, which
is controlled by death receptors; and the intrinsic pathway, which
is mediated by the mitochondria (58). Whether GLP may regulate the two
apoptotic pathways in PCa cells requires further study.
NAG-1 is upregulated by several anticancer drugs,
including NSAIDs, in addition to other dietary compounds, including
resveratrol, epigallocatechin-3-gallate, genistein, conjugated
linoleic acid, diallyl disulfide, green tea catechins and capsaicin
(23,59). Previously, it was demonstrated that
NAG-1 expression is upregulated by GLP in colorectal cancer HCT116
cells and xenograft tumors at the mRNA and protein levels (22). However, whether GLP-induced
apoptosis of HCT116 cells is mediated through NAG-1 induction has
not been investigated. In the present study, using siRNA
transfection, the role of NAG-1 in GLP-induced growth inhibition in
PCa cells was determined. Notably, it was demonstrated that
knocking down NAG-1 expression by siRNA significantly inhibited
GLP-induced apoptosis of PC-3 cells. Furthermore, NAG-1 silencing
inhibited the suppressive effects of GLP on the protein expression
levels of pro-caspase 3, -6 and -9, and inhibited PARP cleavage.
These data suggested that NAG-1 may serve a pivotal role in
GLP-induced apoptosis of PC-3 cells. Consistent with the present
results, Shim and Eling (32)
reported that vitamin E succinate treatment induces growth arrest
and apoptosis, also mediated by NAG-1 induction, in PC-3 cells.
Conjugated linoleic acid (CLA) has been reported to induce
apoptosis and suppress cell proliferation in colorectal cancer
HCT116 cells, and also to induce NAG-1 expression (60). In a previous study, inhibition of
NAG-1 by siRNA was shown to result in the suppression of
CLA-induced apoptosis of HCT116 cells (60); however, Chiu et al (61) demonstrated that NAG-1 induction by
isochaihulactone causes isochaihulactone-induced cell cycle arrest,
but not apoptosis, in LNCaP cells. Wynne et al (62) reported that NAG-1 induction is
critical for NSAID-induced inhibition of cell migration in PCa
cells, which is mediated by the p38 MAPK signaling pathway. Taken
together, these findings suggest that NAG-1 induction by anticancer
agents may target numerous cellular processes other than
apoptosis.
Since the discovery of NAG-1, studies have examined
its role in cancer development and progression. However, the
results have been contradictory, with data from in vitro and
in vivo studies suggesting an anticarcinogenic effect of
NAG-1, whereas a protumorigenic or uncertain role of NAG-1 may be
inferred from the clinical evidence. In particular, efforts have
been made to establish the role of NAG-1 in PCa. Tan et al
(63) demonstrated that NAG-1
causes growth arrest of DU145 cells, which may be the earliest
report suggesting an anticancer effect of NAG-1 in PCa.
Furthermore, overexpres-sion of NAG-1 inhibits the growth of
PC-3-bearing xenograft tumors (64). Husaini et al (24) crossed NAG-1 transgenic mice with
transgenic adenocarcinoma of mouse prostate (TRAMP) mice, and
observed that overexpression of NAG-1 in TRAMP mice significantly
reduces tumor size and tumor grade compared within the TRAMP
control mice. However, NAG-1-overexpressing TRAMP mice develop more
distant organ metastases. In a parallel study, the same study group
reported that deletion of the NAG-1 gene in TRAMP mice
significantly promotes prostate tumor growth and increases the
mortality rate of TRAMP mice (65). Collectively, in vivo and
in vitro studies in general have suggested that NAG-1 exerts
anticarcinogenic activity in PCa.
Clinical evidence, however, does not support the
idea of an antitumorigenic role for NAG-1 in PCa. Previous studies
reported that the serum levels of NAG-1 are significantly increased
in patients with cancer, including PCa (66,67).
Furthermore, NAG-1 levels in plasma are positively associated with
PCa metastasis and tumor grade (68,69).
At present, the exact role of the increased secretion of NAG-1 in
the plasma of patients with PCa is unknown. The mechanism
underlying the paradoxical effects of NAG-1 in PCa from laboratory
and clinical evidence is unclear at present and remains to be
elucidated in future studies.
