Introduction
Cutaneous squamous cell carcinoma (SCC) is the
second most frequent skin cancer after basal cell carcinoma, and
the second leading cause of skin cancer-associated mortality after
malignant melanoma (1,2). Although the majority of SCC tumors
are resected at an early stage, SCC carries a risk of local
recurrence and lymph node metastasis. Investigations into molecules
associated with the unfavorable phenotypes of cancer are important
for the diagnosis and effective treatment of cutaneous SCC.
Toll-like receptor 4 (TLR4) is a transmembrane
protein and member of the Toll-like receptor family. TLR4 is well
known as a key regulator of innate immunity; however, its perturbed
expression has also been observed in a number of types of cancer,
including ovarian cancer (3),
pancreatic cancer (4),
hepatocellular carcinoma (5),
colorectal cancer (6,7), malignant melanoma (8) and skin cancers (9-11).
TLR4 may exert anti-tumor or pro-tumor effects, depending on the
tumor type and whether it is expressed in tumor cells or immune
cells (12-14). TLR4 is expressed in both normal and
pathologic skin cells, and is involved in several skin diseases,
including skin cancers (15-17).
Recent studies have demonstrated that the TLR4 antagonist,
resatorvid, blocks solar UV-induced skin tumorigenesis in mice
ex vivo and in vivo (9,10).
These studies have indicated that TLR4 may exert pro-tumor effects
and may thus be a suitable target with which to prevent
photo-carcinogenesis. Anti-tumor effects of TLR4 in cutaneous SCC,
however, have also been reported (11,18).
In a previous study, TLR4 knockdown by small hairpin RNA (shTLR4)
was shown to induce HaCaT keratinocyte proliferation in
vitro. Conversely, the overexpression of TLR4 in the SCC13 cell
line reduced proliferation compared to TLR4-negative SCC13 cells
in vitro. Moreover, the growth rate of TLR4-overexpressing
SCC13 tumors was attenuated compared to TLR4-negative SCC13 tumors
in a xenograft mouse model (11).
These findings indicate the possibility for an anti-tumor role of
TLR4 in cutaneous SCC. However, whether TLR4 is truly an anti-tumor
or pro-tumor molecule in cutaneous SCC remains to be
determined.
CD44 is a transmembrane glycoprotein that is
expressed in different variant forms in several types of cancer,
including cutaneous SCC (19-21).
CD44 interacts with extracellular matrix ligands to regulate
cell-matrix and cell-cell interactions, and to promote metastasis
(22). Hyaluronan-activated CD44
promotes RhoGTPase signaling, leading to keratinocyte activities,
such as cell adhesion, proliferation and migration (23). A hyaluronan-mediated CD44
interaction with TLR4 has been demonstrated in NDA-MB-123 breast
cancer cells (24); however, the
interaction between CD44 and TLR4 in skin cancers remains
unknown.
In this study, we examined the biological role of
TLR4 in cutaneous SCC. We confirmed the expression and localization
of TLR4 in non-melanocytic skin cancer tissues by
immunohistochemistry and analyzed the biological effects of TLR4
and the expression of CD44 on cutaneous SCC in vitro.
Materials and methods
Formalin-fixed paraffin-embedded (FFPE)
tissue samples
A total of 36 skin tumor, 5 AK, 5 BD and 26 SCC
cases were obtained from the Nippon Medical School Hospital
archives (Tokyo, Japan). The AK and BD cases were obtained between
2015 and 2017. The SCC cases were obtained between 2009 and 2015.
This study was carried out in accordance with the Declaration of
Helsinki, 2013, and the Japanese Society of Pathology Ethics
Committee. The Nippon Medical School Hospital Institutional Review
Board approved this study (approval no. 29-07-788, August 18th,
2017) and written informed consent was obtained from all patients.
All cases were carefully reviewed, and pathological diagnoses were
made according to the WHO classification (25). SCC cases were classified as
well-differentiated (>75%), moderately differentiated (25-75%),
or poorly differentiated (<25%) tumors based on the degree of
keratinization. The clinicopathological data of the SCC cases are
presented in Table I.
 | Table IClinicopathological data of SCC
cases. |
Table I
Clinicopathological data of SCC
cases.
| Case | Age (years) | Sex | Clinical stage | Location | Size (mm) | Depth of invasion
(mm) |
Differentiation |
|---|
| 1 | 86 | M | I | Face | 17 | 7.5 | Well |
| 2 | 83 | M | II | Face | 30 | 7.1 | Well |
| 3 | 53 | M | I | Head | 11 | 5.9 | Well |
| 4 | 70 | M | I | Lip | 6 | 2.9 | Poor |
| 5 | 77 | F | II | Face | 23 | 1.2 | Poor |
| 6 | 73 | M | I | Face | 10 | 1.2 | Mod |
| 7 | 69 | F | II | Lip | 30 | 4.1 | Mod |
| 8 | 92 | F | I | Face | 14 | 3.5 | Mod |
| 9 | 71 | M | I | Extremities
(finger) | 10 | 2.2 | Mod |
| 10 | 89 | M | III | Head | 49 | 9.6 | Well |
| 11 | 76 | F | I | Face | 8 | 0.8 | Well |
| 12 | 86 | M | II | Head | 20 | 7.2 | Poor |
| 13 | 70 | M | II | Extremities
(foot) | 25 | 9.3 | Mod |
| 14 | 92 | F | II | Face | 20 | 2.9 | Mod |
| 15 | 66 | M | I | Genital Area | 9 | 1.6 | Poor |
| 16 | 72 | F | I | Extremities
(finger) | 8 | 3.5 | Mod |
| 17 | 57 | M | III | Genital Area | 11 | 3.3 | Mod |
| 18 | 82 | F | III | Genital Area | 60 | 6.9 | Mod |
| 19 | 95 | F | II | Trunk
(abdomen) | 26 | 2.2 | Mod |
| 20 | 88 | M | I | Face | 13 | 3.3 | Mod |
| 21 | 92 | F | I | Face | 19 | 1.2 | Poor |
| 22 | 89 | F | II | Face | 30 | 1.2 | Poor |
| 23 | 87 | F | II | Face | 30 | 1.7 | Mod |
| 24 | 89 | M | I | Face | 13 | 1.6 | Mod |
| 25 | 79 | F | I | Head | 16 | 6.1 | Well |
| 26 | 65 | M | I | Extremities
(hand) | 8 | 2.3 | Mod |
The AK cases included 3 males and 2 females, and the
age of the patients ranged from 71 to 91 years, with a mean age of
82.2 years. The lesion locations were all found on the face (5/5).
The BD cases included 3 males and 2 females, and the age of the
patients ranged from 62 to 89 years, with a mean age of 74.2 years.
The lesion locations included 4 on the extremities (4/5) and 1 on
the trunk (1/5).
Immunohistochemistry
The FFPE tissue sections were stained for TLR4 or
CD44. Following deparaffinization, the sections were pre-treated in
an autoclave at 121°C for 15 min in 10 mM citrate buffer (pH 6.0).
Endogenous peroxidase was blocked using 0.3% hydrogen peroxide in
methanol for 30 min. The sections were then incubated with an
anti-TLR4 mouse monoclonal antibody (1:100; ab89455; Abcam,
Cambridge, UK) or an anti-human CD44 monoclonal mouse antibody
(1:10,000; BBA10; R&D Systems, Inc. Minneapolis, MN, USA) in
phosphate-buffered saline containing 1% bovine serum albumin at 4°C
overnight. The sections were further incubated with Simple Stain
MAX-PO (M; Nichirei Biosciences Inc., Tokyo, Japan) for 40 min and
peroxidase activity was visualized by 0.02% diaminobenzidine
containing 0.003% hydrogen peroxide for 2 min. The sections were
then counterstained with Mayer’s hematoxylin. We could not collect
normal skin or other tissues as control tissues. However, normal
epidermis and skin appendage adjacent to the tumors were
consistently positive for TLR4; in addition, normal epidermis and
the secretory part of the sweat gland adjacent to the tumors were
consistently positive for CD44 in all of the cases. Thus, the
normal epidermis and skin appendage served as an internal positive
control for TLR4 staining, and the normal epidermis and sweat gland
served as an internal positive control for CD44 staining. Tissues
stained without primary antibody were used as negative controls in
each staining.
Evaluation of the results of
immunohistochemistry
TLR4-stained slides were scanned at ×40
magnification and digitized using a Leica SCN400 slide scanner
(Leica Microsystems, Wetzlar, Germany). The acquired images were
analyzed using HistoQuest cell analysis software 4.0
(TissueGnostics, Vienna, Austria) for automated measurements of
TLR4 expression intensity and to calculate the percentage of
TLR4-positive cells within each slide. Five, randomly selected
fields of view were analyzed.
To assess the TLR4 expression levels, the TLR4
integrated intensity was calculated by multiplying the intensity of
TLR4-stained cells by the percentage of TLR4-stained cells. This
threshold was used for all samples.
