Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains one
of the most lethal types of cancer with little improvement in the
survival rates over the past decades. It is the fourth leading
cause of cancer-related mortality in the USA and in Europe
(1). The prognosis of patients
with this disease remains unfavorable for all stages of the disease
with the 5-year overall survival rate being <5% (2). Surgical resection, followed by
adjuvant therapy, is the only radical therapeutic option; however,
<15% of patients present with operable disease. Patients with
advanced disease at presentation or recurrence may receive
palliative chemotherapy, although the rates of response and
survival benefit is modest. Aggressive tumor biology, as well as
primary or secondary drug resistance (3) contribute significantly towards this
dismal prognosis.
Caveolin-1 (Cav-1) is a 22-kDa molecular weight
integral cell membrane scaffolding protein and a main component of
caveolae, which are transmembrane microdomains composed of
cholesterol and sphingolipids thus known as ‘lipid rafts’. This
protein, in one of two similar isoforms (Cav-1a and Cav-1b), is
associated with the processes of endo- and exocytosis, and with
intracellular signal transduction mechanisms (4). Cav-1 is involved in a number of
biological processes, as well as cellular transformation,
tumorigenesis and metastasis (5).
Cav-1 protein allows for signaling transduction events associated
with the epidermal growth factor receptor (EGFR), HER2/neu, Src,
the focal adhesion-associated protein kinase (FAK) and the
mitogen-activated protein kinase (MAPK) (6). In addition, through its interaction
with the adhesion molecules of the extracellular matrix, the
integrins, it seems to be associated with the induction of the
apoptosis of cancer cells (7,8).
Of note, Cav-1 appears to play a dual role in cancer
biology (9). Its expression in
cancer cells has been associated with an aggressive phenotype and a
poor prognosis in various tumor types, including pancreatic
adenocarcinoma (10-14). It has also been directly linked to
the metastatic potential of pancreatic cancer cells through the
regulation of epithelial to mesenchymal transition (EMT), a
phenomenon closely related to the metastatic potential and
chemoresistance of cancer cells (15,16).
Nevertheless, conflicting results have been presented (17), with the loss of Cav-1 in the tumor
stroma being associated with an adverse clinical outcome in a
variety of cancer types (18-23).
Previous studies have implicated Cav-1 protein as an
important factor in the development of chemo - and radio-resistance
(24,25), while complex interactions seem to
be involved in the role of Cav-1 in the development of multi-drug
resistance (MDR) (26). To date,
there are no consistent data implicating Cav-1 in the development
of chemoresistance in pancreatic adenocarcinoma (27-29).
Moreover, it is not clear whether the expression of Cav-1 in
pancreatic cancer cells or cancer-associated fibroblasts (CAFs) is
predictive of the treatment response or overall prognosis (10,21,30).
The purpose of this study was to examine the role of
Cav-1 in the development of chemoresistance in pancreatic cancer.
Initially, we examined the differential immunohistochemical
expression of Cav-1 between tumor and stromal cells in human
pancreatic cancer tissue specimens. We then examined the response
of human pancreatic cancer cell lines, with differential expression
levels of Cav-1, to chemotherapeutic agents both in vitro
and in vivo using pancreatic cancer animal models developed
in immunodeficient mice. Furthermore, and in order to shed light on
the role of Cav-1 expression in the context of the tumor
microenvironment, we generated and used fibroblasts with a
decreased expression of Cav-1. Our results indicate that expression
of Cav-1 in tumor cells per se may play a minor role in their
tumorigenicity and chemoresistance. However, the decreased
expression of this protein in the tumor microenvironment i.e., in
fibroblasts, seems to result in increased tumorigenic properties of
cancer cells together with increased chemoresistance.
Materials and methods
Materials
RPMI-1640 and DMEM were purchased from Gibco/Thermo
Fisher Scientific (Athens, Greece) and L-glutamine, PBS and trypsin
were purchased from GE Healthcare Life Sciences (GE Healthcare Life
Sciences/Athal, Athens, Greece). Fetal bovine serum was purchased
from Biowest (Biowest/Bioline Scientific, Athens, Greece) and
dimethylsulphoxide (DMSO) from Eastman Kodak (Columbus, GA, USA).
Trichloroacetic acid (TCA), TEMED, hydrochloric acid, SDS, hydrogen
peroxide, glycerol 99.9%, sulphorodamine-B for the in vitro
cytotoxic assay, NP40 and protease inhibitors were obtained from
Sigma-Aldrich (Merck, Chemilab S.A., Athens, Greece).
2-β-mercaptoethanol was purchased from Merck (Chemilab S.A.) while
Ponceau S staining solution and Triton X-100 were from AppliChem
(AppliChem GmbH, Darmstadt, Germany). Glycine ≥99% was purchased
from Roth (Karlsruhe, Germany) while protein electrophoresis
markers, SDS acrylamide 30% and the Quick Start Bradford Dye
reagent 1X for the measurement of protein content of our samples
were purchased from Bio-Rad Laboratories Ltd. (Athens, Greece). All
the chemotherapeutic agents [5-fluorouracil (5-FU), gemcitabine,
doxorubicin, epirubicin, cisplatin, oxaliplatin, docetaxel and
Paclitaxel] were kindly provided by the Oncology Department of the
General University Hospital of Larissa, Larissa, Greece. Cell
culture plastic products were all purchased from Sarstedt (Sarstedt
Ltd., Athens, Greece).
Cell culture
BxPC3 (pancreatic adenocarcinoma), AsPC1 (pancreatic
adenocarcinoma metastatic), PANC-1 (epithelioid carcinoma from
pancreatic duct) and MIAPaCa-2 (pancreatic carcinoma) cancer cell
lines were obtained from ATCC (Manassas, VA, USA). Human dermal
fibroblasts were obtained originally from Thermo Fisher Scientific
(Loughborough, UK). The cancer cells were adapted to proliferate in
RPMI-1640 medium and the fibroblasts in DMEM, supplemented with 5%
heat-inactivated fetal calf serum, 2 mM L-glutamine and
antibiotics. The cultures were grown at 36.7°C in a humidified
incubator with 5% CO2 atmosphere and 95% humidity.
Silencing of Cav-1 in BxPC3 cells
To minimize the differences between various cell
lines, we set out to induce the stable knockdown of Cav-1 in BxPC-3
cells that naturally express high levels of Cav-1. Hence, we
measured their proliferative capacity, their migratory capacity and
chemosensitivity. We induced the stable knockdown through
lentiviral infection, which also allowed tracking the cells
containing the virus due to constitutive green fluorescent protein
(GFP) expression (fluorescent in the green channel). Cav-1
expression was silenced by transduction with short hairpin RNA
(shRNA) mir GIPZ lentiviral particles (Open Biosystems, Surrey,
UK). The cells were seeded at 50% confluence and infected by direct
contact with lentiviral particles diluted 1:50 into 1 ml of
serum-free RPMI-1640 and incubated for 6 h, following which an
additional 1 ml of 10% RPMI-1640 was added and the cells were
incubated for a further 72 h. The transduction efficiency was
evaluated by GFP co-expression by a fluorescence microscope (EVOS™
FL Imaging System; Thermo Fisher Scientific, Loughborough, UK).
Stably transduced cells were then selected in media containing 1.0
µg/ml puromycin (Life Technologies/Thermo Fischer
Scientific, Athens, Greece) for 10 days.
To purify further and homogenize the cellular
populations, cells transduced as described above were sorted on a
BD FACS-Vantage cell sorter (Becton-Dickinson, Oxford, UK) based on
GFP expression. Through this procedure, employing silencing shRNA
for caveolin, the cell line named BxPC3shCAV was finally
generated, while a mock-transfected cell line named
BxPC3mock was also generated to be used as control.
These cells were used for the experiments described further in this
study.
Immortalization of human dermal
fibroblasts and silencing of Cav-1
For immortalization, pBabe hTERT geneticin
retrovirus supernatant (Addgene, Cambridge, MA, USA) was used to
transduce human dermal fibroblasts at 50% confluence. After 24 h,
the cell media were changed and the cells were cultured for a
further 48 h. Selection was carried out for 4 days with 1.0
µg/ml geneticin (Life Technologies; Thermo Fisher
Scientific, Loughborough, UK). The hTERT-immortalized human skin
fibroblasts named hhsF from hereon were used in the further
experiments.
Cav-1 expression was silenced as described above.
Two types of hhsF were developed: The hhsFmock and the
hhsFshCAV with unaffected levels of Cav-1 or with
silenced Cav-1, respectively.
Western blot analysis
For western blot analysis cancer cells were cultured
in 6-well plates at inoculation densities varying from
4×106 to 6×106/ml, depending on the cell
line. After 24 h, the cells were washed twice in ice-cold PBS,
trypsinized, collected by gentle centrifugation, and whole cell
protein extracts were prepared as previously described (31). Protein concentrations were
determined with the Bradford assay, and subsequently, aliquots
containing 30 µg of protein were subjected to gel
electrophoresis on 10% polyacylamide SDS-gels under reducing
conditions, and then transferred onto PVDF membranes (Millipore
Immobilon; Merck S.A. Hellas, Athens, Greece). To confirm protein
transfer, the membranes were stained with Ponceau S solution
(AppliChem GmbH). Finally after the washing membranes to remove the
Ponceau S, the proteins were visualized using an enhanced
chemoluminescence detection system (Amersham ECL or ECL Plus, GE
Healthcare Life Sciences) according to the manufacturer’s
instructions. Cav-1 antibody was purchased from Santa Cruz
Biotechnology (clone N-20, sc-894; Heidelberg, Germany) while,
actin antibody was purchased from Sigma-Aldrich (Life Science
Chemilab S.A., Athens, Greece) and GAPDH antibody from BioLegend
(San Diego, CA, USA). Anti-rabbit and anti-mouse HRP conjugated
secondary antibodies used were purchased from Sigma-Aldrich.
Antibodies were used as follows (all were diluted in Tris-buffered
saline supplemented 0.05% Tween-20 and 5% FCS): CAV-1 at 1:500
(overnight incubation at 4°C), GAPDH at 1:6,000 (2 h at room
temperature), actin at 1:2,000 (2 h at room temperature), and both
secondary antibodies at 1:6,000 (1 h at room temperature).
RNA isolation and RT-qPCR analysis
Total RNA was isolated using the RNeasy Mini kit
(Qiagen, Valencia, CA, USA) according to the manufacturer’s
instructions. One microgram of total RNA from each sample was
retro-transcribed to first-strand cDNA using the SuperScript III
One Step RT-PCR system (Invitrogen/Thermo Fisher Scientific,
Loughborough, UK). Quantitative RT-PCR was performed in triplicates
using SYBR-Green RT-PCR Master Mix kit and the ABI PRISM 7500 Fast
Real-Time PCR System (Applied Biosystems/Thermo Fisher Scientific,
Loughborough, UK) with the following primers: Cav-1,
5′-CGACCCTAAACACCTCAACGA-3′ (forward) and 5′-TCCCTTCTGGTTCTGTCA-3′
(reverse). Quantification was performed using the comparative
CT (cycle-threshold) method employing ribosomal 18S as a
housekeeping gene (ΔΔCq method) (32).
