Introduction
Osteosarcoma is one of the most common malignant
tumors of the musculoskeletal system (1,2) with
a survival rate of <20% when solely treated by surgical
intervention (3). Combinatory
therapeutic approaches, such as surgery together with systemic
chemotherapy, are therefore required for the effective treatment of
this cancer (1,4). Previous research (5) has shown that osteoclasts can be used
as a potential therapeutic target in osteosarcoma, due to their
prominence within the tumor and their critical function in bone
resorption at sites of microfracture or bone destruction.
Osteosarcoma cells originate from cells of an osteoblastic lineage,
which is characterized by cell secretion of receptor activator of
nuclear factor-κB ligand, a surface bound molecule that induces
osteoclast activation. Osteosarcoma may therefore be a suitable
candidate for osteoclast-targeted therapy, with osteosarcoma cells
being used as a cellular model in which novel therapeutic methods
and molecular mechanisms may be researched (5).
Autophagy is central to the pathogenesis of numerous
conditions, including aging, cancer, myopathies, neuronal
degeneration and microbial infection (6,7).
Therefore, an increasing number of novel therapeutic strategies for
osteosarcoma are focusing on the modulation of dysregulated
autophagy (8,9).
Bafilomycin A1 (BafA1), a macrolide antibiotic, is a
known inhibitor of the latter stages of autophagy, inhibiting
fusion between autophagosomes and lysosomes by inhibiting vacuolar
H+ ATPase (10). This
has been demonstrated to result in a marked accumulation of
autophagosomes, concomitant with apoptotic cell death (11,12).
Since the discovery of BafA1 and the identification
of its molecular effects in vitro on autophagy-mediated cell
death, BafA1 may be considered to be a central modulator of both
apoptosis and autophagy. The present study aimed to detect the
effects of BafA1 on cell growth and apoptosis in MG63 cells in
vitro.
Materials and methods
Reagents
The MG63 osteosarcoma cell line was purchased from
the Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). RPMI-1640 medium was purchased from Gibco-BRL
(Rockville, MD, USA); BafA1 was purchased from Biovision (Shanghai,
China); L-glutamine was obtained from Sigma (St. Louis, MO, USA);
and antibodies against p53, p62, Beclin1 and microtubule-associated
protein 1 light chain 3 (LC3) were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA).
Drug preparation
BafA1 was diluted in dimethylsulfoxide (DMSO) to a
final working concentration of 1 μmol/l and DMSO was used as the
vehicle control.
Cell culture and viability assay
MG63 cells were cultured in RPMI-1640 with 10% fetal
bovine serum and 4 mmol/l L-glutamine. For all assays, the cells
were cultured at 37°C in humidified incubators with 5%
CO2 and 95% air. The cell viability was assessed using
the Cell Counting Kit-8 (CCK-8; Dojindo Labotatories, Kumamoto,
Japan) assay. Cells were plated in 96-well plates at a density of
7×104 cells/well and treated with BafA1 for 6, 12 or 24
h. Cell viability was subsequently assessed by the addition of 10
μl CCK-8 solution to each well, 24 h after BafA1 treatment.
Following incubation with CCK-8 for 4 h, the optical absorbance at
570 nm was measured. Each experiment was performed in triplicate
(13).
Detection of mitochondrial potential
(ΔΦ)
The ΔΦ was determined using the KeyGEN Mitochondrial
Membrane Sensor kit (KeyGEN, Nanjing, China) according to the
manufacturer’s instructions. Cells were first treated with BafA1,
harvested and then washed three times with 5 ml phosphate-buffered
saline (PBS), prior to centrifugation and aspiration. The cells
were then re-suspended in 0.5 ml diluted MitoSensor reagent (1
μmol/m1 in incubation buffer; Becton-Dickinson, Heidelberg,
Germany). Following incubation with the fluorescent probe JC-1 for
20–30 min, the cells were washed in 0.2 ml incubation buffer and
resuspended in 40 μl incubation buffer, prior to re-washing and
re-suspension in 1 ml PBS. The cells were then analyzed by flow
cytometry (FACScan; Becton-Dickinson).
Detection of apoptosis
Cells treated with BafA1 for 6–24 h were harvested
and washed three times with 5 ml PBS/0.1% fetal calf serum wash
buffer, centrifuged and aspirated. The cells were then re-suspended
in wash buffer containing 20 μg/ml propidium iodide (PI), 500 μg/ml
RNase and 0.03% Nonidet P-40 and subsequently analyzed by flow
cytometry. The percentage of apoptotic cells was taken as the
percentage of cells with a lower DNA content than that of cells in
the G0-G1 phase in the PI intensity-area
histogram plot (14).