Cells and cell culture
Human skin SCC cell lines, HSC-1 (cat. no. JCRB1015)
(26) and HSC-5 (cat. no.
JCRB1016) (27), were obtained
from the Japanese Collection of Research Bioresources (Osaka,
Japan). An immortalized human keratinocyte cell line, HaCaT (cat.
no. 300493), was purchased from CLS Cell Lines Service GmbH
(Eppelheim, Germany). The HSC-1, HSC-5 and HaCaT cells were grown
in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) medium containing 10% fetal bovine serum (FBS; Nichirei
Biosciences Inc., Tokyo, Japan) at 37°C in a humidified 5%
CO2 atmosphere.
Knockdown of TLR4 expression in HSC-1,
HSC-5 and HaCaT cells
The HSC-1, HSC-5 and HaCaT cells were transfected
using Lipofectamine® RNAiMAX Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) with 5 nM pre-designed TLR4 siRNA (siTLR4;
no. 4390824; Ambion; Thermo Fisher Scientific, Inc.), or 5 nM
negative control siRNA (siCtrl; no. 4390844; Ambion; Thermo Fisher
Scientific, Inc.) according to the manufacturer’s instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A total of 2.5×105 cells were seeded in
60-mm dishes and cultured for 48 h at 37°C in a humidified 5%
CO2 atmosphere. Total RNA was extracted using a FastPure
RNA kit, and 1 µg total RNA was used for reverse
transcription using a SuperScript VILO cDNA Synthesis kit, as per
the manufacturer’s instructions (Thermo Fisher Scientific, Inc.).
RT-qPCR for TLR4, CD44 and 18S rRNA (as an internal standard) was
performed using a StepOnePlus Real-Time PCR system (Thermo Fisher
Scientific, Inc.) with specific primers (18S: Hs 03928990_g1, TLR4:
Hs 00152939_m7, CD44: Hs 00153304_m7, Thermo Fisher Scientific,
Inc.) and a TaqMan probe (Thermo Fisher Scientific, Inc.). The
cycling conditions were as follows: 20 sec at 95̊C, and then 40
cycles of 1 sec at 95̊C, and 20 sec at 60̊C. The RT-qPCR results
are expressed as the ratio of target mRNA to 18S rRNA and analyzed
using the ∆∆Cq method (28).
Western blot analysis
Total proteins were extracted from the cells using
10X Cell Lysis Buffer (no. 9803; Cell Signaling Technology,
Danvers, MA, USA) according to the manufacturer’s instructions. The
supernatants were collected as a cell extract, and the protein
concentration was measured by Pierce 660 nm Protein Assay (Thermo
Fisher Scientific, Inc.). Equal amounts of protein (10 µg)
for each cell extract were loaded onto 5-20% sodium dodecyl
sulfate-polyacrylamide gels and separated by electrophoresis
(SDS-PAGE) and then electrophoretically blotted onto a
polyvinylidene difluoride (PVDF) membrane (Trans-Blot Turbo Mini
PVDF Transfer Packs, Bio-Rad Laboratories, Inc., Richmond, CA,
USA). The blots were blocked for 30 min with 5% skimmed milk in
Tris-buffered saline (TBS) containing 0.01 M Tris-HCl, 150 mM NaCl
and 0.05% Tween-20, and then incubated with an anti-human TLR4
antibody (1:500; ab89455; Abcam) and an anti-human CD44 monoclonal
mouse antibody (1:1,000; no. 3570; Cell Signaling Technology), or
anti-β-actin monoclonal mouse antibody (1:10,000; Clone AC-74;
Sigma-Aldrich, St. Louis, MO, USA) overnight at 4̊C. After 30-min
washing in TBS with 0.01% Triton X-100, the blots were incubated
with a horseradish peroxidase-conjugated secondary antibody
(1:10,000, A106PU; American Qualex Antibodies, San Clemente, CA,
USA) for 1 h at room temperature. Signals were visualized using a
Clarity Max Western ECL Substrate (no. 1705062; Bio-Rad
Laboratories, Inc.) for TLR4 and CD44, and a Super Signal West Pico
Chemiluminescence substrate (Thermo Fisher Scientific, Inc.) for
β-actin. Immunoreactive bands were quantified using Fiji-ImageJ
software version 2.0.0 (https://imagej.nih.gov/ij/). Experiments were
performed in triplicate.
Cell migration and invasion assays
In vitro migration and invasion assays were
carried out by Boyden chamber assay using BioCoat control inserts
and BioCoat Matrigel-coated inserts with BioCoat chambers (BD
Biosciences, San Jose, CA, USA). Following transfection with the
siRNA for 72 h, the cells were harvested and suspended in
serum-free RPMI-1640. The cells were then applied to the surface of
control or Matrigel-coated inserts at a density of 1×105
cells per insert, and culture medium with 10% FBS was added to the
lower chamber to serve as chemoattractant. The cells were incubated
for 24 h (HSC-1 and HSC-5 cells) or 36 h (HaCaT cells) at 37°C in a
humidified 5% CO2 atmosphere, before the migrating and
invading cells were stained with Diff-Quick™ three-step stain kit
(Sysmex Corp., Kobe, Japan). Stained cells on the outer surface in
5 randomly selected fields per insert were counted under a bright
field microscope (Olympus, Tokyo, Japan) with a X20 objective.
Experiments were performed in triplicate.
Immunofluorescence staining
The HSC-1, HSC-5 and HaCaT cells were seeded in
35-mm glass bottomed dishes at 2.0×104 cells/dish and
incubated with the relevant siRNAs for 72 h. The cells were then
fixed with 4% paraformaldehyde/PBS for 15 min and then incubated
with an anti-TLR4 antibody (1:100) or anti-CD44 antibody (1:400) at
4°C overnight. The cells were washed with PBS, and then incubated
with Alexa 488 (1:1,000; A11001; Invitrogen; Thermo Fisher
Scientific, Inc.) or Alexa 568 conjugated secondary antibodies
(1:1,000; A11031; Invitrogen; Thermo Fisher Scientific, Inc.) for 1
h in the dark at room temperature. Following incubation, the cells
were washed with PBS and mounted with Vectashield H-1200 containing
DAPI (Vector Laboratories, Inc., Burlingame, CA, USA). Tissues
stained without primary antibody were used as negative controls in
each staining. The fluorescent images were observed under a Digital
Eclipse C1 TE2000-E confocal microscope (Nikon Insteck Co., Ltd.,
Tokyo, Japan) and analyzed using Digital Eclipse C1 control
software EZ-C1 (Version 3.8; Nikon Insteck Co., Ltd.). For imaging
analysis, the confocal settings such as the laser intensity and
detector sensitivity were unchanged during the acquisition of all
images (X20 objective). The analysis of integrated density related
to the fluorescence signal of TLR4 and CD44 was carried out on 5
randomly selected fields using Fiji-ImageJ software version 2.0.0
(https://imagej.nih.gov/ij/). The total
cell number in each field was counted visually. The quantitative
evaluation of the fluorescence signal was carried out by dividing
the total integrated density by the total cell number of each
field.
