Introduction
Macrophages are the primary effector cells of the
immune system that protect against microbial infection. Upon
stimulation with pathogen-derived molecules, including
lipopolysaccharide (LPS) and flagellin, macrophages secrete a
variety of inflammatory mediators and cytokines (1,2).
These secreted proteins bind tightly to toll-like receptors (TLRs)
and stimulate the formation of signaling complexes resulting in a
rapid defensive response (3–6). The
tight regulation of host immune signaling ensures that the
resulting immune response is appropriate for the continuously
changing microenvironment, as well as for maintenance of
immunological balance. However, inappropriate or prolonged
activation of the immune system is largely responsible for the
pathology of acute and chronic inflammatory conditions, including
septic shock and chronic inflammatory conditions, such as
rheumatoid arthritis, inflammatory bowel disease and chronic
obstructive pulmonary disease (1,7). In
this regard, the secretion of pro-inflammatory mediators by
activated macrophages, including cytokines, growth factors,
hydrolytic enzymes, bioactive lipids, reactive oxygen intermediates
and nitric oxide (NO), are involved in the pathogenesis of tissue
injury (1,3). Therefore, identification of agents
that can regulate the production of pro-inflammatory mediators is
considered an effective strategy for developing therapeutic agents
to treat severe inflammation.
Activated macrophages transcriptionally express
inducible NO synthase (iNOS) in response to various
pro-inflammatory cytokines and bacterial LPS, resulting in the
production of NO via the oxidative deamination of L-arginine at
sites of inflammation (8,9). NO modulates a variety of biological
processes, including inflammation and carcinogenesis (10,11).
Tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 are potent
pro-inflammatory cytokines that are important for stimulating the
secretion of additional inflammatory cytokines. Therefore,
inhibiting the excessive production of these mediators in
macrophages through the inhibition of mRNA and protein expression
of iNOS, TNF-α and IL-6 may be a viable strategy for the
development of novel anti-inflammatory agents.
Several attempts have been made to develop a new
generation of anti-inflammatory agents from natural compounds, as
natural compounds are known to have fewer side effects (12). The latexes of Euphorbia
plants were traditionally considered as toxic substances due to
their irritancy on mucus membranes, including the nose and mouth,
in humans (13). Although
Euphorbia plants have toxic effects in humans, the extracts
of Euphorbia plants are well known to inhibit excessive
inflammation and are used in Chinese medicine (14,15).
Suarez et al demonstrated that intraperitoneal
administration of an aqueous extract of Croton malambo
(Euphorbiaceae) resulted in a significant anti-inflammatory effect
in a rat model of edema (16).
Furthermore, Sangre de Drago (dragon’s blood) from Croton
lechleri (Euphorbiaceae) inhibits inflammation in vitro
and in vivo (17,18). For example, treatment with Sangre
de Drago significantly decreased intracellular generation of
reactive oxygen species in several cell lines and alleviated paw
edema in rats (18). However, the
anti-inflammatory effects of methanol extracts of Euphorbia
cooperi (MEC) remain to be elucidated.
In the present study, the anti-inflammatory effect
of MEC in LPS-stimulated RAW 264.7 macrophages and its underlying
mechanisms were investigated to evaluate the therapeutic potential
of MEC in the treatment of abnormal inflammation.
Materials and methods
Cell culture and reagents
The RAW 264.7 macrophages, a mouse monocytic cell
line, were cultured in Dulbecco’s modified Eagle’s medium
supplemented with 10% fetal bovine serum, 50 U/ml penicillin and 50
μg/ml streptomycin (Gibco-BRL, Grand Island, NY, USA) at 37°C in a
5% CO2 humidified air atmosphere. Rabbit polyclonal
anti-inhibitors of κB (IκB) and mouse anti-tubulin antibodies were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). Rabbit polyclonal anti-inducible iNOS, rabbit polyclonal
anti-phospho IκB, rabbit polyclonal anti-phospho p38
mitogen-activated protein kinase (MAPK), rabbit polyclonal
anti-p38, mouse monoclonal anti-phospho extracellular
signal-regulated kinase (ERK), rabbit polyclonal anti-ERK, rabbit
polyclonal anti-phospho c-Jun N-terminal kinase (JNK) and mouse
monoclonal anti-JNK were purchased from Cell Signaling Technology
Inc. (Danvers, MA, USA). MEC was purchased from the International
Biological Material Research Center (Daejeon, Korea). The above
compounds were dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) and added directly to the
culture media. The final concentrations of DMSO never exceeded
0.1%, which did not affect the assay systems.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The RAW 264.7 macrophages were incubated with MEC
and LPS for 24 h. Following incubation, MTT (0.5 mg/ml) was added
for 3 h at 37°C and the supernatants were carefully removed. The
crystals of viable cells were dissolved in DMSO and absorbance was
measured at 595 nm using a Synergy microplate reader (BioTek
Instruments Inc., Winooski, VT, USA).
Nitrite assay
The RAW 264.7 macrophages were incubated with MEC
and LPS for 24 h. Following incubation, the levels of NO synthesis
were determined by assaying the culture supernatants for nitrite,
the stable reaction product of NO, with molecular oxygen, using the
Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine
dihydrochloride and 2.5% phosphoric acid). The absorbance was
measured at 540 nm using a Synergy microplate reader after 10 min
incubation.
