Introduction
Human platelets are crucial in the primary
hemostasis and repair of the injured endothelium (1,2).
Additionally, platelets also act in the development of vascular
events. Platelets may cause blood vessel occlusion by forming a
thrombus, leading to myocardial infarction and brain strokes.
Platelet activation and aggregation represent the first step in
thrombus formation at an injured vascular site. The initial
adhesion of flowing platelets to the injured vessel wall is
mediated by the interaction of the glycoprotein Ib/IX/V complex
with von Willebrand factor (2).
Following the adhesion of the platelets, the repair of the injured
endothelium is processed by autocrine and paracrine mediators,
including ADP and thromboxane A2. These autacoids
enhance and sustain the initial platelet response. Finally,
glycoprotein IIb/IIIa (αIIbβ3) is activated by platelet activators,
and a hemostatic plug is formed. Thrombus formation is associated
with the release of granule contents, including platelet-derived
growth factor (PDGF)-AB and serotonin, and the release of
inflammatory substances, including soluble CD40 ligand (sCD40L).
These secreted and generated mediators trigger a positive feedback
mechanism that potentiates the platelet activation (3,4).
It has been established that human platelets mainly
contain a synthase (cyclooxygenase) that generates thromboxane
A2 in the metabolism of arachidonic acid (5). Thromboxane A2, which is
produced and released by activated human platelets, is a crucial
second wave mediator that acts as a potent aggregatory agent.
Thromboxane A2 is one of the most significant
physiological activators of human platelets and exerts their
effects by acting on GTP-binding protein-coupled thromboxane (TP)
receptors. It is recognized that thromboxane A2 induces
the activation of the mitogen-activated protein (MAP) kinase
superfamily, including p38 MAP kinase, via TP receptors (6). It has been revealed that the
overproduction of thromboxane A2 by platelets leads to
thrombosis (5). In our previous
study (7), it was demonstrated
that ristocetin, a glycoprotein Ib/IX/V activator, induces the
release of sCD40L via thromboxane A2 generation from
human platelets, and that this release is elevated in
atherosclerotic patients. However, the exact mechanism of action
for thromboxane A2 in platelet activation has not yet
been precisely clarified.
Rac is a member of the Rho family of small
GTP-binding proteins (8). It has
been shown that the Rho-family regulates cytoskeletal
reorganization, particularly in vascular smooth muscle, and gene
expression. While Rac is inactive when bound to GDP, it is
activated upon the exchange of GDP for GTP by guanine nucleotide
exchange factor, leading to downstream signaling. With regard to
Rac in platelets, it has been demonstrated that platelets express
the Rho-family GTPases, including Rac (9). Additionally, in platelets, collagen
and thrombin reportedly stimulate the activation of Rac, which is
important in thrombus formation (9,10).
However, the exact role of Rac in human platelets remains to be
elucidated. In the present study, the involvement of Rac in the
thromboxane A2-induced release of sCD40L and the
secretion of PDGF-AB from human platelets was investigated.
Materials and methods
Materials
Ristocetin and U46619 were purchased from Cayman
Chemical, Co. (Ann Arbor, MI, USA). NSC23766 was purchased from
Tocris Bioscience (Bristol, UK). The phospho-p38 MAP kinase
antibodies and p38 MAP kinase antibodies were obtained from Cell
Signaling, Inc. (Beverly, MA, USA). The enhanced chemiluminescence
(ECL) western blot analysis detection system was purchased from GE
Healthcare (Buckinghamshire, UK). Other materials and chemicals
were obtained from commercial sources.
Preparation of platelets
Human blood was donated from healthy volunteers and
used in a 1/10 volume of 3.8% sodium citrate. Platelet-rich plasma
(PRP) was obtained from the blood samples by centrifugation at 155
× g for 12 min at room temperature. Platelet-poor plasma was
prepared from the residual blood by centrifugation at 2,500 × g for
5 min. All the participants signed an informed consent agreement
after receiving a detailed explanation of the study. This study was
approved by The Committee of Ethics at Gifu University Graduate
School of Medicine (Gifu, Japan).
Measurement of platelet aggregation
induced by U46619
Platelet aggregation using citrated PRP was followed
using a PA-200 aggregometer (Kowa Co., Ltd., Tokyo, Japan), which
is capable of determining the size of platelet aggregates based
upon the particle count using a laser scattering method (small
size, 9–25 μm; medium size, 25–50 μm; large size, 50–70 μm)
(11), at 37°C for 5 min, with a
stirring speed of 800 rpm. The percentage of transmittance of the
isolated platelets was recorded as 0%, and that of the appropriate
platelet-poor plasma, e.g. blank, was recorded as 100%.
Sample preparation following ristocetin
or U46619 stimulation
Each PRP sample was pretreated with NSC23766 for 15
min at 37°C and then stimulated by 1.5 mg/ml ristocetin or 3 μM
U46619 for 5 min (for measurement of the protein expression levels
by western blot analysis) and for 30 min (for measurement of the
levels of PDGF-AB and sCD40L). The platelet aggregation was
terminated by the addition of an ice-cold EDTA (10 mM) solution,
followed by centrifugation at 10,000 × g at 4°C for 2 min. To
perform the western blot analysis, the pellet was washed twice with
phosphate-buffered saline and then lysed and immediately boiled
using a lysis buffer containing 62.5 mM Tris/Cl (pH 6.8), 2% SDS,
50 mM dithiothreitol and 10% glycerol, as described previously
(12). To measure PDGF-AB and
sCD40L, as described later, the supernatant was isolated and stored
at −20°C for subsequent ELISA analysis.