Statistical analysis
The data represent the means ± 95% confidence
interval or the means ± standard error of the mean (SEM). A
Mann-Whitney U-test, two-way ANOVA Kruskal-Wallis test followed by
Dunn’s multiple comparison test, two-way ANOVA followed by Sidak’s
multiple comparisons test, or the multiple test using the
Holm-Sidak method were used to evaluate statistical significance. A
value of P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using GraphPad Prism version 7 (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
TLR4 expression is elevated in SCC
tissues compared to AK and BD
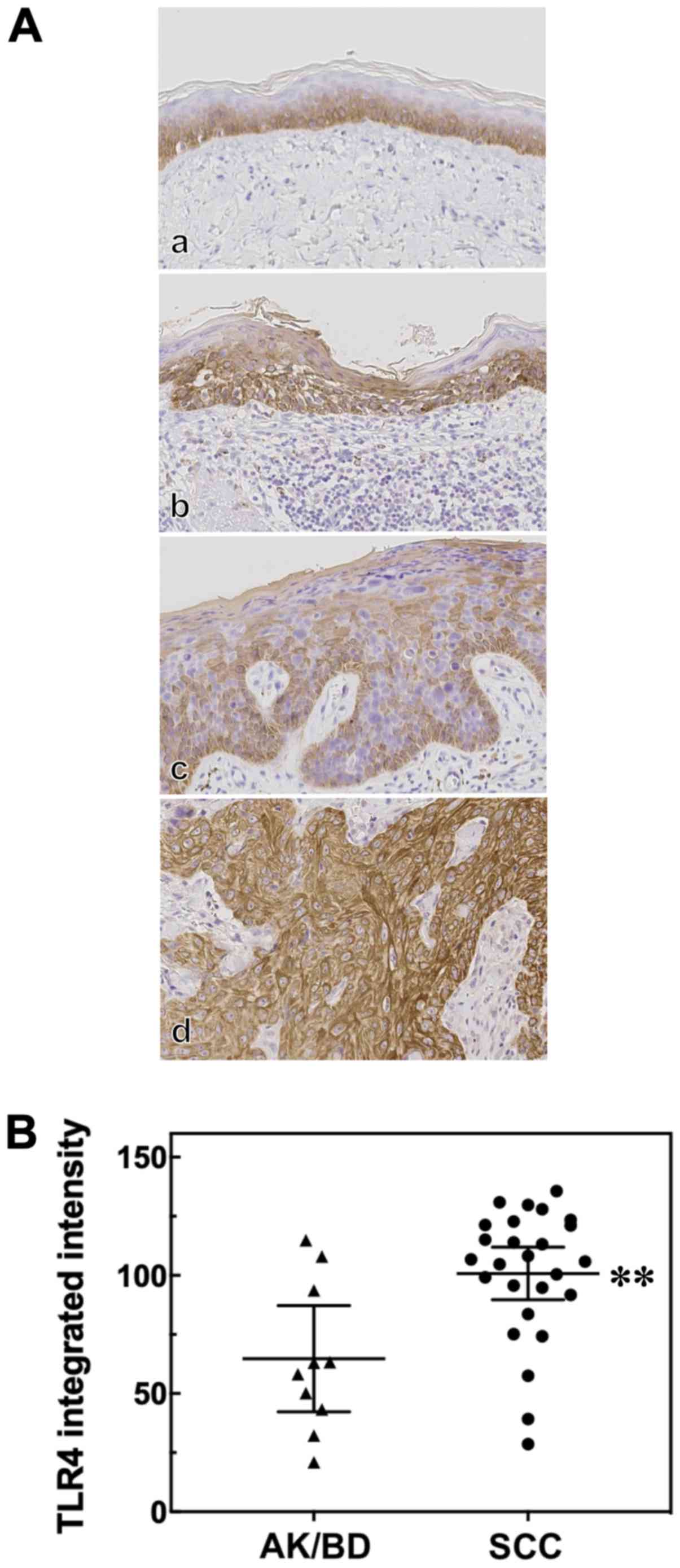
We first analyzed TLR4 expression in AK, BD and SCC
patient tissues by immunohistochemistry (Fig. 1A). We found that the TLR4-positive
cells in the normal epidermis adjacent to the tumors were confined
to one or more layers of the basal epidermis (Fig. 1A, panel a). The AK, BD and SCC
lesions were all TLR4-positive (Fig.
1A, panels b-d, respectively). In AK and BD, TLR4
immunoreactivity was diffusely distributed in the cytoplasm. TLR4
expression in the skin tumors was estimated by calculating the TLR4
integrated intensity. In this study, the number of cases of AK and
BD was only 5 cases each, and the TLR4 integrated intensity score
and TLR4 expression pattern between AK and BD did not differ
significantly. Thus, we compared the SCC group with the AK/BD
group. We found that the TLR4 integrated intensity in SCC was
significantly higher than that in the combined group of AK or BD
cases (Fig. 1B).
TLR4 expression varies depending on the
SCC differentiation level
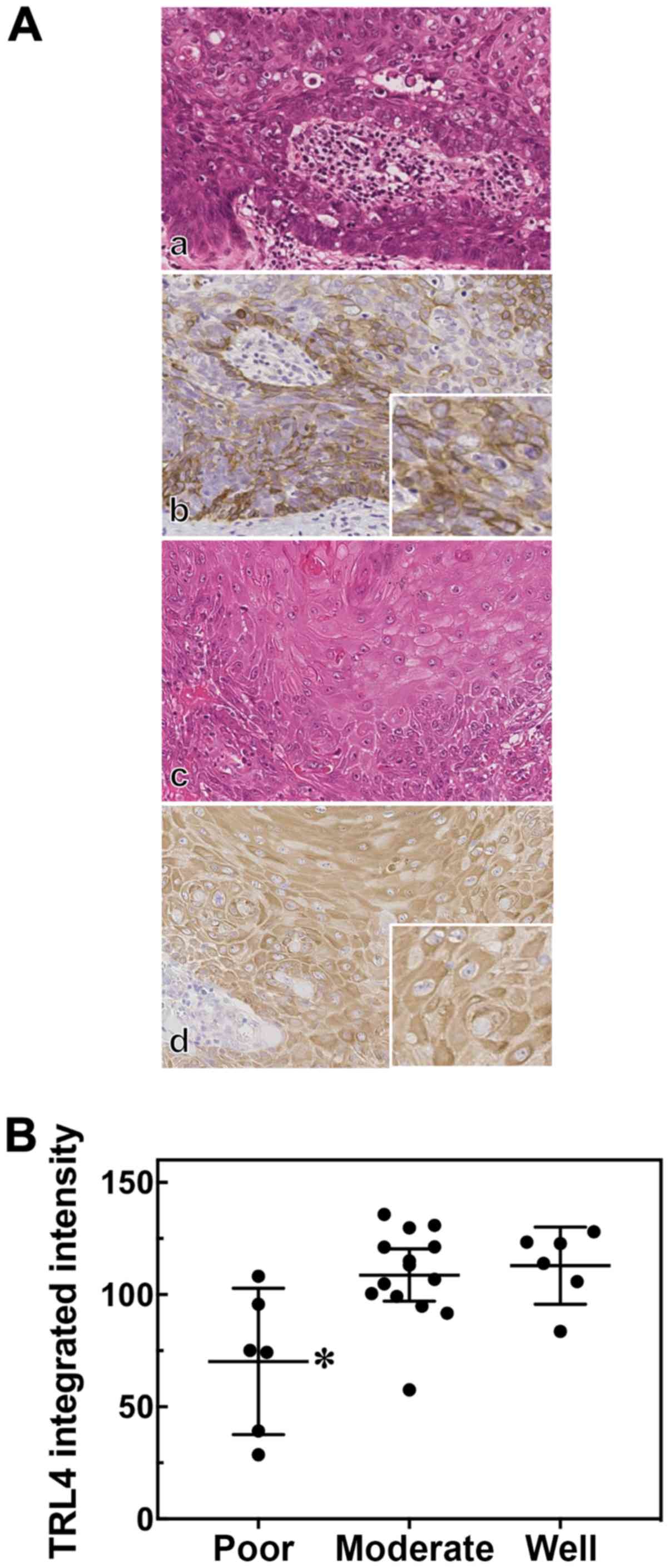
We then assessed TLR4 expression according to 3
levels of SCC differentiation: Poor, moderate and well. The TLR4
immunoreactivity pattern varied among the SCC cases. The poorly
differentiated SCC samples exhibited atypical epithelial neoplastic
cells without evident keratinization (Fig. 2A, panel a). In poorly
differentiated SCC, TLR4 immunoreactivity was sporadic and
localized mainly to the cell membrane (Fig. 2A, panel b, inset). On the other
hand, TLR4 immunoreactivity was diffusely distributed in the
cytoplasm in well-differentiated SCC (Fig. 2A, panels c and d). Furthermore, the
TLR4 integrated intensity score in the poorly differentiated SCC
cases was significantly lower than that in the moderately
differentiated or well-differentiated SCC cases (Fig. 2B).
