Introduction
Periodontitis is a chronic inflammatory disease,
which is characterized by bleeding, destruction of connective
tissues and alveolar bone loss (1–3). In
addition, periodontitis is reported to be implicated in the onset
of a variety of diseases, including diabetes, rheumatoid arthritis
and cardiovascular disease (4).
The occurrence of periodontitis is ascribed to multiple factors. Of
these, with the exception of bacterial infection and host
contributing factors, the role of oxidative stress in the
development and pathogenesis of periodontitis has been elucidated
(5). Therefore, detailed
investigation of the association between oxidative stress and
periodontitis is of crucial importance.
Reactive oxygen species (ROS), which markedly
contribute to cellular oxidative stress, are important signaling
mediators in several biological processes (6). Living organisms have adapted to the
efflux of ROS, in which antioxidants, including vitamin C (Vc) are
important in counteracting the oxidative effects (7). These reactive species are generated
from molecular oxygen and, if they are not cleared by the
antioxidative system, these highly reactive species induce
significant damage in the cellular environment (2). An imbalance between the upregulation
of pro-oxidants and antioxidants can lead to severe impairment of
critical cellular structures, membrane dysfunction and cell death
by necrosis or apoptosis (8).
Subtle alterations in the intracellular redox state initiates
cellular events associated with altered gene expression and may
eventually lead to destruction of or damage to the tooth supporting
tissues secondary to the induction of proinflammatory
processes.
The periodontal ligament (PDL) is an assembly of
specialized tooth supporting tissue fibers that attach root cement
to alveolar bone (9). The PDL is
composed of diverse cell subpopulations, including fibroblasts and
osteoblasts, with PDL cells (PDLCs) constituting the majority.
PDLCs have fibroblast-like features and are able to produce
collagen whilst simultaneously retaining osteoblastic features
(10). The secreted collagens
build up the PDL to ensure the attachment of root cement to
alveolar bone and tissue recovery following injury. Human PDLCs
also produce cytokines and chemokines in conditions of stress,
which are characteristic of leucocytes and macrophages (9). In addition, the abnormal reduction or
structural destruction of PDLCs increase the likelihood of
pathogenesis in the periodontal tissues implying that PDLCs may be
important in initiating periodontal inflammation.
Given the proinflammatory status of periodontal
tissues, ROS can also be linked to the pathogenesis of
periodontitis. Hydrogen peroxide (H2O2) is a
type of ROS, which is generated by almost all types of oxidative
stress and infiltrates cells through their membrane.
H2O2 has a broad spectrum of biological
effects, while the cellular responses to H2O2
may differ in a cell type and concentration-specific manner
(11,12). H2O2 may
induce growth inhibitory effects, whereas in a similar setting it
may also promote proliferation in other cell types (13). In addition a dual association
between apoptosis and the concentration of
H2O2 has been demonstrated, which further
complicates the exact functionality of H2O2
exposure to cellular events (14).
Materials and methods
Cell culture
The study was approved by the Ethics Committee of
the Institute and Hospital of Stomatology, Nanjing University
Medical School (Nanjing, China). Primary human PDLCs were obtained
from premolars, which were extracted from healthy patients aged
between 10 and 18 years with no evidence of gingivitis,
periodontitis or caries. All patients provided informed consent
prior to sample collection. In brief, the human PDL tissues were
obtained from the central tooth root surface using a surgical
scalpel. The obtained tissues were then cultured in Dulbecco’s
modified Eagle’s medium supplemented with 10% fetal bovine serum
and 1% penicillin-streptomycin solution (50 g/ml streptomycin and
5,000 U/ml penicillin; Sigma Aldrich, St. Louis, MO, USA) at 37°C
and 5% CO2 in a humidified atmosphere. The medium was
replaced every 2–3 days. When the cultured PDLCs reached
confluence, they were trypsinized and divided at a ratio of 1:2
with 0.25% trypsin solution (Sigma Aldrich). Cells at passages 4–5
were used in the present study.
MTT assay
The PDLCs were seeded into a 96-well-plate overnight
at 37°C and were then washed with phosphate-buffered saline (PBS;
Kangchen Biotech, Shanghai, China). The cells were treated with
different concentrations of H2O2 (600, 800 or
1,000 μM) for the indicated time periods between 0 and 48 h. MTT
solution (20 μl; 5 mg/ml; Kangchen Biotech) was then added to each
well. After 4 h incubation at 37°C, the media was removed and 100
μl dimethyl sulfoxide (Kangchen Biotech) was added to each well in
the plate to dissolve the purple formazan crystals. The plate was
then agitated for 10 min for solubilization and the
spectrophotometric absorbance at 540 nm was calculated using a
Multilabel Counter (Safire; Tecan Austria GmbH, Grödig,
Austria).
Flow cytometry
The cells were collected at specific time points (3
or 6 h) following H2O2 treatment, washed in
PBS and fixed using 75% ethanol overnight at −20°C. The fixed PDLCs
were then washed with cold PBS and stained using 5 μl annexin
V-fluorescein isothiocyanate (Kangchen Biotech) and propidium
iodide (PI) solutions (0.1% Triton X-100, 100 mg/ml PI and 0.01
mg/ml RNase; Kangchen Biotech) for 30 min at 4°C in the dark. The
successfully stained PDLCs were further analyzed using a FACScan
laser flow cytometer (Becton-Dickinson, Franklin Lakes, NJ,
USA).