NAG-1 regulation at the transcriptional level has
been extensively investigated by our group and others. The promoter
region of NAG-1 has been well characterized, and a number of
cis-acting and trans-acting elements, including Sp1 and two p53
tumor suppressor protein binding sites, have been identified
(70). While certain studies have
suggested that NSAIDs and natural compounds induce NAG-1 expression
in a p53-dependent manner, others have reported that NAG-1 is
induced in a p53-independent manner (71,72).
In the present study, GLP induced NAG-1 in p53-null PC-3 cells,
thus suggesting a p53-independent mode of NAG-1 induction. EGR-1
has been demonstrated to act as a tumor suppressor gene, and its
loss may lead to the progression of colorectal cancer (73). Studies have demonstrated that
EGR-1exhibits its proapoptotic function by directly binding to the
NAG-1 promoter (28,31,74).
Furthermore, it has been demonstrated that EGR-1 activates NAG-1
through interactions with the GC-rich proximal region of the NAG-1
promoter (74,75). In the present study, the mRNA and
protein levels expression of EGR-1 and NAG-1 were both induced by
GLP in a time-and dose-dependent manner. Consistent with these
results, a number of NSAIDs and peroxisome proliferator-activated
receptor γ ligands induce NAG-1 expression at the transcriptional
level through the EGR-1 transcription factor (76,77).
It has previously been reported that capsaicin and damnacanthal
induce NAG-1 expression through the transcription factor
CCAAT-enhancer-binding protein β (78,79).
Taken together, the transcriptional regulation of NAG-1 may be
mediated by several mechanisms. Data from the present study
suggested that NAG-1 was induced in PC-3 cells by upregulating the
transcription factor EGR-1 in a p53-independent manner.
Signal transduction pathways serve an important role
in carcinogenesis, and also serve as potential targets of
chemopreventive agents. At present, the signaling pathways
underlying the effects of GLP on cancer prevention remain poorly
understood, although numerous studies have assessed the downstream
signaling pathways of GLP. In the present study, it was observed
that GLP inhibited the phosphorylation of MAPK/Erk and Akt in a
time- and dose-dependent manner. The present data suggested that
MAPK/Erk and Akt may serve as downstream signaling pathways of GLP
in PCa. Akt is a downstream target of phosphoinositide 3-kinase
(PI3K), and it has been well established that activation of the
PI3K/Akt pathway serves an anti-apoptotic role in carcinogenesis
(80). The MAPK cascade is a
critical pathway for cancer cell survival, and Erk activation has
been demonstrated to promote cancer cell proliferation (81). Yang et al (46) investigated the antitumor activity
of GLP in HL-60 acute myeloid leukemia cells, and observed that GLP
induces apoptosis of these cells. Furthermore, GLP blocks the
MAPK/Erk pathway, and activates the p38 and c-Jun N-terminal kinase
MAPK pathways (46). Together with
the present study, further studies are required to clarify the
signal transduction molecular targets of GLP, which may be
associated with its anticancer effect.