Western blot analysis
A western blot analysis was performed, as described
previously (12). Briefly,
SDS-polyacrylamide gel electrophoresis was performed using the
Laemmli method (13) in 10%
polyacrylamide gel. Proteins were fractionated and transferred onto
polyvinyl difluoride (PVDF) membranes. The membranes were blocked
with 5% skimmed dry milk in Tris-buffered saline with 0.1% Tween 20
(TBST; 20 mM Tris (pH 7.6), 137 mM NaCl and 0.1% Tween 20) for 1 h
prior to incubating them with the indicated primary antibodies.
Phospho-p38 MAP kinase antibodies and p38 MAP kinase antibodies
(polyclonal-rabbit antibodies) were used as primary antibodies.
Peroxidase-labeled anti-mouse IgG (Santa Cruz Biotechnology., Inc,
Santa Cruz, CA, USA) or anti-rabbit IgG antibodies (KPL,
Gaithersburg, MD, USA) were used as secondary antibodies. The
primary and secondary antibodies were diluted to the optimum
concentrations with 5% skimmed dry milk in phosphate-buffered
saline with 0.1% Tween-20. The peroxidase activity on the PVDF
membranes was visualized on X-ray film by means of an ECL western
blot analysis detection system (GE Healthcare), as described in the
manufacturer’s instructions.
Measurement of Rac activity
Following stimulation with 3 μM U46619 for 0, 1, 3
or 5 min, platelet aggregation was terminated by the addition of
ice-cold EDTA (10 mM) solution, followed by centrifugation at
10,000 × g at 4°C for 2 min. The pellet was washed twice with
ice-cold TBS and Rac1 activity was determined using a Rac1
activation assay kit (Millipore, Corp., Temecula, CA, USA), as
described in the manufacturer’s instructions.
Measurement of plasma PDGF-AB and sCD40L
levels
The plasma PDGF-AB and sCD40L levels in the human
samples were determined using PDGF-AB or sCD40-Ligand Quantikine
ELISA kits purchased from R&D systems, Inc., (Minneapolis, MN,
USA), respectively. All assay procedures were performed according
to the manufacturer’s instructions.
Determination
The densitometric analysis was performed using
Molecular Analyst/Macintosh (Bio-Rad Laboratories, Hercules, CA,
USA).
Statistical analysis
All the figures are representative results of five
independent experiments. The data are presented as the mean ±
standard error of the mean. The data were analyzed by Student’s
t-test, and P<0.05 was considered to indicate a statistically
significant difference.
Results
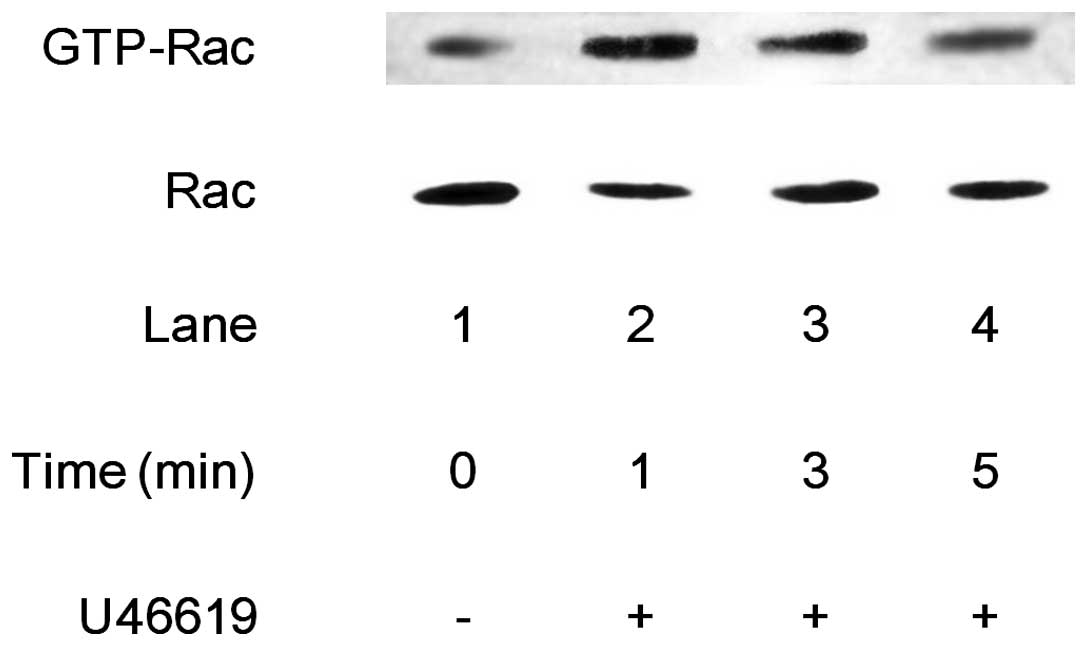
Effect of Rac on the U46619-stimulated
human platelets
The present study investigated whether thromboxane
A2 stimulates the activation of Rac in human platelets.