Western blot analysis
The PDLCs were incubated following treatment with
various concentrations of H2O2 and Vc for
either 3 or 6 h. Following incubation, the PDLCs were harvested and
lysed. The proteins were then quantified using a bicinchoninic acid
protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA)
according to the manufacturer’s instructions. The proteins were
electrophoresed on a 12% SDS-PAGE gel for western blot analysis,
followed by immunoblotting on a polyvinylidene difluoride membrane
(Amersham Biosciences, Pittsburgh, PA, USA). Caspase-3 antibodies
were purchased from Bioworld Technology, Inc. (St. Louis Park, MN,
USA; cat no. BS1518). Mouse monoclonal antibodies for caspase-9 and
PARP were obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA; cat nos. 9508 and 9532, respectively). The proteins were
incubated with the antibodies overnight at 4°C and subsequently
with peroxidase-labeled anti-rabbit or anti-mouse antibodies
(Kangchen Biotech) for 1 h at room temperature. Quantification of
the blots was performed using a chemiluminescent method kit from
Sino-American Biotechnology (Henan, China).
Statistical analysis
Statistical comparisons were performed using Welch’s
t-test or analysis of variance followed by Dunnett’s test.
P<0.05, P<0.01 and P<0.001 were considered to indicate
statistically significant differences.
Results
Identifying the optimal
H2O2 concentration for decreased PDLC
viability
ROS have been suggested to induce marked destruction
in periodontal tissues. To identify whether
H2O2 exerts a toxic effect on PDLCs and to
determine which concentration of H2O2 causes
a significant decrease in PDLC viability, the PDLCs were treated
with different concentrations of H2O2 for the
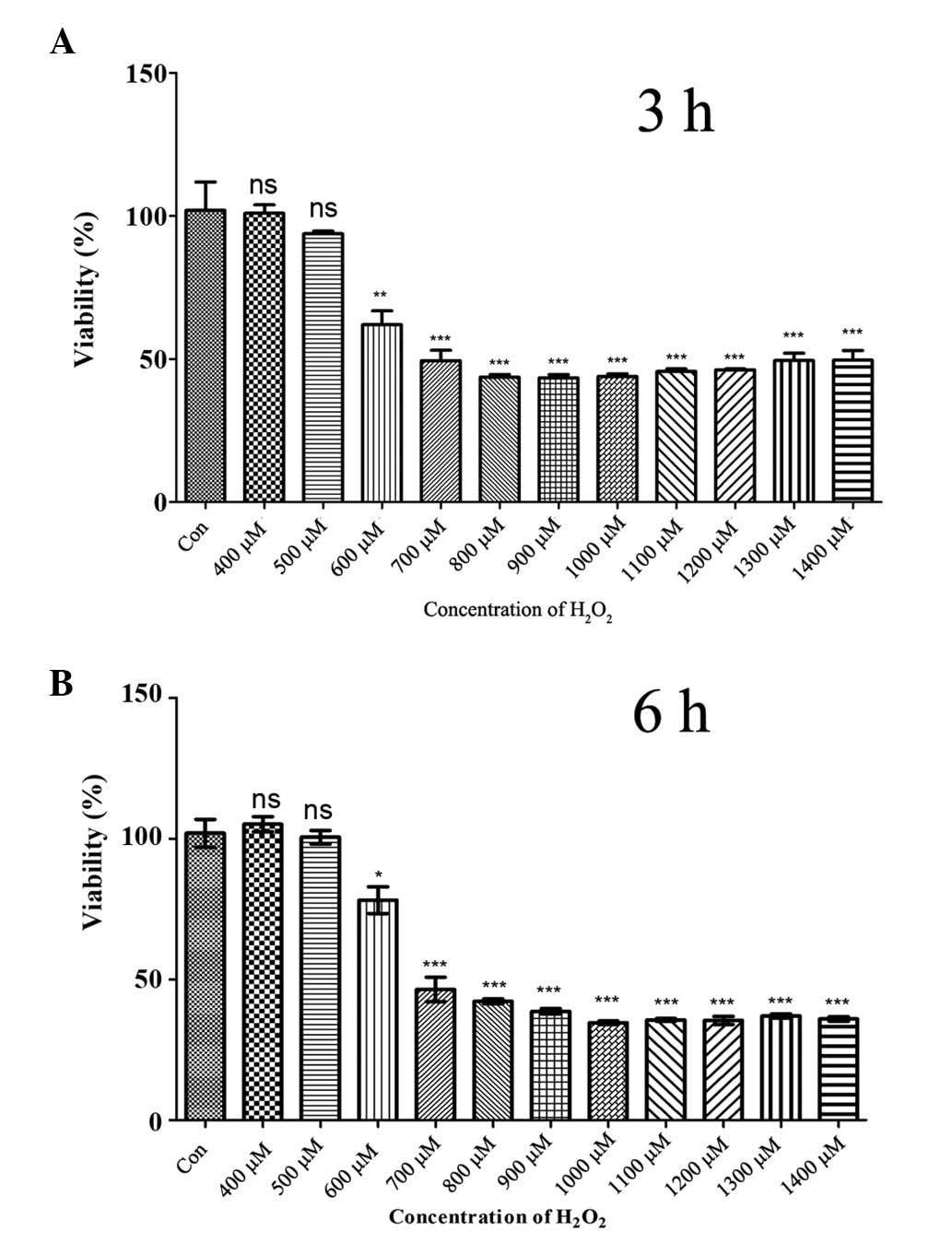
indicated time periods (Fig. 1).
