Introduction
Cardiovascular disease is one of the most common
life-threatening diseases, and it has been predicted that it will
be the leading cause of mortality worldwide by 2030 (1). The predominant cause of mortality in
cardiovascular disease is an acute myocardial infarction (AMI).
Restoration of the blood flow to the ischemic myocardium is the
most effective treatment principle of AMI. Therapeutic strategies,
including thrombolysis and primary angioplasty (2), are frequently used in clinical
practice. Nevertheless, reperfusion is considered to pose a risk,
as it may result in a worsening of the tissue injury (3) and myocardial ischemia reperfusion
injury (MI/RI), which may paradoxically reduce the beneficial
effects of myocardial reperfusion, resulting in contractile
dysfunction and cellular damage (4). The pathogenesis of MI/RI is complex
and the mechanisms involved have not yet been fully elucidated.
Previous studies have shown that active oxygen, calcium overload,
neutrophil infiltration and apoptosis are all involved in the
occurrence of MI/RI (5–9).
Dracocephalum moldavuca L. is a herb from the
Labiatae family. This herb is traditionally used in Uyghur Medicine
for the treatment of coronary heart disease, hypertension,
atherosclerosis and myocardial ischemic disease in China (10). The predominant chemical
constituents of Dracocephalum moldavuca L. are flavonoids,
terpenoids, volatile oils, amino acids, trace elements and
peptides. Tilianin is a predominant effective flavonoid monomer
enriched from Dracocephalum moldavuca L. (11). As this traditional Chinese medicine
monomer has a clear chemical structure and certain pharmacodynamic
advantages, such as improved targeting, the utilization of Chinese
medicine resources to analyze the efficacy of this compound is
currently a key area of investigation. Nam et al (12) suggested that tilianin inhibits
inducible nitric oxide synthase (iNOS) expression and production of
nitric oxide (NO), and may act as an anti-inflammatory agent.
Another previous study revealed tilianin to exert a protective
effect in MI/RI (13), but the
protective mechanism remains largely unknown. Therefore, further
studies are required regarding the protective effects and
mechanisms of tilianin on MI/RI.
The present study aimed to continue to investigate
the cardioprotective effects of tilianin on MI/RI and elucidate its
mechanism of action, in order to provide a theoretical basis for
the clinical treatment of coronary heart disease with tilianin.
Materials and methods
Drugs and reagents
Tilianin was enriched from Dracocephalum
moldavica L. in the laboratory, and 1H-nuclear
magnetic resonance (NMR), 13C-NMR, mass spectrometry and
infrared spectrometry were used to identify the chemical structure.
The purity obtained was >98%, as measured by high-performance
liquid chromatography. The propranolol hydrochloride tablet (PHT)
was purchased from Tianjin Lisheng pharmaceutical Co., Ltd
(Tianjin, China) and sodium pentobarbital was supplied by Beijing
Chemical Reagent Company (Beijing, China). The 2X Taq polymerase
chain reaction (PCR) MasterMix was bought from Tiangen Biological
Technology Co., Ltd (Beijing, China). The β-actin and caspase-3
primers were obtained from Bioneer Corporation (Daejeon,
Korea).
Animals
The animal experiments carried out in the present
study were approved by the Institutional Animal Care and Use
Committee at Xinjiang Uyghur Autonomous Region Experimental Animal
Research Center (Xinjiang, China). A total of 48 male Sprague
Dawley rats, weighing 250–300 g, were provided by the Xinjiang
Uyghur Autonomous Region Experimental Animal Research Center
(certificate no.: XK 2003-0001; Xinjiang, China) and were housed
under conditions of constant temperature and controlled
illumination (light on between 8:30 and 20:30 h). Food and water
were available ad libitum.
In vivo myocardial ischemia and
reperfusion model
All animals were assigned randomly to one of four
groups: Sham group, rats were pretreated with saline, n=8; MI/RI
group, rats were pretreated with saline, n=8; MI/RI + tilianin
groups, high-, medium- and low-dose group rats were pretreated with
tilianin (5.0 mg/kg, 2.5 mg/kg, 1.5 mg/kg respectively), n=24, 8
rats in each group; MI/RI + PHT group, rats were pretreated with
PHT (25.0 mg/kg), n=8. Each treatment was orally administered for
eight days, at a volume of 5 ml/kg weight once a day, and the
surgical procedure was established 10 min after the last
administration.
The rats were anesthetized with sodium pentobarbital
(45 mg/kg injected intraperitoneally), and then intubated and
artificially ventilated using a rodent ventilator (HX-100E;
Chongqing Taimeng Technology Co., Ltd., Chongqing, China). A normal
electrocardiogram was recorded following the placement of
subcutaneous electrodes connected to an electrocardiograph
(BL-420S; Chongqing Taimeng Technology Co., Ltd.).
Ischemia/reperfusion was established as previously described by
Zhao et al (14). Briefly,
myocardial ischemia was induced by ligating the left anterior
descending coronary artery (LAD) with a 3–0 silk suture. After 30
min ischemia, the ligature was released for 2 h, resulting in
myocardial reperfusion (15,16).
Sham-operated animals (sham group) underwent the same surgical
procedures, with the exception that the 3-0 silk was passed around
the left coronary artery but not tied. Successful myocardial
ischemia was confirmed by ST segment elevation in the
electrocardiogram, in addition to visual assessment of regional
cyanosis of the ischemic region in the left ventricle. Reperfusion
was confirmed by ST segment reversal and a color change in the
ventricular surface from cyanotic to hyperemic (16). Arterial blood samples were
collected at the end of the reperfusion, and the blood serum was
separated at 1,200 × g and stored at −70°C. The mouse
hearts were removed following the collection of blood and
immediately placed in cold saline. The intracardiac blood was
rinsed off and the left ventricle tissue was line-clipped under
ligation and cryopreserved.
