Introduction
Malignant pleural mesothelioma (MPM) is a type of
rare, aggressive and chemoradiation-resistant cancer, which is most
commonly caused by asbestos exposure (1-3).
Malignant mesothelioma arises from the mesothelial cells of serous
membranes, including the pleura, peritoneum and pericardium. The
5-year survival rate of MPM is ~10% (4).
The majority of chemotherapeutic agents exhibit
restricted efficacy in MPM treatment. Cisplatin plus pemetrexed is
the most commonly used first-line regimen for MPM chemotherapy,
which is approved by the US Food and Drug Administration. In
patients with MPM, chemotherapy failure is often attributed to
resistance to cytotoxic drug-induced apoptosis; therefore, the
discovery of novel drugs that have proapoptotic effects on MPM
cells or that target sensitive signaling pathways may result in
more effective strategies to improve the outcome of MPM (5,6).
Curcumin is an active ingredient of turmeric and
curry powder, which has been reported to possess anti-inflammatory,
antiviral, antibacterial, antifungal and anti-oxidant properties.
In addition, numerous studies have revealed that curcumin exerts
marked antitumor effects in various types of cancer (7,8).
Furthermore, curcumin, in combination with chemotherapy, has been
investigated in clinical trials for the treatment of patients with
prostate cancer or breast cancer. Although curcumin induces MPM
cell death through pathways associated with pyroptosis (9), autophagy (10) and apoptosis (11,12),
how curcumin induces apoptosis in MPM remains to be elucidated. In
addition, whether curcumin induces angiogenesis in vivo has
yet to be studied.
The present study aimed to identify the antitumor
effects of curcumin, as well as its possible targets and affected
signaling pathways in RN5 murine mesothelioma cells. The results
demonstrated that curcumin significantly inhibited RN5 cell
viability. The antitumor activity of curcumin was also confirmed in
RN5 mouse models, thus indicating that it was highly effective in
inhibiting tumor growth. The molecular basis of these antitumor
effects was also investigated.
Materials and methods
Reagents
Curcumin (cat. no. S1848; Selleck Chemicals,
Houston, TX, USA) was dissolved in dimethyl sulfoxide (DMSO) and
stored at 4°C as a stock solution. Cisplatin (cat. no. S1166;
Selleck Chemicals) was dissolved in normal saline and stored at
room temperature. RPMI-1640 medium, fetal bovine serum (FBS),
penicillin, streptomycin and all other cell culture reagents were
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Primary antibodies against cleaved-poly (ADP-ribose) polymerase
(PARP) (cat. no. 9548), caspase-3 (cat. no. 9665), cleaved
caspase-3 (cat. no. 9664), caspase-8 (cat. no. 4927), cleaved
caspase-8 (cat. no. 8592), caspase-9 (cat. no. 9504), PI3K (cat.
no. 4257), AKT serine/threonine kinase (Akt) (cat. no. 4691),
phosphorylated (p)-Akt (Ser473) (cat. no. 9271), mammalian target
of rapamycin (mTOR) (cat. no. 2972), p-mTOR (Ser2448) (cat. no.
5536), p70 ribosomal protein S6 kinase (p70S6K) (cat. no. 2708),
p-p70S6K (Thr389) (cat. no. 9234) and Histone H3 (cat. no. 4499)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Primary antibodies against β-actin (cat. no. YT0099) and
GAPDH (cat. no. YT5052) were purchased from ImmunoWay Biotechnology
Company (Plano, TX, USA). Primary antibodies against
apoptosis-inducing factor (AIF) (cat. no. ab1998), B-cell lymphoma
(Bcl)-extra large (xL) (cat. no. ab32370), Bcl-2-associated X
protein (Bax) (cat. no. ab32503), cluster of differentiation (CD)31
(cat. no. ab28364) and Ki67 (cat. no. ab15580) were purchased from
Abcam (Cambridge, MA, USA). Horseradish peroxidase (HRP)-conjugated
secondary antibodies [Anti-rabbit immunoglobulin G (IgG),
HRP-linked Antibody #7074 or Anti-mouse IgG, HRP-linked Antibody
#7076] were purchased from Cell Signaling Technology, Inc.
Polyvinylidene difluoride (PVDF) membranes and western blot
luminescence reagents were purchased from EMD Millipore (Billerica,
MA, USA). Cell cycle assay kit and cell apoptosis kit were
purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China).
Alexa Fluor-555-conjugated goat anti-rabbit IgG antibody (cat. no.
A27039), and the nuclear and cytoplasmic extraction kit were
purchased from Thermo Fisher Scientific, Inc.
Cell culture
The RN5 murine malignant mesothelioma cell line
(13), which was kindly gifted by
Dr Marc de Perrot's Laboratory (Toronto General Hospital, Toronto,
ON, Canada), was maintained in RPMI-1640 medium supplemented with
10% FBS and 100 µg/ml penicillin-streptomycin. The cells
were maintained at 37°C in the presence of 5% CO2.
Cytotoxicity assay
The inhibitory effects of curcumin were determined
using the sulforhodamine B (SRB) assay. Briefly, RN5 cells were
seeded at 4×103 cells/well in 96-well flat-bottom plates
for 24 h at 37°C. The cells were then treated with curcumin or
cisplatin at various concentrations for 72 h at 37°C in the
presence of 5% CO2. The optical density (OD) was
measured at 510 nm using a microplate reader. The percentage of
cells that were killed was calculated using the following formula:
100% - ODsample/ODcontrol. Half maximal effective concentration
(EC50) values were calculated using Sigmaplot 12.0
software (Systat Software Inc., San Jose, CA, USA).
Protein extraction and western blot
analysis
Cells were seeded in a 10-cm dish (106
cells/dish) and were incubated at 37°C for 24 h. Cells were then
treated with vehicle control (DMSO 0.1%), various doses of curcumin
(15, 20 and 25 µM) or cisplatin (30 µM) for 24 h,
after which, cells were lysed with extraction buffer (cat. no.
FNN0011; Thermo Fisher Scientific, Inc.) supplemented with protease
inhibitor (cat. no. P8340; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), phosphatase inhibitor (cat. no. 4906845001; Roche
Diagnostics, Basel, Switzerland) and 1 mM phenylmethylsulfonyl
fluoride. Protein concentration was determined using the Pierce™
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). Equal volumes of protein (15-40 µg) were separated by
10% SDS-PAGE and were electrophoretically transferred onto PVDF
membranes. The membranes were blocked with 5% non-fat milk in
Tris-buffered saline (TBS) with 0.1% Tween-20 and were agitated for
1 h at room temperature. Subsequently, the membranes were incubated
overnight at 4°C with specific primary antibodies (1:1,000, in 5%
non-fat dry milk or bovine serum albumin (cat. no. B2064;
Sigma-Aldrich; Merck KGaA) in TBS with 0.1% Tween-20). The
membranes were washed three times with TBS containing 0.1% Tween-20
solution and were then incubated with the secondary antibodies
(1:5,000, in 5% non-fat milk in TBS with 0.1% Tween-20). After
secondary antibody incubation, the membranes were washed three
times with TBS with 0.1% Tween-20 and the proteins were detected
using the Luminata Forte Western HRP substrate (EMD Millipore),
according to the manufacturer's protocol. Band intensity was
analyzed using ImageJ 1.51 software (National Institutes of Health,
Bethesda, MD, USA), according to previous studies (14,15).
Cell cycle analysis
RN5 cells were seeded in a 6-well plate at a cell
density of 2×105 cells/well. The cells were treated with
various concentrations of curcumin for 24 h, whereas cisplatin (30
µM) was used to treat cells as a positive control. After
treatment, cells were harvested, washed twice with PBS and fixed in
70% ethanol at −20°C overnight. After washing, the cells were
suspended in PBS containing 50 µg/ml propidium iodide (PI)
and were incubated at 4°C for at least 4 h. Cell cycle analysis was
performed using flow cytometry. Fluorescence was measured using the
BD FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA) and cell cycle progression was estimated using Modfit LT 4.1
Software (Verity Software House, Inc., Topsham, ME, USA).
