Introduction
Tumor progression and metastasis relies on the
delivery of sufficient oxygen and nutrients via blood vessels
(1). Selectively inducing
thrombosis in tumor vasculature, leading to tumor infarction, is an
effective and promising antitumor strategy (2). Over the past 20 years, encouraging
research into a new vascular targeting therapy has emerged
(3,4). In this strategy, truncated tissue
factor (tTF) is used as a mediator and an extracellular domain of
tissue factor (TF). TF is a major initiator of thrombogenic
cascades (5). As a recombinant
form of TF, tTF only contains the cell surface domain and exhibits
less (1×105) factor X activity compared with TF in the
phospholipid membrane (6). Both
the intrinsic and extrinsic blood coagulation pathways lead to
activation of coagulation factor X (7); activated factor X then converts
prothrombin to thrombin, which finally accumulates in the
generation of fibrin polymers and blood clots. tTF exhibits limited
ability to activate factor X; however, tTF can recover its native
function and initiate local thrombosis once bound to the cell
surface (negatively charged phospholipid) using a targeting agent
(8). This targeting agent may be
an antibody or a peptide ligand. Numerous biochemical conjugates
and recombinant fusion proteins associated with tTF have been
synthesized, and have been demonstrated to exhibit potential
antitumor activity (9,10). However, the use of tTF-ligand has
certain limitations. Due to the low affinity of the targeting
moiety in tumors, the undesirable tumor-specific targets often fail
to induce complete thrombosis (11). Identification of additional
targeting ligands or tumor-specific receptors may be required to
enhance therapeutic efficacy.
Neuropilin-1 (NRP-1) is a non-tyrosine kinase
trans-membrane receptor of 120-130 kDa. This glycoprotein, first
characterized as the receptor of neuronal semaphorin 3A, was
subsequently revealed to be a co-receptor of vascular endothelial
growth factor (VEGF)165 (12).
Therefore, NRP-1 may indirectly enhance the biological activities
of VEGF, including promoting the migration and angiogenesis of
human umbilical vein endothelial cells (HUVECs) (13). Furthermore, NRP-1 expression has
been revealed to be upregulated in a variety of tumor types,
including hepatocellular carcinoma and breast cancer, and has been
associated with poor prognosis (14-16).
Due to the association of NRP-1 with tumor promotion, NRP-1 appears
to be a promising angiogenesis target. However, conjugated
antibody-tTF often cannot induce complete thrombosis due to this
antibody only solving the issue of specific delivery (17,18).
The distribution of vascular targets (antigens or receptors) in the
tumor vasculature is a major factor affecting tTF concentration
(19). In the present study, the
accumulation of tTF in tumor blood vessels was increased using the
streptavidin (SA)-biotin (B) system, which was introduced to
promote coagulation efficiency.
SA is a 60-kDa tetrameric protein generated from
Streptomyces avidinii, and is commercially available at a
high purity and exhibits favorable in vivo stability
(20,21). The binding affinity of SA for B, a
244-kDa vitamin, is high (Kd=10−15 mol/l) (22). Due to the rapid association and
strong interaction between SA and B, these molecules have been
widely used as binding pairs in analysis, drug delivery systems and
pre-targeting radioimmunotherapy (23-28).
In the present study, a novel tumor
vasculature-targeting approach was explored. This strategy
consisted of an SA-conjugated anti-NRP-1 monoclonal antibody
(mAb-SA) and biotinylated tTF (tTF-B). Anti-NRP-1 mAb (mAb), which
was previously generated via the hybridoma technique in the
laboratory (29), was conjugated
to SA to pre-target the NRP-1 receptors on the tumor vascular
endothelial cell surface. mAb-SA diffused into the tumor area, and
tTF-B was subsequently administered and efficiently combined with
mAb-SA to induce local tumor thrombosis. To explore the therapeutic
feasibility of this two-step coagulation approach, in vitro
studies were performed to assess the targeting ability of mAb-SA
and procoagulant activity of tTF-B, and to compare the B/SA binding
capacity between mAb-SA and tTF-B. Live imaging was used to
investigate the distribution and in vivo tumor-binding
ability of mAb-SA. Antitumor activity and coagulation efficiency
was subsequently evaluated via in vivo assessments and
histological analysis.
Materials and methods
Materials
Anti-NRP-1 mAb with a high purity was produced using
the hybridoma technique and preserved in the laboratory after
freeze-drying (29).
2-(N-morpholino) ethane sulfonic acid (MES),
1-ethyl-3-(3-dimethylaminopropyl) carbodi-imide (EDC),
N-hydroxysulfosuccinimide (sulfo-NHS), streptavidin (SA), Sephadex
G200 and a Sephadex G200 column (1.5×22 cm) were purchased from
Sigma-Aldrich (Merck KGaA). GoldBand 3-color Regular Range Protein
Marker (10-180 kDa) was purchased from Shanghai Yeasen
Biotechnology Co., Ltd. Endothelial cell medium (ECM) containing
human epidermal growth factor was purchased from Shanghai Zhong
Qiao Xin Zhou Biotechnology Co., Ltd. DMEM and FBS were purchased
from Invitrogen (Thermo Fisher Scientific, Inc.). Mouse IgG isotype
(cat. no. 0107-01) was obtained from AmyJet Scientific Inc.
Rhodamine B isothiocyanate (RBITC)-conjugated goat anti-mouse IgG
(cat. no. D111097), isopropyl-l-thio-B-d-galactopyranoside (IPTG),
BSA, Hoechst 33258 powder, and hematoxylin and eosin (H&E) were
purchased from Sangon Biotech Co., Ltd. Factors X and VII were
obtained from Sigma-Aldrich (Merck KGaA). pET22b (+) plasmid and
E. coli BL21(DE3) were purchased from Novagen (Merck KGaA).
cDNA encoding for tTF, containing amino acids 1 to 218 of human TF,
was used to generate the tTF expression vector tTF-pET22b (+) via
PCR using the following primers: Forward, 5′-TCC ATG GGC TCT GGC
ACT ACA-3′ and reverse, 5′-GTG CTC GAG TTC TCT GAA TTC C-3′.
NcoI and XhoI restriction enzymes were used to insert
cDNA into the plasmid. Nickel-nitrilotriacetic acid (Ni-NTA)
agarose was purchased from Qiagen, Inc. A B-labeling kit was
obtained from Wuhan Elabscience Biotechnology Co., Ltd. Cyanine-5
(Cy5) NHS-ester was purchased from Tiangen Biotech Co., Ltd.