Measurement of
Na+-K+-ATPase and Ca2+-ATPase
activity
Briefly, the frozen myocardial tissue was weighted
and normal saline was added at a ratio of 1:9. Subsequently, the
tissue homogenates were centrifuged to obtain the supernatant,
followed by measurement of protein concentration using Coomassie
Brilliant Blue kit (Nanjing Jiancheng Bioengineering Institute).
Na+-K+-ATPase and Ca2+-ATPase
activity levels were determined by measuring the release of
inorganic phosphate from ATP using the
Na+-K+-ATPase kit and the
Ca2+-ATPase kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China), according to the manufacturer’s
instructions.
Measurement of NO and NOS activity
NO is chemically reactive and and can quickly
convert to nitrate (NO3−) and nitrite
(NO2 −) in vivo, with
NO2− further converting to
NO3−. In the assay,
NO3− was reduced to
NO2− using nitrate reductase. The absorbance
of NO2− at 550 nm indirectly signifies the NO
concentrations. NOS catalyzes L-Arg and molecular oxygen to produce
NO and pronuclear material, which generates colored compounds.
Thus, the absorbance at the 530 nm wavelength allows for
calculation of the NOS activity. Using the serum and homogenate
supernatant of each group, the NO levels and NOS activity were
determined with a power wave XS2 enzyme-labeled instrument (Bio-Rad
Laboratories, Hercules, CA, USA) using NO and NOS kits according to
the manufacturer’s instructions (Nanjing Jiancheng Bioengineering
Institute).
Measurement of endothelial system-related
factors
The serum levels of endothelin (ET)-1, calcitonin
gene-related peptide (CGRP), thromboxane (TX) B2 and
6-keto prostaglandin (PG) F1a (6-Keto-PGF1a) in the rat
blood samples were measured using the corresponding rat ELISA kits
(Shanghai Xitang Biological Technology Co., Ltd., Shanghai,
China).
Immunohistochemistry
A section of ischemic tissue was fixed in 4%
paraformaldehyde solution. After 24 h, this was dehydrated and then
embedded in paraffin blocks. The blocks were then cut into 5 μm
sections using a rotary microtome (Leica RM 2235; Leica Instrument
Co., Ltd., Beijing, China).
Immunohistochemical procedures were conducted
according to the manufacturer’s instructions of the
streptavidin-peroxidase kit (ZSGB-BIO, Beijing, China). Briefly,
the tissue sections were dewaxed and dehydrated with xylene and
alcohol. The sections were then incubated for 10 min with 3%
H2O2 and an autoclave was used to heat the
samples for 8 min for antigen retrieval. The samples were then
incubated with the following primary antibodies: Bax (B-9; dilution
1:25; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
Bcl-2 (C-2; dilution 1:50; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. Following three washes with phosphate-buffered
saline, the sections were incubated with a non-biotin labeled goat
anti-mouse IgG secondary antibody (ZSGB-BIO) for 30 min and further
developed with 3,3-diaminobenzidine tetrahydrochloride (DAB). The
sections were observed under an Olympus CX21 microscope (Xiamen
Sannuoxinu Electronic Technology Co., Ltd., Xiamen, China). The
immunopositive cell rate was calculated as follows: Immunopositive
cell rate = immunopositive cells/(immunopositive cells +
immunonegative cells) ×100%. The expression levels of the analyzed
proteins were statistically compared using the staining intensity
plus the percentage of positive cells. The score criteria are
listed in Table I (17).
 | Table IImmunohistochemical staining
criteria. |
Table I
Immunohistochemical staining
criteria.
| Staining
intensity | Score | Immunopositive cell
rate (%) | Score |
|---|
| Colorless | 0 | 0 | 0 |
| Buff | 1 | ≤25 | 1 |
| Yellow | 2 | 26–50 | 2 |
| Brown | 3 | 51–75 | 3 |
| N/A | N/A | ≥75 | 4 |
Semi-quantitative (q) PCR analysis
A centrifugal columnar RNAprep Pure Tissue kit
(Tiangen Biological Technology Co., Ltd., Beijing, China) was used
to extract the total RNA from the myocardial tissue. cDNA was first
synthesized by reverse transcription PCR. An appropriate quantity
of total RNA was used to synthesize cDNA using a reverse
transcriptase kit (Thermo Fisher Scientific, Rockford, IL, USA) at
the following conditions: 65°C for 5 min, 42°C for 60 min and 70°C
for 5 min. Subsequently, this cDNA served as a template for the
subsequent qPCR reaction: 94°C for 3 min, 94°C for 30 sec, 53°C for
30 sec, 72°C for 1 min and 72°C for 5 min; 35 cycles. The following
primers were used in the reaction: β-actin forward,
5′-AGCCATGTACGTAGCCATCC-3′ and reverse, 5′-CTCTCAGCTGTGGTGAA-3′;
caspase-3 forward, 5′-TTGGAGCACTGTAGCACACA-3′ and reverse,
5′-ACCACTGAAGGATGGTAGCC-3′. The PCR products were detected by
ionization on a 1.5% agarose gel.