Evaluation of cell proliferation
[bromodeoxyuridine (BrdU) assay]
Cell proliferation was evaluated by determining the
incorporation of BrdU nucleotide in actively proliferating cells,
using a colorimetric ELISA (Abcam, Cambridge, UK). The assay was
performed as per the manufacturer’s instructions. The colored
reaction product was quantified using a spectrophotometer
(microplate reader, BioTek EL-311; BioTek Instruments, Bad
Friedrichshall, Germany).
Wound healing/scratch assay
To determine the effects of Cav-1 on the ability of
cancer cells to migrate in vitro, we utilized the scratch
assay. In the monolayer of cells covering approximately the 80% of
the microtiter plate well surface, a scratch was made using a
sterile 200 µl pipette tip. Following a wash with PBS and
the addition of fresh growth medium, the cells were allowed to
migrate for 24 or 48 h. The cells were photographed using a light
microscope and a ×10 magnification (Axioplan equipped with an
AxioCam, Zeiss Ltd., Cambridge, UK) at various time points i.e., 0,
5, 9 14 and 28 h to analyze the migration of the cells towards the
‘wounded’ area.
Chemotaxis/migration assay
To determine the ability of fibroblasts to induce
the chemotactic migration of the BxPC3 cancer cells, 24 mm
Transwell® with 8.0-µm pore polycarbonate
membrane inserts (Product #3428, Corning®
Transwell®; Sigma-Aldrich; Merck, Chemilab S.A.) was
used. The chemotaxis/migration assay was performed as follows:
At day zero, a 24-well migration plate was
inoculated with the fibroblasts (hhsFmock or
hhsFshCAV) at a density of 20×103 cells/well
in 500 µl of serum-free DMEM, and the cells were allowed to
adapt in a 5% CO2 incubator at 36.7°C for 48 h.
Subsequently, the medium was removed and DMEM supplemented with 10%
FCS (10% DMEM) was added for 12 h to serum-activate the
fibroblasts, as previously described (33). Thereafter, the 10% DMEM medium was
removed, and the fibroblasts were washed twice with PBS and 500
µl of DMEM supplemented with 1% FCS was added. Following a
further 12 h of incubation, a cell suspension of BxPC3 cells at a
density of 60×104 cells/ml in serum-free media was
prepared. A Transwell® insert was then applied to the
fibroblast-containing wells and 250 µl of the BxPC3 cells
suspended in serum-free media were then added to the insert. Wells
containing 1% DMEM underneath the insert were used as negative
controls in order to determine the spontaneous migration of BxPC3
cells under these conditions, while wells with 10% DMEM underneath
the insert served as positive controls. Incubation continued (5%
CO2, 36.7°C) for a further 12 h. Next, the media from
the inside of the insert were carefully aspirated, the inserts
removed from the culture plates and non-migratory cells were
removed from the interior of the inserts with cotton-tipped swabs.
Subsequently, the inserts were transferred to a clean well
containing 400 µl of ice-cold TCA to fix the cells attached
to the outer surface of the insert as described for the
cytotoxicity assay. The fixed inserts were gently washed several
times in distilled water, air-dried and stained with sulforhodamine
B (SRB) as for the in vitro cytotoxic activity assay
described below. After a second wash step to remove any unbound
staining, the inserts were transferred to a clean plate containing
400 µl of unbuffered Tris to extract the dye and 200
µl of the solution was transferred to a 96-well plate and
finally the OD value was measured using a micro-plate reader
(Biotek EL-311; BioTek Instruments). Chemotaxis was calculated as
the % OD value of the inserts as compared to inserts containing no
fibroblasts (negative controls). For chemotaxis, two independent
experiments were performed.
In vitro cytotoxic activity of
chemotherapeutic drugs
The in vitro cytotoxic activity of all
chemotherapeutics tested herein [5-fluo-rouracil (5-FU),
gemcitabine, doxorubicin, epirubicin, cisplatin, oxaliplatin,
docetaxel and Paclitaxel] was determined using the SRB assay, as
previously described (34,35). Cell viability was assessed at the
beginning of each experiment by the trypan blue dye exclusion
method, and was always >97%. For the SRB assay, the cells seeded
into 96-well plates in 100 µl medium at a density of 5,000
cells per well, and incubated under standard conditions for 24 h to
enable the cells to resume exponential growth before addition of
the compounds. In order to measure the starting cell population
[time zero (Tz)], cells in one plate were fixed in situ with
TCA 50%. Compounds were diluted to twice the desired final maximum
test concentration (100 µM for all other drugs, but for
paclitaxel and docetaxel the maximum concentrations tested were 10
µM) with complete medium and 4 or 5 additional 10-fold
dilutions, depending on the drug, were prepared. Aliquots of 100
µl of these different drug dilutions were added to the
appropriate microtiter wells already containing 100 µl of
medium, resulting in the required final drug concentrations. Cell
cultures containing DMSO alone served as negative controls, while
as a positive control, control representative wells were treated
with a corresponding volume of culture medium.
Following drug addition, the plates were incubated
for 48 h at the conditions described above. The experiment was
terminated by the addition of 50 µl of cold 50% (w/v) TCA
(final concentration, 10% TCA). Incubation for 1 h at 4°C and
staining with SRB was carried out as previously described (34,35).
The bound stain was subsequently solubilized with a 10 mM Trizma
base (Sigma-Aldrich; Merck, Chemilab) and the absorbance was read
on an automated plate reader, EL-311 (BioTek Instruments), at a 530
nm wavelength.
Using the absorbance measurements [time zero (Tz),
control growth (C), and test growth in the presence of drug at the
5 concentration levels (Ti)], the growth percentage of the cells
was calculated for each drug concentration using the following
formulas: i) [(Ti-Tz)/(C-Tz)] × 100 for concentrations for which
Ti>/=Tz and ii) [(Ti-Tz)/Tz] × 100 for concentrations for which
Ti<Tz.
In vivo experiments
To generate human-to-mouse pancreatic cancer
xenografts, 5×106 cells from exponentially growing
cultures of BxPC3mock or BxPC3shCAV cells
were injected subcutaneously, according to the British practice of
bilateral implants, at the axillary region of the rear flanks into
6-8-week-old (mean weight, 20 g) female
NOD.CB17-Prkdcscid/J (NOD/SCID) mice from our animal
facility (EL 42 BIO_BR01). During experimentation, all animals were
kept in the Animal Unit of the Department of Pharmacology
(University of Thessaly, Larissa Greece; EL42 BIO_EXP03) under
specific pathogen-free (SPF) conditions, a 12-h/12-h light/dark, a
temperature of 21°C and a relative humidity 50% and allowed access
to water and food ad libitum. For the co-injection of
fibroblasts with BxPC3 (3×105) cells at the exponential
growth phase, hhsFmock or hhsFshCAV
fibroblasts were co-injected with BxPC3 cells at 1:1 or 1:3 ratios,
respectively in plain DMEM, subcutaneously at the axillary region
of the rear flanks of mice (two injections per mouse as described
above for the generation of BxPC3mock or
BxPC3shCAV xenografts). In this experiment a group of
mice received 3×105 fibroblasts alone, as well, to
determine whether the transformed fibroblasts had any tumorigenic
capacity. Each group consisted of 5 mice of matching age and
weight. The mice were then followed for the development of tumors.
At the end of the experiment, which varied depending on the
specific group (i.e., for the generation of BxPC3mock or
BxPC3shCAV the experiment ended at day 65 post-tumor
cell inoculation, for the development of co-injected xenografts
experiments ended at days 26-28 post-tumor cell inoculation) the
mice were euthanized (age, 12-16 weeks; mean weight, 20-22 g) and
the tumors were removed and weighed. Where tumor volume was used
this was calculated according to the formula [(axb2)/2],
where a=length and b=width of the tumor as measured with a
Vernier’s caliper (measurements were performed twice a week).
Animals treated with gemcitabine received an
intraperitoneal injection/week at a 100 mg/kg dose until the end of
experiment. Treatment began 4 days after cell implantation. Weight
loss (assessed twice weekly), neurological disorders, behavioral
and dietary changes were also recorded as indicators of drug
toxicity.
The handling and experimentation of the animals were
conducted in accordance with the Greek laws (PD 56/2013 and
Circular 2215/117550/2013) and the guidelines of the European Union
(2013/63/EU) under a licenced protocol approved by the IACUC and
Greek authorities (Lisence no. 5542/228006, IACUC; Professor Dr N.
Pitsikas, Dr A. Zacharioudaki, Dr J. Chloptsios and Dr A.
Konstantinidis).
Immunohistochemistry
A total of 11 de-identified FFPE (formalin-fixed
paraffin-embedded) blocks of pancreatic cancer tissues, obtained
from the archive of the Pathology Department of The Leeds Teaching
Hospitals Trust (Leeds, UK) were stained for Cav-1. All patients
provided informed consent prior to the collection of the samples
and all procedures were approved by and were in compliance with the
ethical standards of the Leeds Teaching Hospitals NHS Trust
(R&I Ref. no. CO18/113235). The differential expression of
Cav-1 between the tumor and stroma in poorly differentiated (PD)
and well/moderately differentiated (WD) areas was assessed by a
pancreatic pathologist (CV). As previously described by Witkiewicz
et al (30), Cav-1
expression was evaluated as follows: 0 for no staining; 1 for weak
and/or focal (<10% of the cells) staining; 2 for moderate or
strong staining (10-50% of the cells); and 3 for moderate or strong
staining (>50% of the cells).
Immunohistochemical analysis (IHC) of human and
xenograft pancreatic cancer tissues was performed on
3-µm-thick paraffin-embedded sections using a rabbit
poly-clonal anti-Cav-1 antibody (dilution 1:200, anti-cav-1 N-20
rabbit; sc-894; Santa Cruz Biotechnology). Antigen retrieval was
performed according to the suppliers’ instructions. The
immunoreaction was detected using a goat anti-rabbit-ALP (Menarini
Diagnostics, Winnersh-Wokingham, UK) followed by
3,3-diaminobenzidine tetrahydrochloride (DAB; Vector Laboratories,
Peterborough, UK). Negative controls were processed by the omission
of primary antibody. For histological evaluation, sections were
stained with hematoxylin and eosin (H&E; Thermo Fisher
Scientific, Loughborough, UK). The sections were imaged using an
Axioplan Zeiss light microscope (Carl Zeiss Ltd., Cambridge, UK)
equipped with an AxioCam digital camera.
Statistical analysis
Statistical analysis was performed using a Student’s
t-test or one-way ANOVA with Holm-Sidak as a post hoc test where
multiple comparisons were carried out using GraphPad Prism 6.0
software (GraphPad Software, La Jolla, USA). Data are presented as
the means ± SD. Statistical significance was set at P<0.05.