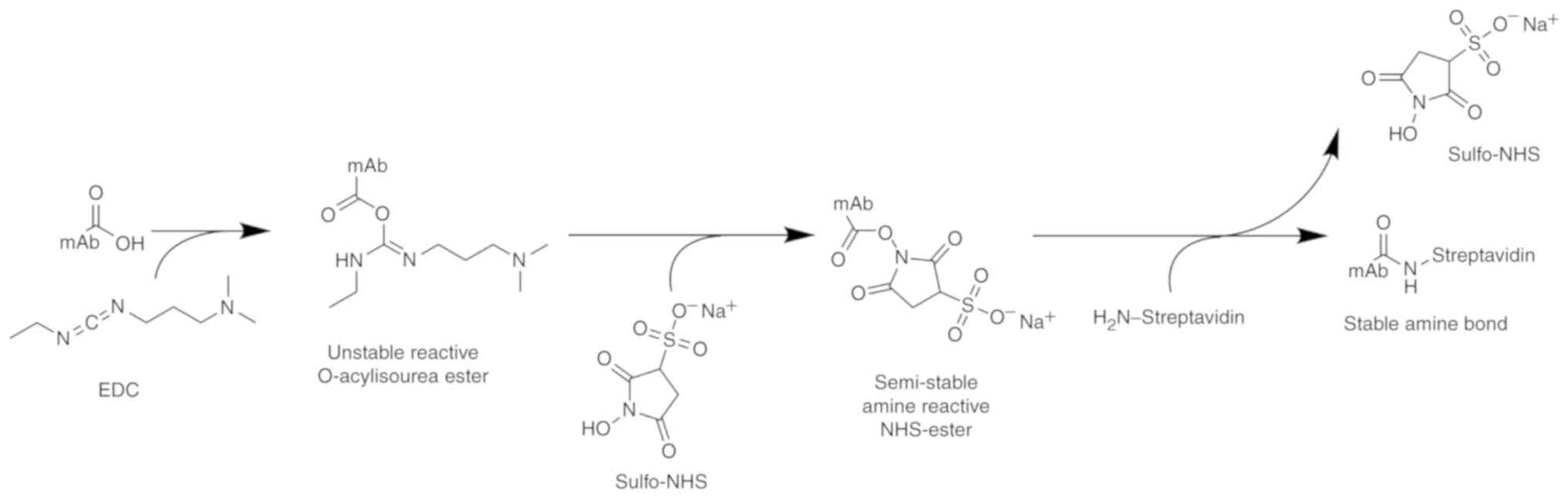
Preparation of mAb-SA conjugate
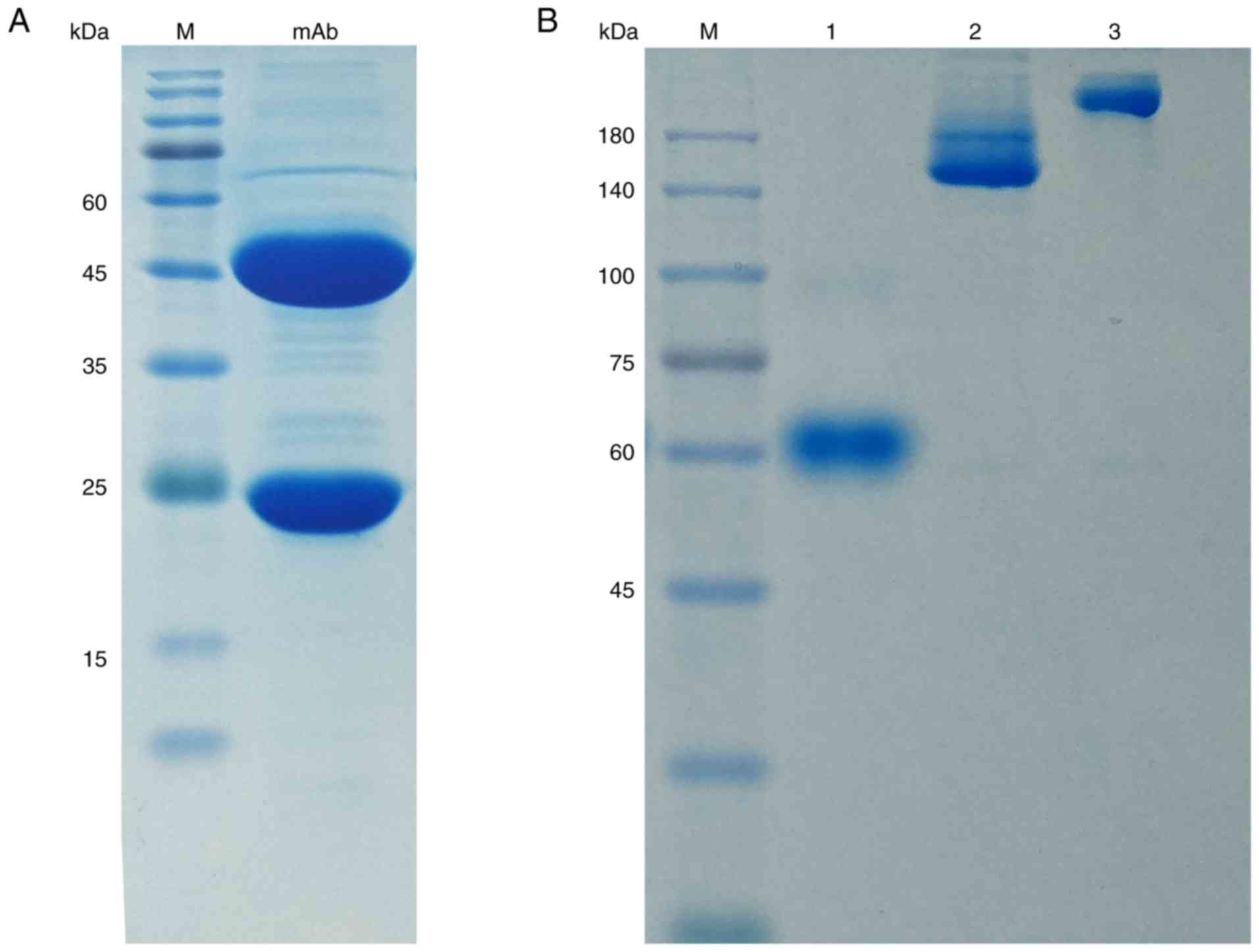
The purity of anti-NRP-1 mAb was first identified
via 12% SDS-PAGE. As presented in Fig.
1, the mAb-SA conjugate was synthesized using a coupling method
(30). The concentration of mAb
was adjusted to 3 mg/ml with reaction buffer (0.1 mol/l MES; 0.5
mol/l NaCl; pH 6.0) according to the improved Bradford method. A
total of 3.83 mg (0.02 mmol) of EDC and 4.34 mg (0.02 mmol) of
sulfo-NHS were weighed and immediately transferred to the reaction
solution (1 ml). The solution was mixed and stirred at room
temperature for 15 min. SA concentration was adjusted to 1.2 mg/ml
with 0.1 mol/l potassium phosphate buffer (pH 7.5), and 1 ml SA was
added to the reaction buffer. Nitrogen gas was purged into the
solution for 3 min and the beaker was then sealed. The reaction was
subsequently left to proceed at room temperature for 2 h, then
purified using a Sephadex G200 column as previously described
(31,32), with some modifications. Briefly,
Sephadex G200 was swelled in a boiling water bath for 2 h and
cooled. Pretreated Sephadex G200 was poured into the column slowly,
with no air mixing in, and equilibrated with pure water (pH 7.5)
for 40 min. The crude product was then slowly added to the column
and eluted with pure water at a rate of 0.2 ml/min. Eluted samples
(1.5 ml/tube) were collected and read with a NanoDrop™ 2000
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.) at 280 nm. All purification steps were conducted at 4°C.
Native 8% PAGE was performed to confirm whether conjugated protein
had been successfully isolated (33).
Cell culture
HUVECs and the human liver cancer cell line HepG2,
which both overexpress NRP-1 (34,35),
were obtained from the American Type Culture Collection. HUVECs
were used for in vitro experiments, and HepG2 cells were
used to establish a mouse tumor model. HepG2 cells were cultured in
high-glucose DMEM supplemented with 1% penicillin- streptomycin and
10% FBS. HUVECs were cultured in ECM containing human epidermal
growth factor. All cells were incubated in a humidified atmosphere
with 5% CO2 at 37°C.
Confocal immunofluorescence
HUVECs (1×105 cells/ml) were seeded into
a 6-well culture plate with one glass cover-slip per well. The
cells were then incubated and subsequently washed with PBS (pH 7.4)
three times, until 50% cloning efficiency had been reached. The
cells were then fixed with 1 ml 4% paraformaldehyde at 4°C for 30
min and washed with PBS three times. A total of 2 ml PBS containing
mAb, mAb-SA or a mouse IgG isotype control (1:5,000) was added, and
the cells were cultured at 37°C for 1 h. After washing, cells were
cultured with a goat anti-mouse RBITC mAb (1:200) at 37°C for an
additional 1 h in the dark. Hoechst 33258 was used to stain cell
nuclei at 37°C for 5 min and the samples were then examined under a
FV1000MPE-B confocal microscope (Olympus Corporation) and
photographed. Five random fields per sample were analyzed
(magnification, ×600).
Flow cytometry
Semiquantitative analysis was conducted to further
assess the ability of the mAb-SA conjugate to target NRP-1. HUVECs
were removed from the culture plate using trypsin and washed with
PBS three times. The cells were then fixed with 1 ml 4%
paraformaldehyde at 4°C for 30 min and washed with PBS three times.
After being resuspended in PBS, cells were incubated with mAb,
mAb-SA or the mouse IgG isotype (1:5,000) control at 37°C for 1 h
and incubated with the anti-mouse RBITC mAb (1:200) at 37°C for a
further 1 h. Each sample of 10,000 cells was analyzed using a
CytoFlex S flow cytometer (Beckman Coulter, Inc.). Results were
analyzed using CytExpert version 2.0 (Beckman Coulter, Inc.).
Production of fusion protein tTF
E. coli (BL21; DE3) containing tTF expression
vector were cultured in Luria broth supplemented with 1%
ampicillin. IPTG was added when the germiculture reached 0.6-0.8 at
600 nm to induce the expression of the fusion protein tTF. After
stimulation for 6 h, bacterial cells were collected and centrifuged
at 12,000 × g at 4°C for 20 min. A total of 5 ml lysis buffer (20
mmol/l Tris/HCl; pH 8.0; 0.5 mol/l NaCl; 2 mol/l urea; 20 ml/l
Triton X-100) per gram (wet weight) was subsequently added. After
incubating for 90 min, cells were centrifuged at 12,000 × g for 20
min at 4°C. The pellet was resuspended and sonicated (sonication
time: 5 sec; interval time: 5 sec) in washing buffer (20 mmol/l
Tris/HCl; pH 8.0; 0.5 mol/l NaCl; 2 mol/l urea; 20 ml/l Triton
X-100) at 4°C for 30 min. A total of 5 ml solubilization buffer (20
mmol/l Tris/HCl; pH 8.0; 8 mol/l urea; 1 mmol/l β-mercaptoethanol;
20 ml/l Triton X-100) per gram (wet weight) was added to dissolve
the inclusion bodies. After incubation at room temperature
overnight, the suspension was centrifuged at 12,000 × g for 20 min
at 4°C. The supernatant was then purified with a Ni-NTA column
according to the protocol of the His-Bind Buffer kit (Novagen;
Merck KGaA). The products were analyzed using 12% SDS-PAGE under
denaturing conditions. The purified fusion protein tTF was then
concentrated using a Centrifugal Filter and freeze-dried for
subsequent use.