Statistical analysis
Data are expressed as the means ± standard
deviation. A Student’s t-test was performed to analyze the
differences between two groups. Data analyses were performed using
the SPSS 17.0 software package (SPSS, Inc., Chicago, IL, USA). A
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of tilianin on
Na+-K+-ATPase and Ca2+-ATPase
activity
The activity of Na+-K+- and
Ca2+-ATPases in myocardial tissues was investigated in
response to MI/RI. As compared with the sham group,
Na+-K+- and Ca2+-ATPase activity
was significantly reduced in the MI/RI group
(Na+-K+-ATPase: sham, 11.97±1.21 versus
MI/RI, 3.96±0.48, P<0.01; Ca2+-ATPase: sham,
3.66±0.28 versus MI/RI, 1.34±0.15, P<0.01). As compared with the
MI/RI group, significant recoveries in
Na+-K+-ATPase and Ca2+-ATPase
activities were observed in the high- and medium-dose tilianin
groups (P<0.01 and P<0.05 respectively), whereas no
significant differences were observed between the low-dose tilianin
group and the MI/RI group. The MI/RI + PHT group also exhibited a
statistically significant increase in ATPase activity, in
comparison with the MI/RI group (P<0.05; Fig. 1A).
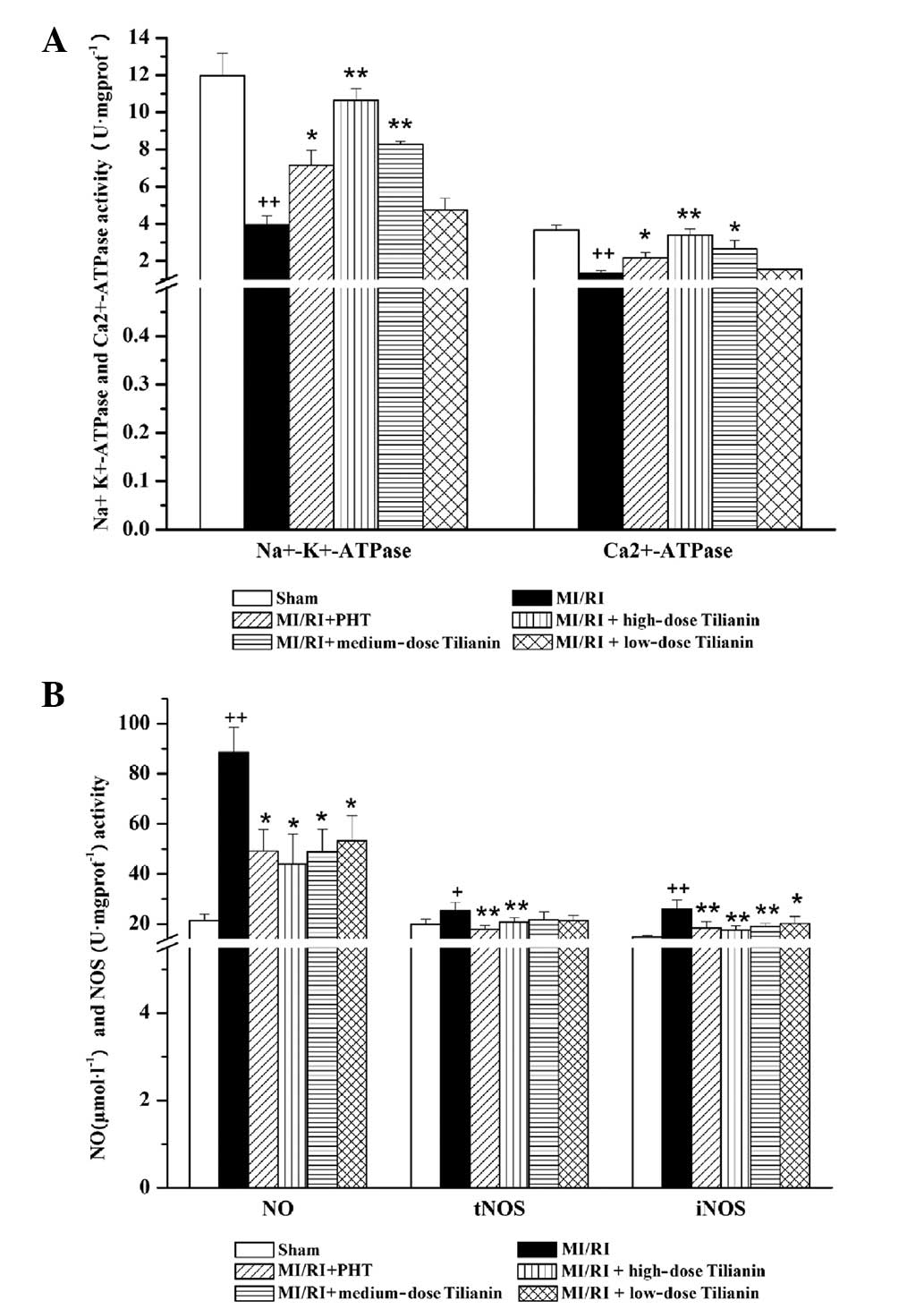 | Figure 1Effect of Tilianin on energy
metabolism enzymes and NO activity in rat cardiac tissues. ATPase
activity, NO level and myocardial NOS activity were measured. (A)
Effect on Na+-K+-ATPase and
Ca2+-ATPase activities following MI/RI in each group.
(B) Effect on NO level and myocardial NOS activity following MI/RI
in each group. ++P<0.01, +P<0.05 vs.
Sham, **P<0.01, *P<0.05 vs. MI/RI. NO,
nitric oxide; MI/RI, myocardial ischemia/reperfusion injury; NOS,
NO synthase; PHT, propranolol hydrochloride tablet; tNOS, total
NOS;.iNOS, inducible NOS. |
Effect of tilianin on NO and NOS
activity
As shown in Fig.
