Introduction
Pituitary adenoma is a common type of neurological
neoplasm and the third most common type of intracranial tumors.
While pituitary adenomas are classified as benign tumors, a
proportion of them exhibit invasive growth towards the surrounding
normal structures, including the skull, cavernous sinus, dura,
sphenoid bone, the third ventricle, and even the pharyngonasal
cavity (1). Although recent studies
have advanced the current knowledge on the pathogenesis of
pituitary adenoma, the precise mechanisms and causes have remained
elusive. Phosphatase and tensin homolog deleted on chromosome 10
(PTEN), the first known tumor suppressor gene with phosphatase
activity, was first cloned and named in 1997 (2,3). In
2007, neural precursor cell expressed developmentally down
regulated 4-1 (NEDD4-1) was identified by Wang et al
(4). NEDD4-1 has an important role
in downregulating the levels of PTEN. It can promote the
degradation of PTEN in the proteasome by adding a molecular marker,
ubiquitin (4–8). To the best of our knowledge, no
previous studies have assessed whether NEDD4-1 is expressed in
pituitary adenomas or whether its expression is associated with
their invasiveness or with PTEN. In the present study, the
expression of NEDD4-1 and PTEN in invasive and non-invasive
pituitary adenomas and normal pituitary tissues were analyzed using
immunohistochemistry, and their correlation was assessed. The
present study aimed to investigate the possible molecular
mechanisms of the genesis and invasiveness of pituitary adenomas,
and to identify biomarkers for early detection as well as
characterization of the biological behavior of pituitary
adenomas.
Materials and methods
Patients and reagents
The specimens for immunohistochemical analysis were
collected from 50 patients with pituitary adenomas who underwent
treatment at the Department of Neurosurgery of Heze Municipal
Hospital (Heze, China) from July 2012 to May 2015. The patient
cohort comprised 27 males and 23 females, with a male-to-female
ratio of 1.17:1. The average age of the patients was 38.7 years
(range, 18–78 years). According to the presence or absence of
invasion of the tumors to the surrounding bones and cavernous
sinus, patients were divided into the invasive group (26 cases) and
the non-invasive group (24 cases) (Table
I). Furthermore, 10 samples of normal pituitary tissues were
obtained through normal autopsy. Rabbit anti-human NEDD4-1
polyclonal antibody (cat. no. bs-3506R-AF350; 1:200 dilution) and
rabbit anti-human PTEN polyclonal antibody (cat. no. DM-0748R,1:300
dilution) were from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA), and secondary antibodies were from a designated
immunohistochemistry kit for rabbit antibodies (Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd., China). All experiments and
use of the specimens in the present study were approved by the
Ethics Committee of Heze Municipal Hospital (Heze, China), and all
patients provided written informed consent prior to enrollment.
 | Table I.Clinical data of patients with
pituitary adenoma. |
Table I.
Clinical data of patients with
pituitary adenoma.
| Characteristic | Invasive group
(n) | Non-invasive group
(n) |
|---|
| Cases | 26 | 24 |
| Age (years) |
|
|
| ≥40 | 20 | 15 |
|
<40 | 6 | 9 |
| Gender |
|
|
| Male | 13 | 14 |
|
Female | 13 | 10 |
| Endocrine type |
|
|
| ACTH | 2 | 5 |
| GH | 6 | 3 |
| PRL | 8 | 10 |
|
Others | 10 | 6 |
| Operative method |
|
|
|
Transcranial | 21 | 6 |
|
Trans-sphenoidal | 5 | 18 |
Immunohistochemical staining
procedures
Paraffin-embedded specimens were cut into 4-µm thick
sections, which were deparaffinized in turpentine, rehydrated in an
ascending ethanol series and incubated in citrate buffer (0.01
mol/l, pH 6.0) with water bath heating (~98°C) for 15 min for
antigen recovery. Following a general streptavidin-biotin complex
immunohistochemical protocol (9),
immunoreactivity was visualized with diaminobenzidine and the
nuclei were counterstained with hematoxylin. Sections were then
subsequently dehydrated in a series of graded alcohols, cleared in
xylene and mounted. Prostate cancer samples were used as the
positive control and slides incubated with phosphate-buffered
saline without antibody were used as the negative control.