Factor X activation
tTF-B was prepared using a B-labeling kit according
to the manufacturer's protocol. A factor X activation assay was
performed to assess the coagulation activity of tTF-B, as
previously described (36).
Various concentrations (0.01-10 µmol/l) of BSA, tTF or tTF-B
were incubated with 100 nmol/l factor VII in Tris-buffered saline
at 37°C for 10 min. Factor X (5 nmol/l) was then added, and the
mixture was incubated for 10 min at room temperature. The reaction
was subsequently quenched using 100 mmol/l EDTA. A total of 2
nmol/l Spectrozyme FXa was added to the mixture, and the absorbance
was detected at 405 nm after 3 min.
ELISA
A competitive ELISA was performed to determine the
B/SA-binding capacity of mAb-SA/tTF-B. A 96-well plate was coated
with tTF-B (100 µl/well; 20 µg/ml) in coating buffer
(7.5 mM sodium carbonate; 17.4 mM sodium bicarbonate; pH 9.6) and
incubated overnight at 4°C. The plate was then washed three times
with PBS-Tween solution (0.01 mol/l, pH 7.4, 0.05% Tween-20), and
10% FBS was then used to block the unbound sites at 37°C for 1 h.
After washing the plate five times, HRP-labeled SA (100
µl/well; 200 µg/ml) and was added to each well to
serve as the competitor. Serially diluted mAb-SA or SA (100
µl/well; 200-12.5 µg/ml) was then added separately.
After incubation for 1 h at 37°C, the enzyme substrate
orthophenylenediamine, in 50 mM phosphate-citrate buffer (pH 5.0
with fresh 30% hydrogen peroxide) and at a concentration of 0.4
mg/ml, was added (100 µl/well). The plate was subsequently
incubated at room temperature for 20 min, and 1 M
H2SO4 was added to quench the reaction (50
µl/well). Absorbance values were measured at 490 nm. The
inhibition rate was calculated according to a previous study
(37) to determine the
immunoreactivity of mAb-SA.
Mouse tumor models
Female BALB/c nude mice (n=60, 6-8 weeks old, 18-22
g) were purchased from the Experimental Animal Center of Xiamen
University, of which 52 were ultimately used for experiments. HepG2
cells were dissociated in a culture plate using 10% trypsin at 37°C
for 3 min, and subsequently resuspended in PBS. Each mouse was then
subcutaneously injected in the right flank with 100 µl HepG2
cells (1×106). Animals were housed in the Experimental
Animal Center under specific pathogen-free conditions: Temperature,
22-25°C; humidity, 50-60%; 12-h light/dark cycle. Mice had free
access to water and Purina 5L79 rodent chow. Animal health and
behavior were monitored daily. Animal welfare was in accordance
with institutional guidelines. When the mean tumor volume
[calculated as (length x width2)/2)] reached 150-250
mm3, those mice were used for live imaging, antitumor
activity studies and histological analysis after being
acclimatized. The largest subcutaneous tumor observed in the
present study was 1.9 cm in diameter at its widest point, and no
mice exhibited multiple subcutaneous tumors. According to a
previous study (38), the humane
endpoints were determined based on the mouse weight loss (>20%
of total body weight) or mouse activity assessment (hunching,
stationary, ruffling and poor grooming), and mice were euthanized
via cervical dislocation and dissected. The duration of the animal
experiment was ~25 days. Then, all remaining mice were euthanized
by cervical dislocation, and no mice were found dead. All animal
experiments performed in the present study were approved by the
Ethics Committee of Xiamen University.
Fluorescent labeling
SA, anti-NRP-1 mAb and mAb-SA were labeled with
fluorescein. The concentration of Cy5 NHS-ester used was 1 mg/ml in
DMSO. According to the total amount of proteins (SA, anti-NRP-1 mAb
or mAb-SA), 0.01 mg fluorescein per mg protein were mixed at room
temperature for 2 h. Dialysis was performed to remove unlabeled Cy5
NHS-ester for 4 h at room temperature and subsequently at 4°C
overnight with 0.15 mol/l NaCl solution. The whole process was
performed in the dark.
In vivo imaging
To investigate the in vivo distribution of
mAb-SA, nude mice with subcutaneous xenografts were randomly
divided into four groups (n=3/group). Mice in each group were
intravenously injected with 100 µl saline, SA-Cy5,
anti-NRP-1 mAb-Cy5, or mAb-SA-Cy5 via the tail vein. After the mice
were anesthetized in 1.5% isoflurane, the Cy5 fluoro-chrome was
then detected in mice using an Imaging IVIS-200 system
(PerkinElmer, Inc.) at 0.5, 3, 6, 12, 24, 36, 48 and 72 h. The nude
mice were sacrificed after imaging. Tumor tissues and major organs,
including the heart, liver, spleen, lungs, kidneys and brain, were
isolated from the mAb-SA-Cy5 treatment group, and their fluorescent
signal intensity was measured using Living Image version 4.3
(Caliper Life Sciences, Inc.; PerkinElmer, Inc.).
Antitumor efficacy evaluation
Nude mice bearing 150±50 mm3 HepG2 tumors
were randomly assigned to four groups (n=10/group). Mice in each
group received four intravenous administrations of saline, mAb-SA,
tTF-B and mAb-SA:tTF-B, at 3-day intervals, at a dose of 5 mg/kg.
In the mAb-SA:tTF-B treatment group, tTF-B was injected 24 h after
each administration of mAb-SA. Tumor size was measured every second
day. Tumor tissues were surgically removed and weighed after 12
days of treatment.
Histological analysis
To evaluate the tumor vascular targeting ability of
the mAb-SA:tTF-B composite system and its effects on intravascular
thrombosis in vivo, tumors from different treatment groups
and major organs from the mAb-SA:tTF-B group were fixed in 4%
formaldehyde solution at room temperature for 48 h before embedding
in paraffin and cutting into 5-7-µm sections for Harris
H&E staining. Tissues were stained with hematoxylin and eosin
at room temperature for 3 min and 20 min, respectively. Five random
fields per sample were examined under a light microscope
(magnification, ×200).
Statistical analysis
All data in the present study were analyzed using
Prism 6.0 (GraphPad Software, Inc.) and presented as the mean ±
standard deviation. One-way ANOVA with Tukey's multiple comparison
post hoc test was performed to assess the differences among groups.
P<0.05 was considered to indicate statistically significant
differences.
Results
Preparation of mAb-SA conjugate via EDC
reaction and purification using gel-filtration
As presented in Fig.
2A, the purity of anti-NRP-1 mAb reached ~95%. To separate the
desired synthetic product from any impurities, including the
cross-linked byproduct, any unreacted antibodies and the unreacted
SA, the crude products were purified using a Sephadex G200
gel-filtration column, which was washed using pure water (pH 7.5).
To avoid dissociation to monomeric species, the conjugate was
analyzed using native 8% PAGE under a non-boiled condition. The
bands shown in Fig. 2B correspond
to (from left to right) unreacted SA, unreacted mAb and conjugate
products after purification. The final purified conjugate was
well-defined and clear.
