Introduction
para-Phenylenediamine (p-PD) is used
in hair dye formulations and it is estimated that two-thirds of the
hair dye formulations that are marketed contain p-PD
(1). Various azo dyes used by the
industry also contain p-PD. Upon azo reduction of these
compounds by environmental or intestinal microorganisms,
p-PD is released. When p-PD is ingested, it is
absorbed and redistributed to target sites to exert its effects
(2,3). Epidemiological studies have indicated
that increased usage of permanent hair dye may increase the risk of
bladder cancer, non-Hodgkin’s lymphoma, multiple myeloma, and
haematopoietic cancer (4,5). Besides personal usage, professional
hairdressers and dye industry workers are frequently exposed to
dyes containing p-PD, and epidemiological studies have
demonstrated that those who are frequently exposed to this chemical
compound have incurred a higher risk of various cancers (1,4). In
addition, Sontag (6) demonstrated
that the incidence of kidney tumours increased with exposure to
p-PD in rats. Thus, in the current study, the mechanism by
which p-PD induces apoptosis in normal rat kidney proximal
tubular epithelial (NRK52E) cells was investigated. This cell line
is a commonly used cell line for in vitro evaluation of
apoptotic pathways. For example, it was demonstrated in NRK52E
cells that Numb protects against puromycin aminonucleoside-induced
apoptosis by inhibiting the Notch signalling pathway (7). In addition, urografin was
demonstrated to induce apoptosis in NRK52E cells via upregulated
glucose-regulated protein 78 (GRP78) and GRP94 expression,
procaspase-12 cleavage and phosphorylation of protein kinase-like
endoplasmic reticulum kinase and eukaryotic initiation factor 2α
(8).
Focal adhesion kinase (FAK) phosphorylation is the
pivotal integrin-mediated signalling event, since this cytoplasmic
tyrosine kinase acts as the scaffold for several effector
molecules, such as phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt) and the Ras/Raf/c-Jun N-terminal kinase (JNK)
cascades (9,10). FAK activation has been associated
with survival signals through the activation of the PI3K/Akt and
Ras/Raf/JNK pathways (11,12,13).
PI3K/Akt is intimately involved in cell survival, as it regulates
the activity of several Bcl-2 family members (14,15,16).
Protein tyrosine kinases (PTKs) are activated by and form complexes
with growth factor receptor-bound protein 2 and Son of sevenless,
resulting in activation of Ras, and subsequent activation of the
Raf and JNK cascade survival pathways (17,18).
For example, evodiamine-induced oxidative stress and cell cycle
arrest was demonstrated to act through the PTK/Ras/Raf/JNK pathway
in HeLa human cervical carcinoma cells (19), and oridonin has been indicated to
induce G2/M phase cell cycle arrest and apoptosis via
inhibition of the PTK/Ras/Raf/JNK survival pathway in L929 murine
fibrosarcoma cells (20).
Previous studies have demonstrated that p-PD
induces apoptosis via p53 in addition to intrinsic and extrinsic
pathways in MDCK cells (21,22).
Huang et al (23) also
demonstrated that p-PD induces DNA damage and the expression
of mutant p53 and COX-2 proteins in SV-40 immortalized human
uroepithelial cells. In the present study, the roles of the
PTK/Ras/Raf/JNK and PI3K/Akt signalling pathways on
p-PD-treated NRK-52E cells was investigated in relation to
cell death.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), foetal
bovine serum (FBS) and trypsin-EDTA were purchased from Gibco Life
Technologies (Grand Island, NY, USA). p-PD, dimethyl
sulfoxide (DMSO), Triton X-100, Tergitol NP-40, EDTA, Tris-HCl,
trypan blue, phosphate-buffered saline (PBS), goat anti-rabbit
Immunoglobulin G horseradish peroxidase (HRP)-conjugated polyclonal
secondary antibodies, dithiothreitol (DTT), sodium dodecyl sulphate
(SDS), ammonium acetate, Tris-borate-EDTA buffer, Bradford reagent
and phenylmethyl sulfonyl fluoride were purchased from
Sigma-Aldrich (St. Louis, MO, USA). The Annexin-V-FLUOS Staining
kit was purchased from Roche Diagnostics GmbH (Mannheim, Germany).
Proteinase K, ribonuclease A (RNase A) were obtained from BD
Pharmingen (San Diego, CA, USA). Rabbit anti-rat monoclonal
antibodies for Ras, SAPK-JNK, Akt, Bcl-2, Bcl-xL, Bad, tubulin and
phospho SAPK-JNK and mouse anti-rat c-Raf were perchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Amersham ECL-Plus
Western Blotting Reagents and polyvinylidine fluoride (PVDF)
membranes were obtained from GE Healthcare Bio-Sciences
(Pittsburgh, PA, USA). All of the chemicals were of the highest
grade commercially available.
Cell culture and treatment
The NRK-52E normal rat renal tubular epithelial cell
line was obtained from the American Type Culture Collection
(Manassas, VA, USA). The cells were maintained as a monolayer in
DMEM with 2.0 mM L-glutamine adjusted to contain 3.7 g/l sodium
bicarbonate and 4.5 g/l glucose. The medium was supplemented with
1% penicillin (100 U/ml; Sigma-Aldrich), streptomycin (10,000
μg/ml; Sigma-Aldrich) and 10% FBS. Cells were cultured in
25-cm2 tissue culture-treated flasks at 37°C and 5%
CO2 in humidified chambers.
The stock solution of p-PD (100 mg/ml) was
dissolved in DMSO and different concentrations were prepared in the
culture medium with a final DMSO concentration of 0.1%.
Cell viability assay
The NRK-52E cells were treated with various
concentrations of p-PD (50, 100, 200 and 300 μg/ml), or 0.1%
DMSO for the control, for 24 h at 37°C. A trypan blue exclusion
protocol was used to determine the cell viability. Briefly, ~10 μl
cell suspension in PBS was mixed with 40 μl trypan blue, and the
numbers of stained (dead cells) and unstained cells (live cells)
were examined under a Nikon Eclipse TS-100F inverted microscope
(Nikon Corp., Tokyo, Japan) (24).
Cell cycle analysis
The NRK-52E cells were cultured in 25-cm2
culture flasks and treated with different concentrations of
p-PD (50, 100, 200 and 300 μg/ml) for 24 h. Subsequent to
exposure, the cells were collected, washed with PBS and fixed with
ice-cold 70% ethanol overnight at 4°C. The cells were washed with
PBS, stained with 1 ml fluorochrome solution from the
Annexin-V-FLUOS Staining kit [containing 20 μg/ml propidium iodide
(PI) and 10 μg/ml RNase A] for 15 min in dark conditions and
analysed using a BD FACSCalibur flow cytometer (E97500679; BD
Biosciences, Franklin Lakes, NJ, USA).