Results
IHC reveals the variable expression of
Cav-1 in the epithelial and stromal component of pancreatic cancer
tissue specimens
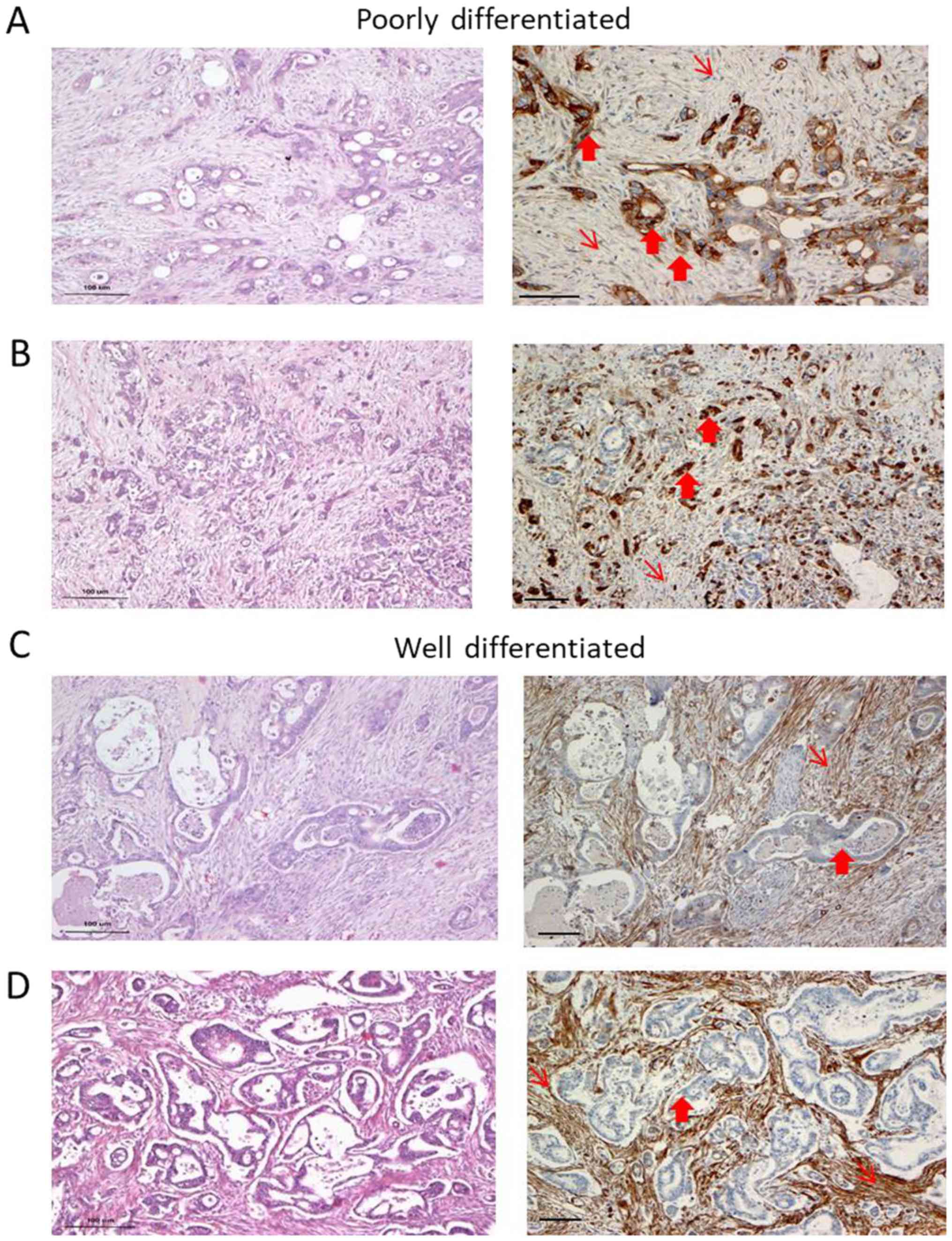
We observed heterogeneity in Cav-1 expression with
respect to the degree of differentiation. In the
well-differentiated (WD) areas of the samples, Cav-1 staining in
the cancer cells was negative or exhibited a focal/weak protein
expression in 9 out of the 11 cases. By contrast, the stroma of the
WD areas exhibited a strong expression of Cav-1 (Fig. 1 and Table I). Cancer cells in the poorly
differentiated (PD) areas were moderately to strongly
Cav-1-positive in 6 out of the 7 cases examined. Immunostaining was
predominantly cytoplasmic, while membranous staining was apparent
only in the tumor cells with clear cell morphology. The fibroblasts
were weakly stained in the PD areas, particularly in areas where
cancer cells were strongly positive (Fig. 1A and C). There seemed to be an
inverse pattern of Cav-1 expression, with strong staining in the
cancer cells and weak or no staining in the stroma-associated
fibroblasts or vice versa (Table
I).
 | Table ICaveolin-1 expression in patient
archival samples. |
Table I
Caveolin-1 expression in patient
archival samples.
| Poorly
differentiated | Moderately/well
differentiated |
|---|
|
|---|
| Case no. | Cav-1 expression
| Cav-1 expression
|
|---|
| Cancer | Stroma | Cancer | Stroma |
|---|
| 1 | 3 | 1 | 1 | 3 |
| 2 | 2 | 2 | 0 | 2 |
| 3 | No PD areas | | 2 | 3 |
| 4 | No PD areas | | 2 | 1 but scarce
stroma |
| 5 | No PD areas | | 1 | 3 |
| 6 | 1 | 3 | 0 | 3 |
| 7 | 3 | 2 | 0 | 1 but scarce
stroma |
| 8 | 3 | 1 | 1 | 3 |
| 9 | 2 | 2 | 1 | 3 |
| 10 | 3 | 1 | 1 | 3 |
| 11 | No PD areas | | 0 | 3 |
Generation of pancreatic cancer cells and
fibroblasts in which Cav-1 is knocked down
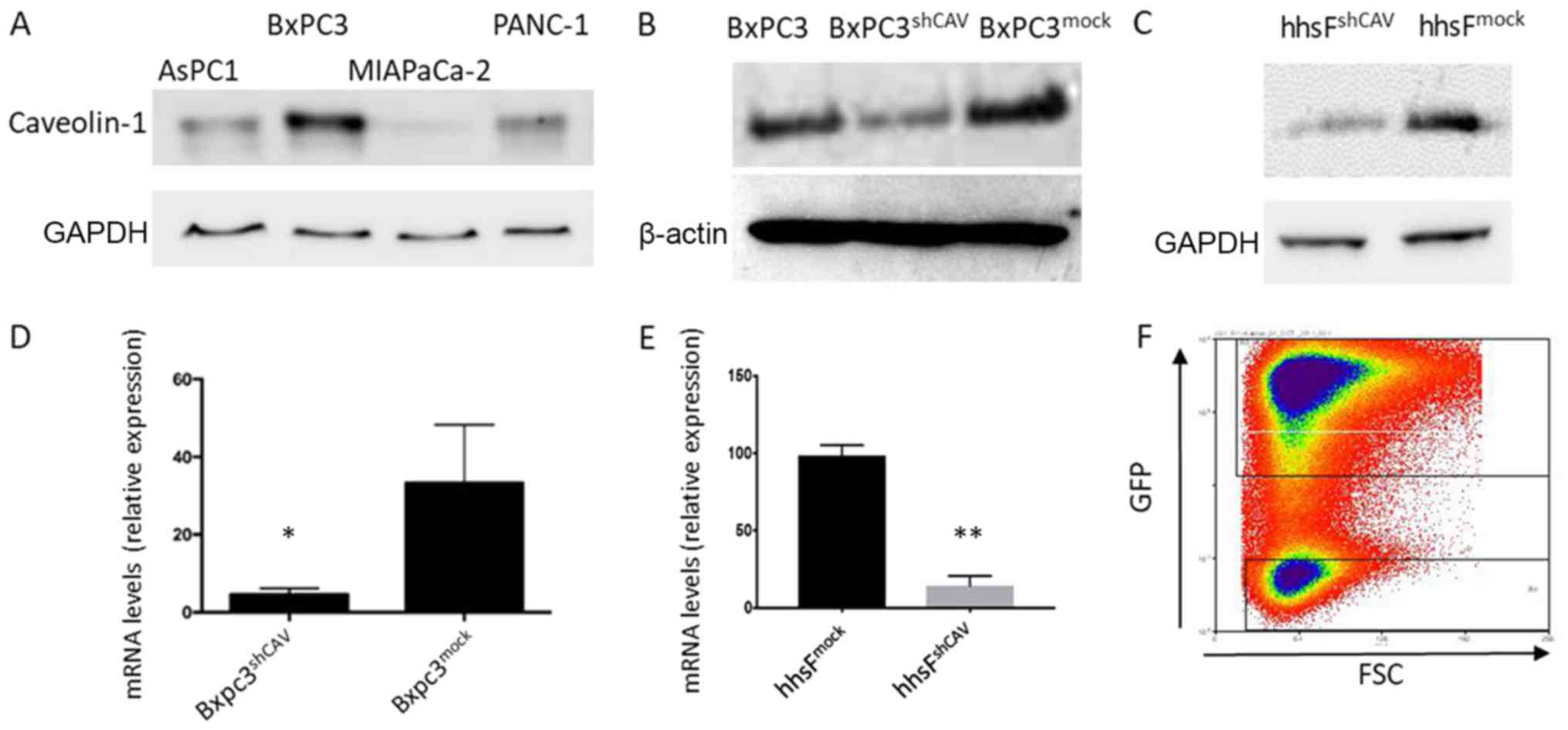
In order to select the most appropriate cell line
for our hypothesis, we screened several pancreatic cell lines for
Cav-1 expression (Fig. 2A) and we
found that the BxPC3 cells expressed the highest levels of Cav-1.
We thus selected these cells for use in the subsequent experiments.
The lentivirus-induced introduction of shRNA into the BXPC3 cells
resulted in a decreased mRNA and protein expression of Cav-1, as
shown in Fig. 2B and D. The
concomitant introduction of GFP allowed for the discrimination and
separation of cells with the highest viral integration/expression
and consequent lower Cav-1 expression (named BxPC3shCAV)
with the aid of cell sorting (Fig.
2F). As a control, the BxPC3mock cell line was
generated with unaffected levels of Cav-1. Lentiviral particles
expressing scrambled shRNA (mock) did not alter the Cav-1 levels,
confirming that the decreased expression of Cav-1 in
BxPC3shCAV was due to the specific effects of shRNA
(Fig. 2B and D). Fibroblasts with
silenced (hhsFshCAV) or unaffected (hhsFmock)
Cav-1 expression were generated from hTERT immortalized human skin
fibroblasts following the methodology described above for the BxPC3
cells (Fig. 2C and E).