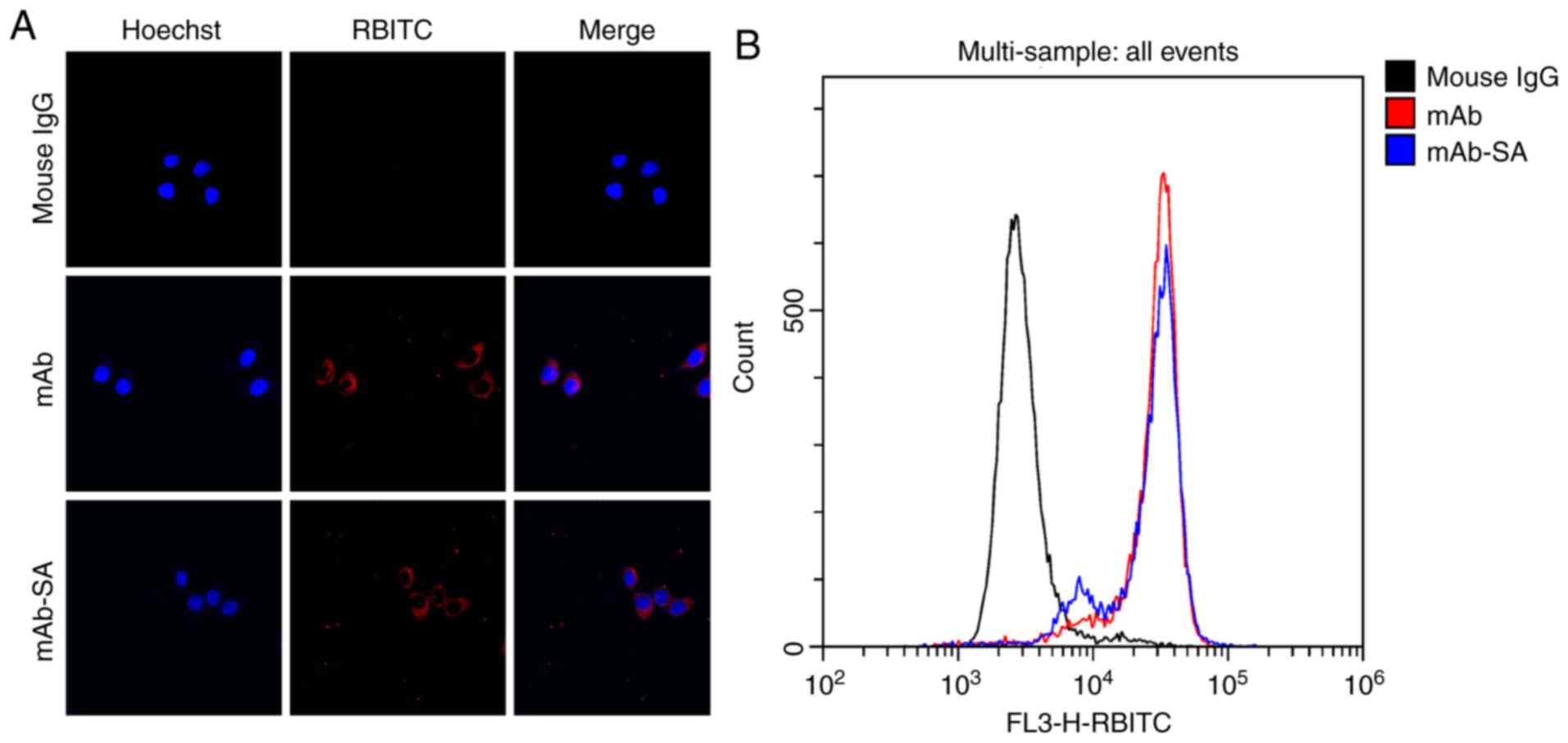
Confocal immunofluorescence and flow
cytometry demonstrate the in vitro NRP-1 targeting ability of
mAb-SA
Confocal immunofluorescence and flow cytometry were
performed to determine whether mAb-SA maintained biological
targeting activity. As presented in Fig. 3A, red fluorescence of RBITC was
observed on the surface membrane of HUVECs treated with mAb or
mAb-SA. This result indicated that mAb-SA was able to bind with
NRP-1 on the cell surface. The results of flow cytometry (Fig. 3B) were similar to those of the
confocal experiment. The anti-NRP-1 mAb and mAb-SA groups exhibited
significant HUVEC-binding activity. The NRP-1-binding capacity of
mAb-SA was almost equivalent to that of mAb.
Preparation and purification of tTF
fusion protein
To optimize the culture conditions, various
concentrations (0-1 mmol/l) of IPTG were used. 12% SDS-PAGE was
performed to evaluate the induced fusion protein yield (Fig. 4). A maximum yield was observed when
the IPTG concentration reached 0.8 mmol/l. The fusion protein was
expressed in the form of inclusion bodies. The inclusion bodies
were washed with washing buffer and subsequently dissolved in
denaturation buffer. A nickel affinity column was used to purify
the soluble products. As presented in Fig. 4, the objective fusion protein was
~34 kDa with ~99% purity.
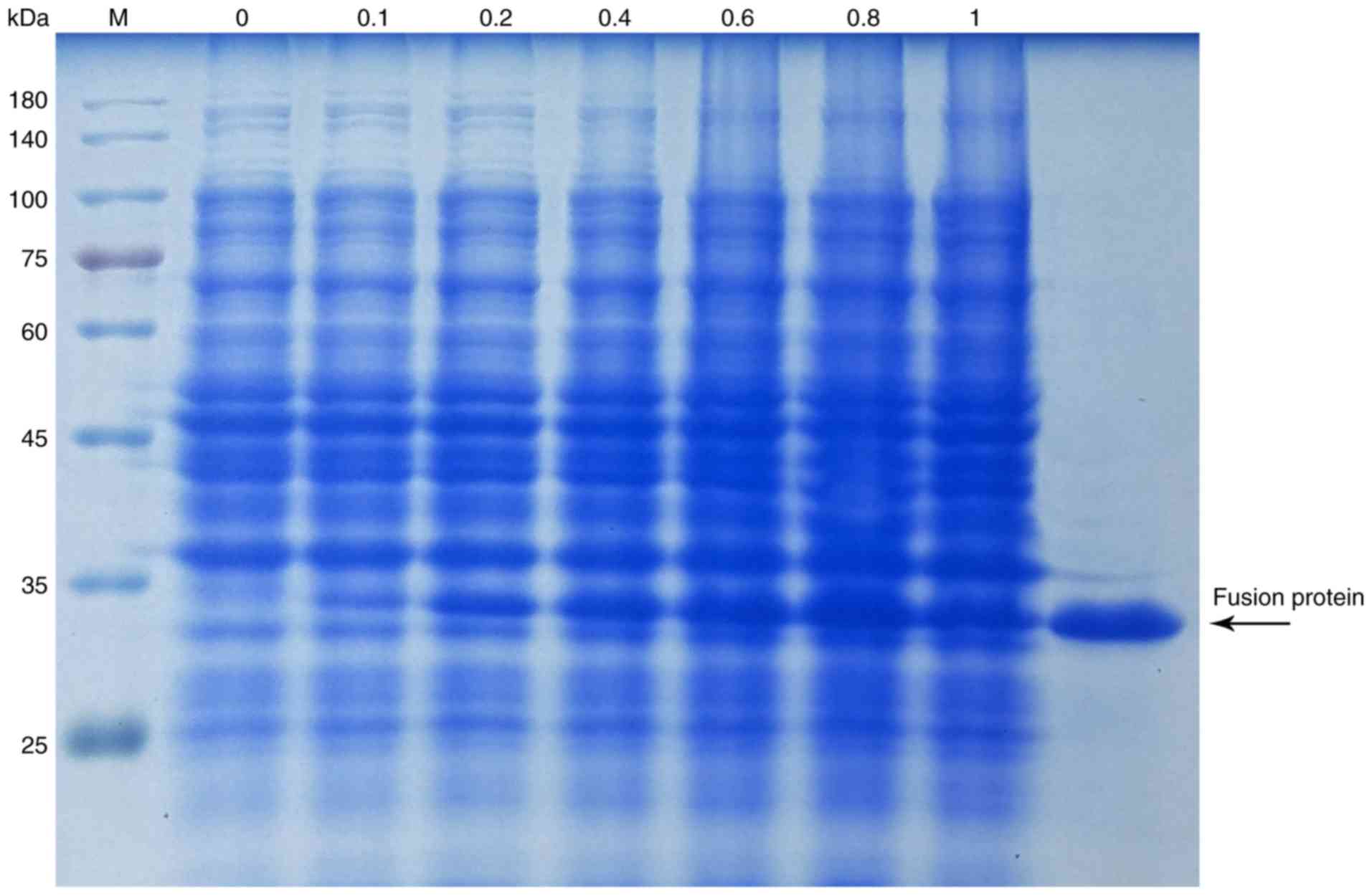 | Figure 4SDS-PAGE analysis of the expression
and purification of the fusion protein tTF. Lane 2-8, total
proteins induced by IPTG at a concentration of 0, 0.1, 0.2, 0.4,
0.6, 0.8 and 1 mmol/l, respectively; lane 9, final fusion protein
tTF purified with a nickel affinity column. tTF, truncated tissue
factor; M, protein marker. |
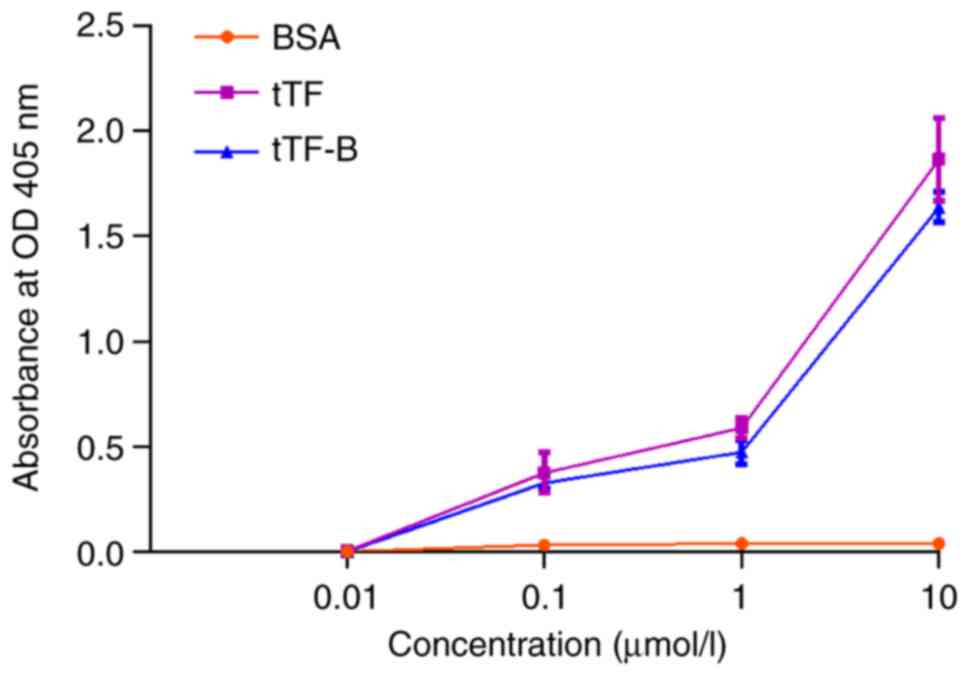
tTF-B promotes blood coagulation in
vitro
A factor X activation assay was performed to
evaluate the retention of blood coagulation activity in tTF-B. As
presented in Fig. 5, tTF and tTF-B
exhibited similar factor X activity at the corresponding
concentrations, indicating that tTF-B retained the tTF moiety when
inducing blood coagulation.