The results demonstrated a significant decrease in PDLC viability 3
h post-H2O2 treatment at concentrations
>600 μM (Fig. 1A). At 600 μM
H2O2, ~50% of the PDLCs were lost and the
toxic effects of H2O2 were relatively stable
at higher levels of H2O2 (Fig. 1A). Extending the treatment time to
6 h led to similar results (Fig.
1B) demonstrating that >600 μM H2O2
caused PDLC death within at least 6 h. Therefore,
H2O2 concentrations of 600, 800 and 1,000 μM
were selected for further investigation.
High levels of H2O2
result in morphological alterations and apoptosis in PDLCs
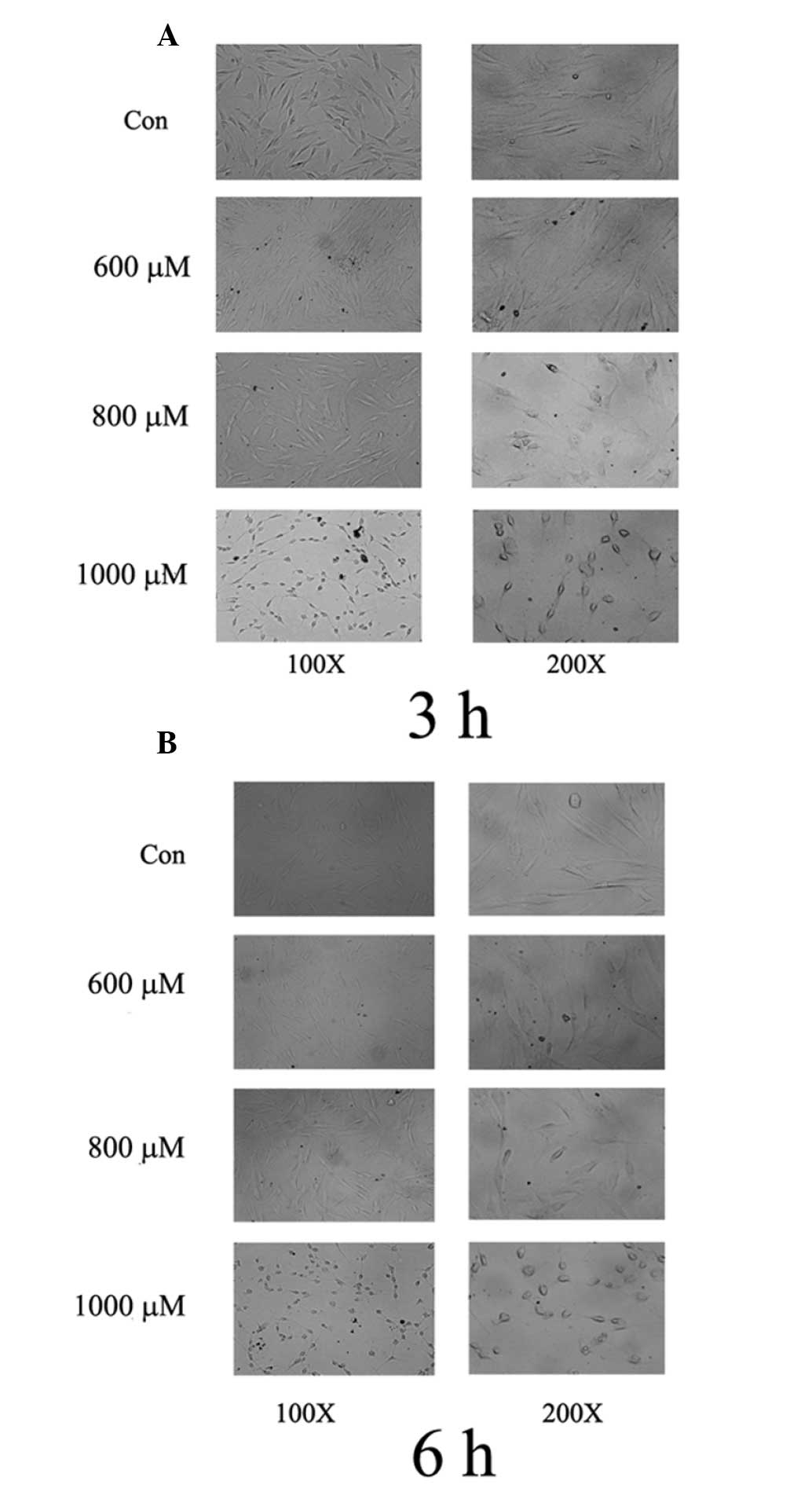
To evaluate the cytotoxic effect of
H2O2 on cell morphology, the PDLCs were
treated with different concentrations of H2O2
(600–1,000 μM) for 3 or 6 h. Subsequently, morphological
alterations in the PDLCs were monitored. The untreated cells
remained intact (Fig. 2A),
however, when the PDLCs were treated with high levels of
H2O2 (800 and 1,000 μM), the basic structure
of the cells was lost, particularly at 1,000 μM
H2O2 (Fig.
2A). The cytotoxic effects on morphology were more evident 6 h
post-stimulation (Fig. 2B) and the
proportion of cells exhibiting shrinkage and loss of intact
structure generally increased with increased
H2O2 levels (Fig. 2A and B). Taken together, these
results suggested that H2O2 induced PDLC
death through apoptosis in a dose- and time-dependent manner.
Optimal concentrations of Vc antagonizes
H2O2-induced cell death
Several studies have suggested that the high degree
of free radicals generated by bacterial stimulation function as an
integrated part of host defense to infection and result in marked
oxidative damage of periodontal tissues (2,3,11).