Downregulation of Cav-1 expression
results in a marginal increase in DNA synthesis and tumor cell
proliferation, and in the increased migration/motility of BxPC3
cells
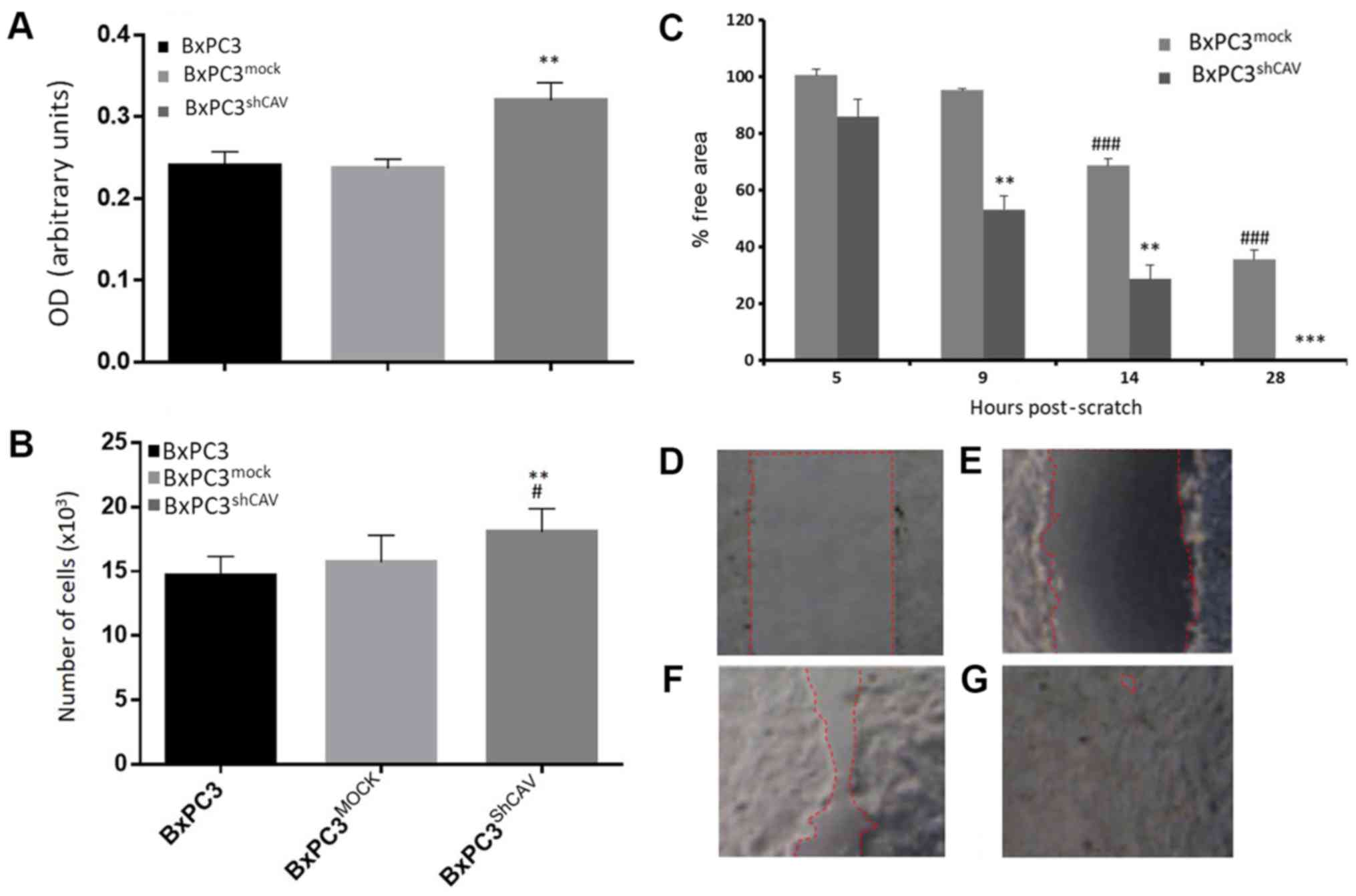
We first examined whether Cav-1 downregulation in
pancreatic cancer cells affected the proliferation rate of these
cells. By the SRB method and the BrdU DNA incorporation method,
beginning with an inoculation density of 5,000 cells/well, a
moderate increase in DNA synthesis (Fig. 3A) and in the proliferation of
BxPC3shCAV as compared to BxPC3 and BxPC3mock
cells were observed (Fig. 3B).
These results suggest that Cav-1 may, to a certain extent, act as
negative regulator of the growth of pancreatic cancer cells under
the prevailing experimental conditions. Furthermore, as shown in
Fig. 3C, the downregulation of
Cav-1 in the BxPC3 cells resulted in a statistically significant
increase in the migratory capacity of the BxPC3shCAV
cells as compared to the BxPC3mock cells (P<0.01),
suggesting again that Cav-1 may act as a brake on the proliferative
and migratory capacity of pancreatic cancer cells.
Cav-1 downregulation in fibroblasts
increases the migratory and chemotactic ability of BxPC3 cells
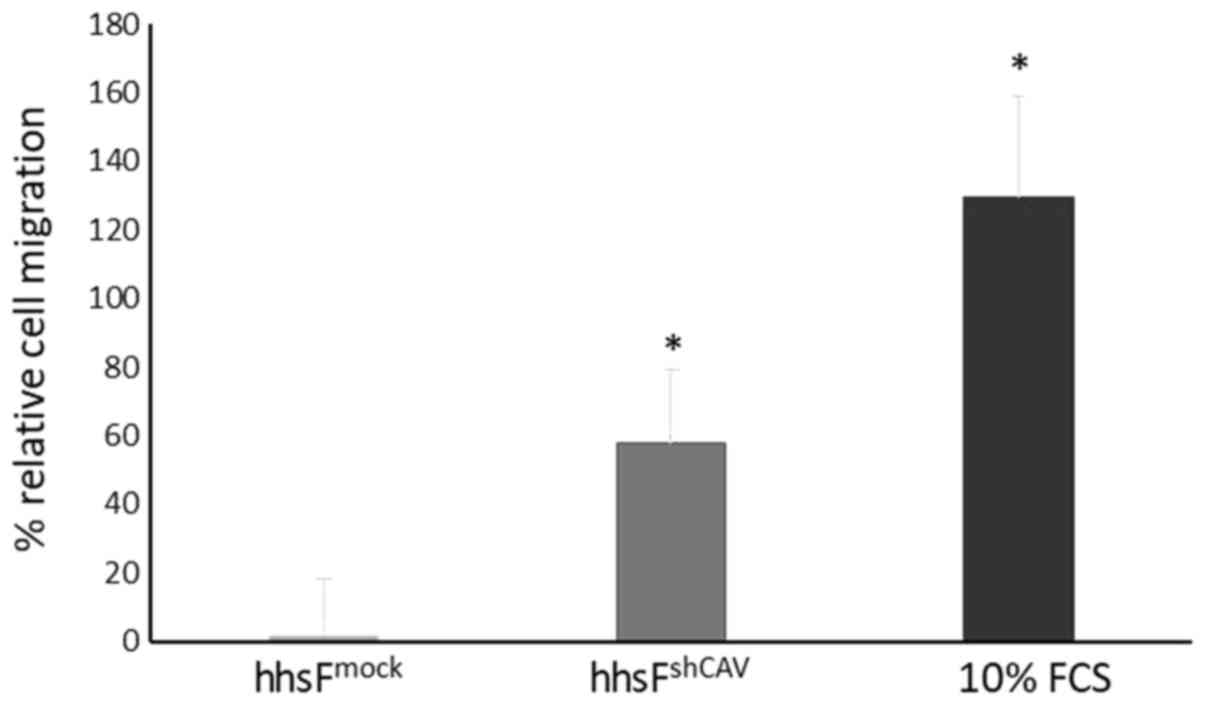
To further determine the effects of Cav-1 levels not
in the cancer cells, but in the context of the tumor
microenvironment, a Transwell migration/chemotaxis assay was
performed (Fig. 4) where the
effect of fibroblasts in which Cav-1 was silenced on the
chemotactic migration of normal (non-Cav 1 silenced) BxPC3 cells
was examined. In this experiment, the silencing of Cav-1 in the
fibroblasts (hhsFshCAV) resulted in an increased
migration of BxPC3 cancer cells (60±21%) through the micropores of
the Transwell as compared to the mock-infected fibroblasts
(hhsFmock, P<0.05, paired t-test). These results
suggest that the absence of Cav-1 in fibroblasts may favor the
metastatic potential of tumor cells in the context of the tumor
microenvironment. As the tumor cells were not in contact with the
fibroblasts, the secretion of agents from the fibroblasts may have
acted as chemoattractants to induce the migration of tumor cells
towards the surrounding stroma.