Cell apoptosis assay
RN5 cells were seeded in a 6-well plate at a cell
density of 2×105 cells/well. The cells were treated with
various concentrations of curcumin for 24 h, whereas cisplatin (30
µM) was used to treat cells as a positive control. The cells
were then collected and washed twice with cold PBS. Annexin V-PI
(AV-PI) double staining was performed according to the
manufacturer's protocol. Apoptosis was analyzed using the BD
FACSCanto II flow cytometer (BD Biosciences) and FlowJo version
10.0.7 (FlowJo LLC, Ashland, OR, USA).
In vivo tumor models
To evaluate the in vivo effects of curcumin,
the effects of curcumin on tumor growth were investigated in
tumor-bearing mice. The animal study was approved by the Committee
on the Ethics on Animal Experimentation of The Second Hospital of
Shandong University [no. KYLL-2017(LW) 011, approved February 3,
2017; Jinan, China]. A total of 20 male C57BL/6J mice (weight,
20-25 g; age, 6 weeks) were purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China) and were
housed under pathogen-free conditions. The mice were maintained in
a temperature-controlled room (22-24°C) with a relative humidity
between 55 and 65% under a 12-h light/dark cycle. Food and water
were freely available. RN5 cells (5×106 cells in 100
µl PBS) were injected subcutaneously into the dorsal flank
of each mouse. When the tumors reached volumes of 60
mm3, the animals were randomly assigned into four
groups. The mice were treated five times every 5 days with
intraperitoneal administration of i) 200 µl solvent
(PBS/cremophor/DMSO/ethanol, 15:3:1:1); ii) curcumin at a low dose
(200 mg/kg); or iii) curcumin at a high dose (500 mg/kg). As a
positive control, another group of mice was treated with cisplatin
at a dose of 5 mg/kg. Cisplatin was dissolved in normal saline at a
final concentration of 1 mg/ml. Body weight was measured every day
in order to monitor the toxicity of the treatments. Tumor volumes
were determined using the following formula: a × b2/2,
where 'a' is the longest diameter and 'b' is the shortest diameter.
The mice were euthanized by CO2 inhalation when the
tumor in the solvent-treated group grew to 15 mm in diameter. The
tumor samples were then collected and were fixed in 10% formalin
neutral buffer solution for at least 24 h at room temperature.
Sections were then paraffin-embedded.
Preparation of nuclear and cytoplasmic
extracts for western blot analysis
RN5 cells were seeded into a 10-cm Petri dish
(106 cells/dish) and were incubated at 37°C for 24 h.
Cells were then treated with vehicle control (DMSO 0.1%), three
doses of curcumin (15, 20 and 25 µM) for 24 h. After
treatment, cells were trypsinized, and nuclear and cytoplasmic
fractions were isolated according to the manufacturer's protocol,
using NE-PER® Nuclear and Cytoplasmic Extraction
reagents (Thermo Fisher Scientific, Inc.). After isolation of
nuclear and cytoplasmic fractions, western blotting was performed
using antibodies specific for the nuclear fraction of AIF and
Histone H3.
Immunohistochemistry and histological
analysis
Paraffin- embedded sections (5 µm) of tumor
tissues were mounted on gelatin-coated histological slides,
deparaffinized with xylene, and rehydrated in a descending series
of alcohol (100-70%) and distilled water at room temperature for 5
min. Antigen retrieval was performed by microwaving the slides in
10 mM citrate buffer (pH 6.0) for 20 min. Endogenous peroxidase
activity was quenched by incubation with 3%
H2O2 for 20 min, after which, the sections
were blocked with 1.5% goat serum (Beyotime Institute of
Biotechnology, Shanghai, China) for 60 min at room temperature to
prevent non-specific binding. The sections were then incubated
overnight at 4°C with primary antibodies against CD31 and Ki67
(1:100). The slides were washed three times with PBS containing
0.1% Tween-20 (5 min/wash) and were incubated with a secondary
antibody (1:10,000) [Goat anti-Rabbit IgG H&L (HRP), cat. no.
ab205718; Abcam] for 1 h at room temperature. Immunohistochemical
staining for CD31 and Ki67 was further performed using the
avidin-biotin peroxidase complex method, with diaminobenzidine
(DAB) as a chromogenic substrate. Immunostained sections were
counterstained with hematoxylin staining solution (Beyotime
Institute of Biotechnology) for 40 sec at room temperature, and
were then examined by light microscopy.
To semi-quantitatively analyze tumor cell
proliferation and apoptosis in tumor sections, the H-score was used
to evaluate the expression of Ki67-positive cells, as previously
reported (16,17). The H-score was calculated using the
following formula: (0 × percentage of negative staining) + (1 ×
percentage of weak staining) + (2 × percentage of moderate
staining) + (3 × percentage of strong staining). Staining intensity
was classified as follows: Negative staining (no staining), weak
staining (light yellow), moderate staining (yellowish brown) and
strong staining (tan). The values ranged between 0 and 300.
CD31-positive area was used to analyze angiogenesis.
TUNEL staining
For TUNEL staining of tumor tissue samples,
proteolytic digestion was conducted by incubating the sections for
10 min at room temperature with proteinase K (10 µg/ml),
after which, they were fixed in 4% paraformaldehyde solution for 5
min at room temperature. After rinsing with PBS, the samples were
permeabilized in equilibration buffer for 5 min. Samples were then
incubated with recombinant terminal deoxynucleotidyl transferase
reaction mix for 60 min at 37°C, washed in saline sodium citrate
solution for 15 min, and then quenched with 0.3%
H2O2 in PBS for 5 min. After washing with
PBS, the samples were incubated with HRP-labeled streptavidin for
30 min at room temperature and DAB was applied as a chromogenic
substrate. The sections were then dehydrated through a graded
series of alcohol, immersed in xylene and mounted with coverslips.
The sections were examined under an Olympus BX43 light microscope
(Olympus Corporation, Tokyo, Japan). The H-score was used to
evaluate the expression of TUNEL-positive cells, as
aforementioned.
Immunofluorescence
RN5 cells were seeded onto glass coverslips at
2×105 cells in a 6-well plate overnight at 37°C in the
presence of 5% CO2. Subsequently, cells were treated
with 15, 20 or 25 µM curcumin at 37°C for 24 h, after which,
cells were rinsed with cold PBS and fixed with 4% paraformaldehyde
for 30 min at room temperature. Fixed cells were washed with PBS
and were permeabilized with 1% Triton X-100 in PBS for 10 min.
Cells were then blocked with goat serum for 30 min at room
temperature and were incubated with AIF primary antibodies (1:500)
overnight at 4°C. After washing with PBS three times (5 min/wash),
fixed cells were incubated with Alexa Fluor-555-conjugated goat
anti-rabbit IgG antibody at room temperature for 1 h. After washing
three times with 0.1% Tween-20 in PBS, the coverslips were mounted
with mounting medium containing DAPI (Abcam). Cells were examined
under an Olympus BX43 fluorescence microscope (Olympus
Corporation).
Statistical analysis
The data are presented as the means ± standard
deviation. All of the statistical analyses were performed using
Sigmaplot 12.0. Statistical comparisons between the control and
treatment groups, as well as differences within the experimental
groups at different doses, were conducted using one-way analysis of
variance followed by least-significant difference or Tukey's tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Curcumin inhibits cell viability and
induces G2/M phase arrest
To investigate the inhibitory effects of curcumin on
RN5 cell viability, RN5 cells were treated with increasing
concentrations of curcumin. Cisplatin was used as a positive
control. Cell viability was determined following treatment with
increasing concentrations of curcumin for 72 h (Fig. 1A). The results demonstrated that
curcumin induced a significant inhibition in RN5 cell viability in
a dose-dependent manner, as measured by the SRB assay at 72 h,
relative to control cells (Fig.
1A). The EC50 value of curcumin at 72 h was 19.1±1.3
µM, whereas the EC50 value of cisplatin was
7.09±1.14 µM (Fig. 1B).
These findings indicated that curcumin may inhibit MPM cell
viability in a dose-dependent manner. In addition, 5 µM
cisplatin killed ~50% of RN5 cells after 72 h of treatment, whereas
20 µM curcumin provoked similar effects, thus suggesting
that cisplatin is more cytotoxic to RN5 cells than curcumin.
