Introduction
Atherosclerosis, one of the leading causes of
morbidity and mortality worldwide, is a chronic inflammatory
disease and a disorder of lipid metabolism (1). The accumulation of excess cholesterol
has been recognized as a crucial event in the development of
atherosclerosis (2); therefore,
preventing or reversing cholesterol accumulation may be effective
protective strategies against atherosclerosis. A growing body of
evidence suggests that high density lipoprotein (HDL) has an
important role in the removal of cholesterol from atherosclerotic
plaques and the transport of the excess cholesterol back to the
liver for its subsequent elimination as bile acids and neutral
steroids. This process is termed reverse cholesterol transport
(RCT) and is one of the major protective mechanisms against the
development of atherosclerosis (3–5).
Cholesterol efflux from macrophage-derived foam
cells is an initial and key step in RCT (6), and serves as an integrated measure of
HDL quantity and quality (7). This
cholesterol efflux involves numerous genes, including ATP-binding
cassette (ABC) A1 and G1 (8).
ABCA1 is a member of the ABC superfamily and is the defective gene
in Tangier disease. ABCA1 has been reported to have an important
role in the prevention of atherosclerosis through facilitating
cholesterol efflux from macrophages to lipid-poor apolipoproteinA-I
(apoA-I), and decreasing cholesterol accumulation in macrophages
(9). Similar to ABCA1, ABCG1 is
capable of promoting cholesterol efflux from macrophages to mature
HDL particles, but not to apoA-I (10).
Liver X receptor (LXR) α, a member of the nuclear
hormone receptor superfamily, has a crucial role in cholesterol
metabolism (11). Upon activation,
LXRα induces numerous genes, which are involved in cholesterol
efflux, absorption, transport and excretion. ABCA1 and ABCG1 have
been identified as direct targets of LXRα (12).
Fibroblast growth factor (FGF)21 is a member of the
FGF superfamily and is predominantly secreted by the liver and
adipose tissue. FGF21 has gained attention for its multiple
metabolic functions (13). FGF21
was first identified to stimulate glucose uptake in mouse 3T3-L1
adipocytes, which was observed to be additive and independent of
insulin (14). FGF21 has also been
shown to improve function and survival of pancreatic β-cells
(15) and to decrease glucagon
secretion (16), which together
enhance insulin sensitivity. In addition, FGF21 has been found to
increase energy expenditure in mice with free access to food
(17) and promote weight loss in
diabetic primates (18).
Furthermore, FGF21 exerts favorable effects on lipid metabolism in
animal models. FGF21 administration has been found to reduce the
levels of plasma triglycerides (TGs), free fatty acids (FFAs) and
cholesterol in genetically compromised diabetic and obese rodents
(19). Moreover, in diabetic
monkeys, FGF21 treatment has been shown to reduce the levels of TG
and LDL and increase those of HDL (20). The therapeutic role of recombinant
human FGF21 in type 2 diabetes and dyslipidemia is under
investigation. The disruption of glucose and lipid metabolism are
important processes in the pathogenesis of atherosclerosis, and
FGF21 has numerous benefits on glucose and lipid metabolism.
Therefore, FGF21 may represent a promising therapeutic hormone for
the treatment of atherosclerosis.
The present study investigated the effect of FGF21
on cholesterol efflux, and ABCA1 and ABCG1 mRNA and protein
expression in oxidized low-density lipoprotein (ox-LDL)-stimulated
macrophage foam cells. The mechanism by which FGF21 induces these
effects was also investigated.
Materials and methods
Materials
Recombinant Human FGF-21 was obtained from Shanghai
Jinan Co., Ltd. (Shanghai, China). Phorbol-12-myristate-13-acetate
(PMA), bovine serum albumin (BSA) and horseradish peroxidase
(HRP)-conjugated anti-rabbit secondary antibodies were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS)
was purchased from Gibco-BRL (Carlsbad, CA, USA). The total RNA
extraction reagent RNAiso Plus, a PrimeScript™ RT reagent kit and a
SYBR® Green PCR kit were purchased from Takara Bio, Inc.