The damage mediated by free radicals can be mitigated by the
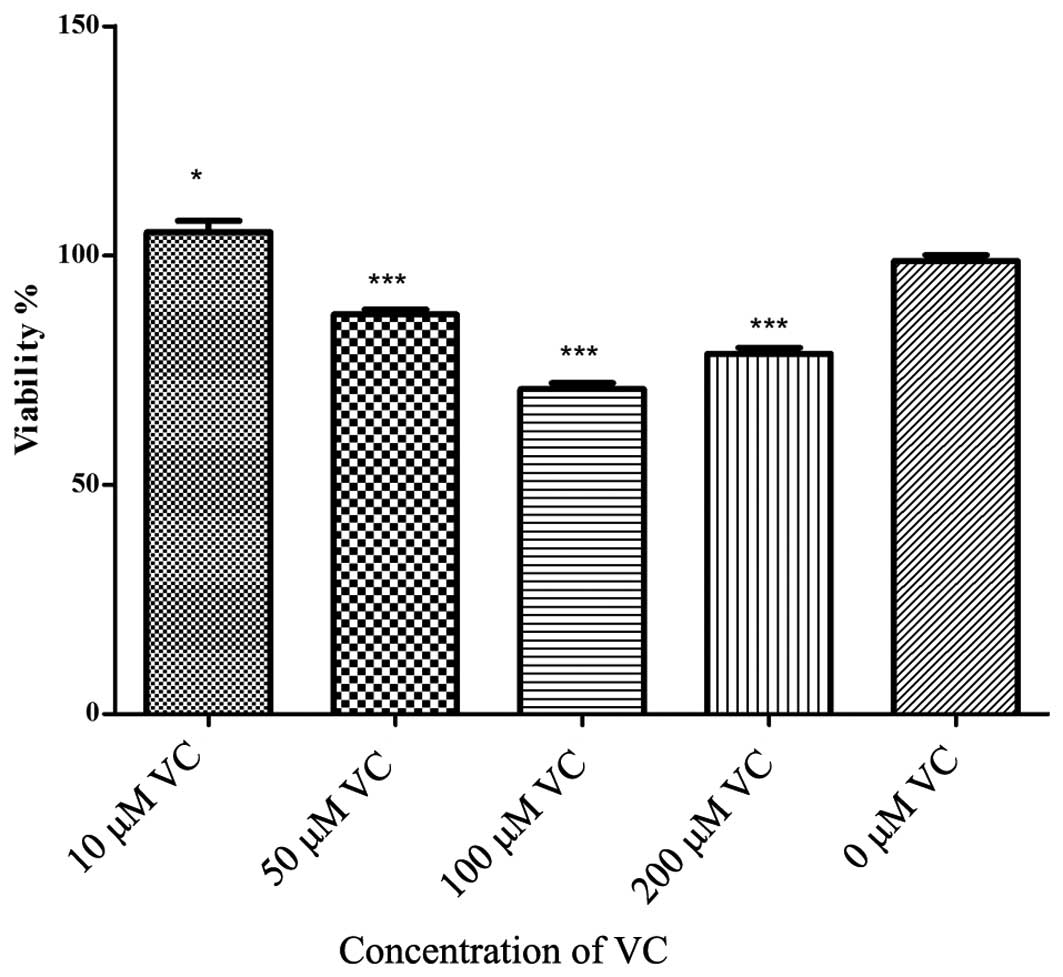
antioxidant defense system. To gain further insights into the
potential protective effect of antioxidants, PDLCs were treated
with different concentrations of Vc and the viability of cells was
evaluated (Fig. 3). Relatively
high levels of Vc (50–200 μM) were found to be cytotoxic, even
without additional H2O2 treatment, with the
exception of 10 μM Vc (Fig. 3).
These results suggested that Vc exerted pro-survival effects on
H2O2-treated cells at specific concentrations
only.