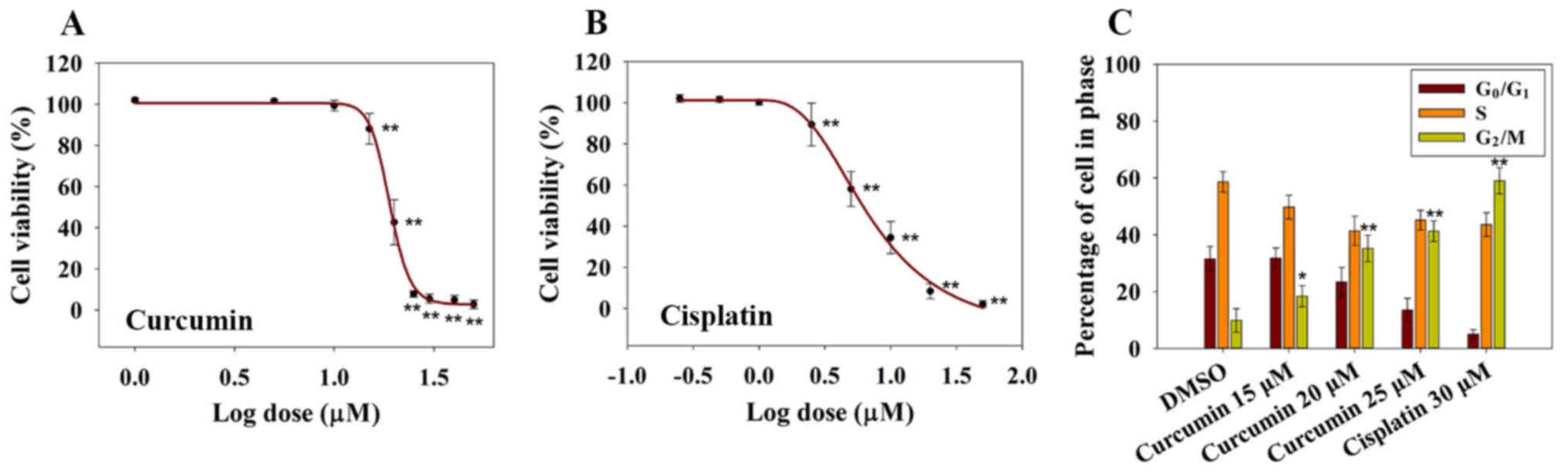 | Figure 1Cytotoxic and cell cycle-altering
effects of curcumin on RN5 cells. (A) Effects of curcumin on the
viability of RN5 cells. RN5 cells were treated with curcumin at
concentrations of 1, 5, 10, 15, 20, 25, 30, 40 or 50 µM for
72 h. Data are presented as the means ± standard deviation from
three independent experiments. **P<0.01 vs. the 1
µM (log value, 0) curcumin group. (B) Effects of cisplatin
on the viability of RN5 cells. RN5 cells were treated with
cisplatin at concentrations of 0.25, 0.5, 1, 2.5, 5, 10, 20 or 50
µM for 72 h. Data are presented as the means ± standard
deviation from three independent experiments.
**P<0.01 vs. the 0.25 µM (log value, −0.602)
cisplatin group. (C) Cell cycle analysis of RN5 cells treated with
curcumin (15, 20 and 25 µM) and cisplatin (30 µM) for
24 h. Data are presented as the means ± standard deviation from
three independent experiments. *P<0.05 and
**P<0.01 vs. the DMSO group. DMSO, dimethyl
sulfoxide. |
Previous studies have reported that curcumin induces
G1/S or G2/M cell cycle arrest in numerous
types of human cancer cells (18,19).
In order to investigate whether curcumin induced a cell cycle
arrest in RN5 cells, cell cycle distribution was examined following
treatment with curcumin for 24 h. As shown in Fig. 1C, <10% of RN5 cells were in
G2/M phase in the DMSO groups. Conversely, 18, 35 and
41% of cells were in G2/M phase in the 15, 20 and 25
µM curcumin groups after 24 h, respectively. These findings
indicated that 25 µM curcumin resulted in an obvious
G2/M cell phase arrest. Curcumin may inhibit cell
viability in a dose-dependent manner and may induce G2/M
cell phase arrest.
AIF mediates curcumin-induced apoptotic
cell death
It is well accepted that cell cycle arrest at
G2/M phase leads to apoptotic cell death. The present
study assessed curcumin-induced RN5 cell apoptosis using flow
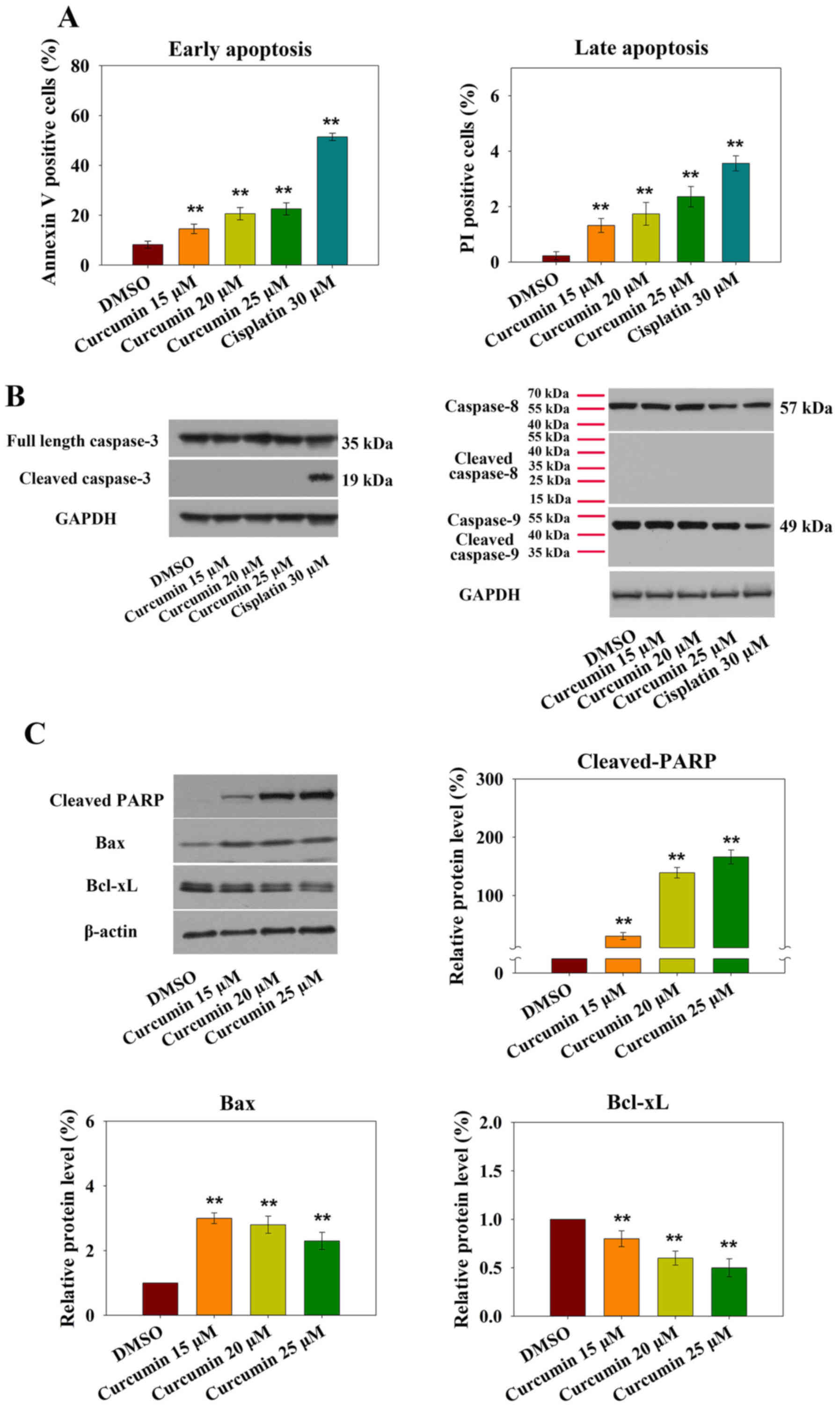
cytometry. AV-PI staining indicated that treatment with 15
µM curcumin markedly increased early and late apoptosis.