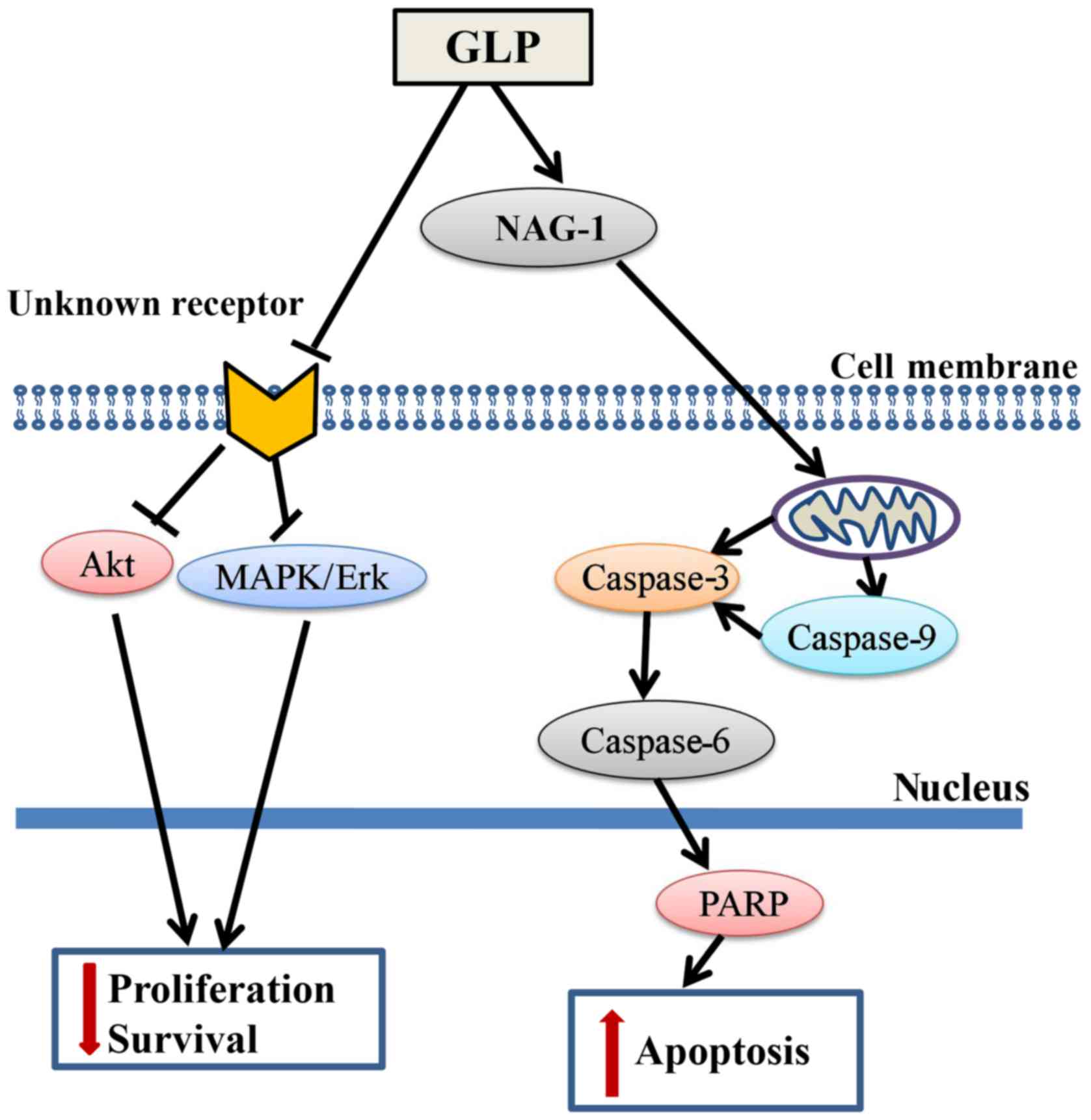
In conclusion, the present study demonstrated that
GLP significantly inhibited PCa cell proliferation via
transcriptional upregulation of NAG-1 and downregulation of the
MAPK/Erk and Akt signaling pathways (Fig. 7). The results of the present study
identified NAG-1 as a molecular target of GLP-induced apoptosis,
which may provide a novel approach for the study of GLP as an
anticancer agent against PCa and other cancer types. To the best of
our knowledge, this is the first study to demonstrate that GLP may
inhibit the proliferation of PCa cells with the involvement of
NAG-1 induction. However, the definitive role of NAG-1 in
GLP-induced apoptosis and/or other cellular processes in PCa
requires further examination in future studies.