Evaluation of immunohistochemical
staining
For each sample, 10 microscopic fields
(magnification, ×200) were selected from the tissue sections with
the strongest immune response. A total of 100 cells per field were
counted and averaged. The percentage of positively stained cells
was recorded. Staining was rated according to the color intensity
and the percentage of positive cells. Positive staining was defined
as the staining intensity being markedly higher than that of the
background. Staining was classified into four categories according
to the number of stained cells and the staining intensity: Negative
[- (i.e., the staining intensity was not significantly different
from the background)]; weakly positive [+ (i.e., the percentage of
positively stained cells was <10%, or the staining intensity was
weakly positive or only individual cells were positive to strongly
positive)]; moderately positive [++ (i.e., the percentage of
positive cells was 10–60%)]; strongly positive [+++ (i.e., the
percentage of positively stained cells was >60%, or the staining
intensity was moderately to strongly positive and only a few cells
were weakly positive]. The known positive prostate section was
selected as the positive control.
Statistical analysis
SPSS 18.0 software was used for statistical
analysis. The two-samples t-test and analysis of variance were used
to assess differences between groups. The χ2 test and
exact test on a four-fold table were used to determine differences
in the positive staining rate between normal pituitary tissues and
invasive, as well as non-invasive, pituitary adenomas. The Line ×
List χ2 test and the one-sided test were used for
correlation analyses. α=0.05 was considered as the level of
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
NEDD4-1 expression in pituitary
tissues/adenoma increases with the degree of malignancy
Immunohistochemistry revealed that NEDD4-1 was
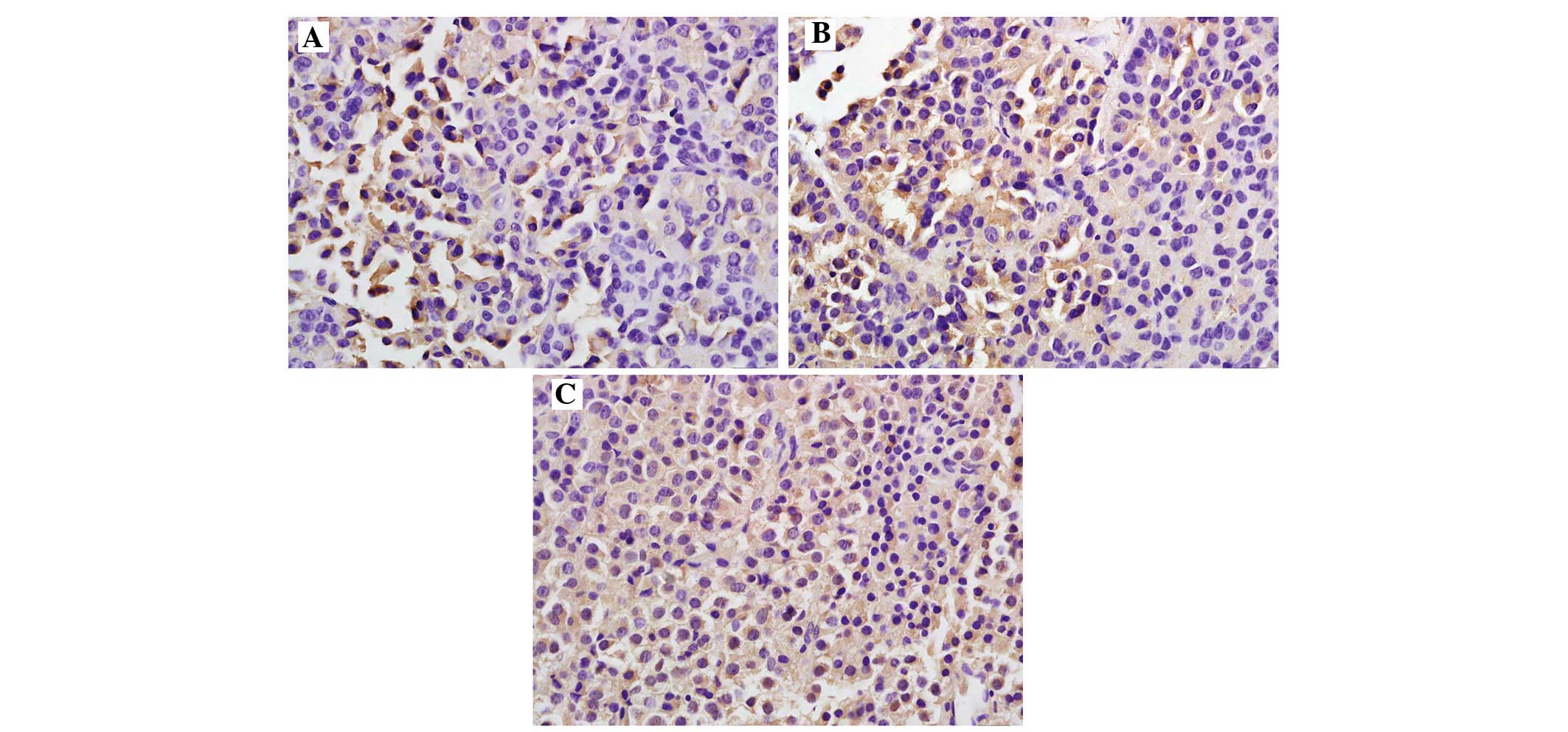
expressed in the cytoplasm and/or the nucleus (Fig. 1). The positive rate of NEDD4-1
expression in the normal control group [10.0% (1/10)] was
significantly lower compared with that in the experimental group
[52.0% (26/50); P<0.05] (Table
II). The positive rate of NEDD4-1 was 37.5% (9/24) in the
non-invasive pituitary adenomas group and 65.3% (17/26) in the
invasive adenomas group, thereby revealing a significantly
increasing trend with the increasing degree of tumor invasiveness
(P<0.05); the differences were also significant compared with
the control group (P<0.05).