Enzyme-linked immunosorbent assay
(ELISA)
The RAW 264.7 macrophages were stimulated with LPS
and MEC for 24 h. Following stimulation, the supernatants were
obtained and the quantities of TNF-α and IL-6 in the culture
supernatants were determined using sandwich ELISA, which used
monoclonal antibodies specific to each mediator. Prior to the
application of samples, the plate was pre-coated with coating
antibody in the supplied buffer. Following overnight incubation at
4°C, the plate was washed and assay diluents (1X) were treated for
1 h. The samples were then loaded into each well and incubated for
2 h at room temperature. They were then treated with biotinylated
secondary antibody and horseradish peroxidase-streptavidin
solutions for 1 h and 30 min, respectively and substrate solution
was added to the washed plate. After 10 min incubation in the dark,
1N H3PO4 treatment was applied and the
optical density of the individual wells was determined at 450 nm
using a Synergy microplate reader.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total RNA was prepared from the cells and
reverse-transcribed into complementary DNA (cDNA), following which
PCR amplification of the cDNA was performed. The sequences of PCR
primers used in the present study were as follows: mouse iNOS,
forward 5′-GCA TGG AAC AGT ATA AGG CAA ACA-3′ and reverse 5′-GTT
TCT GGT CGA TGT CAT GAG CAA-3′; TNF-α, forward 5′-GTG CCA GCC GAT
GGG TTG TAC C-3′ and reverse 5′-AGG CCC ACA GTC CAG GTC ACT G3′;
IL-6, forward 5′-TCT TGG GAC TGA TGC TGG TGA C-3′ and reverse
5′-CAT AAC GCA CTA GGT TTG CCG A-3′ and GAPDH, forward 5′-GTC TTC
ACC ACC ATG GAG AAG G-3′ and reverse 5′-CCT GCT TCA CCA CCT TCT TGC
C-3′. The PCR was run for 20–25 cycles of 94°C (30 sec), 60°C (30
sec) and 72°C (30 sec) using a Bioer’s thermal cycler (Bioer
Technology Co., Hangzhou, China). Following amplification, the
RT-PCR products (10 μl) were separated in 1.5% (w/v) agarose gels
and stained with ethidium bromide.
Transient transfection and luciferase
assay
The nuclear factor-kappa B (NF-κB) and activator
protein-1 (AP-1) promoters containing the luciferase gene were
purchased from Agilent Technologies (Santa Clara, CA, USA). HEK 293
cells were transiently transfected using polyethyleneimine
according to the manufacturer’s instructions (Polysciences Inc.,
Warrington, PA, USA) and transfected cells were stimulated with
phorbol 12-myristate 13-acetate (PMA) in the presence or absence of
MEC for 24 h. The cells were harvested and the luciferase
activities were assayed according to the manufacturer’s
instructions (Promega Corporation, Madison, WI, USA).
Preparation of total cell lysates
LPS-stimulated RAW 264.7 cells were treated with MEC
for the indicated time periods (15 min and 24 h) and washed with
ice-cold phosphate-buffered saline. The cells were lysed in lysis
buffer containing 0.5% NP-40, 0.5% Triton X-100, 150 mM sodium
chloride, 20 mM trisaminomethane-hydrochloride (Tris-HCl; pH 8.0),
1 mM ethylenediaminetetraacetic acid, 1% glycerol, 1 mM
phenylmethylsulfonyl fluoride and 1 μg/ml aprotinin, collected into
microtubes and then centrifuged at 15,500 × g for 30 min at 4°C.
The supernatants were prepared in new microtubes.
Western blot analysis
Protein concentration was measured using the
Bradford method. Aliquots of the cell lysates were separated on a
10% sodium dodecyl sulfate-polyacrylamide gel in a Mini-Protein II
gel apparatus (Bio-Rad, Richmond, CA, USA) and transferred onto
nitrocellulose membranes (GE Healthcare, Milwaukee, WI, USA) with
transfer buffer [(192 mM glycine, 25 mM Tris-HCl; pH 8.8 and 20%
MeOH (v/v)]. Following inhibition of the non-specific sites with 5%
bovine serum albumin solution, the membrane was incubated overnight
at 4°C with the primary antibodies (1:1,000). Each membrane was
further incubated for 1 h with secondary peroxidase-conjugated goat
polyclonal immunoglobulin G (IgG; 1:5,000). The target proteins
were detected using an enhanced chemiluminescence solution.
Statistical analysis
Differences between the experimental conditions were
assessed using Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference. In all instances,
the means of data from three independent experiments were
analyzed.
Results
Effects of MEC on the viability of
activated macrophages
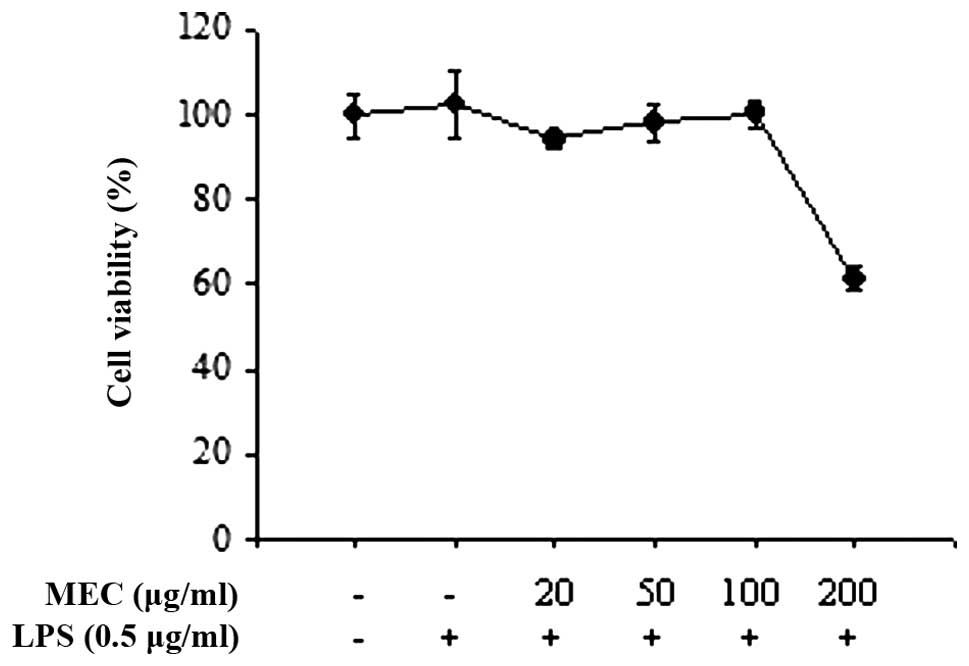
To determine the maximal effective concentration of
MEC that has minimal cytotoxicity, RAW 264.7 macrophages were
treated with the indicated concentrations of MEC (20, 50, 100 and
200 μg/ml) for 24 h in the presence of LPS. Cell viability was
determined by the ability of the cells to metabolically reduce a
tetrazolium salt to a formazan dye. MEC had little effect on cell
viability at doses of ≤100 μg/ml either in the absence or presence
of 0.5 μg/ml LPS (Fig. 1).