Abbreviations:
|
GLP
|
Ganoderma lucidum
polysaccharide
|
|
NAG-1
|
nonsteroidal anti-inflammatory
drug-activated gene-1
|
|
EGR-1
|
early growth response-1
|
Acknowledgments
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81473397).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KW performed the majority of the study and wrote the
manuscript. KN conducted the flow cytometry assay. DC, YW and HP
helped with GLP extraction and purification. XW designed the
experiments and edited the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaidman BZ, Wasser SP, Nevo E and Mahajna
J: Androgen receptor-dependent and -independent mechanisms mediate
Ganoderma lucidum activities in LNCaP prostate cancer cells. Int J
Oncol. 31:959–967. 2007.PubMed/NCBI
|
|
3
|
Baillargeon J, Platz EA, Rose DP, Pollock
BH, Ankerst DP, Haffner S, Higgins B, Lokshin A, Troyer D,
Hernandez J, et al: Obesity, adipokines, and prostate cancer in a
prospective population-based study. Cancer Epidemiol Biomarkers
Prev. 15:1331–1335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hettel D and Sharifi N: HSD3B1 status as a
biomarker of androgen deprivation resistance and implications for
prostate cancer. Nat Rev Urol. 15:191–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View Article : Google Scholar
|
|
6
|
Mahajna J, Dotan N, Zaidman BZ, Petrova RD
and Wasser SP: Pharmacological values of medicinal mushrooms for
prostate cancer therapy: The case of Ganoderma lucidum. Nutr
Cancer. 61:16–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bigler D, Gulding KM, Dann R, Sheabar FZ,
Conaway MR and Theodorescu D: Gene profiling and promoter reporter
assays: Novel tools for comparing the biological effects of
botanical extracts on human prostate cancer cells and understanding
their mechanisms of action. Oncogene. 22:1261–1272. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Darzynkiewicz Z, Traganos F, Wu JM and
Chen S: Chinese herbal mixture PC SPES in treatment of prostate
cancer (Review). Int J Oncol. 17:729–736. 2000.PubMed/NCBI
|
|
9
|
Halicka H, Ardelt B, Juan G, Mittelman A,
Chen S, Traganos F and Darzynkiewicz Z: Apoptosis and cell cycle
effects induced by extracts of the Chinese herbal preparation PC
SPES. Int J Oncol. 11:437–448. 1997.PubMed/NCBI
|
|
10
|
Kubota T, Hisatake J, Hisatake Y, Said JW,
Chen SS, Holden S, Taguchi H and Koeffler HP: PC-SPES: A unique
inhibitor of proliferation of prostate cancer cells in vitro and in
vivo. Prostate. 42:163–171. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de la Taille A, Hayek OR, Burchardt M,
Burchardt T and Katz AE: Role of herbal compounds (PC-SPES) in
hormone-refractory prostate cancer: Two case reports. J Altern
Complement Med. 6:449–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de la Taille A, Hayek OR, Buttyan R,
Bagiella E, Burchardt M and Katz AE: Effects of a phytotherapeutic
agent, PC-SPES, on prostate cancer: A preliminary investigation on
human cell lines and patients. BJU Int. 84:845–850. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DiPaola RS, Zhang H, Lambert GH, Meeker R,
Licitra E, Rafi MM, Zhu BT, Spaulding H, Goodin S, Toledano MB, et
al: Clinical and biologic activity of an estrogenic herbal
combination (PC-SPES) in prostate cancer. N Engl J Med.
339:785–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pfeifer BL, Pirani JF, Hamann SR and
Klippel KF: PC-SPES, a dietary supplement for the treatment of
hormone-refractory prostate cancer. BJU Int. 85:481–485. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pirani JF: The effects of phytotherapeutic
agents on prostate cancer: An overview of recent clinical trials of
PC SPES. Urology. 58(Suppl 1): 36–38. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Small EJ, Frohlich MW, Bok R, Shinohara K,
Grossfeld G, Rozenblat Z, Kelly WK, Corry M and Reese DM:
Prospective trial of the herbal supplement PC-SPES in patients with
progressive prostate cancer. J Clin Oncol. 18:3595–3603. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaudhary UB, Rashid M and Keane TE:
PC-SPES withdrawal response. Acta Oncol. 43:772–773. 2004.
View Article : Google Scholar
|
|
18
|
Cordell GA: PC-SPES: A brief overview.
