Introduction
Epithelial ovarian cancer (EOC) is the fifth most
common lethal cancer in women. The majority of the patients present
with advanced-stage disease and, therefore, an unfavorable
prognosis (1). EOC is not a single
disease entity. Histological subtyping is a reasonable first
stratification; however, the identification of prognostic and
predictive biomarkers remains a major challenge in order to
establish targeted therapies. The five major
histological/morphological types of ovarian carcinoma are as
follows: High-grade serous carcinoma (68%), clear cell (12%),
endometrioid (11%), mucinous (3%) and low-grade serous carcinoma
(3%) (2).
Despite high response rates to first-line
chemotherapy, resistance to treatment frequently develops. Indeed,
~80% of the patients with International Federation of Gynecology
and Obstetrics (FIGO) stage II–IV EOC will progress during or after
adjuvant chemotherapy (3). Therefore,
there is a need for new therapeutic approaches, as well as
prognostic and predictive markers. Identification of new prognostic
factors may help to distinguish different biological subgroups. In
EOC, this is particularly important for the group of patients who
develop recurrent disease. These patients often do not benefit from
current treatment modalities and may suffer from severe
therapy-related side effects. Future research should be focused on
establishing more targeted and individualized treatment strategies
in biologically distinct subgroups.
Trefoil factor 3 (TFF3) is an estrogen-regulated
oncogene (4). TFF3 expression has
been found to be associated with prognostic factors in a multitude
of different types of cancer, including estrogen receptor-positive
breast cancer (4). Furthermore, TFF3
has been found to be involved in gastric cancer progression
(5). TFF3 is part of the marker panel
that was selected for the calculator for ovarian carcinoma subtype
prediction, introduced by Kölbel et al in 2011, as being
most predictive of ovarian carcinoma subtype in both the archival
and the tumor bank cohorts (2). The
selection of TFF3 was based on comprehensive gene expression
profiling data (2,6). To the best of our knowledge, data on
TFF3 in ovarian cancer are scarce. Therefore, the expression of the
TFF3 in 91 ovarian cancer patients was investigated to determine
its effect on prognosis and platinum sensitivity in ovarian
cancer.
Patients and methods
Patients and treatment
The study included patients with primary EOC treated
between 1995 and 2008 at the Department of Obstetrics and
Gynecology of Goethe University (Frankfurt, Germany). A total of 91
patients were retrospectively analyzed. Formalin-fixed,
paraffin-embedded (FFPE) tissue samples were obtained from the
Department of Pathology. The patient characteristics are listed in
Table I. Clinical and pathological
factors were evaluated by reviewing medical charts and pathology
records. The Local Research Ethics Committee approved studies of
human tissue and the samples were processed anonymously. Clinical
outcome was assessed from the date of surgery to the date of death
or until the end of 2009. Only patients with histologically proven
EOC were included. The majority of the patients had advanced-stage
disease (FIGO III–IV). All the patients received primary surgery
followed by platinum- and taxane-based chemotherapy.
 | Table I.Patients characteristics. |
Table I.
Patients characteristics.
| Characteristics | Number (n) | % |
|---|
| Age, years |
|
|
|
>50 | 74 | 81.3 |
| ≤50 | 17 | 18.6 |
| Grade |
|
|
| G3 | 62 | 68.1 |
| G1 and
G2 | 29 | 31.9 |
| FIGO stage |
|
|
| I | 15 | 16.5 |
| II | 7 | 8.2 |
| III | 54 | 58.8 |
| IV | 15 | 16.5 |
| Histological
subtype |
|
|
|
Serous | 70 | 76.9 |
|
Other | 21 | 23.1 |
| Residual tumor,
cm |
|
|
| 0 | 43 | 47.3 |
| 0–1 | 19 | 20.9 |
|
>1 | 29 | 31.9 |
| Platinum
sensivity |
|
|
|
Sensitive | 55 | 60.4 |
|
Resistant | 32 | 35.2 |
| Not
applicable | 4 | 4.4 |
| Progression-free
survival, months |
|
|
|
Median |
| 16.9 |
|
Range |
| 14.0–19.7 |
| Overall survival,
months |
|
|
|
Median |
| 51.6 |
|
Range |
| 42.9–60.2 |
Tissue samples and
immunohistochemistry
The tissue samples were processed as previously
described (7). Routine
histopathological sections stained with hematoxylin and eosin were
used for primary diagnosis and second reviewing (M.O.). Diagnosis
and grading were performed according to the current World Health
Organization criteria (8,9). Following mounting on Superfrost Plus
slides (Fisherbrand, Fisher Scientific, Hampton, NH, USA), 2-µm
paraffin sections were dewaxed in xylene and rehydrated with
graduated ethanol treatment. For antigen retrieval, the sections
were incubated for 20 min in a microwave oven (800 W) using citrate
buffer (10 mM; pH 6.0). The monoclonal anti-TFF3 antibody (ab57752,
Lot GR71649-1; Abcam, Cambridge, UK,) was used at a dilution of
1:100. Incubation with the antibody for 1 h at room temperature was
performed. For negative controls, the primary antibody was omitted.
