Introduction
Coronary atherosclerosis heart disease (coronary
heart disease) is a leading cause of mortality in the 21st century,
among which acute myocardial infarction (AMI) is one of the most
serious clinical manifestations associated with high occurrence
rate, high mortality and poor long-term prognosis (1,2). The
early, continuous and full opening of infarct-associated vessels to
recover blood perfusion in the ischemic myocardium is the most
important principle of treatment for AMI. In recent years, with the
wide application of intravenous thrombolysis, percutaneous coronary
interventions (PCI), coronary artery bypass grafting and other
strategies, there has been an evident improvement in the symptoms
and prognosis for patients with coronary heart disease and AMI
(3). However, despite the numerous
strategies available, it is common that clinical symptoms do not
improve as expected. On the contrary, numerous patients emerge with
reperfusion arrhythmia, increased myocardial infarction area and
cardiac insufficiency. As a result, the concept of myocardial
ischemia reperfusion injury has emerged. Attempting to relieve the
occurrence of reperfusion injury has become a prominent obstacle in
the prevention and treatment of ischemic heart disease.
Myocardial ischemia reperfusion injury is a highly
complex process, and currently there are several key pathogenic
mechanisms that are considered to be involved, including oxidative
stress injury, intracellular calcium overload, cell apoptosis,
cellular energy loss and activation of neutrophil inflammatory
reaction (4). Ischemia reperfusion
injury also involves multiple regulatory mechanisms, including the
activation and inactivation of signaling pathways (5). In processes including ischemic
pre-adaptation, ischemic post-adaptation and pharmacological
pretreatment, the confluence of numerous pathways is triggered by
the heart to release endogenous active substances, activating
various intracellular signal transduction systems to adjust cardiac
function, which has a protective effect on the myocardium (5).
Proanthocyanidins are highly efficient free radical
scavengers, which are widely used in the clinic to delay senility,
regulate blood fat and to reduce the development of atherosclerosis
and tumor growth (6). Several
studies have identified that proanthocyanidins have a protective
effect on myocardial ischemia reperfusion, but the endogenous
mechanism underlying this effect is has yet to be elucidated. In
the present study, myocardial cells cultured in vitro were
exposed to acute anoxia-reoxygenation in order to simulate
myocardial ischemia-reperfusion injury. Pretreatment with
proanthocyanidins was performed to study their ability to relieve
anoxia-reoxygenation injury and to investigate the specific
manifestation mode of this injury. Furthermore, it was investigated
whether the protective effects of proanthocyanidins on
anoxia-reoxygenation injury in myocardial cells proceeded via the
phosphatidylinositol-3-kinase/Akt and glycogen synthase kinase
(P13K/Akt/GSK)-3β signaling pathway and the mitochondrial ATP
potassium (mitoKATP) channel.
Materials and methods
Experimental animals and reagents
Sprague Dawley rats, aged 1–3 days, were provided by
the Animal Center of Shandong University (Jinan, Shandong, China).
This study was approved by the Ethics Committee of Shandong
University (Jinan, China). Several regents were used, including
Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen Life
Technologies, Carlsbad, CA, USA), trypsin (Tiangen Biotech
(Beijing) Co., Ltd., Beijing, China), dichlorofluorescein probe
(Sigma, St. Louis, MO, USA), MTT proliferation detection kit
(Sigma, San Jose, CA, USA), a terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) kit (Tiangen Biotech (Beijing) Co.,
Ltd.), Annexin V-fluorescein isothiocyanate (FITC) cell apoptosis
detection kit (Invitrogen Life Technologies), LY294002 and
5-hydroxy decanoic acid (5-HD; Sigma, CA, USA), mouse anti-rat Akt
antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Furthermore, mouse anti-rat glycogen synthase kinase (GSK)-3β and
caspase-3 antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA).
Isolation of neonatal cardiomyocytes
Isolation of neonatal cardiomyocytes is a
technically more simple procedure than cell isolation from adult
hearts, as it does not require aorta cannulation and perfusion. A
two-step procedure was employed, consisting of enzyme digestion and
mechanical agitation of the ventricular tissue followed by
purification of the cardiomyocyte population. The enzymatic
digestion and purification of cardiomyocytes was performed as
previously described (7).
Cell culture
For the culture of neonatal myocardial cells, two
methods were employed, including a re-differentiation and a rapid
attachment method as previously described (8,9). The
redifferentiation method was performed to ensure that the neonatal
cells regained a typical morphology and shape, concurrent with slow
cell attachment to the substrate. The rapid attachment method
resulted in an improved retention of in vivo myocyte
morphology and functionality, as well as ease of use in
experiments.
Establishment of the anoxia-reoxygenation
model
The experiment was started when the myocardial cells
grew close to confluence, exhibiting synchronous growth cycles.
Ischemia simulation solution components included
NaH2PO4 0.9 mmol/l, NaHCO3 6.0
mmol/l, CaCl2 1.8 mmol/l, MgSO4 1.2 mmol/l,
sodium lactate 40 mmol/l,
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) 20
mmol/l, NaCl 98.5 mmol/l, KCl 10.0 mmol/l at pH 6.8. Furthermore,
cells were presaturated in an atmosphere of 95% N2 and
5% CO2 for 1 h to create an oxygen pressure of
P(O2)≤4.0 Kpa, to allow the cells to contain high
concentrations of K+, lactic acid and H+, a
low oxygen concentration, and no glucose or other energy
substrates. Cultured cells were kept in this anoxia solution under
anoxic conditions for 3 h. The solution was then replaced with
reoxygenation solution containing NaH2PO4 0.9
mmol/l, NaHCO3 20 mmol/l, CaCl2 1.0 mmol/l,
MgSO4 1.2 mmol/l, glucose 5.5 mmol/l, HEPES 20 mmol/l,
NaCl 129.5 mmol/l, KCl 5.0 mmol/l, and the pH was adjusted to the
reoxygenation condition of 95% oxygen saturation for 3 h.
Trial grouping
The present study was divided into five groups
according to an experimental scheme. The control group (CN)
consisted of myocardial cells cultured under normal conditions. The
anoxia-reoxygenation group (AR) was composed of the
anoxia/reoxygenation injury model in myocardial cells subjected to
anoxia for 3 h and reoxygenation for 3 h. In the proanthocyanidin
(Shenfu Inc., Shanxi, China) pretreatment groups (PC), the culture
medium was added with a final concentration of 100 mg/l of the
proanthocyanidins and the cells were incubated for 2 h prior to
exposure to anoxic and reoxygenation conditions. In the LY294002
(blocker of the PIK3/Akt channel) group, culture medium was added
containing LY294002 at concentration of 15 μmol/l,
proanthocyanidins were added 30 min later, then anoxia and
reoxygenation were conducted. In the 5-HD [an inhibitor of
mitochondrial ATP-sensitive potassium (mitoKATP) channels] group,
the culture medium was added to 5-HD at a concentration of 100
μmol/l, proanthocyanidins were added 30 min later and then anoxia
and reoxygenation were conducted.