(Shiga, Japan). Reagents for western blot analysis were purchased
from Beyotime Institute of Biotechnology (Shanghai, China). Ox-LDL
was obtained from Beijing Xiesheng Engineering Co., Ltd. (Beijing,
China). LXRα-short interfering (si)RNA was synthesized by Shanghai
Genechem Co., Ltd (Shanghai, China). ABCA1, ABCG1 and LXRα specific
antibodies were purchased from Wuhan Boster Biological Engineering
Co., Ltd (Wuhan, China). All other chemicals were of the highest
grade available from commercial sources.
Cell culture
Human THP-1 cells were cultured at a density of
1×106 cells/well in RPMI-1640 medium supplemented with
10% FBS and 1% penicillin/streptomycin at 37°C in a 5%
CO2 incubator. THP-1 monocytes were treated with 160
nmol/l PMA for 24 h to facilitate the differentiation of monocytes
into macrophages. Following PMA treatment, macrophages were
incubated with 80 mg/l ox-LDL in serum-free RPMI-1640 medium
containing 0.3% BSA for 24 h in order to generate foam cells.
Measurement of cellular cholesterol
efflux
Human macrophages were cultured as indicated.
Macrophages were then labeled with 0.3 μCi/ml
[3H]cholesterol in serum-free RPMI-1640 medium
containing 0.2% BSA for 24 h. After 24 h, cells were washed with
phosphate-buffered saline (PBS) and incubated in serum-free
RPMI-1640 medium containing 0.2% BSA in the absence or presence of
10, 50 or 100 ng/ml FGF21 for 24 h. Cells were then washed with PBS
and incubated in RPMI-1640 medium containing 0.2% BSA with 10 μg/ml
apoA-I or 50 μg/ml HDL for 24 h. The efflux medium was obtained at
the doses designated and centrifuged at 500 × g for 3 min. to
remove any floating cells. Cell monolayers were washed twice with
PBS and cellular lipids were extracted with isopropanol. Media and
cell-associated [3H]cholesterol were then measured using
a liquid scintillation counting method (21). Cholesterol efflux was expressed as
the percentage of counts in the medium relative to the total count
in the medium and cells combined.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted using the RNAiso Plus
reagent (Takara Bio, Inc., Shiga, Japan) in accordance with the
manufacturer’s instructions. The PCR primer sequences used were as
follows: Forward: 5′-CTGGTT CTATGCCCGCT TGA-3′ and reverse:
5′-TCTGCATTCCAC CTGACAGC-3′ for ABCA1; forward: 5′-CTGCTGCCGCAT
CTCACTG-3′ and reverse: 5′-TCCCTTCTGCCTTCA TCCTTC-3′ for ABCG1;
forward: 5′-CTGTGCCTGACATTC CTCCT-3′ and reverse:
5′-CATCCTGGCTTCCTCTCTGA-3′ for LXRα; and forward:
5′-GAGTCAACGGATTTGGTCGT-3′ and reverse: 5′-TTGATTTTGGAGGGATCTCG-3′
for GAPDH. qPCR was performed using SYBR® Premix Ex Taq™
II in a Bio-Rad LightCycler with an iQ3.1 Realtime PCR system
(Bio-Rad, Hercules, CA, USA). GAPDH was used as an internal
control. Quantitative measurements were analyzed using the ΔΔCt
method.