Treatment of RN5 cells with 15, 20 and 25 µM curcumin for 24
h induced an increase in the percentage of AV- and PI-positive
cells (Fig. 2A).
Apoptosis is induced via two main routes, involving
either the caspase-dependent or caspase-independent pathway. It has
previously been demonstrated that mitochondria or activation of the
death receptor pathway converge to induce the activation of
caspases, which are the final executioners of cell death (20). However, treatment of RN5 cells with
increasing doses of curcumin did not affect the expression levels
of activated caspase-3, -8 or -9 after 24 h (Fig. 2B).
Increased levels of the proapoptotic protein Bax and
decreased levels of the anti-apoptotic protein Bcl-xL were observed
in response to curcumin (Fig. 2C).
Furthermore, cleaved-PARP expression was also increased (Fig. 2C). These results demonstrated that
curcumin-induced apoptosis may be mediated by the mitochondrial
pathway.
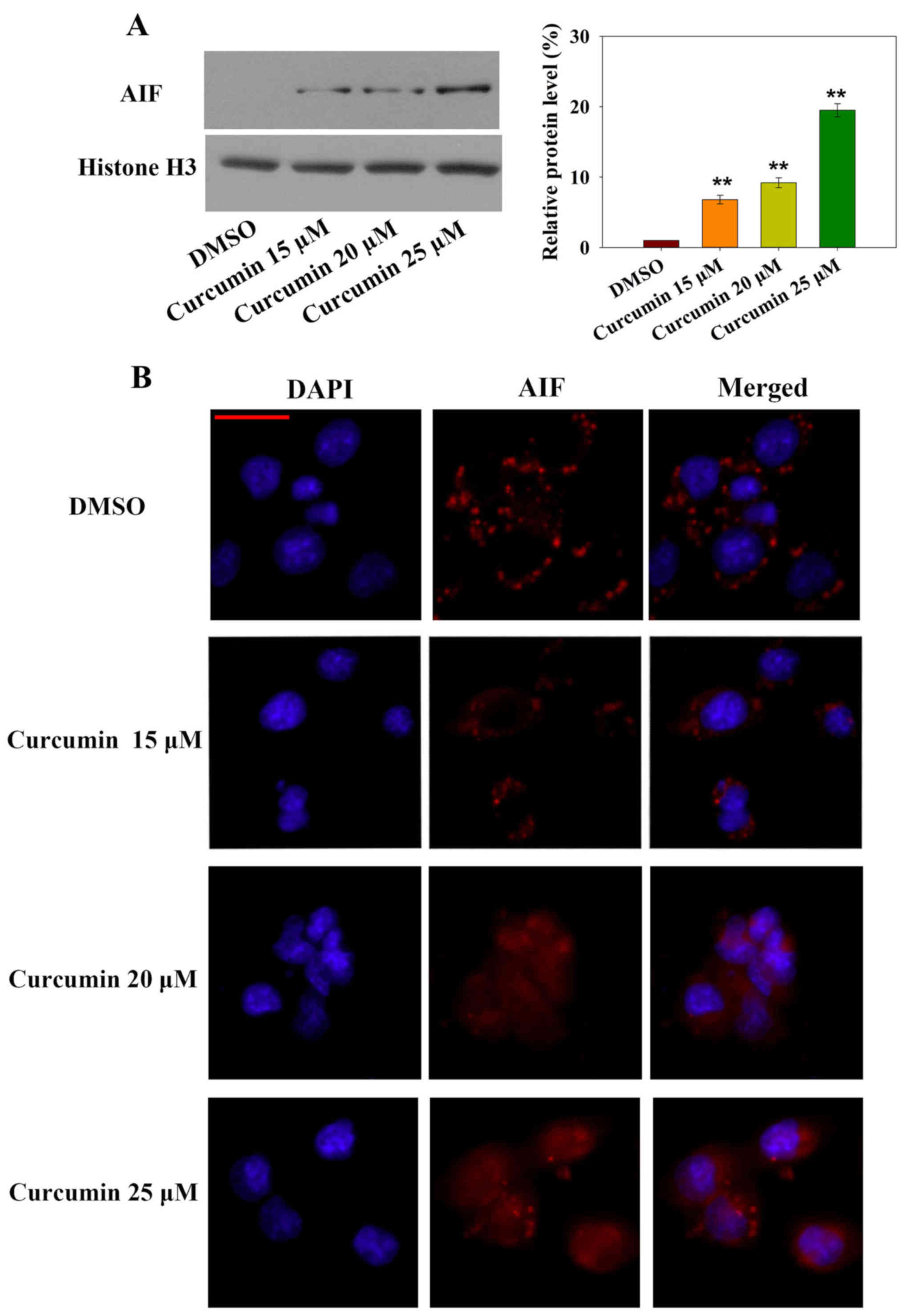
Notably, caspase-independent forms of apoptosis are
mediated by AIF. To explore whether AIF nuclear translocation was
involved in curcumin-induced apoptosis, subcellular AIF protein
expression was measured by western blotting and AIF nuclear
translocation was detected by immunofluorescence (Fig. 3A and B). Nuclear AIF expression was
significantly increased after 24 h curcumin treatment in a
dose-dependent manner. In addition, AIF-positive immunofluorescence
was increased in the nucleus in a dose-dependent manner. These
results indicated that the caspase-independent AIF nuclear
translocation-mediated apoptotic pathway may be involved in
curcumin-induced cell death.
Curcumin induces apoptosis through
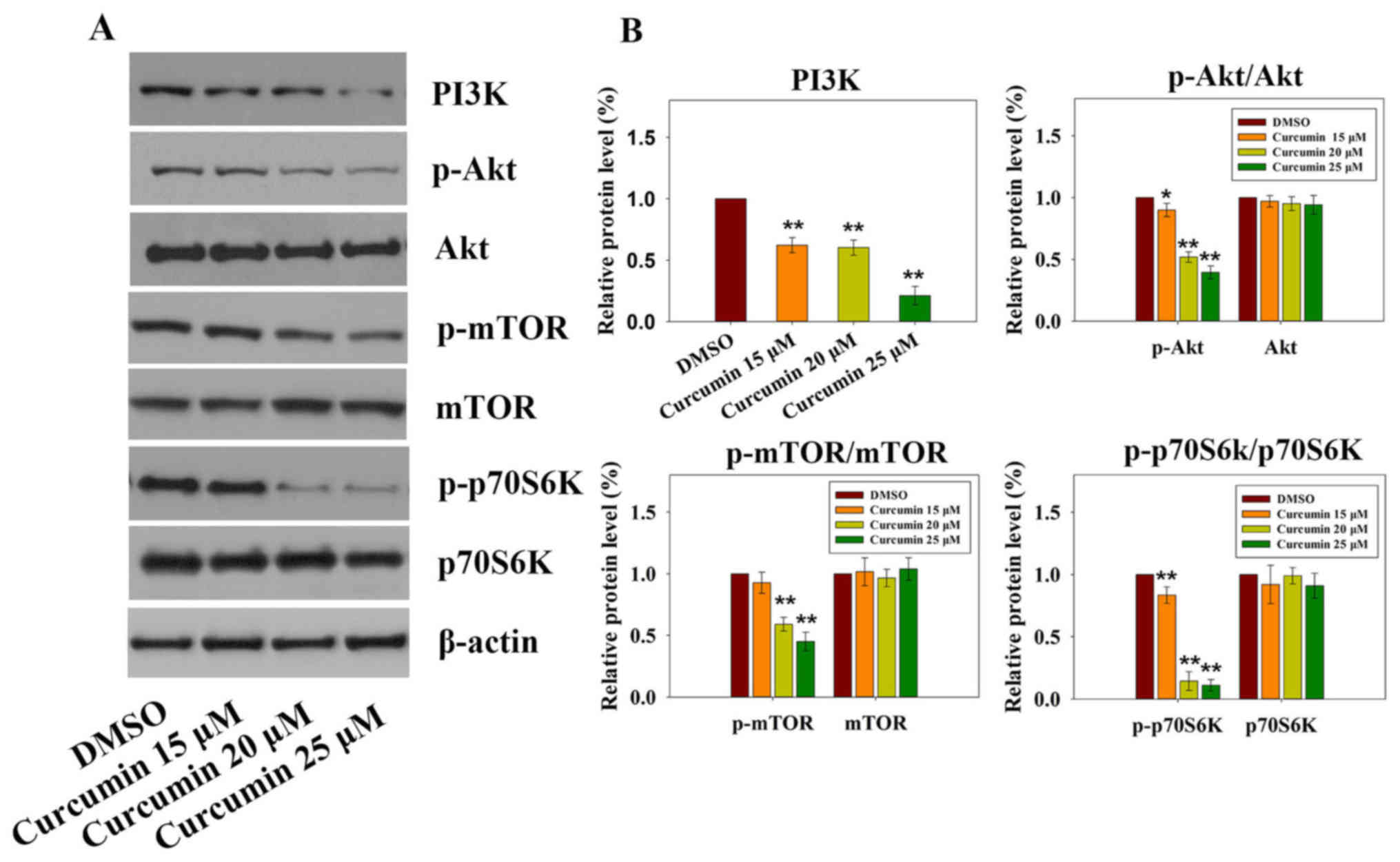
suppression of the PI3K-Akt-mTOR signaling pathway
The PI3K-Akt-mTOR signaling pathway has a critical
role in various cellular processes (21), including survival, proliferation,
apoptosis and angiogenesis. Previous studies on cancer have
revealed that curcumin inhibits the PI3K-Akt-mTOR signaling pathway
(22,23). Targeting the PI3K-Akt-mTOR
signaling pathway, which is downstream of several activated
receptor tyrosine kinases (RTKs) in mesothelioma, seems to be an
emerging therapeutic strategy (24). As shown in Fig. 4A and B, treatment with increasing
doses of curcumin resulted in significant downregulation of PI3K,
p-Akt, p-mTOR and p-p70S6K; however, no significant differences
were detected with regards to the total expression levels of Akt,
mTOR and p70S6K. These results suggested that the PI3K-Akt-mTOR
signaling pathway may be involved in curcumin-induced
apoptosis.
