Introduction
Gastric cancer (GC) is one of the most common causes
of cancer-related mortality, with >700,000 mortalities worldwide
every year (1). Although the
prognosis of early GC is generally favorable, a large proportion of
cases of GC are diagnosed in the locally advanced or disseminated
stage. Chemotherapy remains an important therapeutic modality for
patients with advanced GC. However, due to multi-drug resistance
and adverse side-effects, the objective response to conventional
treatments is not satisfactory in the majority of patients with
metastatic GC. The overall 5-year survival rate of GC patients is
only 20–25% (2). Therefore, more
effort must be devoted to developing novel therapeutic or
preventive strategies and/or agents that target cancer
metastasis.
Transforming growth factor-β (TGF-β) is a
multifunctional cytokine that controls a number of different cell
functions and regulates several important processes during tumor
progression, including proliferation, apoptosis, angiogenesis,
invasion, and metastasis (3).
TGF-β exerts a growth inhibition function in premalignant cells,
but promotes tumor metastasis in later stages of cancer. Increased
expression levels of TGF-β have been detected in numerous types of
tumor, including GC, and are correlated with the invasion and
metastasis of GC; thus it is a potential marker for the
identification of GC patients with a poor prognosis (4–6).
Furthermore, TGF-β treatment may increase the metastatic potential
of the BGC823 and SGC7901 GC cell lines (7). This suggests that the TGF-β signaling
pathway has an important role in cancer invasion and metastasis and
that its modulation may be a novel strategy for treatment of GC
metastasis.
It has previously been reported that flavonoid
compounds may have a potential inhibitory effect on cancer
progression (8,9). Of these substances, baicalein, a
flavonoid derived from the root of Scutellaria baicalensis,
exerted strong anti-metastatic activity against various types of
cancer cell, including breast (10), skin (11), bladder (12), liver (13) and bone (14) cancers. However, the mechanisms by
which baicalein exerts its anti-metastatic effects are largely
unknown. The aim of the present study was to use the AGS human GC
cell line to evaluate the effect of baicalein on the migration and
invasion of cancer cells, and to investigate the possible molecular
mechanisms of its anti-metastatic effects.
Materials and methods
Cell culture and baicalein treatment
The AGS human GC cell line was purchased from
Nanjing KeyGEN Biotech.Co., Ltd. (Nanjing, China). Cells were
cultured with RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified incubator at 37°C under 5% CO2 in air. Cell
culture materials were purchased from Life Technologies (Grand
Island, NY, USA). For baicalein treatment, an appropriate
concentration of baicalein (Enzo, Farmingdale, NY, USA) was added
to the culture medium to achieve the indicated concentrations and
then incubated with cells for indicated time points. A
dimethylsulfoxide solution (Solarbio, Beijing, China)without
baicalein was used as the control.
Wound-healing assay
Cells were seeded into a 6-well plate and allowed to
grow in complete medium until approximately confluent. Cell
monolayers were wounded using a 250-μl tip and washed twice with
phosphate-buffered saline to remove cell debris. The cells were
then incubated in culture medium in the absence or presence of
baicalein (0, 25 or 50 μM) for 24 h. Images of treated cells moving
within the wound were captured using a Leica DM IL LED inverted
microscope and the average width of the wound was analyzed at the
indicated times by LAS software v.4.0 (Leica Microsystems GmbH,
Wetzlar, Germany).
Cell invasion and migration assays
Transwell chambers with a 6.5-mm diameter and an
8.0-μm pore polycarbonate membrane (Corning Life Sciences, New
York, NY, USA) were coated with Matrigel (20 μg/insert; BD
Bioscience, Bedford, MA, USA) for 6 h to form a basement membrane
prior to use. Following treatment with baicalein (25 or 50 μM) for
24 h, the surviving cells were harvested and seeded in the upper
chamber at a density of 30,000 cells/well in 200 μl free-serum
culture medium. Culture medium containing 10% FBS (600 μl) was
added in the lower chamber as a chemoattractant. After incubation
for 24 h at 37°C, all of the non-invaded cells were removed from
the upper face of the membrane with a cotton swab. The invaded
cells on the lower face of membrane were then fixed for 10 min with
4% paraformaldehyde (Genmed Scientifics, Inc., Arlington, MA, USA)
and stained with 0.2% crystal violet for 15 min. Cells that had
invaded through the membrane were counted using the inverted
microscope (x200 magnification).