Western blot analysis
Cells were washed twice with PBS and harvested in
lysis buffer (10 mM HEPES, pH 7.9; 10 mM KCl, 1.5 mM
MgCl2, 0.5% Nonidet P-40, 1 μg/ml leupeptin, 10 μg/ml
aprotinin and 1 mM phenylmethylsulfonyl fluoride) for western blot
analysis. Nuclei were pelleted at 5000 × g for 5 min at 4°C and the
resulting supernatant was used as the cytosolic fraction. Nuclei
were resuspended in lysis buffer, sheared for 15 sec using a
microprobe sonicator (Hangzhou Success Ultrasonic Equipment Co.,
Ltd., Hangzhou, China). and incubated on ice for 5 min. Subsequent
to centrifugation at 12,000 × g for 5 min at 4°C, the supernatant
was collected as nuclear extracts. Cellular lysates or nuclear
protein extracts (40 μg) were separated using 10% SDS-PAGE and
transferred onto Immobilon®-P membranes (Millipore,
Billerica, MA, USA). Membranes were blocked in 5% skimmed milk in
Tris-buffered saline with Tween-20 (0.1% Tween-20, pH 7.4) and
incubated with human antibodies against ABCA1, ABCG1 and LXRα.
Membranes were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit polyclonal antibody (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China).
GAPDH was used as an internal control. Proteins were visualized
using an enhanced chemiluminescence detection system (Amersham
International, Buckinghamshire, UK).
Transfection for LXRα silencing
siRNA specific to human LXRα and a nonsilencing
control siRNA were synthesized by the Biology Engineering
Corporation (Shanghai, China). Cells were transfected with each
siRNA as described previously (22,23).
Annealed oligos for shRNA expression were cloned into the
pSilencer™ 2.1-U6 vector (Biology Engineering Corporation,
Shanghai, China) linearized with BamHI and HindIII.
The oligonucleotide sequences used to construct siRNA were as
follows: forward
5′-ACTGAAGCGGCAAGAGGAGTTCAAGAGACTCCTCTTGCCGCTTCAGTTTTTTT-3′ and
reverse
5′-AATTAAAAAAAACTGAAGCGGCAAGAGGAGTCTCTTGAACTCCTCTTGCCGCTTCAGTGGCC-3′.
The products were transformed into competent Escherichia
coli DH5α cells (Beijing TransGen Biotech Co., Ltd., Beijing,
China). and cultured on Luria-Bertani plates with 100 μg/ml
ampicillin. Ampicillin-resistant colonies were selected using
restriction digestion and verified using DNA sequencing. For
transfection with siRNA, foam cells were plated in 96-well plates
at a density of 1×106 cells/well. After 24 h, cells were
transfected with LXRα siRNA in OptiMEM® with 5 μg/ml
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
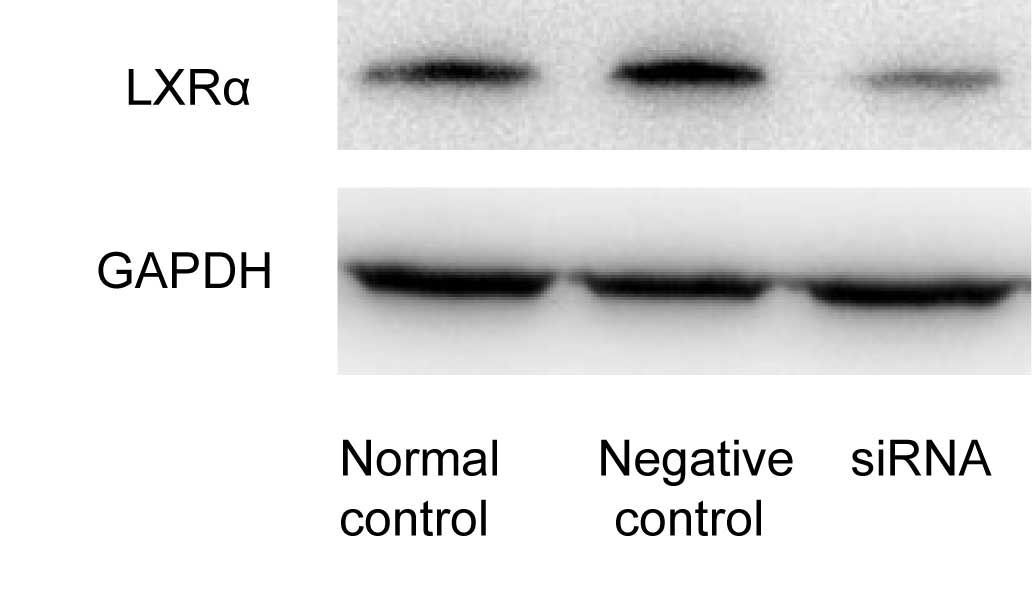
USA). After 48 h of transfection, western blot analysis revealed
that LXRα siRNA suppressed the protein expression of LXRα by 80%,
compared with the cells transfected with the control siRNA
(Fig. 1).