1B, serum NO levels and myocardial NOS activity were
significantly increased following MI/RI surgery, as compared with
the sham group [NO: sham, 21.38±2.66 versus MI/RI, 88.49±10.11,
P<0.01; Total (t)NOS: sham, 19.87±2.08 versus MI/RI, 25.40±3.24,
P<0.05; iNOS: sham, 14.88±0.51 versus MI/RI, 26.04±3.51,
P<0.01]. In comparison with the MI/RI group, significant
decreases (P<0.01 and P<0.05) were identified in the serum NO
levels and myocardial iNOS activity upon administration of tilianin
to MI/RI rats at the three dose levels analyzed. Only the high-dose
tilianin group significantly reduced tNOS activity, as compared
with the MI/RI group (P<0.01)
Effect of tilianin on endothelial
system-related factors
The tilianin drug groups exhibited dose-dependent
reductions in the levels of serum ET-1 and TXB2, and
increases in the levels of serum CGRP and 6-Keto-PGF1a,
as compared with the MI/RI group. The high- and medium-dose groups
significantly differed from the model group (ET-1: MI/RI,
34.63±4.32 vs. MI/RI + high-dose tilianin, 21.64±2.82, P<0.01;
CGRP: MI/RI, 1,479.58±235.29 vs. MI/RI + high-dose tilianin,
2,036.06±261.24, P<0.01; TXB2: MI/RI, 2,480.05±317.55
versus MI/RI + high-dose tilianin, 1,717.45±241.44, P<0.01;
6-Keto-PGF1a: MI/RI, 30.13±4.72 versus MI/RI + high-dose
tilianin, 79.56±7.90, P<0.01). However, the MI/RI + PHT group
exhibited reduced ET-1 and TXB2 levels and increased
CGRP and 6-Keto-PGF1a levels as compared with all the
tilianin-treated groups. (Fig.
2)
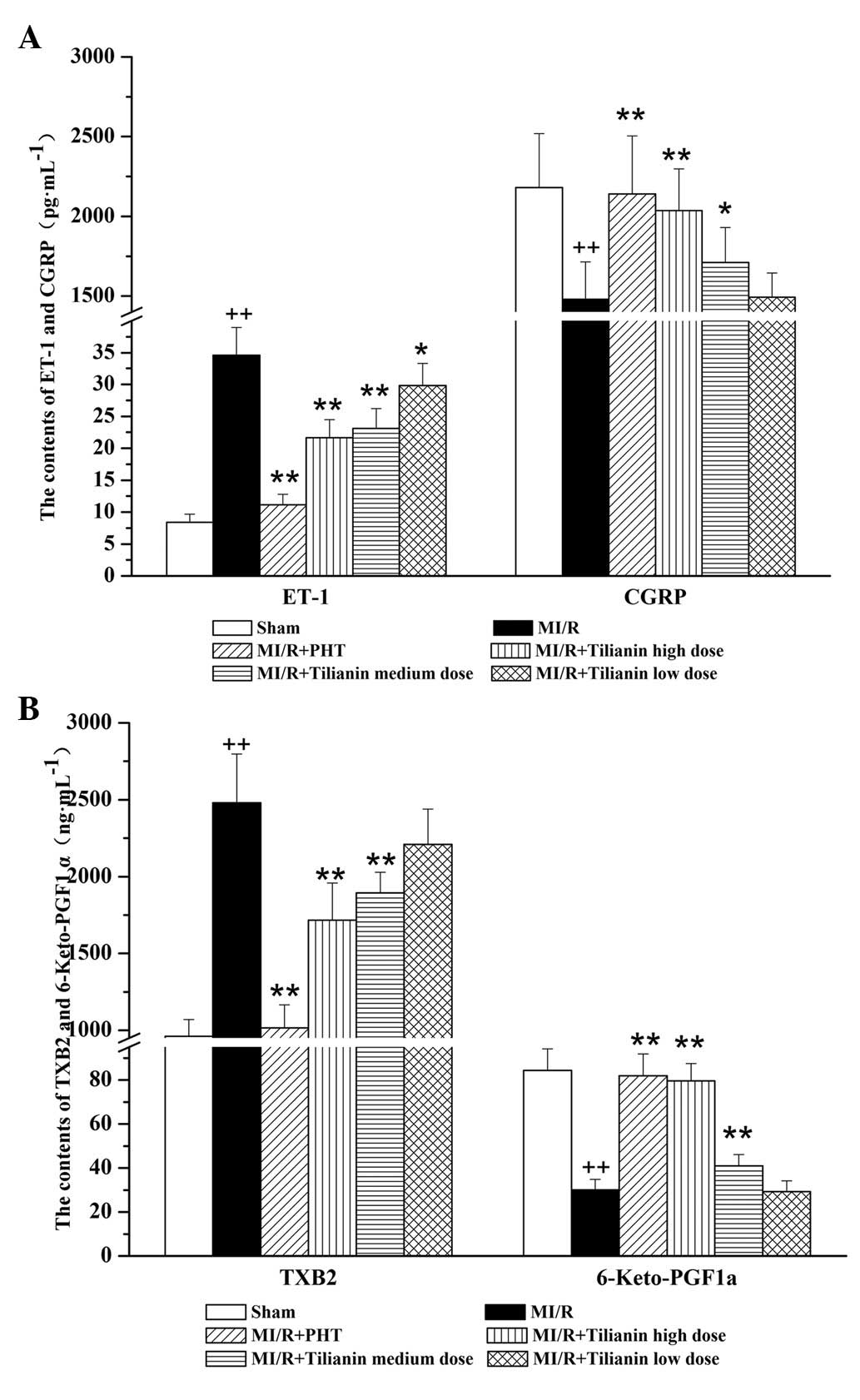 | Figure 2Effect of Tilianin on endothelial
system-related factors, measured by ELISA. (A) Serum levels of ET-1
and CGRP in each group. (B) Serum level of TXB2 and
6-Keto-PGF1a in each group. ++P<0.01 vs.