However, significant cytotoxicity was observed at MEC
concentrations ≥100 μg/ml. These data indicated that low doses of
MEC (100 μg/ml) do not affect the viability of RAW 264.7
macrophages. Therefore, concentrations ≤100 μg/ml were used in the
subsequent experiments.
Effects of MEC on the production of
LPS-induced iNOS and NO
To determine the anti-inflammatory effect of MEC,
the release of NO was examined by treating the RAW 264.7
macrophages with MEC in the absence or presence of LPS. Culture
supernatants were collected after 24 h incubation and the quantity
of nitrite accumulated in the culture media was estimated using
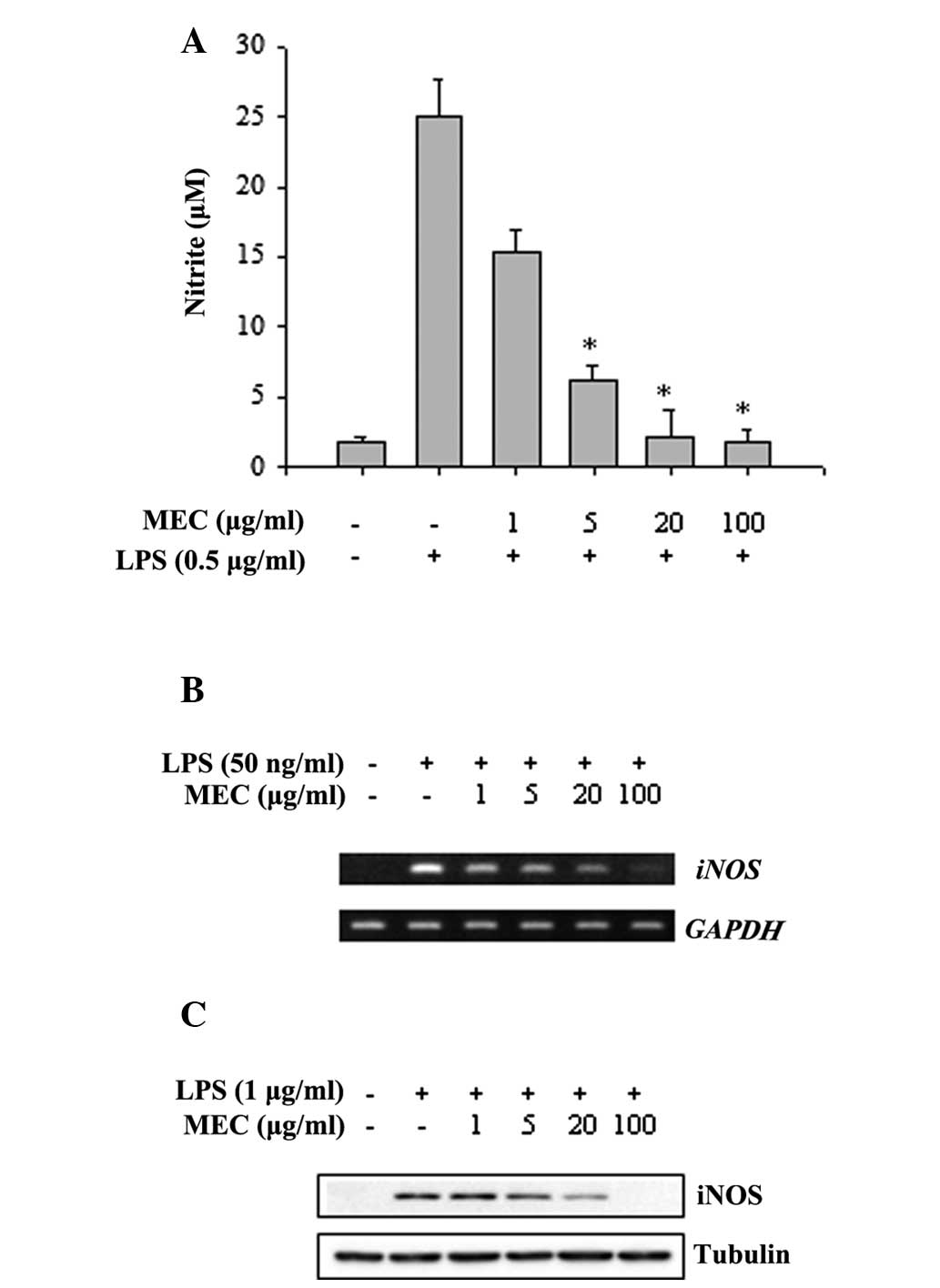
Griess reagent as an indicator of NO release. As shown in Fig. 2A, the nitrite concentration in the
media was increased markedly in the LPS-activated RAW 264.7
macrophages compared with the unstimulated cells. When the RAW
264.7 macrophages were treated with various concentrations of MEC,
the levels of LPS-stimulated nitrite production decreased
significantly in a dose-dependent manner (Fig. 2A). Since nitrite is a product of
iNOS activation, the effects of MEC on iNOS mRNA and protein
in RAW 264.7 macrophages were measured using RT-PCR and western
blot analysis, respectively. As shown in Fig. 2B, treatment of RAW 264.7
macrophages with various concentrations of MEC markedly reduced the
LPS-stimulated increase in the level of iNOS mRNA expression
in a dose-dependent manner. Furthermore, MEC treatment reduced the
LPS-induced increase of iNOS protein expression in these cells
(Fig. 2C). These results indicated
that MEC reduced NO production by inhibiting the expression of iNOS
in LPS-activated macrophages.