 | Table II.Protein expression of neural precursor
cell expressed developmentally downregulated 4–1 in normal
pituitary tissues and in pituitary adenoma (invasive and
non-invasive). |
Table II.
Protein expression of neural precursor
cell expressed developmentally downregulated 4–1 in normal
pituitary tissues and in pituitary adenoma (invasive and
non-invasive).
|
|
|
| Positive (n) |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | Cases (n) | Negative (n) | + | ++ | +++ | Total positives
(n) | Positive rate
(%) |
|---|
| Normal | 10 | 9 | 1 | 0 | 0 | 1 | 10.0 |
| Non-invasive | 24 | 15 | 5 | 4 | 0 | 9 | 37.5a |
| Invasive | 26 | 9 | 4 | 6 | 7 | 17 | 65.3b |
PTEN protein expression in pituitary
tissues/adenoma decreases with increasing degree of malignancy
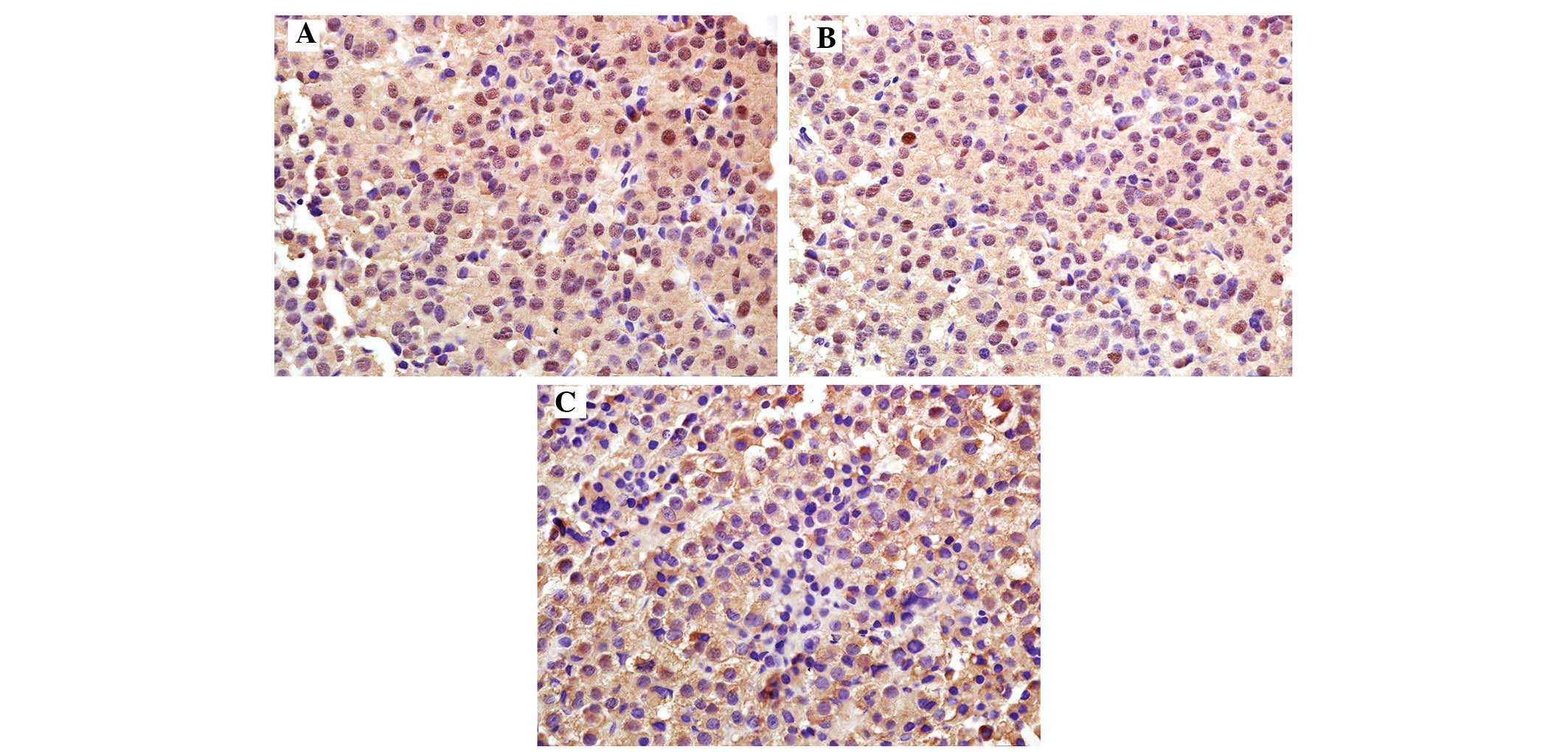
Immunohistochemistry revealed that PTEN was mainly
expressed in the cytoplasm, displaying as a brownish-yellow stain
(Fig. 2). The rate of PTEN
expression in the normal control group [100.0% (10/10)] was
significantly higher compared with that in the experimental group
[54.0% (27/50); P<0.05] (Table
III). The positive rate of PTEN was 83.3% (20/24) in the
non-invasive pituitary adenoma group and 26.9% (7/26) in the
invasive pituitary adenoma group, thereby revealing a significantly
decreasing trend with the increasing degree of tumor invasiveness
(P<0.05); the differences were also significant compared with
the control group (P<0.05).
 | Table III.Protein expression of phosphatase and
tensin homolog deleted on chromosome 10 in normal pituitary tissues
and in pituitary adenoma (invasive and non-invasive). |
Table III.
Protein expression of phosphatase and
tensin homolog deleted on chromosome 10 in normal pituitary tissues