Integr Cancer Ther. 1:271–286. 2002. View Article : Google Scholar
|
|
19
|
Bishop KS, Kao CH, Xu Y, Glucina MP,
Paterson RR and Ferguson LR: From 2000years of Ganoderma lucidum to
recent developments in nutraceuticals. Phytochemistry. 114:56–65.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang J, Slivova V, Valachovicova T,
Harvey K and Sliva D: Ganoderma lucidum inhibits proliferation and
induces apoptosis in human prostate cancer cells PC-3. Int J Oncol.
24:1093–1099. 2004.PubMed/NCBI
|
|
21
|
Sliva D, Labarrere C, Slivova V, Sedlak M,
Lloyd FP Jr and Ho NW: Ganoderma lucidum suppresses motility of
highly invasive breast and prostate cancer cells. Biochem Biophys
Res Commun. 298:603–612. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Na K, Li K, Sang T, Wu K, Wang Y and Wang
X: Anticarcinogenic effects of water extract of sporoderm-broken
spores of Ganoderma lucidum on colorectal cancer in vitro and in
vivo. Int J Oncol. 50:1541–1554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Baek SJ and Eling TE: The diverse
roles of nonsteroidal anti-inflammatory drug activated gene
(NAG-1/GDF15) in cancer. Biochem Pharmacol. 85:597–606. 2013.
View Article : Google Scholar :
|
|
24
|
Husaini Y, Qiu MR, Lockwood GP, Luo XW,
Shang P, Kuffner T, Tsai VW, Jiang L, Russell PJ, Brown DA, et al:
Macrophage inhibitory cytokine-1 (MIC-1/GDF15) slows cancer
development but increases metastases in TRAMP prostate cancer prone
mice. PLoS One. 7:e438332012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Piyanuch R, Sukhthankar M, Wandee G and
Baek SJ: Berberine, a natural isoquinoline alkaloid, induces NAG-1
and ATF3 expression in human colorectal cancer cells. Cancer Lett.
258:230–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang SU, Shin YS, Hwang HS, Baek SJ, Lee
SH and Kim CH: Tolfenamic acid induces apoptosis and growth
inhibition in head and neck cancer: Involvement of NAG-1
expression. PLoS One. 7:e349882012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tesei A, Rosetti M, Ulivi P, Fabbri F,
Medri L, Vannini I, Bolla M, Amadori D and Zoli W: Study of
molecular mechanisms of pro-apoptotic activity of NCX 4040, a novel
nitric oxide-releasing aspirin, in colon cancer cell lines. J
Transl Med. 5:522007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woo SM, Min KJ, Kim S, Park JW, Kim DE,
Chun KS, Kim YH, Lee TJ, Kim SH, Choi YH, et al: Silibinin induces
apoptosis of HT29 colon carcinoma cells through early growth
response-1 (EGR-1)-mediated non-steroidal anti-inflammatory
drug-activated gene-1 (NAG-1) up-regulation. Chem Biol Interact.
211:36–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baek SJ, Kim KS, Nixon JB, Wilson LC and
Eling TE: Cyclooxygenase inhibitors regulate the expression of a
TGF-beta superfamily member that has proapoptotic and
antitumorigenic activities. Mol Pharmacol. 59:901–908. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Yoshioka H, Kamitani H, Watanabe T and
Eling TE: Nonsteroidal anti-inflammatory drug-activated gene
(NAG-1/GDF15) expression is increased by the histone deacetylase
inhibitor trichostatin A. J Biol Chem. 283:33129–33137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shim M and Eling TE: Vitamin E succinate
induces NAG-1 expression in a p38 kinase-dependent mechanism. Mol
Cancer Ther. 7:961–971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dillard PR, Lin MF and Khan SA:
Androgen-independent prostate cancer cells acquire the complete
steroidogenic potential of synthesizing testosterone from
cholesterol. Mol Cell Endocrinol. 295:115–120. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tai S, Sun Y, Squires JM, Zhang H, Oh WK,
Liang CZ and Huang J: PC3 is a cell line characteristic of
prostatic small cell carcinoma. Prostate. 71:1668–1679. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Auyeung KK, Cho CH and Ko JK: A novel
anticancer effect of Astragalus saponins: Transcriptional
activation of NSAID-activated gene. Int J Cancer. 125:1082–1091.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baek SJ, Kim JS, Jackson FR, Eling TE,
McEntee MF and Lee SH: Epicatechin gallate-induced expression of
NAG-1 is associated with growth inhibition and apoptosis in colon
cancer cells. Carcinogenesis. 25:2425–2432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jutooru I, Chadalapaka G, Chintharlapalli
S, Papineni S and Safe S: Induction of apoptosis and nonsteroidal
anti-inflammatory drug-activated gene 1 in pancreatic cancer cells
by a glycyrrhetinic acid derivative. Mol Carcinog. 48:692–702.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soto-Cerrato V, Viñals F, Lambert JR,
Kelly JA and Pérez-Tomás R: Prodigiosin induces the proapoptotic
gene NAG-1 via glycogen synthase kinase-3beta activity in human
breast cancer cells. Mol Cancer Ther. 6:362–369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li K, Na K, Sang T, Wu K, Wang Y and Wang
X: The ethanol extracts of sporoderm-broken spores of Ganoderma
lucidum inhibit colorectal cancer in vitro and in vivo. Oncol Rep.