Differential inhibitory effect of MEC on
the production of IL-6 and TNF-α in activated macrophages
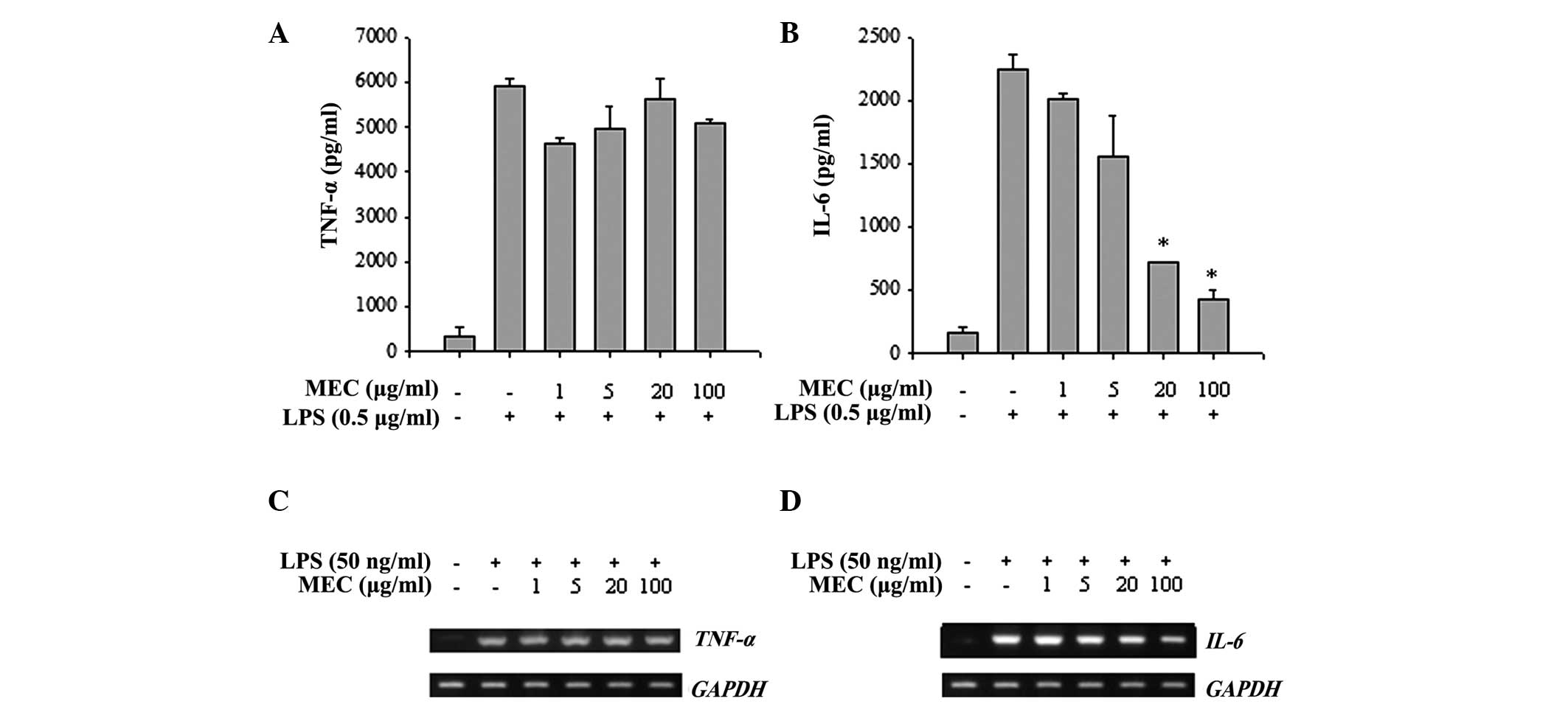
Since stimulation of macrophages with LPS
consequently induces the production of pro-inflammatory cytokines,
including TNF-α and IL-6, the anti-inflammatory effects of MEC on
cytokine production were evaluated in LPS-stimulated macrophages.
As shown in Fig. 3A and B,
LPS-stimulated RAW 264.7 macrophages produced large quantities of
TNF-α and IL-6. Notably, MEC treatment reduced the LPS-stimulated
production of IL-6 in a dose-dependent manner but did not affect
the production of TNF-α. Furthermore, the LPS-induced increase in
IL-6 mRNA expression was also markedly inhibited by MEC
treatment, whereas the mRNA level of TNF-α was not affected
(Fig. 3C and D). These results
indicated that MEC selectively regulated the production of IL-6 by
inhibiting IL-6 mRNA expression.