Statistical analysis
All experiments were performed at least three times.
Data are presented as the mean ± standard deviation. Results were
analyzed by one-way analysis of variance and Student’s t-tests,
using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
FGF21 enhances apoA-I- and HDL-mediated
cholesterol efflux from THP-1 macrophage-derived foam cells
The effect of FGF21 on cholesterol efflux in THP-1
macrophage-derived foam cells was analyzed using a liquid
scintillation counter with apoA-I or HDL as a cholesterol acceptor.
A dose course study was performed. Treatment with FGF21 at 50 and
100 ng/ml was found to enhance apoA-I-mediated cholesterol efflux
compared with the control group (P<0.05), and FGF21 at 100 ng/ml
was found to enhance HDL-mediated cholesterol efflux compared with
the control group (P<0.05).
FGF21 upregulates the expression of ABCA1
and ABCG1 in THP-1 macrophage-derived foam cells
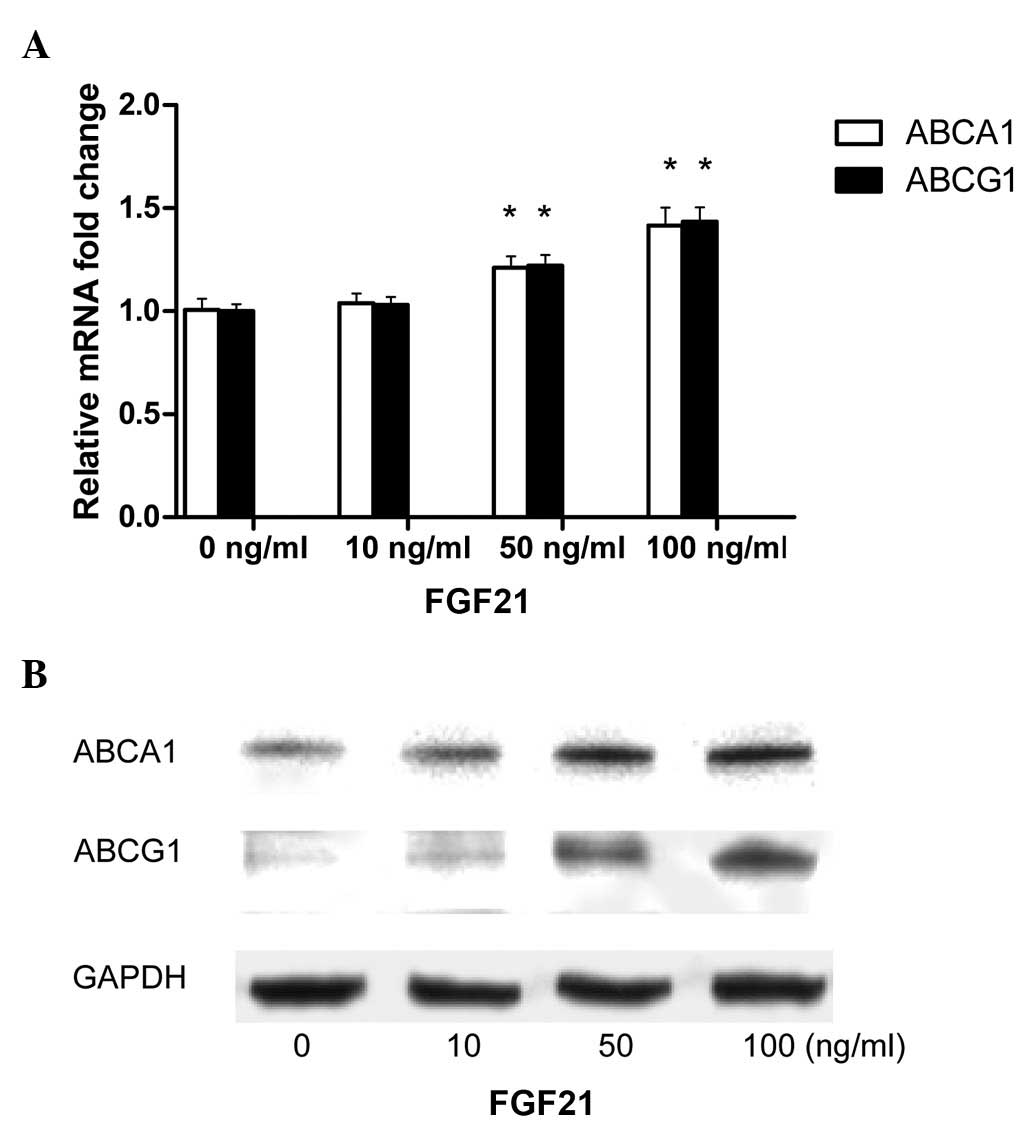
ABCA1 and ABCG1 have been identified to have a key
role in cholesterol efflux and foam cell formation in macrophages.
In order to determine whether FGF21 enhances cholesterol efflux
through modifying ABCA1 and ABCG1 expression, the expression of
ABCA1 and ABCG1 was assessed using qPCR and western blot analyses
in FGF21-treated THP-1 macrophage-derived foam cells. At 50 and 100
ng/ml, FGF21 was observed to significantly upregulate the mRNA
expression of ABCA1 and ABCG1 (Fig.
3A). Similar results were observed for ABCA1 and ABCG1 protein
expression (Fig. 3B).
LXRα mediates the FGF21-induced
upregulation of ABCA1 and ABCG1 in THP-1 macrophage-derived foam