Measurement of reactive oxygen species
(ROS)
ROS levels were measured by flow cytometry.
Following treatment, cells were trypsinized and centrifuged (2 min
at 20,000 × g), and the cell pellet was treated with
2′,7′-dichlorodihydrofluorescein diacetate stain (1:200),
resuspended and incubated at 37°C for 20 min in the dark. Cells not
treated with 2′,7′-dichlorodihydrofluorescein diacetate were used
as negative controls and stained cells treated with 100 μl hydrogen
peroxide (30% w/v hydrogen peroxide) incubated for 10 min served as
positive controls. ROS levels were measured using a flow cytometer
(Becton-Dickinson, Franklin Lakes, NJ, USA) and quantified by
determining the mean fluorescence for each treatment.
TUNEL assay
Potential DNA fragmentation was examined by the
TUNEL apoptosis detection kit (Chemicon, Temecula, CA, USA). The
specific procedure of the TUNEL assay was performed as previously
described (10).
Flow cytometric analysis
Binding of annexin V-FITC and uptake of propidium
iodide (PI) into the cells were assessed using a FACScan flow
cytometer (Becton-Dickinson). Briefly, cells were harvested,
resuspended and incubated with Annexin V-FITC and PI (5 μg/ml) in
the dark at room temperature for 15 min. Fluorescence was measured
through a 530/30 band filter (FL-1) to monitor Annexin V-FITC
binding and through a 585/42 band filter (FL-2) to monitor PI
uptake.
Western blot analysis
Western blotting was employed to analyze the
expression levels of caspase-3, p-Akt, GSK-3β and p-GSK-3β. The
assay was performed as previously described (11).
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD), variance analysis was used to compare multiple groups and the
q test was used to analyze inter-group differences. Analysis was
performed using SASS 6.12 statistical software (SPSS, Inc.,
Chicago, IL, USA) and P<0.01 was considered to indicate a
statistically significant difference between values.
Results
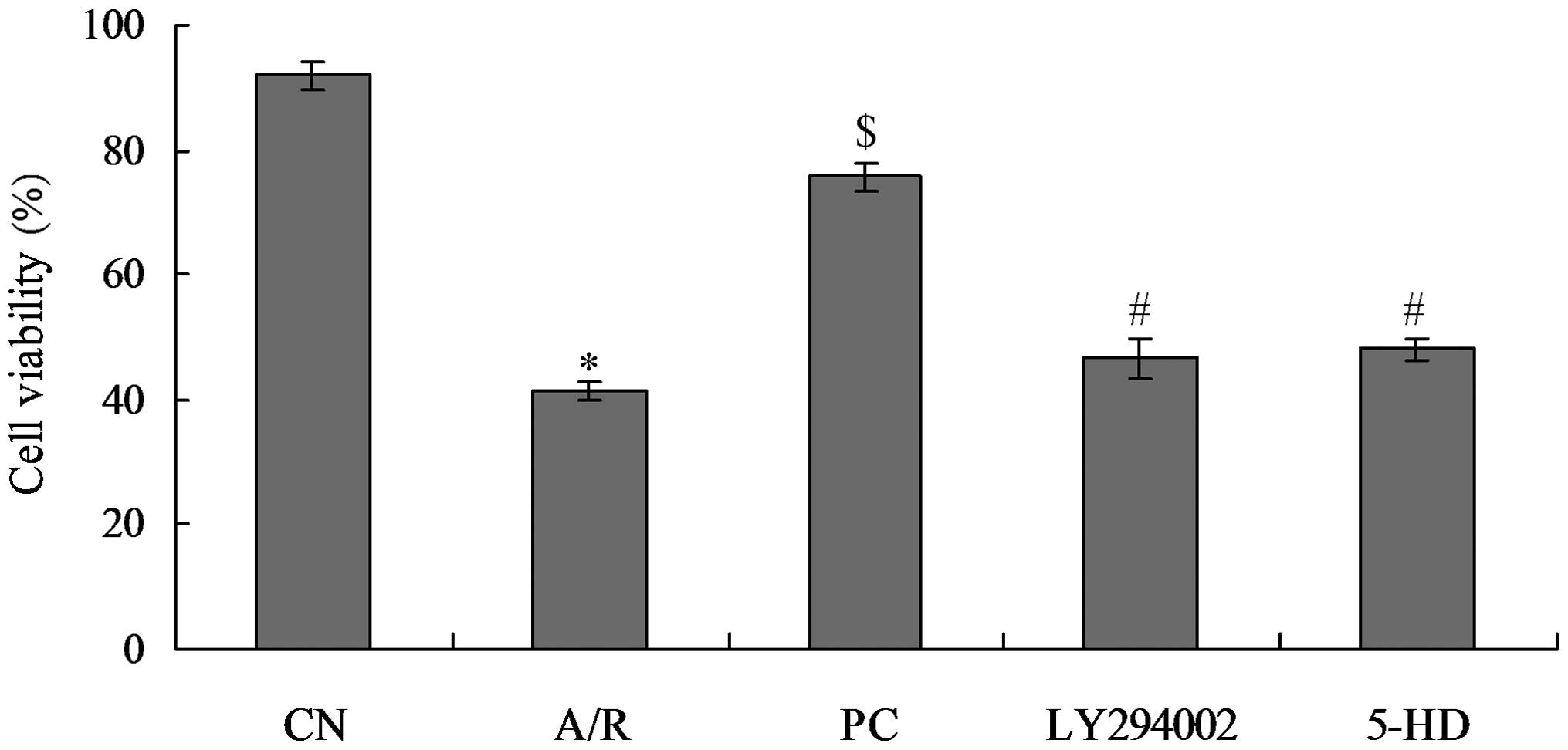
Myocardial cell survival rate in each
group
Myocardial cells were evidently impaired in the A/R
group (41.33±1.45%), and the cell survival rate was significantly
reduced as compared with that in the control group (91.95±2.27%;
P<0.05). Cell survival rate in the PC group (75.64±2.01%), was
significantly higher than that in the A/R group (P<0.05). The
cell survival rate in the LY294002 group (46.56±3.3%) was
significantly lower than that in the PC group (P<0.05) as
compared with that in A/R group, where there were no significant
differences. The cell survival rate in the 5-HD group (48.17±1.7%)
was significantly lower than that in the PC group (P<0.05),
while there was no significant difference from that in the A/R
group. There was no significant difference between the cell
survival rate in the LY294002 group and that in the 5-HD group
(Fig. 1).