The general procedure of the migration assay was
similar to that of the invasion assay, with the exception that the
membranes in each chamber were not coated with Matrigel and the
number of cells seeded in the upper chamber was 50,000
cells/well.
Quantitative polymerase chain reaction
(qPCR)
Following treatment with baicalein for 24 h, total
RNA was extracted from cells using RNAiso reagent (Takara, Dalian,
China) according to the manufacturer’s instructions. cDNA was
synthesized from 1 μg total RNA using an PrimeScript™ RT Reagent
kit (Takara). qPCR analysis was performed using SYBR®
Select Master mix (Applied Biosystems, Foster City, CA, USA) with a
7500 Fast Real-Time PCR system with (v.2.0.5 software; Applied
Biosystems). The following cycling conditions were used: 40 cycles
of 50°C for 2 min, 95°C for 2 min, 58°C for 15 sec and 72°C for 1
min. The data were analyzed using the 2−ΔΔCt method and
the ABI 7500 PCR system software (Applied Biosystems). The gene
expression levels were normalized to GAPDH and presented as a
relative fold change compared with the control. All reactions were
independently repeated three times and the mean was calculated. The
sequences of the primers used are presented in Table I.
 | Table IqPCR primers. |
Table I
qPCR primers.
| Sequence of primers
(5′-3′) |
|---|
| TGF-β | S:
CCGACTACTACGCCAAGGAG
A: TGTGTACTCTGCTTGAACTTGTC |
| Smad4 | S:
CATCTGAGTCTAATGCTACC
A: CAACAGTCCTTCACTATGG |
| N-cadherin | S:
AAGAACGCCAGGCCAAACAAC
A: CTGGCTCAAGTCATAGTCCTGGTCT |
| Vimentin | S:
CCTCTTCCAAACTTTTCCTCCCT
A: AAGTTTCGTTGATAACCTGTCCATC |
| ZEB1 | S:
AAGAATTCACAGTGGAGAGAAGCCA
A: CGTTTCTTGCAGTTTGGGCATT |
| ZEB2 | S:
TGTCATTAGAAGAGGCGTAA
A: GCAGAGCAGGTTAGAACT |
| GAPDH | S:
CATCAGCAATGCCTCCTGCAC
A: TGAGTCCTTCCACGATACCAAAGTT |
Western blotting analysis
After treatment with baicalein for 24 h, the cells
were lysed in RIPA buffer (Pierce, Rockford, IL, USA) with Complete
Protease Inhibitors and PhosSTOP Phosphatase Inhibitor Cocktail
tablets (Roche, Mannheim, Germany). The lysates were then clarified
by centrifugation at 4°C for 15 min at 30,270 × g. The
concentrations of the proteins in the supernatants were detected
using a BCA Protein Assay kit (Pierce, Rockford, IL, USA). Equal
amounts of the proteins were then separated by 10% SDS-PAGE gels
and transferred onto the polyvinyl difluoride (PVDF) membranes
(Roche). The PVDF membranes were blocked with 3% bovine serum
albumin for 1 h and then probed overnight at 4°C with the primary
antibodies listed below. Following three washes with Tris-buffered
saline with Tween 20, the membranes were incubated with the
appropriate horseradish peroxidase (HRP)-conjugated secondary
antibody for 1 h at room temperature. The bands were detected using
the Enhanced Chemiluminescent substrate (Pierce, Rockford, IL,
USA). The primary antibodies used were as follows: N-cadherin
(1:1,000, #MA5-15633; Pierce), vimentin (1:1,000, #21488; Signalway
Antibody, College Park, MD, USA), ZEB1 (#ab155249), ZEB2 (1:1,000,
#ab25837; Abcam, Hong Kong, China), TGF-β (#3709), Smad4 (#9515)
and β-actin (#4967, 1:1,000; Cell Signaling Technology, Inc.,
Beverly, MA, USA). The following secondary antibodies used were:
HRP-conjugated goat anti-mouse IgG and goat anti-rabbit IgG,
purchased from Cell Signaling Technology, Inc. (1:2,000).