Selective inhibition of MAPK
phosphorylation by MEC in activated macrophages
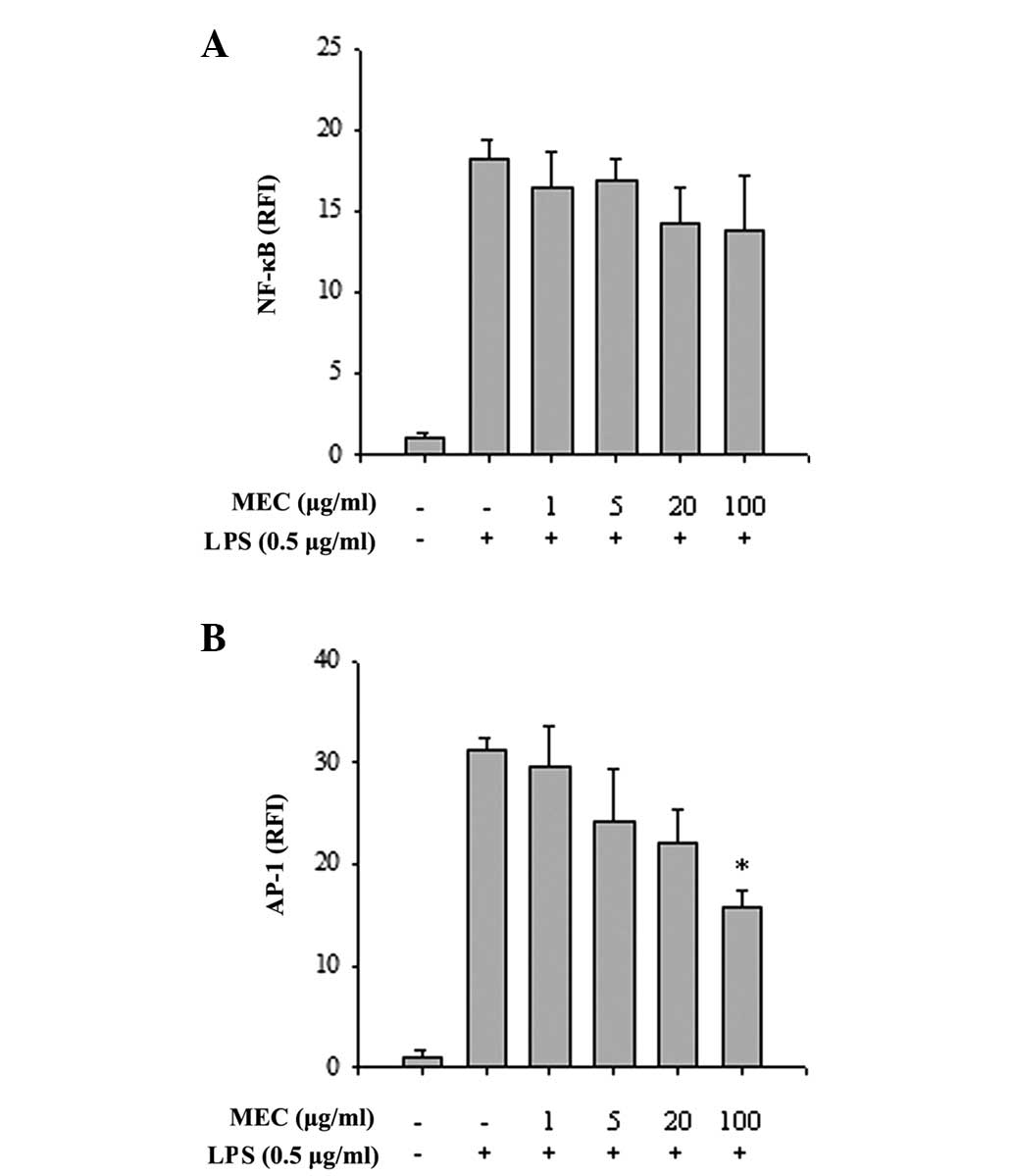
To elucidate the mechanisms underlying the
anti-inflammatory effect of MEC, the activities of the
inflammation-associated transcription factors, NF-κB and AP-1, were
measured. HEK 293 cells transiently transfected with NF-κB or AP-1
luciferase reporter constructs were treated with PMA, either in the
absence or presence of MEC and luciferase activity was determined.
MEC had no apparent effect on PMA-stimulated NF-κB activity
(Fig. 4A), however, the constructs
were markedly stimulated by PMA and the PMA-activated AP-1
transcriptional activity was inhibited by MEC in a dose-dependent
manner (Fig. 4B). To investigate
this further, the effects of MEC on the LPS-induced phosphorylation
of IκB and MAPKs in RAW 264.7 cells were analyzed using western
blot analysis. LPS treatment markedly induced the phosphorylation
of IκB and MAPKs, whereas MEC inhibited the increased
phosphorylation of JNK and p38 in a dose-dependent manner. By
contrast, the LPS-stimulated phosphorylation of IκB and ERK was not
affected by MEC treatment (Fig.
5). The results indicated that inflammatory responses activated
through MAPK signaling pathways, particularly those involving JNK
and p38, are sensitive to inhibition by MEC.