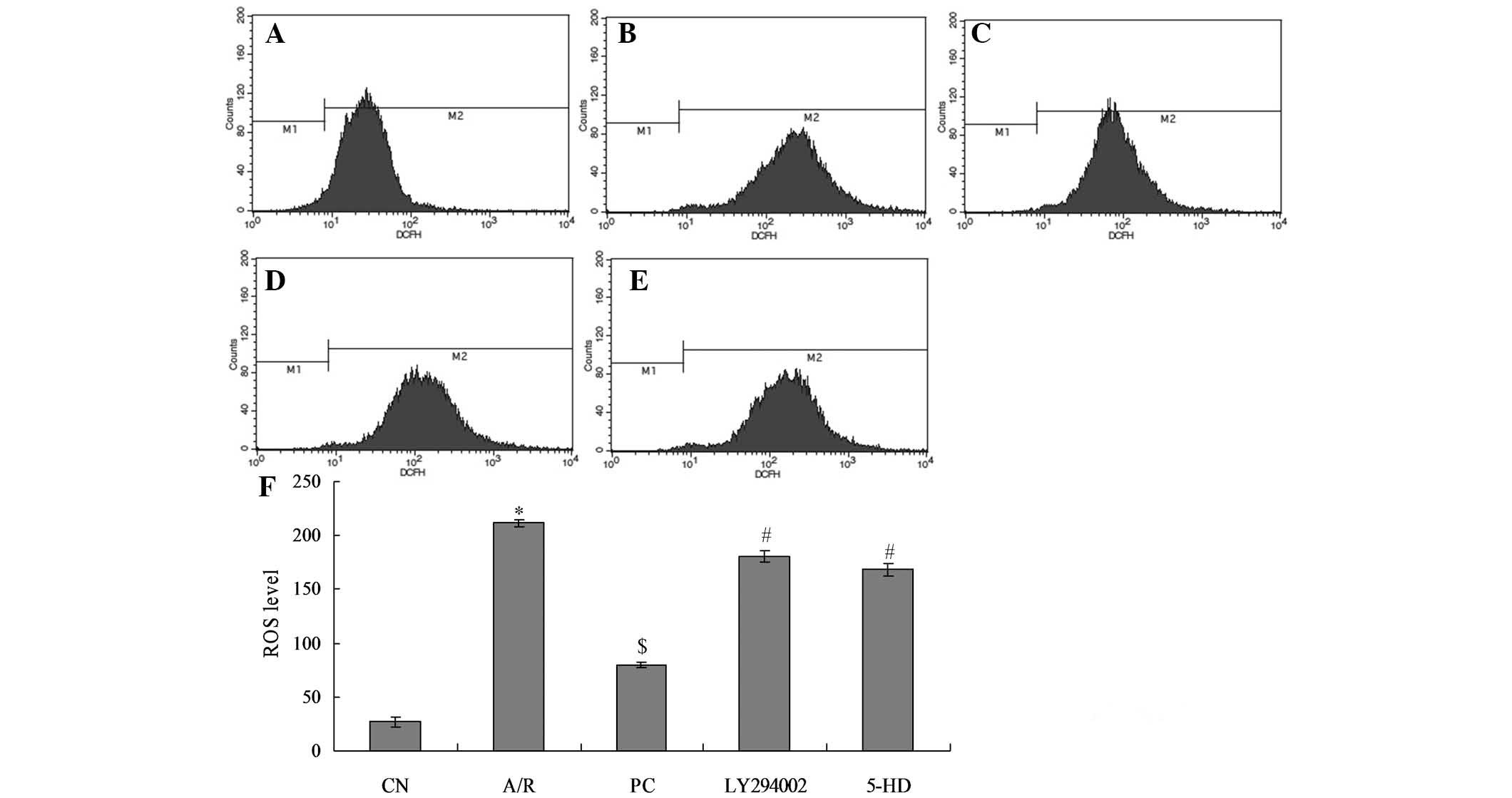
ROS levels in myocardial cells in each
treatment group
In the A/R group (fluorescence intensity, 211.69),
ROS levels were significantly increased as compared with those in
the CN group (27.06; P<0.01). ROS levels were decreased in the
PC group (80.11) as compared with those in the A/R group, which
were significantly different (P<0.01). ROS levels were
significantly increased in the LY294002 group (180.45) as compared
with those in the PC group (P<0.01), while there was no
significant difference from those in the A/R group. ROS levels
increased significantly in the 5-HD group (168.35) as compared with
those in the PC group (P<0.01); however, there was no
significant difference from those in the A/R group. There were no
significant differences between the ROS level of cells in the
LY294002 and 5-HD groups (Fig.
2).