CD44 expression in well- and poorly
differentiated SCC
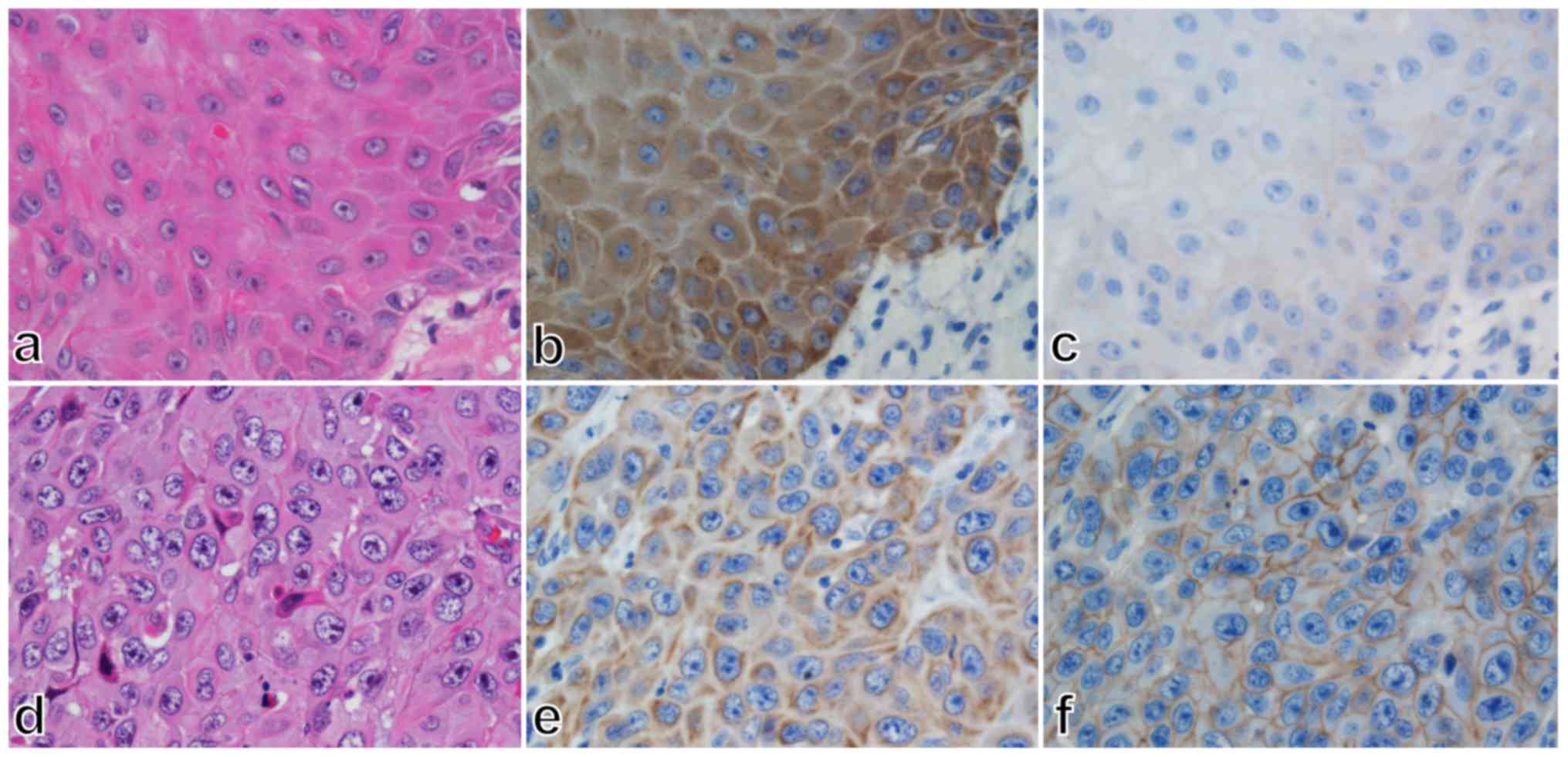
In addition, we assessed CD44 immunoreactivity in
some cases of well- and poorly differentiated SCC. CD44
immunoreactivity tended to be low in the well-differentiated SCC,
whose TLR4 immunoreactivity was diffusely distributed in the
cytoplasm (Fig. 3, panels a-c).
Conversely, CD44 immunoreactivity tended to be high and localized
to the cell membrane in poorly differentiated SCC, whose TLR4
immunoreactivity was low and localized to the cell membrane
(Fig. 3, panels d-f).
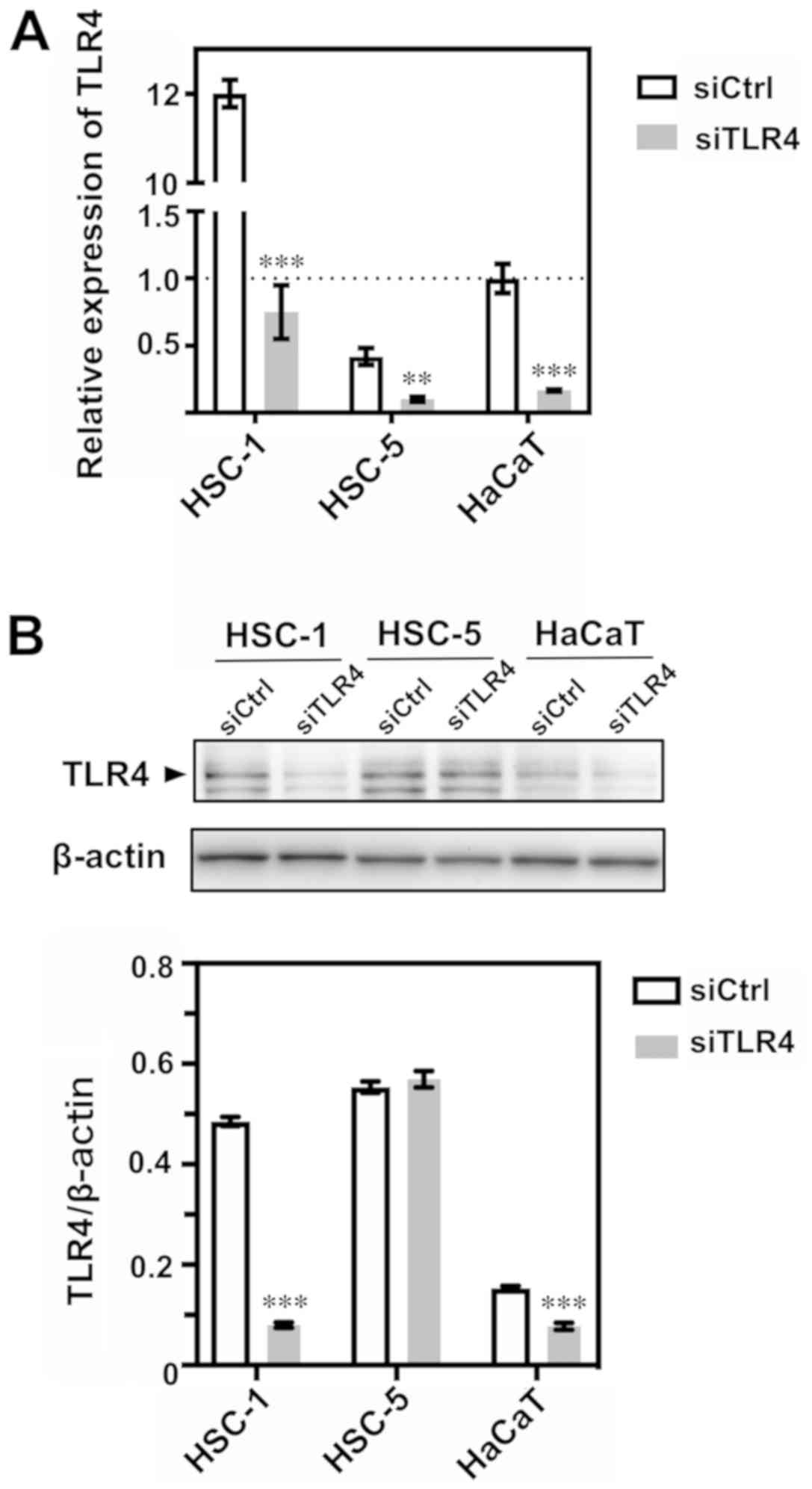
TLR4 mRNA and protein expression in
siTLR4-transfected cells
We used 2 human cutaneous SCC cell lines, HSC-1 and
HSC-5, and the immortalized human keratinocyte cell line, HaCaT, to
assess the TLR4 mRNA and protein expression levels. We found that
TLR4 mRNA relative expression in the HSC-1 cells was ~12-fold
higher compared with the control siRNA-transfected HaCaT cells. We
then knocked down TLR4 using siRNA in the HSC-1, HSC-5 and HaCaT
cells (Fig. 4A) and verified the
decreased TLR4 expression at the mRNA level. TLR4 protein
expression was also successfully decreased in the TLR4
siRNA-transfected HSC-1 and HaCaT cells. However, in the
transfected HSC-5 cells, the degree of knockdown appeared to be
low, and no significant difference was observed (Fig. 4B).
