Introduction
Oral squamous cell carcinoma (OSCC) is one of the
most common malignancy types globally, with an annual incidence of
354,864 cases according to a paper published in 2018 (1). The majority of OSCC cases in the oral
mucosa evolve from oral premalignant lesions (2). Although erythroplakia has an
increased malignant transformation rate, oral leukoplakia is more
common in the clinic (3). Oral
leukoplakia is one of the most common types of precancerous lesion
(4), but its pathogenesis and
carcinogenesis have not yet been thoroughly elucidated. Generally,
the clinical treatment of oral leukoplakia includes photodynamic
therapy, microwave therapy and surgical resection (5). However, oral leukoplakia is prone to
recur and transform into carcinoma in situ, which notably
affects the quality of life of patients in a negative way (6).
Malignant transformation is not only attributable to
the cancer cells, as the complex biological interactions between
the tumor and the stroma-tumor microenvironment (TME) also
contribute to this transformation (7,8). The
TME involves different types of cells, including mesenchymal stem
cells (MSCs), fibroblasts, immune cells and endothelial cells
(9). During carcinogenesis, MSCs
sustain tumor cell growth and downregulate antitumor effector
lymphocytes (10). MSCs also
inhibit the antitumor immune response through carcinoma-associated
fibroblasts or bone marrow stromal cells (11). However, the nature of the
association between MSCs and tumor cells is controversial (12). In mouse xeno-graft models, tumor
growth could be inhibited following the injection of MSCs (13,14).
MSC may inhibit tumor growth by increasing inflammatory
infiltration, inhibiting angiogenesis and suppressing
tumor-associated signaling pathways (15). MSCs derived from oral mucosa have a
distinct neural crest origin and possess superior immunomodulation,
exhibiting a number of unique stem cell-like properties, including
enhanced proliferative capacity, compared with MSCs derived from
bone marrow and other postnatal tissues (16,17).
Therefore, MSCs derived from oral tissues may have different
properties. In oral leukoplakia, whether local MSCs participate in
the process of carcinogenesis has not yet been thoroughly
elucidated.
Cell-to-cell interactions are direct and indirect,
and the exchange of extracellular vesicles has profound effects
(18). Exosomes are vesicles
secreted by the majority of cells and are rich in protein, mRNA,
microRNA (miRNA) and lipids that mediate cell-to-cell signaling
(19-21). The contents in an exosome vary
according to cell type and environment (22). Recent studies demonstrated that
exosomes encapsulating microRNAs are delivered to regulate
recipient cells through serum (23,24)
or saliva (25). Thus, exosomes
are promising prognostic biomarkers and are targets for the
treatment of various types of disease.
In the present study, it was proposed that exosomes
secreted by MSCs are influenced by abnormal microenvironments and
MSCs derived from oral leukoplakia with dysplasia (LK-MSC) may be
the pivotal factor in the process of oral carcinogenesis.
Additionally, the exosomal miR-8485 was also investigated and the
tumor promotion function was identified.
Materials and methods
Clinical samples
Oral mucosal tissues were obtained from clinical
patients in the Peking University School of Stomatology (Beijing,
China) from September 2016. to September 2018. The patients were
suffering from mucosal cysts or the third molar extraction (n=16),
oral dysplasia (n=17) and oral carcinoma (n=15). The inclusion
criteria were as follows: For oral normal mucosal tissues, biopsy
results indicated that there was no inflammation; for dysplasia
tissues, the clinical diagnosis was oral leukoplakia or erythema
and the biopsy results were mild to moderate hyperplasia; and for
carcinoma tissues, the clinical diagnosis was carcinoma and biopsy
results were early infiltration or carcinoma in situ. All
patients provided informed consent. The patient information is
presented in Table I. The present
study was approved by the Ethics Committee of the Peking University
School of Stomatology (Beijing, China).
 | Table IClinical characteristic of the
patients for reverse transcription-quantitative polymerase chain
reaction. |
Table I
Clinical characteristic of the
patients for reverse transcription-quantitative polymerase chain
reaction.
| Group (n) | Median age ±
standard deviation (years) | Male, n | Female, n |
|---|
| Normal (16) | 38±11 | 6 | 10 |
| Dysplasia (17) | 51±14 | 10 | 7 |
| Carcinoma (15) | 56±13 | 4 | 11 |
Cell culture
Human MSCs were isolated and identified according to
a previous study (26). Briefly,
the primary tissues were treated by dispase (2 mg/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in sterile PBS and
the mesenchyme part was separated and cut into 2 mm3,
and then cultured in a 60-mm dish in MSC medium (Sciencell Research
Laboratories, Inc., San Diego, CA, USA) for 5-7 days at 37°C. The
MSCs were obtained from three donors for each type and the patient
information is presented in Table
II. Cells at passages 3-5 were used for the subsequent
experiments. The DOK cell line (oral hyperplasia cell line) was
purchased from the Cell Laboratory of Central South University
(Changsha, China) and the SCC15 cell line (oral carcinoma cell
line) was provided by the Department of Pathology, Peking
University School of Stomatology (Beijing, China). DOK cells were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and SCC15 cells were cultured in Dulbecco's
modified Eagle's medium: F12 (DMEM:F12; Gibco; Thermo Fisher
Scientific, Inc.). All cells were cultured at 37°C in a humidified
5% CO2 atmosphere with 10% fetal bovine serum (FBS).
 | Table IIClinical characteristic of the
patients for primary cell culture. |
Table II
Clinical characteristic of the
patients for primary cell culture.
| Patient | Sex | Age, years |
|---|
| N-MSC-1 | Female | 31 |
| N-MSC-2 | Female | 35 |
| N-MSC-3 | Male | 42 |
| LK-MSC-1 | Female | 32 |
| LK-MSC-2 | Male | 40 |
| LK-MSC-3 | Female | 54 |
| Ca-MSC-1 | Male | 29 |
| Ca-MSC-2 | Female | 48 |
| Ca-MSC-3 | Male | 55 |
Osteogenic and adipogenic
differentiation
The MSCs were characterized by differentiation into
osteogenic and adipogenic lineages according to the protocols of
our previous study (26). For
osteogenic differentiation, 10 nM dexamethasone, 10 mM
β-glycerophosphate, 0.1 mM L-ascorbic acid-2-phosphate and 2 mM
glutamine (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were
supplemented in α-minimum Eagle's medium (α-MEM; Gibco; Thermo
Fisher Scientific, Inc.) for cell culture at 37°C in a humidified
5% CO2 atmosphere. For adipogenic differentiation, 1
µM dexamethasone, 0.5 mM 3-isobutyl-ethylxanthine, 10 mg/ml
insulin, 60 mM indomethacin and 2 mM glutamine were supplemented in
α-MEM. Alizarin Red S and Oil Red O staining assays were performed
respectively for 30 min at room temperature, to detect osteogenic
and adipogenic differentiation. The staining was imaged with a
light microscope (Olympus IX51; Olympus Corporation, Tokyo, Japan)
at ×10 magnification.
Cell proliferation assay
An IncuCyte® live-cell imager (Essen
Bioscience, Ann Arbor, MI, USA) was utilized for the proliferation
assay. In brief, cells (MSC, 1×103; SCC15,
5×103; and DOK, 3×103) were seeded in a
96-well-plate with 100 µl α-MEM (MSCs), DMEM:F12 (SCC15) or
RPMI-1640 (DOK cells), medium containing 10% FBS/well and incubated
at 37°C in a humidified 5% CO2 atmosphere for 2-5 days.
Fresh medium was applied every day. The plate was scanned, and
phase-contrast images were captured at ×10 magnification with a
light microscope (Nikon Corporation, Tokyo, Japan). The ratios of
cell growth confluence were analyzed.
Wound healing assay
The cells (MSC, 1×104; SCC15,
3×104; DOK, 3×104) were seeded in a
96-well-plate with 100 µl α-MEM, DMEM:F12 or RPMI-1640
medium containing 10% FBS at 37°C and then when a confluence of 90%
was reached, a uniform wound ~800 µm in width was inflicted
by a woundmaker (Essen Bioscience). To remove all cellular debris,
the cells were washed twice with PBS. Subsequently, the plates were
cultured in serum-free medium for 6 h (SCC15 cells, DMEM:F12
medium) or 24 h (MSC, α-MEM medium; and DOK cells, RPMI-1640
medium) at 37°C. The closure of the wounds was evaluated using a
light microscope (Nikon Corporation) at ×10 magnification and the
relative mobility width was calculated.