Sham, **P<0.01, *P<0.05 vs. MI/RI.
ET-1, endothelin 1; CGRP, calcitonin gene-related peptide,
TXB2, thromboxane B2; 6-Keto-PGF1a, 6-keto
prostaglandin F1a; MI/RI, myocardial ischemia/reperfusion injury;
PHT, propranolol hydrochloride tablet. |
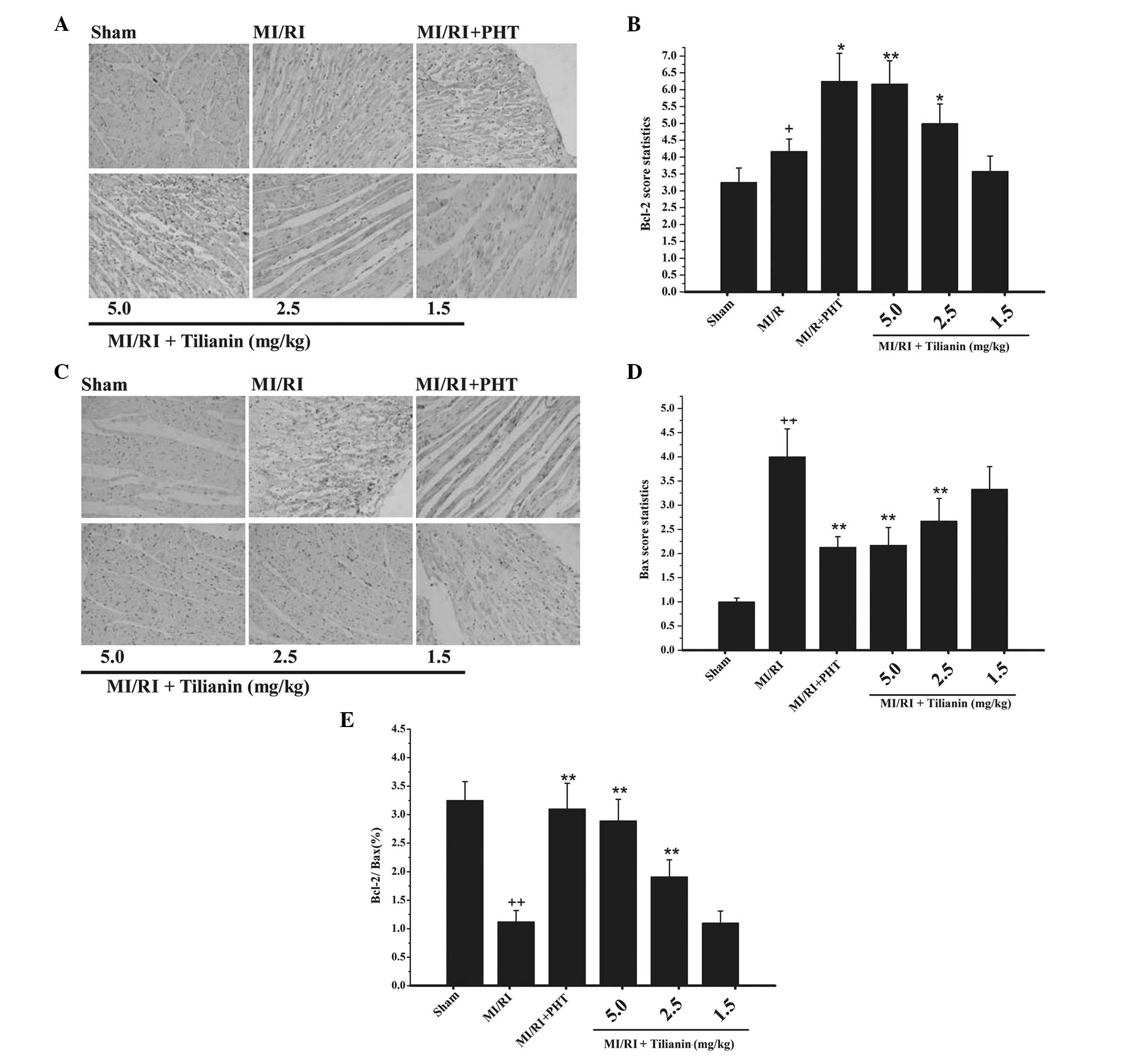
Effect of tilianin on Bcl-2 and Bax
expression levels
Bcl-2 protein was mainly accumulated in the
cytoplasm. Immunohistochemical DAB coloration revealed diffuse
distribution or brown granules in the cell cytoplasm. In the sham
group, a few particles of Bcl-2 protein were detected (Fig. 3A). In the model group, positive
staining of Bcl-2 protein was significantly increased in the
cytoplasm (P<0.05). As compared with the model group, the
tilianin drug groups exhibited significantly increased Bcl-2
protein expression levels (P<0.01 and P<0.05 for the high-
and medium-dose groups, respectively; Fig. 3B).
The expression levels of Bax protein were similar to
those of Bcl-2 protein, with an accumulation of Bax observed in the
cytoplasm. Immunohistochemical staining revealed the sham group to
exhibit almost no Bax protein expression (Fig. 3C); however, the Bax protein
expression levels in the model group were significantly increased
(P<0.01; Fig. 3D), as compared
with those of the sham group These data indicate that myocardial
damage promotes apoptotic protein expression. Compared with the
model group, the high- and medium-dose tilianin drug groups
exhibited significantly decreased Bax protein expression levels
(P<0.01). In addition, the high- and medium-dose tilianin drug
groups exhibited significantly increased Bcl-2/Bax ratio, as
compared with the model group (P<0.01; Fig. 3E).