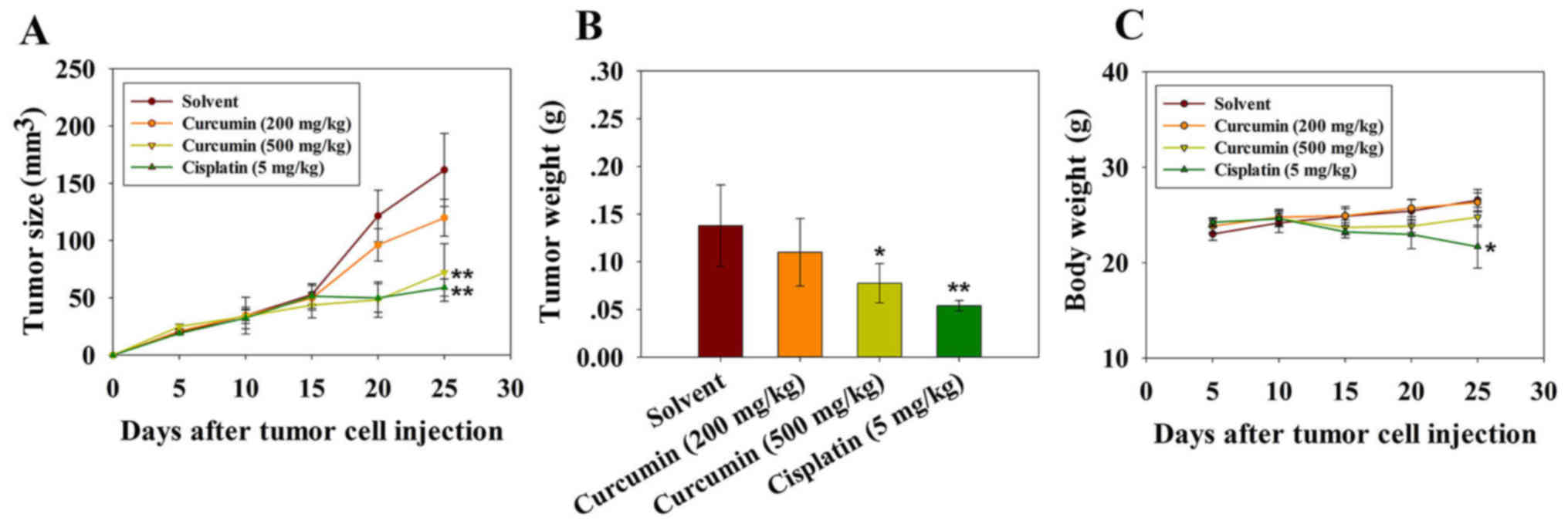
Curcumin inhibits tumor growth and
angiogenesis in vivo
Tumor volume was significantly reduced following
three injections of cisplatin and 500 mg/kg curcumin. The mean
volumes of subcutaneous tumors in the cisplatin- and high dose
curcumin-treated mice were decreased compared with in the vehicle
control group (Fig. 5A and B). The
experiment was terminated and the mice were sacrificed on day 25
following tumor cell injection. The mean tumor volumes in mice
treated with cisplatin and a high dose of curcumin were 58 and 72
mm3, respectively, whereas the tumor volumes in mice
treated with the solvent and a low dose of curcumin were 161 and
120 mm3 on day 25. The body weight of the mice was also
monitored, in order to evaluate the potential side effects of
curcumin. As shown in Fig. 5C,
there was no obvious body weight loss in mice treated with curcumin
throughout the experiment, thus indicating that curcumin did not
exert evident systemic toxicity at the doses used in this
investigation. Body weight loss in mice treated with cisplatin
reflected the toxicity of the drug towards normal tissue (Fig. 5C). These results suggested that
curcumin may inhibit tumor growth in vivo with no obvious
toxicity.