cells
It is well established that LXRα activation induces
the expression of ABCA1 and ABCG1. The following experiments were
performed in order to examine the role of LXRα in FGF21-treated
macrophages. qPCR and western blot analyses were performed to
assess whether FGF21 alters LXRα expression. FGF21 was found to
induce an increase in LXRα expression at the mRNA (Fig. 4A) and protein (Fig. 4B) levels. LXRα expression was then
silenced using siRNA to examine the role of LXRα in the
FGF21-induced upregulation of ABCA1 and ABCG1. The upregulation of
ABCA1 and ABCG1 mRNA expression induced by FGF21 was observed to be
attenuated in the LXRα-siRNA-treated foam cells compared with those
solely treated with FGF21 (Fig.
4C). Similar results were obtained in the upregulation of ABCA1
and ABCG1 protein expression (Fig.
4D). FGF21-induced apoA-I- and HDL-mediated cholesterol efflux
was also attenuated in foam cells treated with LXRα-siRNA compared
with those solely treated with FGF21 (Fig. 4E and F). These findings suggest
that the FGF21-induced activation of LXRα may be responsible for
the effect of FGF21 on cholesterol efflux and ABCA1 and ABCG1
expression.
Discussion
To the best of our knowledge, the present study is
the first study to demonstrate that macrophages are a target of
FGF21, and preliminary data suggest that FGF21 may have a
protective effect against atherosclerosis. FGF21 was found to
enhance apoA-I- and HDL-mediated cholesterol efflux in THP-1
macrophage-derived foam cells. FGF21 was also observed to
upregulate the mRNA and protein expression of ABCA1 and ABCG1.