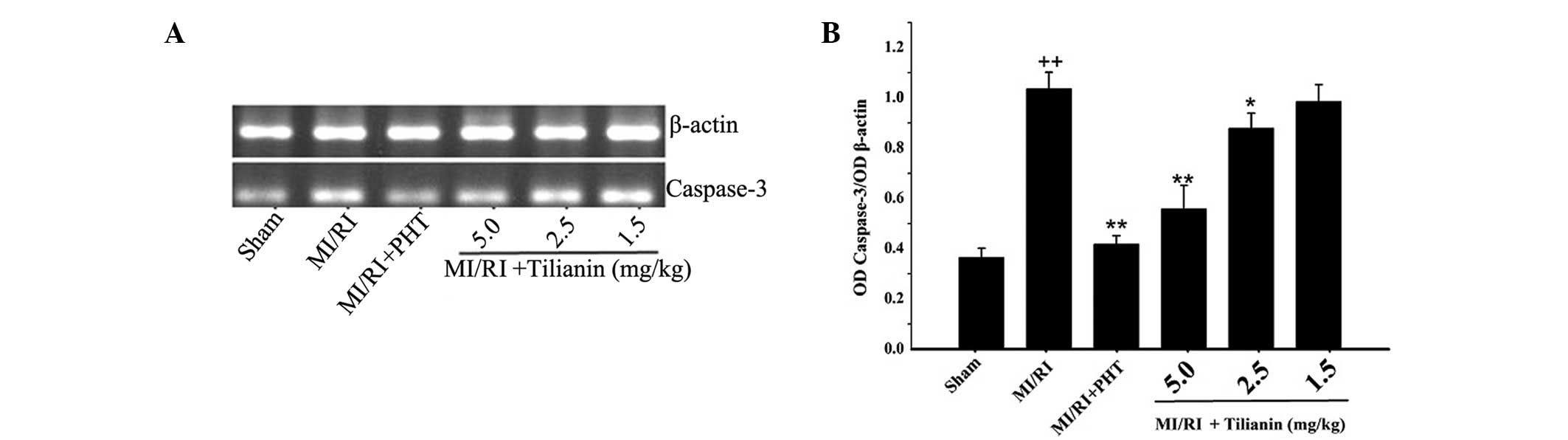
Effect of tilianin on caspase-3 mRNA
expression levels
Compared with the sham group, the expression levels
of caspase-3 mRNA in the MI/RI group were significantly increased
(sham, 0.37±0.037 versus MI/RI, 1.04±0.07, P<0.01). As compared
with the MI/RI group, the tilianin high- and medium-dose groups
exhibited significantly reduced caspase-3 mRNA expression levels
(high-dose tilianin, 0.56±0.09, P<0.01; medium-dose tilianin,
0.88±0.06, P<0.05). In addition, he MI/RI + PHT group exhibited
decreased expression levels of caspase-3 mRNA, as compared with
those of the MI/RI group (MI/RI + PHT, 0.42±0.03, P<0.01). The
results indicated that tilianin exerted a protective effect on the
cardiovascular tissue that underwent MI/RI, by reducing the
expression levels of caspase-3 mRNA (Fig. 4).
Discussion
The present study generated three notable findings:
(i) Pretreatment with tilianin attenuated calcium overload and
energy metabolism disorders in MI/RI, a condition that
predominantly manifests through increased
Na+-K+-ATPase and Ca2+-ATPase
activity; (ii) reduced endothelial system-related factors,
including NO and endothelial NOS, significantly contributed to the
cardioprotective effect of tilianin; (iii) tilianin was
demonstrated to protect against MI/RI by inhibiting apoptosis.
Traditional Chinese herbs are used worldwide and
analyses of the effective components are being widely investigated.
A number of studies (18,19) have confirmed that Dracocephalum
moldavuca L. total flavonoid exerts a protective effect on
MI/RI. Tilianin is an effective ingredient extracted from
Dracocephalum moldavuca L. Previous studies have confirmed
that the cardioprotective effects of tilianin in MI/RI are
associated with reduced levels of intracellular enzymes that are
released into the blood, attenuation of the overproduction of
reactive oxygen species and reductions in the release of
inflammatory factors, such as IL-1, IL-6 and tumor necrosis
factor-α (13).
Na+-K+-ATPase is an integral
membrane protein that maintains the normal physiological gradient
across the cell membrane. This is achieved through coupling ATP
hydrolysis to the transport of Na+ and K+.
The Na+-K+-ATPase enzyme is comprised of two
subunits, both of which have numerous isoforms and ion-pumping
functions (20). For the transport
of calcium ions, Ca2+-ATPase is the predominant active
transport protein that can regulate intracellular calcium levels in
numerous cell types. The Ca2+-ATPase has an important
role in maintaining the cation gradient for homeostatic control of
cellular properties, and is also crucial for the contractility and
excitability of muscles (21,22).
In the present study, Na+-K+-ATPase and
Ca2+-ATPase activity was found to be reduced in the
MI/RI group, but a significant recovery was observed in the
tilianin drug group (Fig. 1). The
results suggested that tilianin may protect the myocardium by
increasing ATP enzyme activity, maintaining homeostasis of the
intracellular environment and rectifing energy metabolism.
A number of studies have confirmed the
cardioprotective effects of NO during MI/RI. In addition, the
effects of NO on MI/RI that have been demonstrated include pro- and
antiapoptotic effects, depending on the source of NO (23,24).