Statistical analysis
All experiments were repeated at least three times.
Data were presented as the mean ± standard deviation. The
differences between groups were analyzed with Student’s t-test or
one-way analysis of variance using SPSS 19.0 software (IBM, Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
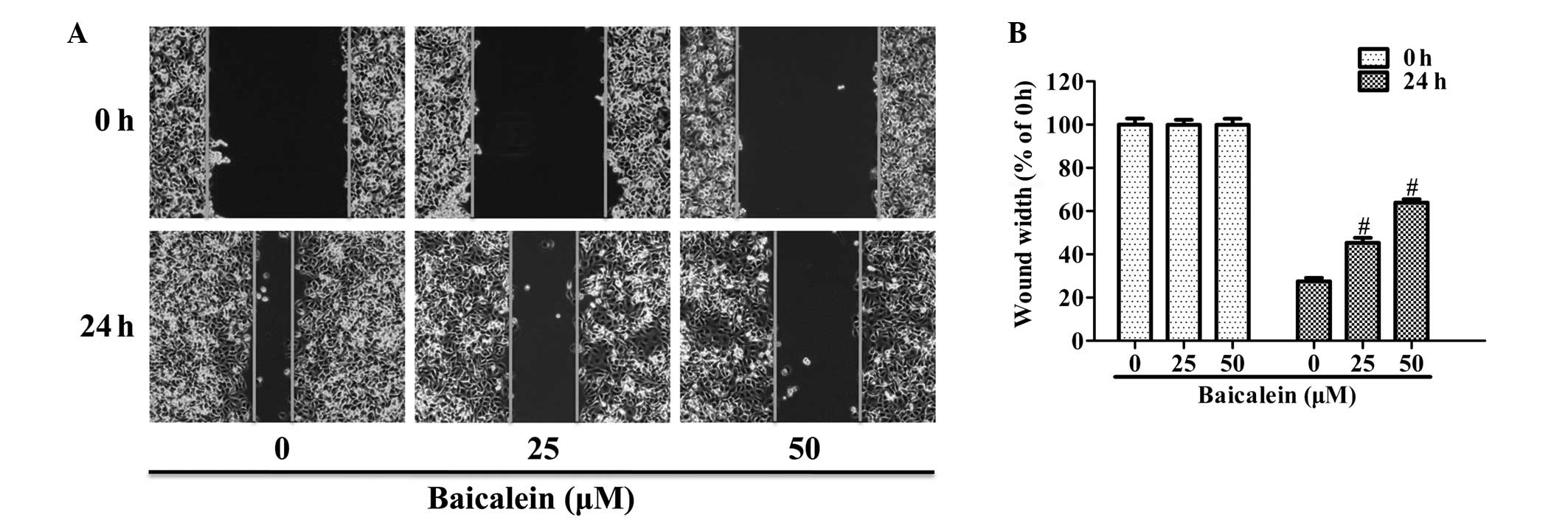
Baicalein inhibits the motility of AGS
human GC cells
The effect of baicalein on cell motility was tested
by performing a wound-healing assay. Confluent monolayers of cells
were scraped to form a wound and then stimulated with 25 or 50 μM
baicalein for 24 h. Fig. 1 shows
the effect of the treatment with baicalein after 24 h. Treatment
with 25 or 50 μM baicalein inhibited the wound width in AGS tissue
by up to 45.3 or 64.0% respectively, suggesting that baicalein
significantly inhibits AGS cell motility (Fig. 1B).