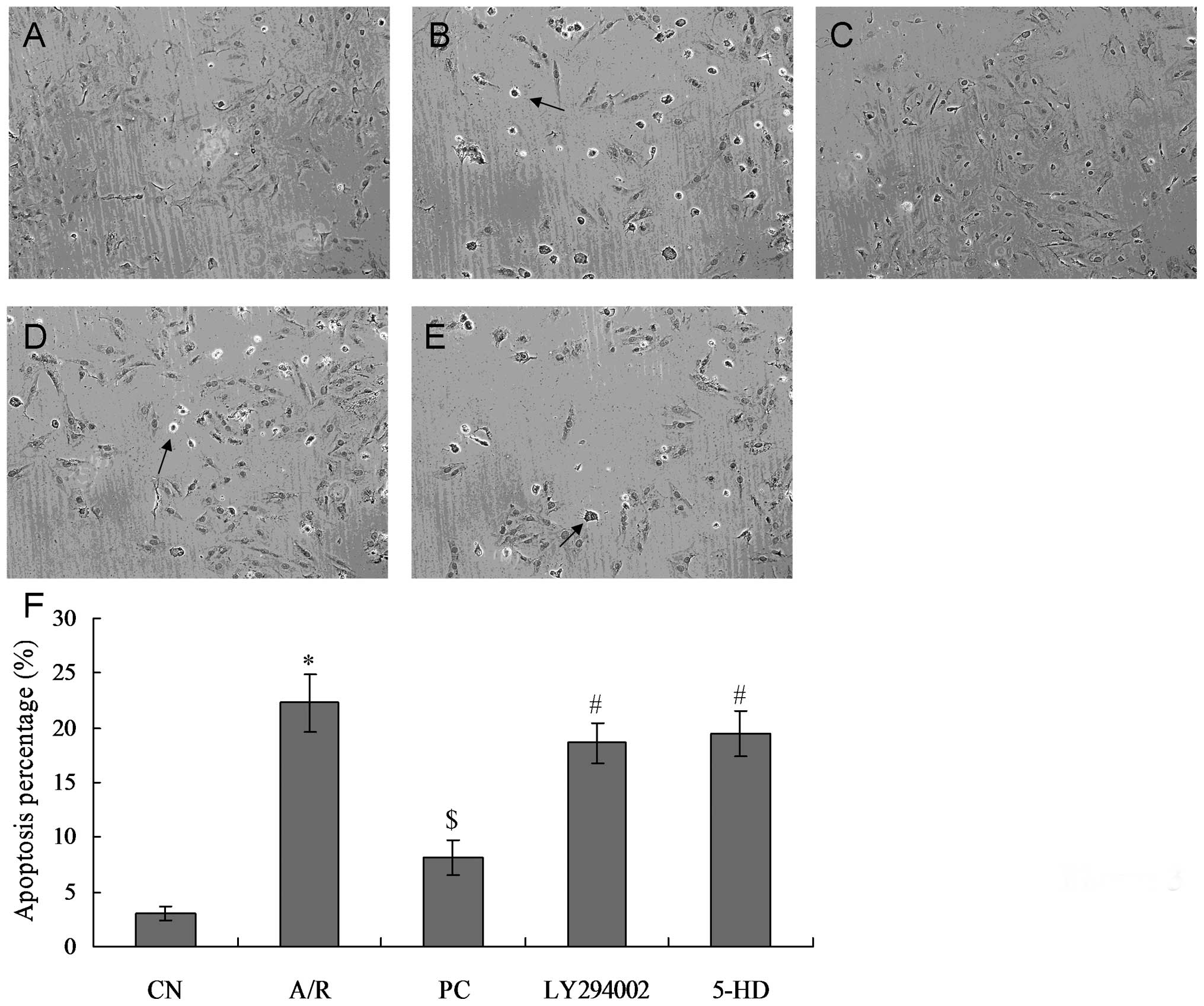
TUNEL results
As compared with the CN group (3.1±0.65%), the
percentage of TUNEL-positive cells in the A/R group (25.3±2.64%)
was significantly increased (P<0.05). As compared with the A/R
group, TUNEL-positive cells in the PC group (10.2±1.59%) were
significantly decreased (P<0.01). TUNEL-positive cells in the
LY294002 group (18.6+1.79%) were significantly increased as
compared with those in the PC group (P<0.01). TUNEL-positive
cells in the 5-HD group (19.5±2.03%) were also significantly
increased when compared with the PC group (P<0.01). There were
no significant differences between the A/R and the 5-HD groups,
between the LY294002 and 5-HD groups and between the LY294002 and
A/R groups (Fig. 3).
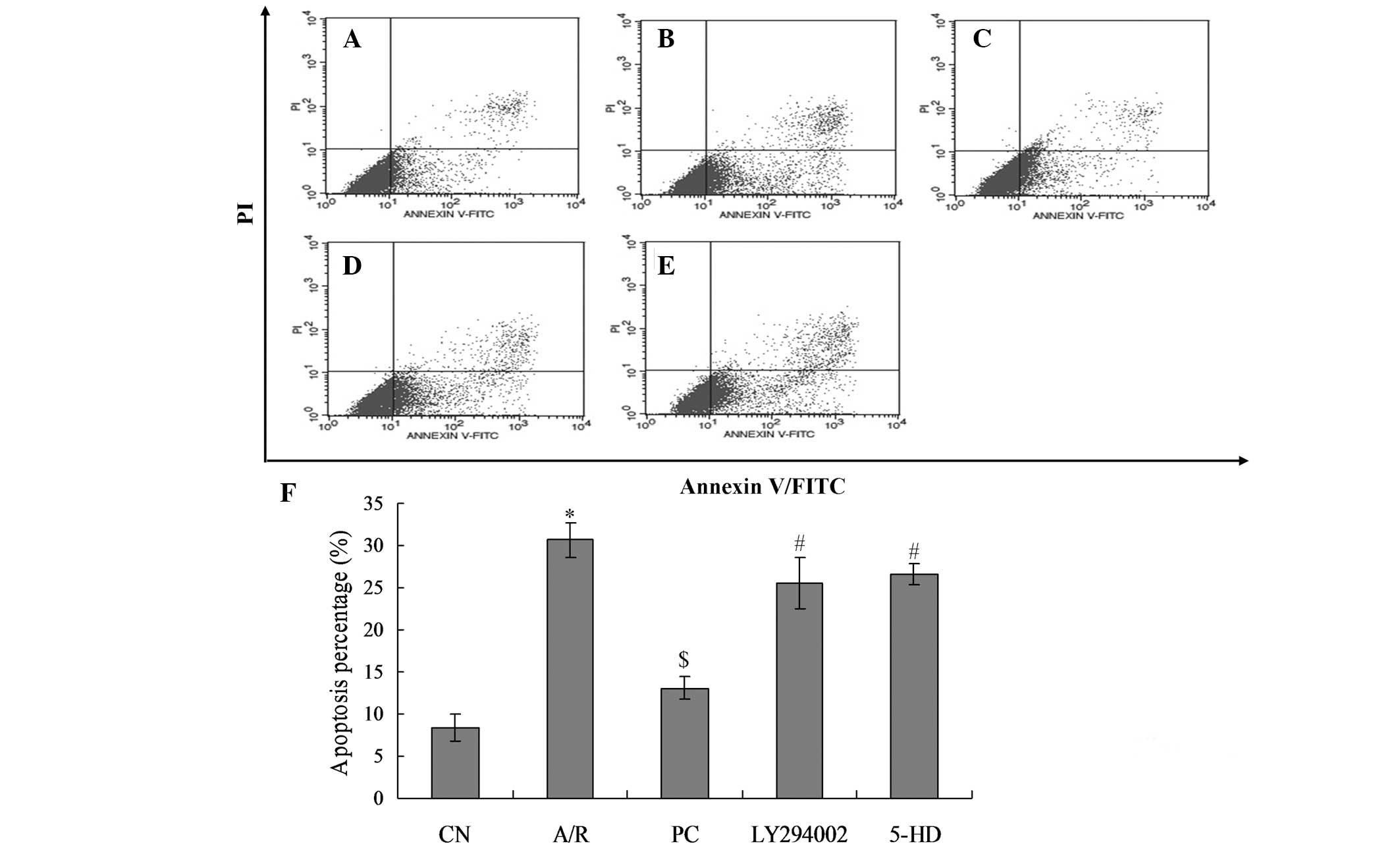
Apoptotic rates of myocardial cells
Flow cytometric analysis was used to detect the
Annexin V/FITC and PI staining and determine apoptotic rates. In
the Fig. 4, cells in the bottom
right of the dot plot represent cells in late apoptosis, while
those in the top right of the graph represent cells in early
apoptosis. Apoptotic cells in the A/R group (30.70%) were
significantly increased as compared with those in the CN group
(8.36%; P<0.01). The percentage of apoptotic cells was
significantly decreased in the PC group (13.11%) as compared with
that in the A/R group (P<0.01). Furthermore, apoptotic cells in
the LY294002 (25.48%) and 5-DH groups were significantly increased
when compared with those in the PC group (P<0.05). There was no
significant difference between the LY294002 and 5-DH groups.