Effect of Cav-1 expression on the
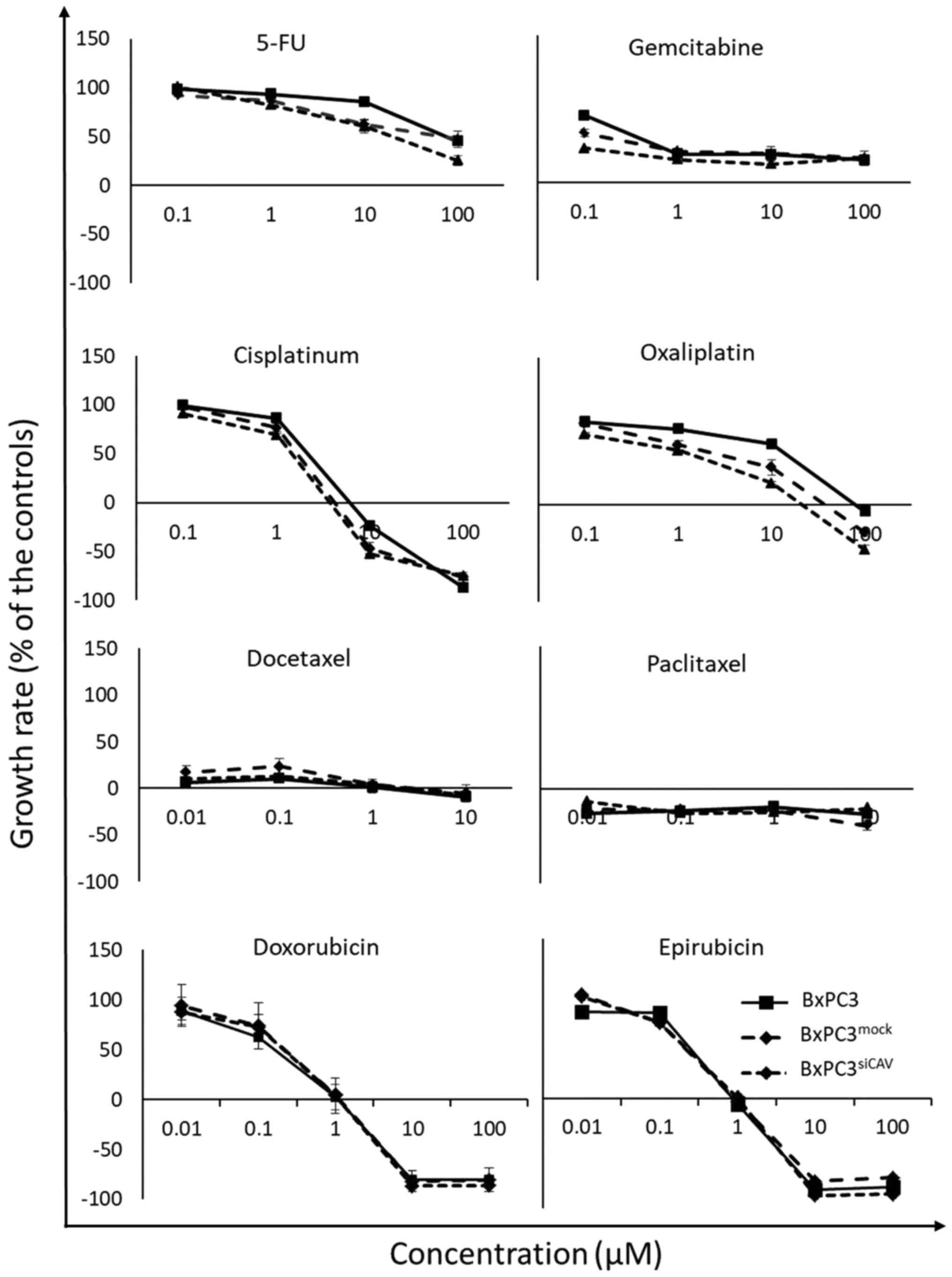
chemosensitivity of cancer cells
To examine the effect of Cav-1 expression on the
chemo-sensitivity of human the pancreatic cancer BxPC3 cells, the
BxPC3mock and BxPC3shCAV cells were exposed
to various chemotherapeutic agents i.e., 5-fluorouracil (5-FU),
gemcitabine, doxorubicin, epirubicin, cisplatin, oxaliplatin,
docetaxel and Paclitaxel. The sensitivity of all 3 cell lines
(BxPC3, BxPC3mock and BxPC3shCAV) seemed to
be unaffected by the levels of expression of Cav-1 to the
chemotherapeutic agents that were tested, as shown by the
corresponding growth curves (Fig.
5).
Decreased Cav-1 levels in the stroma
promote the growth of BxPC3 tumor xenografts
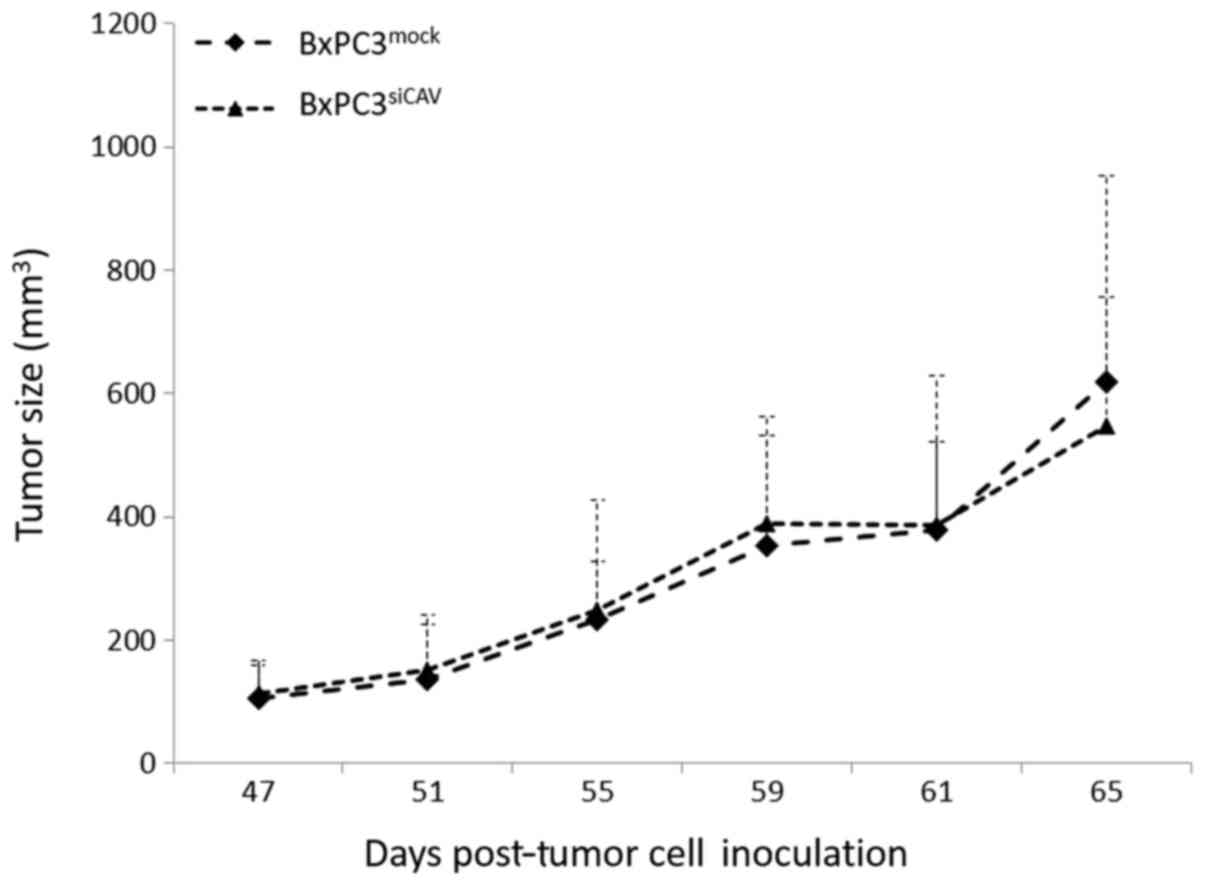
We then examined whether the protein expression
levels of Cav-1 can affect the tumorigenic capacity and/or the
chemoresistance of BxPC3 in xenografts. Towards this aim, we first
sought to compare the tumorigenic and growth characteristics of the
two cell lines, BxPC3mock and BxPC3shCAV,
when inoculated into NOD/SCID mice. The two xenografts from the
transfected cells demonstrated no difference between the growth
rates of BxPC3mock- and BxPC3shCAV-derived
tumors (Fig. 6).
Following the in vitro observation that low
levels of fibroblast Cav-1 expression resulted in increased cancer
cell motility/migration (Fig. 4),
we determined whether fibroblast Cav-1 expression can affect the
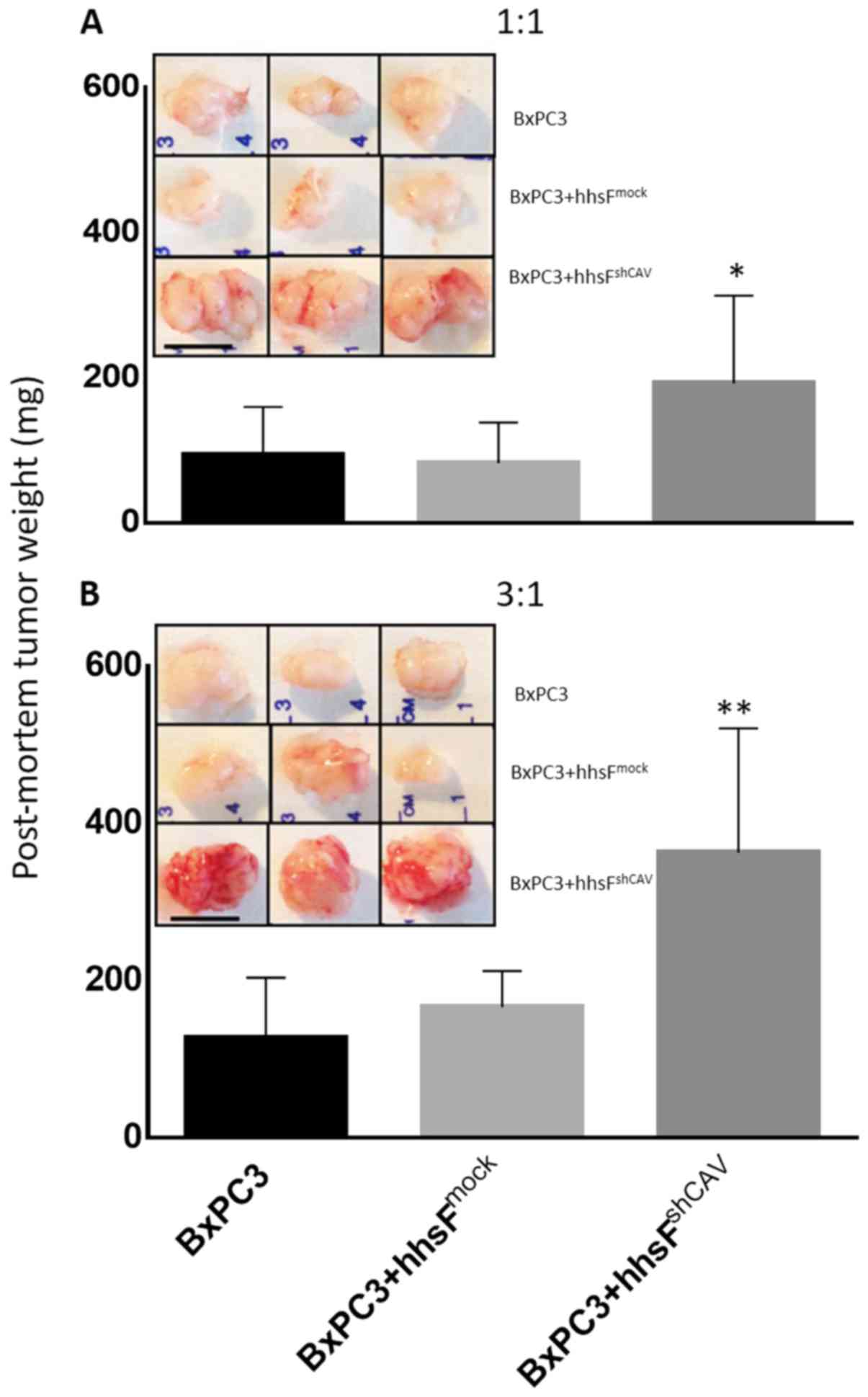
growth of BxPC3 when grown as xenografts. As shown in Fig. 7, the co-injection of fibroblasts
with BxPC3 cells significantly affected the growth of tumors in a
Cav-1-dependent manner. The tumors that developed from BxPC3 cells
co-injected with hhsFmock (BxPC3 + hhsFmock
tumors) cells exhibited similar growth as the tumors derived from
the BxPC3 cells alone. However, the co-injection of BxPC3 cells
with hhsFshCAV (BxPC3 + hhsFshCAV tumors),
resulted in an almost 3-fold increase in tumor weight compared to
both the BxPC3- and the BxPC3 + hhsFmock-derived tumors
(130±98 and 167±42 mg, respectively vs. 360±138 mg for the BxPC3 +
hhsFshCAV tumors; P<0.01). At 6 days post-inoculation
of 3×105 BxPC3 cells into NOD/SCID mice, at a 1:1 ratio
of cancer cells:fibroblasts, the following was observed: No tumor
development in the BxPC3-only inoculated mice (0/10); 1 tumor
palpable in 10 mice that received BxPC3 + hhsFmock
(1/10), and 3 tumors palpable in mice that were co-injected with
BxPC3 and hhsFshCAV cells (3/10). A week later, at day
13, the ratio was 3/10 for BxPC3 and for BxPC3 +
hhsFmock as compared to 7/10 for the BxPC3 +
hhsFshCAV-derived tumors. Similarly, increasing the
inoculation density of cancer cells to 1×106 (cancer
cells:fibroblasts ratio: 3:1) resulted in the development of tumors
in the following numbers of mice: 0/10 for BxPC3, 1/10 for BxPC3 +
hhsFmock and 7/10 for BxPC3 + hhsFshCAV at
day 6 and 5/10, 6/10 and 9/10 tumors at day 13, respectively (data
not shown). The post-mortem weights of the tumors that had
developed by day 28 post-inoculation of the cells revealed a
substantial difference between the BxPC3-derived tumors that
developed from the co-injection with hhsFshCAV
fibroblasts as compared to the BxPC3-only and BxPC3 +
hhsFmock-derived tumors, both at the cancer
cells:fibroblasts ratios of 1:1 and 3:1 (P<0.05 or 0.01,
respectively; Fig. 7). Mice
injected with fibroblasts alone did not develop any tumors (data
not shown).