Furthermore, LXRα was found to be involved in facilitating these
FGF21-induced effects.
A growing body of evidence has shown that FGF21 has
numerous benefits on glucose and lipid metabolism and insulin
sensitivity (13). Therefore,
FGF21 may be beneficial for the treatment of metabolic diseases,
including non-alcoholic fatty liver disease, type 2 diabetes and
dyslipidemia, suggesting that FGF21 may have the potential to
prevent the development of atherosclerosis. Thus, the present study
aimed to investigate whether FGF21 has the capacity to reverse
cholesterol transport, a process that is particularly important in
atherosclerosis and the regression of atherosclerotic plaques.
Despite being a small fraction of the overall
cholesterol efflux mediated by the RCT pathway, the efflux of
cholesterol from macrophages has a significant inverse association
with the risk of coronary artery disease, particularly
atherosclerosis, independent of HDL cholesterol levels (6,7). In
the present study, FGF21-treated foam cells were found to exhibit a
dose-dependent increase in apoA-I- and HDL-mediated cholesterol
efflux.
Cholesterol efflux involves several important
transporters and mechanisms (6).
ABCA1 and ABCG1 are two of the most important proteins involved in
mediating cholesterol efflux from macrophages to extracellular
acceptors, including apoA-I and HDL. Therefore, ABCA1 and ABCG1
have important roles in the formation and metabolism of HDL
(24). It is well established that
extracellular apoA-I is a determinant of ABCA1-dependent
cholesterol efflux. Moreover, HDL contributes to ABCG1-dependent
cholesterol efflux (6). In the
present study, FGF21 was found to enhance apoA-I- and HDL-mediated
cholesterol efflux; thus, the effect of FGF21 on ABCA1 and ABCG1
was also investigated. In FGF21-treated cells, the increase in
apoA-I- and HDL-mediated cholesterol efflux was observed to be
consistent with an upregulation of ABCA1 and ABCG1 mRNA and protein
expression. Therefore, FGF21-mediated upregulation of ABCA1 and
ABCG1 may have anti-atherosclerotic effects, through enhancing
cholesterol efflux.
Numerous studies have revealed that LXRα has a
significant role in lipid metabolism and that increases in LXRα in
lipid-loaded macrophages upregulate ABCA1 and ABCG1 (9,12,22,23).
Therefore, the present study investigated the effect of FGF21 on
the expression of LXRα. The FGF21-induced upregulation of ABCA1 and
ABCG1 expression was concurrent with an increase in LXRα
expression. LXRα-siRNA was then used to determine the role of LXRα
in the FGF21-induced upregulation of ABCA1 and ABCG1. Knockdown of
LXRα using siRNA was found to attenuate the FGF21-mediated
upregulation of ABCA1 and ABCG1. Furthermore, the increase in
cholesterol efflux induced by FGF21 was attenuated with the
LXRα-siRNA. These results suggest that LXRα has a key role in
conferring the therapeutic benefits of FGF21 in improving lipid
metabolism. Despite the unique pathway identified in the present
study, the specific mechanism by which FGF21 affects cholesterol
efflux requires further investigation.
The inverse correlation between plasma HDL levels
and the risk of atherosclerosis has been extensively investigated
(25,26). However, a recent study suggests
that analysis of HDL function may be more beneficial than an
assessment of HDL plasma levels (27). Although HDL has numerous beneficial
functions, the protective effect of HDL against atherosclerosis is
largely attributed to its capacity to promote cholesterol efflux
from macrophage foam cells (28).
FGF21-treatment has been reported to increase HDL plasma levels in
diabetic monkeys (20). Notably,
the present study found that FGF21 promoted RCT through enhancing
cholesterol efflux, which is the most important function of HDL.