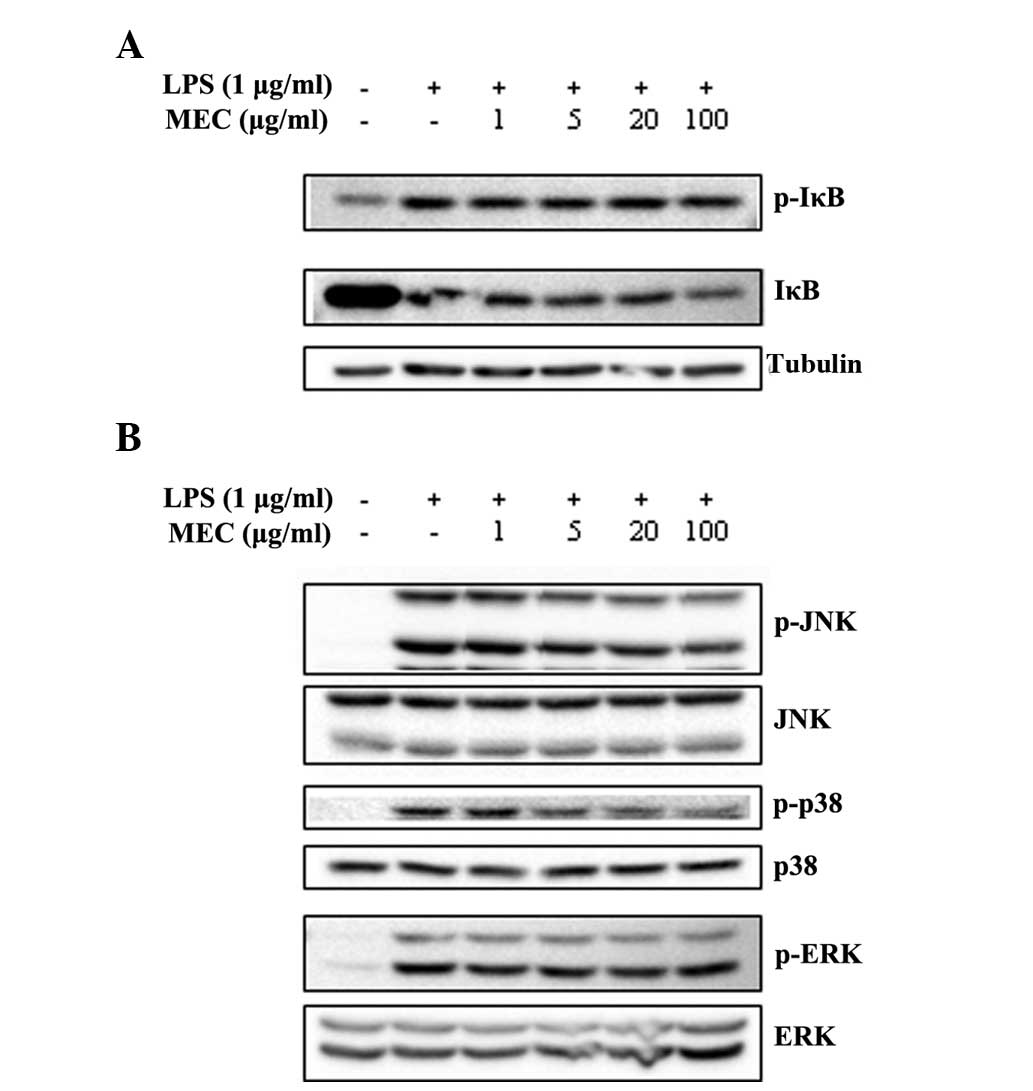 | Figure 5Inhibitory effect of MEC on the
activation of NF-κB and MAPKs. RAW 264.7 macrophages were
pretreated with various concentrations of MEC (1, 5, 20 and 100
μg/ml) for 1 h and then stimulated with LPS for 15 min. Total cell
lysates were prepared and western blot analysis was performed. The
expression level of (A) p-IκB, IκB and (B) p-p38, p38, p-ERK, ERK,
p-JNK and JNK was detected by each specific antibody and tubulin
was used as a loading control. MEC, methanol extracts from
Euphorbia cooperi; NF-κB, nuclear factor-κB; MAPK,
mitogen-activated protein kinase; LPS, lipopolysaccharide; p-,
phosphorylated; ERK, extracellular signal-regulated kinase; JNK,
c-Jun N-terminal kinase; IκB, inhibitor of κB. |
Discussion
The excessive production of iNOS by external stimuli
is recognized as an important pathophysiological consequence in
numerous inflammatory disorders (11). Aberrant expression of iNOS leads to
abnormal levels of NO production (8,19,20)
and several studies have indicated that increased expression of
iNOS is associated with carcinogenesis and severe inflammatory
diseases, including sepsis and arthritis (10,21,22).
Since iNOS/NO are central to the inflammatory process, a number of
studies have attempted to identify novel anti-inflammatory agents
that inhibit the expression of iNOS and to elucidate the underlying
mechanism. The present study demonstrated that MEC inhibited the
production of NO in RAW 264.7 macrophages by inhibiting the
expression of iNOS in a non-cytotoxic manner. These results
suggested that MEC contains anti-inflammatory phytochemicals.
Pro-inflammatory cytokines are key mediators of
apoptosis and innate immune reactions. Excessive levels of
pro-inflammatory cytokines can induce tissue injury and potentiate
septic shock (23,24). Therefore, agents that inhibit the
production and action of pro-inflammatory cytokines may inhibit the
progression of inflammatory diseases. The pro-inflammatory
cytokines, TNF-α and IL-6, are major pathogenic factors for a
number of inflammatory diseases, including rheumatoid arthritis,
and anti-IL-6 receptor antibody is currently used as a therapeutic
agent in the clinical treatment of this disease (24–26).
The present study demonstrated that MEC significantly inhibited the
production of IL-6, but not TNF-α, in LPS-stimulated RAW 264.7
macrophages. Several studies have demonstrated that these cytokines
are not simultaneously inhibited by natural compounds (27,28).