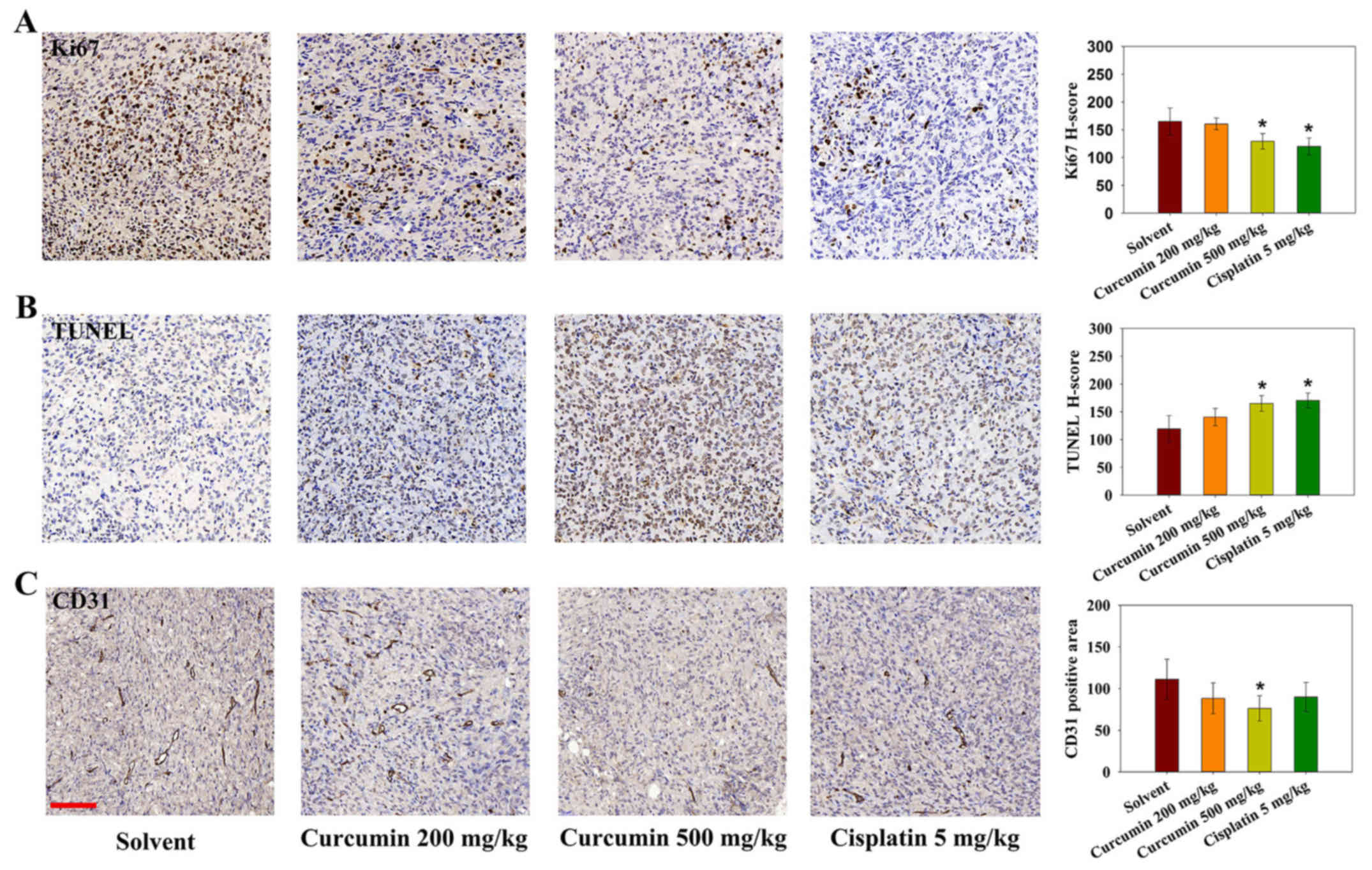
As tumor cell proliferation and inhibition of
apoptosis are attributable to tumor growth, Ki-67
immunohistochemistry and TUNEL analysis were performed on tumor
sections. As shown in Fig. 6A and
B, treatment with cisplatin or a high dose of curcumin affected
the number of proliferating Ki-67-positive cells. In addition,
using TUNEL cell death detection, it was revealed that tumors from
the cisplatin and high-dose curcumin groups contained an increased
number of TUNEL-positive apoptotic cells. CD31 immunostaining was
also conducted to evaluate tumor angiogenesis following curcumin
treatment in mice. The positive area of CD31 was significantly
lower in the high dose curcumin-treated group (Fig. 6C). These results indicated that
curcumin may inhibit tumor growth through inducing apoptosis, and
inhibiting cell proliferation and angiogenesis.
Discussion
The present study demonstrated that curcumin induced
apoptotic cell death of RN5 murine cells via the AIF-dependent
pathway and suppression of the PI3K-Akt-mTOR signaling pathway
(Fig. 7). Further analysis
indicated that curcumin may suppress tumor growth and angiogenesis
without systemic toxicity in vivo. These results indicated
that curcumin may be a promising anti-mesothelioma agent.
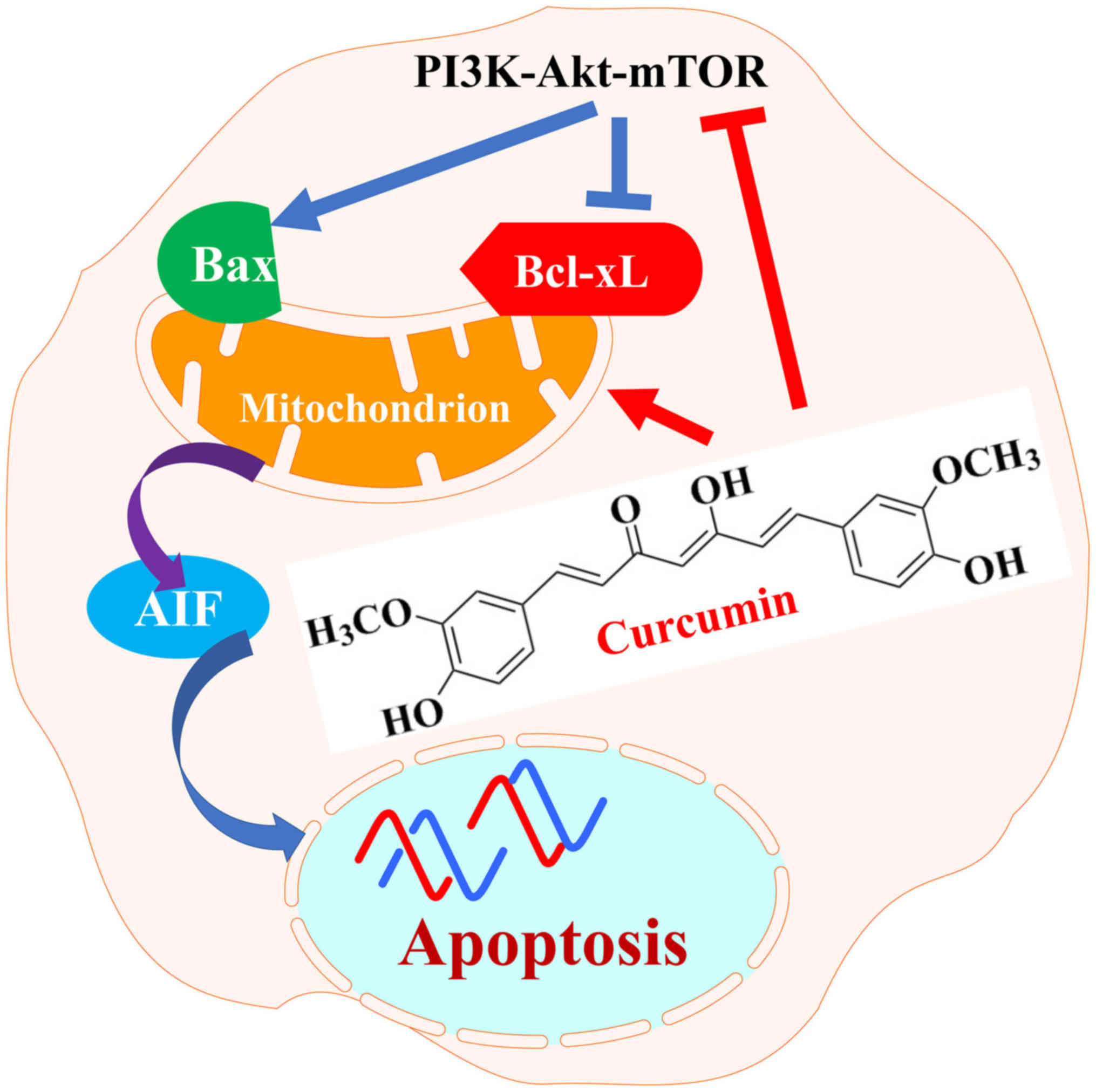 | Figure 7Schematic diagram indicating how
curcumin exerts anticancer effects on RN5 murine mesothelioma
cells. Curcumin suppressed PI3K-Akt-mTOR signaling pathway by
downregulating PI3K, p-Akt, p-mTOR and p-p70S6K and induced
apoptosis through mitochondrial, caspase-independent and
AIF-dependent pathways. AIF, apoptosis-inducing factor; Akt, AKT
serine/threonine kinase; Bax, B-cell lymphoma-2-associ-ated X
protein; Bcl-xL, B-cell lymphoma-extra large; mTOR, mammalian
target of rapamycin; p, phosphorylated; P70S6k, p70 ribosomal
protein S6 kinase; PI3K, phosphoinositide 3-kinase. |
Curcumin treatment for 24 h markedly upregulated Bax
expression and PARP cleavage, and downregulated Bcl-xL. Bcl-2
family members, which mediate cleavage of PARP, are major
regulators of the mitochondrial apoptotic pathway. The present
findings demonstrated that curcumin-induced RN5 cell death may be
caused by the mitochondrial apoptotic pathway. It has previously
been reported that curcumin induces apoptosis through the death
receptor and mitochondrial pathways (25). The caspase cascade serves a vital
role in apoptosis. In numerous cell types, including in
mesothelioma cells, curcumin-stimulated apoptosis has been revealed
to be induced via the caspase-mediated pathway (12,22,25).