Total cell protein extraction and western
blotting
Cells were cultured for 24–48 h prior to lysis in
buffer containing 50 mmol/l Tris-HCl (pH 8.0), 150 mmol/l NaCl, 1%
(v/v) Triton X-100 and a protease inhibitor cocktail (1:100
dilution; Sigma, Shanghai, China). The protein concentration was
determined using Bradford reagent. The proteins were then separated
by electrophoresis using 8.5% polyacrylamide gels and transferred
onto nitrocellulose membranes. The membranes were subsequently
exposed to anti-Beclin 1 (1:1,000), -p53 (1:2,000), -p62 (1:1,000)
and -LC3 (1:1,000) antibodies and incubated at 4°C overnight, prior
to exposure to horseradish peroxidase-conjugated secondary antibody
(1:3,000) for 1 h at room temperature. β-actin (1:5,000; Sigma) was
used as a loading control. Membranes were developed using an
enhanced chemiluminescence detection system (Denville Scientific,
Inc., Plainfield, NJ, USA) and exposed to X-ray films (15).
Transmission electron microscopy
Following treatment with BafA1, the cells were fixed
in ice-cold 2.5% glutaraldehyde in 0.1 M PBS and stored at 4°C,
prior to further processing. The cells were post-fixed in 1% osmium
tetroxide in ice-cold 2.5% glutaraldehyde in 0.1 M PBS and then
dehydrated through an alcohol series prior to embedding in Epon™