U46619 (3 μM), a selective TP agonist (14), markedly increased the GTP-Rac
levels time-dependently in human platelets (Fig. 1).
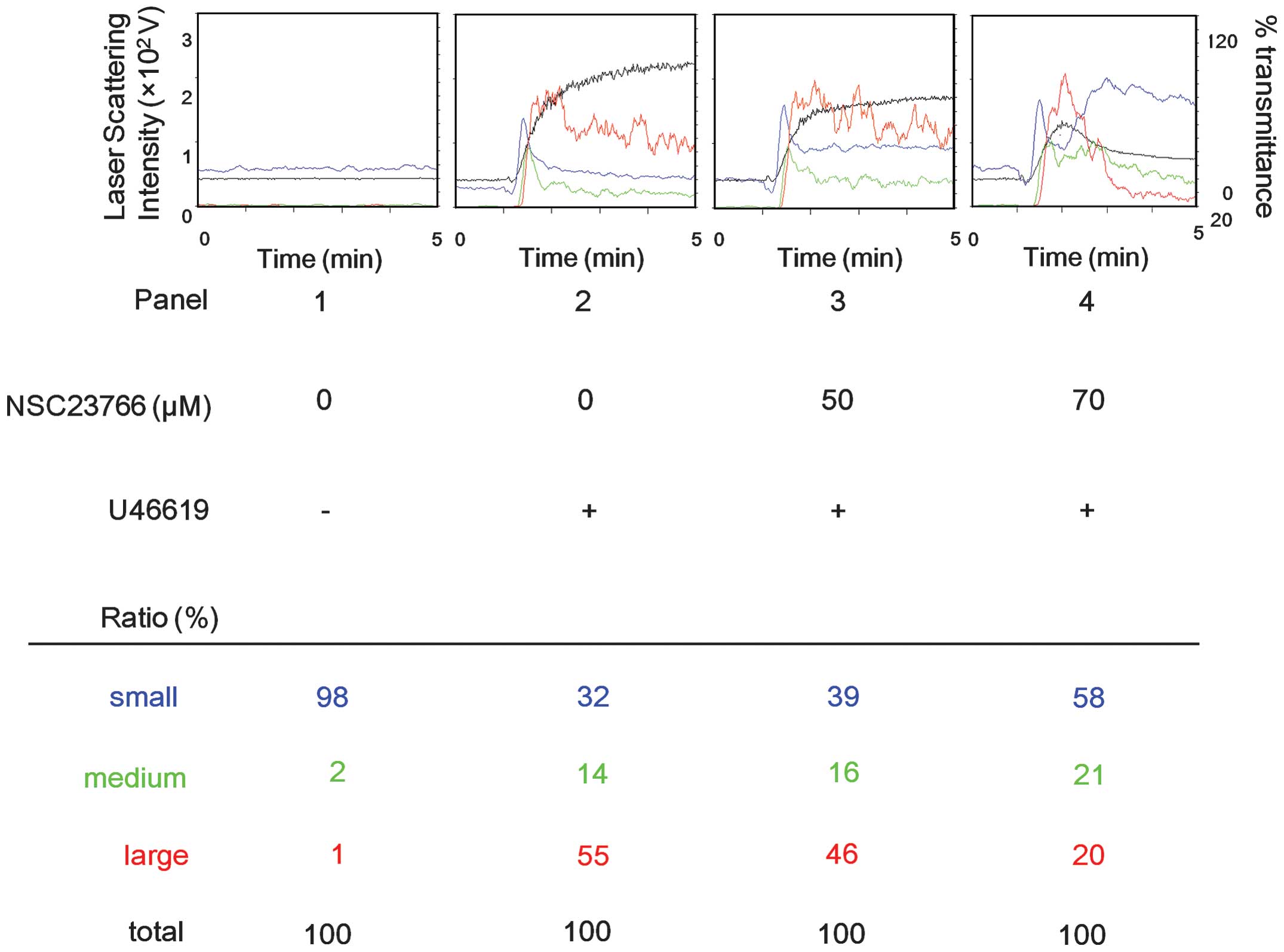
Effect of NSC23766 on platelet
aggregation induced by U46619 stimulation
Next, the effect of NSC23766, a selective inhibitor
of Rac1-guanine nucleotide exchange factor interaction (15), on the platelet aggregation
stimulated by U46619 was examined using an aggregometer with laser
scattering methods. NSC23766 markedly suppressed the U46619-induced
platelet aggregation in a dose-dependent manner in the range
between 50 and 70 μM (Fig. 2).
According to an analysis of the size of the platelet aggregates,
large aggregates (50–70 μm) were dose-dependently decreased by
NSC23766. By contrast, NSC23766 markedly increased the small (9–25
μm) and medium (25–50 μm) aggregates (Fig. 2).