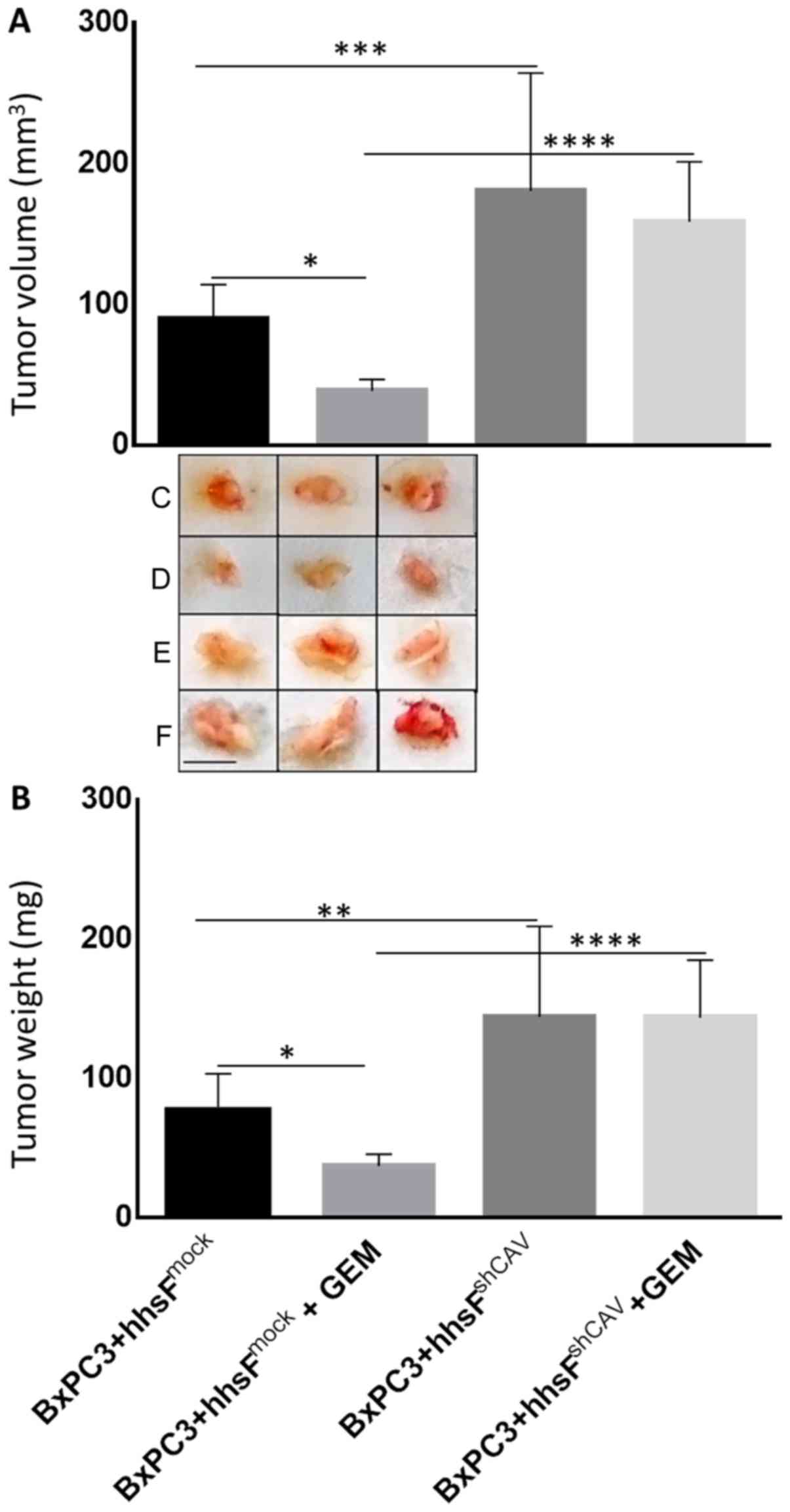
Finally, to examine the effect of Cav-1 expression
in fibroblasts on the chemosensitivity of pancreatic cancer cells
exposed to gemcitabine, co-injection experiments as described above
were undertaken following the administration of gemcitabine.
Beginning on day 4 post-cells’ inoculation, the mice were injected
intraperitoneally with either 100 mg/kg gemcitabine or an
equivalent volume of saline as the control, on a weekly basis for 3
weeks (till the end of the experiment). As shown in Fig. 8, tumors that developed from the
co-injection of BxPC3 and hhsFshCAV fibroblasts were
substantially more resistant as compared to those developed from
the co-inoculation of hhsFmock fibroblasts (P<0.01vs.
BxPC3 + hhsFmock + gemcitabine). These tumors exhibited
no response at all when compared to the corresponding untreated
tumors (i.e., vs. BxPC3 + hhsFshCAV).
Taken together, these results demonstrate that
although the levels of Cav-1 seem to have a minor effect on
cellular proliferation and none regarding the chemosensitivity of
BxPC3 cancer cells in vitro, the lack of Cav-1 expression in
fibroblasts within the cancer microenvironment may affect more
substantially both the growth and chemoresistance of the tumor
cells.
Immunohistochemical analysis of
xenografts does not identify differences in the amount of
stroma
To determine the contribution of the stroma to the
tumor weight in xenografts from the co-injection experiments of
explanted xenografts, IHC was performed. The amount of stroma did
not differ across the experimental conditions, indicating that
differences in tumor size are not attributed to differences in
stromal content (data now shown).
Discussion
In this study, we initially observed that there was
an inverse association between Cav-1 expression in cancer cells and
stromal fibroblasts. Despite the small number of cases, it was
consistently observed that in areas of well-differentiated tumors,
fibroblasts stained strongly for Cav-1, while cancer cells were
negative or stained only weakly. By striking contrast, in areas of
poorly differentiated tumors, the cancer cells exhibited a high
expression of Cav-1 and were surrounded by fibroblasts with a low
Cav-1 expression. These results are in accordance with those of
previous studies, which linked Cav-1 expression in cancer cells to
poor differentiation (30,36). However, to the best of our
knowledge, this is the first study showing an opposite pattern of
Cav-1 expression between tumor and stromal cells in pancreatic
cancer. These results are in agreement with those of a previous
study on colorectal cancer, reporting the differential expression
of Cav-1 between stroma and cancer cells (37). Alshenawy and Ali reported that the
overexpression of Cav-1 in cancer cells was associated with adverse
prognostic features, while a higher stromal expression was
associated with small non-metastasizing tumors and thus a better
prognosis (37). The exact
mechanisms behind this observation are not yet clear; however, it
has been suggested that regional differences in hypoxia or acidity
may explain tumor heterogeneity (38,39).
To determine whether this differential expression of
Cav-1 in cancer cells and the surrounding stroma have an influence
on tumor biology, we examined the effects of decreased levels of
Cav-1 expression on cell proliferation and chemosensitivity in a
human pancreatic cancer cell line with a high Cav-1 expression.
Four commonly used human pancreatic cancer cell lines, i.e., BxPC3
(moderate to poor differentiation) (40), AsPC1, PANC-1 and MIAPaCa-2 (all 3
of poor differentiation) were tested for caveolin expression
(Fig. 1A). In our laboratory,
BxPC3 cells were found to express the highest levels of Cav-1 with
the MIAPaCa-2 cells showing minimal expression. We herein though
need to point out that the data from previous studies regarding the
expression of Cav-1 in MIAPaCa-2 cells have been controversial, as
in the study by Salem et al (29), it was reported a low expression of
the protein, in agreement with our data, while Chatterjee et
al (36) reported high levels
of Cav-1 expression in MIAPaCa-2 cells. In studies using mice to
compare these cell lines for their tumorigenicity (xenograft
studies), it was suggested that BxPC3 may be more aggressive as
compared to PANC1, AsPC1 and MIAPaCa-2 cells (40). Thus, we decided to proceed with the
BxPC3 cell line in this study.
The results revealed that the decreased expression
of Cav-1 only marginally increased the cellular proliferation and
DNA synthesis, suggesting that Cav-1 may act as a tumor suppressor
to a certain extent. The downregulation of the protein resulted in
the increased migratory capability of the BxPC3 cancer cell line in
the wound healing assay. However, we need to point out herein that
this effect may be the result of the increased proliferation of the
cells.
Our results are in agreement with those reported in
other studies regarding the role of Cav-1 in the migration and
invasion of pancreatic cancer cell lines (16,29,41).
In support of the inhibitory effects of Cav-1 on the migration and
invasion of pancreatic cancer cells, Han and Zhu (41) reported that the knockdown of Cav-1
promoted the activity of matrix metalloprotease (MMP)2 and 9.
However, these observations are not supported by Chatterjee et
al (36), who reported
opposite results, suggesting that the overexpression of Cav-1 may
act as a promoting factor for cancer cell proliferation, invasion
and migration. The use of different cell lines or other methods to
down- or upregulate Cav-1 and to address migration and invasion may
potentially explain these differences.