812 (Electron Microscopy Sciences, Hatfield, PA, USA). The cells
were next sectioned using an ultramicrotome (Leica, Wetzlar,
Germany). Finally, the sections (500 nm) were stained with uranyl
acetate and lead citrate and examined by transmission electron
microscopy (Philips CM120; Philips, Eindhoven, The
Netherlands).
Statistical analysis
The data are presented as the mean ± standard
deviation. A Student’s t test was used for statistical analysis and
a P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
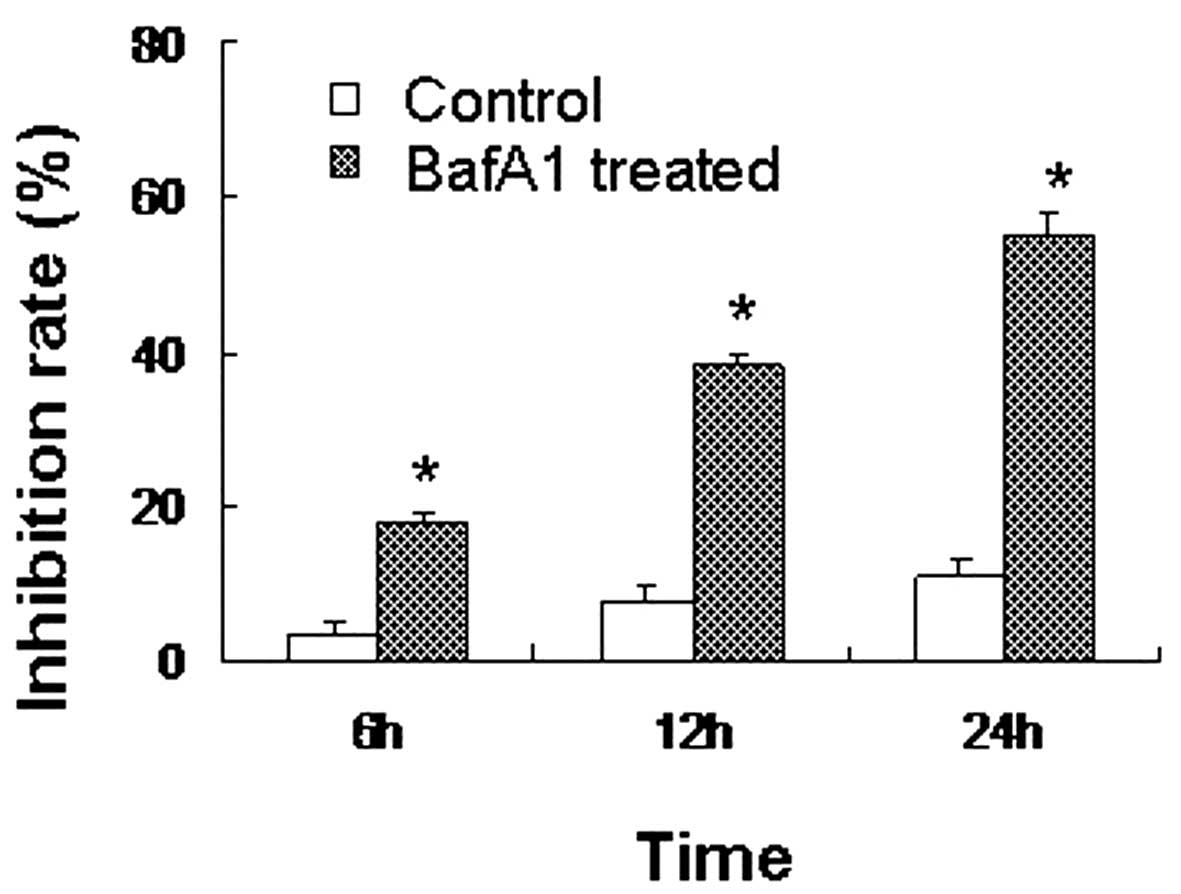
BafA1 inhibits MG63 cell viability
The CCK-8 assay revealed that the inhibition rate of
MG63 cells treated with BafA1 (1 μmol/l) was significant1y higher
than that of the controls (only vehicle used) (P<0.05). BafA1
inhibited the proliferation of the MG63 cells, with the rate of
inhibition reaching 18±0.57% after 6 h of incubation. The
inhibition rate increased to 39±2.82 and 56±3.91% by 12 and 24 h,
respectively (Fig. 1).
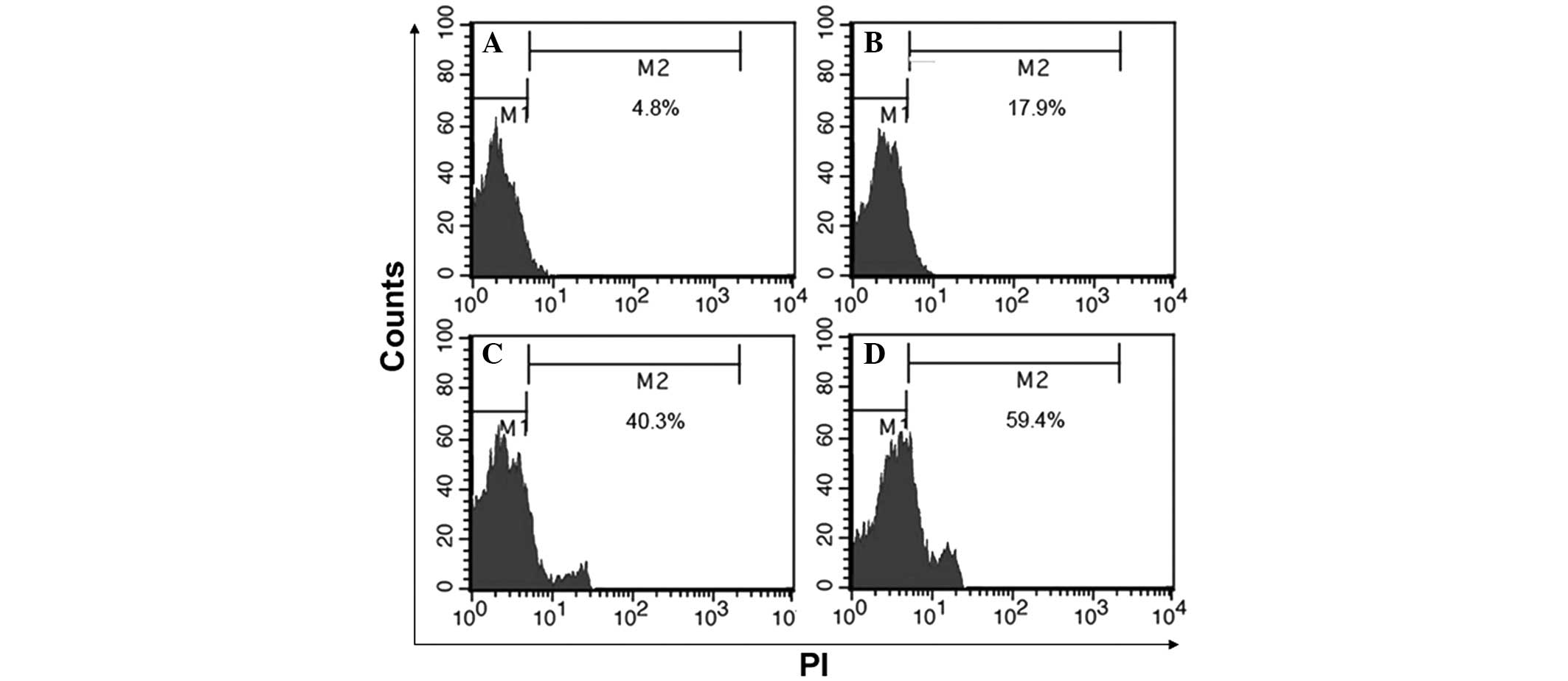
BafA1 induces mitochondrial
dysfunction
MG63 cells showed a collapse in the Δψ after 6 h of
exposure to BafA1 (1 μmol/l), with a maximum being reached by 24 h
(Fig. 2). These data therefore
indicated that BafA1 could induce mitochondrial dysfunction and
apoptosis in MG63 cells.