Optimal Vc antagonizes the adverse effect
of H2O2
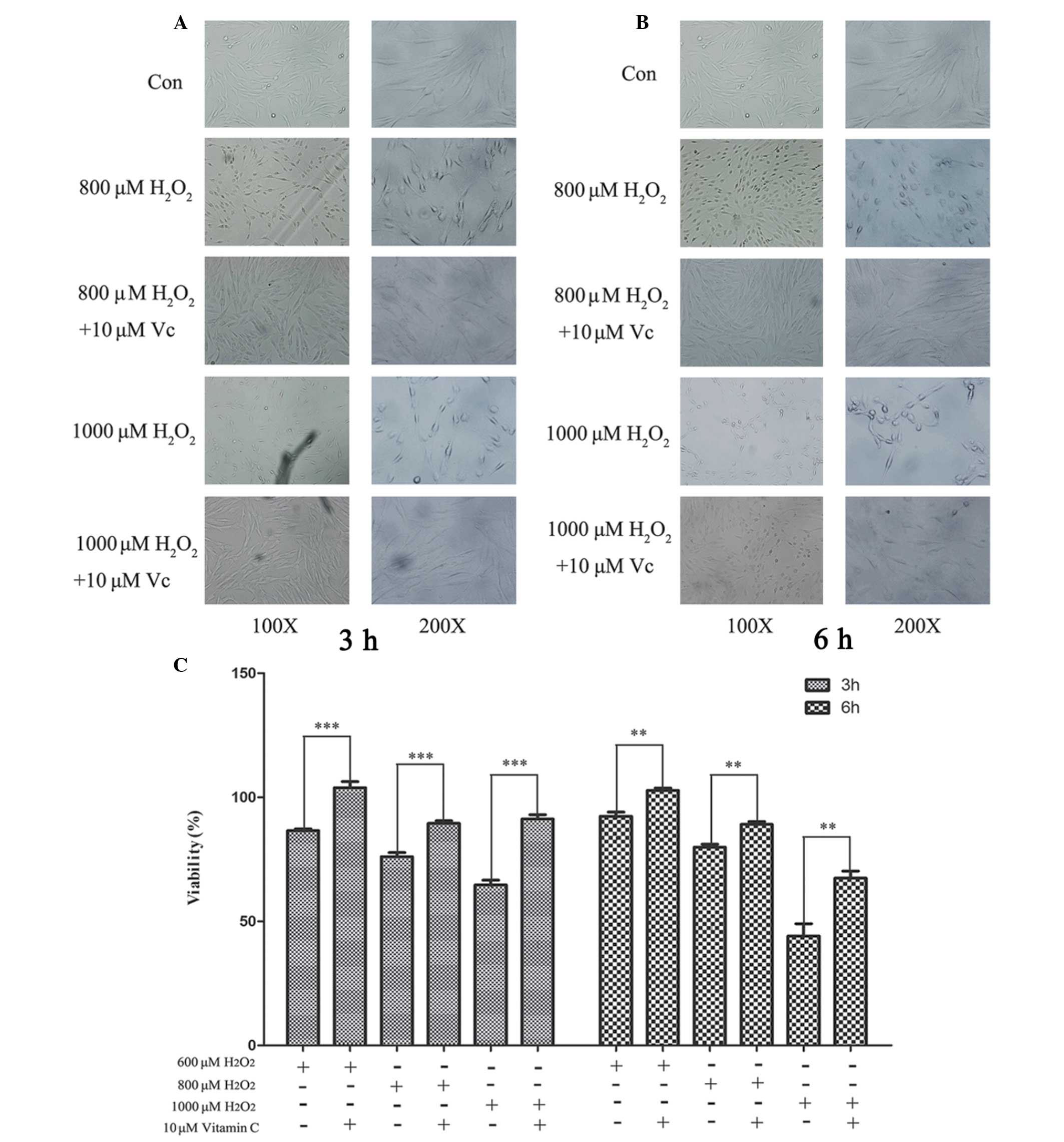
To understand the role of Vc, PDLCs were treated
with different concentrations of Vc together with 800 or 1,000 μM
H2O2. The viability of the PDLCs was
monitored at 3 and 6 h. PDLCs did not retain their intact structure
when treated with H2O2 for 3 or 6 h (Fig. 4A and B). However, Vc administration
partially reversed the adverse effect of H2O2
treatment and this effect was more marked when the cells were
treated with 1,000 μM H2O2 (Fig. 4A and B). The antioxidative effect
of Vc was also evaluated using an MTT assay. The results
demonstrated a clear cytoprotective effect of Vc even at <600 μM
H2O2 for 3 h. (Fig. 4C). This antioxidative effect was
even more clear when the PDLCs were treated with 1,000 μM
H2O2 (Fig.
4C). The viability of challenged PDLCs was increased at all
H2O2 concentrations 3 and 6 h post
stimulation (Fig. 4C). These
results suggested that optimal Vc administration increased the
viability of PDLCs under oxidative stress and exerted a
cytoprotective effect.
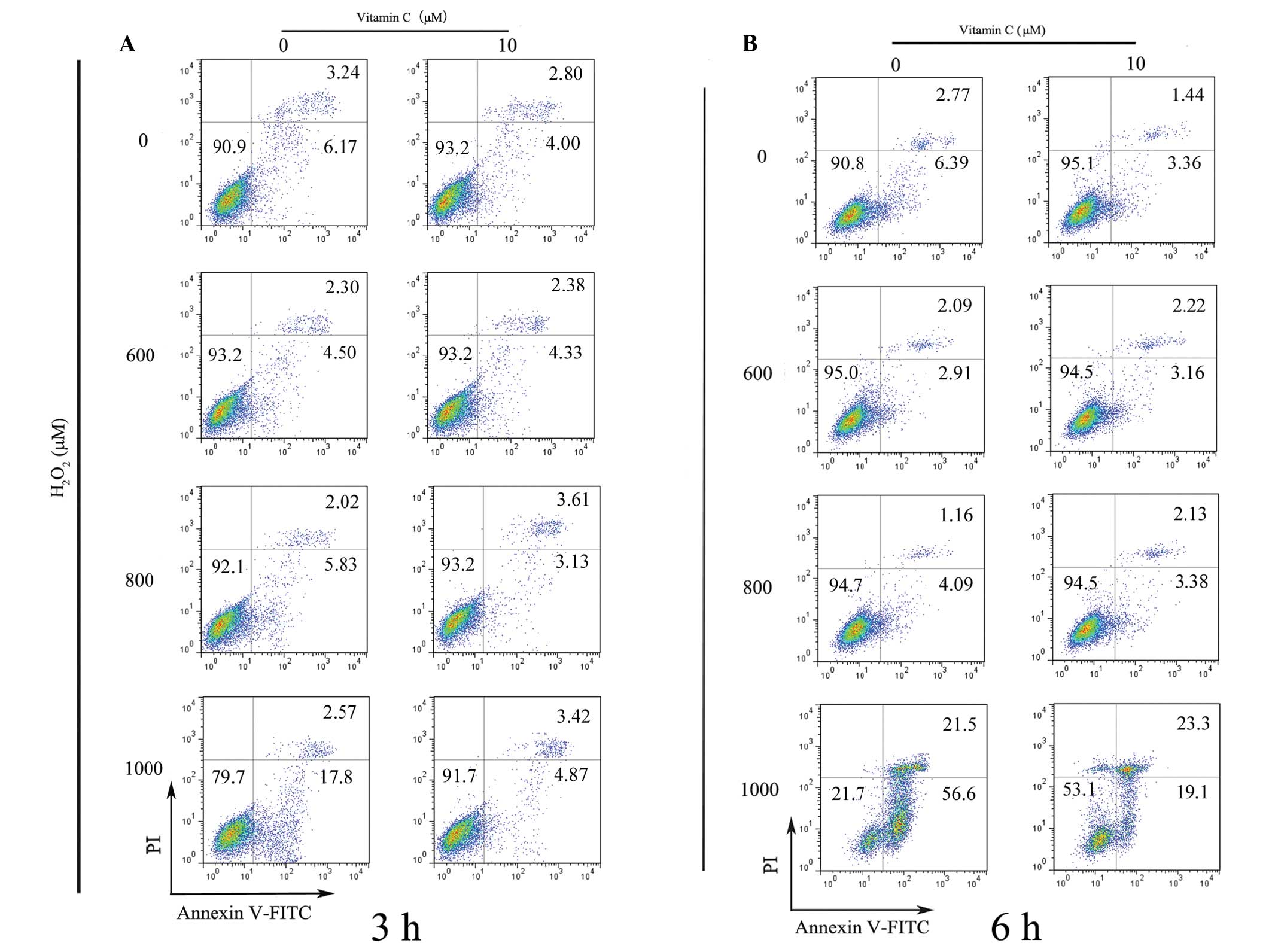
Optimal Vc rescues PDLCs under oxidative
stress by decreasing apoptosis
The results of the present study demonstrated that