Caspase-3 is downstream of the activator caspases and acts to
cleave various targets. In the present study, cleaved caspase-3
expression was significantly upregulated by cisplatin treatment,
whereas cleaved caspase-3, -8 and -9 expression was not detected
following curcumin treatment. The caspase-3 and -9 antibodies used
in this study can detect both full length and cleaved caspase
fragments. In addition, antibodies against caspase-8 and cleaved
caspase-8 were used to detect endogenous levels of caspase-8 (45 or
57 kDa), and active caspase-8 [p18 and cleavage products containing
the pro-domain with the p18 subunit (p43)]. Incubation with the
cleaved caspase-8 antibody did not detect any signal following RN5
cell treatment, even in cells treated with cisplatin. AIF is a
mitochondrial intermembrane space-localized flavoprotein that is
released by mitochondria and translocated to the nucleus in
response to death stimuli. AIF serves key roles in
caspase-independent apoptosis. To further confirm whether
curcumin-induced apoptosis was mediated by AIF, AIF expression was
detected in the nucleus by western blotting, and AIF translocation
from the mitochondria to the nucleus was analyzed by
immunofluorescence. The results indicated that AIF was translocated
from the cytoplasm to the nucleus, and its expression was increased
with increasing doses of curcumin. Several studies have reported
that X-rays and other natural agents induce AIF-dependent apoptosis
(26,27). The present study demonstrated that
curcumin induced apoptosis through activation of the
caspase-independent pathway in RN5 cells.
The PI3K-Akt-mTOR signaling pathway is activated in
cancer and is known to have a key role in a wide range of cellular
processes, including apoptosis inhibition, cell proliferation and
angiogenesis. Activation of the PI3K-AKT pathway in tumor cells can
also increase vascular endothelial growth factor (VEGF) secretion,
via hypoxia-inducible factor 1-dependent and -independent
mechanisms. The PI3K-Akt-mTOR signaling pathway is downstream of
several hyperactive RTKs in mesothelioma, thus indicating that this
signaling pathway may be a promising target for the treatment of
this disease. Dual PI3K-mTOR inhibitors inhibit Akt activation
through the mTOR complex 1/P70S6K negative feedback loop (28). At present, >50 drugs have been
developed based on inhibiting this signaling pathway (29).
On the basis of the curcumin-induced effects
detected in RN5 cells, the present study aimed to determine the
effects of curcumin on tumor-bearing mice. Treatment with a high
dose of curcumin, 500 mg/kg, inhibited tumor growth with low
toxicity. Previous studies have used similar doses of curcumin
(30,31). Furthermore, even low doses of
cisplatin (5 mg/kg) exerted evident side effects. Resistance to
cytotoxic drug-induced apoptosis is a main limitation in current
mesothelioma chemotherapy. Some targeted chemotherapy agents have
still not shown promise in clinical trials. Epidermal growth factor
receptor (EGFR) overexpression has been detected in >50% of
mesothelioma specimens (32);
however, erlotinib and gefitinib, as first-generation tyrosine-
kinase inhibitors (TKIs) that specifically target EGFR, have failed
to exhibit significant activity in clinical trials. The proposed
mechanism underlying resistance to these TKIs may be associated
with activation of the PI3K-Akt downstream pathway. Curcumin
inhibits drug-resistant cancer cell proliferation due to its
regulation of numerous targets involved in drug resistance,
including its ability to downregulate the multidrug-resistance gene
(33,34), and inhibit the nuclear factor
(NF)-κB signaling pathway (35) or
the NF-κB transcription factor (36).
Angiogenesis is an independent prognostic factor in
MPM (37). The VEGF signaling
pathway is associated with MPM growth; 31.5 and 66.7% cases of MPM
express VEGF and VEGF-C, respectively (38). These previous findings indicated
that VEGF inhibitors may be promising therapeutic agents. The
addition of nintedanib to the pemetrexed plus cisplatin strategy in
the treatment of MPM is associated with an improvement in
progression-free survival (39).
In addition, another VEGF inhibitor, cediranib, is in phase I/II
clinical trials (40). Despite
curcumin inhibiting angiogenesis through the VEGF-VEGF receptor 2
signaling pathway in some types of cancer (41), the mechanism underlying
curcumin-induced inhibition of angiogenesis in MPM remains to be
elucidated.
In conclusion, the present results demonstrated that
curcumin induced apoptosis via the mitochondrial pathway and was
involved in caspase-independent apoptotic signaling. In addition,
curcumin inhibited the PI3K-Akt-mTOR signaling pathway, and
suppressed tumor cell viability and angiogenesis, without evident
toxicity in vivo. The present study indicated that a
connection exists between the PI3K pathway and angiogenesis in
mesothelioma. These findings may provide a promising application
for curcumin, and indicated that the synergistic therapy of
curcumin plus platinum-based cytotoxic agents may be promising in
MPM treatment.
Acknowledgments
The authors of the present study would like to thank
Mr. Samuel (Jia Wei) Liu (Honours Life Sciences, McMaster
University, Hamilton, ON, Canada), and Ms. Alice Chen (Medical
Sciences, Western University, London, ON, Canada) for editing this
manuscript.
Funding
The present study was supported by grants from the
Natural Science Foundation of Shandong Province (grant no.
ZR2015HM054), the Key Research and Development Program of Shandong
Province (grant nos. 2015GSF118144 and 2016ZDJS07A15), the Shandong
Province Science and Technology Department Non-profit Program
(grant no. 2014kjhm0107) and the Youth Talent Innovation Foundation
of the Second Hospital of Shandong University (grant no.
2018YT17).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XZ, XD, CZ and YH conceived and designed this study.
CZ, YH, NJ, JL and WZ performed the experiments. XZ, LW, BC and DT
analyzed and interpreted the data. CZ, XD, LW and MdP wrote the
paper. MdP participated in general supervision of this study,
discussion and interpretation of the results, and provided the MPM
cell line. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was carried out in accordance with
the National Institutes of Health guidelines outlined in the Guide
for the Care and Use of Laboratory Animals. The animal study was
approved by the Committee on the Ethics on Animal Experimentation
of the Second Hospital of Shandong University [grant no.
KYLL-2017(LW) 011, approved on February 3, 2017].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robinson BWS, Musk AW and Lake RA:
Malignant mesothelioma. Lancet. 366:397–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scherpereel A, Astoul P, Baas P, Berghmans
T, Clayson H, de Vuyst P, Dienemann H, Galateau-Salle F, Hennequin
C, Hillerdal G, et al: European Respiratory Society/European
Society of Thoracic Surgeons Task Force: Guidelines of the European
Respiratory Society and the European Society of Thoracic Surgeons
for the management of malignant pleural mesothelioma. Eur Respir J.
35:479–495. 2010. View Article : Google Scholar
|
|
3
|
Perrot M, Wu L, Wu M and Cho BCJ:
Radiotherapy for the treatment of malignant pleural mesothelioma.
Lancet Oncol. 18:e532–e542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Milano MT and Zhang H: Malignant pleural
mesothelioma: A population-based study of survival. J Thorac Oncol.
5:1841–1848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaku Y, Nagaya H, Tsuchiya A, Kanno T,
Gotoh A, Tanaka A, Shimizu T, Nakao S, Tabata C, Nakano T, et al:
Newly synthesized anticancer drug HUHS1015 is effective on
malignant pleural mesothelioma. Cancer Sci. 105:883–889. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altomare DA, You H, Xiao GH, Ramos-Nino
ME, Skele KL, De Rienzo A, Jhanwar SC, Mossman BT, Kane AB and
Testa JR: Human and mouse mesotheliomas exhibit elevated AKT/PKB
activity, which can be targeted pharmacologically to inhibit tumor
cell growth. Oncogene. 24:6080–6089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anand P, Sundaram C, Jhurani S,
Kunnumakkara AB and Aggarwal BB: Curcumin and cancer: An 'old-age'
disease with an 'age-old' solution. Cancer Lett. 267:133–164. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller JM, Thompson JK, MacPherson MB,
Beuschel SL, Westbom CM, Sayan M and Shukla A: Curcumin: A double
hit on malignant mesothelioma. Cancer Prev Res (Phila). 7:330–340.
2014. View Article : Google Scholar
|
|
10
|
Yamauchi Y, Izumi Y, Asakura K, Hayashi Y
and Nomori H: Curcumin induces autophagy in ACC-MESO-1 cells.