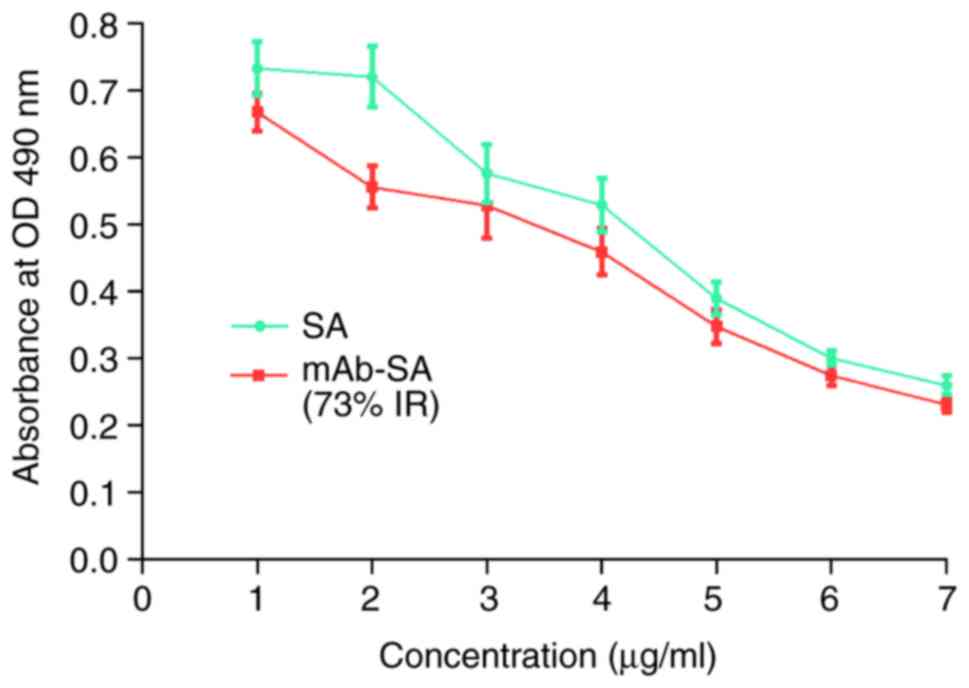
Verification of B/SA binding ability
A competitive ELISA was used to detect the in
vitro binding ability of tTF-B and mAb-SA (Fig. 6). The results revealed that the EDC
reaction did not impair the B-binding ability of SA. The
tTF-B-binding capacity of the mAb-SA conjugate was 73% compared
with that of unmodified SA. Therefore, the in vitro use of
the mAb-SA:tTF-B system may also be of value for in vivo
studies.
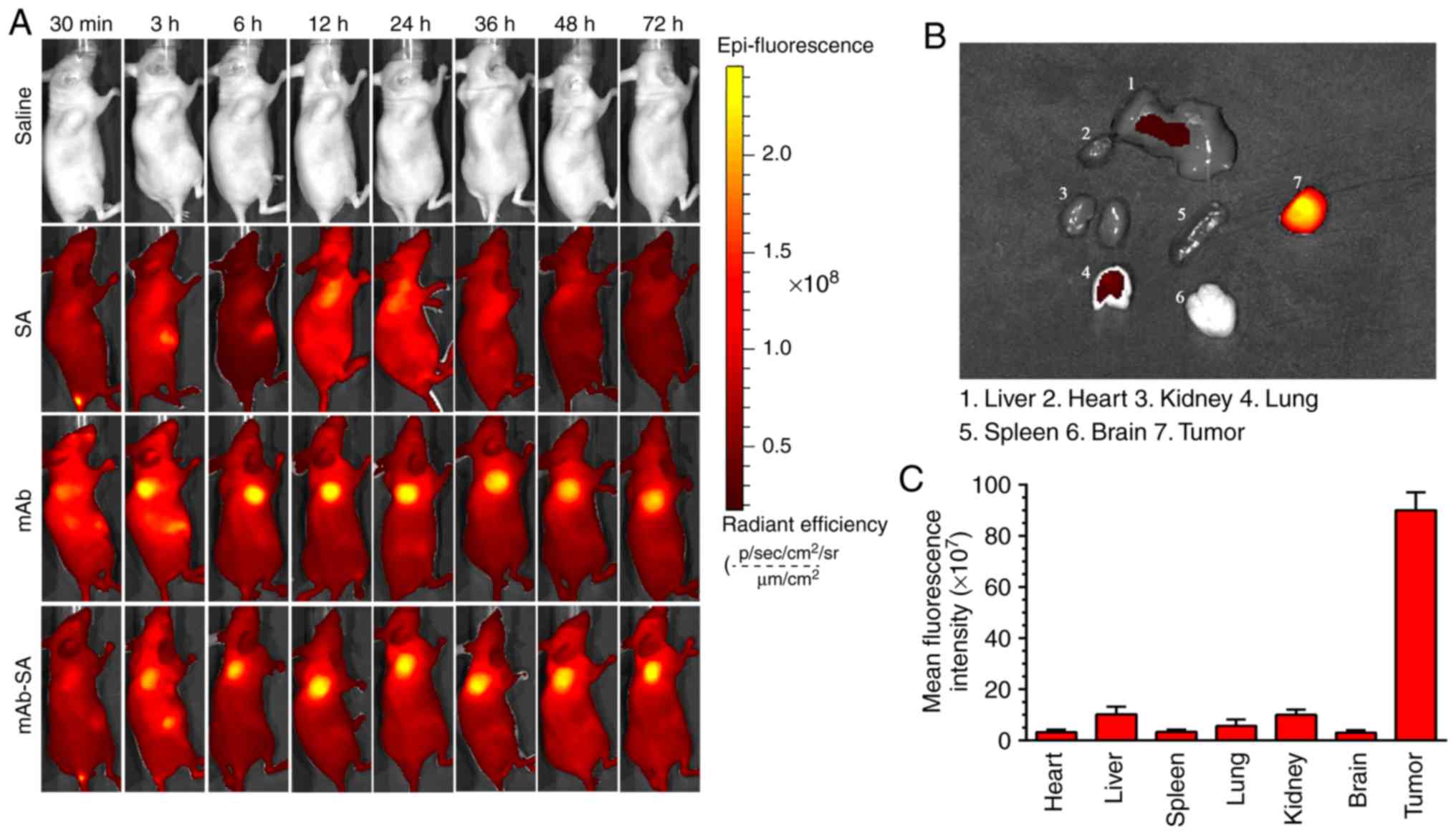
In vivo distribution of mAb-SA
Live imaging was performed to assess the
distribution of mAb-SA in tumor-bearing mice. A variety of
Cy5-labeled reagents and saline (blank control) were intravenously
injected, and the fluorescent signals were detected at various time
points. As presented in Fig. 7A,
Cy5-labeled mAb and mAb-SA were detected in the tumor area at 3 h
following intravenous injection. The peaks in fluorescent signals
were similar at 24 h; however, no SA-Cy5 fluorescent signal was
observed in tumor tissues. The fluorescent signals in the mAb and
mAb-SA-Cy5 treatment groups remained strong at 72 h (Fig. 7B and C). Weak Cy5 fluorescence
signals were detected in the major organs; the fluorescence
intensity ratio of tumor to non-tumor tissue was ~8.83-29.80 in
mice injected with mAb-SA-Cy5. The results of the present study
suggested that the mAb-SA conjugate was able to selectively target
tumor tissues, but was not retained by normal organs.