Annexin-V staining
The NRK52E cells were cultured in 60-mm
tissue-culture dishes. The culture medium was replaced with fresh
medium as cells reached 70% confluence, then different
concentrations of p-PD (50, 100, 200 and 300 μg/ml) were
added prior to 24-h culture. Levels of apoptosis were determined by
staining with the Annexin-V kit (25). Following incubation, floating or
adherent cells that were later trypsinised were pooled and
centrifuged for 5 min at 1,000 × g. Pelleted cells were washed with
PBS. Next, cells were centrifuged for 5 min at 1,000 × g and
resuspended in 100 μl Annexin-V-Fluos and PI labelling solution
(from the Annexin-V kit) for 10 min. The stained cells were
analysed by flow cytometry, where the fluorescence emission was
measured at 530 nm. The percentage cell apoptosis was calculated
using BD Multiset™ 2.2, BD FACStation™ 5.2.1, ModFit LT 3.0 and
CellQuest software (BD Biosciences).
Detection of intracellular reactive
oxygen species (ROS)
The NRK52E cells were treated with 100 μg/ml
p-PD for 1, 2 or 3 h, and controls were treated with 0.1%
DMSO. All cells were stained with 10 μM 2′,7′-dichlorofluorescin
diacetate (DCFH-DA; Sigma-Aldrich) for 30 min. Subsequent to
washing with PBS, the fluorescence intensity was detected by a
Tecan Infinite 200 PRO fluorescence plate reader (Tecan Group Ltd.,
Maennedorf, Switzerland) with excitation and emission wavelengths
of 488 and 525 nm, respectively.
Western blot analysis
Western blot analysis was performed according to the
methods of a previous study (26).
The culture medium was replaced with fresh medium as cells reached
70% confluence, then different concentrations of p-PD (50,
100, 200 and 300 μg/ml) were added and cells were cultured for 24
h. Next, adherent and floating cells were collected and homogenised
in a lysis buffer (10 mM Tris-HCl, pH 8.0; 0.32 mM sucrose; 5 mM
EDTA; 2 mM DTT; 1 mM phenylmethyl sulfonyl fluoride; and 1% Triton
X-100) and centrifuged at 10,621 × g (5427R; Eppendorf, Hamburg,
Germany) for 10 min. The supernatants were collected and assayed
for protein concentration using the Bradford protein assay method
(27). An equal quantity of
protein per sample was subjected to 10% SDS-polyacrylamide gel
electrophoresis. Following electrophoresis, the proteins were
transferred to the PVDF membranes by electroblotting and incubated
with diluted primary antibodies for 1 h at 25°C. The membranes were
washed, incubated for 30 min at 25°C with the HRP-conjugated
secondary antibodies and subsequently washed extensively prior to
detection by chemiluminescence with the ECL-Plus kit. The proteins
were visualised by exposing the blots to film (Kodak, Rochester,
NY, USA). The western blot data were quantified using Image J
software (http://imagej.nih.gov/ij/).
Statistical analysis
Results are expressed as the mean ± standard
deviation from at least three independent experiments. Statistical
analysis was performed using Student’s t-test.
*P<0.05 was considered to indicate a statistically
significant difference. The error bars denote standard
deviation.
Results
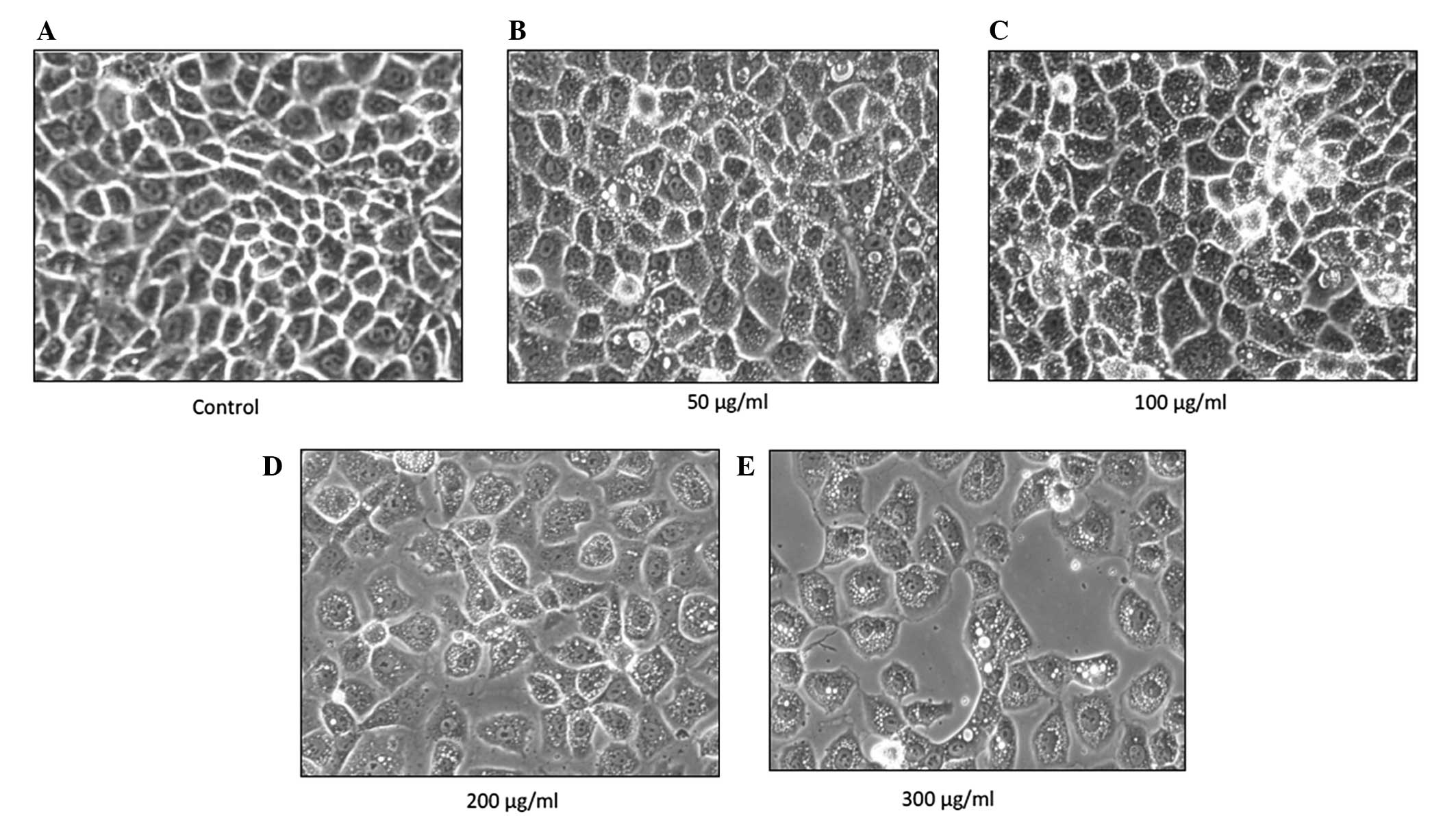
p-PD alters cell morphology
The NRK-52E cells were treated with four different
concentrations of p-PD (50, 100, 200 and 300 μg/ml) for 24
h, then observed under an inverted microscope. The control cells
retained their normal, clear plasma membrane. There was a uniform
cell distribution with neighbouring cells closely connected to each
other and clear cell nuclei (Fig.