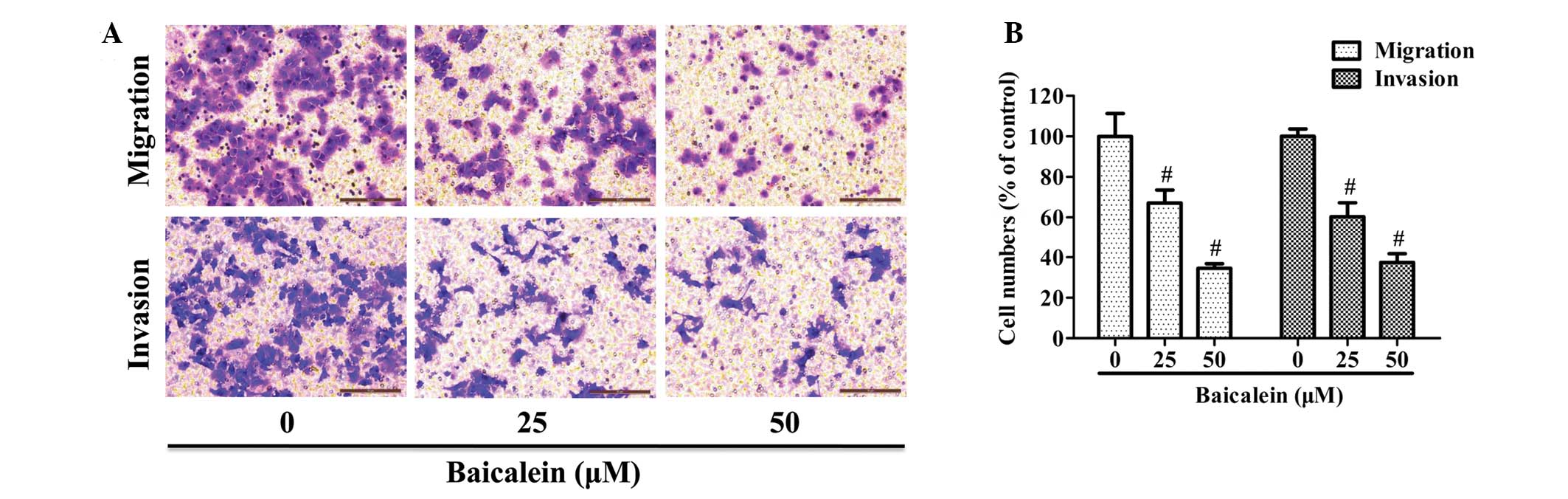
Baicalein inhibits AGS cell migration and
invasion
A Transwell chamber coated with or without Matrigel
was employed to respectively study the migratory or invasive
capacity of AGS cells. As shown in Fig. 2A, the number of cells that migrated
or invaded into the lower chamber was significantly reduced by
baicalein treatment in a dose-dependent manner. Quantification
analysis indicated that treatment with 25 or 50 μM baicalein
reduced the migration of AGS by 32.8 and 65.4%, respectively, as
well as inhibiting cell invasion by 39.7 and 62.6%, respectively
(Fig. 2B).
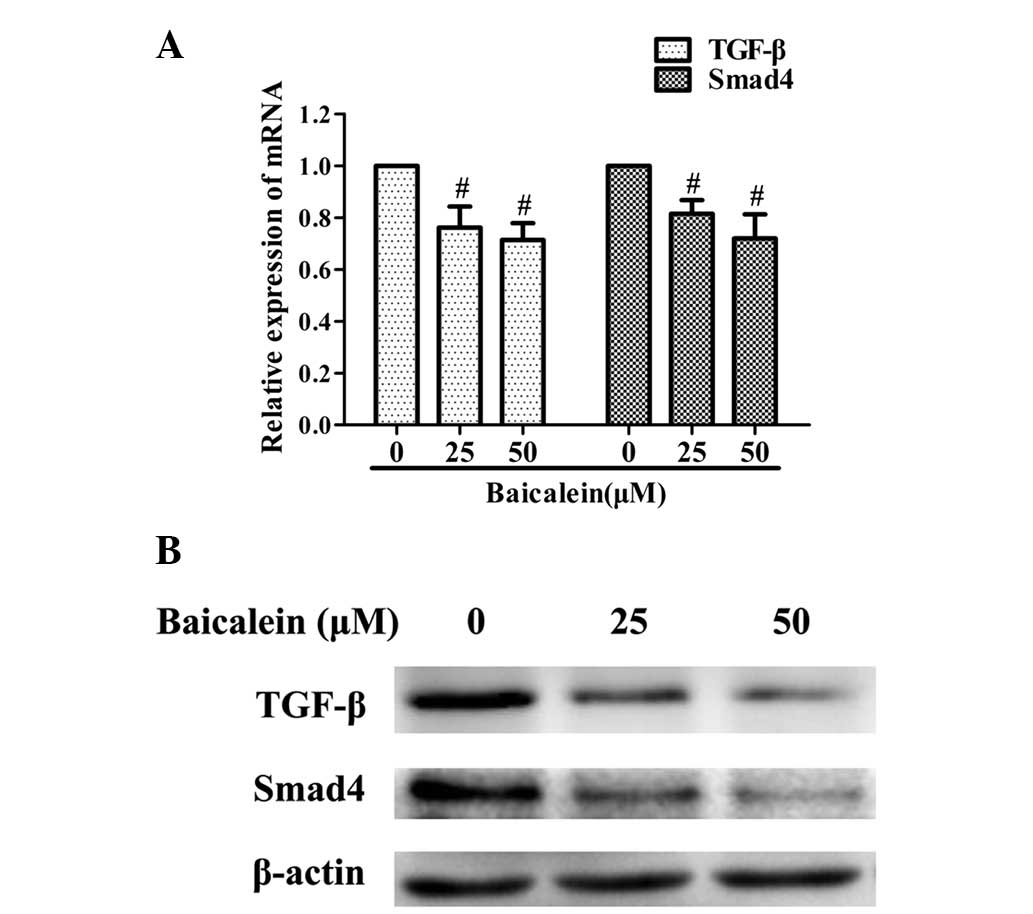
Baicalein inhibits the TGF-β/Smad4
signaling pathway in AGS cells
To investigate the possible mechanisms mediating the
anti-metastatic activities of baicalein, qPCR and western blot
analyses were performed to examine its effect on the expression of
TGF-β and Smad4. The mRNA and protein expression levels of TGF-β
and Smad4 were markedly downregulated by baicalein when compared
with those of the controls (Fig.
3).