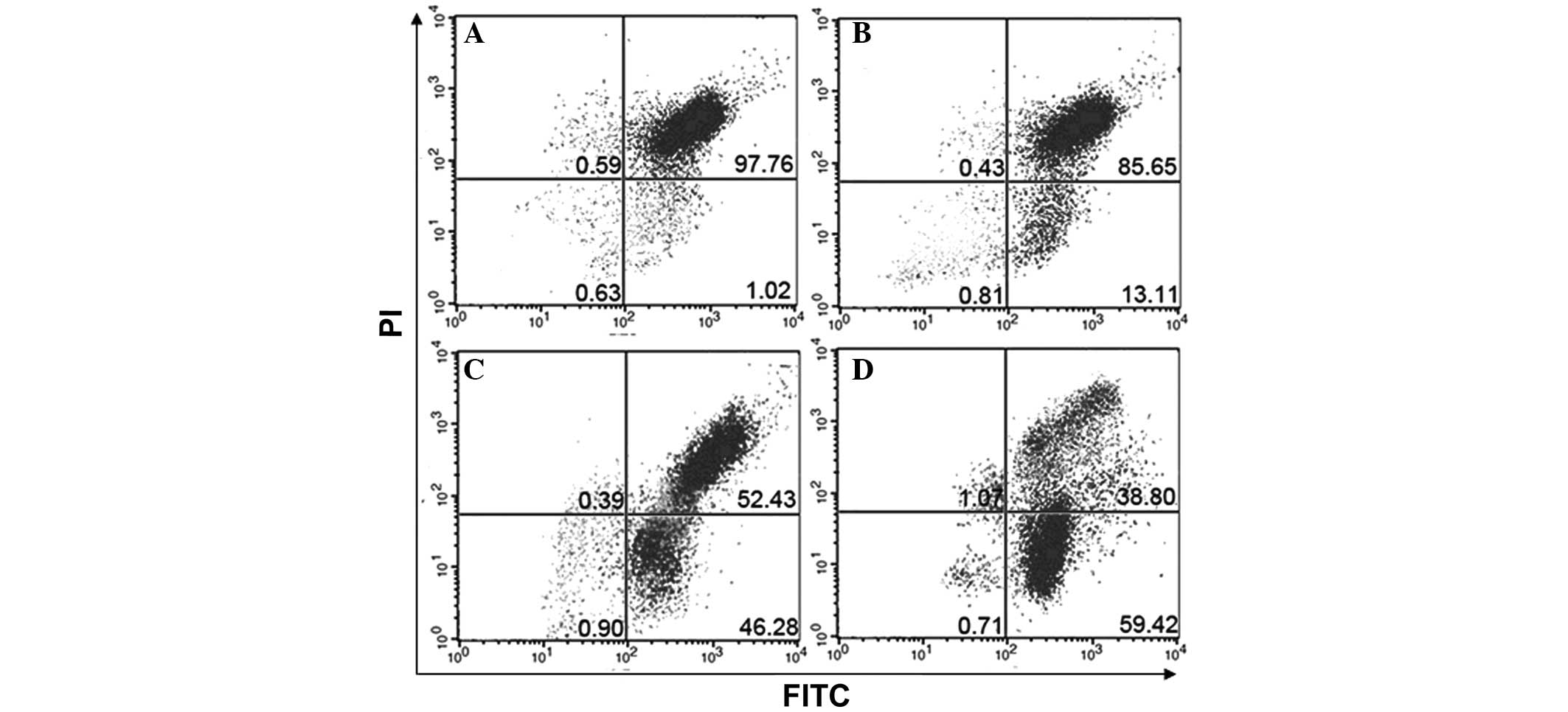
BafA1 induces apoptosis in MG63
cells
The rate of apoptosis in MG63 cells was assessed by
flow cytometry at 6, 12 and 24 h after exposure to BafA1 (1
μmol/l). BafA1-induced cellular apoptosis was evident after 6, 12
and 24 h of treatment (Fig.