Cell invasion assay
A cell invasion assay was conducted using a
Transwell chamber (8 µm, 24-well insert; Corning Inc.,
Corning, NY, USA). The Transwell membranes were coated with
Matrigel (cat. no. 356234; BD Biosciences; Becton, Dickinson and
Company, Franklin Lakes, NJ, USA) prior to the seeding of SCC15 or
DOK cell suspensions (1×106 cells/ml) with medium (SCC15
cells, DMEM:F12 medium; and DOK cells, RPMI-1640 medium) without
FBS in the upper chamber. After culturing for 12 h, the cells
invading into the lower chambers, which contained 10% FBS and
RPMI-1640 medium for DOK cells and DMEM:F12 for SCC15 cells, were
fixed with methanol at room temperature for 20 min, stained with
0.1% crystal violet at room temperature for 5 min and observed in
≥6 fields using a light microscope (Olympus BX51; Olympus
Corporation) at ×20 magnification in order to count the cells.
Exosome isolation, characterization and
uptake
Exosomes were collected from the supernatants of the
MSCs cultured for 48 h in α-MEM containing 10% FBS and centrifuged
for 16 h at 100,000 × g at 4°C. The exosomes were then collected by
density gradient differential centrifugation (27). Briefly, the supernatants were
centrifuged at 300 × g for 10 min, 2,000 × g for 20 min and 10,000
× g for 30 min all at 4°C to remove the cell debris and large
vehicles. Subsequently, the supernatants were passed through
centrifugal filters at 5,000 × g for 30 min at 4°C. The
concentrated supernatants were centrifuged at 100,000 × g for 70
min at 4°C in a 30% sucrose/D2O solution, and were then
washed and purified with PBS by centrifugation at 5,000 × g for 30
min repeated 3 times at 4°C in centrifugal filters. The shape of
exosomes was observed with an electron microscope (JEOL, Ltd.,
Tokyo, Japan) at ×350,000 magnification. The concentration of the
total exosome proteins was quantified using a Bicinchoninic Acid
(BCA; Thermo Fisher Scientific, Inc.) protein assay. Exosomes were
labeled by PKH26 (Sigma-Aldrich; Merck KGaA), according to the
manufacturer's protocols. Briefly, 100 µg exosomes suspended
in PBS were added into 1 ml Dilute C, and then 4 µl PKH26
was added into Dilute C and mixed for 5 min at room temperature.
Subsequently, 2 ml 0.5% bovine serum albumin (Huaxingbio
Biotechnology, Beijing, China) was added to bind excess dye. The
labeled exosome suspensions were then centrifuged at 100,000 × g
for 70 min at 4°C and resuspended with 100 µl PBS. The
labeled exosomes were incubated with SCC15 cells for 24 h at 37°C.
Actin-Tracker Green [F-actin-fluorescein isothiocyanate (FITC);
Beyotime Institute of Biotechnology, Haimen, China] was used to
label the cytoskeleton at room temperature for 20 min and DAPI was
used to label the cellular nuclei at room temperature for 5 min,
respectively. The uptake images were captured with a LSM 5 Exciter
confocal laser scanning microscope (Carl Zeiss AG, Oberkochen,
Germany) at ×100 magnification. In total, 100 µg/ml exosomes
were predicted to be the best concentration and were therefore
applied in the subsequent experiments.
Western blotting
The proteins was extracted by
radioimmunoprecipitation assay lysis buffer (cat. no. R0020;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). The protein concentration was determined by BCA method. A
total of 30 µg proteins were loaded and separated on 10%
SDS-PAGE. The proteins were transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% evaporated milk at room temperature for 1 h, the
membrane was incubated with the mouse antibodies cluster of CD63
(1:1,000; cat. no. ab59479; Abcam, Cambridge, MA, USA) and CD9
(1:1,000; cat. no. ab92726; Abcam) and the rabbit antibody p53
(1:1,000; cat. no. 2527; Cell Signaling Technology, Inc., Danvers,
MA, USA) at 4°C overnight. The mouse antibody GAPDH (1:5,000; cat.
no. HX1828; Huaxingbio Biotechnology) was used as reference protein
at 4°C overnight. Goat FITC-conjugated anti-mouse IgG (1:10,000;
cat. no. HX2032; Huaxingbio Biotechnology) or goat anti-rabbit IgG
(1:5,000; cat. no. CW0103; CWbio, Beijing, China) was used as the
secondary antibody at room temperature for 30 min. The results were
detected with a chemiluminescence reagent (CWbio).
3D coculture model
A total of 5×105 MSCs were embedded in 1 ml rat tail
collagen type-I (Beijing Solarbio Science & Technology Co.,
Ltd.). After the collagen had solidified, the gel was overlaid with
5×105 SCC15 cells and then cultured at 37°C, 24 h later,
the gel was lifted at the cell-air interface with a Transwell
chamber (8 µm, 24-well insert; Corning Inc.) and cultured
for 5 days at 37°C with the stimulation of transforming growth
factor (TGF)-β1 (5 ng/ml; Proteintech Group, Inc., Chicago, IL,
USA). Meanwhile, GW4869 (10 mM; cat. no. HY-19363/CS-6865;
MedChemExpress, Monmouth Junction, NJ, USA), an inhibitor of
exosomal secretion, was added to the coculture models.
Subsequently, the cocultures were fixed with 4% paraformaldehyde
for 20 min at room temperature for the following staining
procedures.
Immunohistochemical staining and
haematoxylin and eosin (H&E) staining
The tissues were fixed by 4% paraformaldehyde at 4°C
for 24 h and the paraffin-embedded sections (5 µm) were then
dewaxed in xylene and dehydrated in ethanol (100, 95, 90, 80 and
70%). For immunohistochemical staining, the tissue sections were
placed into sodium citrate buffer (OriGene Technologies, Inc.,
Beijing, China) and heated to 120°C for 1 min for antigen
retrieval. Subsequently, 3% hydrogen peroxide was applied as
blocking reagent at room temperature for 10 min. CK-Pan (1:1,000;
cat. no. 4545; Cell Signaling Technology, Inc.) and vimentin
(1:1,000; cat. no. ab92547; Abcam) primary antibodies were applied
to the tissue sections overnight at 4°C. The endogenous peroxidase
activity was blocked by 3% hydrogen peroxide pretreatment. The
sections were then incubated with peroxidase-conjugated mouse
anti-goat secondary antibody (ready to use; cat. no. PV-9000;
OriGene Technologies, Inc.) for 30 min at room temperature, and DAB
staining at room temperature for 30 sec was used for staining. For
H&E staining, the tissue sections were stained followed by
standard protocols (28) after
dewaxing in xylene and dehydrated in ethanol (100, 95, 90, 80 and
70%). The results were observed with a light microscope (Olympus
BX51; Olympus Corporation) at ×20 magnification.
Reverse transcription-quantitative
polymerase chain reaction (PCR) and microarray analysis
Total RNA was extracted by TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) from the 6-well plates
and tissue RNA was obtained, according to standard protocols
(29). An All-in-One™ First-Strand
cDNA Synthesis kit (cat. no. QP008; GeneCopoeia, Inc., Rockville,
MD, USA) was used to reverse transcribe the miRNAs. Subsequently,
quantitative PCR was performed and the expression of miR-8485 (cat.
no. HmiRQP4656; GeneCopoeia, Inc.) was normalized to the expression
of U6 [Sangon Biotech Co., Ltd., Shanghai, China; sequence (5′-3′),
forward primer: CTCGCTTCGGCAGCACA; and reverse primer: AACGCTT
CACGAATTTGCGT]. The experiment was performed with an Applied
Biosystems 7500 instrument. A 20 µl reaction system was used
with 10 µl 2X SYBR® Green (Roche Applied Science,
Rotkreuz, Switzerland), 1 µl primer, 1 µl 2X
Universal Adapter (cat. no. QP029; GeneCopoeia, Inc.), 2 µl
cDNA and 2 µl H2O. Reactions were incubated at
95°C for 10 min; 95°C for 15 sec; 60°C for 30 sec; 72°C for 20 sec
for 40 cycles; 95°C for 15 sec; 60°C for 1 min; 95°C for 15 sec;
60°C for 15 sec. The results were analyzed using the
2-∆∆Cq method (30).
Microarray analysis was performed by Sangon Biotech Co., Ltd.,
according to standard Agilent protocols (31-33).
miRNA transfection
DOK or SCC15 cells were pretreated with 50 nM
miR-8485 mimics (cat. no. miR1180323091501) or negative control
(NC-mimics; cat. no. miR1N0000001), as well as 100 nM miR-8485
inhibitor (cat. no. miR2180709052553) or the corresponding negative
control (NC-inhibitor; cat. no. miR2N0000001) (Guangzhou RiboBio
Co., Ltd., Guangzhou, China) for 48 h at 37°C.