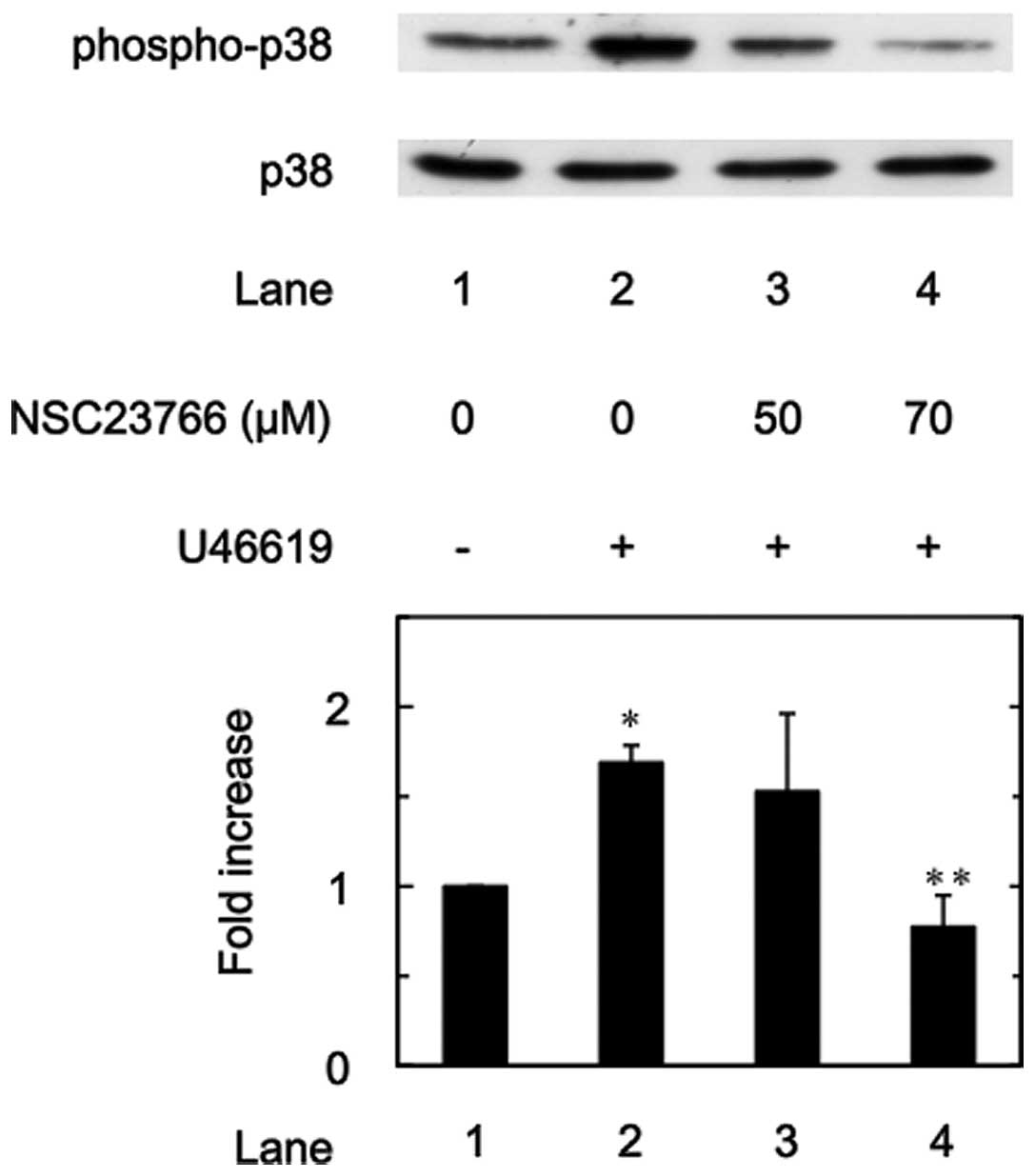
Effects of NSC23766 on the U46619-induced
phosphorylation of p38 MAP kinase
It is generally recognized that the MAP kinase
superfamily, including p38 MAP kinase, is activated downstream of
TP-mediated responses (6).
Therefore, the present study examined the effect of NSC23766 on the
U46619-induced phosphorylation of p38 MAP kinase in human
platelets. It was demonstrated that U46619 induced the
phosphorylation of p38 MAP kinase in the human platelets. In
addition, NSC23766 significantly reduced the U46619-stimulated
phosphorylation of p38 MAP kinase (Fig. 3).