1A). Following p-PD treatment, the cells were enlarged,
forming a variety of shapes and sizes. The cells lost contact with
neighbouring cells and cytoplasmic vacuolisation occurred (Fig. 1B and C). In addition, cell
shrinkage and blebbing of the plasma membrane were also observed at
higher p-PD concentrations (Fig. 1D and E).
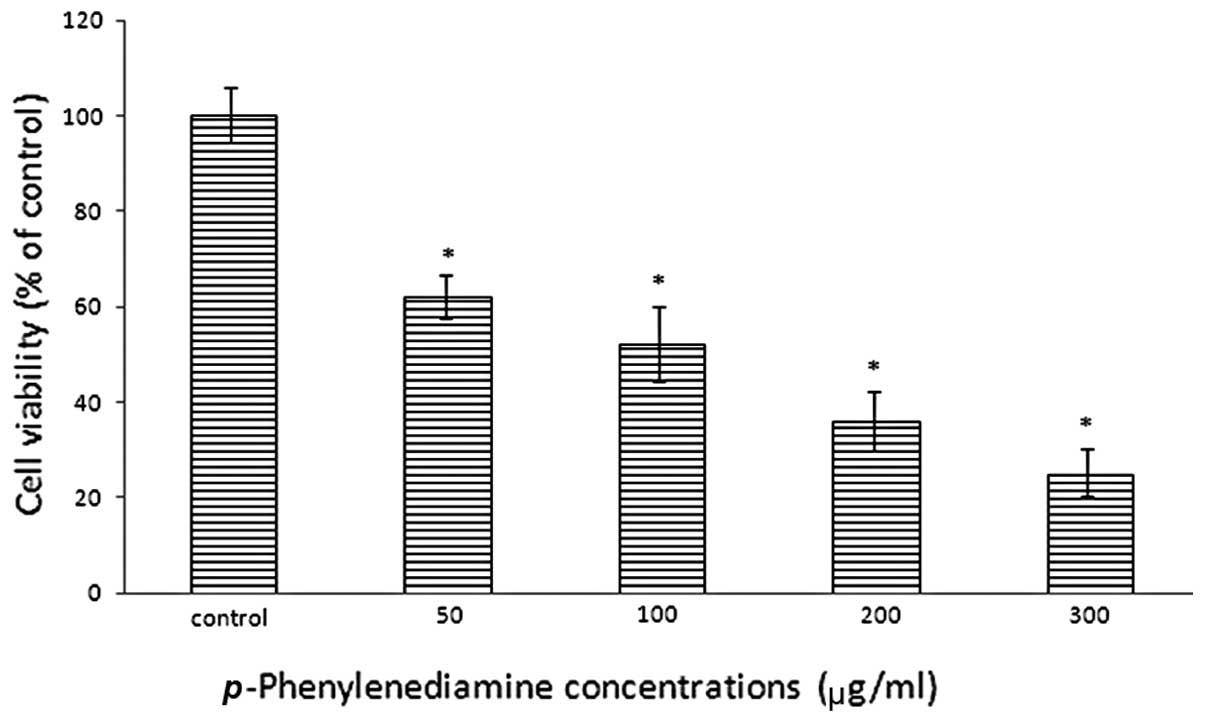
p-PD reduces cell viability
The cell viability of p-PD-treated NRK-52E
cells was examined with a trypan blue exclusion assay subsequent to
a 24 h incubation period. The results demonstrated reduced cell
viability in NRK-52E cells that were treated with p-PD; and
as the concentration increased, cell viability reduced. Treatments
of 50, 100, 200 and 300 μg/ml led to cell viabilities of 62.2,
52.5, 36.8 and 25.4% of the control (Fig. 2), respectively.