Furthermore, the percentage of apoptotic cells in the LY294002 and
5-DH groups demonstrated no significant difference when compared
with that in the A/R group (P>0.05).
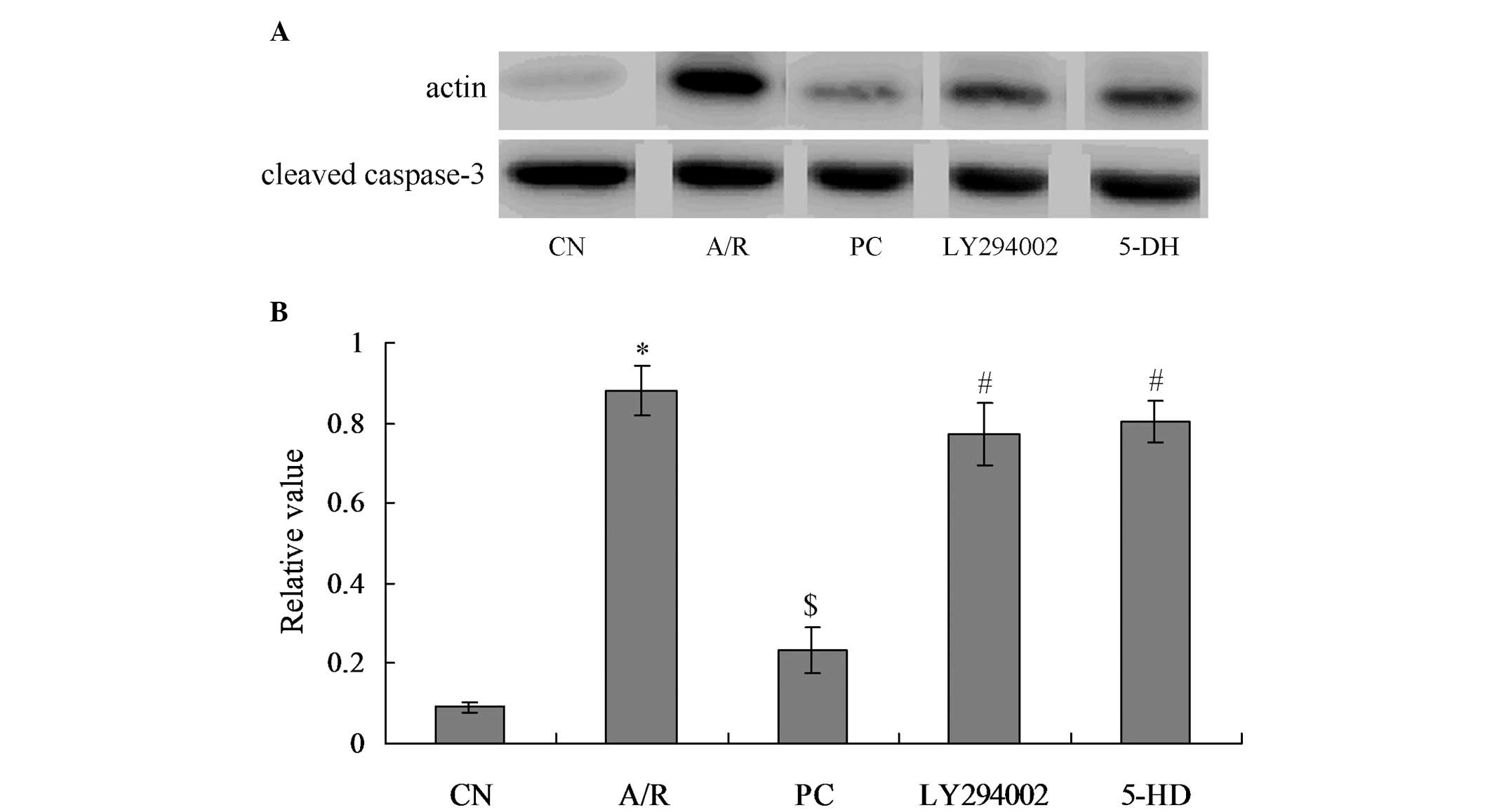
Activated caspase-3 levels in each
group
The levels of activated caspase-3 protein (cleaved
caspase-3) reflected the degree of apoptosis in myocardial cells,
which showed a positive correlation. From Fig. 5, it was identified that caspase-3
in the A/R group were significantly activated as compared with
those in the CN group (P<0.05). When treated with
proanthocyanidins, caspase-3 activation was significantly inhibited
in the PC group, and activated caspase-3 level was significantly
decreased, compared with the A/R group (P<0.05). Furthermore,
activated caspase-3 level in the LY294002 and 5-DH groups was
significantly increased, when compared with the PC group
(P<0.05). However, there was no significant difference between
the LY294002 and 5-DH groups.