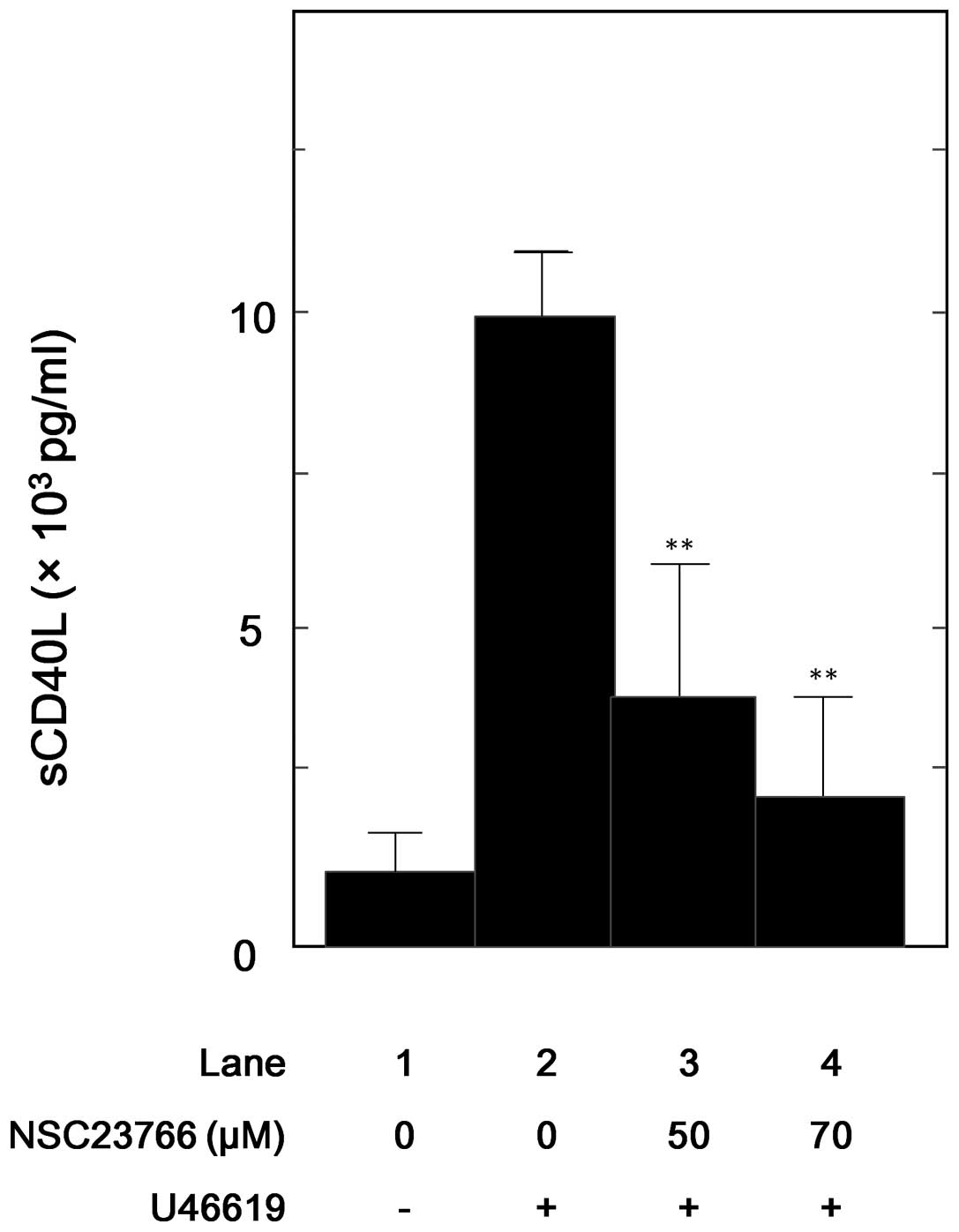
Effect of NSC23766 on the U46619-induced
release of sCD40L from human platelets
In addition, the effect of NSC23766 on the
U46619-stimulated sCD40L release from human platelets was examined.
NSC23766 significantly inhibited the U46619-stimulated release of
sCD40L (Fig. 4), and this
inhibitory effect of NSC23766 was dose-dependent in the range
between 50 and 70 μM. NSC23766 (70 μM) caused ~85% suppression in
the U46619 effect.
Effect of NSC23766 on the U46619-induced
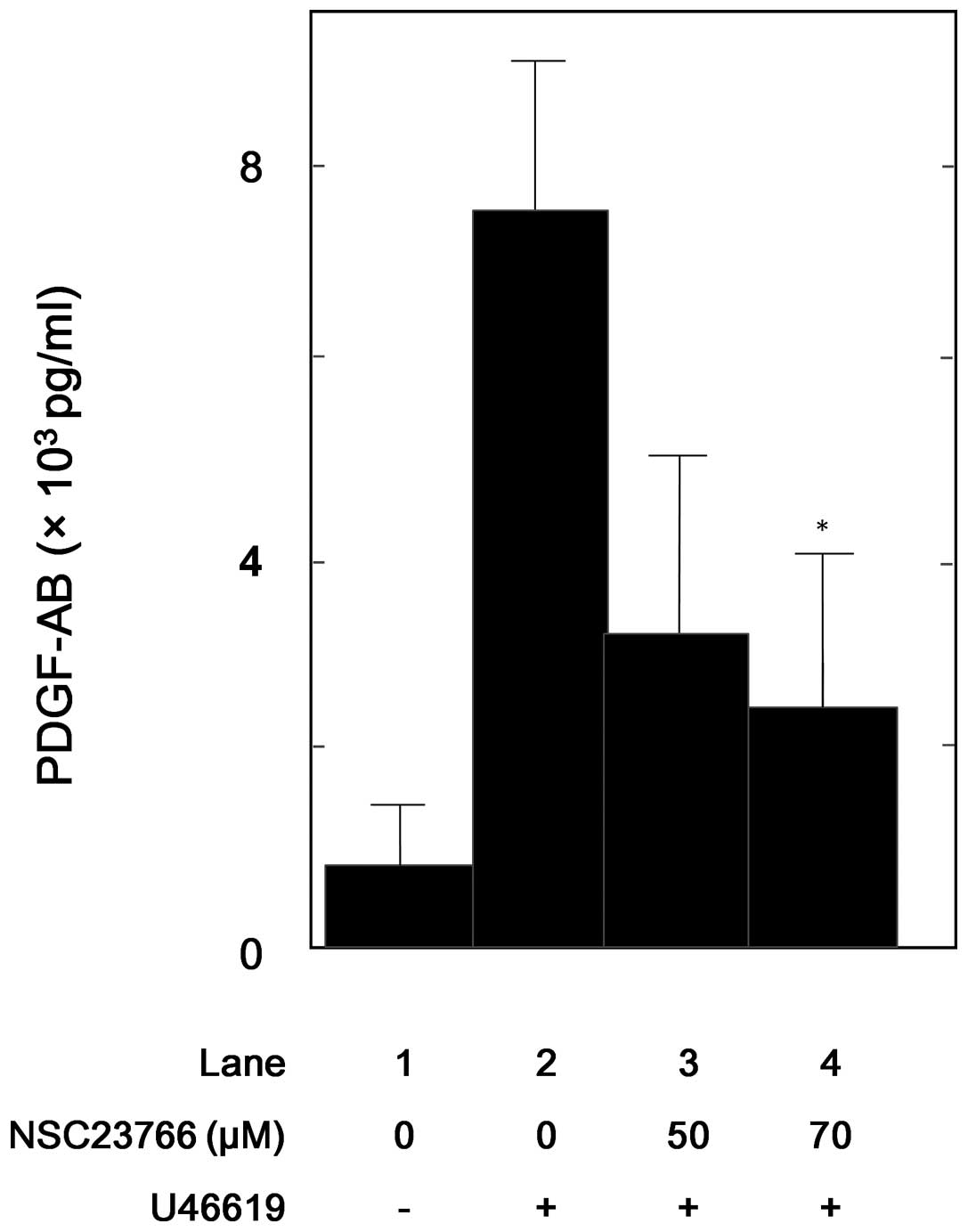
PDGF-AB secretion from human platelets
To investigate whether Rac is implicated in
thromboxane A2-induced platelet granule secretion, the
effect of NSC23766 on the U46619-induced secretion of PDGF-AB from
human platelets was then examined. NSC23766 significantly
suppressed the U46619-induced PDGF-AB secretion from human
platelets in a dose-dependent manner in the range between 50 and 70
μM (Fig. 5). NSC23766 (70 μM)
caused ~75% suppression in the U46619 effect.
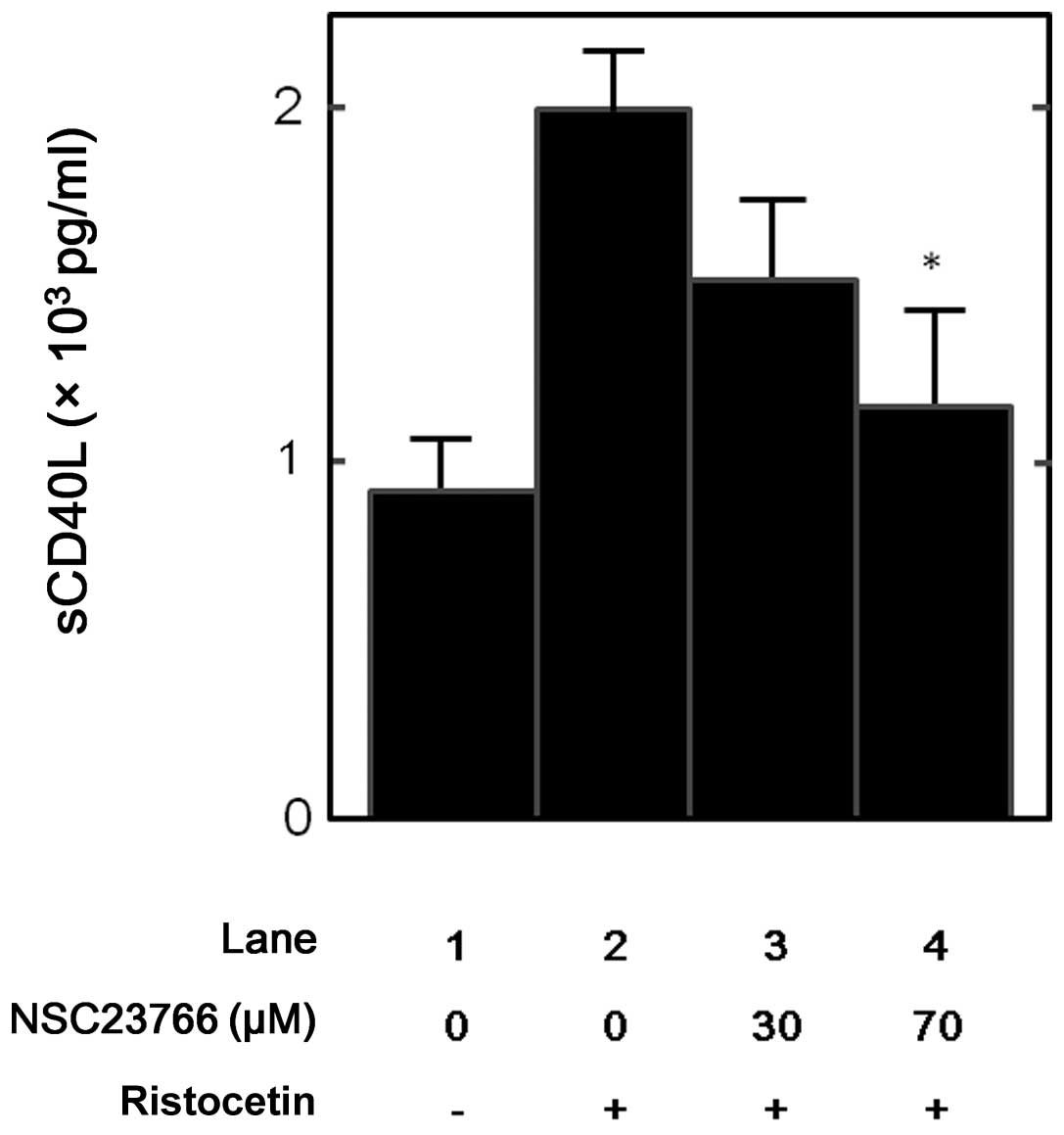
Effect of NSC23766 on the
ristocetin-induced release of sCD40L from human platelets
In our previous study (7), it was demonstrated that glycoprotein
Ib/IX/V activation stimulates sCD40L release via thromboxane
A2 generation from human platelets. Furthermore, the
effect of NSC23766 on the sCD40L release stimulated by ristocetin,
an activator of glycoprotein Ib/IX/V from human platelets was
examined. NSC23766 (70 μM) significantly suppressed the
ristocetin-stimulated release of sCD40L (Fig. 6) and caused ~80% inhibition in the
ristocetin-effect.
Discussion
The present study investigated whether Rac is
involved in the thromboxane A2-stimulated activation of
human platelets. It was revealed that U46619, a selective
thromboxane A2 receptor agonist (14), markedly increased the levels of