For secondary antibody incubation, the Dako REAL Detection System
Alkaline Phosphatase/RED (REF K5005, Lot 20023341; Dako, Glostrup,
Denmark) was applied, according to the manufacturer's instructions.
The sections were counterstained with hematoxylin (Gill no. 3, Lot
060M4356; Sigma-Aldrich, St. Louis, Missouri, USA). Expression
levels for cytoplasmic TFF3 were scored semi-quantitatively based
on the product of staining intensity (SI) and percentage of
positive cells (PP) using the immunoreactive score (IRS) as follows
(10): IRS=SIxPP. SI was assigned as
0, negative; 1, weak; 2, moderate; or 3, strong. PP was defined as
0, negative; 1, <10%; 2, 11–50%; 3, 51–80%; or 4, >80%
positive cells. All assessments were performed blinded with respect
to clinical patient data.
Statistical analysis
For statistical analysis a cut-off value was defined
according to the IRS, i.e., scores of 0–3 (negative and low) were
collectively defined as a low score, whereas scores ≥3 were defined
as a high TFF3 expression score. The Chi-square and Fisher's exact
tests were used to assess the associations between TFF3 expression
of tumors and clinicopathological parameters. For those patients
with available follow-up data, Kaplan-Meier curves were constructed
and the log-rank test was used to determine the univariate
significance of the variables. Cox regression analysis was
performed to determine hazard ratios. All reported P-values were
two-sided and P-values of ≤0.05 were considered to indicate
statistically significant differences. All analyses were performed
using the SPSS software package, version 22.0 (IBM SPSS, Armonk,
NY, USA).
Results
Patient characteristics
A total of 91 patients were included in this study.
All the patients underwent primary debulking surgery including
hysterectomy, bilateral salpingo-oophorectomy, infragastric
omentectomy, appendectomy (mucinous subtype), systematic pelvic and
para-aortic lymphadenectomy and, in distinct cases, peritonectomy
and resection of affected tissues with intended resection of all
visible tumors. In the majority of the patients (n=62; 68%) optimal
debulking, i.e., achieving an optimal postoperative residual tumor
of ≤1 cm, according to the National Comprehensive Cancer Network
guidelines (11), could be
achieved.
The median follow-up time was 33 months. The
Kaplan-Meier estimate for median progression-free survival (PFS)
for the entire group was 16.9 months [95% confidence interval (CI):
14.0–19.7] and for overall survival (OS) 51.6 months (95% CI:
42.9–60.2). The corresponding 5-year PFS and OS rates were 17.9±5.1
and 34.9±6.2%, respectively. In the group of serous ovarian
cancers, the PFS was 15.4 months (95% CI: 13.7–17.0) and the OS was
44.4 months (95% CI: 26.0–62.1). The patient characteristics for
the entire cohort are displayed in Table
I.
TFF3 expression according to different
clinicopathological characteristics
Immunohistochemistry revealed higher expression
levels of TFF3 (IRS score ≥4) in 25 tumor samples (27.5%). In the
cohort of 91 patients, no significant difference in TFF3 expression
was found based on age, FIGO stage or residual tumor. In the group
of serous carcinomas, there was a significant association with
lower expression of TFF3 when compared with other subtypes.
Furthermore, a significant correlation of TFF3 expression and grade
was observed. Among TFF3-negative tumors (IRS=0–3), 25.8% were G1
or G2 and 74.2% were G3, while among TFF3-positive tumors, grade
was equally distributed between the two groups (48 vs. 52%,
respectively). The correlation of TFF3 negativity with higher grade
was statistically significant (P=0.05 Fisher's exact test; Table II).
 | Table II.Clinical characteristics according to
TFF3 expression. |
Table II.
Clinical characteristics according to
TFF3 expression.