However, the mechanisms underlying these effects are not completely
understood. Previous studies have indicated that the beneficial
effects of NO from NOS are mediated by the regulation of vascular
tone, superoxide radical scavenging, and the inhibition of
neutrophil adherence and infiltration (9,25).
The results of the present study were contrary to those of previous
studies, showing that myocardial NO expression levels and NOS
activity was markedly increased following MI/RI injury. However,
the rats of the tilianin drug-treatment groups exhibited
significantly lower levels of NO and NOS (Fig. 1). These data indicate that tilianin
may protect against MI/RI, by reducing myocardial NOS activity and
decreasing the production of NO, therefore reducing the cytotoxic
effects of NO.
The endothelial system-related factors ET-1 and CGRP
were investigated following tilianin administration. ET-1 is one of
the most potent endogenous vasoconstrictor peptides so far
identified (26); CGRP is a
vasoactive peptide that exhibits a variety of physiological
functions, including vasodilation (27). Together, ET-1 and CGRP are a pair
of vasoconstriction and vasodilation factors that are mainly
regulated through endothelial function. In addition, the dynamic
balance of TXA2/PGI2 is the main regulatory
system in maintaining angiectasis and normal platelet function.
However, these molecules are unstable in vivo due to a short
half-life. Therefore, the concentrations of TXA2 and
PGI2 are determined by monitoring the levels of the
respective hydrolysis products, TXB2 and
6-Keto-PGF1a. In the tilianin drug groups, reduced serum
ET-1 and TXB2 levels, and increased serum CGRP and
6-Keto-PGF1a levels were observed, as compared with the
the MI/RI group. The results suggest that tilianin further protects
against MI/RI by maintaining the balance of endothelial
function-related factors.
Cardiomyocyte apoptosis is a predominant pathogenic
mechanism underlying MI/RI injury (28). Apoptosis following
ischaemia-reperfusion (I/R) has been determined to be associated
with increased levels of Bax protein and a decreased Bcl-2/Bax
ratio (29). The overexpression of
Bcl-2 in mice has been shown to significantly inhibit apoptosis and
decrease the infarct size in the mouse heart following I/R
(30). Bax and Bcl2 are important
mitochondrial regulators during cardiomyocyte apoptosis (31). Bcl-2 regulates the opening of mPTP
in opposition to Bax, blocking cytochrome c release, inhibiting
caspase activity and reducing cell apoptosis (32). Therefore, the roles of Bcl-2 and
Bax proteins in the antiapoptotic effects of tilianin were
investigated in the present study. The results showed that tilianin
preconditioning significantly increased Bcl-2 expression levels and
reduced Bax levels, with a corresponding increase in the Bcl-2/Bax
ratio. The results indicated that changes in the ratio of
proapoptotic to antiapoptotic proteins may also contribute to the
antiapoptotic and cardioprotective effects of tilianin in I/R
injury (Fig. 3).
Caspases are critical enzymes in the induction and
execution of apoptosis, and can induce cellular destruction through
the formation of apoptotic bodies. The caspase family has numerous
members, and the effector caspase, caspase-3, is associated with
the apoptotic cascade pathway (33), and is therefore widely used as an
indicator of apoptosis. The results of the present study revealed
that tilianin exhibited cardioprotective effects by inhibiting
caspase-3 activity, and thus the activation of the cleavage process
(Fig. 4).
In conclusion, these results demonstrate that
tilianin exerts potent cardioprotective effects in rats with MI/RI.
The effects of anti-MI/RI observed, included relief of calcium
overload, correction of energy metabolism, improvement in
endothelial function and inhibition of cell apoptosis. This
suggests that tilianin is a potentially useful drug that may be
applied clinically for the prevention or treatment of MI/RI.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81160525 and 81360671)
and the Xinjiang Production and Construction Corps Applied Basic
Research Plan for Doctor (no. 2013BB014).
References
|
1
|
Kreatsoulas C and Anand SS: The impact of
social determinants on cardiovascular disease. Can J Cardiol.
26:8C–13C. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM: New directions
for protecting the heart against ischemia-reperfusion injury:
targeting the Reperfusion injury Salvage Kinase (RISK)-pathway.
Cardiovasc Res. 61:448–460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambrosio G and Tritto I: Reperfusion
injury: experimental evidence and clinical implications. Am Heart
J. 138:S69–S75. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yellon DM and Baxter GF: Protecting the
ischemic and reperfused myocardium in acute myocardial infarction:
distant dream or near reality? Heart. 83:381–387. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J, Zhang H and Zhang C: Role of
inflammation in the regulation of coronary blood flow in ischemia
and reperfusion: mechanisms and therapeutic implications. J Mol
Cell Cardiol. 52:865–872. 2012. View Article : Google Scholar
|
|
6
|
Gottlieb RA: Cell death pathways in acute
ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther.
16:233–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koenitze JR and Freeman BA: Redox
signaling in inflammation: interactions of endogenous electrophiles
and mitochondria in cardiovascular disease. Ann NY Acad Sci.
12:45–52. 2010. View Article : Google Scholar
|
|
8
|
Lakshmi SV, Padmaja G, Kuppusamy P and
Kutala VK: Oxidative stress in cardiovascular disease. Indian J
Biochem Biophys. 46:421–440. 2009.
|
|
9
|
Liou SF, Ke HJ, Hsu JH, et al:
San-Huang-Xie-Xin Tang prevents rat hearts from
ischemia/reperfusion-induced apoptosis through eNOS and MAPK
pathways. Evid Based Complement Alternat. 2011:9150512011.
|
|
10
|
Feng Changgen and Li Qiong: The research
of Dracocephalum moldavuca L. chemical constituents and
pharmacological activities. J Tradit Chin Med. 25:1552003.
|
|
11
|
Yuan Yong, Xing Jian Guo, Zhang Yong Jun,
et al: Determination the content of tilianin in Dracocephalum
moldavuca L. by HPLC. Chin J Exp Tradit Med Formulae. 16:68–69.