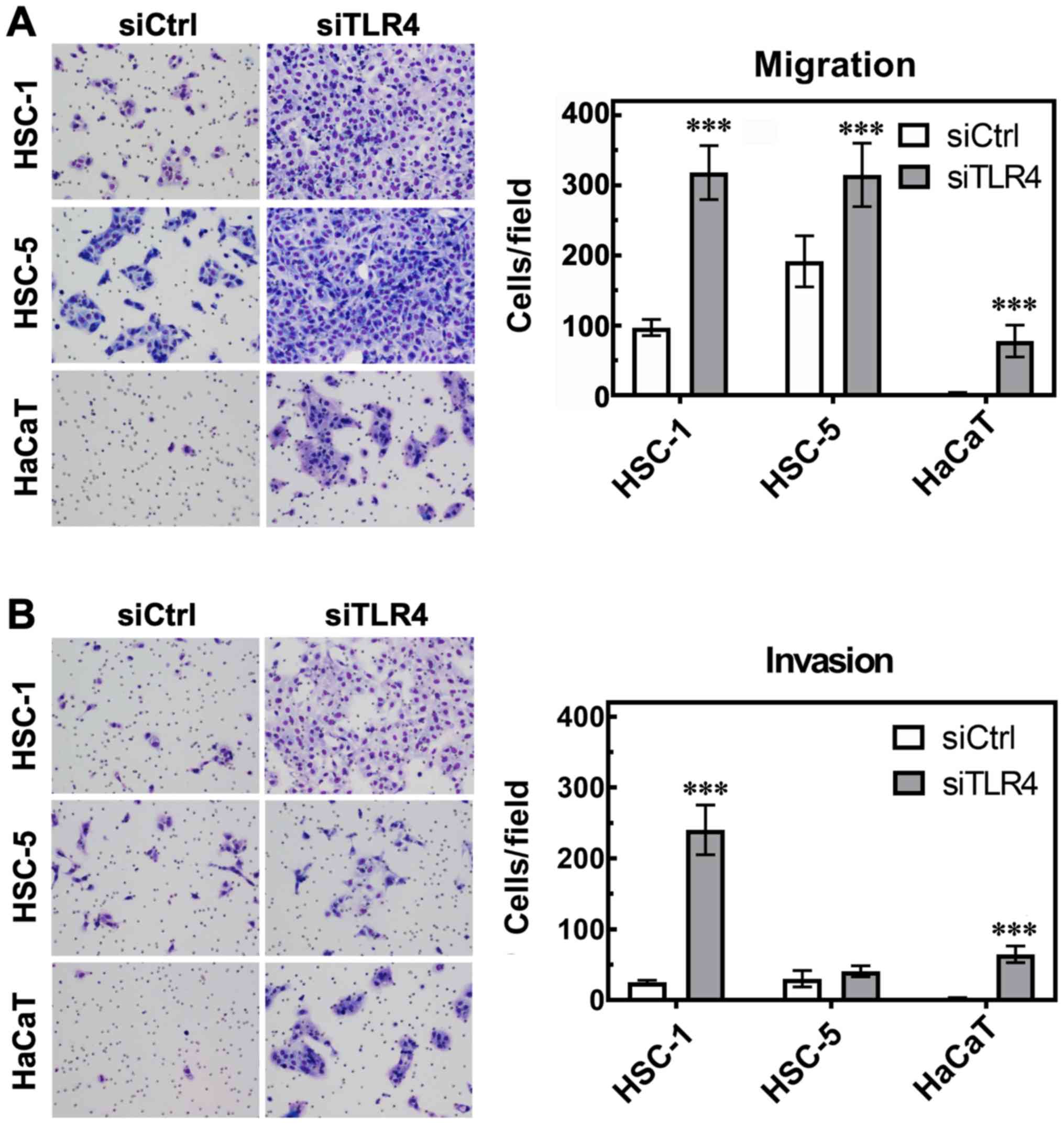
TLR4 knockdown enhances the migration and
invasion of SCC cells
We then assessed the effects of TLR4 on cell
migration and invasion by Boyden chamber assay. We found that
transfection with TLR4 siRNA enhanced the migration of the HSC-1,
HSC-5 and HaCaT cells compared to the control siRNA-transfected
cells (Fig. 5A), and enhanced the
invasion of the HSC-1 and HaCaT cells compared to the control
siRNA-transfected cells (Fig. 5B).
The HSC-1 cells exhibited the most prominent response in terms of
migration and invasion following TLR4 knockdown compared to the
HSC-5 and HaCaT cells.
TLR4 knockdown enhances CD44 mRNA and
protein expression
We then found that the decreased TLR4 expression
enhanced CD44 mRNA relative expression in the HSC-1, HSC-5 and
HaCaT cells (Fig. 6A). We also
detected the increased expression of the CD44 80-kDa isoform
(CD44s) in TLR4 siRNA-transfected treated HSC-1 and HaCaT cells
(Fig. 6B). In addition, we
detected a 140-kDa CD44 variant (CD44v) in the HSC-5 and HaCaT
cells; CD44v expression was also enhanced upon TLR4 siRNA
transfection (Fig. 6B).
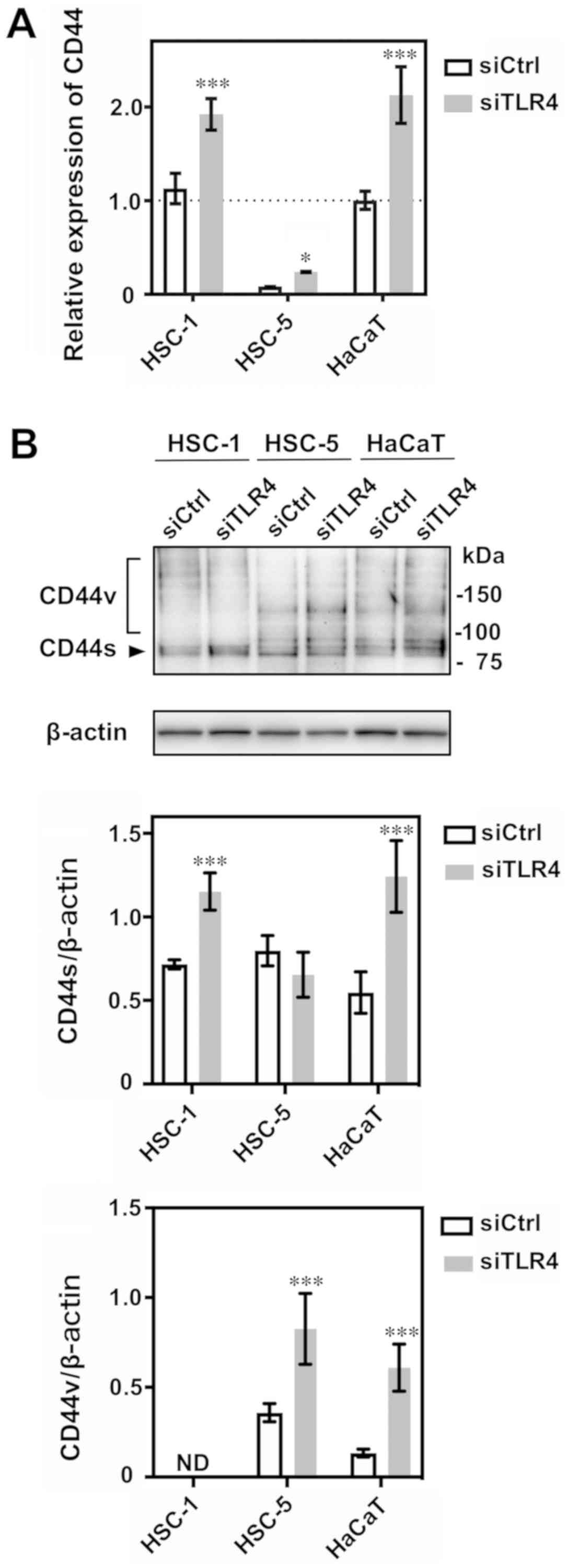 | Figure 6CD44 mRNA and protein expression in
siTLR4-transfected cells. (A) The relative expression of CD44 mRNA
was analyzed by RT-qPCR following transfection of the HSC-1, HSC-5
and HaCaT cells with siTLR4. The relative expression of CD44 mRNA
is indicated as a percentage of that of siCtrl-treated HaCaT cells.
The data represent the means ± 95% confidence interval of
triplicate measurements in 3 independent experiments. Statistical
significance between siTLR4 or siCtrl groups and among cell lines
was determined by two-way ANOVA followed by Sidak’s multiple
comparisons test. HSC-1: ***P<0.001; HSC-5:
*P=0.0358; HaCaT: ***P<0.001. (B) Western
blot analysis of CD44 protein expression following transfection
with siTLR4 in HSC-1, HSC-5 and HaCaT cells. The data represent the
mean ± 95% confidence interval of triplicate measurements in 3
independent experiments. Statistical significance between siTLR4 or
siCtrl groups and among cell lines was determined by two-way ANOVA
followed by Sidak’s multiple comparisons test. CD44s/β-actin:
HSC-1, ***P<0.001; HSC-5, P=0.2111; HaCaT,
***P<0.001. CD44v/β-actin: HSC-1, not detected (ND);
HSC-5, ***P<0.001; HaCaT, ***P<0.001.
CD44s: CD44 standard form, CD44v: CD44 variant iso-forms (140 kDa).
TLR4, Toll-like receptor 4; SCC, squamous cell carcinoma; siTLR4,
siRNA against TLR4; siCtrl, control siRNA. |
Reduced TLR4 expression enhances CD44
expression at the cell membrane
Finally, we performed immunofluorescence to monitor
TLR4 and CD44 cellular localization by confocal microscopy. We
found that TLR4 (green) was diffusely expressed in the cytoplasm
and cell membrane in all cell types (Fig. 7A, panels a-c). Upon transfection
with TLR4 siRNA, TLR4 expression in the cytoplasm was decreased,
particularly in the HSC-1 cells compared to the siRNA
control-transfected cells; TLR4 expression in the cell membrane
seemed unaffected (Fig. 7A, panels
d-f). In the quantitative evaluation, TLR4 expression was
significantly decreased in the TLR4 siRNA-transfected HSC-1 and
HaCaT cells (Fig. 8A) Moreover,
the TLR4 siRNA-transfected cells exhibited an increased number of
filopodia protrusions and TLR4 expression in the cell membrane
extended into these protrusions (Fig.