Cav-1 expression in pancreatic cancer cells has been
related to a decreased sensitivity to ionizing radiation (42), while chemosensitivity is preserved
through EMT inhibition (29). In
this study, a variety of chemotherapeutic agents commonly used in
pancreatic cancer were tested in vitro. It was demonstrated
that lower levels of the protein did not affect the in vitro
chemosensitivity of the BxPC3 cells to all the tested
chemotherapeutics. Finally, in an effort to shed some light on the
capacity of the Cav-1 to affect tumorigenicity of cancer cells, we
performed an in vivo experiment by injecting
BxPC3mock and BxPC3shCAV cells into SCID
mice. This experiment clearly demonstrated that Cav-1 expression in
the injected cells played no role in overall tumor growth.
Tumor development involves a stromal
microenvironment that contains fibroblasts, macrophages and other
cell types. It is now widely accepted that cancer-associated
fibroblasts play a major role in both tumor initiation and
progression. In this context, it has been proposed that the absence
of Cav-1 may be a characteristic of a cancer-associated fibroblast
phenotype (43,44). Pancreatic cancer stromal cells have
been shown to express low levels of Cav-1 (11), and in a cohort of 45 patients, the
expression levels of this protein were found to be related with the
TNM stage, HER-2/neu amplification and the overall prognosis of the
disease (21).
We then set out to determine whether differences in
Cav-1 expression in the tumor microenvironment have a direct
influence on the phenotype of pancreatic cancer cells. Human dermal
fibroblasts, manipulated to express decreased levels of Cav-1
compared to the control, induced the increased invasion of
pancreatic cancer cells in vitro. Since the two types of
cells were not in direct contact, these findings suggest that the
increased invasiveness of the tumor cells may be driven by secreted
chemotactic agents. Such an effect has already been demonstrated in
breast cancer (45); however, to
the best of our knowledge, this is the first report to demonstrate
that Cav-1 silencing in fibroblasts may directly regulate the
invasiveness of pancreatic tumor cells in a paracrine manner. In
support of this observation, it has been suggested that
cancer-associated fibroblasts (CAFs) with a decreased expression of
Cav-1 may provide nutritional support to the cancer cells by the
phenomenon of reverse Warburg effect (46).
Subsequently, we examined the in vivo effect
of Cav-1 silencing in fibroblasts. From these in vivo
experiments it was evident that the cancer cells gained
developmental advantage when they were co-injected with fibroblasts
that had a decreased expression of Cav-1. Indeed, an almost 3-fold
increase in tumor weight was observed in the tumors that were
developed by the co-injection of fibroblasts with silenced Cav-1
and BxPC3 cells, as compared to BxPC3- and
hhsFmock-derived tumors. These results outweighed the
need to perform co-culture experiments in vitro. Similar
results have been reported by Capozza et al (47) in a murine model of melanoma, in
which the downregulation of Cav-1 in CAFs promoted the development
of melanoma cells through paracrine Sonic Hedgehog signaling.
Similarly, Bonuccelli et al (45) reported that fibroblasts with
silenced Cav-1 expression enhanced the growth of MDA-MB231 breast
cancer xenografts.
Once we obtained clear evidence that the
downregulation of Cav-1 expression in fibroblasts could provide a
growth advantage to cancer cells in vivo, we set out to
determine whether the absence of Cav-1 in fibroblasts of the tumor
microenvironment may play a role in the development of
chemoresistance. According to our findings, the downregulation of
Cav-1 in stromal fibroblasts triggered the development of
chemoresistance when mice were treated with gemcitabine. Indeed,
tumors derived from the inoculation with fibroblasts with silenced
Cav-1 expression and BxPC3 cells alone did not respond to
gemcitabine chemotherapy. These data suggest a possible link
between stromal Cav-1 expression and chemo-resistance to
gemcitabine, which is a standard drug used in the treatment of
pancreatic cancer. In accordance to our initial clinical
observation, we could argue that hhsFshCAV represent the
stroma in the poorly differentiated pancreatic cancer area and the
fibroblasts with a decreased expression of Cav-1 provide survival
gain and chemoresistance to the pancreatic cancer cells.
The stroma has been regarded for a long time as a
potential barrier to the diffusion of chemotherapeutic agents into
tumors. Strategies to deplete the tumor-related stroma have
therefore been considered as a rational approach to improving
response to chemotherapy (48).
Unfortunately, this approach has not been translated into
successful clinical trials. Recent studies have actually suggested
that stroma depletion may accelerate cancer development, thus
reducing survival (49,50). Furthermore, the recent observation
that CAFs lead the scavenging of gemcitabine is a further possible
mechanism of treatment failure in pancreatic cancer (51). Methods to restore the stromal
expression of Cav-1 (52) may
represent another potential therapeutic strategy for pancreatic
cancer by increasing the sensitivity to chemotherapy.
Despite some limitations to our study, mainly
pertaining to the use of dermal immortalized fibroblasts instead of
CAFs and the use of a single cell line, our data support the
hypothesis that Cav-1 expression in the stromal component of
pancreatic cancer may influence cancer cell growth and the
development of resistance to chemotherapy. Nevertheless, xenograft
models with the use of non-tumor associated fibroblasts have been
used successfully for the study of chemotherapy resistance
(53). Our group aims to further
shed light onto the role of Cav-1 in pancreatic cancer by
developing patient derived xenografts and trying to isolate
cancer-associated normal fibroblasts that could be used to address
the role of Cav-1 in clinically more relevant settings.
In conclusion, the data presented herein suggest
that Cav-1 expressed in the stroma rather than in the tumor cells
has an impact on tumor development and chemoresistance in
pancreatic cancer. However, further studies are warranted to
investigate whether the manipulation of Cav-1 expression in CAFs
may represent a valid and effective therapeutic approach in
pancreatic cancer.
Funding
Dr Kamposioras was supported by the European
Society of Medical Oncology (ESMO) Translational Research
Fellowship. This study was supported by the Hellenic Society of
Medical Oncology (HeSMO) Fellowship program.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors’ contributions
KK, KD, AA, FDG, CNP, NS, GV, SPP conceived and
designed the experiments. KK, CT, CV, AD, VL, GM performed the
experiments. KK, KD, FDG, CV, NS analyzed the data. KK, KD, FDG CP,
NS, GV, SPP wrote and modified the manuscirpt. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided informed consent prior to the
collection of the samples and all procedures were approved by and
were in compliance with the ethical standards of the Leeds Teaching
Hospitals NHS Trust (R&I Ref. no. CO18/113235). The handling
and experimentation of the animals were conducted in accordance
with the Greek laws (PD 56/2013 and Circular 2215/117550/2013) and
the guidelines of the European Union (2013/63/EU) under a licenced
protocol approved by the IACUC and Greek authorities (Lisence no.
5542/228006, IACUC; Professor Dr N. Pitsikas, Dr A. Zacharioudaki,
Dr J. Chloptsios and Dr A. Konstantinidis).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
Not applicable.
References
|
1
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2014. Ann Oncol. 25:1650–1656. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ducreux M, Cuhna AS, Caramella C,
Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van
Laethem JL, Conroy T, et al: ESMO Guidelines Committee: Cancer of
the pancreas: ESMO Clinical Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 26(Suppl 5): v56–v68. 2015.
View Article : Google Scholar
|
|
3
|
Andersson R, Aho U, Nilsson BI, Peters GJ,
Pastor-Anglada M, Rasch W and Sandvold ML: Gemcitabine
chemoresistance in pancreatic cancer: Molecular mechanisms and
potential solutions. Scand J Gastroenterol. 44:782–786. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams TM and Lisanti MP: The Caveolin
genes: From cell biology to medicine. Ann Med. 36:584–595. 2004.
View Article : Google Scholar
|
|
5
|
Chen T, Liu L, Xu HX, Wang WQ, Wu CT, Yao
WT and Yu XJ: Significance of caveolin-1 regulators in pancreatic
cancer. Asian Pac J Cancer Prev. 14:4501–4507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boscher C and Nabi IR: Caveolin-1: Role in
cell signaling. Adv Exp Med Biol. 729:29–50. 2012. View Article : Google Scholar
|
|
7
|
Del Pozo MA and Schwartz MA: Rac, membrane
heterogeneity, caveolin and regulation of growth by integrins.
Trends Cell Biol. 17:246–250. 2007. View Article : Google Scholar
|
|
8
|
Wary KK, Mariotti A, Zurzolo C and
Giancotti FG: A requirement for caveolin-1 and associated kinase
Fyn in integrin signaling and anchorage-dependent cell growth.
Cell. 94:625–634. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goetz JG, Lajoie P, Wiseman SM and Nabi
IR: Caveolin-1 in tumor progression: The good, the bad and the
ugly. Cancer Metastasis Rev. 27:715–735. 2008. View Article : Google Scholar
|
|
10
|
Suzuoki M, Miyamoto M, Kato K, Hiraoka K,
Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh
T, et al: Impact of caveolin-1 expression on prognosis of
pancreatic ductal adenocarcinoma. Br J Cancer. 87:1140–1144. 2002.
View Article : Google Scholar
|
|
11
|
Tanase CP, Dima S, Mihai M, Raducan E,
Nicolescu MI, Albulescu L, Voiculescu B, Dumitrascu T, Cruceru LM,
Leabu M, et al: Caveolin-1 overexpression correlates with tumour
progression markers in pancreatic ductal adenocarcinoma. J Mol
Histol. 40:23–29. 2009. View Article : Google Scholar
|
|
12
|
Nam KH, Lee BL, Park JH, Kim J, Han N, Lee
HE, Kim MA, Lee HS and Kim WH: Caveolin 1 expression correlates
with poor prognosis and focal adhesion kinase expression in gastric
cancer. Pathobiology. 80:87–94. 2013. View Article : Google Scholar
|
|
13
|
Han F, Zhang J, Shao J and Yi X:
Caveolin-1 promotes an invasive phenotype and predicts poor
prognosis in large cell lung carcinoma. Pathol Res Pract.
210:514–520. 2014. View Article : Google Scholar
|
|
14
|
Ando T, Ishiguro H, Kimura M, Mitsui A,
Mori Y, Sugito N, Tomoda K, Mori R, Harada K, Katada T, et al: The
overexpression of caveolin-1 and caveolin-2 correlates with a poor
prognosis and tumor progression in esophageal squamous cell
carcinoma. Oncol Rep. 18:601–609. 2007.
|
|
15
|
Huang C, Qiu Z, Wang L, Peng Z, Jia Z,
Logsdon CD, Le X, Wei D, Huang S and Xie K: A novel FoxM1-caveolin
signaling pathway promotes pancreatic cancer invasion and
metastasis. Cancer Res. 72:655–665. 2012. View Article : Google Scholar
|
|
16
|
Lin M, DiVito MM, Merajver SD, Boyanapalli
M and van Golen KL: Regulation of pancreatic cancer cell migration
and invasion by RhoC GTPase and caveolin-1. Mol Cancer. 4:212005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye Y, Miao SH, Lu RZ and Zhou JW:
Prognostic value of caveolin-1 expression in gastric cancer: A
meta-analysis. Asian Pac J Cancer Prev. 15:8367–8370. 2014.