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.) was
used to transfer the mimics or inhibitors into the cells, according
to the manufacturer's instructions.
Statistical analysis
All data conforming to a normal distribution is
expressed as the mean ± standard deviation. For all other data, the
median ± standard deviation is expressed. A Student's t-test, least
significant difference test of one-way analysis of variance or the
Mann-Whitney U test was applied to evaluate differences among
groups using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL,
USA). All in vitro data was repeated at least 3 independent
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification and comparison of MSCs
derived from normal oral mucosa (N-MSCs), LK-MSCs and oral
carcinoma (Ca-MSCs)
N-MSCs, LK-MSCs and Ca-MSCs were successfully
generated according to the methods of our previous study (26). For osteogenic and adipogenic
differentiation, all of the MSCs formed mineralized nodules and oil
droplets, and there was no statistically significant difference
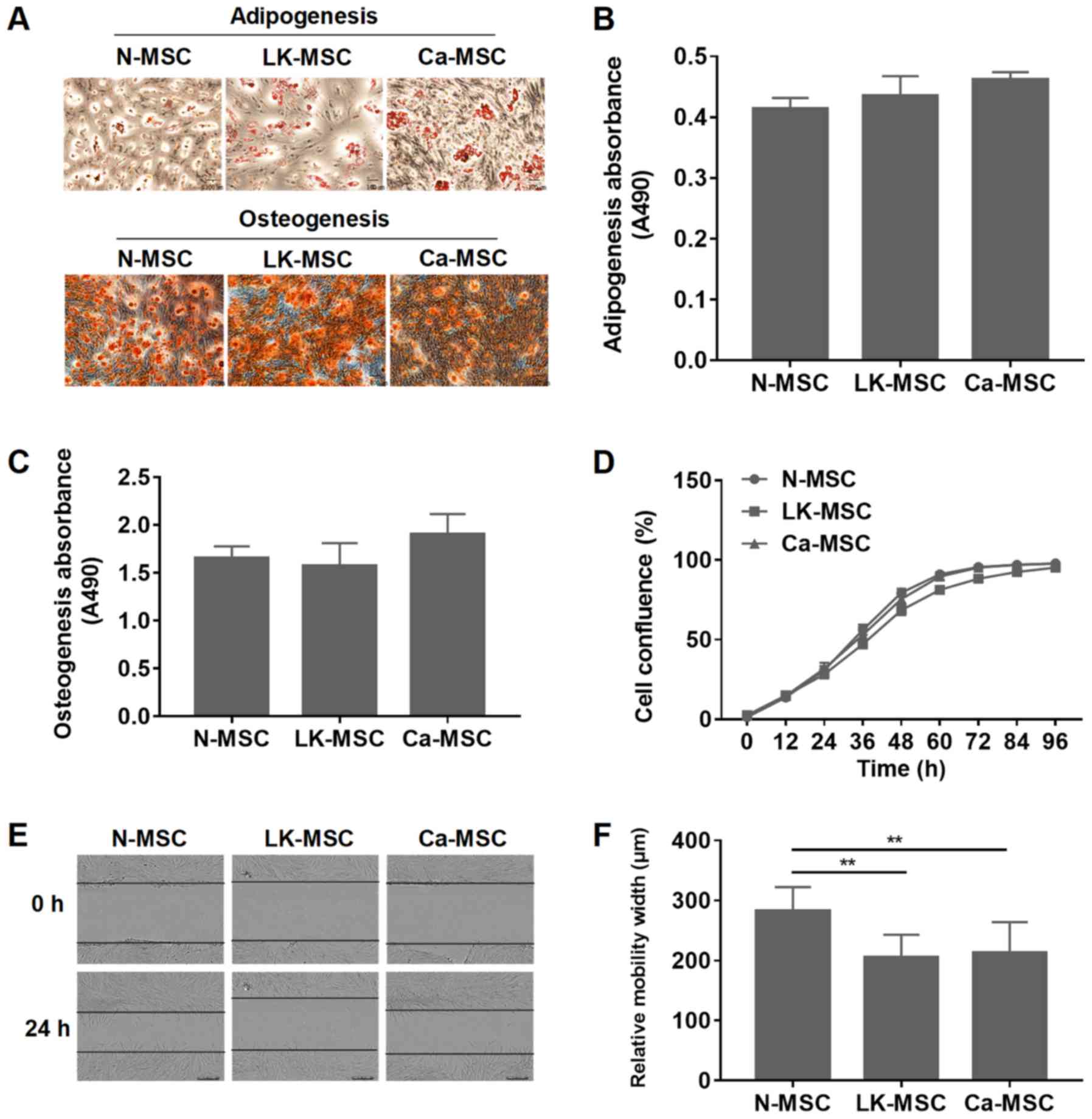
among the three groups (P>0.05; Fig. 1A-C). However, the proliferation
rate of the LK-MSCs was reduced at 24-72 h, compared with N-MSCs
and Ca-MSCs (Fig. 1D). The wound
healing assay demonstrated that compared with the N-MSCs, the
migration rates of the LK-MSCs and Ca-MSCs were significantly
reduced; however, there was no significant difference between those
of the LK-MSCs and Ca-MSCs (P>0.05; Fig. 1E and F).
Characterization of MSC-derived
exosomes
In oral premalignant lesions, the interaction
between epithelial cells and MSCs is probably via paracrine
signaling with cytokines or extracellular vesicles. Exosomes are
small membrane vesicles (diameter, 30-200 nm) that are
constitutively released via fusion with the cell membrane (22). In order to investigate whether
exosomes participate in the interaction between MSCs and epithelial
cells, exosomes from MSCs were isolated and characterized in the
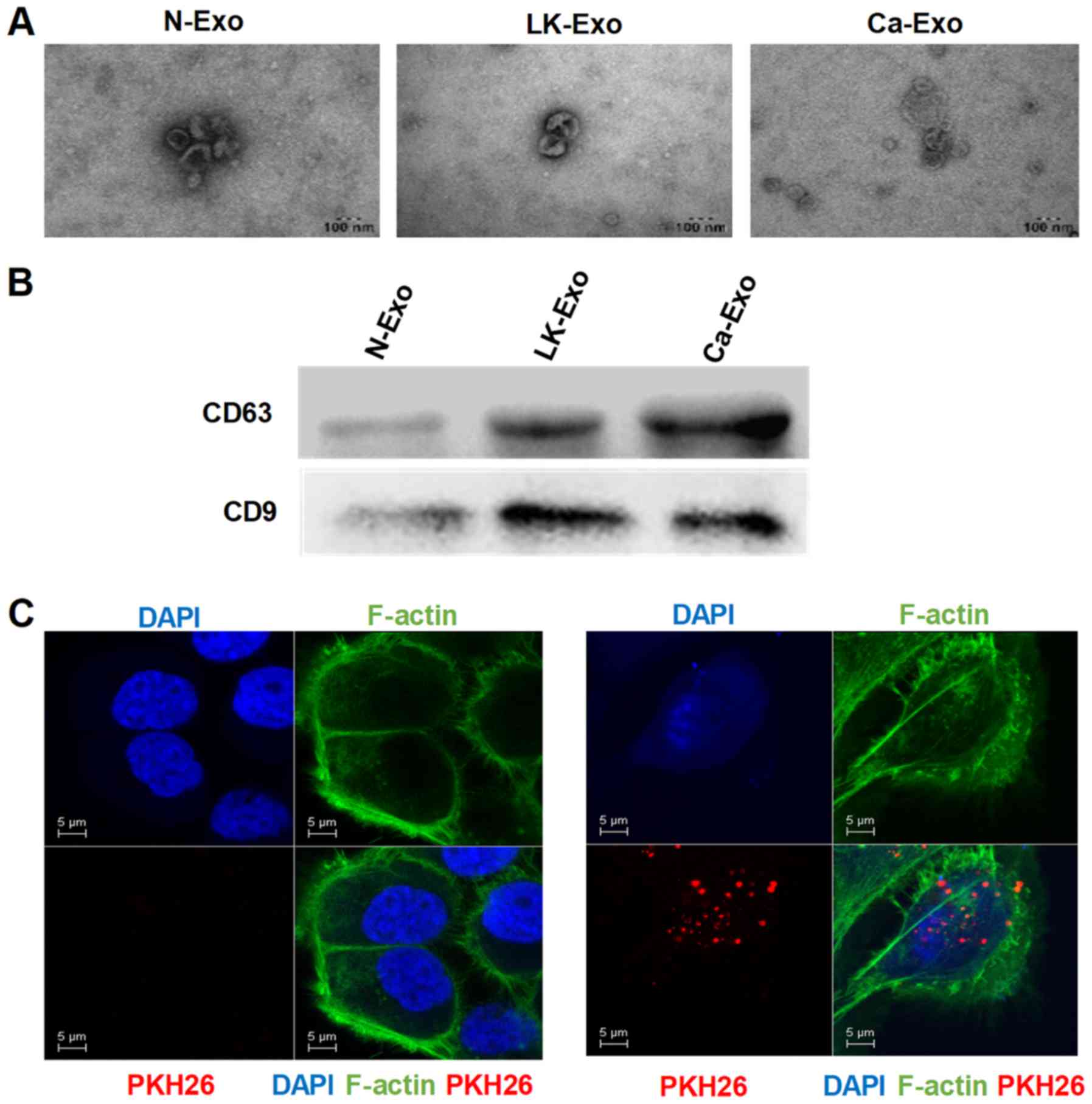
present study. The electron microscopy results revealed that the
exosomes had a cup-shaped morphology with diameters of <100 nm
(Fig. 2A). Additionally, CD63 and
CD9 were enriched among the exosome proteins (Fig. 2B). To confirm the uptake of
exosomes, the fluorescent dye PKH26 was applied to label the
exosomes. The PKH26-labeled exosomes were localized in the
cytoplasm of the SCC15 cells (Fig.