Phytother Res. 26:1779–1783. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masuelli L, Benvenuto M, Di Stefano E,
Mattera R, Fantini M, De Feudis G, De Smaele E, Tresoldi I, Giganti
MG, Modesti A, et al: Curcumin blocks autophagy and activates
apoptosis of malignant mesothelioma cell lines and increases the
survival of mice intraperitoneally transplanted with a malignant
mesothelioma cell line. Oncotarget. 8:34405–34422. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Rishi AK, Wu W, Polin L, Sharma S,
Levi E, Albelda S, Pass HI and Wali A: Curcumin suppresses growth
of mesothelioma cells in vitro and in vivo, in part, by stimulating
apoptosis. Mol Cell Biochem. 357:83–94. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blum W, Pecze L, Felley-Bosco E,
Worthmüller-Rodriguez J, Wu L, Vrugt B, de Perrot M and Schwaller
B: Establishment of immortalized murine mesothelial cells and a
novel mesothelioma cell line. In Vitro Cell Dev Biol Anim.
51:714–721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang C, Zhai S, Li X, Zhang Q, Wu L, Liu
Y, Jiang C, Zhou H, Li F, Zhang S, et al: Synergistic action by
multi-targeting compounds produces a potent compound combination
for human NSCLC both in vitro and in vivo. Cell Death Dis.
5:e11382014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang C, Zhai S, Wu L, Bai Y, Jia J, Zhang
Y, Zhang B and Yan B: Induction of size-dependent breakdown of
blood-milk barrier in lactating mice by TiO2
nanoparticles. PLoS One. 10:e01225912015. View Article : Google Scholar
|
|
16
|
Detre S, Saclani Jotti G and Dowsett M: A
"quickscore" method for immunohistochemical semiquantitation:
Validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jordan RC, Lingen MW, Perez-Ordonez B, He
X, Pickard R, Koluder M, Jiang B, Wakely P, Xiao W and Gillison ML:
Validation of methods for oropharyngeal cancer HPV status
determination in US cooperative group trials. Am J Surg Pathol.
36:945–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SJ, Krauthauser C, Maduskuie V,
Fawcett PT, Olson JM and Rajasekaran SA: Curcumin-induced HDAC
inhibition and attenuation of medulloblastoma growth in vitro and
in vivo. BMC Cancer. 11:1442011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subramaniam D, May R, Sureban SM, Lee KB,
George R, Kuppusamy P, Ramanujam RP, Hideg K, Dieckgraefe BK,
Houchen CW, et al: Diphenyl difluoroketone: A curcumin derivative
with potent in vivo anticancer activity. Cancer Res. 68:1962–1969.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fruman DA and Rommel C: PI3K and cancer:
Lessons, challenges and opportunities. Nat Rev Drug Discov.
13:140–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu H, Wang C, Yang D, Wei Z, Xu J, Hu Z,
Zhang Y, Wang W, Yan R and Cai Q: Curcumin regulates proliferation,
autophagy, and apoptosis in gastric cancer cells by affecting PI3K
and P53 signaling. J Cell Physiol. 233:4634–4642. 2018. View Article : Google Scholar
|
|
23
|
Banik U, Parasuraman S, Adhikary AK and
Othman NH: Curcumin: The spicy modulator of breast carcinogenesis.
J Exp Clin Cancer Res. 36:982017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Echeverry N, Ziltener G, Barbone D, Weder
W, Stahel RA, Broaddus VC and Felley-Bosco E: Inhibition of
autophagy sensitizes malignant pleural mesothelioma cells to dual
PI3K/mTOR inhibitors. Cell Death Dis. 6:e17572015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu A, Huang JJ, Li RL, Lu ZY, Duan JL, Xu
WH, Chen XP and Fan JP: Curcumin as therapeutics for the treatment
of head and neck squamous cell carcinoma by activating SIRT1. Sci
Rep. 5:134292015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang S, Huang J, Liu P, Li J and Zhao S:
Apoptosis-inducing factor (AIF) nuclear translocation mediated
caspase-independent mechanism involves in X-ray-induced MCF-7 cell
death. Int J Radiat Biol. 93:270–278. 2017. View Article : Google Scholar
|
|
27
|
Cho H-D, Lee J-H, Moon K-D, Park K-H, Lee
M-K and Seo K-I: Auriculasin-induced ROS causes prostate cancer
cell death via induction of apoptosis. Food Chem Toxicol.
111:660–669. 2018. View Article : Google Scholar
|
|
28
|
Rodrik-Outmezguine VS, Chandarlapaty S,
Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J,
Guichard S and Rosen N: mTOR kinase inhibition causes
feedback-dependent biphasic regulation of AKT signaling. Cancer
Discov. 1:248–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pons-Tostivint E, Thibault B and
Guillermet-Guibert J: Targeting PI3K signaling in combination
cancer therapy. Trends Cancer. 3:454–469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fetoni AR, Paciello F, Mezzogori D, Rolesi
R, Eramo SL, Paludetti G and Troiani D: Molecular targets for
anticancer redox chemotherapy and cisplatin-induced ototoxicity:
The role of curcumin on pSTAT3 and Nrf-2 signalling. Br J Cancer.
113:1434–1444. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dienstmann R, Rodon J, Serra V and
Tabernero J: Picking the point of inhibition: A comparative review
of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 13:1021–1031.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bronte G, Incorvaia L, Rizzo S, Passiglia
F, Galvano A, Rizzo F, Rolfo C, Fanale D, Listì A, Natoli C, et al:
The resistance related to targeted therapy in malignant pleural
mesothelioma: Why has not the target been hit yet? Crit Rev Oncol
Hematol. 107:20–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi BH, Kim CG, Lim Y, Shin SY and Lee
YH: Curcumin downregulates the multidrug-resistance mdr1b gene by
inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett.
259:111–118. 2008. View Article : Google Scholar
|
|
34
|
Chearwae W, Anuchapreeda S, Nandigama K,
Ambudkar SV and Limtrakul P: Biochemical mechanism of modulation of
human P-glycoprotein (ABCB1) by curcumin I, II, and III purified
from Turmeric powder. Biochem Pharmacol. 68:2043–2052. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bava SV, Puliyappadamba VT, Deepti A, Nair
A, Karunagaran D and Anto RJ: Sensitization of taxol-induced
apoptosis by curcumin involves downregulation of nuclear
factor-kappaB and the serine/threonine kinase Akt and is
independent of tubulin polymerization. J Biol Chem. 280:6301–6308.
2005. View Article : Google Scholar
|
|
36
|
Dhandapani KM, Mahesh VB and Brann DW:
Curcumin suppresses growth and chemoresistance of human
glioblastoma cells via AP-1 and NFkappaB transcription factors. J
Neurochem. 102:522–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Edwards JG, Cox G, Andi A, Jones JL,
Walker RA, Waller DA and O'Byrne KJ: Angiogenesis is an independent
prognostic factor in malignant mesothelioma. Br J Cancer.
85:863–868. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ohta Y, Shridhar V, Bright RK, Kalemkerian
GP, Du W, Carbone M, Watanabe Y and Pass HI: VEGF and VEGF type C
play an important role in angiogenesis and lymphangiogenesis in
human malignant mesothelioma tumours. Br J Cancer. 81:54–61. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grosso F, Steele N, Novello S, Nowak AK,
Popat S, Greillier L, John T, Leighl NB, Reck M, Taylor P, et al:
Nintedanib plus pemetrexed/cisplatin in patients with malignant
pleural mesothelioma: Phase II results from the randomized,
placebo-controlled LUME-meso trial. J Clin Oncol. 35:3591–3600.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yap TA, Aerts JG, Popat S and Fennell DA:
Novel insights into mesothelioma biology and implications for
therapy. Nat Rev Cancer. 17:475–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fu Z, Chen X, Guan S, Yan Y, Lin H and Hua
ZC: Curcumin inhibits angiogenesis and improves defective
hematopoiesis induced by tumor-derived VEGF in tumor model through
modulating VEGF-VEGFR2 signaling pathway. Oncotarget.
6:19469–19482. 2015. View Article : Google Scholar : PubMed/NCBI
|