One possibility for the differential regulation of IL-6 and TNF-α
by MEC is that IL-6 and TNF-α possess a different promoter binding
region for transcription factors. Previous studies have revealed
that the signal transducer and activator of transcription (STAT)
protein binding region is contained in the IL-6 promoter region,
but not in the TNF-α promoter (29), and MEC may be a regulator of STAT
signaling.
Several intracellular signaling pathways are
associated with the increased expression of iNOS and
pro-inflammatory cytokines (2,20,30).
In particular, MAPKs, including p38, ERK and JNK are important in
the production of various inflammatory mediators (7,30).
LPS treatment of murine macrophages significantly enhances the
production of inflammatory mediators via MAPK phosphorylation and
stimulation of the downstream signaling pathway (2,5,6).
These studies imply that inhibition of p38, ERK and JNK
phosphorylation may be a potential target pathway for the
alleviation of severe inflammatory states. However, several studies
have suggested that MAPK signaling cascades may be differently
involved in the response of anti-inflammatory compounds in
macrophages (31–33). In particular, a study by Watters
et al demonstrated that the MEK/ERK pathway is not essential
for the production of iNOS and IL-1β in macrophages (34). In the present study, MEC inhibited
the LPS-induced phosphorylation of p38 and JNK, but not ERK, in a
dose-dependent manner. However, the total MAPK levels were
unchanged. Collectively, these results suggested that the activity
of MAPKs, including p38 and JNK, rather than the expression of
MAPKs, is the key regulatory mechanism underlying the MEC-mediated
inhibition of inflammatory mediators.
NF-κB is the other major regulatory signaling
molecule for inflammation. Following LPS stimulation, which leads
to the phosphorylation and degradation of IκB in the cytosol, NF-κB
subunits are freely translocated into the nucleus (35,36).
The nuclear translocated NF-κB subunits, p65 and p50, regulate the
production of various inflammatory mediators, including TNF-α, IL-6
and NO (37,38). In the present study, stimulation of
macrophages by LPS led to the activation of NF-κB and MAPKs.
However, MEC had no effect on the activity of NF-κB or on the
degradation of IκB in the LPS-stimulated RAW 264.7 macrophages.
NF-κB and MAPK signaling share TLR4 adaptor molecules and accessory
molecules, including myeloid differentiation primary response 88
(MyD88), toll/IL-1 receptor domain-containing adapter-inducing
interferon-β (TRIF), tumor-necrosis factor receptor-associated
factor 6 (TRAF6)and IL receptor-associated kinase 1. However, the
downstream signaling molecules of MyD88, TRIF and TRAF6 are quite
different in the NF-κB and MAPK signaling pathways, therefore, the
present study hypothesized that MEC may selectively regulate JNK
and p38, but not the TLR4 accessory molecules.
The present study examined the regulation of
inflammatory mediators by MEC in activated macrophages. Since
macrophages are important in the pathogenesis of numerous
inflammatory diseases, the MEC-mediated selective regulation of
inflammatory mediators suggests that MEC may have therapeutic
potential against inflammatory diseases. However, further studies
are required to analyze the major components that are responsible
for the reduction of inflammatory mediators and to elucidate the
exact mechanism underlying the difference in the production of
pro-inflammatory cytokines, IL-6 and TNF-α.
Acknowledgements
This study was supported by the National Research
Foundation of Korea grant funded by the Korean government (Ministry
of Education, Science and Technology; grant nos.
NRF-2012R1A2A2A01047338 and NRF-2013R1A1A2062389).
References
|
1
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar
|
|
2
|
Schroder K, Sweet MJ and Hume DA: Signal
integration between IFNgamma and TLR signalling pathways in
macrophages. Immunobiology. 211:511–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aderem A and Underhill DM: Mechanisms of
phagocytosis in macrophages. Annu Rev Immunol. 17:593–623. 1999.
View Article : Google Scholar
|
|
4
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cario E, Rosenberg IM, Brandwein SL, Beck
PL, Reinecker HC and Podolsky DK: Lipopolysaccharide activates
distinct signaling pathways in intestinal epithelial cell lines
expressing Toll-like receptors. J Immunol. 164:966–972. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanada T and Yoshimura A: Regulation of
cytokine signaling and inflammation. Cytokine Growth Factor Rev.
13:413–421. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nathan C and Xie QW: Regulation of
biosynthesis of nitric oxide. J Biol Chem. 269:13725–13728.
1994.PubMed/NCBI
|
|
9
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clancy RM, Amin AR and Abramson SB: The
role of nitric oxide in inflammation and immunity. Arthritis Rheum.
41:1141–1151. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moncada S: Nitric oxide: discovery and
impact on clinical medicine. J R Soc Med. 92:164–169.
1999.PubMed/NCBI
|
|
12
|
Dinarello CA: Anti-inflammatory agents:
present and future. Cell. 140:935–950. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eke T, Al-Husainy S and Raynor MK: The
spectrum of ocular inflammation caused by Euphorbia plant
sap. Arch Ophthalmol. 18:13–16. 2000. View Article : Google Scholar
|
|
14
|
Lazarini CA, Uema AH, Brandão GM,
Guimarães AP and Bernardi MM: Croton zehntneri essential oil:
effects on behavioral models related to depression and anxiety.
Phytomedicine. 7:477–481. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thyagarajan SP, Subramanian S,
Thirunalasundari T, Venkateswaran PS and Blumberg BS: Effect of
Phyllanthus amarus on chronic carriers of hepatitis B virus.