2C), indicating that exosomes can be internalized by tumor
cells.
LK-MSC- and Ca-MSC-derived exosomes
enhance the proliferation, migration and invasion abilities of
epithelial cells
To identify the function of the exosomes, SCC15 and
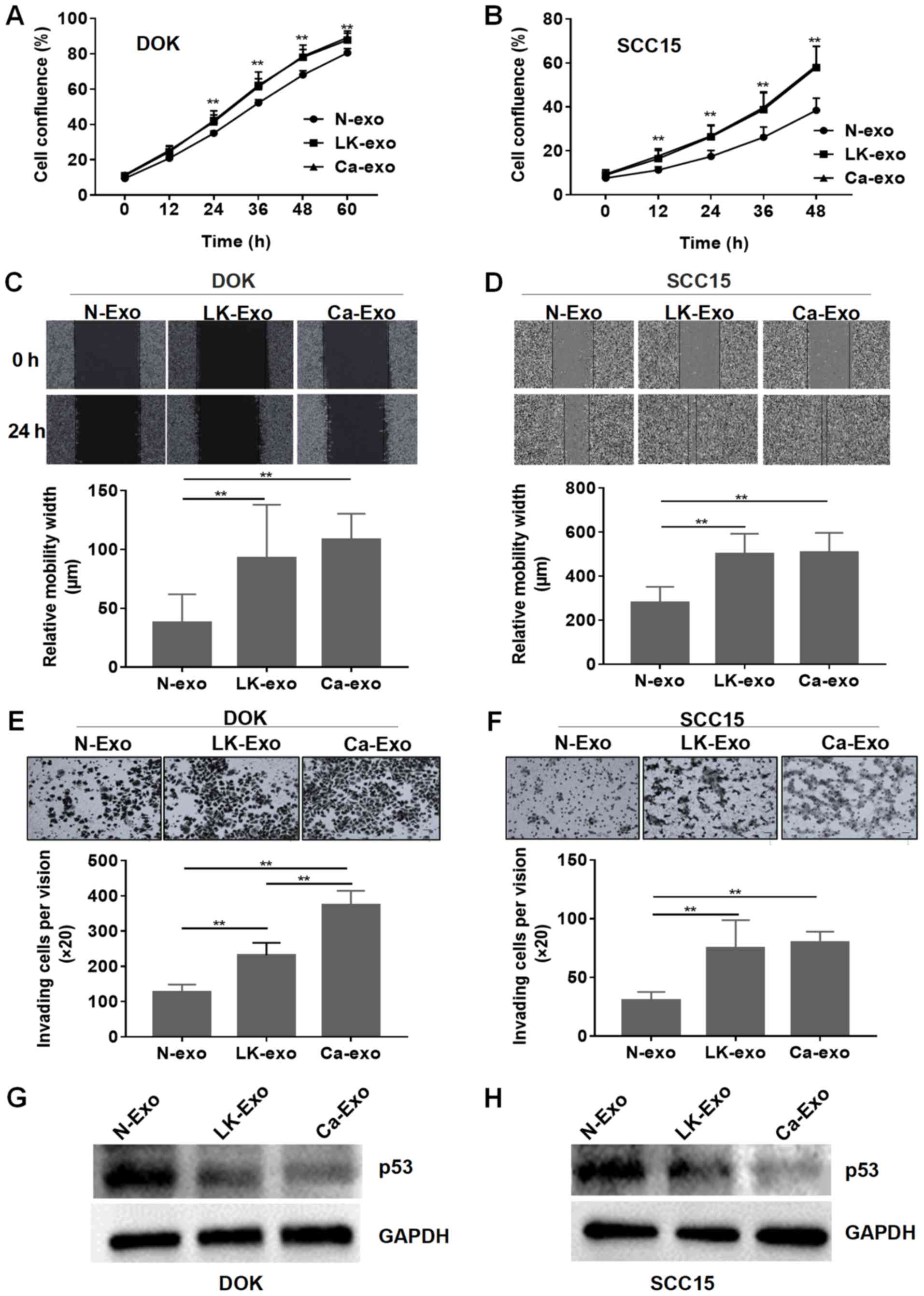
DOK cells were treated with them separately. The cell proliferation
assay demonstrated that the exosomes from the LK-MSCs (LK-exo) and
Ca-MSCs (Ca-exo) accelerated the proliferation of DOK and SCC15
cells, compared with the exosomes derived from the N-MSCs (N-exo)
(P<0.01; Fig. 3A and B).
However, there was no significant difference between the LK-MSC and
Ca-MSC groups (P>0.05).
Subsequently, the role of the exosomes secreted by
MSCs in inducing migration and invasion was investigated. The wound
healing assay demonstrated that the relative migration widths of
the SCC15 and DOK cells pretreated with LK-exo and Ca-exo were
significantly increased, compared with the N-exo-treated group
(P<0.01; Fig. 3C and D). The
Transwell cell invasion experiment revealed that the LK-exo and
Ca-exo groups had an increased number of invading cells, compared
with the N-exo group (Fig. 3E and
F). The p53 tumor suppressor gene is widely reported to be
implicated in oral carcinogenesis (34-36).
The western blot assay in the present study demonstrated that the
LK-exo- and Ca-exo-pretreated SCC15 and DOK cells exhibited reduced
expression of p53, compared with the N-exo-pretreated group
(Fig. 3G and H). These results
implied that exosomes secreted by LK-MSCs have similar functions to
those secreted by Ca-MSCs, which indicates that exosomes secreted
by residual LK-MSCs may be a cause of the recurrence of oral
leukoplakia and may accelerate the process of malignant
transformation, which will be further identified in animal
models.