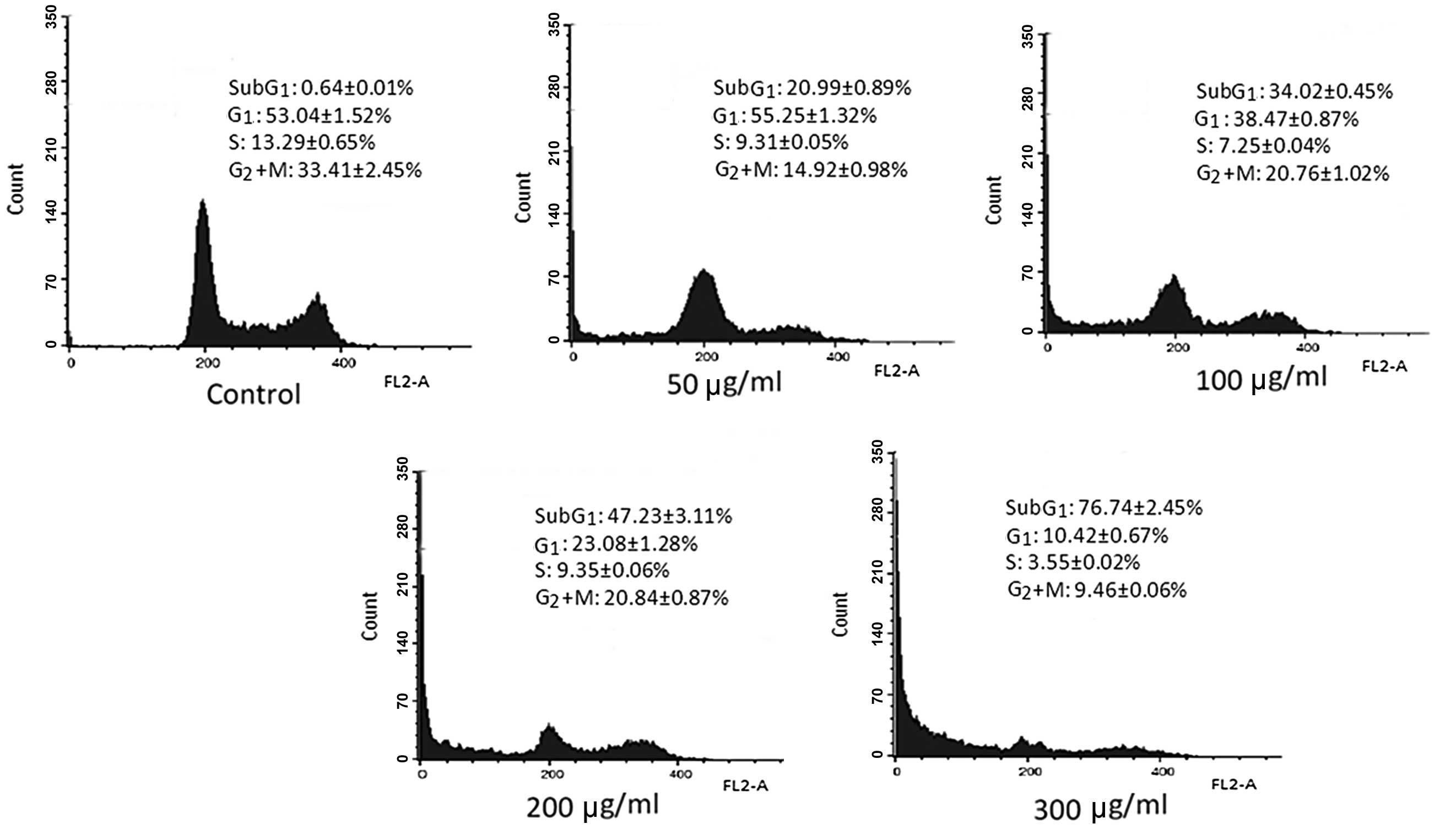
p-PD alters cell cycle progression and
inhibits mitosis
p-PD was previously demonstrated to induce
cell death. To determine the nature of the cell death (necrotic or
apoptotic) changes in the DNA content of the p-PD-treated
cells were detected using the PI staining method. The cell cycle
results indicated that the cell cycle distribution of control cells
was as follows: 0.64±0.01% in the sub-G1 phase;
53.04±1.52% in the G1 phase; 13.29±0.65% in the S phase;
and 33.41±2.45% in the G2+M phase.
In cells exposed to 50 μg/ml p-PD, the
percentage of cells in the sub-G1 phase increased to
20.99±0.89%, compared with 34.02±0.45% following exposure to 100
μg/ml p-PD. When the p-PD concentrations increased to
200 and 300 μg/ml, the percentages of cells in the
sub-G1 phase further increased to 47.23±3.11 and
76.74±2.45%, respectively (Fig.
3).
p-PD also induced a reduction in the numbers
of cells in the G2+M phase compared with the control
cells. Compared with 33.41±2.45% in the control cells, the cells
treated with p-PD at concentrations of 50, 100, 200 and 300
μg/ml presented 14.92±0.98, 20.76±1.02, 20.84±0.87 and 9.46±0.06%
of cells in the G2+M phase, respectively. This result
demonstrates a reduction in mitosis in the treated cells compared
with the control cells (Fig.
3).
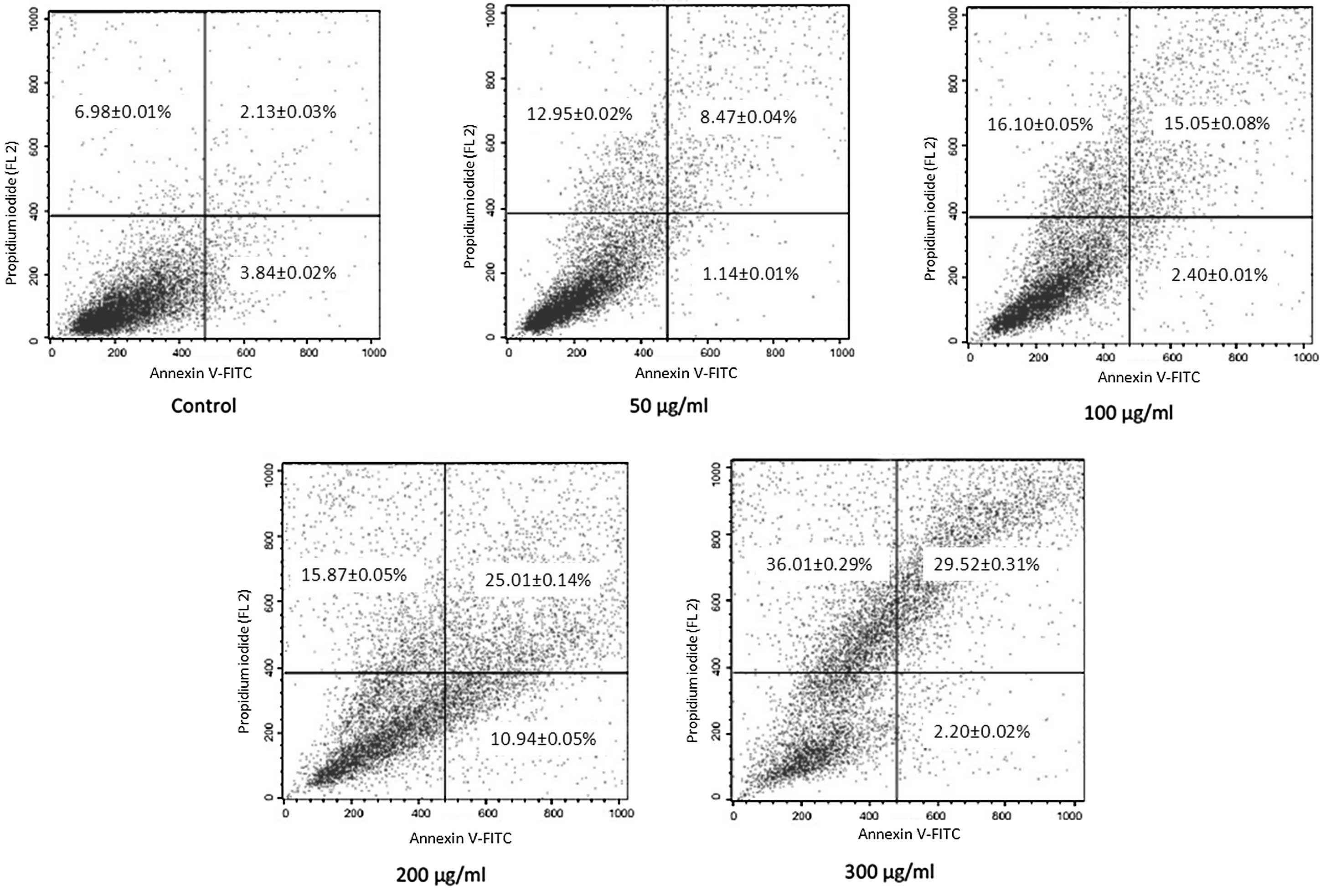
Annexin-V staining and flow
cytometry
The induction of apoptosis by p-PD was
further confirmed by Annexin-V staining. Following incubation with
p-PD at a concentration of 50, 100, 200 or 300 μg/ml for 24
h, the percentages of Annexin V+/PI+ cells
increased to 8.47±0.04, 15.05±0.08, 25.01±0.14 and 29.52±0.31%,
respectively, compared with the control group (2.13±0.03%).