and in pituitary adenoma (invasive and non-invasive).
|
|
|
| Positive (n) |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Group | Cases (n) | Negative (n) | + | ++ | +++ | Total positives
(n) | Positive rate
(%) |
|---|
| Normal | 10 | 0 | 2 | 3 | 5 | 10 | 100.0 |
| Non-invasive | 24 | 4 | 5 | 5 | 10 | 20 | 83.3a |
| Invasive | 26 | 19 | 4 | 2 | 1 | 7 | 26.9b |
Inverse correlation of NEDD4-1 and
PTEN protein expression in pituitary adenomas
Among the 26 cases of pituitary adenomas which
stained positive for NEDD4-1, four were positive for PTEN.
Furthermore, among the 27 cases of pituitary adenomas which stained
positive for PTEN, 24 were negative for NEDD4-1. Statistical
analysis revealed a negative correlation between the two proteins
(γ=−0.711, P<0.05) (Table
IV).
 | Table IV.Correlation of NEDD4-1 and PTEN
expression in pituitary adenomas. |
Table IV.
Correlation of NEDD4-1 and PTEN
expression in pituitary adenomas.
|
| PTEN |
|
|---|
|
|
|
|
|---|
| NEDD4-1 | Positive (n) | Negative (n) | Total (n) |
|---|
| Positive | 4 | 22 | 26 |
| Negative | 23 | 1 | 24 |
| Total | 27 | 23 | 50 |
Discussion
In general, the growth of pituitary adenomas is
limited; however, in certain cases, they may invade the surrounding
brain tissues, blood vessels and nerves, and destroy the sellar
bone, thereby showing characteristics of malignant tumors that
require complete surgical resection (10–12).
According to the concepts of modern molecular biology of tumors,
the occurrence and development of tumors constitutes a
multi-factorial, multi-step and complex biological evolutionary
process involving the mutation of multiple genes, and pituitary
adenoma is no exception (13–15).
PTEN is among the tumor suppressor genes with the highest
vulnerability to mutations (16,17).