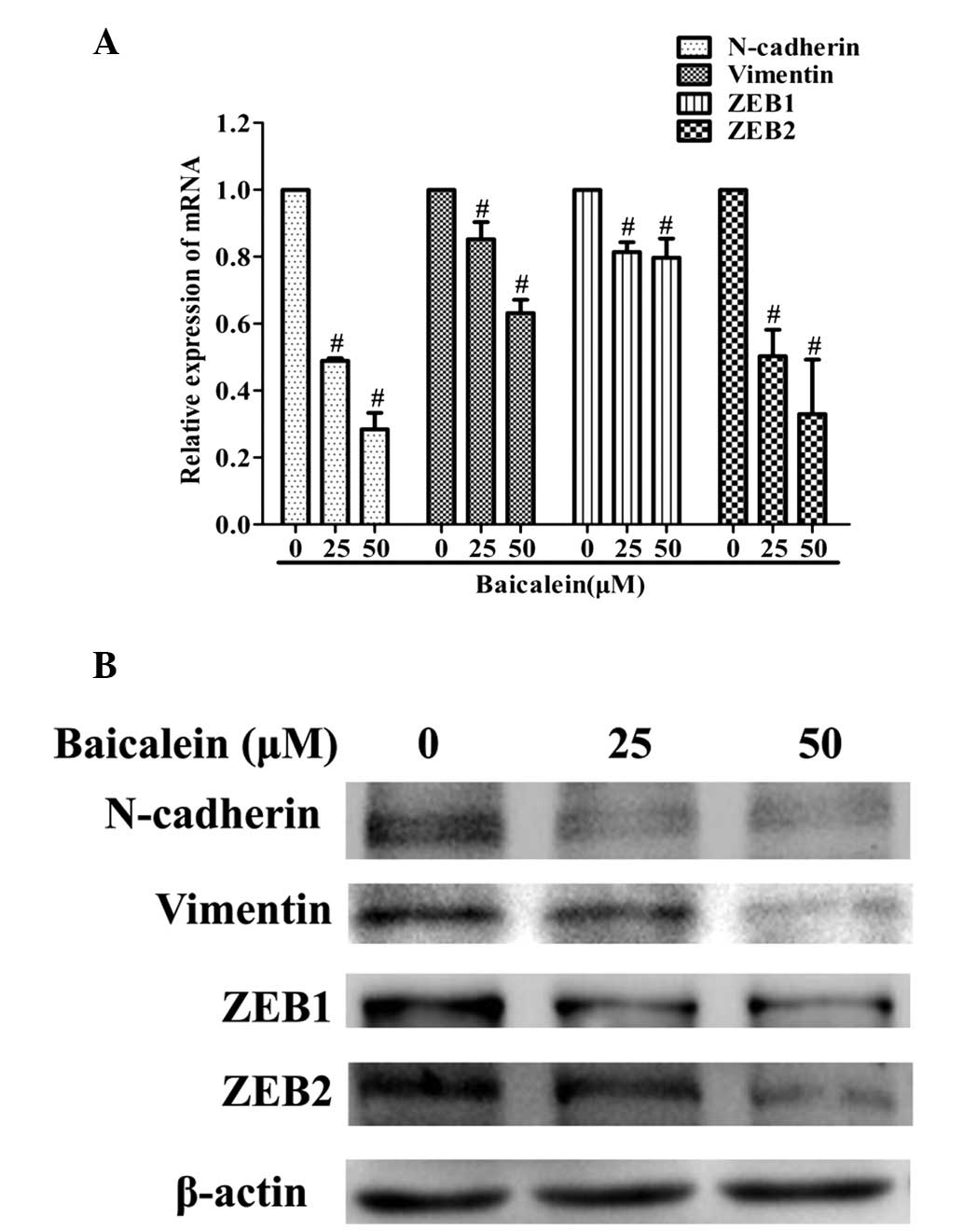
Baicalein reduces the expression levels
of N-cadherin, vimentin, ZEB1 and ZEB2 in AGS cells
Subsequently, the effect of baicalein on the
expression levels of metastasis-associated N-cadherin, vimentin,
ZEB1, ZEB2 was investigated. These are critical downstream target
genes of the TGF-β signaling pathway. As shown in Fig. 4, baicalein treatment markedly
decreased the expression of N-cadherin, vimentin, ZEB1 and ZEB2, at
the transcriptional and translational levels.
Discussion
In the majority of GC cases, local invasion and
distant metastases are the main causes of treatment failure and
mortality. Therefore, there is an urgent requirement to develop
novel and more effective strategies for preventing and treating the
metastatic stage of this disease. Baicalein, a natural flavonoid
extracted from the root of Scutellaria baicalensis, has been
considered as a potential anti-metastatic agent for inhibition of
cancer cell motility, migration and invasion. It has been
demonstrated that baicalein exerts its anti-tumor activities in
multiple types of cancer through modulation of multiple signaling
pathways (10,11,13).
However, the molecular mechanisms by which baicalein exerts its
anti-metastatic effects are yet to be elucidated.
The present study used the AGS human GC cell line to
demonstrate that baicalein was able to inhibit cell motility,
migration and invasion. To investigate the molecular mechanism
underlying these effects of baicalein on AGS cells, the alteration
of TGF-β signaling was analyzed. TGF-β is a multifunctional
cytokine with dual roles in cancer progression. In the early stages
of cancer, it serves a tumor suppressing function either by
inhibiting cellular proliferation or by promoting cellular
apoptosis. However, in the later stages of cancer, cancer cells
become resistant to the growth inhibitory activity of TGF-β and
even overexpress TGF-β, which promotes invasion and metastasis by
stimulating cell motility, tumor angiogenesis and inducing
immunosuppression. Disruption of the tumor autocrine TGF-β
signaling has been found to inhibit metastasis (15,16).