3).
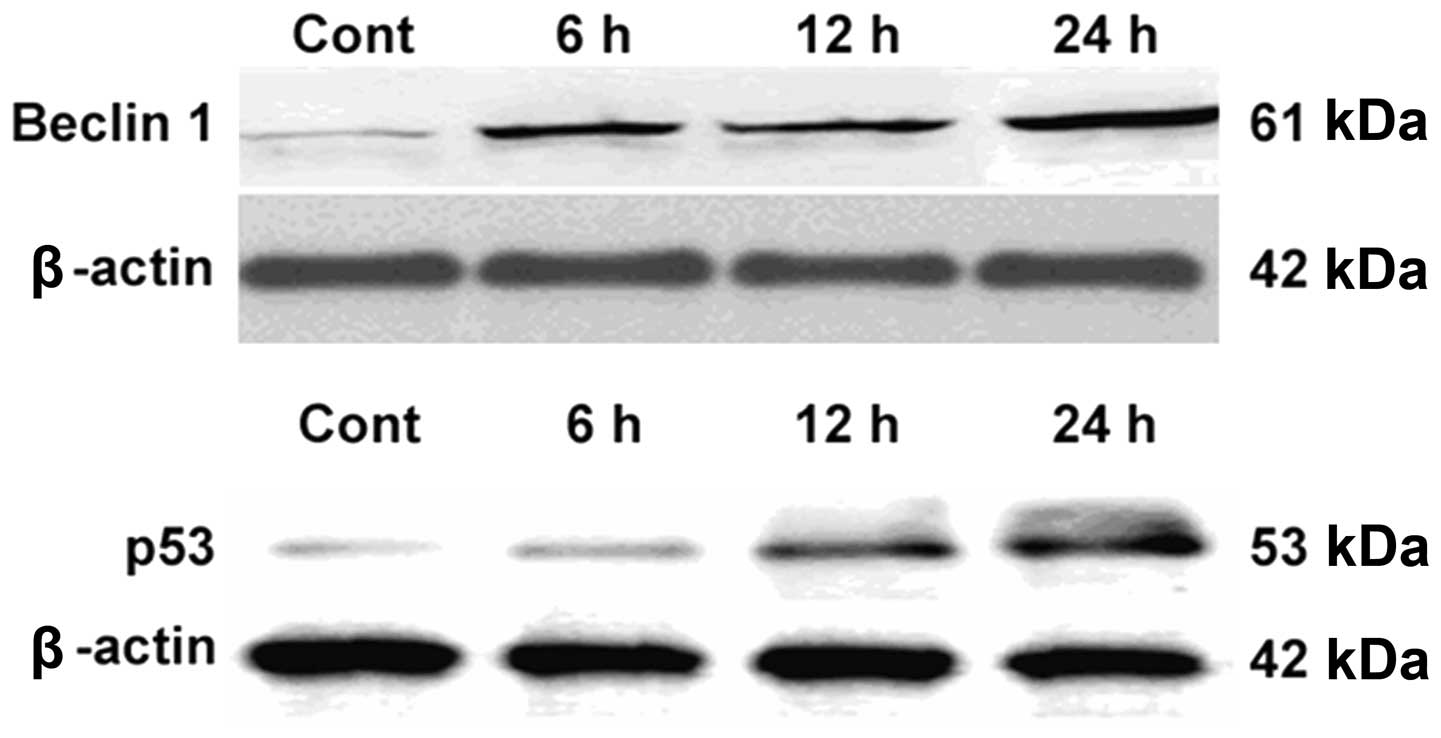
BafA1 increases Beclin 1 and p53 protein
expression levels in MG63 cells
Western blot analysis was used to assess the effect
of BafA1 on the expression of the apoptosis-related proteins Beclin
1 and p53. The results showed that the basal level of p53 protein
in the untreated MG63 cells was low. Following incubation with
BafA1 (1 μmol/l), the protein expression level of p53 and Beclin 1
was significantly increased 6–24 h after exposure (Fig. 4).