38:2803–2813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kladar NV, Gavarić NS and Božin BN:
Ganoderma: Insights into anticancer effects. Eur J Cancer Prev.
25:462–471. 2016. View Article : Google Scholar
|
|
41
|
Hsieh TC and Wu JM: Suppression of
proliferation and oxidative stress by extracts of Ganoderma lucidum
in the ovarian cancer cell line OVCAR-3. Int J Mol Med.
28:1065–1069. 2011.PubMed/NCBI
|
|
42
|
Cao QZ and Lin ZB: Ganoderma lucidum
polysaccharides peptide inhibits the growth of vascular endothelial
cell and the induction of VEGF in human lung cancer cell. Life Sci.
78:1457–1463. 2006. View Article : Google Scholar
|
|
43
|
Gill BS, Navgeet and Kumar S: Ganoderma
lucidum targeting lung cancer signaling: A review. Tumour Biol.
39:1010428317707437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Loganathan J, Jiang J, Smith A, Jedinak A,
Thyagarajan-Sahu A, Sandusky GE, Nakshatri H and Sliva D: The
mushroom Ganoderma lucidum suppresses breast-to-lung cancer
metastasis through the inhibition of pro-invasive genes. Int J
Oncol. 44:2009–2015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li A, Shuai X, Jia Z, Li H, Liang X, Su D
and Guo W: Ganoderma lucidum polysaccharide extract inhibits
hepatocellular carcinoma growth by downregulating regulatory T
cells accumulation and function by inducing microRNA-125b. J Transl
Med. 13:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang G, Yang L, Zhuang Y, Qian X and Shen
Y: Ganoderma lucidum polysaccharide exerts anti-tumor activity via
MAPK pathways in HL-60 acute leukemia cells. J Recept Signal
Transduct Res. 36:6–13. 2016. View Article : Google Scholar
|
|
47
|
Zhao X, Zhou D, Liu Y, Li C, Zhao X, Li Y
and Li W: Ganoderma lucidum polysaccharide inhibits prostate cancer
cell migration via the protein arginine methyltransferase 6
signaling pathway. Mol Med Rep. 17:147–157. 2018.
|
|
48
|
Hsieh T, Chen SS, Wang X and Wu JM:
Regulation of androgen receptor (AR) and prostate specific antigen
(PSA) expression in the androgen-responsive human prostate LNCaP
cells by ethanolic extracts of the Chinese herbal preparation,
PC-SPES. Biochem Mol Biol Int. 42:535–544. 1997.PubMed/NCBI
|
|
49
|
Geliebter J, Tiwari R and Wu JM: PC-SPES
in prostate cancer. N Engl J Med. 340:567–568. 1999.PubMed/NCBI
|
|
50
|
Liu J, Tamura S, Kurashiki K, Shimizu K,
Noda K, Konishi F, Kumamoto S and Kondo R: Anti-androgen effects of
extracts and compounds from Ganoderma lucidum. Chem Biodivers.