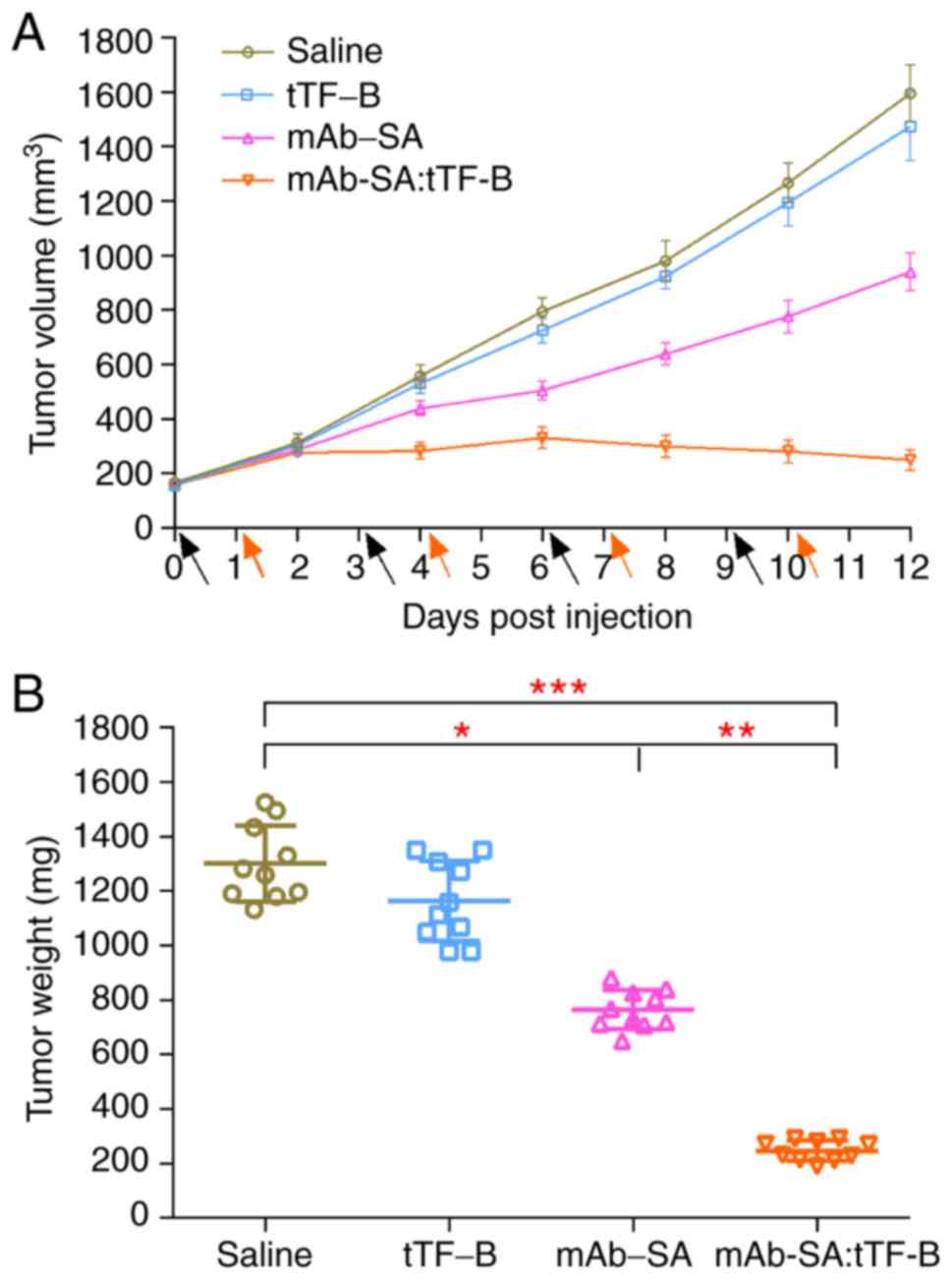
Antitumor activity of the mAb-SA:tTF-B
system
The anti-tumor activity of the mAb-SA:tTF-B system
was evaluated in HepG2 xenograft-bearing nude mice. Tumor growth
curves are presented in Fig. 8A.
Overall, tumors in mice treated with mAb-SA:tTF-B exhibited
decreased growth compared with other treatment groups. Tumor
regression was also only observed following the third treatment on
day 6. This indicated that the two-step coagulation approach was
able to effectively inhibit the growth of HepG2 xenografts and may
result in tumor regression. In addition, the results revealed that
mAb-SA alone could markedly affect tumor growth rate (mAb-SA vs.
saline, P<0.05). These results are supported by the final tumor
weights (Fig. 8B) of mice in
different treatment groups after treatment for 12 days.
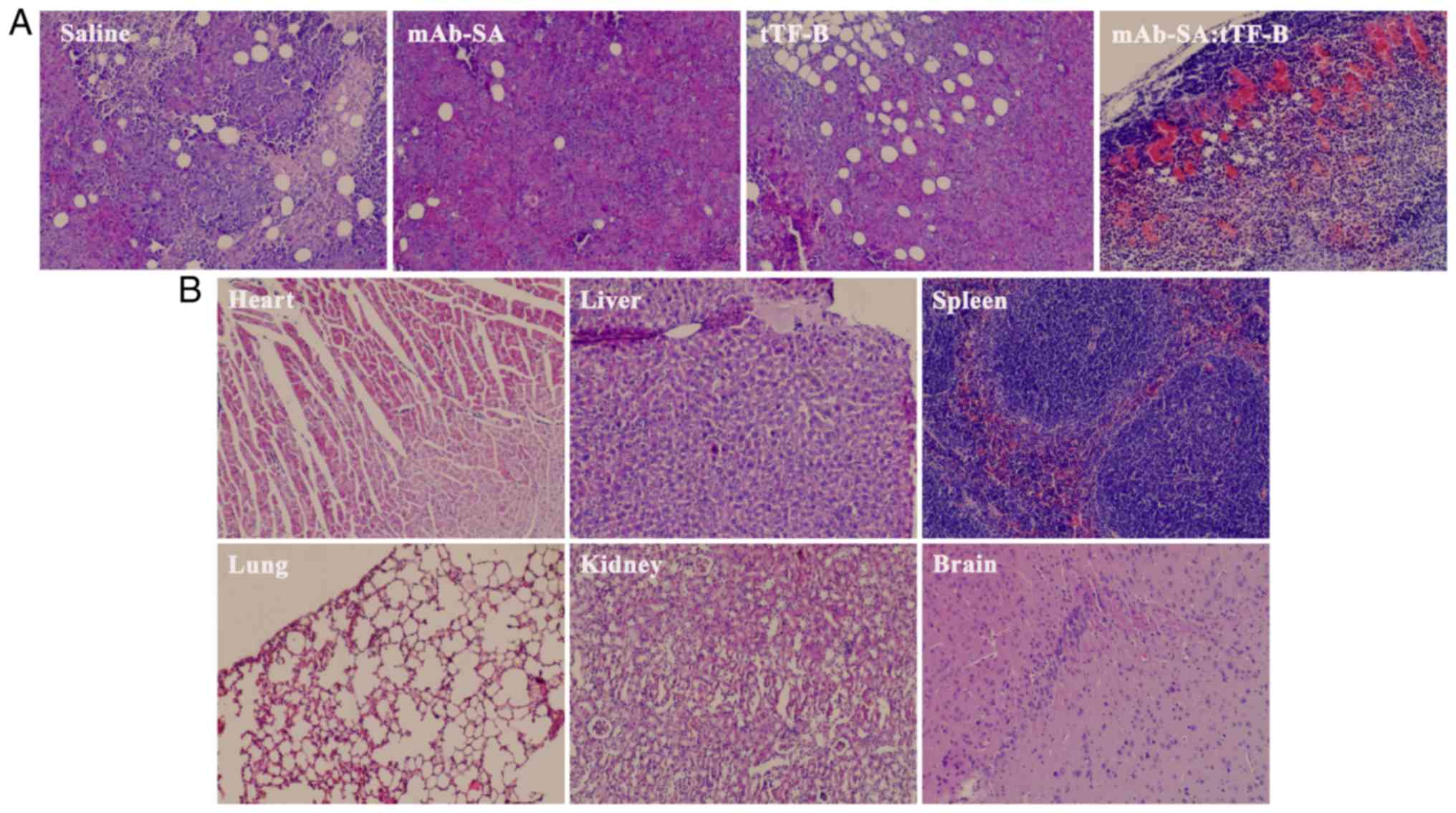
In vivo intravascular thrombosis is
observed following mAb-Sa:tTF-B treatment
H&E staining was performed to observe
histological changes in the tumor tissues of mice from the
different treatment groups. Extensive thrombosis (estimated at
~80%) in blood vessels and necrosis were observed in the tumor
tissues of mice treated with mAb-SA:tTF-B (Fig. 9A). In contrast, decreased
intravascular embolization was exhibited in the other treatment
groups. These results confirmed that the mAb-SA conjugate retained
tumor vasculature-targeting ability and induced intravascular
thrombosis in vivo by directing tTF-B to the vascular
endothelial cell surface, thereby causing inhibition of tumor
growth and promoting tumor regression. Major organs from the
mAb-SA:tTF-B treatment group did not exhibit any visible ectopic
embolism or overt tissue injury (Fig.