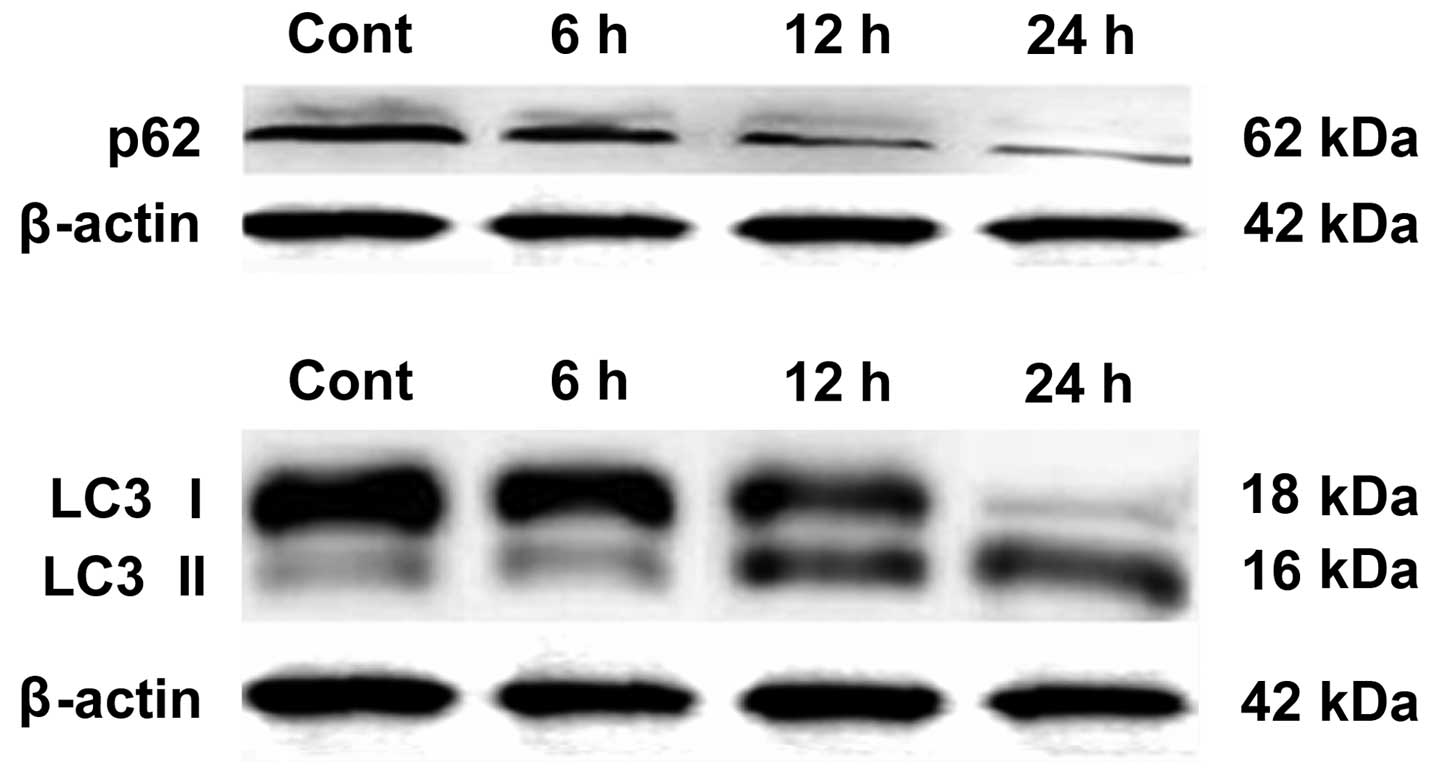
BafA1 downregulates the expression of p62
and LC3-I
Western blot analysis was used to assay whether
BafA1 (1 μmol/l) affected the expression of the autophagy-related
proteins LC3-I, LC3-II and p62. The results showed that, prior to
exposure of MG63 cells to BafA1, the basal levels of p62 and LC3
protein in MG63 cells were high. Following incubation for 6–24 h
with BafA1 (1 μmol/l), the expression levels of p62 and LC3-I were
significantly decreased, whereas the protein levels of LC3-II were
increased (Fig. 5).