6:231–243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ferreira IC, Heleno SA, Reis FS, Stojkovic
D, Queiroz MJ, Vasconcelos MH and Sokovic M: Chemical features of
Ganoderma polysaccharides with antioxidant, antitumor and
antimicrobial activities. Phytochemistry. 114:38–55. 2015.
View Article : Google Scholar
|
|
52
|
Guo L, Xie J, Ruan Y, Zhou L, Zhu H, Yun
X, Jiang Y, Lü L, Chen K, Min Z, et al: Characterization and
immunostimulatory activity of a polysaccharide from the spores of
Ganoderma lucidum. Int Immunopharmacol. 9:1175–1182. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 7:72015. View Article : Google Scholar
|
|
56
|
Shang D, Li Y, Wang C, Wang X, Yu Z and Fu
X: A novel polysaccharide from Se-enriched Ganoderma lucidum
induces apoptosis of human breast cancer cells. Oncol Rep.
25:267–272. 2011.
|
|
57
|
Liang Z, Yi Y, Guo Y, Wang R, Hu Q and
Xiong X: Chemical characterization and antitumor activities of
polysaccharide extracted from Ganoderma lucidum. Int J Mol Sci.
15:9103–9116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dasgupta A, Nomura M, Shuck R and Yustein
J: Cancer's Achilles' Heel: Apoptosis and necroptosis to the
rescue. Int J Mol Sci. 18:182016. View Article : Google Scholar
|
|
59
|
Liggett JL, Zhang X, Eling TE and Baek SJ:
Anti-tumor activity of non-steroidal anti-inflammatory drugs:
Cyclooxygenase-independent targets. Cancer Lett. 346:217–224. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lee SH, Yamaguchi K, Kim JS, Eling TE,
Safe S, Park Y and Baek SJ: Conjugated linoleic acid stimulates an
anti-tumorigenic protein NAG-1 in an isomer specific manner.
Carcinogenesis. 27:972–981. 2006. View Article : Google Scholar
|
|
61
|
Chiu SC, Wang MJ, Yang HH, Chen SP, Huang
SY, Chen YL, Lin SZ, Harn HJ and Pang CY: Activation of NAG-1 via
JNK signaling revealed an isochaihulactone-triggered cell death in
human LNCaP prostate cancer cells. BMC Cancer. 11:1462011.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wynne S and Djakiew D: NSAID inhibition of
prostate cancer cell migration is mediated by Nag-1 Induction via
the p38 MAPK-p75(NTR) pathway. Mol Cancer Res. 8:1656–1664. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Tan M, Wang Y, Guan K and Sun Y:
PTGF-beta, a type beta transforming growth factor (TGF-beta)
superfamily member, is a p53 target gene that inhibits tumor cell
growth via TGF-beta signaling pathway. Proc Natl Acad Sci USA.
97:109–114. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lambert JR, Kelly JA, Shim M, Huffer WE,
Nordeen SK, Baek SJ, Eling TE and Lucia MS: Prostate derived factor
in human prostate cancer cells: Gene induction by vitamin D via a
p53-dependent mechanism and inhibition of prostate cancer cell
growth. J Cell Physiol. 208:566–574. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Husaini Y, Lockwood GP, Nguyen TV, Tsai
VW, Mohammad MG, Russell PJ, Brown DA and Breit SN: Macrophage
inhibitory cytokine-1 (MIC-1/GDF15) gene deletion promotes cancer
growth in TRAMP prostate cancer prone mice. PLoS One.
10:e01151892015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Brown DA, Ward RL, Buckhaults P, Liu T,
Romans KE, Hawkins NJ, Bauskin AR, Kinzler KW, Vogelstein B and
Breit SN: MIC-1 serum level and genotype: Associations with
progress and prognosis of colorectal carcinoma. Clin Cancer Res.
9:2642–2650. 2003.PubMed/NCBI
|
|
67
|
Welsh JB, Sapinoso LM, Kern SG, Brown DA,
Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, et
al: Large-scale delineation of secreted protein biomarkers
overexpressed in cancer tissue and serum. Proc Natl Acad Sci USA.