| Characteristics | TFF3-negative (IRS
0–3); n (%) | TFF3-positive (IRS
4–12); n (%) | P-value | Total (n=91) |
|---|
| Residual tumor,
cm |
|
| 0.64 |
|
| 0 | 30 (69.8) | 13 (30.2) |
| 43 |
|
>0 | 36 (75.0) | 12 (25.0) |
| 48 |
| Grade |
|
| 0.05 |
|
| 1, 2 | 17 (58.6) | 12 (41.4) |
| 29 |
| 3 | 49 (79.0) | 13 (21.0) |
| 62 |
| Age, years |
|
| 0.77 |
|
|
>50 | 53 (71.6) | 21 (28.4) |
| 74 |
| ≤50 | 13 (76.5) | 4 (23.5) |
| 17 |
| FIGO stage |
|
| 0.29 |
|
| I,
II | 14 (63.6) | 8 (36.4) |
| 22 |
| III,
IV | 52 (75.4) | 17 (24.6) |
| 69 |
| Histological
subtype |
|
| 0.01 |
|
|
Serous | 56 (80) | 14 (20) |
| 70 |
|
Other | 10 (47.6) | 11 (52.4) |
| 21 |
| Platinum
response |
|
| n.a. |
|
|
Sensitive | 39 (70.9) | 16 (29.1) |
| 55 |
|
Resistant | 24 (75.0) | 8 (25.0) |
| 32 |
|
Unknown/n.a. | 3 (75.0) | 1 (25.0) |
| 4 |
Association of TFF3 expression with
survival
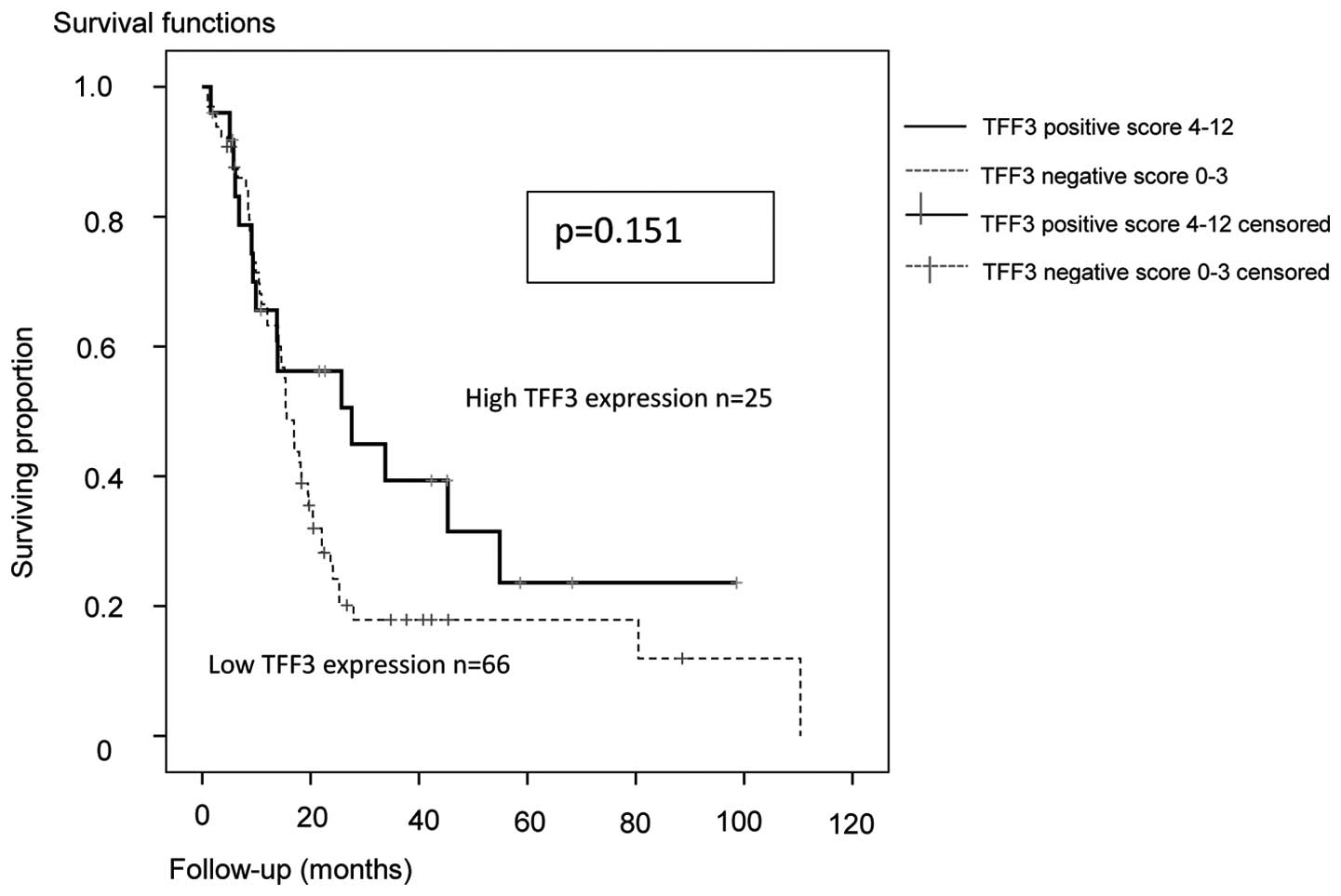
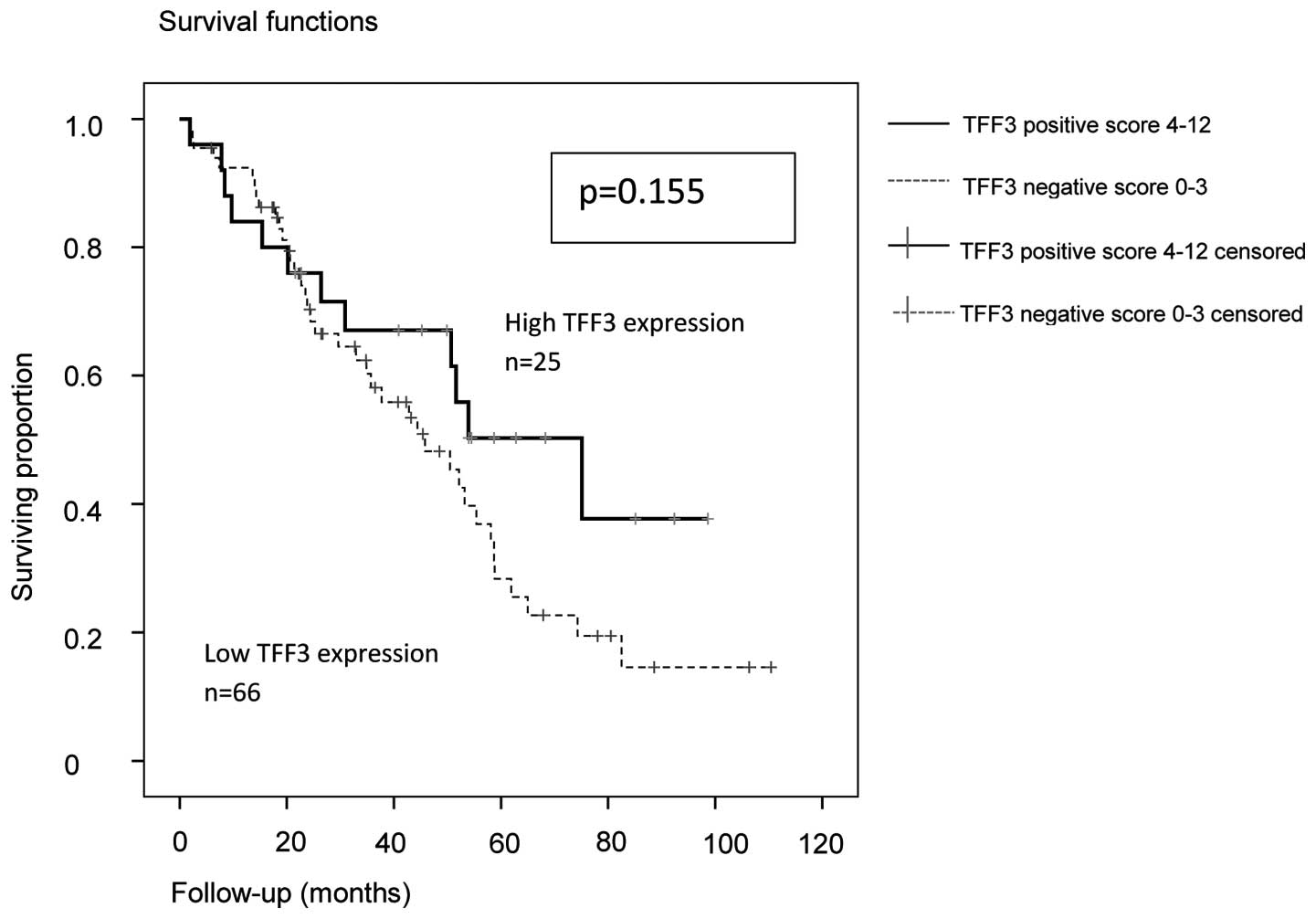
In the Kaplan-Meier analysis of the entire cohort,
the median PFS and OS were increased in TFF3-positive patients
(Figs. 1 and 2). However, this was statistically not
significant (P=0.151 and P=0.155, respectively; log-rank