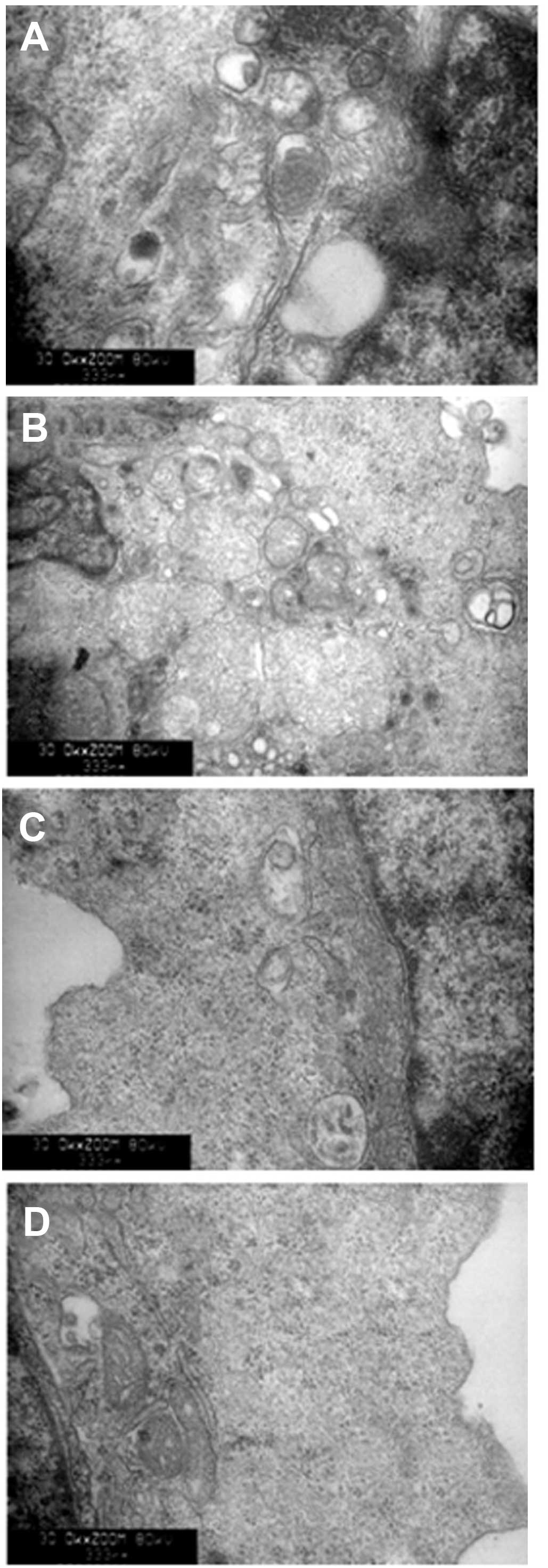
Autophagic activation of lysosomes and
impairment of mitochondria with BafA1 treatment
As shown in Fig. 6,
transmission electron microscopy was used to identify the
ultrastructural changes in MG63 cells following BafA1 (1 μmol/l)
treatment. The control cells showed a round shape and the
organelles, nuclei and chromatin had a normal appearance (Fig. 6A). By contrast, the BafA1-treated
cells exhibited typical signs of apoptosis (Fig. 6B–D). The mitochondria exhibited
vacuolization and swelling with a complete loss of cristae
(Fig. 6C). Numerous isolated
membranes, likely deriving from ribosome-free endoplasmic
reticulum, were observed. These isolated membranes were elongated
and curved, engulfing the cytoplasmic fraction and organelles
(Fig. 6C). Prolongation of BafA1
treatment (1 μmol/l) resulted in apoptosis, as well as the loss of
organelles and cytoplasm vacuolization (Fig. 6C and D).
Discussion
BafA1 was shown in vitro to significantly
induce Δψ collapse. It has been reported that the mitochondrial
permeability transition represents an important cellular event,
initiating apoptotic cell death (16). These observations were confirmed by
flow cytometry. Taken together, these data indicate that BafA1 can
trigger apoptosis in MG63 cells.
Western blot analysis was used to elucidate the
possible mechanisms underlying BafA1-mediated apoptosis. It was
observed that p53 protein expression levels were increased
following BafA1 treatment in MG63 cells. In addition, levels of
other indicators of autophagy, including LC3-II and Beclin1, were
increased, whereas those of p62 were decreased, similar to the
results of Paglin et al (17). Due to the toxic cellular stress
imposed by ectopic BafA1 application, p53 was observed to become
activated; activated p53 may be hypothesized to subsequently act as
a transcription factor to regulate downstream genes and promote
apoptosis. It is therefore concluded that BafA1 can inhibit
autophagy and enhance p53 expression in MG63 cells, resulting in
the promotion of MG63 cell apoptosis.