Additionally, Annexin V+/PI− cells were also increased
to 12.95±0.02, 16.10±0.05, 15.87±0.05 and 36.01±0.29%,
respectively, compared with the control group (6.98±0.01%)
(Fig. 4).
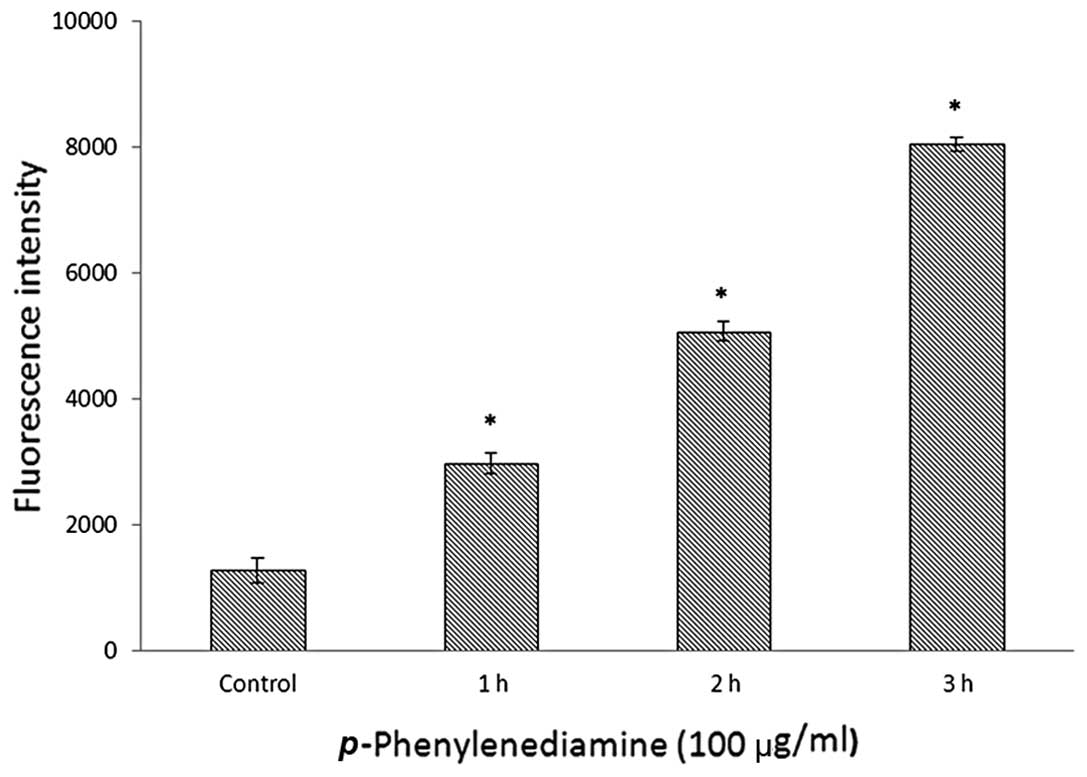
p-PD induces intracellular ROS
generation
The effects of p-PD on the production of
intracellular ROS were examined following DCFH-DA staining. The
control cells exhibited low-level ROS generation (Fig. 5), and the cells treated with 100
μg/ml p-PD for 1 h presented a ROS level twice as high as
controls. Following 2-h treatment, the ROS level had increased
four-fold compared with the control cells. The intracellular ROS
level increased markedly in the cells that were treated with 100
μg/ml p-PD for 3 h (Fig.
5).
Effects of p-PD on the protein
expression levels of Ras, Raf, SAPK-JNK, Akt, Bcl-2, Bcl-xL and
Bad
To assess the molecular mechanism underlying
p-PD-induced apoptosis, the expression levels of the
survival proteins Ras, Raf, Bcl-2 and Bcl-XL were assessed at 24 h
following p-PD treatments of 50, 100, 200 and 300 μg/ml. The
results demonstrated that the expression levels of the Ras and Raf
survival proteins were reduced by p-PD in a dose-dependent
manner (Fig. 6). However, Akt,
Bcl-2 and Bcl-xL protein expression levels were not markedly
altered compared with controls.