9B).
Discussion
Vascular targeting therapy was developed to
selectively induce thrombosis in the vasculature of solid tumors.
There are several advantages of this antitumor strategy (39). A local occlusion in the tumor
vasculature may cause subsequent cell death due to the deprivation
of adequate oxygen and nutrient supply. Drugs can also directly
target universal tumor vascular endothelium cells in the blood; it
has been reported that tumor vascular endothelial cells are
unlikely to transform or acquire mutations (40). Therefore, this targeting therapy
may be widely applicable. Conversely, conventional therapies,
including chemotherapy and radiotherapy, although widely used, may
exert cytotoxic effects on normal tissues (41,42).
Numerous reports have demonstrated that the tTF ligand, which
targets a specific antigen, can markedly inhibit tumor growth and
promote tumor regression by selectively inducing intratumoral
thrombosis (43-45); tTF has been conjugated with various
peptides to improve its efficacy, including SP5.2, NGR peptide and
arginylglycylaspartic acid, or their derivatives. However, these
fusion proteins require multiple administrations to achieve
complete thrombosis in the tumor vasculature, which prevents the
use of this strategy in clinical trials. This may be attributed to
the target's low affinity for the targeting moiety and poor
distribution (46). Therefore, to
improve the antitumor efficacy of this ligand-directed approach, it
is important to find a suitable tTF ligand delivery moiety and
ensure it has a suitable tumor-specific receptor, which is also
expressed in solid tumors.
NRP-1 is highly expressed in the endothelial cells
of a variety of tumors, and exerts a positive effect on tumor
angiogenesis, progression and metastasis (47-48).
Therefore, the anti-NRP-1 mAb has been previously demonstrated to
be a promising delivery method that may be used to target tumor
vessels (14). To increase the
concentration of tTF in tumor blood vessels and minimize its
distribution in normal tissues, the present study introduced the
SA/B system, which is characterized by high binding affinity.
In this approach, the preparation of mAb-SA
conjugate was performed using EDC and sulfo-NHS. Sephadex G200
column chromatography was used to purify and unify the products.
Native SDS-PAGE analysis was subsequently conducted to characterize
the mAb-SA conjugate that was synthesized. The results indicated
that a homogeneous conjugate product was obtained, with a
theoretical molecular weight of ~210 kDa. A B-labeling kit was used
to prepare tTF-B after the fusion protein tTF had been generated
and purified. The in vitro activity of the mAb-SA:tTF-B
system was tested prior to in vivo investigations. In
vitro experiments, including confocal immunofluorescence, flow
cytometry and factor X activation, indicated that mAb-SA preserved
the antibody's binding ability in HUVECs, which highly express
NRP-1 (35). The results also
demonstrated that tTF-B retained the ability to induce blood
coagulation. Furthermore, the mAb-SA:tTF-B system was assessed
using competitive ELISA, which demonstrated stable binding between
mAb-SA and tTF-B. A subcutaneous tumor-bearing nude mouse model was
established to identify the in vivo feasibility of the
mAb-SA:tTF-B system. The in vivo distribution and
tumor-targeting efficacy of mAb-SA was evaluated using live
imaging. An intense fluorescent signal was observed in the tumor
area after 3 h; this fluorescence persisted for 72 h. Conversely,
weak fluorescence was observed in healthy organs. This suggested
that mAb-SA was able to selectively target tumor tissues by
interacting with NRP-1 on the vascular endothelial cell surface. In
the antitumor activity studies, the mAb-SA conjugate was used to
localize tumors, with the conjugate accumulating for 24 h until the
concentration reached its peak. tTF-B was subsequently administered
and captured by SA. Data from the growth inhibition assay
demonstrated that tumor size decreased after day 6, and that the
mean tumor volume and weight on day 12 were reduced compared with
the other treatment groups. Histological analysis revealed that the
mAb-SA:tTF-B system induced extensive thrombosis in tumor vessels.
No visible ectopic embolism or injury was observed in normal
organs, which reflects the robust biological targeting ability of
this system.
In conclusion, an antitumor composite system based
on vascular targeting was successfully constructed in the present
study. In vitro experiments and in vivo studies
demonstrated that the mAb-SA:tTF-B system effectively inhibited
tumor growth and promoted tumor regression by selectively targeting
tumor blood vessels and inducing complete vascular infarction. The
introduction of the SA/B system is a promising approach for
improving tTF-ligand coagulation efficiency and antitumor activity.
Therefore, the present study may provide novel insight for the
development of vasculature-targeting antitumor treatments.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant. nos. 81773770) and the Science
and Technology Foundation of Fujian Province, China (grant nos.
2018R1036-1, 2018R1036-3 and 2019R1001-2).
Availability of data and materials
The datasets generated and analyzed during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
PX drafted the manuscript. PX, MZ and SW performed
the experiments. JY, FL, TW and PX designed the study. TL, CL, LiW
and LaW performed data analysis. JY and PX contributed to the
manuscript revisions. All authors reviewed the manuscript. All
authors have read and approved the final version of the manuscript
for publication.
Ethics approval and consent to
participate
All the animal experiments performed in this study
were approved by the Ethics Committee of Xiamen University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Folkman J: The role of angiogenesis in
tumor growth. Semin Cancer Biol. 3:65–71. 1992.PubMed/NCBI
|
|
2
|
Blumberg N: Tumor angiogenesis factor.
Speculations on an approach to cancer chemotherapy. Yale J Biol
Med. 47:71–81. 1974.PubMed/NCBI
|
|
3
|
Milowsky MI, Nanus DM, Kostakoglu L,
Sheehan CE, Vallabhajosula S, Goldsmith SJ, Ross JS and Bander NH:
Vascular targeted therapy with anti-prostate-specific membrane
antigen monoclonal antibody J591 in advanced solid tumors. J Clin
Oncol. 25:540–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bose D, Meric-Bernstam F, Hofstetter W,
Reardon DA, Flaherty KT and Ellis LM: Vascular endothelial growth
factor targeted therapy in the perioperative setting: Implications
for patient care. Lancet Oncol. 11:373–382. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kessler T, Bieker R, Padró T, Schwöppe C,
Persigehl T, Bremer C, Kreuter M, Berdel WE and Mesters RM:
Inhibition of tumor growth by RGD peptide-directed delivery of
truncated tissue factor to the tumor vasculature. Clin Cancer Res.
11:6317–6324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwöppe C, Kessler T, Persigehl T,
Liersch R, Hintelmann H, Dreischalück J, Ring J, Bremer C, Heindel
W, Mesters RM and Berdel WE: Tissue-factor fusion proteins induce
occlusion of tumor vessels. Thromb Res. 125(Suppl 2): S143–S150.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Furie B and Furie BC: The molecular basis
of coagulation. Cell. 53:505–518. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Persigehl T, Ring J, Bremer C, Heindel W,
Holtmeier R, Stypmann J, Claesener M, Hermann S, Schäfers M, Zerbst
C, et al: Non-invasive monitoring of tumor-vessel infarction by
retargeted truncated tissue factor tTF-NGR using multi-modal
imaging. Angiogenesis. 17:235–246. 2014. View Article : Google Scholar
|
|
9
|
Alessi P, Ebbinghaus C and Neri D:
Molecular targeting of angiogenesis. Biochim Biophys Acta.
1654:39–49. 2004.PubMed/NCBI
|
|
10
|
Archer R, Wakabayashi M, Sevilla R,
Summers S, King S and Aimes R: Targeting truncated tissue factor
with tumor vascula-ture specific monoclonal antibodies: Developing
coaguligands as cancer therapeutics. Cancer Res. 67:14–18.
2007.
|
|
11
|
Kessler T, Schwöppe C, Liersch R,
Schliemann C, Hintelmann H, Bieker R, Berdel WE and Mesters RM:
Generation of fusion proteins for selective occlusion of tumor
vessels. Curr Drug Discov Technol. 5:1–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wise LM, Veikkola T, Mercer AA, Savory LJ,
Fleming SB, Caesar C, Vitali A, Makinen T, Alitalo K and Stacker
SA: Vascular endothelial growth factor (VEGF)-like protein from orf
virus NZ2 binds to VEGFR2 and neuropilin-1. Proc Natl Acad Sci USA.