2010.
|
|
12
|
Nam KH, Choi JH, Seo YJ, et al: Inhibitory
effects of tilianin on the expression of inducible nitric oxide
synthase in low density lipoprotein receptor deficiency mice. Exp
Mol Med. 38:445–452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Xinhong, Cao Wenjiang, Fan Xinmei, et
al: The protective effects of Tilianin on myocardial
ischemia-reperfusion injury in rats. Chin J Exp Tradit Med
Formulae. 13:169–172. 2013.
|
|
14
|
Zhao Q, Shao L, Hu X, Wu G, et al: Lipoxin
A4 preconditioning and postconditioning protect
myocardial ischemia/reperfusion injury in rats. Mediators Inflamm.
2013:2313512013.
|
|
15
|
Wei Q, Yin Y, Xi M, et al: Antioxidant
properties of magnesium lithospermate B contribute to the
cardioprotection against myocardial ischemia/reperfusion injury in
vivo and in vitro. J Tradit Chin Med. 33:85–91. 2013. View Article : Google Scholar
|
|
16
|
Ahmed LA, Salem HA, Attia AS and Agha AM:
Pharmacological preconditioning with nicorandil and pioglitazone
attenuates myocardial ischemia/reperfusion injury in rats. Eur J
Pharmacol. 663:51–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun C, Liu C, Li S, et al: Overexpression
of GEFT, a Rho family guanine nucleotide exchange factor, predicts
poor prognosis in patients with rhabdomyosarcoma. Int J Clin Exp
Pathol. 7:1606–1615. 2014.PubMed/NCBI
|
|
18
|
Zhao J, Xue W and Liang S: Protective
effects of dracocephalum moldavica extracts on
hypoxia/reoxygenation injury of cardiomyocytes in cultured neonatal
rat. J Zhenzhou University (Med Sci). 2010:485–487. 2010.
|
|
19
|
Fan XM, Cao WJ, Xing JG, et al: Study on
the protective effect of Dracocephalum total flavones against
myocardial ischemia-reperfusion injury in rats. Chin Tradit Patent
Med. 8:1625–1629. 2013.(In Chinese).
|
|
20
|
Rajadurai M and Stanely Mainzen Prince P:
Preventive effect of naringin on cardiac markers,
electrocardiographic patterns and lysosomal hydrolases in normal
and isoproterenol-induced myocardial infarction in Wistar rats.
Toxicol. 230:178–188. 2007. View Article : Google Scholar
|
|
21
|
Tian D, Dmitrieva RI, Doris PA, et al:
Protein kinase M zeta regulation of Na/K ATPase: a persistent
neuroprotective mechanism of ischemic preconditioning in
hippocampal slice cultures. Brain Res. 1213:127–139. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yorozuya T, Adachi N, Dote K, et al:
Enhancement of Na+K+-ATPase and
Ca2+-ATPase activities in multi-cycle ischemic
preconditioning in rabbit hearts. Eur J Cardiothorac Surg.
26:981–987. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang G, Fang Z, Liu Y, et al: Protective
effects of Chinese traditional medicine Buyang Huanwu decoction on
myocardial injury. Evid Based Complement Alternat Med.
2011:9303242011. View Article : Google Scholar :
|
|
24
|
Razavi HM, Hamilton JA and Feng Q:
Modulation of apoptosis by nitric oxide: implications in myocardial
ischemia and heart failure. Pharmacol Ther. 106:147–162. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu YN, Zhou ZM and Chen P: Evidence that
hydroxysafflor yellow A protects the heart against
ischaemia-reperfusion injury by inhibiting mitochondrial
permeability transition pore opening. Clin Exp Pharmacol Physiol.
35:211–216. 2008.
|
|
26
|
Benigni A, Perico N and Remuzzi G:
Endothelin antagonists and renal protection. J Cardiovasc
Pharmacol. 35(4 Suppl 2): S75–S78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Hoon JN, Pickkers P, Smits P, et al:
Calcitonin gene-related peptide: exploring its vasodilating
mechanism of action in humans. Clin Pharmacol Ther. 73:312–321.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji L, Fu F, Zhang L, Liu W, Cai X, et al:
Insulin attenuates myocardial ischemia/reperfusion injury via
reducing oxidative/nitrative stress. Am J Physiol Endocrinol Metab.
298:E871–E880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moudgil R, Menon V, Xu Y, et al:
Postischemic apoptosis and functional recovery after angiotensin II
type 1 receptor blockade inisolated working rat hearts. J
Hypertens. 19:1121–1129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Chua CC, Ho YS, et al:
Overexpression of Bcl-2 attenuates apoptosis and protects against
myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ
Physiol. 280:H2313–H2320. 2001.PubMed/NCBI
|
|
31
|
Kumar D and Jugdutt BI: Apoptosis and
oxidants in the heart. J Lab Clin Med. 142:288–297. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishihara M, Miura T, Miki T, et al:
Modulation of the mitochondrial permeability transition pore
complex in GSK-3beta-mediated myocardial protection. J Mol Cell
Cordial. 43:564–570. 2007. View Article : Google Scholar
|
|
33
|
Zhang Q, Xiang J, Wang X, et al:
Beta(2)-adrenoceptor agonist clenbuterol reduces infarct size and
myocardial apoptosis after myocardial ischaemia/reperfusion in
anaesthetized rats. Br J Pharmacol. 160:1561–1572. 2010. View Article : Google Scholar : PubMed/NCBI
|