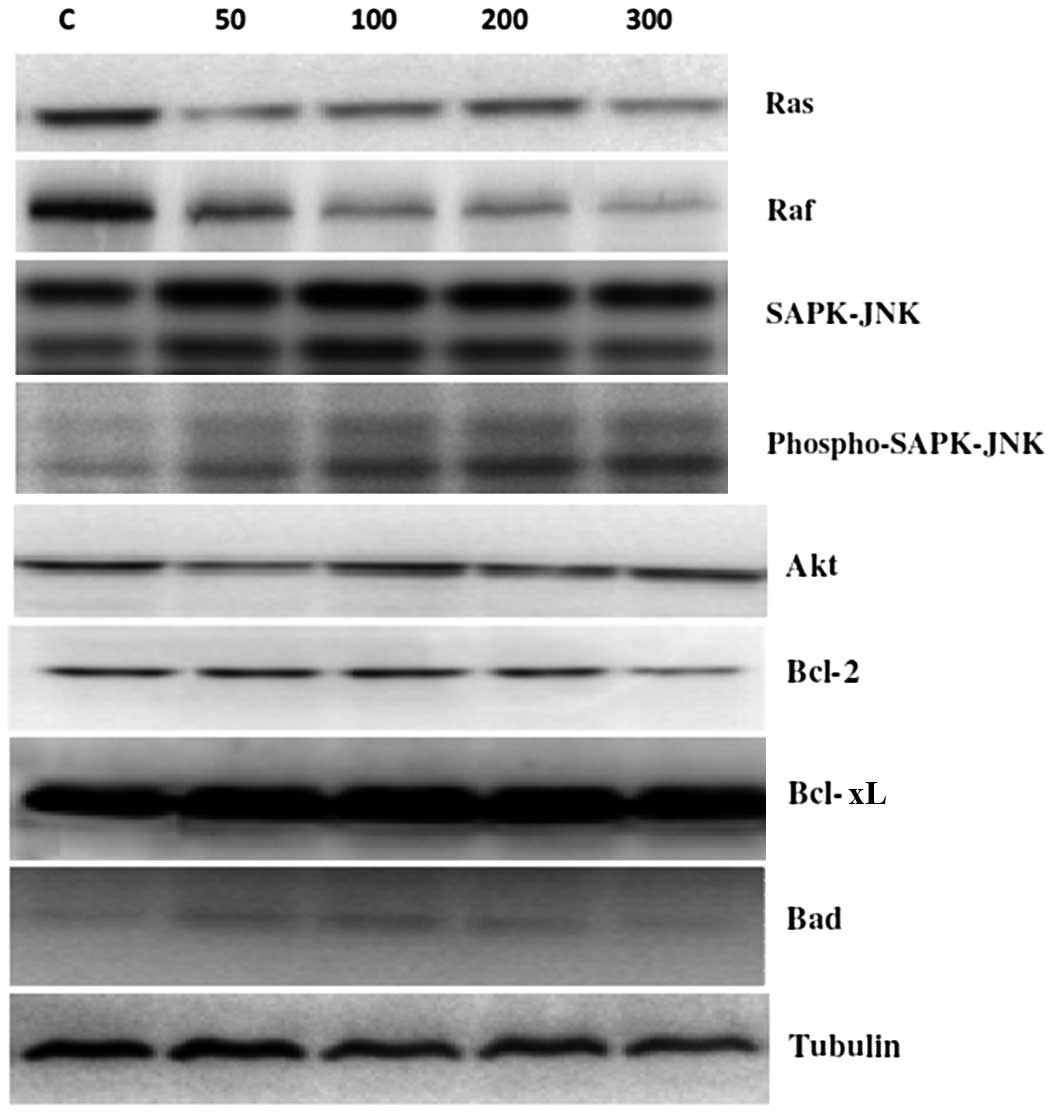 | Figure 6Effects of p-PD on signalling
pathways in NRK52E cells. NRK52E cells were treated with different
doses of p-PD (50, 100, 200 and 300 μg/ml) for 24 h. The
protein expression levels of Ras, Raf, SAPK-JNK, Akt, Bcl-2, Bcl-xL
and Bad were evaluated by western blot analysis. p-PD,
para-phenylenediamine; SAPK, stress-activated protein
kinase; JNK, c-Jun N-terminal kinase Akt, protein kinase B. |
With regards to the apoptotic proteins, SAPK-JNK
expression level was not markedly altered, and the phosphorylated
SAPK-JNK expression levels markedly increased as p-PD
concentrations increased (Fig. 6).
In addition, p-PD had no effect on the Bad expression level
with p-PD treatment, compared with the control cells.
Tubulin was used as a loading control.
Discussion
Carcinogenesis is a process resulting from genetic
alterations leading to mutations of oncogenes or tumour suppressor
genes that drive the progressive transformation of normal cells
into malignant cells (28,29). At the molecular level, genetic
mutations are able to alter translated proteins and thereby disrupt
downstream signalling pathways that are essential for apoptosis,
cell cycle and other cellular processes (30,31).
Cadmium, a causative agent in various types of cancer, elevates
intracellular free calcium ion ([Ca2+]i) levels, leading
to neuronal apoptosis partly by activating mitogen-activated
protein kinases (MAPK) and mammalian target of rapamycin pathways
(32). Additionally, cadmium
exposure leads to the induction of the ERK signalling pathway,
which alters gene expression in osteoblasts, and apoptotic death in
Saos-2 cells (33). Chronic
exposure to arsenic can lead to the development of various types of
cancer; it downregulates Akt and c-Fos protein expression, and
induces apoptosis in glutathione-deficient cells (34).
p-PD is a potential carcinogen that is widely
used in permanent hair dye (35,36),
and it has been reported that incidences of kidney tumours increase
in rats following exposure to p-PD (6). Therefore, in the present study, the
molecular mechanism underlying p-PD-induced apoptosis was
investigated in NRK-52E cells, and to the best of our knowledge, it
was demonstrated for the first time that p-PD-induces cell
death in a dose-dependent manner in NRK-52E cells (Fig. 2). It was confirmed that this cell
death was due to apoptosis, as indicated in Fig. 3. Cell cycle analysis demonstrated a
reduction in the number of cells in the G2+M phase in
addition to an increase in the number of cells in the
sub-G1 phase in the treated cells, when compared with
the respective percentages in the control group. This finding
indicates that p-PD induced cell cycle arrest. In addition,
Annexin-V staining demonstrated that the number of apoptotic cells
increased following p-PD exposure in a dose-dependent manner
(Fig. 4). In previous studies, it
has been established that oxidised p-PD induces the
production of ROS, leading to an imbalance between production and
the removal of ROS and overwhelming oxidative stress that
eventually induces apoptosis (37,38).
ROS generated primarily by the mitochondria are
highly reactive metabolites that are produced during normal cell
metabolism (39). Curtin et
al (40) reported that the
increases in intracellular ROS levels may lead to apoptosis. The
underlying mechanism may involve the direct interaction and
destruction of cellular proteins, lipids and DNA, and/or indirect
interference with normal cellular signalling pathways and gene
regulation (41). Consistent with
these findings, the present results demonstrated that intracellular
ROS levels increased significantly in the p-PD-treated
NRK-52E cells in a dose-dependent manner (Fig. 5). High levels of intracellular ROS
cause disruption of the mitochondrial membrane potential, release
of cytochrome c with subsequent activation of the caspase
cascade and ultimately, programmed cell death (42,43).
Additionally, intracellular ROS can catalytically inactivate
protein tyrosine phosphatases through the oxidation of active-site
cysteine residues, which negatively regulate receptor tyrosine
kinase (RTK) activity and downstream signalling, and hence allow
sustained PTK phosphorylation and activation (44).