In summary, the present study revealed a new
mechanism associated with autophagy inhibition-induced impairment
of cell proliferation and induction of cell death of cancer cells.
Further investigation of the association between autophagy and
apoptosis may unveil novel strategies for tumor therapy.
Acknowledgements
This study was supported in part by a grant from the
National Science Foundation of China (no. 81171730).
References
|
1
|
Raymond AK, Ayala AG and Knuutila S:
Conventional osteosarcoma. World Health Organization Classification
of Tumours. Pathology and Genetics of Tumours of Soft Tissue and
Bone. Fletcher CDM, Unni KK and Mertens F: IARC Press; Lyon: pp.
264–270. 2002
|
|
2
|
Xiao H, Chen L, Luo G, Son H, Prectoni JH
and Zheng W: Effect of the cytokine levels in serum on
osteosarcoma. Tumour Biol. 35:1023–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scully SP, Ghert MA, Zurakowski D,
Thompson RC and Gebhardt MC: Pathologic fracture in osteosarcoma :
prognostic importance and treatment implications. J Bone Joint Surg
Am. 84-A:49–57. 2002.PubMed/NCBI
|
|
4
|
Gelderblom H, Jinks RC, Sydes M, Bramwell
VH, van Glabbeke M, Grimer RJ, Hogendoorn PC, McTiernan A, Lewis
IJ, Nooij MA, Taminiau AH and Whelan J; European Osteosarcoma
Intergroup. Survival after recurrent osteosarcoma: data from 3
European Osteosarcoma Intergroup (EOI) randomized controlled
trials. Eur J Cancer. 47:895–902. 2011. View Article : Google Scholar
|
|
5
|
Akiyama T, Dass CR and Choong PF: Novel
therapeutic strategy for osteosarcoma targeting osteoclast
differentiation, bone-resorbing activity, and apoptosis pathway.
Mol Cancer Ther. 7:3461–3469. 2008. View Article : Google Scholar
|
|
6
|
Malicdan MC, Noguchi S, Nonaka I, Saftig P
and Nishino I: Lysosomal myopathies: an excessive build-up in
autophagosomes is too much to handle. Neuromuscul Disord.
18:521–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Orvedahl A and Levine B: Eating the enemy
within: autophagy in infectious diseases. Cell Death Differ.
16:57–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar
|
|
9
|
Carew JS, Nawrocki ST and Cleveland JL:
Modulating autophagy for therapeutic benefit. Autophagy. 3:464–467.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto A, Tagawa Y, Yoshimori T,
Moriyama Y, Masaki R and Tashiro Y: Bafilomycin: A1 prevents
maturation of autophagic vacuoles by inhibiting fusion between
autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E
cells. Cell Struct Funct. 23:33–42. 1998. View Article : Google Scholar
|
|
11
|
Boya P, González-Polo RA, Casares N, et
al: Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol.
25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shacka JJ, Klocke BJ, Shibata M, Uchiyama
Y, Datta G, Schmidt RE and Roth KA: Bafilomycin A1 inhibits
chloroquine-induced death of cerebellar granule neurons. Mol
Pharmacol. 69:1125–1136. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi X, Chen Z, Liu D, Cen J and Gu M:
Expression of Dlk1 gene in myelodysplastic syndrome determined by
microarray, and its effects on leukemia cells. Int J Mol Med.
22:61–68. 2008.PubMed/NCBI
|
|
14
|
Alvarez-Tejado M, Naranjo-Suarez S,
Jiménez C, Carrera AC, Landázuri MO and del Peso L: Hypoxia induces
the activation of the phosphatidylinositol 3-kinase/Akt cell
survival pathway in PC12 cells: protective role in apoptosis. J
Biol Chem. 276:22368–22374. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qi X, Jiang J, Zhu M and Wu Q: Human corin
isoforms with different cytoplasmic tails that alter cell surface
targeting. J Biol Chem. 286:20963–20969. 2011. View Article : Google Scholar
|
|
16
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paglin S, Hollister T, Delohery T, et al:
A novel response of cancer cells to radiation involves autophagy
and formation of acidic vesicles. Cancer Res. 61:439–444.
2001.PubMed/NCBI
|