Interaction between MSCs and tumor cells
in a 3D coculture model
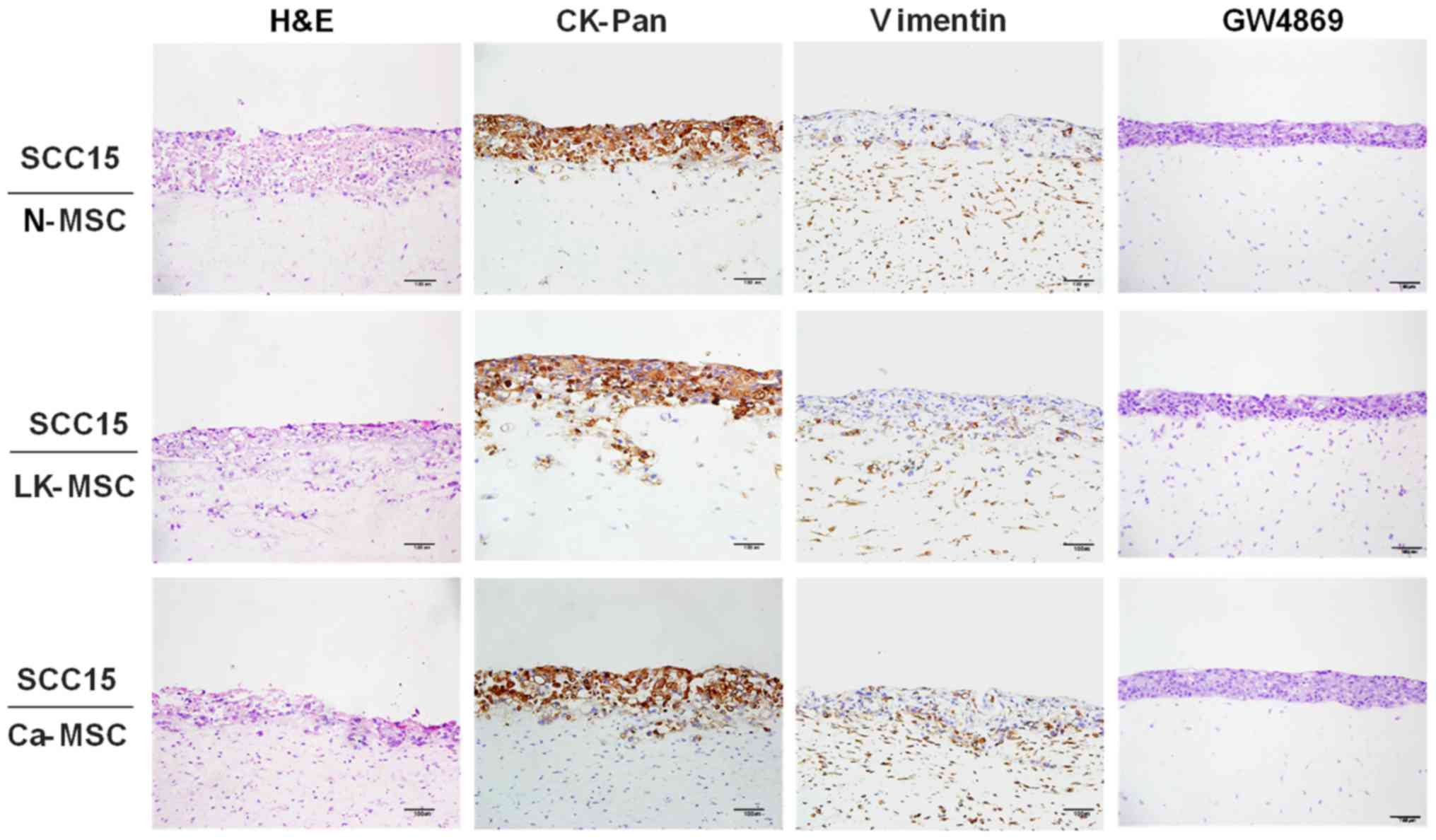
In order to fully understand the interaction between
MSCs and epithelial cells, a TGF-β1-3D-coculture model was prepared
to mimic in vivo interactions (37). Immunohistochemical staining of
CK-Pan and vimentin was conducted to clarify the boundaries between
the MSCs and SCC15 cells. Notably, the LK-MSCs and Ca-MSCs,
particularly LK-MSCs, were more susceptible to TGF-β1 stimulation,
compared with N-MSCs, promoting SCC15 cell invasion via collagen
hydrolysis and fracture, with CK-Pan expressed in a deeper part of
the MSCs; however, the integrity of the basement membrane was
maintained in the N-MSC group. Furthermore, the enhancing nature of
LK-MSC- and Ca-MSC-derived exosomes was blocked by the exosomal
secretion inhibitor GW4869, as they exhibited similar H&E
staining to the N-MSC group (Fig.
4).
Exosomes derived from LK-MSCs and Ca-MSCs
contain increased miR-8485, compared with N-MSCs
To investigate whether miRNAs contained in exosomes
from different MSCs function differently, microarray analysis was
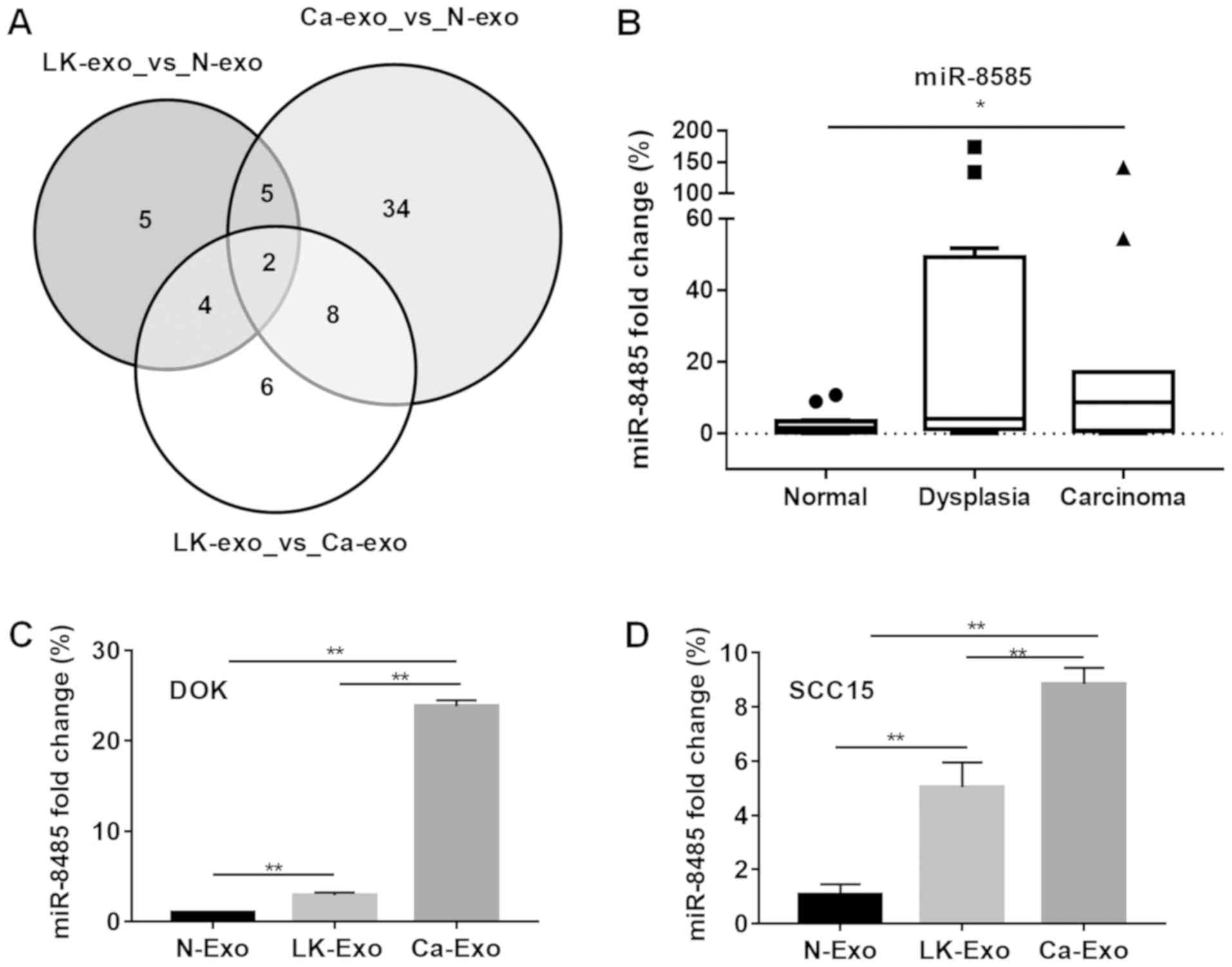
performed. The results demonstrated that there were 16
differentially-expressed miRNAs between the N-exo and LK-exo
groups, 49 between the N-exo and Ca-exo groups, and 20 between the
LK-exo and Ca-exo groups. Among these miRNAs, the expression levels
of miR-4433a and miR-8485 were different between all three groups
(Fig. 5A). Therefore, miR-8485,
which may participate in manipulating tumor cells, was selected for
the subsequent experiments. The clinical tissues and cells were
treated with the exosomes. The expression levels of miR-8485 were
elevated in the oral carcinoma tissues (P<0.05) and oral
dysplasia tissues, compared with the normal mucosa tissues
(Fig. 5B). Furthermore, the cells
treated with LK-exo and Ca-exo expressed significantly increased
levels of miR-8485, compared with the N-exo group (P<0.01;
Fig. 5C and D).