Mantel-Cox) although there was a trend for a better PFS.
In the entire group, the median PFS in TFF3-positive
patients was 27.6 months (95% CI: 1.4–53.8) vs. 15.6 months (95%
CI: 13.6–17.6) in TFF3-negative patients. The OS for the entire
group in TFF3-positive patients was 75.1 months (95% CI:
40.4–109.8) vs. 45.8 months (95% CI: 30.5–61.6) in TFF3-negative
cases.
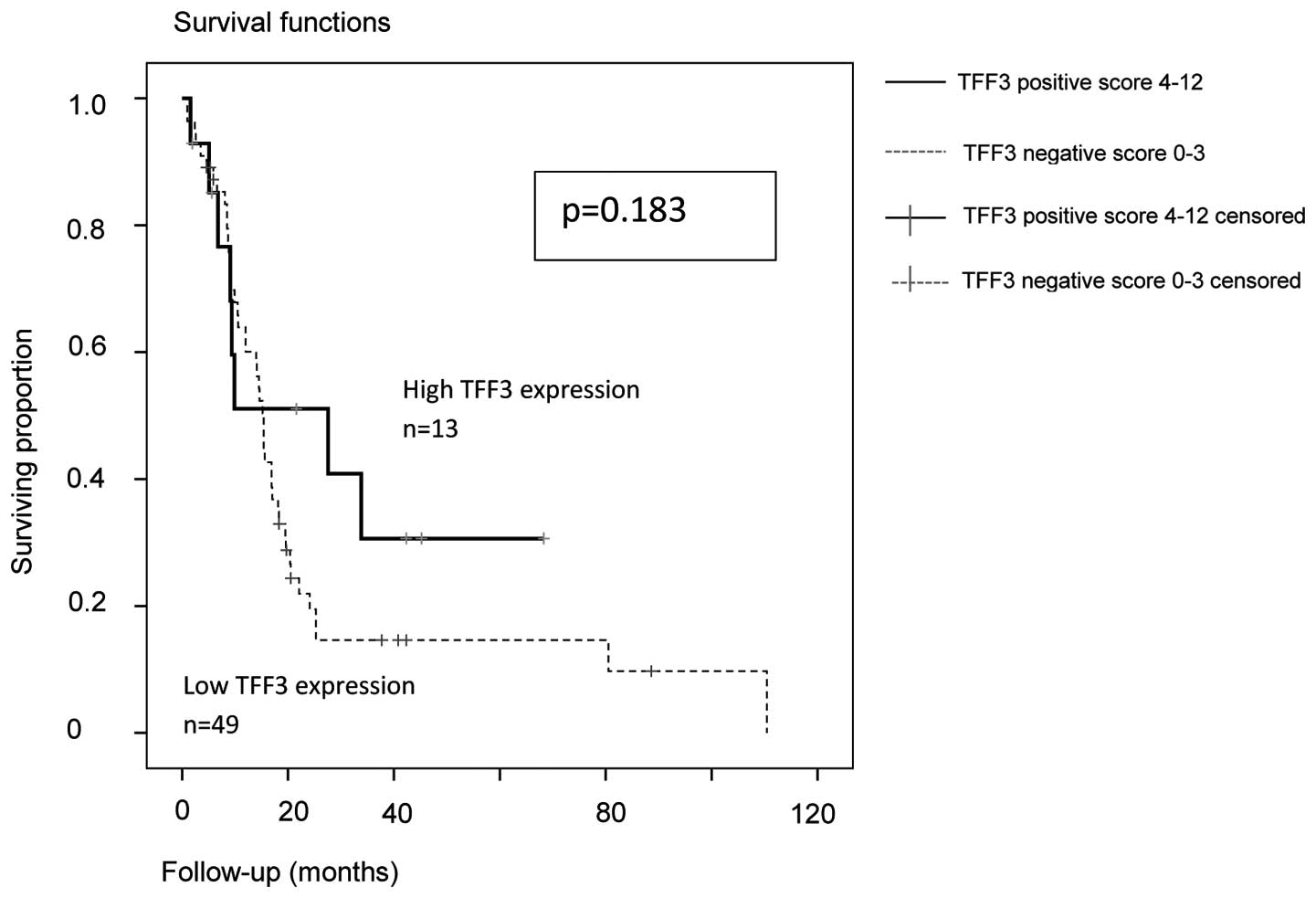
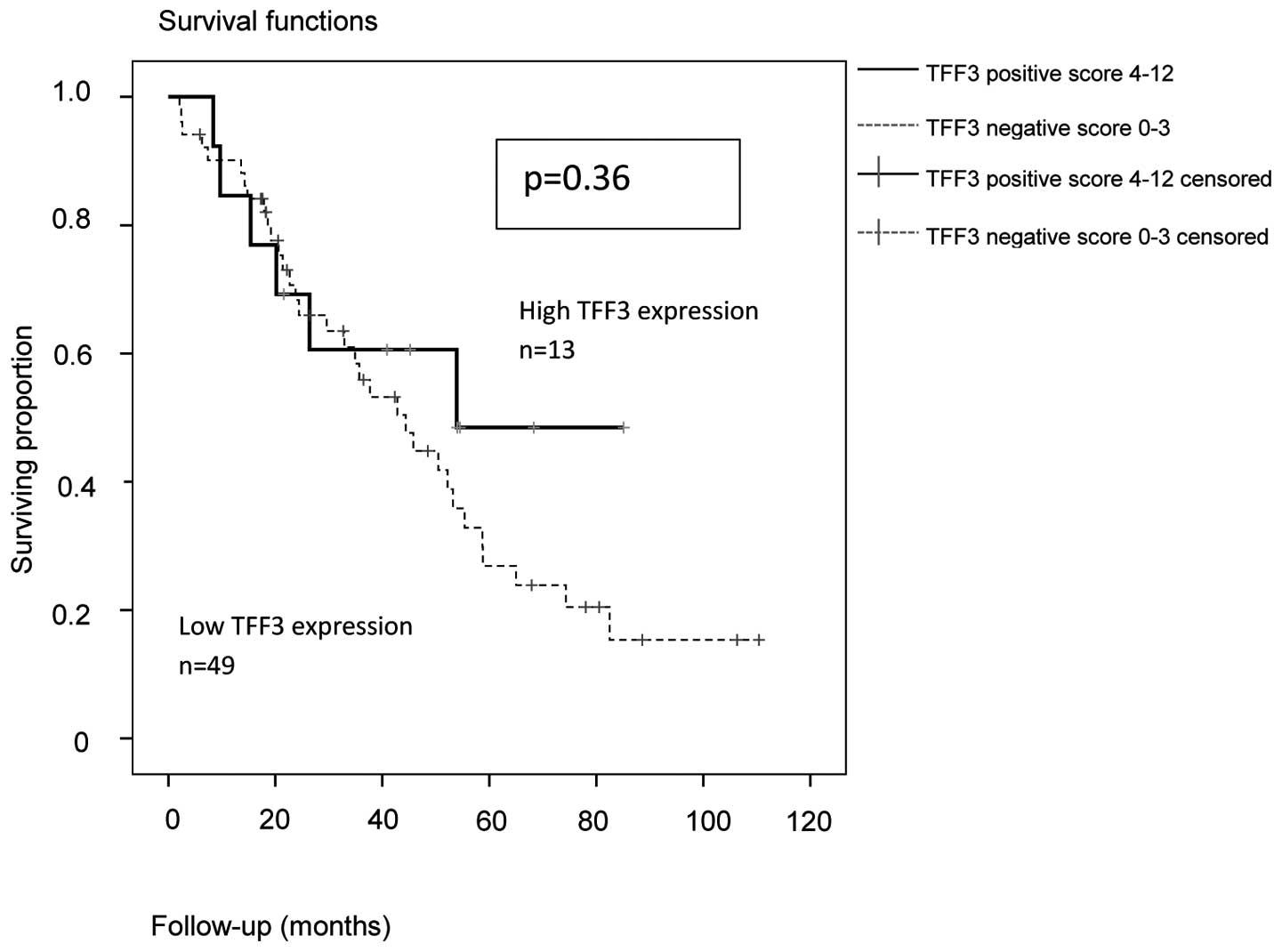
By analyzing the subgroups of serous (n=70) and
high-grade serous (n=62) ovarian carcinomas, the trend for a better
PFS and OS in patients with high expression of TFF3 was confirmed.