GTP-Rac time-dependently in human platelets. In addition, NSC23766,
which is a selective inhibitor of Rac1-guanine nucleotide exchange
factor interaction (15),
attenuated the U46619-induced platelet aggregation. According to
the size of the platelet aggregates observed using a laser
scattering method, the ratio of large platelet aggregates (50–70
μm) was dose-dependently decreased by NSC23766, while the ratios of
small (9–25 μm) and medium (25–50 μm) platelet aggregates were
markedly increased. Based on these findings, it is possible that
thromboxane A2-induced Rac activation is involved in the
aggregation of human platelets.
In our previous study (7), it was shown that ristocetin, an
activator of GPIb/IX/V, stimulates sCD40L release via thromboxane
A2 production in human platelets, and that this release
is elevated in atherosclerotic patients. Therefore, the involvement
of Rac in the thromboxane A2-stimulated release of
sCD40L and PDGF-AB in human platelets was investigated in the
present study. NSC23766 significantly suppressed the sCD40L release
stimulated by thromboxane A2. In addition, the
thromboxane A2-induced granule secretion of PDGF-AB was
demonstrated to be significantly decreased by NSC23766. It is
generally recognized that thromboxane A2 induces the
activation of the MAP kinase superfamily through TP thromboxane
A2 receptors (6). In
the present study, it was revealed that NSC23766 markedly
attenuated the thromboxane A2-induced phosphorylation of
p38 MAP kinase. Furthermore, it was demonstrated that the
ristocetin-induced release of sCD40L was inhibited by NSC23766.
Taking our findings into account as a whole, it is most likely that
Rac activated by thromboxane A2 functions at a point
upstream from p38 MAP kinase in human platelets and that it
regulates the stimulation of sCD40L release and PDGF-AB