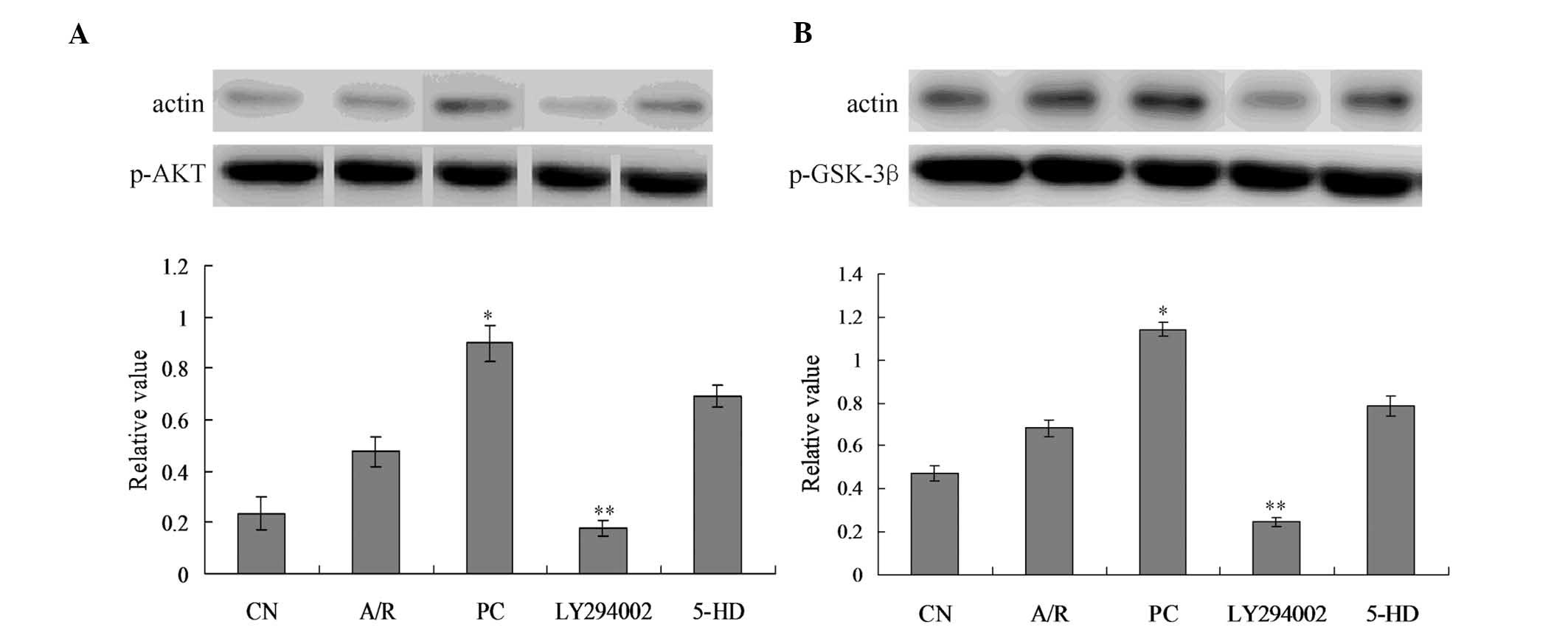
p-Akt and p-GSK-3β expression
analysis
p-Akt protein expression was moderately increased in
the A/R group, but there was no significant difference when
compared with the CN group (Fig.
6A). p-Akt protein expression increased significantly in PC
group when compared with the A/R group (P<0.05). p-Akt protein
expression decreased significantly in the LY294002 group when
compared with the PC group (P<0.05). There was no significant
difference between the PC and 5-DH group (P>0.05). Furthermore,
there was no significant difference of t-Akt expression between
every group (data not shown).
p-GSK-3β protein expression was slightly increased
in the A/R group, but there was no significant difference when
compared with the CN group (Fig.
6B). p-Akt protein expression increased significantly in the PC
group when compared with the A/R group (P<0.05). p-GSK-3β
protein expression decreased significantly in LY294002 group when
compared with the PC group (P<0.05). There was no significant
difference in p-GSK-3β expression between PC group and 5-DH group
(P>0.05). Furthermore, there was no significant difference of
t-GSK-3β among every group (data not shown).
Discussion
Oxidative stress is important in myocardial
anoxia-reoxygenation injury, where excessive ROS production is
derived from the mitochondrial electron transport chain, activated
neutrophils and the enzyme xanthine oxidase (12,13).
ROS have a highly potent chemical activity, and their active
electrons easily militate against the components of cells,
enhancing cell membrane lipid peroxidation, restraining the
function of cellular proteins and damaging nucleic acids and
chromosomes, resulting in overall damage to cellular structure and
functioning with harmful consequences (14,15).
In the present study, it has been identified that the myocardial
cell survival rate decreased following anoxia-reoxygenation injury,
ROS production was enhanced and the apoptotic rate of myocardial
cells increased. Cell apoptosis, also known as programmed cell
death (PCD), is induced by the activation of signal transduction
pathways, which trigger karyocyte-dependent activation of
endogenous DNA incision enzymes. Under certain physiological or
pathological conditions, this process is able to independently
regulate gene expression, inducing characteristic morphological and
biochemical changes to normal cellular functioning. Caspase-3 has a
key function in the implementation of apoptosis. Apoptotic stimuli
induce a reduction in the mitochondrial transmembrane potential,
triggering cytochrome C release into the cytoplasm and the
subsequent activation of a caspase-mediated apoptosis reaction,
which drives the rapid execution of cell death (16). Apoptosis is critical in the
pathological process underlying myocardial ischemia-reperfusion
injury. Zhao et al (17)
identified that inhibiting myocardial apoptosis significantly
reduced the area of myocardial infarction, by not only relieving
the necrotic lesion of ischemia-reperfusion, but also by improving
the mechanical contractile function of the heart.
When the mitochondrial outer membrane ruptures and
the mitochondrial content, including apoptosis inducing factor
(AIF) and cytochrome C, is released, this disrupts the electron
transfer chain and directly induces cell death (18,19).
Through the study of the association between ROS generation and the
opening of the mitochondrial permeability transition pore (mPTP)
during the process of myocardial anoxia-reoxygenation injury with
apoptosis, it has been identified that the generation of ROS occurs
earlier than the opening of the mPTP. This indicates that ROS may
cause the opening of the mPTP, as single anoxia does not induce the
opening of the mPTP. However, large amounts of ROS are generated
following reoxygenation and the mPTP opens. A rapid and wide
opening of the mPTP may cause excessive expansion of mitochondria
and uncoupling of oxidative phosphorylation, which causes
hydrolysis of large amounts of ATP, leading to cell death (20). Accordingly, stabilizing the
permeability transition pore in the mitochondrial membrane and
reducing the production of ROS are important in preventing cellular
apoptosis. Bergmann et al (21) identified that pravastatin
pretreatment reduces the generation of ROS in
anoxia-reoxygenation-injured myocardial cells, as well as the rate
of cellular apoptosis. The results of the present study revealed
that proanthocyanidins effectively inhibited the production of ROS
induced by acute anoxia-reoxygenation, which subsequently inhibited
apoptosis. These data are consistent with earlier studies,
confirming that the antiapoptotic effect of proanthocyanidins
proceeds via inhibition of the mitochondrial pathway, which reduces
caspase-3 activity.