View Article : Google Scholar
|
|
18
|
Witkiewicz AK, Dasgupta A, Sotgia F,
Mercier I, Pestell RG, Sabel M, Kleer CG, Brody JR and Lisanti MP:
An absence of stromal caveolin-1 expression predicts early tumor
recurrence and poor clinical outcome in human breast cancers. Am J
Pathol. 174:2023–2034. 2009. View Article : Google Scholar
|
|
19
|
Ma X, Liu L, Nie W, Li Y, Zhang B, Zhang J
and Zhou R: Prognostic role of caveolin in breast cancer: A
meta-analysis. Breast. 22:462–469. 2013. View Article : Google Scholar
|
|
20
|
Zhao Z, Han FH, Yang SB, Hua LX, Wu JH and
Zhan WH: Loss of stromal caveolin-1 expression in colorectal cancer
predicts poor survival. World J Gastroenterol. 21:1140–1147. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan T, Lu H, Ji H, Li Y, Guo J, Chen X
and Wu T: Loss of stromal caveolin-1 expression: A novel tumor
microenvironment biomarker that can predict poor clinical outcomes
for pancreatic cancer. PLoS One. 9:e972392014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia Y, Wang N, Wang J, Tian H, Ma W, Wang
K, Tan B, Zhang G, Yang S, Bai B, et al: Down-regulation of stromal
caveolin-1 expression in esophageal squamous cell carcinoma: A
potent predictor of lymph node metastases, early tumor recurrence,
and poor prognosis. Ann Surg Oncol. 21:329–336. 2014. View Article : Google Scholar
|
|
23
|
Zhao X, He Y, Gao J, Fan L, Li Z, Yang G
and Chen H: Caveolin-1 expression level in cancer associated
fibroblasts predicts outcome in gastric cancer. PLoS One.
8:e591022013. View Article : Google Scholar
|
|
24
|
Nakatani K, Wada T, Nakamura M, Uzawa K,
Tanzawa H and Fujita S: Expression of caveolin-1 and its
correlation with cisplatin sensitivity in oral squamous cell
carcinoma. J Cancer Res Clin Oncol. 131:445–452. 2005. View Article : Google Scholar
|
|
25
|
Tirado OM, MacCarthy CM, Fatima N, Villar
J, Mateo-Lozano S and Notario V: Caveolin-1 promotes resistance to
chemotherapy-induced apoptosis in Ewing’s sarcoma cells by
modulating PKCalpha phosphorylation. Int J Cancer. 126:426–436.
2010. View Article : Google Scholar
|
|
26
|
Wang Z, Wang N, Liu P, Peng F, Tang H,
Chen Q, Xu R, Dai Y, Lin Y, Xie X, et al: Caveolin-1, a
stress-related oncotarget, in drug resistance. Oncotarget.
6:37135–37150. 2015.PubMed/NCBI
|
|
27
|
Hehlgans S and Cordes N: Caveolin-1: An
essential modulator of cancer cell radio-and chemoresistance. Am J
Cancer Res. 1:521–530. 2011.
|
|
28
|
Hehlgans S, Eke I, Storch K, Haase M,
Baretton GB and Cordes N: Caveolin-1 mediated radioresistance of 3D
grown pancreatic cancer cells. Radiother Oncol. 92:362–370. 2009.
View Article : Google Scholar
|
|
29
|
Salem AF, Bonuccelli G, Bevilacqua G,
Arafat H, Pestell RG, Sotgia F and Lisanti MP: Caveolin-1 promotes
pancreatic cancer cell differentiation and restores membranous
E-cadherin via suppression of the epithelial-mesenchymal
transition. Cell Cycle. 10:3692–3700. 2011. View Article : Google Scholar
|
|
30
|
Witkiewicz AK, Nguyen KH, Dasgupta A,
Kennedy EP, Yeo CJ, Lisanti MP and Brody JR: Co-expression of fatty
acid synthase and caveolin-1 in pancreatic ductal adenocarcinoma:
Implications for tumor progression and clinical outcome. Cell
Cycle. 7:3021–3025. 2008. View Article : Google Scholar
|
|
31
|
Dimas K, Hatziantoniou S, Tseleni S, Khan
H, Georgopoulos A, Alevizopoulos K, Wyche JH, Pantazis P and
Demetzos C: Sclareol induces apoptosis in human HCT116 colon cancer
cells in vitro and suppression of HCT116 tumor growth in
immunodeficient mice. Apoptosis. 12:685–694. 2007. View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Iyer VR, Eisen MB, Ross DT, Schuler G,
Moore T, Lee JC, Trent JM, Staudt LM, Hudson J Jr, Boguski MS, et
al: The transcriptional program in the response of human
fibroblasts to serum. Science. 283:83–87. 1999. View Article : Google Scholar
|
|
34
|
Tsimplouli C, Demetzos C,
Hadzopoulou-Cladaras M, Pantazis P and Dimas K: In vitro activity
of dietary flavonol congeners against human cancer cell lines. Eur
J Nutr. 51:181–190. 2012. View Article : Google Scholar
|
|
35
|
Dimas K, Demetzos C, Vaos B, Marselos M
and Kokkinopoulos D: Cytotoxic and antiproliferative effects of
heptaacetyltiliroside on human leukemic cell lines. Leuk Res.
23:1021–1033. 1999. View Article : Google Scholar
|
|
36
|
Chatterjee M, Ben-Josef E, Thomas DG,
Morgan MA, Zalupski MM, Khan G, Andrew Robinson C, Griffith KA,
Chen CS, Ludwig T, et al: Caveolin-1 is associated with tumor
progression and confers a multi-modality resistance phenotype in
pancreatic cancer. Sci Rep. 5:108672015. View Article : Google Scholar :
|
|
37
|
Alshenawy HA and Ali MA: Differential
caveolin-1 expression in colon carcinoma and its relation to
E-cadherin-β-catenin complex. Ann Diagn Pathol. 17:476–482. 2013.
View Article : Google Scholar
|
|
38
|
Erkan M, Kurtoglu M and Kleeff J: The role
of hypoxia in pancreatic cancer: A potential therapeutic target.
Expert Rev Gastroenterol Hepatol. 10:301–316. 2016. View Article : Google Scholar
|
|
39
|
Verbeke C: Morphological heterogeneity in
ductal adenocarcinoma of the pancreas - Does it matter.
Pancreatology. 16:295–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Deer EL, González-Hernández J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar
|
|
41
|
Han F and Zhu HG: Caveolin-1 regulating
the invasion and expression of matrix metalloproteinase (MMPs) in
pancreatic carcinoma cells. J Surg Res. 159:443–450. 2010.
View Article : Google Scholar
|
|
42
|
Cordes N, Frick S, Brunner TB, Pilarsky C,
Grützmann R, Sipos B, Klöppel G, McKenna WG and Bernhard EJ: Human
pancreatic tumor cells are sensitized to ionizing radiation by
knockdown of caveolin-1. Oncogene. 26:6851–6862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Di Vizio D, Morello M, Sotgia F, Pestell
RG, Freeman MR and Lisanti MP: An absence of stromal caveolin-1 is
associated with advanced prostate cancer, metastatic disease and
epithelial Akt activation. Cell Cycle. 8:2420–2424. 2009.
View Article : Google Scholar
|
|
44
|
Martinez-Outschoorn UE, Pavlides S,
Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco
G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, et al: Tumor
cells induce the cancer associated fibroblast phenotype via
caveolin-1 degradation: Implications for breast cancer and DCIS
therapy with autophagy inhibitors. Cell Cycle. 9:2423–2433. 2010.
View Article : Google Scholar
|
|
45
|
Bonuccelli G, Whitaker-Menezes D,
Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, Witkiewicz AK,
Vander Heiden MG, Migneco G, Chiavarina B, et al: The reverse
Warburg effect: Glycolysis inhibitors prevent the tumor promoting
effects of caveolin-1 deficient cancer associated fibroblasts. Cell
Cycle. 9:1960–1971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pavlides S, Tsirigos A, Vera I, Flomenberg
N, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG,
et al: Loss of stromal caveolin-1 leads to oxidative stress, mimics
hypoxia and drives inflammation in the tumor microenvironment,
conferring the ‘reverse Warburg effect’: A transcriptional
informatics analysis with validation. Cell Cycle. 9:2201–2219.
2010. View Article : Google Scholar
|
|
47
|
Capozza F, Trimmer C, Castello-Cros R,
Katiyar S, Whitaker- Menezes D, Follenzi A, Crosariol M, Llaverias
G, Sotgia F, Pestell RG, et al: Genetic ablation of Cav1
differentially affects melanoma tumor growth and metastasis in
mice: Role of Cav1 in Shh heterotypic signaling and
transendothelial migration. Cancer Res. 72:2262–2274. 2012.
View Article : Google Scholar
|
|
48
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar
|
|
49
|
Özdemir BC, Pentcheva-Hoang T, Carstens
JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C,
Novitskiy SV, et al: Depletion of carcinoma-associated fibroblasts
and fibrosis induces immunosuppression and accelerates pancreas
cancer with reduced survival. Cancer Cell. 25:719–734. 2014.
View Article : Google Scholar
|
|
50
|
Rhim AD, Oberstein PE, Thomas DH, Mirek
ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP,
Tattersall IW, et al: Stromal elements act to restrain, rather than
support, pancreatic ductal adenocarcinoma. Cancer Cell. 25:735–747.
2014. View Article : Google Scholar
|
|
51
|
Hessmann E, Patzak MS, Klein L, Chen N,
Kari V, Ramu I, Bapiro TE, Frese KK, Gopinathan A, Richards FM, et
al: Fibroblast drug scavenging increases intratumoural gemcitabine
accumulation in murine pancreas cancer. Gut. 67:497–507. 2018.
View Article : Google Scholar :
|
|
52
|
Bocci G, Fioravanti A, Orlandi P, Di
Desidero T, Natale G, Fanelli G, Viacava P, Naccarato AG, Francia G
and Danesi R: Metronomic ceramide analogs inhibit angiogenesis in
pancreatic cancer through up-regulation of caveolin-1 and
thrombos-pondin-1 and down-regulation of cyclin D1. Neoplasia.
14:833–845. 2012. View Article : Google Scholar
|
|
53
|
Ma Y, Lin Z, Fallon JK, Zhao Q, Liu D,
Wang Y and Liu F: New mouse xenograft model modulated by
tumor-associated fibroblasts for human multi-drug resistance in
cancer. Oncol Rep. 34:2699–2705. 2015. View Article : Google Scholar
|