96:3071–3076. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gagnon ML, Bielenberg DR, Gechtman Z, Miao
HQ, Takashima S, Soker S and Klagsbrun M: Identification of a
natural soluble neuropilin-1 that binds vascular endothelial growth
factor: In vivo expression and antitumor activity. Proc Natl Acad
Sci USA. 97:2573–2578. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bergé M, Allanic D, Bonnin P, de Montrion
C, Richard J, Suc M, Boivin JF, Contrerès JO, Lockhart BP, Pocard
M, et al: Neuropilin-1 is upregulated in hepatocellular carcinoma
and contributes to tumour growth and vascular remodelling. J
Hepatol. 55:866–875. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seifi-Alan M, Shams R, Bandehpour M,
Mirfakhraie R and Ghafouri-Fard S: Neuropilin-1 expression is
associated with lymph node metastasis in breast cancer tissues.
Cancer Manag Res. 10:1969–1974. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang X, Molema G, King S, Watkins L,
Edgington TS and Thorpe PE: Tumor infarction in mice by
antibody-directed targeting of tissue factor to tumor vasculature.
Science. 275:547–550. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thorpe PE: Vascular targeting agents as
cancer therapeutics. Clin Cancer Res. 10:415–427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu P, Yan J, Sharifi J, Bai T, Khawli LA
and Epstein AL: Comparison of three different targeted tissue
factor fusion proteins for inducing tumor vessel thrombosis. Cancer
Res. 3:5046–5053. 2003.
|
|
20
|
Saga T, Weinstein JN, Jeong JM, Heya T,
Lee JT, Le N, Paik CH, Sung C and Neumann RD: Two-Step targeting of
experimental lung metastases with biotinylated antibody and
radiolabeled streptavidin. Cancer Res. 54:2160–2165.
1994.PubMed/NCBI
|
|
21
|
Zhang M, Sakahara H, Yao Z, Saga T,
Nakamoto Y, Sato N, Nakada H, Yamashina I and Konishi J:
Intravenous avidin chase improved localization of radiolabeled
streptavidin in intraperito-neal xenograft pretargeted with
biotinylated antibody. Nucl Med Biol. 24:61–64. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakahara H and Saga T: Avidin-biotin
system for delivery of diagnostic agents. Adv Drug Deliv Rev.
37:89–101. 1999. View Article : Google Scholar
|
|
23
|
Liu X, Yang Q, Nakamura C and Miyake J:
Avidin-biotin- immobilized liposome column for chromatographic
fluorescence on-line analysis of solute-membrane interactions. J
Chromatogr B Biomed Sci Appl. 750:51–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Medina LA, Calixto SM, Klipper R, Phillips
WT and Goins B: Avidin/biotin-liposome system injected in the
pleural space for drug delivery to mediastinal lymph nodes. J Pharm
Sci. 93:2595–2608. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nobs L, Buchegger F, Gurny R and Allémann
E: Biodegradable nanoparticles for direct or two-step tumor
immunotargeting. Bioconjug Chem. 17:139–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kheirolomoom A, Dayton PA, Lum AF, Little
E, Paoli EE, Zheng H and Ferrara KW: Acoustically-active
microbubbles conjugated to liposomes: Characterization of a
proposed drug delivery vehicle. J Control Release. 118:275–284.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goldenberg DM, Sharkey RM, Paganelli G,
Barbet J and Chatal JF: Antibody pretargeting advances cancer
radioimmu-nodetection and radioimmunotherapy. J Clin Oncol.
24:823–834. 2006. View Article : Google Scholar
|
|
28
|
Urbano N, Papi S, Ginanneschi M, De Santis
R, Pace S, Lindstedt R, Ferrari L, Choi S, Paganelli G and Chinol
M: Evaluation of a new biotin-DOTA conjugate for pretargeted
antibody-guided radioimmunotherapy (PAGRIT). Eur J Nucl Med Mol
Imaging. 34:68–77. 2007. View Article : Google Scholar
|
|
29
|
Zeng F, Luo F, Lv S, Zhang H, Cao C, Chen
X, Wang S, Li Z, Wang X, Dou X, et al: A monoclonal antibody
targeting neuropilin-1 inhibits adhesion of MCF7 breast cancer
cells to fibronectin by suppressing the FAK/p130cas signaling
pathway. Anticancer Drugs. 25:663–672. 2014.PubMed/NCBI
|
|
30
|
Chen F, Nielsen S and Zenobi R:
Understanding chemical reactivity for homo- and heterobifunctional
protein cross-linking agents. J Mass Spectrom. 48:807–812. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nwe K, Milenic DE, Ray GL, Kim YS and
Brechbiel MW: Preparation of cystamine core dendrimer and
antibody-dendrimer conjugates for MRI angiography. Mol Pharm.
9:374–381. 2012. View Article : Google Scholar
|
|
32
|
Neerman MF, Zhang W, Parrish AR and
Simanek EE: In vitro and in vivo evaluation of a melamine dendrimer
as a vehicle for drug delivery. Int J Pharm. 281:129–132. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chan EC and Ho PC: Preparation and
characterization of immunogens for antibody production against
metanephrine and normetanephrine. J Immunol Methods. 266:143–154.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu ZC, Shen HX, Chen C, Ma L, Li WZ, Wang
L and Geng ZM: Neuropilin-1 promotes primary liver cancer
progression by potentiating the activity of hepatic stellate cells.
Oncol Lett. 15:2245–2251. 2018.PubMed/NCBI
|
|
35
|
Wang L, Zeng H, Wang P, Soker S and
Mukhopadhyay D: Neuropilin-1-mediated vascular permeability
Factor/Vascular endothelial growth Factor-dependent endothelial
cell migration. J Biol Chem. 278:48848–48860. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen X, Lv H, Ye M, Wang S, Ni E, Zeng F,
Cao C, Luo F and Yan J: Novel superparamagnetic iron oxide
nanoparticles for tumor embolization application: Preparation,
characterization and double targeting. Int J Pharm. 426:248–255.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hylarides MD, Mallett RW and Meyer DL: A
robust method for the preparation and purification of
Antibody/streptavidin conjugates. Bioconjug Chem. 12:421–427. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liao MY, Kuo MY, Lu TY, Wang YP and Wu HC:
Generation of an anti-EpCAM antibody and epigenetic regulation of
EpCAM in colorectal cancer. Int J Oncol. 46:1788–1800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gottstein C, Wels W, Ober B and Thorpe PE:
Generation and characterization of recombinant vascular targeting
agents from hybridoma cell lines. Biotechniques. 30:190–194. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu Z, Rao B, Chen S and Duanmu J:
Selective and effective killing of angiogenic vascular endothelial
cells and cancer cells by targeting tissue factor using a factor
VII-targeted photodynamic therapy for breast cancer. Breast Cancer
Res Treat. 126:589–600. 2011. View Article : Google Scholar
|
|
41
|
Rades D, Fehlauer F, Bajrovic A, Mahlmann
B, Richter E and Alberti W: Serious adverse effects of amifostine
during radiotherapy in head and neck cancer patients. Radiother
Oncol. 70:261–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Monsuez JJ, Charniot JC, Vignat N and
Artigou JY: Cardiac side-effects of cancer chemotherapy. Int J
Cardiol. 144:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dienst A, Grunow A, Unruh M, Rabausch B,
Nör JE, Fries JW and Gottstein C: Specific occlusion of murine and
human tumor vasculature by VCAM-1-targeted recombinant fusion
proteins. J Natl Cancer Inst. 97:733–747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bieker R, Kessler T, Schwöppe C, Padró T,
Persigehl T, Bremer C, Dreischalück J, Kolkmeyer A, Heindel W,
Mesters RM and Berdel WE: Infarction of tumor vessels by
NGR-peptide-directed targeting of tissue factor: Experimental
results and first-in-man experience. Blood. 113:5019–5027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lv S, Ye M, Wang X, Chen X, Dou X, Dai Y,
Dai Y, Zeng F, Luo L, Wang C, et al: A recombined fusion protein
SP5.2/tTF induce thrombosis in tumor blood vessel. Neoplasma.
62:531–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schmidt LH, Stucke-Ring J, Brand C,
Schliemann C, Harrach S, Muley T, Herpel E, Kessler T, Mohr M,
Görlich D, et al: CD13 as target for tissue factor induced tumor
vascular infarction in small cell lung cancer. Lung Cancer.
113:121–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pan Q, Chanthery Y, Liang WC, Stawicki S,
Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, et al:
Blocking neuro-pilin-1 function has an additive effect with
anti-VEGF to inhibit tumor growth. Cancer Cell. 11:53–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Grandclement C and Borg C: Neuropilins: A
new target for cancer therapy. Cancers (Basel). 3. pp. 1899–1928.
2011, View Article : Google Scholar
|