The PI3K/Akt/GSK-3β signalling pathway is the most
important signal transduction pathway in myocardial ischemic
pretreatment. The protective effect of ischemic pretreatment on the
myocardium is predominantly due to the activation of the PI3K/Akt
signal transduction pathway (22).
Efthymiou et al (23)
identified that administering atorvastatin reduced the myocardial
infarction area (MI) in the ischemia reperfusion model of the
normal myocardium of rats in vitro. Furthermore, these
effects were eliminated by the PI3K/Akt inhibitor, indicating that
the protective effect of atorvastatin on the myocardium was
mediated by the PI3K/Akt pathway, inhibiting apoptosis and
regulating the transport of glucose in correlation with glycogen
synthesis. In addition, studies have demonstrated that GSK-3β is
associated with the opening of the mPTP.
In the myocardial cells of the anoxia-reoxygenation
model, the addition of diazoxide prior to cell anoxia reduced
intracellular calcium overload, mitochondrial membrane potential
and oxidative stress induced-apoptosis. By contrast, the addition
of the mitoKATP channel blocker 5-HD eliminated this protective
effect (24). One study revealed
that oxytocin is protective against myocardial ischemia-reperfusion
injury by reducing the area of myocardial infarction and myocardial
enzyme release, and this effect was inhibited by 5-HD and
atractyloside (a mPTP-opening agent). ROS produced in the process
of anoxia-reoxygenation regulate the permeability of the mPTP and
thus reduce apoptosis, while mitoKATP regulates the production of
ROS (25). The mitoKATP channel
has a protective role in anoxia-reoxygenation injury; however, the
precise location of interference with the process remains to be
identified. A number of studies have hypothesized that the mitoKATP
channel is the initiating factor in the protection mechanism of
ischemia-reperfusion injury, while others consider it to be the
final effector (26–28).
Previous studies have identified that
proanthocyanidins have a protective effect on myocardial ischemia
reperfusion. Sato et al (29) demonstrated that grape seed extract
promotes the recovery of cardiac contractile function following
ischemia and reperfusion, as a significant reduction in the
myocardial infarct area was observed and it appeared to directly
scavenge peroxyl radicals, thus leading to the inhibition of cell
apoptosis. In one study, proanthocyanidins attenuated the
isoproterenol-induced activation of various enzymes in the
mitochondria, including lysosomal enzyme, isocitrate dehydrogenase,
cytochrome C oxidase, NADH dehydrogenase, and reduced the area of
myocardial infarction (30). In
the present study, myocardial cells were exposed to
anoxia-reoxygenation conditions in vitro in order to
simulate myocardial ischemia-reperfusion injury. This model did not
consider the effect of neural, humoral and other hybrid cell
factors present in in vivo and in vitro perfusion
models. Following proanthocyanidin treatment, the myocardial cells
exhibited an increase in overall survival, a reduction in ROS
levels and a decrease in the apoptotic rate, all of which showed
significant differences. These data demonstrated that pretreatment
with proanthocyanidins had a protective effect on myocardial cells
with anoxia-reoxygenation injury. The addition of the
PI3K/Akt-specific inhibitor LY294002 and the mitoKATP
channel-specific blocker 5-HD prior to pretreatment with
proanthocyanidins eliminated the protective effects of
proanthocyanidin, including the reduction in myocardial
intracellular ROS and apoptotic rate. These data suggested that the
PI3K/Akt/GSK-3β signaling pathway and mitoKATP channels were
activated by the proanthocyanidins, which are likely to be the
mechanisms underlying the protective effect of proanthocyanidins on
myocardial anoxia-reoxygenation injury. Following the addition of
the P13K blocker, the protein levels of p-Akt and p-GSK-3β were
significantly reduced, which were significantly different when
compared with those in the PC group. Following administration of
the mitoKATP channel-specific blocker, PI3K and phosphorylated
GSK-3β showed no significant difference, which indicated that the
mitoKATP channel may have an important role downstream of the
P13K/Akt/GSK-3β pathway.
In conclusion, pretreatment with proanthocyanidins
had a protective effect on myocardial cell anoxia-reoxygenation
injury in rat cells. This effect was associated with the activation
of the PI3K/Akt/GSK-3β signaling pathway and the opening of
mitoKATP channels, which may have an important role downstream of
the PI3K pathway.
Acknowledgements
This study was supported by a grant from the
Independent Innovation Foundation of Shandong University (grant no.
2012TS153).
References
|
1
|
Zhang LN, Liu PP, Zhou J, Huang RS, Yuan
F, Fei LJ, Huang Y, Xu L, Hao LM, Qiu XJ, et al: Positive
correlation between variants of lipid metabolism-related genes and
coronary heart disease. Mol Med Rep. 8:260–266. 2013.PubMed/NCBI
|
|
2
|
Zhang S, Liu X, Goldstein S, Li Y, Ge J,
He B, Fei X, Wang Z and Ruiz G: Role of JAK/STAS signaling pathway
in the pathogenesis of acute myocardial infarction in rats and its
effect on NF-κB expression. Mol Med Rep. 7:93–98. 2013.PubMed/NCBI
|
|
3
|
Rao SV, Hess CN, Dai D, Green CL, Peterson
ED and Douglas PS: Temporal trends in percutaneous coronary
intervention outcomes among older patients in the United States. Am
Heart J. 166:273–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang N, Min, Li D, He P and Zhao L:
Geranylgeranylacetone protects against myocardial ischemia and
reperfusion injury by inhibiting high-mobility group box 1 protein
in rats. Mol Med Rep. 5:521–524. 2012.PubMed/NCBI
|
|
5
|
Turer AT and Hill JA: Pathogenesis of
myocardial ischemia-reperfusion injury and rationale for therapy.
Am J Cardiol. 106:360–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu Z, Du P, Meiser P and Jacob C:
proanthocyanidins: oligomeric structures with unique biochemical
properties and great therapeutic promise. Nat Prod Commun.