NEDD4-1 is a newly discovered ubiquitin ligase of PTEN, with HECT
(homologous to the E6-AP carboxyl terminus) domains. NEDD4-1 has a
role in the multi-ubiquitination of PTEN and degradation of its
proteasome (4,18,19). In
the present study, the expression of PTEN and NEDD4-1 in invasive
and non-invasive pituitary adenomas and normal pituitary tissues
was explored using immunohistochemistry and their correlation was
analyzed.
Although the genetic status of PTEN was not
evaluated in the present study, it is likely that inactivation of
PTEN may have led to the loss of protein expression detected by
immunohistochemistry. Of note, the loss of PTEN protein expression
is an important negative prognostic indicator (20–22).
Immunohistochemical analysis may be the best method for evaluating
the PTEN status, as it is able to detect PTEN loss due to various
causes, including the inactivation of the gene product (23–25). By
using immunohistochemistry, the present study revealed that the
rate of PTEN-positive cells was significantly different between
patients with non-invasive pituitary adenomas [83.3% (20/24)] and
those with invasive pituitary adenomas [26.9% (7/26); P<0.05],
whereas 100% of cells in normal pituitary tissues were
PTEN-positive. This result indicated that the expression intensity
of PTEN was reduced with the increase in the degree of tumor
invasiveness. It can be speculated that deletion of the PTEN gene
is one of the reasons of decreased protein levels in pituitary
adenomas compared with normal pituitary tissues.
The positive rate of NEDD4-1 expression in normal
pituitary tissues was 10.0%, which was significantly lower compared
with that in pituitary adenomas (52.0%; P<0.05). Furthermore,
the NEDD4-1-positive rate in the non-invasive pituitary adenoma
group (37.5%) was significantly lower compared with that in the
invasive group (65.3%; P<0.05), indicating that NEDD4-1 may be
associated with the degree of malignancy or loss of histological
differentiation. It was speculated that, with an increasing degree
of malignancy, NEDD4-1 protein participated in PTEN protein
degradation by polyubiquitylation, thus resulting in increased
NEDD4-1 protein expression. Therefore, the overexpression of
NEDD4-1 is likely to have an important role in the development of
pituitary adenoma.
To the best of our knowledge, the present study was
the first to assess NEDD4-1 and PTEN expression in pituitary
adenoma. Among 26 cases of NEDD4-1-positive pituitary adenoma, four
were PTEN-positive, whereas, among 27 cases of PTEN-positive
pituitary adenoma, 24 were NEDD4-1-negative. Statistical analysis
revealed a negative correlation between NEDD4-1 and PTEN (γ=−0.711;
P<0.05). With the increasing degree of invasiveness of pituitary
adenoma, the NEDD4-1-positive rate was increased, whereas the
PTEN-positive rate was decreased. A possible underlying mechanism
is that increased expression of NEDD4-1 leads to an increase in
PTEN ubiquitination, thus causing its degradation in the
proteasome.
In conclusion, the present study showed that NEDD4-1
and PTEN may have an important role in the occurrence and
development of pituitary adenoma, which was associated with the
degree of invasiveness and prognosis of pituitary adenoma. The two
proteins may thus be utilized as diagnostic and prognostic
biomarkers, as well as indicators for determining the biological
characteristics of pituitary adenoma. However, the specific
mechanisms of the roles of NEDD4-1 and PTEN in pituitary adenomas
remain to be fully elucidated by further experimental studies.
References
|
1
|
Palumbo T, Faucz FR, Azevedo M, Xekouki P,
Iliopoulos D and Stratakis CA: Functional screen analysis reveals
miR-26b and miR-128 as central regulators of pituitary
somatomammotrophic tumor growth through activation of the PTEN-AKT
pathway. Oncogene. 32:1651–1659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li DM and Sun H: TEP1, encoded by a
candidate tumor suppressor locus, is a novel protein tyrosine
phosphatase regulated by transforming growth factor-β. Cancer Res.
57:2124–2129. 1997.PubMed/NCBI
|
|
3
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Trotman LC, Koppie T, Alimonti A,
Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo
C, et al: NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN.
Cell. 128:129–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu DN, Pei DS, Wang Q and Zhang GY:
Down-regulation of PTEN by sodium orthovanadate inhibits ASK1
activation via PI3-K/AKT during cerebral ischemia in rat
hippocampus. Neurosci Lett. 404:98–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castellino RC and Durden DL: Mechanisms of
disease: The PI3K-AKT-PTEN signaling node - an intercept point for
the control of angiogenesis in brain tumors. Nat Clin Pract Neurol.