This suggests that FGF21 may have the potential to protect against
atherosclerosis.
In conclusion, the present study demonstrated that
FGF21 increases apoA-I- and HDL-mediated cholesterol efflux in
ox-LDL-stimulated macrophage foam cells, potentially through
LXRα-dependent upregulation of ABCA1 and ABCG1. This suggests a
direct association between FGF21 treatment and inhibition of
atherosclerosis. While these findings support a promising role for
FGF21 in cholesterol efflux, its regulation, function and clinical
application require further investigation in order to establish its
specific pathophysiological role in atherosclerosis.
Acknowledgements
The authors would like to thank the Chongqing Key
Laboratory of Metabolism on Lipid and Glucose for providing the
THP-1 cells, as well as Professor Qin Zhou for the advice and
technical support. This study was supported by the Department of
Cardiology, First Affiliated Hospital, Chongqing Medical
University, China.
References
|
1
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maxfield FR and Tabas I: Role of
cholesterol and lipid organization in disease. Nature. 438:612–621.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rader DJ, Alexander ET, Weibel GL,
Billheimer J and Rothblat GH: The role of reverse cholesterol
transport in animals and humans and relationship to
atherosclerosis. J Lipid Res. 50(Suppl): S189–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenson RS, Brewer HB Jr, Chapman MJ,
Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT and
Schaefer EJ: HDL measures, particle heterogeneity, proposed
nomenclature, and relation to atherosclerotic cardiovascular
events. Clin Chem. 57:392–410. 2011. View Article : Google Scholar
|
|
5
|
Lewis GF and Rader DJ: New insights into
the regulation of HDL metabolism and reverse cholesterol transport.
Circ Res. 96:1221–1232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenson RS, Brewer HB Jr, Davidson WS,
Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips
MC, Rader DJ, et al: Cholesterol efflux and atheroprotection:
advancing the concept of reverse cholesterol transport.
Circulation. 125:1905–1919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khera AV, Cuchel M, de la Llera-Moya M,
Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage
ML, Wilensky RL, et al: Cholesterol efflux capacity, high-density
lipoprotein function, and atherosclerosis. N Engl J Med.
364:127–135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu XH, Fu YC, Zhang DW, Yin K and Tang CK:
Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu YW, Wang Q, Ma X, Li XX, Liu XH, Xiao
J, Liao DF, Xiang J and Tang CK: TGF-beta1 up-regulates expression
of ABCA1, ABCG1 and SR-BI through liver X receptor alpha signaling
pathway in THP-1 macrophage-derived foam cells. J Atheroscler
Thromb. 17:493–502. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ni ZL, Zhao SP and Wu Z: ABCG1 - a
potential therapeutic target for atherosclerosis. Med Hypotheses.
69:214–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao C and Dahlman-Wright K: Liver X
receptor in cholesterol metabolism. J Endocrinol. 204:233–240.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang SL, Chen WJ, Yin K, Zhao GJ, Mo ZC,
Lv YC, Ouyang XP, Yu XH, Kuang HJ, Jiang ZS, et al: PAPP-A
negatively regulates ABCA1, ABCG1 and SR-B1 expression by
inhibiting LXRα through the IGF-I-mediated signaling pathway.
Atherosclerosis. 222:344–354. 2012.PubMed/NCBI
|
|
13
|
Woo YC, Xu A, Wang Y and Lam KS:
Fibroblast growth factor 21 as an emerging metabolic regulator:
clinical perspectives. Clin Endocrinol (Oxf). 78:489–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kharitonenkov A, Shiyanova TL, Koester A,
Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers
JS, Owens RA, et al: FGF-21 as a novel metabolic regulator. J Clin
Invest. 115:1627–1635. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wente W, Efanov AM, Brenner M,
Kharitonenkov A, Köster A, Sandusky GE, Sewing S, Treinies I,
Zitzer H and Gromada J: Fibroblast growth factor-21 improves
pancreatic beta-cell function and survival by activation of
extracellular signal-regulated kinase 1/2 and Akt signaling
pathways. Diabetes. 55:2470–2478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu J, Stanislaus S, Chinookoswong N, Lau
YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, et al:
Acute glucose-lowering and insulin-sensitizing action of FGF21 in
insulin-resistant mouse models - association with liver and adipose
tissue effects. Am J Physiol Endocrinol Metab. 297:E1105–E1114.