7:381–388. 2012.PubMed/NCBI
|
|
7
|
Louch WE, Sheehan KA and Wolska BM:
Methods in cardiomyocyte isolation, culture, and gene transfer. J
Mol Cell Cardiol. 51:288–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cantin M, Ballak M, Beuzeron-Mangina J,
Anand-Srivastava MB and Tautu C: DNA synthesis in cultured adult
cardiocytes. Science. 214:569–570. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piper HM, Probst I, Schwartz P, Hütter FJ
and Spieckermann PG: Culturing of calcium stable adult cardiac
myocytes. J Mol Cell Cardiol. 14:397–412. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Dong CF, Shi Q, Shi S, Wang GR,
Lei YJ, Xu K, An R, Chen JM, Jiang HY, et al: Cytosolic prion
protein induces apoptosis in human neuronal cell SH-SY5Y via
mitochondrial disruption pathway. BMP Rep. 42:444–449. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang W, Shi Q, Xu K, Gao C, Chen C, Li XL,
et al: Familial CJD associated PrP mutants within the transmembrane
region induced Ctm-PrP retention in ER and trigger apoptosis by ER
stress in SH-SY5Y cells. PLoS One. 6:e146022011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HJ, Kang PF, Wu WJ, Tang Y, Pan QQ,
Ye HW, Tang B, Li ZH and Gao Q: Changes in cardiac mitochondrial
aldehyde dehydrogenase 2 activity in relation to oxidative stress
and inflammatory injury in diabetic rats. Mol Med Rep. 8:686–690.
2013.PubMed/NCBI
|
|
13
|
Decoursey TE and Ligeti E: Regulation and
termination of NADPH oxidase activity. Cell Mol Life Sci.
62:2173–2193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marczin N, E1-Habashi N, Hoare GS, Bundy
RE and Yacoub M: Antioxidants in myocardial ischemia-reperfusion
injury: therapeutic potential and basic mechanisms. Arch Biochem
Biophys. 420:222–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Fan S, Song D, Wang Z, Ma S, Li S,
Li X, Xu M, Xu M and Wang X: Long-term streptozotocin-induced
diabetes in rats leads to severe damage of brain blood vessels and
neurons via enhanced oxidative stress. Mol Med Rep. 7:431–440.
2013.PubMed/NCBI
|
|
16
|
Scarabelli TM, Stephanou A, Pasini E, et
al: Minocycline inhibits caspase activation and reactivation,
increases the ratio of XIAP to smac/DIABLO, and reduces the
mitochondrial leakage of cytochrome C and smac/DIABLO. J Am Coll
Cardiol. 43:865–874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao ZQ, Morris CD, Budde JM, Wang NP,
Muraki S and Sun HY: Inhibition of myocardial apoptosis reduces
infarct size and improves regional contractile dysfunction during
reperfusion. Cardiovasc Res. 59:132–142. 2003. View Article : Google Scholar
|
|
18
|
Arguad L, Gateau-Roesh O, Raisky O,
Loufouat J, Robert D and Ovize M: Postconditioning inhibits
mitochondrial permeability transition. Circulation. 111:194–197.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang X and Huang N: Berberine induces
selective apoptosis through the AMPK-mediated mitochondrial/caspase
pathway in hepatocellular carcinoma. Mol Mep Rep. 8:505–510.
2013.
|
|
20
|
Assaly R, de Tassigny AD, Paradis S,
Jacquin S, Berdeaux A and Morin D: Oxidative stress, mitochondrial
permeability transition pore opening and cell death during
hypoxia-reoxygenation in adult cardiomyocytes. Eur J Pharmacol.
675:6–14. 2012. View Article : Google Scholar
|
|
21
|
Bergmann MW, Rechner C, Freund C, Baurand
A, El Jamali A and Dietz R: Statins inhibit reoxygenation-induced
cardiomyocyte apoptosis: role for glycogen synthase kinase 3beta
and transcription factor beta-catenin. J Mol Cell Cardiol.
37:681–690. 2004. View Article : Google Scholar
|
|
22
|
Liu ZL, Mao JH, Peng AF, Yin QS, Zhou Y,
Long XH and Huang SH: Inhibition of fatty acid synthase suppresses
osteosarcoma cell invasion and migration via downregulation of the
PI3K/Akt signaling pathway in vitro. Mol Med Rep. 7:608–612.
2013.PubMed/NCBI
|
|
23
|
Efthymiou CA, Mocanu MM and Yellon DM:
Atorvastatin and myocardial reperfusion injury: new pleiotropic
effect implicating multiple prosurvival sinaling. J Cardiovasc
Pharmac. 45:247–252. 2005. View Article : Google Scholar
|
|
24
|
Abdallah Y, Wolf C, Meuter K, Piper HM,
Reusch HP and Ladilov Y: Preconditioning with diazoxide prevents
reoxygenation-induced rigor-type hypercontracture. J Mol Cell
Cardiol. 48:270–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alizadeh AM, Faghihi M, Khori V, Sohanaki
H, Pourkhalili K, Mohammadghasemi F and Mohsenikia M: Oxytocin
protects cardiomyocytes from apoptosis induced by
ischemia-reperfusion in rat heart: role of mitochondrial
ATP-dependent potassium channel and permeability transition pore.
Peptides. 36:71–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grossini E, Pollesello P, Bellofatto K, et
al: Protective effects elicited by levosimendan against liver
ischemia/reperfusion injury in anesthetized rats. Liver Transpl.
20:361–375. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amani M, Jeddi S, Ahmadiasl N, Usefzade N
and Zaman J: Effect of HEMADO on level of CK-MB and LDH enzymes
after ischemia/reperfusion injury in isolated rat heart.
Bioimpacts. 3:101–104. 2013.PubMed/NCBI
|
|
28
|
Zeng Z, Huang HF, He F, et al: Diazoxide
attenuates ischemia/reperfusion injury via upregulation of heme
oxygenase-1 after liver transplantation in rats. World J
Gastroenterol. 18:1765–1772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato M, Maulik G, Ray PS, Bagchi D and Das
DK: Cardioprotective effects of grape seed proanthocyanidin against
ischemic reperfusion injury. J Mol Cell Cardiol. 31:1289–1297.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu X, Qiu J, Zhao S, You B, Ji X, Wang Y,
Cui X, Wang Q and Gao H: Grape seed proanthocyanidin extract
alleviates ouabain-induced vascular remodeling through regulation
of endothelial function. Mol Med Rep. 6:949–954. 2012.
|