miR-8485 promotes the proliferation,
migration and invasion abilities of epithelial cells
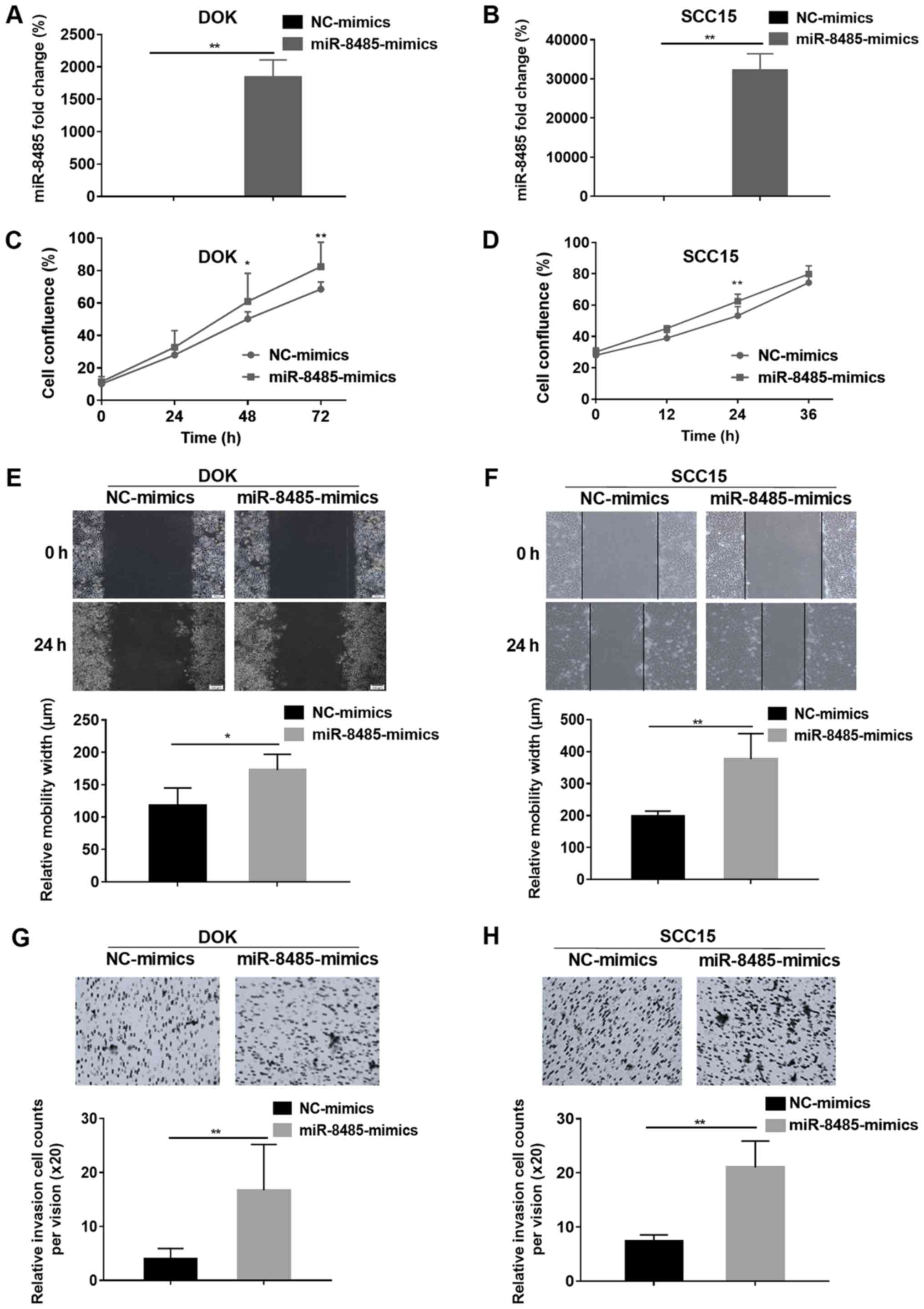
Based on the aforementioned results, miR-8485 mimics
were transfected into DOK and SCC15 cells and the transfection
efficiency was assessed by a reverse transcription-quantitative PCR
assay (Fig. 6A and B). Compared
with the NC-mimics group, the miR-8485 mimics caused rapid growth
of the DOK (48 and 72 h) and SCC15 cells (24 h) (P<0.01;
Fig. 6C and D). Overexpression of
miR-8485 promoted migration of the DOK cells (P<0.05) and SCC15
cells (P<0.01) (Fig. 6E and F).
Furthermore, the cell invasion assay demonstrated that the miR-8485
mimics increased the invasive ability of the DOK and SCC15 cells
(P<0.01; Fig. 6G and H).
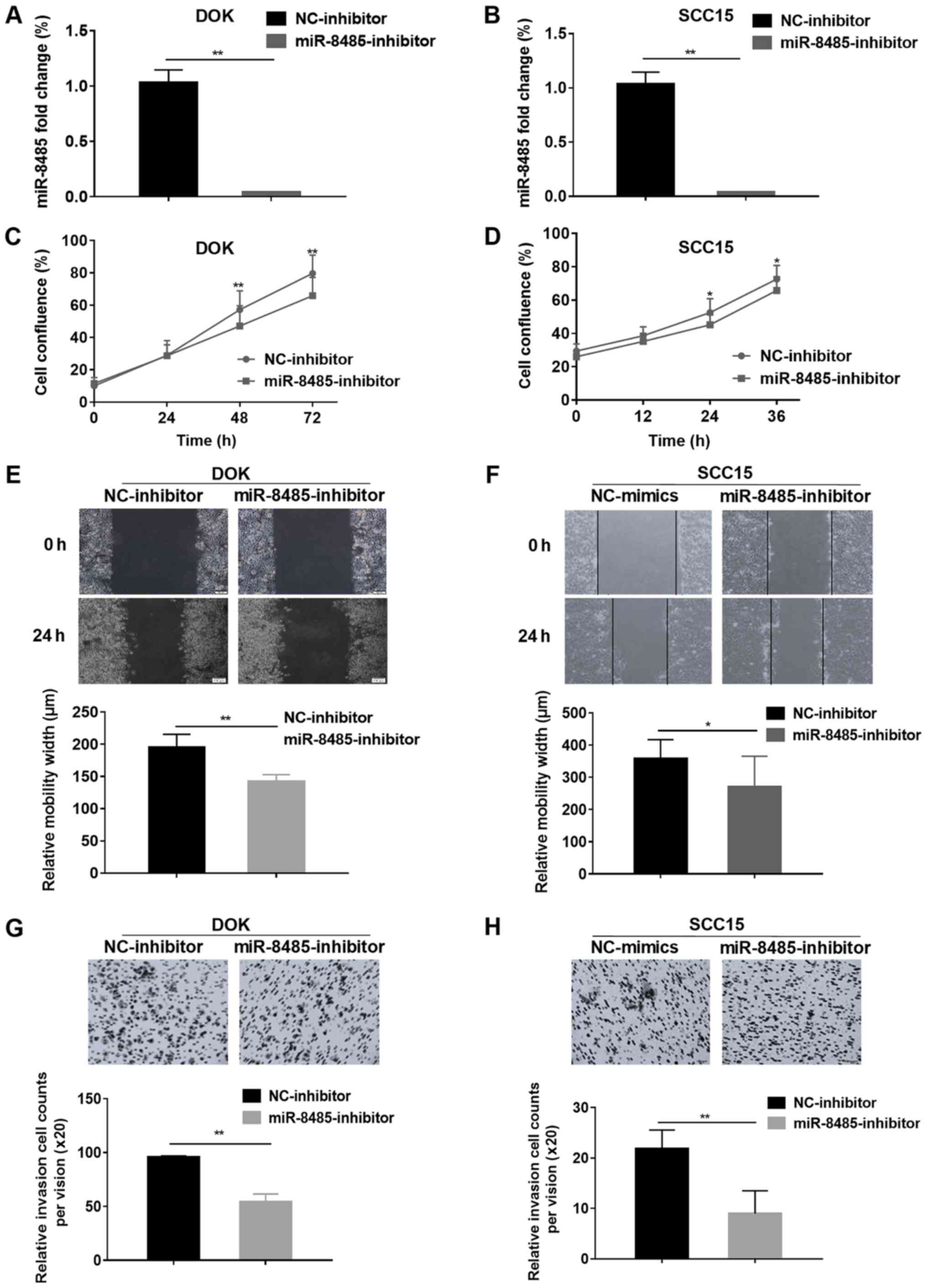
To further confirm the effects of miR-8485, DOK and
SCC15 cells were transfected with a miR-8485 inhibitor (Fig. 7A and B). As expected, the DOK and
cells transfected with the miR-8485 inhibitor exhibited marked
inhibition of proliferation at 48 and 72 h, and the SCC15 cells at
24 and 36 h (Fig. 7C and D).