Among the signaling molecules, Smad proteins exhibit a central role
in the manifestation of the biological activities of TGF-β. Smad
proteins are classified into three subtypes: Receptor-regulated
Smads (R-Smads), common-partner Smads (Co-Smads), and inhibitory
Smads (I-Smads). Numerous studies have demonstrated that Smad4, the
only Co-Smad in mammals, is essential for TGF-β-induced tumor cell
invasion (17–20). The present study revealed that
baicalein inhibits TGF-β and Smad4 expression at the mRNA and
protein levels. Thus, it is possible that baicalein may suppress GC
cell invasion and metastasis through the inhibition of TGF-β/Smad4
signaling pathway.
Several studies have shown that TGF-β signaling
enhances cancer cell migration and invasion through multiple
adhesion molecules, cytoskeletal proteins and transcriptional
factors (21–23). N-cadherin is a calcium-dependent
cell adhesion molecule which functions as an invasion promoter in
cancer. Expression of N-cadherin renders cells more motile and
invasive (24). Vimentin is a type
III mesenchymal filament, a mammalian structural cytoskeletal
protein with elevated and aberrant expression that correlates well
with upregulated cell invasion and migration in malignancy
(25). ZEB1 and ZEB2 are members
of the zinc finger E-box binding transcriptional factor family and
have been found to be upregulated in GC. Knockdown of ZEB1 and ZEB2
reduces GC cell migration and invasion (26–28).
The downstream signaling targets of TGF-β were
investigated in the present study to deduce which were involved in
the anti-metastastic effects of baicalein on AGS cells. It was
revealed that the expression levels of N-cadherin, vimentin, ZEB1
and ZEB2 in AGS cells were repressed by baicalein treatment,
suggesting that baicalein may counteract the invasive and
metastatic phenotype partly via TGF-β/Smad4-induced downregulation
of N-cadherin, vimentin, ZEB1 and ZEB2.
In conclusion, this study identified that baicalein
modulates TGF-β, and Smad4 expression levels, as well as
N-cadherin, vimentin, ZEB1 and ZEB2 expression levels, which may
have inhibited the motility, migration and invasion of AGS cells.
These results suggest that baicalein may be a potential anticancer
agent for prevention and treatment of GC metastasis. Further
studies in clinically relevant animal models should be conducted to
exploit its potential benefits against metastatic GC in future.
Acknowledgements
This study was sponsored by the Key Clinical
Specialty Discipline Construction Program of Fujian, China and the
Special Funds of the Finance Department of Fujian Province
(2012B013)
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
3
|
Derynck R and Akhurst RJ: Differentiation
plasticity regulated by TGF-beta family proteins in development and
disease. Nat Cell Biol. 9:1000–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito H, Tsujitani S, Oka S, et al: The
expression of transforming growth factor-beta1 is significantly
correlated with the expression of vascular endothelial growth
factor and poor prognosis of patients with advanced gastric
carcinoma. Cancer. 86:1455–1462. 1999. View Article : Google Scholar
|
|
5
|
Maehara Y, Kakeji Y, Kabashima A, et al:
Role of transforming growth factor-beta 1 in invasion and
metastasis in gastric carcinoma. J Clin Oncol. 17:607–614.
1999.PubMed/NCBI
|
|
6
|
Ijichi H, Ikenoue T, Kato N, et al:
Systematic analysis of the TGF-beta-Smad signaling pathway in
gastrointestinal cancer cells. Biochem Biophys Res Commun.
289:350–357. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang KS, Hu ZL, Li JH, Xiao DS and Wen JF:
Enhancement of metastatic and invasive capacity of gastric cancer
cells by transforming growth factor-beta1. Acta Biochim Biophys Sin
(Shanghai). 38:179–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parajuli P, Joshee N, Rimando AM, Mittal S
and Yadav AK: In vitro antitumor mechanisms of various Scutellaria
extracts and constituent flavonoids. Planta Med. 75:41–48. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li-Weber M: New therapeutic aspects of
flavones: the anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Ling Y, Chen Y, et al: Flavonoid
baicalein suppresses adhesion, migration and invasion of MDA-MB-231
human breast cancer cells. Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu B, Li J, Huang D, et al: Baicalein
mediates inhibition of migration and invasiveness of skin carcinoma
through Ezrin in A431 cells. BMC Cancer. 11:5272011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu JY, Tsai KW, Li YZ, et al:
Anti-bladder-tumor effect of baicalein from Scutellaria
baicalensis Georgi and its application in vivo. Evid Based
Complement Alternat Med. 2013:5797512013.PubMed/NCBI
|
|
13
|
Chiu YW, Lin TH, Huang WS, et al:
Baicalein inhibits the migration and invasive properties of human
hepatoma cells. Toxicol Appl Pharmacol. 255:316–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Song L, Cai L, Wei R, Hu H and
Jin W: Effects of baicalein on apoptosis, cell cycle arrest,
migration and invasion of osteosarcoma cells. Food Chem Toxicol.
53:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Picon A, Gold LI, Wang J, Cohen A and
Friedman E: A subset of metastatic human colon cancers expresses
elevated levels of transforming growth factor beta1. Cancer
Epidemiol Biomarkers Prev. 7:497–504. 1998.
|
|
16
|
Fransvea E, Angelotti U, Antonaci S and
Giannelli G: Blocking transforming growth factor-beta up-regulates
E-cadherin and reduces migration and invasion of hepatocellular
carcinoma cells. Hepatology. 47:1557–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takano S, Kanai F, Jazag A, et al: Smad4
is essential for down-regulation of E-cadherin induced by TGF-beta
in pancreatic cancer cell line PANC-1. J Biochem. 141:345–351.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wiercinska E, Naber HP, Pardali E, van der
Pluijm G, van Dam H and ten Dijke P: The TGF-β/Smad pathway induces
breast cancer cell invasion through the up-regulation of matrix
metalloproteinase 2 and 9 in a spheroid invasion model system.
Breast Cancer Res Treat. 128:657–666. 2011.
|
|
19
|
Deckers M, van Dinther M, Buijs J, et al:
The tumor suppressor Smad4 is required for transforming growth
factor beta-induced epithelial to mesenchymal transition and bone
metastasis of breast cancer cells. Cancer Res. 66:2202–2209. 2006.
View Article : Google Scholar
|
|
20
|
Shiou SR, Datta PK, Dhawan P, et al:
Smad4-dependent regulation of urokinase plasminogen activator
secretion and RNA stability associated with invasiveness by
autocrine and paracrine transforming growth factor-beta. J Biol
Chem. 281:33971–33981. 2006. View Article : Google Scholar
|
|
21
|
Araki K, Shimura T, Suzuki H, et al:
E/N-cadherin switch mediates cancer progression via TGF-β-induced
epithelial-to-mesenchymal transition in extrahepatic
cholangiocarcinoma. Br J Cancer. 105:1885–1893. 2011.PubMed/NCBI
|
|
22
|
Cheng JC, Auersperg N and Leung PC:
TGF-beta induces serous borderline ovarian tumor cell invasion by
activating EMT but triggers apoptosis in low-grade serous ovarian
carcinoma cells. PLoS One. 7:e424362012. View Article : Google Scholar
|
|
23
|
Dai M, Al-Odaini AA, Fils-Aimé N, et al:
Cyclin D1 cooperates with p21 to regulate TGFβ-mediated breast
cancer cell migration and tumor local invasion. Breast Cancer Res.
15:R492013.PubMed/NCBI
|
|
24
|
Derycke LD and Bracke ME: N-cadherin in
the spotlight of cell-cell adhesion, differentiation,
embryogenesis, invasion and signalling. Int J Dev Biol. 48:463–476.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Satelli A and Li S: vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia B, Liu H, Kong Q and Li B:
Overexpression of ZEB1 associated with metastasis and invasion in
patients with gastric carcinoma. Mol Cell Biochem. 366:223–229.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okugawa Y, Toiyama Y, Tanaka K, et al:
Clinical significance of Zinc finger E-box Binding homeobox 1
(ZEB1) in human gastric cancer. J Surg Oncol. 106:280–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai YH, Tang YP, Zhu HY, et al: ZEB2
promotes the metastasis of gastric cancer and modulates epithelial
mesenchymal transition of gastric cancer cells. Dig Dis Sci.
57:1253–1260. 2012. View Article : Google Scholar
|