For the subgroup of high-grade serous ovarian cancer (n=62), the
PFS (P=0.183) and OS (P=0.36) results are shown in Figs. 3 and 4:
In TFF3-positive serous and high-grade serous ovarian cancers, the
median PFS was 27.6 months (95% CI: 0–55.7) vs. 15.2 months in
TFF3-negative cases (95% CI: 13.8–16.6). The median OAS was 53.9
months in TFF3-positive (95% CI: Non-applicable) vs. 44.4 months in
TFF3-negative cases (95% CI: 30.5–58.3).
Univariate Cox regression
analysis
The univariate Cox regression analysis revealed a
statistically significant effect of residual tumor on OS and PFS
(P<0.001 and P<0.001, respectively) and of FIGO stage on OS
and PFS (P=0.001 and P<0.001, respectively). However, TFF3
positivity did not exert an effect on OS and PFS in the univariate
Cox regression analysis (P=0.158 and P=0.156, respectively)
(Table III).
 | Table III.Univariate Cox regression analysis of
overall and progression-free survival. |
Table III.
Univariate Cox regression analysis of
overall and progression-free survival.
| A, Overall
survival |
|---|
|
|---|
| Variables | P-value | HR | 95% CI |
|---|
| Histology, other
vs. serous | 0.102 | 0.548 | 0.266 | 1.126 |
| Residual tumor, ≥0
vs. 0 cm | 0.000 | 3.714 | 2.010 | 6.865 |
| FIGO stage, III and
IV vs. I and II | 0.001 | 1.085 | 1.036 | 1.137 |
| TFF3-positive (IRS
4–12) vs. -negative (IRS 0–3) | 0.158 | 0.626 | 0.327 | 1.200 |
| Age, ≤50 vs. >50
years | 0.079 | 0.504 | 0.234 | 1.084 |
| Grade, G3 vs. G1
and G2 | 0.141 | 0.949 | 0.884 | 1.018 |
|
| B, Progression-free
survival |
|
| Variables | P-value | HR | 95% CI |
|
| Histology, other
vs. serous |
0.043 | 0.531 | 0.288 | 0.981 |
| Residual tumor, ≥0
vs. 0 cm | <0.001 | 3.165 | 1.887 | 5.308 |
| FIGO stage, III and
IV vs. I and II | <0.001 | 1.065 | 1.031 | 1.101 |
| TFF3-positive (IRS
4–12) vs. -negative (IRS 0–3) |
0.156 | 0.654 | 0.364 | 1.175 |
| Age, ≤50 vs. >50
years |
0.445 | 0.775 | 0.403 | 1.491 |
| Grade, G3 vs. G1
and G2 |
0.095 | 0.949 | 0.892 | 1.009 |
Multivariate Cox regression
analysis
The multivariate Cox regression analysis for OS and
PFS included age (>50 vs. ≤50 years), grade (G1 or G2 vs. G3),
FIGO stage (I and II vs. III and IV), pathology (serous vs. others)
and residual tumor (0 vs. >0 cm). Only FIGO stage and residual
tumor exhibited a statistically significant correlation with poor
OS (P=0.041 and P=0.043, respectively). TFF3 expression was not
found to be significantly correlated with PFS or OS in all patients
in the multivariate analysis (P=0.249) (Table IV).
 | Table IV.Multivariate Cox regression analysis
of standard variables among ovarian cancers for progression-free
and overall survival. |
Table IV.