secretion.
It has been firmly established that the major
product among eicosanoids in human platelets is thromboxane
A2, and various platelet activators, including ADP,
stimulate thromboxane A2 production (1,2).
Thromboxane A2 potently induces the activation of
GPIIb/IIIa (integrin αIIbβ3) through signal transduction from
GTP-binding proteins coupled to TP in human platelets (6). The materials stored in the specific
granules, including dense-granules and α-granules, are secreted
from activated human platelets. While dense-granules contain small
non-protein molecules, including ADP, α-granules contain large
adhesive and healing proteins, such as PDGF-AB (16). It is generally known that PDGF-AB
secreted from α-granules is a potent mitogenic growth factor that
mainly acts on connective tissue cells, such as vascular smooth
muscle cells, resulting in the promotion of arteriosclerosis
(17). By contrast, activated
human platelets release inflammatory mediators of atherosclerosis,
including CD40L. It is recognized that CD40L exists in the
cytoplasm of resting human platelets, and is promptly translocated
to the surface following platelet activation by agonists, such as
ADP and collagen (18,19). The CD40L expressed on the surface
of activated platelets undergoes a cleavage that generates a
functional soluble fragment termed sCD40L. It has been revealed
that sCD40L released from platelets induces inflammatory responses
via CD40, which is expressed on vascular endothelial cells and
neutrophils (20). The elevation
of plasma sCD40L levels is reportedly associated with an increased
risk of cardiovascular events in patients with acute coronary
syndrome (21). In the present
study, it was demonstrated that the thromboxane
A2-induced release of sCD40L and the secretion of
PDGF-AB from α-granules were significantly suppressed by NSC23766.