Lancet. 1:764–766. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suarez AI, Compagnone RS, Salazar-Bookaman
MM, et al: Antinociceptive and anti-inflammatory effects of
Croton malambo bark aqueous extract. J
Ethnopharmacol. 88:11–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pereira U, Garcia-Le Gal C, Le Gal G, et
al: Effects of sangre de drago in an in vitro model of cutaneous
neurogenic inflammation. Exp Dermatol. 19:796–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Risco E, Ghia F, Vila R, Iglesias J,
Alvarez E and Cañigueral S: Immunomodulatory activity and chemical
characterisation of sangre de drago (dragon’s blood) from Croton
lechleri. Planta Med. 69:785–794. 2003.PubMed/NCBI
|
|
19
|
Nathan C and Xie QW: Nitric oxide
synthases: roles, tolls, and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szabo C and Thiemermann C: Regulation of
the expression of the inducible isoform of nitric oxide synthase.
Adv Pharmacol. 34:113–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bosca L, Zeini M, Traves PG and Hortelano
S: Nitric oxide and cell viability in inflammatory cells: a role
for NO in macrophage function and fate. Toxicology. 208:249–258.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kröncke KD, Fehsel K and Kolb-Bachofen V:
Inducible nitric oxide synthase in human diseases. Clin Exp
Immunol. 113:147–156. 1998.
|
|
23
|
Guadagni F, Ferroni P, Palmirotta R,
Portarena I, Formica V and Roselli M: Review. TNF/VEGF cross-talk
in chronic inflammation-related cancer initiation and progression:
an early target in anticancer therapeutic strategy. In Vivo.
21:147–161. 2007.PubMed/NCBI
|
|
24
|
Nishimoto N and Kishimoto T: Interleukin
6: from bench to bedside. Nat Clin Pract Rheumatol. 2:619–626.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kavanaugh A: Interleukin-6 inhibition and
clinical efficacy in rheumatoid arthritis treatment - data from
randomized clinical trials. Bull NYU Hosp Jt Dis. 65:S16–S20.
2007.PubMed/NCBI
|
|
26
|
Straub RH, Harle P, Yamana S, et al:
Anti-interleukin-6 receptor antibody therapy favors adrenal
androgen secretion in patients with rheumatoid arthritis: a
randomized, double-blind, placebo-controlled study. Arthritis
Rheum. 54:1778–1785. 2006. View Article : Google Scholar
|
|
27
|
Samavati L, Rastogi R, Du W, Huttemann M,
Fite A and Franchi L: STAT3 tyrosine phosphorylation is critical
for interleukin 1 beta and interleukin-6 production in response to
lipopolysaccharide and live bacteria. Mol Immunol. 46:1867–1877.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu ZG, Jin H, Yu PJ, Tian YX, Zhang JJ
and Wu SG: Mollugin inhibits the inflammatory response in
lipopolysaccharide-stimulated RAW264.7 macrophages by blocking the
janus kinase-signal transducers and activators of transcription
signaling pathway. Biol Pharm Bull. 36:399–406. 2013. View Article : Google Scholar
|
|
29
|
Lee C, Lim HK, Sakong J, Lee YS, Kim JR
and Baek SH: Janus kinase-signal transducer and activator of
transcription mediates phosphatidic acid-induced interleukin
(IL)-1beta and IL-6 production. Mol Pharmacol. 69:1041–1047.
2006.PubMed/NCBI
|
|
30
|
Stalinska K, Guzdek A, Rokicki M and Koj
A: Transcription factors as targets of the anti-inflammatory
treatment. A cell culture study with extracts from some
Mediterranean diet plants. J Physiol Pharmaco. 56:S157–S169.
2005.
|
|
31
|
Burk DR, Senechal-Willis P, Lopez LC,
Hogue BG and Daskalova SM: Suppression of
lipopolysaccharide-induced inflammatory responses in RAW 264.7
murine macrophages by aqueous extract of Clinopodium vulgare
L. (Lamiaceae). J Ethnopharmacol. 126:397–405. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Bargouti M, Zughaier S, et al:
Osteoinductive LIM mineralization protein-1 suppresses activation
of NF-kappaB and selectively regulates MAPK pathways in
pre-osteoclasts. Bone. 46:1328–1335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shan J, Fu J, Zhao Z, et al: Chlorogenic
acid inhibits lipopolysaccharide-induced cyclooxygenase-2
expression in RAW264.7 cells through suppressing NF-kappaB and
JNK/AP-1 activation. Int Immunopharmacol. 9:1042–1048. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Watters JJ, Sommer JA, Pfeiffer ZA, Prabhu
U, Guerra AN and Bertics PJ: A differential role for the
mitogen-activated protein kinases in lipopolysaccharide signaling:
the MEK/ERK pathway is not essential for nitric oxide and
interleukin 1beta production. J Biol Chem. 277:9077–9087. 2002.
View Article : Google Scholar
|
|
35
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000.PubMed/NCBI
|
|
36
|
Karin M and Delhase M: The I kappa B
kinase (IKK) and NF-kappa B: key elements of proinflammatory
signalling. Semin Immunol. 12:85–98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Surh YJ, Chun KS, Cha HH, et al: Molecular
mechanisms underlying chemopreventive activities of
anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS
through suppression of NF-kappa B activation. Mutat Res.
480–481:243–268. 2001.
|
|
38
|
Verma IM, Stevenson JK, Schwarz EM, Van
Antwerp D and Miyamoto S: Rel/NF-kappa B/I kappa B family: intimate
tales of association and dissociation. Genes Dev. 9:2723–2735.
1995. View Article : Google Scholar : PubMed/NCBI
|