treatment of PDLCs with >10μM Vc consistently exhibited a robust
cytoprotective effect. Subsequent investigation was performed using
flow cytometry to determine whether Vc had a potential
anti-apoptotic function. The results demonstrated that 1,000 μM
H2O2 was capable of initiating apoptosis in
the PDLCs, while Vc protected the PDLCs from
H2O2 exposure at 3 h post stimulation
(Fig. 5A). In the cells treated
with lower concentrations of H2O2 (600 and
800 μM), apoptosis was not attenuated by the addition of Vc at 3 h,
possibly due to the limited time scale (Fig. 5A). Similar results were observed
when the apoptotic effects were evaluated at 6 h (Fig. 5B). The apoptosis observed following
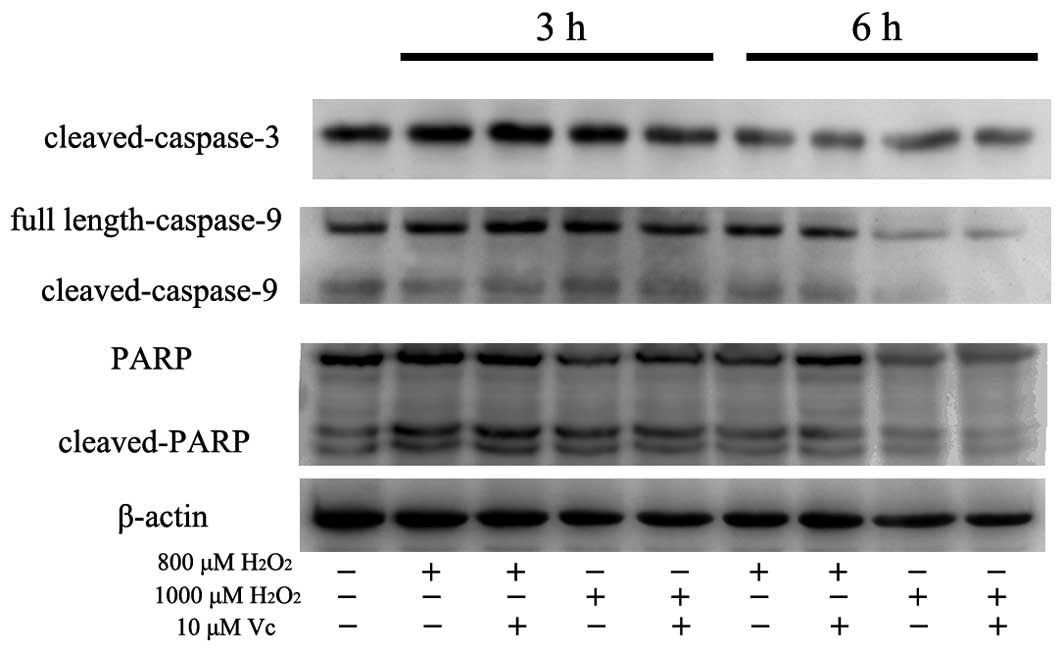
H2O2 exposure at 800 and 1,000 μM was also
verified by the occurrence of cleaved caspase-3, caspase-9 and PARP
using western blot analysis (Fig.
6). However, following the addition of 10 μM Vc, the level of
cleaved caspases-3, caspases-9 and PARP all decreased implying
impairment of the apoptotic response (Fig. 6). These results suggested that
optimal Vc administration antagonized the oxidative effect of
H2O2 and effectively improved the survival of
PDLCs at high levels of H2O2, even at earlier
time points.