7A, panels d-f, arrowhead). We then detected that CD44 was
mainly localized to the cell membrane in all cell types (Fig. 7B, panels a-c). Transfection with
TLR4 siRNA increased CD44 expression and enhanced filopodia
protrusions compared to the control siRNA-transfected cells
(Fig. 7B, panels d-f). All images
are single optical sections, but not compressed stacks, and we
selected a slice with the most obvious filopodia protrusions.
Therefore, there was a height difference between the protrusions
and nuclei (blue) in some cells. In the quantitative evaluation,
CD44 expression was significantly increased in the TLR4
siRNA-transfected HSC-1 cells (Fig.
8B).
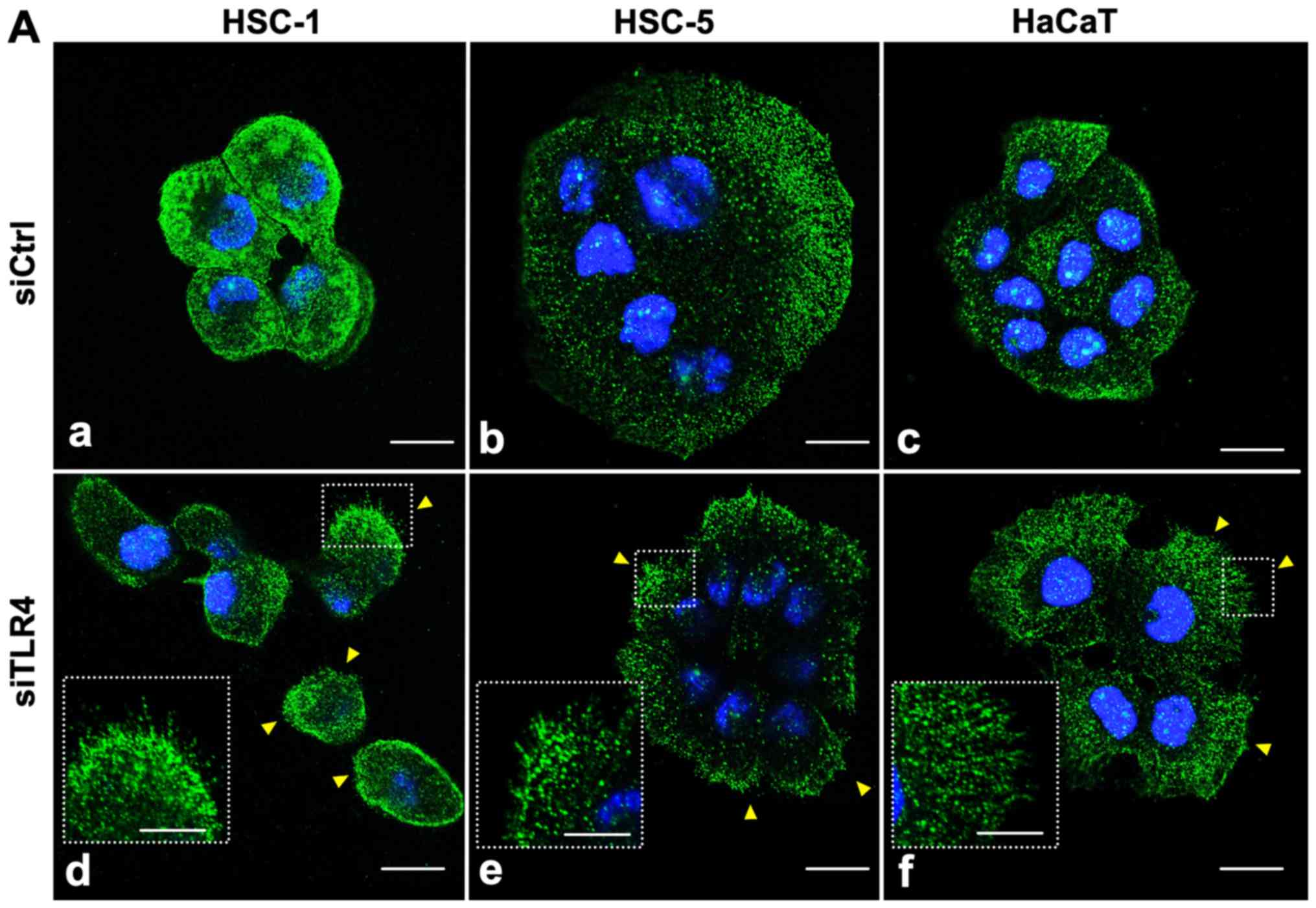 | Figure 7Immunofluorescence staining of TLR4
and CD44 in siTLR4-trans-fected cells. HSC-1, HSC-5 and HaCaT cells
were transfected with siCtrl (panels a, b and c, respectively) or
siTLR4 (panels d, e and f, respectively), and then stained with a
(A) TLR4 or (B) CD44 antibody. The nuclei were stained with DAPI
(blue). (A) TLR4 knockdown in HSC-1 cells decreased TLR4 expression
(green) in the cytoplasm and enhanced the formation of filopodia
protrusions (arrowheads) compared to siCtrl cells. (B) TLR4
knockdown increased CD44 expression (red) in the cell membrane and
increase the formation of filopodia protrusions (arrowheads)
compared to siCtrl cells. The insets show a high-power view of
filopodia protrusions. All images show a single optical section.
Scale bar, 20 µm. TLR4, Toll-like receptor 4; SCC, squamous
cell carcinoma; siTLR4, siRNA against TLR4; siCtrl, control
siRNA. |
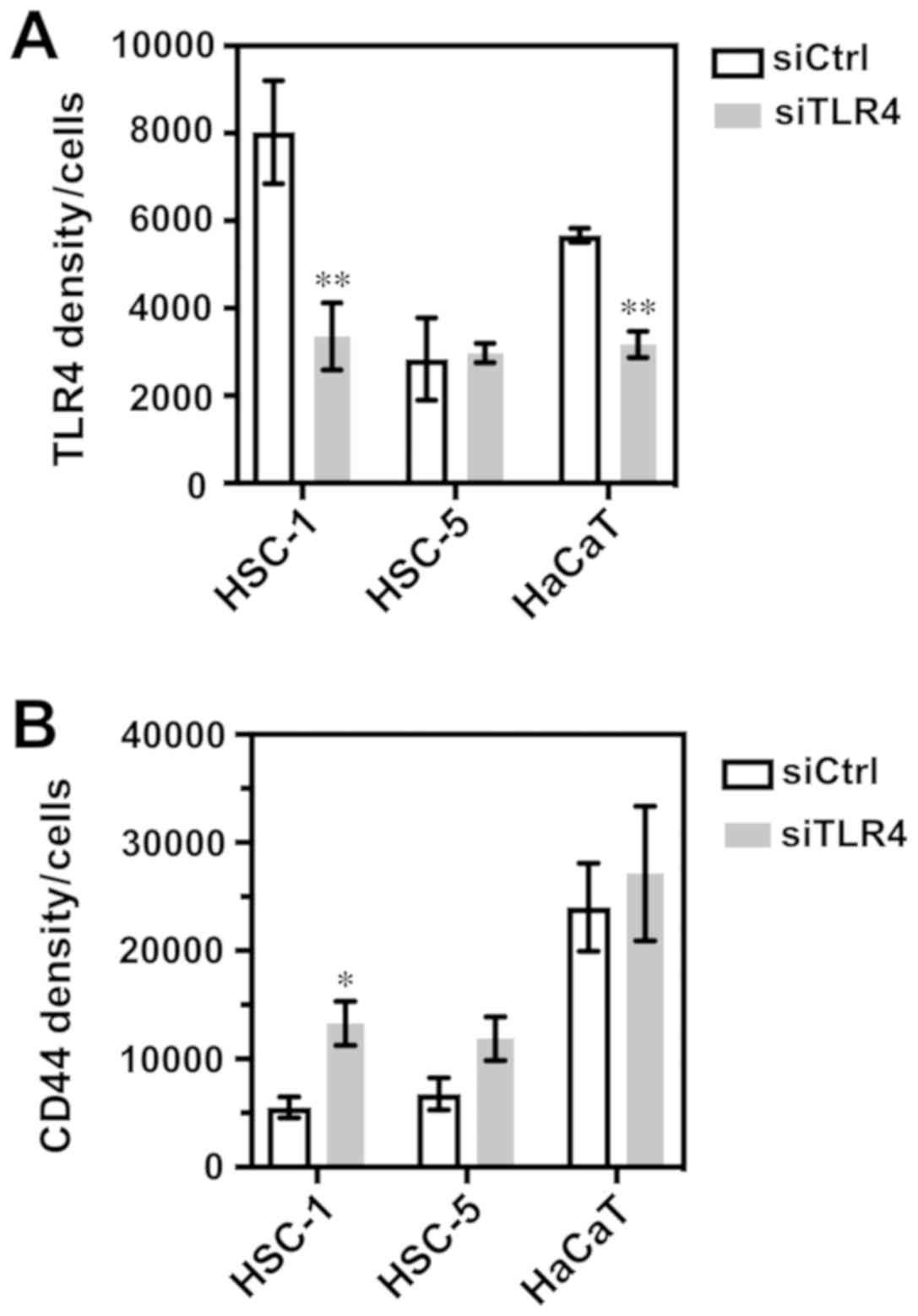 | Figure 8Quantitative evaluation of
Immunofluorescence staining. The analysis of the fluorescence
signal of TLR4 and CD44 was carried out on 5 randomly selected
fields per X20 objective sample. (A) TLR4 integrated density/cells:
HSC-1, **P=0.0077; HSC-5, P=0.6873; HaCaT,
**P=0.0073. (B) CD44 integrated intensity/cells: HSC-1,
*P=0.0177; HSC-5, P=0.1099; HaCaT, P=0.6683. The data
represent the means ± SEM of triplicate measurements in three
independent experiments. Statistical significance between siTLR4 or
siCtrl groups and among cell lines was determined by multiple test
using the Holm-Sidak method. TLR4, Toll-like receptor 4; SCC,
squamous cell carcinoma; siTLR4, siRNA against TLR4; siCtrl,
control siRNA. |
Discussion
This study examined the expression and localization
of TLR4 in non-melanocytic skin cancers AK, BD and SCC. We
determined the biological role of TLR4 in SCC in the HSC-1 and
HSC-5 SCC cells and HaCaT human keratinocytes. We first
quantitatively evaluated TLR4 expression in AK, BD and SCC using
the pathological tissue samples. TLR4 was expressed not only in the
basal epidermis of normal skin, adjacent to the tumor, but also in
all tumor lesions (Fig. 1A). The
TLR4 integrated intensity score of SCC group was significantly
higher than that of the combined AK/BD group (Fig. 1B).