Additionally, the miR-8485 inhibitor inhibited the migrative and
invasive ability of the DOK and SCC15 cells in the wound healing
assay and Matrigel cell invasion assay (P<0.01; Fig. 7E-H).
Discussion
Oral leukoplakia is one of the common oral
potentially malignant disorders worldwide, and the malignant
transformation rate of oral leukoplakia varies between 0.13-34%
(38). To date, studies on the
etiology of oral leukoplakia carcinogenesis have primarily focused
on local irritation factors, including abuse of tobacco and
alcohol, infection factors, such as human papillomavirus (39), and epithelial cell factors,
including oxidative stress injury on DNA (40). However, recent studies demonstrated
that microenvironmental factors serve a critically important role
in tumor development (9,41). MSCs are a cellular component of the
TME, and the interaction between MSCs and tumor cells is
bidirectional (12). In normal
tissues, MSCs maintain normal structure and function, as well as
organizational homeostasis (42).
However, during the process of malignant transformation, MSCs are
vulnerable and acquire the abnormal phenotypes of tumor cells,
thereby sustaining cancer cell growth and tumor progression
(43). In the present study, MSCs
derived from normal mucosa, oral leukoplakia and oral carcinoma
in situ tissues were separated. Compared with the N-MSCs,
the LK-MSCs and Ca-MSCs exhibited a decreased migration capacity.
The functions of MSCs are notably diverse and depend on the
tissue-specific origins and the special microenvironment in which
MSCs are embedded (44). During
the process of carcinogenesis, MSC heterogeneity is characterized
by altered proliferative capacity and aging properties, which may
also include epigenetic changes (45). The result indicates that there is a
process of functional transformation in MSCs during the TME
maturing.
In the 3D coculture models, which were affected by
oral epithelial dysplasia or carcinoma in situ, the LK-MSCs
and Ca-MSCs exhibited clear compatibility with the tumor cells.
Furthermore, the LK-MSCs were more vulnerable and sensitive to the
TGF-β1 stimulation, thus promoting the migration and invasion
capacity of the tumor cells via the hydrolysis and rupture of
collagen type I. Therefore, MSCs are affected by epithelial cells,
and thus MSCs could regulate epithelial cells by negative
feedback.
As previously described, the exosome is capable of
mediating intercellular communication between cells (46). Exosomes secreted by MSCs
orchestrate various autocrine and paracrine functions, including
receptor-binding, direct fusion or endocytosis by target cells
(47), thereby transforming the
biological behavior of epithelial cells (48,49).
The exosome contents of MSCs cocultured with tumor cells differs
from normal MSCs (50). Blocking
the secretion of exosomes using the exosomal inhibitor GW4869
reverses the development of diseases caused by MSCs (51,52).
Additionally, with regards to histocompatibility and
reproducibility, exosomes have favorable application prospects in
cell-free treatments (53).
According to the present study, exosomes derived from MSCs from
normal mucosa or that suffering from oral leukoplakia with
dysplasia and carcinoma exhibit dissimilar effects. The exosomes
isolated from LK-MSCs exhibited similar effects to the Ca-MSCs,
promoting proliferation and migration, and reducing the expression
of p53 in SCC15 and DOK cells in vitro. Additionally, the
application of GW4869 reversed the promoting function of the
LK-MSC- and Ca-MSC-derived exosomes.
MicroRNAs are preferentially encapsulated by
exosomes (54). Exosomal microRNAs
have various effects on tumor biological behaviors, including
proliferation, migration and invasion (55), apoptosis and chemoresistance
(8) and epigenetic modification of
TME (56). Considering the
importance of exosomal microRNAs, the N-exo, LK-exo and Ca-exo
groups were subjected to microarray analysis in the present study.
miR-8485, a rarely reported gene that may be associated with tumor
development, was selected. In order to identify the function of
miR-8485 in the cells, transfection of mimics and inhibitors was
utilized. miR-8485 promoted the proliferation, migration and
invasion of the tumor cells in vitro, indicating that
miR-8485 is a novel microRNA associated with malignant
transformation.
In summary, the present study clarifies the function
of MSCs associated with both dysplastic oral keratinocyte cells and
tumor cells. LK-MSCs share similar effects with Ca-MSCs, and are
more susceptible to the surrounding environment. LK-MSCs may be
involved in the recurrence of oral leukoplakia and malignant
transformation by secreting exosomes. Additionally, the observation
that the exosomes contained miR-8485 demonstrates that miR-8485
acts as an oncogene in oral carcinogenesis and therefore is a
potential novel biomarker for clinical treatment. The present study
emphasizes the importance of MSCs derived from premalignant lesions
and the exosomes they secrete. As it is difficult to convert tumor
cells to normal cells, intervention with MSC-derived exosomes from
premalignant lesions may be an excellent choice to reduce the
malignant transformation rate during clinical therapy.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81771071 and
81772873), Beijing Natural Science Foundation (grant nos. 7172240
and 7182181), Nonprofit Industry Research Specific Fund of National
Health and Family Planning Commission of China (grant no.
201502018) and Foundation of Capital Health Development (grant no.
2014-2-4102).
Availability of data and materials
All data used or analyzed in this study are included
in this article.
Authors' contributions
WL performed the majority of the experiments and
wrote the manuscript. HL and YW made notable contributions to the
design, data interpretation and the manuscript revision. YH, ZZ, XJ
and XW were involved in the validation of data and responsible for
the statistic analysis. JJ, QW and XG helped with the collection of
tissue samples and ZC, ML and GW involved in the table drafting of
the manuscript. All authors have read and approved the
manuscript.
Ethics approval and consent to
participate
Experiments using tissue samples from human subjects
were approved by the Ethics Committee of the School of Stomatology,
Peking University. (Beijing, China). All patients provided written
informed consent for the whole study.
Patient consent for publication
All participants provided written informed consent
for the publication of the study.
Competing interest
The authors declare that they have no competing
interest.
Acknowledgments
Not applicable.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouquot JE and Whitaker SB: Oral
leukoplakia - rationale for diagnosis and prognosis of its clinical
subtypes or 'phases'. Quintessence Int. 25:133–140. 1994.PubMed/NCBI
|
|
3
|
Kao SY, Mao L, Jian XC, Rajan G and Yu GY:
Expert consensus on the detection and screening of oral cancer and
precancer. Chin J Dent Res. 18:79–83. 2015.PubMed/NCBI
|
|
4
|
van der Waal I, Schepman KP, van der Meij
EH and Smeele LE: Oral leukoplakia: A clinicopathological review.
Oral Oncol. 33:291–301. 1997. View Article : Google Scholar
|
|
5
|
Vohra F, Al-Kheraif AA, Qadri T, Hassan
MI, Ahmed A, Warnakulasuriya S and Javed F: Efficacy of
photodynamic therapy in the management of oral premalignant
lesions. A systematic review. Photodiagn Photodyn Ther. 12:150–159.
2015. View Article : Google Scholar
|
|
6
|
Bewley AF and Farwell DG: Oral leukoplakia
and oral cavity squamous cell carcinoma. Clin Dermatol. 35:461–467.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Au Yeung CL, Co NN, Tsuruga T, Yeung TL,
Kwan SY, Leung CS, Li Y, Lu ES, Kwan K, Wong KK, et al: Exosomal
transfer of stroma-derived miR21 confers paclitaxel resistance in
ovarian cancer cells through targeting APAF1. Nat Commun.
7:111502016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hui L and Chen Y: Tumor microenvironment:
Sanctuary of the devil. Cancer Lett. 368:7–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poggi A, Musso A, Dapino I and Zocchi MR:
Mechanisms of tumor escape from immune system: Role of mesenchymal
stromal cells. Immunol Lett. 159:55–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poggi A and Giuliani M: Mesenchymal
stromal cells can regulate the immune response in the tumor
microenvironment. Vaccines (Basel). 4. pp. 42016
|
|
12
|
Klopp AH, Gupta A, Spaeth E, Andreeff M
and Marini F III: Concise review: Dissecting a discrepancy in the
literature: do mesenchymal stem cells support or suppress tumor
growth? Stem Cells. 29:11–19. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khakoo AY, Pati S, Anderson SA, Reid W,
Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, et al:
Human mesenchymal stem cells exert potent antitumorigenic effects
in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Cao F, De A, Cao Y, Contag C,
Gambhir SS, Wu JC and Chen X: Trafficking mesenchymal stem cell
engraftment and differentiation in tumor-bearing mice by
bioluminescence imaging. Stem Cells. 27:1548–1558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rhee KJ, Lee JI and Eom YW: Mesenchymal
Stem Cell-Mediated Effects of Tumor Support or Suppression. Int J
Mol Sci. 16:30015–30033. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boink MA, van den Broek LJ, Roffel S,
Nazmi K, Bolscher JG, Gefen A, Veerman EC and Gibbs S: Different
wound healing properties of dermis, adipose, and gingiva
mesenchymal stromal cells. Wound Repair Regen. 24:100–109. 2016.