Discussion
Oxidative stress is central to periodontal tissue
damage and periodontitis, either due to excess ROS
production/activity, antioxidant deficiency or indirectly as a
result of activating specific transcription factors that lead to a
pro-inflammatory state. A significant association between abnormal
oxidative status and periodontal diseases has been demonstrated
implying that oxidative stress may be a determinant in the
progression of periodontitis (13). Therefore, detailed investigation of
the oxidative status in periodontal tissues may provide crucial
insights into the pathological and pharmaceutical intervention of
periodontitis. In the present study, the role of
H2O2 was examined and
H2O2 was found to induce the apoptosis of
PDLCs in a dose- and time-dependent manner (Figs. 2 and 3). Furthermore, Vc supplementation
partially reversed the adverse effect of H2O2
(Figs. 4, 5 and 6),
however, the addition of Vc did not always improve survival of the
PDLCs, supporting the existence of an optimal level of Vc to
decrease PDLC death (Fig. 3).
Several studies have associated oxidative stress
with the pathogenesis of periodontitis (15–17).
For example, high levels of ROS in the gingivae and saliva are
associated with the progression of periodontal diseases (14). In addition, excess ROS and a marked
reduction in antioxidant levels in gingival crevicular fluid are
responsible for chronic periodontitis (14,16).
The local assembly of pro-inflammatory lymphocytes and the release
of ROS are major triggers of host defense for those suffering from
periodontitis (18). Evidence from
animal studies has also demonstrated a significant association
between higher levels of ROS and periodontitis (14). Despite these findings, to the best
of our knowledge, no studies have established a role of ROS levels
in PDLCs or investigated the mechanisms by which ROS may affect the
viability of PDLCs. The present study demonstrated that
H2O2 may decrease the viability of PDLCs by
inducing programed cell death in a dose- and time-dependent manner.
This apoptosis was verified using flow cytometric analysis and the
occurrence of cleaved caspase-3, caspase-9 and PARP (Fig. 6). These observations suggest that
at least the mitochondrial apoptotic pathway may be involved in
eliminating PDLCs exposed to H2O2. This
mechanistic investigation provides insights into how increased ROS
levels may lead to periodontal tissue destruction and supports the
hypothesis that ROS excess further exacerbates periodontal tissue
damage by inducing the apoptosis of PDLCs. These findings also
demonstrated the importance of ROS control in periodontitis.
Therefore, administration of antioxidants may be favorable in the
treatment of periodontitis, which prompted the present study to
investigate the pharmacological effects of Vc exposure.
Few studies, to the best of our knowledge, have
examined the role of the antioxidative effect of Vc in PDLCs.
Therefore, the current study investigated how oxidative status
affected the viability of PDLCs and whether stressed cells can be
recovered by administration of the antioxidant Vc. Notably, Vc was
found to rescue H2O2-challenged PDLCs in a
dose-dependent manner. However, the viability of
H2O2-challenged PDLCs did not monotonically
increase with increasing Vc concentration. These results suggested
that there may be an optimal Vc concentration for the maximal
survival of PDLCs in conditions of H2O2
stress. Vc is able to either promote or inhibit apoptosis in a dose
and cell type-specific manner (19–24).
For example, Vc exacerbated rather than inhibited
H2O2-induced apoptosis in epithelial and
mesenchymal cells(19).
Additionally, Vc alone has also been observed to exhibit cell
type-specific effects in the regulation of apoptosis (19) and has a cytotoxic effect on the
human gastric cancer cell line AGS, possibly by downregulating
14–3–3σ proteins (20). In human
breast cancer cells, Vc acts through apoptosis inducing factor to
initiate apoptosis (24).
Therefore, the interpretation of Vc-induced cytotoxic effects may
be complex and may depend on cell milieus, dose used and the
availability of other cofactors. A dose-dependent effect of Vc
addition has been demonstrated in L6 muscle cells, where micromolar
quantities of Vc increased apoptosis but millimolar doses were
notably protective (25).
Therefore, the clinical use of antioxidants, particularly Vc,
requires cautious administration, as the addition of Vc may be
cytotoxic under certain conditions. In the present study, it was
clear that optimal use of Vc lowered the risk of cellular apoptosis
at as early as 3 h post stimulation (Figs. 4 and 5) and this observation provides a deeper
understanding of clinical intervention for the treatment of
periodontitis.
Several experimental limitations in the present
study must be noted. Although the mitochondrial apoptotic pathway
was found to be involved in inducing the apoptosis of PDLCs, the
importance of other apoptotic pathways requires further
investigation. In addition, whether PDLCs are subject to other
programs of cell death may be examined in future studies. The
mechanism underlying the decreased survival of PDLCs by excess Vc
also requires investigation. Although the present study did not
enable the inference of a causal association between
H2O2 exposure and the development of
periodontitis, the importance of optimal Vc administration was
evident, even without prior knowledge of the exact association.
In conclusion, the present study demonstrated that
H2O2 disrupted the morphology and decreased
the viability of PDLCs via apoptotic pathways. In addition, the
optimal dose of Vc in treating periodontitis was revealed to be
important, as excess Vc usage may otherwise induce cell death and
exacerbate the progression of periodontitis. These results also
clarified evidence that ROS exert toxic effects on PDLCs. These
findings may broaden current knowledge of the pathogenesis of
periodontitis and may have significant implications for therapeutic
intervention.