2009. View Article : Google Scholar
|
|
17
|
Hotta Y, Nakamura H, Konishi M, Murata Y,
Takagi H, Matsumura S, Inoue K, Fushiki T and Itoh N: Fibroblast
growth factor 21 regulates lipolysis in white adipose tissue but is
not required for ketogenesis and triglyceride clearance in liver.
Endocrinology. 150:4625–4633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inagaki T, Lin VY, Goetz R, Mohammadi M,
Mangelsdorf DJ and Kliewer SA: Inhibition of growth hormone
signaling by the fasting-induced hormone FGF21. Cell Metab.
8:77–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coskun T, Bina HA, Schneider MA, Dunbar
JD, Hu CC, Chen Y, Moller DE and Kharitonenkov A: Fibroblast growth
factor 21 corrects obesity in mice. Endocrinology. 149:6018–6027.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kharitonenkov A, Wroblewski VJ, Koester A,
Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB and Etgen
GJ: The metabolic state of diabetic monkeys is regulated by
fibroblast growth factor-21. Endocrinology. 148:774–781. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma AZ, Zhang Q and Song ZY: TNFa alter
cholesterol metabolism in human macrophages via PKC-θ-dependent
pathway. BMC Biochem. 14:202013.PubMed/NCBI
|
|
22
|
Chen SG, Xiao J, Liu XH, Liu MM, Mo ZC,
Yin K, Zhao GJ, Jiang J, Cui LB, Tan CZ, et al: Ibrolipim increases
ABCA1/G1 expression by the LXRα signaling pathway in THP-1
macrophage-derived foam cells. Acta Pharmacol Sin. 31:1343–1349.
2010.
|
|
23
|
Yan JQ, Tan CZ, Wu JH, Zhang DC, Chen JL,
Zeng BY, Jiang YP, Nie J, Liu W, Liu Q, et al: Neopterin negatively
regulates expression of ABCA1 and ABCG1 by the LXRα signaling
pathway in THP-1 macrophage-derived foam cells. Mol Cell Biochem.
379:123–131. 2013.PubMed/NCBI
|
|
24
|
Jessup W, Gelissen IC, Gaus K and
Kritharides L: Roles of ATP binding cassette transporters A1 and
G1, scavenger receptor BI and membrane lipid domains in cholesterol
export from macrophages. Curr Opin Lipidol. 17:247–257. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharrett AR, Ballantyne CM, Coady SA,
Heiss G, Sorlie PD, Catellier D and Patsch W: Coronary heart
disease prediction from lipoprotein cholesterol levels,
triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL
density subfractions: The Atherosclerosis Risk in Communities
(ARIC) Study. Circulation. 104:1108–1113. 2001. View Article : Google Scholar
|
|
26
|
Gordon DJ and Rifkind BM: High-density
lipoprotein - the clinical implications of recent studies. N Engl J
Med. 321:1311–1316. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Otocka-Kmiecik A, Mikhailidis DP, Nicholls
SJ, Davidson M, Rysz J and Banach M: Dysfunctional HDL: a novel
important diagnostic and therapeutic target in cardiovascular
disease? Prog Lipid Res. 51:314–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meurs I, Van Eck M and Van Berkel TJ:
High-density lipoprotein: key molecule in cholesterol efflux and
the prevention of atherosclerosis. Curr Pharm Des. 16:1445–1467.
2010. View Article : Google Scholar : PubMed/NCBI
|