AK is considered as a pre-cancerous SCC lesion, and
BD is essentially equivalent to and used interchangeably with the
term SCC in situ. These lesions both progress and evolve to
give rise to invasive SCC (29). A
previous study reported a significant increase in TLR4 expression
in keratinocytes in FFPE tissue samples during the progression from
normal skin to AK; the study also detected this increase in
expression at a later stage of SCC progression in tissue microarray
samples (9). These results
indicate that TLR4 expression may be involved in cutaneous SCC
formation and progression.
Subsequently, focusing on the TLR4 expression
pattern in SCC, we found an association between the SCC
differentiation degree and TLR4 expression levels. In poorly
differentiated SCC, the TLR4 integrated intensity score was
significantly lower than in moderately differentiated or
well-differentiated SCC cases (Fig.
2B). We also found that TLR4 immunoreactivity was largely
localized to the cell membrane in poorly differentiated SCC
(Fig. 2A, panel b, inset). In
addition, the CD44 immunoreactivity tended to be high and localized
to the cell membrane in poorly differentiated SCC (Fig. 3). Other histological features, such
as the site of location, tumor size, or depth of invasion were not
associated with TLR4 expression (data not shown). The poor
differentiation of SCC is independently associated with local
recurrence, lymph node metastasis, and disease-specific death
(30). The difference in TLR4
expression may, therefore, be associated with unfavorable outcomes
in poorly differentiated SCC. However, in this study, the number of
patients with TLR4 staining of AK, BD, and poorly differentiated
SCC, as well as the number of patients with CD44 staining of SCC
was small. Thus, further clinicopathological studies using a
greater number of cases are required to confirm the biological role
of TLR4 and CD44 in skin tumors.
An interaction between TLR4 and CD44 was previously
demonstrated in breast cancer cells (24). In cutaneous SCC, Karvinen et
al reported that poorly-differentiated SCC tumors showed an
irregular CD44 staining pattern and reduced expression with areas
of missing or low intensity (19);
conversely, well-differentiated SCC exhibited homogeneous CD44
staining and moderate intensity (19). Although the interaction between
TLR4 and CD44 in cutaneous cancers is unclear, these reports and
the findings of this study suggest that an irregular CD44
expression and localization may be associated with a perturbed TLR4
expression and localization in poorly differentiated SCC.
In this study, we analyzed the biological role of
TLR4 and the association between TLR4 and CD44 in cutaneous SCC
cells. The knockdown of TLR4 expression by siRNA accelerated cell
migration and invasion compared to the control siRNA-transfected
HSC-1 and HaCaT cells (Fig. 5).
Notably, TLR4 expression in the TLR4 siRNA-transfected HSC-1 cells
was mainly reduced in the cytoplasm, and to a certain degree, TLR4
expression remained detectable in the cell membrane (Fig. 7A, panels a and d). A similar TLR4
expression and localization pattern was observed in the poorly
differentiated SCC tissues (Fig.
2A, panel b). Furthermore, the knockdown of TLR4 increased CD44
expression (Figs. 6, 7B and 8B) and the filopodia protrusions
formation (Fig. 7A, panels d-f and
B, panels d-f). Filopodia are thin, finger-like membrane
protrusions that extend out from the cell edge and are involved in
cell migration and cell invasion (31). These results suggest that reduced
TLR4 expression may enhance the malignant features of cutaneous
SCC. In the future, we aim to focus on investigating the role of
TLR4 in epithelial-mesenchymal transition, which is deeply
implicated in cancer cell migration and invasiveness.
In this study, the TLR4 protein levels appeared to
still be high in the TLR4 siRNA-transfected HSC-5 cells, while the
mRNA levels were significantly decreased. However, the lack of the
successful knockdown of TLR4 protein in HSC-5 cells may have
affected the cell migration and CD44 expression results. These
results may be due to cell biological differences between HSC-1 and
HSC-5 cells, such as differences in stability between mRNA and
protein, or difference in protein degradation processes.
HSC-1 is a cell line derived from poorly
differentiated human skin SCC on the hand (26). HSC-5 is a cell line derived from
well-differentiated human skin SCC on the auricle (27). However, the differential ability of
keratinization was lacking in both cell lines, and the patterns of
expression levels of cytokeratin proteins of HSC-1 and HSC-5
differed. In addition, the HSC-1 cells were grown and they
exhibited features of keratinization, such as horn peals and
individual cell keratinization in a xenograft mouse model (26), while HSC-5 transplantation was not
successful (27). The difference
in reactivity to TLR4 siRNA between the HSC-1 and HSC-5 cells may
be caused by these cell biological differences. Additional work
using other SCC cell lines will provide more evidence to support
our results.
TLR4 may exert anti-tumor or pro-tumor effects.
However, a number of studies have described a pro-tumor role of
TLR4 expression in cancer cells (4-6,13,32,33).
In addition, a dual role of TLR4 in breast cancer cells has been
identified: TLR4 activation inhibits TP53 wild-type cell
growth but promotes TP53 mutant cell growth by regulating
proliferation (34). Notably, the
findings of this study indicate an anti-tumor role of TLR4
expression in SCC cells. This may be explained by the increased
CD44 expression in response to the decreased TLR4 expression.
CD44 is a transmembrane glycoprotein that is highly
expressed in a number of types of cancer and cancer stem cells.
CD44 interacts with several extracellular matrix ligands, including
hyaluronan or hyaluronic acid, osteopontin, collagen and
fibronectin to induce actin cytoskeleton regulation, cell migration
and invasion (22,35). CD44s and CD44v have overlapping,
yet distinct roles in cancer cell proliferation, adhesion,
migration and invasion (22,35).
In this study, we found that CD44s expression increased in the TLR4
siRNA-transfected HSC-1 and HaCaT cells. The CD44v isoform was also
expressed in the HSC-5 and HaCaT cells and was similarly enhanced
following TLR4 siRNA transfection (Fig. 6B). These findings indicate that
both CD44s and CD44v expression may contribute to cell migration
and invasive ability in cutaneous SCC, depending on the cell types.
Only a few studies to date have examined the association between
TLR4 and CD44 in cancer cells, at least to the best of our
knowledge. Bourguignon et al reported that low molecular
weight hyaluronan stimulated the association between CD44 and TLRs
(TLR2 and 4), followed by concomitant recruitment of AFAP-110 and
MyD88 that promotes tumor-cell invasion in breast tumor cells
(24). We suggest a possibility
that TLR4 and CD44 may be associated through a negative feedback
mechanism in cutaneous SCC. Overexpression experiments for TLR4
will be required to support this hypothesis.
To the best of our knowledge, this is the first
study to report an association between TLR4 and CD44 that
contributes to tumor migration and invasion in cutaneous SCC. We
found that decreased TLR4 expression levels are associated with
enhanced malignant features in human SCC tissue samples and
cultured SCC cell line. TLR4 may thus play an important anti-tumor
role in suppressing aggressive cutaneous SCC cellular
behaviors.
Funding
This study was supported, in part, by Grants-in-Aid
for the Clinical Rebiopsy Bank Project for Comprehensive Cancer
Therapy development to ZN from the Ministry of Education, Culture,
Sports, Science and Technology, Japan (MEXT), 2013-2017 (S13110022)
and a Grant-in-Aid for scientific research (C, no. 16K00406 to MK)
from JSPS KAKENHI.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
MK and ZN participated in the design of the study.