PTKs serve a key role in the transmission of various
signals from cell-surface receptors to the nucleus. PTKs can be
divided into the transmembrane (T)RTKs and non-RTKs (45). Ras links RTKs and non-RTKs to
downstream serine/threonine kinases, including the MAPKs (46). The activation of the Ras/Raf/MAPK
pathway has been demonstrated to induce growth arrest in several
cell types. Oridonin induces apoptosis in L929 cells through
inhibition of the PTK/Ras/Raf/JNK pathway (20). In addition, PKT/Ras/Raf/JNK
inhibition-derived ROS/NO production contributed to G2/M phase cell
cycle arrest in evodiamine-treated human cervix carcinoma HeLa
cells (19). Consistent with these
findings, in the current study it was demonstrated that the
Ras/Raf/JNK pathway is able to promote apoptosis by inducing
p-PD in NRK52E cells. Additionally, anti-carcinogenic
compounds, UV- and gamma-irradiation have previously been indicated
to induce apoptosis via a JNK-dependent pathway (47–50).
Oxidative stress stimulates multiple intracellular
signal transduction pathways such as Akt-Bad. Akt, which is
downstream of PI3K, regulates mechanically driven and
receptor-ligand signalling (51).
Activation of the PI3K/Akt can lead to Bad phosphorylation at
specific serine residues. Phosphorylated Bad binds 14-3-3ζ proteins
in the cytosol that sequester and tag Bad for subsequent
degradation (52). Alternatively,
pro-apoptotic proteins can be retained in the cytosol by binding to
anti-apoptotic proteins, such as Bcl-2 and Bcl-xL (53). An increase in Bcl-2 and Bcl-xL
expression prevents cytochrome c release from the
mitochondria, thereby inhibiting activation of caspases, such as
caspase-9 and caspase-3, and preventing apoptosis (54,55).
In the present study, it was demonstrated that there were no
changes in the levels of Bcl-2, Bcl-xL and Bad proteins compared
with controls (Fig. 7). These findings suggest that the molecular
mechanism triggered by p-PD-induced cell death is
independent of the PI3K/Akt/Bad pathway.
In conclusion, the results of the present study
demonstrated that p-PD induced apoptosis in NRK52E cells; in
addition, DCFH-DA staining confirmed that apoptosis was induced due
to oxidative stress. Furthermore, the results indicated that
p-PD induced apoptosis via the PTK-Ras-Raf-JNK pathway,
which upregulated SAPK/JNK protein expression levels and
downregulated Ras and Raf protein expression levels. However,
p-PD was found to induce apoptosis independent of PI3K/Akt
pathway, as Akt, Bcl-2, Bcl-XL and Bad protein expression levels
were not significantly altered compared with the control. Future
studies are required in order to further elucidate the role of
p-PD in tumorigenesis.
Acknowledgements
The present study was supported by grants
FRGS/2/2010/ST/IMU/03/1(SKK) from Jabatan Pengajian Tinggi Malaysia
and BMS 102-2010 (10) from the
International Medical University, Kuala Lumpur, Malaysia.
References
|
1
|
Gago-Dominguez M, Castelao JE, Yuan JM, Yu
MC and Ross RK: Use of permanent hair dyes and bladder-cancer risk.
Int J Cancer. 91:575–579. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung KT and Stevens SE Jr: Degradation of
azo dyes by environmental microorganisms and helminths. Environ
Toxicol Chem. 12:2121–2132. 1993.
|
|
3
|
Chung KT, Stevens SE Jr and Cerniglia CE:
The reduction of azo dyes by the intestinal microflora. Crit Rev
Microbiol. 18:175–190. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thun MJ, Altekruse SF, Namboodiri MM,
Calle EE, Myers DG and Heath CW Jr: Hair dye use and risk of fatal
cancers in US women. J Natl Cancer Inst. 86:210–215. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rauscher GH, Shore D and Sandler DP: Hair
dye use and risk of adult acute leukemia. Am J Epidemiol.
160:19–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sontag JM: Carcinogenicity of
substituted-benzenediamines (phenylenediamines) in rats and mice. J
Natl Cancer Inst. 66:591–602. 1981.PubMed/NCBI
|
|
7
|
Ding X, Zhu F, Li T, Zhou Q, Hou FF and
Nie J: Numb protects renal proximal tubular cells from puromycin
aminonucleoside-induced apoptosis through inhibiting Notch
signaling pathway. Int J Biol Sci. 7:269–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu CT, Sheu ML, Tsai KS, Weng TI, Chiang
CK and Liu SH: The role of endoplasmic reticulum stress-related
unfolded protein response in the radiocontrast medium-induced renal
tubular cell injury. Toxicol Sci. 114:295–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stupack DG and Cheresh DA: Get a ligand,
get a life: integrins, signaling and cell survival. J Cell Sci.
115:3729–3738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guan JL and Shalloway D: Regulation of
focal adhesion-associated protein tyrosine kinase by both cellular
adhesion and oncogenic transformation. Nature. 358:690–692. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Frisch SM, Vuori K, Ruoslahti E and
Chan-Hui PY: Control of adhesion-dependent cell survival by focal
adhesion kinase. J Cell Biol. 134:793–799. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilmore AP, Metcalfe AD, Romer LH and
Streuli CH: Integrin-mediated survival signals regulate the
apoptotic function of Bax through its conformation and subcellular
localization. J Cell Biol. 149:431–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao JH, Reiske H and Guan JL: Regulation
of the cell cycle by focal adhesion kinase. J Cell Biol.
143:1997–2008. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurenova E, Xu LH, Yang X, Baldwin AS Jr,
et al: Focal adhesion kinase suppresses apoptosis by binding to the
death domain of receptor-interacting protein. Mol Cell Biol.
24:4361–4371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leverrier Y, Thomas J, Mathieu AL, Low W,
Blanquier B and Marvel J: Role of PI3-kinase in Bcl-X induction and
apoptosis inhibition mediated by IL-3 or IGF-1 in Baf-3 cells. Cell
Death Differ. 6:290–296. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee BH and Ruoslahti E: Alpha5beta1
integrin stimulates Bcl-2 expression and cell survival through Akt,
focal adhesion kinase, and Ca2+/calmodulin-dependent
protein kinase IV. J Cell Biochem. 95:1214–1223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abe M, Suzuki K, Inagaki O, Sassa S and
Shikama H: A novel MPL point mutation resulting in
thrombopoietin-independent activation. Leukemia. 16:1500–1506.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Wu LJ, Tashino S, Onodera S and
Ikejima T: Protein tyrosine kinase pathway-derived ROS/NO
productions contribute to G2/M cell cycle arrest in
evodiamine-treated human cervix carcinoma HeLa cells. Free Radic
Res. 44:792–802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera
S and Ikejima T: Oridonin induces G2/M arrest and apoptosis via
activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK
survival pathway in murine fibrosarcoma L929 cells. Arch Biochem
Biophys. 490:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen SC, Chen CH, Chern CL, Hsu LS, Huang
YC, Chung KT and Chye SM: p-Phenylenediamine induces p53-mediated
apoptosis in Mardin-Darby canine kidney cells. Toxicol In Vitro.