Taking these findings into account, it is possible that activated
Rac induced by thromboxane A2 in human platelets may be
involved in the progression of atherosclerosis and inflammation
through PDGF-AB secretion and sCD40L release.
In conclusion, the results of this study indicate
that Rac regulates thromboxane A2-induced p38 MAP kinase
activation in human platelets, resulting in the stimulation of
sCD40L release and PDGF-AB secretion.
Acknowledgements
The authors would like to thank Mrs. Yumiko Kurokawa
for her technical assistance. This study was supported in part by a
Grant-in-Aid for Scientific Research (grant nos. 20591825 and
23592249) from the Ministry of Education, Science, Sports and
Culture of Japan and the Research Funding for Longevity Sciences
(22–4) from the National Center for Geriatrics and Gerontology,
Japan.
References
|
1
|
Davi G and Patrono C: Platelet activation
and atherothrombosis. N Engl J Med. 357:2482–2494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stegner D and Nieswandt B: Platelet
receptor signaling in thrombus formation. J Mol Med (Berl).
89:109–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kahner BN, Shankar H, Murugappan S, Prasad
GL and Kunapuli SP: Nucleotide receptor signaling in platelets. J
Thromb Haemost. 4:2317–2326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Z, Delaney MK, O’Brien KA and Du X:
Signaling during platelet adhesion and activation. Arterioscler
Thromb Vasc Biol. 30:2341–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vanhoutte PM: COX-1 and vascular disease.
Clin Pharmacol Ther. 86:212–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakahata N: Thromboxane A2:
physiology/pathophysiology, cellular signal transduction and
pharmacology. Pharmacol Ther. 118:18–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Enomoto Y, Adachi S, Matsushima-Nishiwaki
R, Doi T, Niwa M, Akamatsu S, Tokuda H, Ogura S, Yoshimura S, Iwama
T and Kozawa O: Thromboxane A(2) promotes soluble CD40 ligand
release from human platelets. Atherosclerosis. 209:415–421. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takai Y, Sasaki T and Matozaki T: Small
GTP-binding proteins. Physiol Rev. 81:153–208. 2001.PubMed/NCBI
|
|
9
|
Pleines I, Elvers M, Strehl A, Pozgajova
M, Vargo-Szabo D, May F, Chrostek-Grashoff A, Brakebusch C and
Nieswandt B: Rac1 is essential for phospholipase C-gamma2
activation in platelets. Pflugers Arch. 457:1173–1185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soulet C, Gendreau S, Missy K, Benard V,
Plantavid M and Payrastre B: Characterisation of Rac activation in
thrombin-and collagen-stimulated human blood platelets. FEBS Lett.
507:253–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tohgi H, Takahashi H, Watanabe K, Kuki H
and Shirasawa Y: Development of large platelet aggregates from
small aggregates as determined by laser-light scattering: effects
of aggregant concentration and antiplatelet medication. Thromb
Haemost. 75:838–843. 1996.
|
|
12
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alphaB-crystallin by cyclic AMP in C6 rat glioma cells. J
Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertelé V, Di Minno G and de Gaetano G:
U-46619, a stable analogue of prostaglandin H2, induces retraction
of human platelet-rich plasma clots. Thromb Res. 18:543–545.
1980.PubMed/NCBI
|
|
15
|
Gao Y, Dickerson JB, Guo F, Zheng J and
Zheng Y: Rational design and characterization of a Rac
GTPase-specific small molecule inihitor. Proc Natl Acad Sci USA.
101:7618–7623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rendu F and Brohard-Bohn B: The platelet
release reaction: granules’ constituents, secretion and functions.
Platelets. 12:261–273. 2001.
|
|
17
|
Heldin CH and Westermark B: Mechanism of
action and in vivo role of platelet-derived growth factor. Physiol
Rev. 79:1283–1316. 1999.PubMed/NCBI
|
|
18
|
Hermann A, Rauch BH, Braun M, Schrör K and
Weber AA: Platelet CD40 ligand (CD40L) - subcellular localization,
regulation of expression, and inhibition by clopidogrel. Platelets.
12:74–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
André P, Nannizzi-Alaimo L, Prasad SK and
Phillips DR: Platelet-derived CD40L: the switch-hitting player of
cardiovascular disease. Circulation. 106:896–899. 2002.PubMed/NCBI
|
|
20
|
Henn V, Slupsky JR, Gräfe M,
Anagnostopoulos I, Förster R, Müller-Berghaus G and Kroczek RA:
CD40 ligand on activated platelets triggers an inflammatory
reaction of endothelial cells. Nature. 391:591–594. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heeschen C, Dimmeler S, Hamm CW, van den
Brand MJ, Boersma E, Zeiher AM and Simoons ML; CAPTURE Study
Investigators. Soluble CD40 ligand in acute coronary syndromes. N
Engl J Med. 348:1104–1111. 2003. View Article : Google Scholar : PubMed/NCBI
|