3:682–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tena-Suck ML, Ortiz-Plata A and de la Vega
HA: Phosphatase and tensin homologue and pituitary
tumor-transforming gene in pituitary adenomas. Clinical-pathologic
and immunohistochemical analysis. Ann Diagn Pathol. 12:275–282.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng ZZ, Chen JW, Yang ZR, Lu GZ and Cai
ZG: Expression of PTTG1 and PTEN in endometrial carcinoma:
Correlation with tumorigenesis and progression. Med Oncol.
29:304–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Li M, Tian X, Li Q, Lu Q, Jia Q,
Zhang L, Yan J and Li X and Li X: Golgi phosphoprotein 3 inhibits
the apoptosis of human glioma cells in part by downregulating N-myc
downstream regulated gene 1. Med Sci Monit. 22:3535–3543. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Whiteman DC, Zhou XP, Cummings MC, Pavey
S, Hayward NK and Eng C: Nuclear PTEN expression and
clinicopathologic features in a population-based series of primary
cutaneous melanoma. Int J Cancer. 99:63–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmed S Faisal, Marsh DJ, Weremowicz S,
Morton CC, Williams DM and Eng C: Balanced translocation of 10q and
13q, including the PTEN gene, in a boy with a human chorionic
gonadotropin-secreting tumor and the Bannayan-Riley-Ruvalcaba
syndrome. J Clin Endocrinol Metab. 84:4665–4670. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu LM, Li YL, Yin YH, Hou GQ, Zhu R, Hua
XL, Xu JR and Chen ZA: Usefulness of dual-energy computed
tomography imaging in the differential diagnosis of sellar
meningiomas and pituitary adenomas: Preliminary report. PLoS One.
9:e906582014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cao XR, Lill NL, Boase N, Shi PP, Croucher
DR, Shan H, Qu J, Sweezer EM, Place T, Kirby PA, et al: Nedd4
controls animal growth by regulating IGF-1 signaling. Sci Signal.
1:ra52008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Treier M, Staszewski LM and Bohmann D:
Ubiquitin-dependent c-Jun degradation in vivo is mediated by the
delta domain. Cell. 78:787–798. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franke TF, Kaplan DR, Cantley LC and Toker
A: Direct regulation of the Akt proto-oncogene product by
phosphatidylinositol 3,4-bisphosphate. Science. 275:665–668. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Balas B, Christ-Roberts CY, Kim
RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, et al:
Peripheral disruption of the Grb10 gene enhances insulin signaling
and sensitivity in vivo. Mol Cell Biol. 27:6497–6505. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yim EK, Peng G, Dai H, Hu R, Li K, Lu Y,
Mills GB, Meric-Bernstam F, Hennessy BT, Craven RJ and Lin SY: Rak
functions as a tumor suppressor by regulating PTEN protein
stability and function. Cancer Cell. 15:304–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Drinjakovic J, Jung H, Campbell DS,
Strochlic L, Dwivedy A and Holt CE: E3 ligase Nedd4 promotes axon
branching by down-regulating PTEN. Neuron. 65:341–357. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trotman LC, Wang X, Alimonti A, Chen Z,
Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo
C, Erdjument-Bromage H, et al: Ubiquitination regulates PTEN
nuclear import and tumor suppression. Cell. 128:141–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maccario H, Perera NM, Gray A, Downes CP
and Leslie NR: Ubiquitination of PTEN (phosphatase and tensin
homolog) inhibits phosphatase activity and is enhanced by membrane
targeting and hyperosmotic stress. J Biol Chem. 285:12620–12628.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Shi Y, Wang J, Huang G and Jiang
X: Crucial role of the C-terminus of PTEN in antagonizing
NEDD4-1-mediated PTEN ubiquitination and degradation. Biochem J.
414:221–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee JR, Oestreich AJ, Payne JA, Gunawan
MS, Norgan AP and Katzmann DJ: The HECT domain of the ubiquitin
ligase Rsp5 contributes to substrate recognition. J Biol Chem.
284:32126–32137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim RH, Peters M, Jang Y, Shi W, Pintilie
M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, et al: DJ-1, a
novel regulator of the tumor suppressor PTEN. Cancer Cell.
7:263–273. 2005. View Article : Google Scholar : PubMed/NCBI
|