View Article : Google Scholar
|
|
17
|
Xu X, Chen C, Akiyama K, Chai Y, Le AD,
Wang Z and Shi S: Gingivae contain neural-crest- and
mesoderm-derived mesen-chymal stem cells. J Dent Res. 92:825–832.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Melzer C, von der Ohe J and Hass R:
Concise Review: Crosstalk of mesenchymal stroma/stem-like cells
with cancer cells Provides therapeutic potential. Stem Cells.
36:951–968. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xue X, Wang X, Zhao Y, Hu R and Qin L:
Exosomal miR-93 promotes proliferation and invasion in
hepatocellular carcinoma by directly inhibiting
TIMP2/TP53INP1/CDKN1A. Biochem Biophys Res Commun. 502:515–521.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JE, Eom JS, Kim WY, Jo EJ, Mok J, Lee
K, Kim KU, Park HK, Lee MK and Kim MH: Diagnostic value of
microRNAs derived from exosomes in bronchoalveolar lavage fluid of
early-stage lung adenocarcinoma: A pilot study. Thorac Cancer.
9:911–915. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong L, Bao Q, Hu C, Wang J, Zhou Q, Wei
L, Tong L, Zhang W and Shen Y: Exosomal miR-675 from metastatic
osteosarcoma promotes cell migration and invasion by targeting
CALN1. Biochem Biophys Res Commun. 500:170–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colombo M, Raposo G and Théry C:
Biogenesis, secretion, and intercellular interactions of exosomes
and other extracellular vesicles. Annu Rev Cell Dev Biol.
30:255–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sohn W, Kim J, Kang SH, Yang SR, Cho JY,
Cho HC, Shim SG and Paik YH: Serum exosomal microRNAs as novel
biomarkers for hepatocellular carcinoma. Exp Mol Med. 47:e1842015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tengda L, Shuping L, Mingli G, Jie G, Yun
L, Weiwei Z and Anmei D: Serum exosomal microRNAs as potent
circulating biomarkers for melanoma. Melanoma Res. 28:295–303.
2018.PubMed/NCBI
|
|
25
|
Machida T, Tomofuji T, Ekuni D, Maruyama
T, Yoneda T, Kawabata Y, Mizuno H, Miyai H, Kunitomo M and Morita
M: MicroRNAs in salivary exosome as potential biomarkers of aging.
Int J Mol Sci. 16:21294–21309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji X, Zhang Z, Han Y, Song J, Xu X, Jin J,
Su S, Mu D, Liu X, Xu S, et al: Mesenchymal stem cells derived from
normal gingival tissue inhibit the proliferation of oral cancer
cells in vitro and in vivo. Int J Oncol. 49:2011–2022. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang WW, Yang LQ, Zhao F, Chen CW, Xu LH,
Fu J, Li SL and Ge XY: Epiregulin promotes lung metastasis of
salivary adenoid cystic carcinoma. Theranostics. 7:3700–3714. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008. pdb prot4986. 2008
|
|
29
|
Rio DC, Ares M Jr, Hannon GJ and Nilsen
TW: Purification of RNA using TRIzol (TRI reagent). Cold Spring
Harb Protoc. 2010:pdb prot5439. 2010. View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Yin H and Killeen K: The fundamental
aspects and applications of Agilent HPLC-Chip. J Sep Sci.
30:1427–1434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
López-Romero P: Pre-processing and
differential expression analysis of Agilent microRNA arrays using
the AgiMicroRna Bioconductor library. BMC Genomics. 12:642011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sinevici N and O'sullivan J: Oral cancer:
Deregulated molecular events and their use as biomarkers. Oral
Oncol. 61:12–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verma R, Singh A, Jaiswal R, Chandra A,
Verma R and Tak J: Association of Ki-67 antigen and p53 protein at
invasive tumor front of oral squamous cell carcinoma. Indian J
Pathol Microbiol. 57:553–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang M, Li J, Wang L, Tian Z, Zhang P, Xu
Q, Zhang C, Wei F and Chen W: Prognostic significance of p21, p27
and survivin protein expression in patients with oral squamous cell
carcinoma. Oncol Lett. 6:381–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tkach M and Théry C: Communication by
extracellular vesicles: Where we are and where we need to go. Cell.
164:1226–1232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Warnakulasuriya S and Ariyawardana A:
Malignant transformation of oral leukoplakia: A systematic review
of observational studies. J Oral Pathol Med. 45:155–166. 2016.
View Article : Google Scholar
|
|
39
|
Chen X and Zhao Y: Human papillomavirus
infection in oral potentially malignant disorders and cancer. Arch
Oral Biol. 83:334–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Singla S, Singla G, Zaheer S, Rawat DS and
Mandal AK: Expression of p53, epidermal growth factor receptor,
c-erbB2 in oral leukoplakias and oral squamous cell carcinomas. J
Cancer Res Ther. 14:388–393. 2018.PubMed/NCBI
|
|
41
|
Yuan Y, Jiang YC, Sun CK and Chen QM: Role
of the tumor microenvironment in tumor progression and the clinical
applications (Review). Oncol Rep. 35:2499–2515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hass R: Retrodifferentiation - a mechanism
for cellular regeneration? Biol Chem. 390:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cortini M, Massa A, Avnet S, Bonuccelli G
and Baldini N: Tumor-activated mesenchymal stromal cells promote
osteosarcoma stemness and migratory potential via IL-6 secretion.
PLoS One. 11:e01665002016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Melzer C, Yang Y and Hass R: Interaction
of MSC with tumor cells. Cell Commun Signal. 14:202016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Majore I, Moretti P, Hass R and Kasper C:
Identification of subpopulations in mesenchymal stem cell-like
cultures from human umbilical cord. Cell Commun Signal. 7:62009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kourembanas S: Exosomes: Vehicles of
intercellular signaling, biomarkers, and vectors of cell therapy.
Annu Rev Physiol. 77:13–27. 2015. View Article : Google Scholar
|
|
47
|
Cocucci E, Racchetti G and Meldolesi J:
Shedding microvesicles: Artefacts no more. Trends Cell Biol.
19:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sharma A: Role of stem cell derived
exosomes in tumor biology. Int J Cancer. 142:1086–1092. 2018.
View Article : Google Scholar
|
|
49
|
Shi S, Zhang Q, Xia Y, You B, Shan Y, Bao
L, Li L, You Y and Gu Z: Mesenchymal stem cell-derived exosomes
facilitate nasopharyngeal carcinoma progression. Am J Cancer Res.
6:459–472. 2016.PubMed/NCBI
|
|
50
|
Schepers K, Campbell TB and Passegué E:
Normal and leukemic stem cell niches: Insights and therapeutic
opportunities. Cell Stem Cell. 16:254–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Richards KE, Zeleniak AE, Fishel ML, Wu J,
Littlepage LE and Hill R: Cancer-associated fibroblast exosomes
regulate survival and proliferation of pancreatic cancer cells.
Oncogene. 36:1770–1778. 2017. View Article : Google Scholar :
|
|
52
|
Wang B, Yao K, Huuskes BM, Shen HH, Zhuang
J, Godson C, Brennan EP, Wilkinson-Berka JL, Wise AF and Ricardo
SD: Mesenchymal stem cells deliver exogenous microRNA-let7c via
exosomes to attenuate renal fibrosis. Mol Ther. 24:1290–1301. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Phinney DG and Pittenger MF: Concise
Review: MSC-derived exosomes for cell-free therapy. Stem Cells.
35:851–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hannafon BN and Ding WQ: Intercellular
communication by exosome-derived microRNAs in cancer. Int J Mol
Sci. 14:14240–14269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang M, Zhao C, Shi H, Zhang B, Zhang L,
Zhang X, Wang S, Wu X, Yang T, Huang F, et al: Deregulated
microRNAs in gastric cancer tissue-derived mesenchymal stem cells:
Novel biomarkers and a mechanism for gastric cancer. Br J Cancer.
110:1199–1210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gajos-Michniewicz A, Duechler M and Czyz
M: MiRNA in melanoma-derived exosomes. Cancer Lett. 347:29–37.
2014. View Article : Google Scholar : PubMed/NCBI
|