100:3410–3415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Selander KS, Brown DA, Sequeiros GB,
Hunter M, Desmond R, Parpala T, Risteli J, Breit SN and
Jukkola-Vuorinen A: Serum macrophage inhibitory cytokine-1
concentrations correlate with the presence of prostate cancer bone
metastases. Cancer Epidemiol Biomarkers Prev. 16:532–537. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Senapati S, Rachagani S, Chaudhary K,
Johansson SL, Singh RK and Batra SK: Overexpression of macrophage
inhibitory cytokine-1 induces metastasis of human prostate cancer
cells through the FAK-RhoA signaling pathway. Oncogene.
29:1293–1302. 2010. View Article : Google Scholar
|
|
70
|
Baek SJ, Horowitz JM and Eling TE:
Molecular cloning and characterization of human nonsteroidal
anti-inflammatory drug-activated gene promoter. Basal transcription
is mediated by Sp1 and Sp3. J Biol Chem. 276:33384–33392. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kang SU, Lee BS, Lee SH, Baek SJ, Shin YS
and Kim CH: Expression of NSAID-activated gene-1 by EGCG in head
and neck cancer: Involvement of ATM-dependent p53 expression. J
Nutr Biochem. 24:986–999. 2013. View Article : Google Scholar
|
|
72
|
Zhong Y, Krisanapun C, Lee SH, Nualsanit
T, Sams C, Peungvicha P and Baek SJ: Molecular targets of apigenin
in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur
J Cancer. 46:3365–3374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Krones-Herzig A, Mittal S, Yule K, Liang
H, English C, Urcis R, Soni T, Adamson ED and Mercola D: Early
growth response 1 acts as a tumor suppressor in vivo and in vitro
via regulation of p53. Cancer Res. 65:5133–5143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Baek SJ, Kim JS, Moore SM, Lee SH,
Martinez J and Eling TE: Cyclooxygenase inhibitors induce the
expression of the tumor suppressor gene EGR-1, which results in the
up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol.
67:356–364. 2005. View Article : Google Scholar
|
|
75
|
Lim JH, Park JW, Min DS, Chang JS, Lee YH,
Park YB, Choi KS and Kwon TK: NAG-1 up-regulation mediated by EGR-1
and p53 is critical for quercetin-induced apoptosis in HCT116 colon
carcinoma cells. Apoptosis. 12:411–421. 2007. View Article : Google Scholar
|
|
76
|
Baek SJ, Wilson LC, Hsi LC and Eling TE:
Troglitazone, a peroxisome proliferator-activated receptor gamma
(PPAR gamma) ligand, selectively induces the early growth
response-1 gene independently of PPAR gamma. A novel mechanism for
its anti-tumorigenic activity. J Biol Chem. 278:5845–5853. 2003.
View Article : Google Scholar
|
|
77
|
Chintharlapalli S, Papineni S, Baek SJ,
Liu S and Safe S:
1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome
proliferator-activated receptor gamma agonists but decrease HCT-116
colon cancer cell survival through receptor-independent activation
of early growth response-1 and nonsteroidal anti-inflammatory
drug-activated gene-1. Mol Pharmacol. 68:1782–1792. 2005.PubMed/NCBI
|
|
78
|
Lee SH, Krisanapun C and Baek SJ:
NSAID-activated gene-1 as a molecular target for capsaicin-induced
apoptosis through a novel molecular mechanism involving GSK3beta,
C/EBPbeta and ATF3. Carcinogenesis. 31:719–728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Nualsanit T, Rojanapanthu P, Gritsanapan
W, Lee SH, Lawson D and Baek SJ: Damnacanthal, a noni component,
exhibits anti-tumorigenic activity in human colorectal cancer
cells. J Nutr Biochem. 23:915–923. 2012. View Article : Google Scholar
|
|
80
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
81
|
Burotto M, Chiou VL, Lee JM and Kohn EC:
The MAPK pathway across different malignancies: A new perspective.
Cancer. 120:3446–3456. 2014. View Article : Google Scholar : PubMed/NCBI
|