EM and RO performed the histological examination and analysis. EM,
KK, YK, KT, TF, and TK participated in data collection and
performed research. EM wrote the manuscript, and MK, RO, SK, KI,
TS, RW, HS, and ZN critically revised the manuscript and
participated in the analysis and interpretation of the data. All
authors have reviewed, edited, and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The Nippon Medical School Hospital Institutional
Review Board approved this study (approval no. 29-07-788, August
18th, 2017) and written informed consent was obtained from all
patients.
Patient consent for publication
All participants provided written informed consent
for the study.
Competing interests
The authors have no competing interests with respect
to this study.
Abbreviations:
|
TLR4
|
Toll-like receptor 4
|
|
SCC
|
squamous cell carcinoma
|
|
AK
|
actinic keratosis
|
|
BD
|
Bowen’s disease
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
Acknowledgments
Not applicable.
References
|
1
|
Burton KA, Ashack KA and Khachemoune A:
Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and
Metastatic Disease. Am J Clin Dermatol. 17:491–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karia PS, Han J and Schmults CD: Cutaneous
squamous cell carcinoma: Estimated incidence of disease, nodal
metastasis, and deaths from disease in the United States, 2012. J
Am Acad Dermatol. 68:957–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou M, McFarland-Mancini MM, Funk HM,
Husseinzadeh N, Mounajjed T and Drew AF: Toll-like receptor
expression in normal ovary and ovarian tumors. Cancer Immunol
Immunother. 58:1375–1385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Wu C, Ma J, Yang Y, Man X, Wu H and
Li S: Toll-like receptor 4 promotes angiogenesis in pancreatic
cancer via PI3K/AKT signaling. Exp Cell Res. 347:274–282. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dong YQ, Lu CW, Zhang L, Yang J, Hameed W
and Chen W: Toll-like receptor 4 signaling promotes invasion of
hepatocellular carcinoma cells through MKK4/JNK pathway. Mol
Immunol. 68:671–683. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye K, Wu Y, Sun Y, Lin J and Xu J: TLR4
siRNA inhibits proliferation and invasion in colorectal cancer
cells by downregulating ACAT1 expression. Life Sci. 155:133–139.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zou Y, Qin F, Chen J, Meng J, Wei L, Wu C,
Zhang Q, Wei D, Chen X, Wu H, et al: sTLR4/MD-2 complex inhibits
colorectal cancer in vitro and in vivo by targeting LPS.
Oncotarget. 7:52032–52044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eiró N, Ovies C, Fernandez-Garcia B,
Álvarez-Cuesta CC, González L, González LO and Vizoso FJ:
Expression of TLR3, 4, 7 and 9 in cutaneous malignant melanoma:
Relationship with clinicopathological characteristics and
prognosis. Arch Dermatol Res. 305:59–67. 2013. View Article : Google Scholar
|
|
9
|
Janda J, Burkett NB, Blohm-Mangone K,
Huang V, Curiel-Lewandrowski C, Alberts DS, Petricoin EF III,
Calvert VS, Einspahr J, Dong Z, et al: Resatorvid-based
Pharmacological Antagonism of Cutaneous TLR4 Blocks UV-induced
NF-κB and AP-1 Signaling in Keratinocytes and Mouse Skin. Photochem
Photobiol. 92:816–825. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blohm-Mangone K, Burkett NB, Tahsin S,
Myrdal PB, Aodah A, Ho B, Janda J, McComas M, Saboda K, Roe DJ, et
al: Pharmacological TLR4 Antagonism Using Topical Resatorvid Blocks
Solar UV-Induced Skin Tumorigenesis in SKH-1 Mice. Cancer Prev Res
(Phila). 11:265–278. 2018. View Article : Google Scholar
|
|
11
|
Iotzova-Weiss G, Freiberger SN, Johansen
P, Kamarachev J, Guenova E, Dziunycz PJ, Roux GA, Neu J and
Hofbauer GFL: TLR4 as a negative regulator of keratinocyte
proliferation. PLoS One. 12:e01856682017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szatmary Z: Molecular biology of toll-like
receptors. Gen Physiol Biophys. 31:357–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mai CW, Kang YB and Pichika MR: Should a
Toll-like receptor 4 (TLR-4) agonist or antagonist be designed to
treat cancer? TLR-4: Its expression and effects in the ten most
common cancers. Onco Targets Ther. 6:1573–1587. 2013.PubMed/NCBI
|
|
14
|
Dajon M, Iribarren K and Cremer I:
Toll-like receptor stimulation in cancer: A pro- and anti-tumor
double-edged sword. Immunobiology. 222:89–100. 2017. View Article : Google Scholar
|
|
15
|
Miller LS: Toll-like receptors in skin.
Adv Dermatol. 24:71–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng H, Deng Y, Xie Y, Liu H and Gong F:
Expression and significance of HMGB1, TLR4 and NF-κB p65 in human
epidermal tumors. BMC Cancer. 13:3112013. View Article : Google Scholar
|
|
17
|
Burns EM and Yusuf N: Toll-like receptors
and skin cancer. Front Immunol. 5:1352014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yusuf N, Nasti TH, Long JA, Naseemuddin M,
Lucas AP, Xu H and Elmets CA: Protective role of Toll-like receptor
4 during the initiation stage of cutaneous chemical carcinogenesis.
Cancer Res. 68:615–622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karvinen S, Kosma V-MM, Tammi MII and
Tammi R: Hyaluronan, CD44 and versican in epidermal keratinocyte
tumours. Br J Dermatol. 148:86–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartmann-Petersen S, Tammi RH, Tammi MI
and Kosma VM: Depletion of cell surface CD44 in nonmelanoma skin
tumours is associated with increased expression of matrix
metallopro-teinase 7. Br J Dermatol. 160:1251–1257. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Erfani E, Roudi R, Rakhshan A, Sabet MN,
Shariftabrizi A and Madjd Z: Comparative expression analysis of
putative cancer stem cell markers CD44 and ALDH1A1 in various skin
cancer subtypes. Int J Biol Markers. 31:e53–e61. 2016. View Article : Google Scholar
|
|
22
|
Senbanjo LT and Chellaiah MA: CD44: A
Multifunctional Cell Surface Adhesion Receptor Is a Regulator of
Progression and Metastasis of Cancer Cells. Front Cell Dev Biol.
5:182017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bourguignon LYW: Matrix
hyaluronan-activated CD44 signaling promotes keratinocyte
activities and improves abnormal epidermal functions. Am J Pathol.
184:1912–1919. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bourguignon LYW, Wong G, Earle CA and Xia
W: Interaction of low molecular weight hyaluronan with CD44 and
toll-like receptors promotes the actin filament-associated protein
110-actin binding and MyD88-NFκB signaling leading to
proinflammatory cytokine/chemokine production and breast tumor
invasion. Cytoskeleton (Hoboken). 68:671–693. 2011. View Article : Google Scholar
|
|
25
|
Elder DE, Massi D, Scolyer R and Willemze
R: WHO Classification of Skin Tumours Fourth Edition. WHO
Classification of Tumours. 11. IARC; Lyon: 2018
|
|
26
|
Kondo S and Aso K: Establishment of a cell
line of human skin squamous cell carcinoma in vitro. Br J Dermatol.
105:125–132. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hozumi Y, Kondo S, Shimoura T and Aso K:
Human squamous cell carcinoma from skin: Establishment and
characterization of a new cell line (HSC-5). J Dermatol.
17:143–148. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Yanofsky VR, Mercer SE and Phelps RG:
Histopathological variants of cutaneous squamous cell carcinoma: A
review. J Skin Cancer. 2011:2108132011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Motaparthi K, Kapil JP and Velazquez EF:
Cutaneous Squamous Cell Carcinoma: Review of the Eighth Edition of
the American Joint Committee on Cancer Staging Guidelines,
Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol.
24:171–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jacquemet G, Hamidi H and Ivaska J:
Filopodia in cell adhesion, 3D migration and cancer cell invasion.
Curr Opin Cell Biol. 36:23–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dang S, Peng Y, Ye L, Wang Y, Qian Z, Chen
Y, Wang X, Lin Y, Zhang X, Sun X, et al: Stimulation of TLR4 by
LMW-HA induces metastasis in human papillary thyroid carcinoma
through CXCR7. Clin Dev Immunol. 2013:7125612013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu L, Wang L and Chen S: Dual character of
Toll-like receptor signaling: Pro-tumorigenic effects and
anti-tumor functions. Biochim Biophys Acta. 1835:144–154. 2013.
|
|
34
|
Haricharan S and Brown P: TLR4 has a
TP53-dependent dual role in regulating breast cancer cell growth.
Proc Natl Acad Sci USA. 112:E3216–E3225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen C, Zhao S, Karnad A and Freeman JW:
The biology and role of CD44 in cancer progression: Therapeutic
implications. J Hematol Oncol. 11:642018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
Union for International Cancer Control (UICC); Geneva: 2016
|