Acknowledgements
This study was supported by the Key Project
Supported by the Medical Science and Technology Development
Foundation, Nanjing Department of Health (grant no. ZKX11003) and
the Natural Scientific Foundation of China (grant no.
81271155).
References
|
1
|
Fialkow L, Wang Y and Downey GP: Reactive
oxygen and nitrogen species as signaling molecules regulating
neutrophil function. Free Radic Biol Med. 42:153–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chapple IL and Matthews JB: The role of
reactive oxygen and antioxidant species in periodontal tissue
destruction. Periodontol 2000. 43:160–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Newsholme P, Rebelato E, Abdulkader F,
Krause M, Carpinelli A and Curi R: Reactive oxygen and nitrogen
species generation, antioxidant defenses, and beta-cell function: a
critical role for amino acids. J Endocrinol. 214:11–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar
|
|
5
|
Nibali L and Donos N: Periodontitis and
redox status: a review. Curr Pharm Des. 19:2687–2697. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wrzaczek M, Brosché M and Kangasjärvi J:
ROS signaling loops - production, perception, regulation. Curr Opin
Plant Biol. 16:575–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akman S, Canakci V, Kara A, Tozoglu U,
Arabaci T and Dagsuyu IM: Therapeutic effects of alpha lipoic acid
and vitamin C on alveolar bone resorption after experimental
periodontitis in rats: a biochemical, histochemical, and
stereologic study. J Periodontol. 84:666–674. 2013. View Article : Google Scholar
|
|
8
|
Ouyang L, Shi Z, Zhao S, et al: Programmed
cell death pathways in cancer: a review of apoptosis, autophagy and
programmed necrosis. Cell Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jönsson D, Nebel D, Bratthall G and
Nilsson BO: The human periodontal ligament cell: a fibroblast-like
cell acting as an immune cell. J Periodontal Res. 46:153–157.
2011.PubMed/NCBI
|
|
10
|
Somerman MJ, Young MF, Foster RA, Moehring
JM, Imm G and Sauk JJ: Characteristics of human periodontal
ligament cells in vitro. Arch Oral Biol. 35:241–247. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burgoyne JR, Oka S, Ale-Agha N and Eaton
P: Hydrogen peroxide sensing and signaling by protein kinases in
the cardiovascular system. Antioxid Redox Signal. 18:1042–1052.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Novotny GW, Lundh M, Backe MB, et al:
Transcriptional and translational regulation of cytokine signaling
in inflammatory beta-cell dysfunction and apoptosis. Arch Biochem
Biophys. 528:171–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
D’Aiuto F, Nibali L, Parkar M, Patel K,
Suvan J and Donos N: Oxidative stress, systemic inflammation, and
severe periodontitis. J Dent Res. 89:1241–1246. 2010.
|
|
14
|
Lin X, Sun T, Cai M and Shen P:
Cell-death-mode switch from necrosis to apoptosis in hydrogen
peroxide treated macrophages. Sci China Life Sci. 53:1196–1203.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsai CC, Chen HS, Chen SL, et al: Lipid
peroxidation: a possible role in the induction and progression of
chronic periodontitis. J Periodontal Res. 40:378–384. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chapple IL, Milward MR and Dietrich T: The
prevalence of inflammatory periodontitis is negatively associated
with serum antioxidant concentrations. J Nutr. 137:657–664.
2007.PubMed/NCBI
|
|
17
|
Konopka T, Król K, Kopeć W and Gerber H:
Total antioxidant status and 8-hydroxy-2′-deoxyguanosine levels in
gingival and peripheral blood of periodontitis patients. Arch
Immunol Ther Exp (Warsz). 55:417–422. 2007.
|
|
18
|
Darveau RP: Periodontitis: a polymicrobial
disruption of host homeostasis. Nat Rev Microbiol. 8:481–490. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumar D, Moore RM, Elkhwad M, Silver RJ
and Moore JJ: Vitamin C exacerbates hydrogen peroxide induced
apoptosis and concomitant PGE2 release in amnion epithelial and
mesenchymal cells, and in intact amnion. Placenta. 25:573–579.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagappan A, Park KI, Park HS, et al:
Vitamin C induces apoptosis in AGS cells by down-regulation of
14-3-3sigma via a mitochondrial dependent pathway. Food Chem.
135:1920–1928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dhar-Mascareño M, Cárcamo JM and Golde DW:
Hypoxia-reoxygenation-induced mitochondrial damage and apoptosis in
human endothelial cells are inhibited by vitamin C. Free Radic Biol
Med. 38:1311–1322. 2005.PubMed/NCBI
|
|
22
|
Gobbo MG, Ribeiro DL, Taboga SR, de
Almeida EA and Góes RM: Oxidative stress markers and apoptosis in
the prostate of diabetic rats and the influence of vitamin C
treatment. J Cell Biochem. 113:2223–2233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Crott JW and Fenech M: Effect of vitamin C
supplementation on chromosome damage, apoptosis and necrosis ex
vivo. Carcinogenesis. 20:1035–1041. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong SW, Jin DH, Hahm ES, et al: Ascorbate
(vitamin C) induces cell death through the apoptosis-inducing
factor in human breast cancer cells. Oncol Rep. 18:811–815.
2007.PubMed/NCBI
|
|
25
|
Orzechowski A, Łokociejewska M, Muras P
and Hocquette JF: Preconditioning with millimolar concentrations of
vitamin C or N-acetylcysteine protects L6 muscle cells
insulin-stimulated viability and DNA synthesis under oxidative
stress. Life Sci. 71:1793–1808. 2002. View Article : Google Scholar
|