Multivariate Cox regression analysis
of standard variables among ovarian cancers for progression-free
and overall survival.
| A, Progression-free
survival |
|---|
|
|---|
| Variables | P-value | HR | 95% CI |
|---|
| Age, >50 vs. ≤50
years | 0.458 | 1.310 | 0.642 | 2.672 |
| Grade, G1 and G2
vs. G3 | 0.678 | 1.137 | 0.619 | 2.088 |
| Histology, serous
vs. other | 0.880 | 1.053 | 0.540 | 2.051 |
| Residual tumor, 0
vs. >0 cm | 0.041 | 0.511 | 0.269 | 0.971 |
| FIGO stage, I and
II vs. III and IV | 0.043 | 0.397 | 0.162 | 0.970 |
| TFF3-negative (IRS
0–3) vs. -positive (IRS 4–12) | 0.105 | 1.673 | 0.898 | 3.118 |
|
| B, Overall
survival |
|
| Variables | P-value | HR | 95% CI |
|
| Age, >50 vs. ≤50
years | 0.229 | 1.643 | 0.732 | 3.686 |
| Grade, G1 and G2
vs. G3 | 0.528 | 1.243 | 0.632 | 2.446 |
| Histology, serous
vs. other | 0.645 | 0.829 | 0.375 | 1.837 |
| Residual tumor, 0
vs. >0 cm | 0.054 | 0.492 | 0.240 | 1.012 |
| FIGO stage, I and
II vs. III and IV | 0.027 | 0.259 | 0.078 | 0.856 |
| TFF3-negative (IRS
0–3) vs. -positive (IRS 4–12) | 0.754 | 1.117 | 0.559 | 2.229 |
Discussion
Ovarian carcinomas of different histological types
originate from different precursor cells; thus, they retain
specific gene and biomarker expression profiles (2). Ovarian cancer subtyping remains one of
the major challenges in order to establish prognostic and
predictive markers. Apart from histological subtype and the
recently established binary grading system that forms subgroups of
high-grade and low-grade serous EOC, very few predictive or
prognostic biomarkers have been identified in order to better
describe the clinical course of the disease, as well as sensitivity
to different therapeutic approaches. Furthermore, genetic tumor
profiling may reveal several prognostic and predictive genetic
signatures. However, these signatures have yet to be validated. In
this context, TFF3 was found to be involved in certain
signatures.
The TFF family, which comprises three proteins
(TFF1, TFF2 and TFF3), is involved in the development and
progression of various types of cancers (12).
However, the role of TFF3 in ovarian cancer has not
been fully elucidated (13). In
breast cancer cells, TFF3 expression is generally positively
associated with mammary carcinoma of the estrogen receptor-positive
subtype, and TFF3 was recently identified as a promoter of tumor
angiogenesis (4). In breast cancer,
TFF3 expression is associated with poor survival, lymph node
dissemination and distant metastasis. Furthermore, TFF3 may promote
gastric cancer and its expression is associated with increased
microvessel density in gastric as well as breast cancer (4,5). High TFF3
mRNA levels in colorectal cancer tissues are associated with
distant metastasis, recurrence and poor survival (12). Other authors investigated serum TFF3
levels in gastrointestinal cancer patients and found a correlation
of high serum levels with advanced-stage disease and poor response
to chemotherapy (12). TFF3 is
an estrogen receptor-related gene in breast and ovarian cancer.
Apart from the effect of TFF3 on endocrine therapy, TFF3 expression
may also be associated with chemoresistance in breast cancer
(14). As immunohistochemical
staining of the estrogen receptor does not affect the clinical
management of ovarian cancer, it is not part of the routine
histopathological work-up of ovarian cancer. Thus, in the present
study, we did not focus on the correlation of TFF3 expression with
the estrogen receptor. However, further studies are required in
order to elucidate the effect of simultaneous estrogen receptor and
TFF3 expression in ovarian cancer.
This retrospective analysis of 91 patients with EOC
shows that loss of TFF3 expression may be correlated with disease
progression. To the best of our knowledge, this is the first report
on the prognostic effect of TFF3 expression in this tumor
entity.
Interestingly, in our patient collective, there was
a trend for improved prognosis in TFF3-positive patients; however,
this trend was not significant (P=0.151 and P=0.155 for OS and PFS,
respectively). This tendency of differences in OS and PFS in
TFF3-positive vs. -negative patients appears to be contradictory to
the results of other tumor entities, which reported TFF3 to be a
marker of poor prognosis (12). Above
all, a correlation of higher TFF3 expression with poor prognosis
was found in gastrointestinal tumors that were, in this respect,
most intensively evaluated. Furthermore, TFF3 appears to be a
potential marker for screening in this tumor entity. Although
gastrointestinal tumors and EOC share certain common
characteristics, TTF3 expression was hardly investigated in ovarian
cancer. Only a very recent study demonstrated an effect of TFF3
expression on clinical course in EOC. The authors demonstrated a
significant correlation of high TFF3 expression and lower risk of
recurrence (15). However, that study
by Jatoi et al (15) was
designed to focus specifically on interactions between several
potential prognostic markers, rather than analyze the impact of
single markers. The results confirmed our observation of TFF3 as a
protective marker against recurrence of EOC, in contrast to
gastrointestinal tumors. Based on our results, two hypotheses may
be conjured: TFF3-negative ovarian cancers may be more aggressive,
regardless of stage and histopathological subtype; however, TFF3
positivity may be associated with higher chemosensitivity, which
may explain the improved survival due to a better response to
chemotherapy. In our patient collective, no significant correlation
was observed between TFF3 expression and sensitivity to platinum
agents. In order to elucidate this question, in vitro essays
and further studies are required. In our patient collective, all
histopathological subtypes were included. However, histological
subtypes other than serous EOC were underrepresented. Under the
assumption that TFF3 is part of the subtype markers for different
histopathological entities of ovarian cancer, larger studies are
required in order to clearly differentiate between the different
histological subtypes (2). In
particular, the subgroup of mucinous EOC should be further
investigated due to its similarities to gastrointestinal tumors.
Based on the dualistic model of ovarian carcinogenesis proposed by
Kurman and Shih, we performed a subgroup analysis of high-grade
serous ovarian cancers (type II tumors) (16). In our patient collective, the subgroup
analyses of high-grade serous ovarian cancers yielded identical
results. However, these results must be interpreted with caution,
as the differences in OS and PFS between TFF3-positive and
-negative patients were not significant.
A significant difference in TFF3 expression based on
grade (G1 or 2 vs. G3) was observed, namely G3 tumors were less
likely to express TFF3 (P=0.05). To the best of our knowledge,
there are no reported data on a correlation between grade and TFF3
overexpression to date. Further studies with larger patient
collectives and subgroup analyses are required, as our patient
collective was heterogeneous regarding histological subtype, stage
and therapy.
Acknowledgements
We would like to thank Samira Adel and Katherina
Kourtis for expert technical assistance. Furthermore, we would like
to thank Prof. Dr. Dr. M. L. Hansmann Senckenberg (Institute of
Pathology, University of Frankfurt), for providing the FFPE tissue
samples.
References
|
1
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Köbel M, Kalloger SE, Lee S, Duggan MA,
Kelemen LE, Prentice L, Kalli KR, Fridley BL, Visscher DW, Keeney
GL, et al: Biomarker-based ovarian carcinoma typing: A histologic
investigation in the ovarian tumor tissue analysis consortium.
Cancer Epidemiol Biomarkers Prev. 22:1677–1686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
du Bois A, Reuss A, Pujade-Lauraine E,
Harter P, Ray-Coquard I and Pfisterer J: Role of surgical outcome
as prognostic factor in advanced epithelial ovarian cancer: A
combined exploratory analysis of 3 prospectively randomized phase 3
multicenter trials: By the arbeitsgemeinschaft gynaekologische
onkologie studiengruppe ovarialkarzinom (AGO-OVAR) and the groupe
d'Investigateurs Nationaux Pour les Etudes des cancers de l'Ovaire
(GINECO). Cancer. 115:1234–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lau WH, Pandey V, Kong X, Wang XN, Wu Z,
Zhu T and Lobie PE: Trefoil factor-3 (TFF3) stimulates de novo
angiogenesis in mammary carcinoma both directly and indirectly via
IL-8/CXCR2. PLoS One. 10:e01419472015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiao P, Ling H, Lan G, Liu J, Hu H and
Yang R: Trefoil factors: Gastrointestinal-specific proteins
associated with gastric cancer. Clin Chim Acta. 450:127–134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalloger SE, Köbel M, Leung S, Mehl E, Gao
D, Marcon KM, Chow C, Clarke BA, Huntsman DG and Gilks CB:
Calculator for ovarian carcinoma subtype prediction. Mod Pathol.
24:512–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Engels K, Knauer SK, Metzler D, Simf C,
Struschka O, Bier C, Mann W, Kovács AF and Stauber RH: Dynamic
intracellular survivin in oral squamous cell carcinoma: Underlying
molecular mechanism and potential as an early prognostic marker. J
Pathol. 211:532–540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silverberg SG: Histopathologic grading of
ovarian carcinoma: A review and proposal. Int J Gynecol Pathol.
19:7–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tavassoli FA and Devilee P: Tumours of the
ovary and peritoneumPathology and Genetics of Tumours of the Breast
and Female Genital Organs. IARC Press; Lyon: 2003
|
|
10
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
11
|
NCCN Clinical Practice Guidelines in
Oncology (NCCN Guidelines®): Ovarian Cancer Including
Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 2.
2011, https://www.tri-kobe.org/nccn/guideline/gynecological/english/ovarian.pdfAccessed
July 1, 2016.
|
|
12
|
Morito K, Nakamura J, Kitajima Y, Kai K,
Tanaka T, Kubo H, Miyake S and Noshiro H: The value of trefoil
factor 3 expression in predicting the long-term outcome and early
recurrence of colorectal cancer. Int J Oncol. 46:563–568.
2015.PubMed/NCBI
|
|
13
|
Walker G, MacLeod K, Williams AR, Cameron
DA, Smyth JF and Langdon SP: Estrogen-regulated gene expression
predicts response to endocrine therapy in patients with ovarian
cancer. Gynecol Oncol. 106:461–468. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Chen C, Yang B, Xu Q, Wu F, Liu F,
Ye X, Meng X, Mougin B, Liu G, et al: Estrogen receptor-related
genes as an important panel of predictors for breast cancer
response to neoadjuvant chemotherapy. Cancer Lett. 302:63–68. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jatoi A, Vierkant RA, Hawthorne KM, Block
MS, Ramus SJ, Larson NB, Fridley BL and Goode EL: Clinical and
emergent biomarkers and their relationship to the prognosis of
ovarian cancer. Oncology. 90:59–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kurman RJ and Shih IeM: The dualistic
model of ovarian carcinogenesis: Revisited, revised and expanded.
Am J Pathol. 186:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|