20:801–807. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen SC, Chen CH, Tioh YL, Zhong PY, Lin
YS and Chye SM: Para-phenylenediamine induced DNA damage and
apoptosis through oxidative stress and enhanced caspase-8 and -9
activities in Mardin-Darby canine kidney cells. Toxicol In Vitro.
24:1197–1202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang YC, Hung WC, Kang WY, Chen WT and
Chai CY: p-Phenylenediamine induced DNA damage in SV-40
immortalized human uroepithelial cells and expression of mutant p53
and COX-2 proteins. Toxicol Lett. 170:116–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pettit GR, Hoard MS, Doubek DL, Schmidt
JM, Pettit RK, Tackett LP and Chapuis JC: Antineoplastic agents
338: The cancer cell growth inhibitory. Constituents of Terminalia
arjuna (Combretaceae). J Ethnopharmacol. 53:57–63. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu HJ, Wang JS, Guo QL, Jiang Y and Liu
GQ: Reversal of P-glycoprotein mediated multidrug resistance in
K562 cell line by a novel synthetic calmodulin inhibitor, E6. Biol
Pharm Bull. 28:1974–1978. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haendeler J, Zeiher AM and Dimmeler S:
Vitamin C and E prevent lipopolysaccharide-induced apoptosis in
human endothelial cells by modulation of Bcl-2 and Bax. Eur J
Pharmacol. 317:407–411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ernst O and Zor T: Linearization of the
bradford protein assay. J Vis Exp. 38(Pt II): 19182010.PubMed/NCBI
|
|
28
|
Balmain A, Gray J and Ponder B: The
genetics and genomics of cancer. Nat Genet. 33(Suppl): 238–244.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hahn WC and Weinberg RA: Modelling the
molecular circuitry of cancer. Nat Rev Cancer. 2:331–341. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen S, Xu Y, Xu B, Guo M, Zhang Z, Liu L,
et al: CaMKII is involved in cadmium activation of MAPK and mTOR
pathways leading to neuronal cell death. J Neurochem.
119:1108–1118. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Arbon KS, Christensen CM, Harvey WA and
Heggland SJ: Cadmium exposure activates the ERK signaling pathway
leading to altered osteoblast gene expression and apoptotic death
in Saos-2 cells. Food Chem Toxicol. 50:198–205. 2012. View Article : Google Scholar :
|
|
34
|
Habib GM: Arsenite causes down-regulation
of Akt and c-Fos, cell cycle dysfunction and apoptosis in
glutathione-deficient cells. J Cell Biochem. 110:363–371.
2010.PubMed/NCBI
|
|
35
|
Kelsh MA, Alexander DD, Kalmes RM and
Buffler PA: Personal use of hair dyes and risk of bladder cancer: a
meta-analysis of epidemiologic data. Cancer Causes Control.
19:549–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McFadden JP, White IR, Frosch PJ, Sosted
H, Johansen JD and Menne T: Allergy to hair dye. BMJ. 334:2202007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Atukeren P, Yavuz B, Soydinc HO, Purisa S,
Camlica H, Gumustas MK and Balcioglu I: Variations in systemic
biomarkers of oxidative/nitrosative stress and DNA damage before
and during the consequent two cycles of chemotherapy in breast
cancer patients. Clin Chem Lab Med. 48:1487–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeraatpishe A, Oryan S, Bagheri MH,
Pilevarian AA, et al: Effects of Melissa officinalis L. on
oxidative status and DNA damage in subjects exposed to long-term
low-dose ionizing radiation. Toxicol Ind Health. 27:205–212. 2011.
View Article : Google Scholar
|
|
39
|
Reddy PH: Amyloid precursor
protein-mediated free radicals and oxidative damage: implications
for the development and progression of Alzheimer’s disease. J
Neurochem. 96:1–13. 2006. View Article : Google Scholar
|
|
40
|
Curtin JF, Donovan M and Cotter TG:
Regulation and measurement of oxidative stress in apoptosis. J
Immunol Methods. 265:49–72. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan PH: Reactive oxygen radicals in
signaling and damage in the ischemic brain. J Cereb Blood Flow
Metab. 21:2–14. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ling YH, Liebes L, Zou Y and Perez-Soler
R: Reactive oxygen species generation and mitochondrial dysfunction
in the apoptotic response to Bortezomib, a novel proteasome
inhibitor, in human H460 non-small cell lung cancer cells. J Biol
Chem. 278:33714–33723. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qiu JH, Asai A, Chi S, Saito N, Hamada H
and Kirino T: Proteasome inhibitors induce cytochrome
c-caspase-3-like protease-mediated apoptosis in cultured cortical
neurons. J Neurosci. 20:259–265. 2000.PubMed/NCBI
|
|
44
|
Chiarugi P and Cirri P: Redox regulation
of protein tyrosine phosphatases during receptor tyrosine kinase
signal transduction. Trends Biochem Sci. 28:509–514. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baselga J and Arteaga CL: Critical update
and emerging trends in epidermal growth factor receptor targeting
in cancer. J Clin Oncol. 23:2445–2459. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Khosravi-Far R, Solski PA, Clark GJ, Kinch
MS and Der CJ: Activation of Rac1, RhoA, and mitogen-activated
protein kinases is required for Ras transformation. Mol Cell Biol.
15:6443–6453. 1995.PubMed/NCBI
|
|
47
|
Chen YR, Wang W, Kong AN and Tan TH:
Molecular mechanism of c-Jun N-terminal kinase-mediated apoptosis
induced by anticarcinogenic isothiocyanates. J Biol Chem.
273:1769–1775. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu K, Zhao Y, Li GC and Yu WP: c-Jun
N-terminal kinase is required for vitamin E succinate-induced
apoptosis in human gastric cancer cells. World J Gastroenterol.
10:1110–1114. 2004.PubMed/NCBI
|
|
49
|
Wang TH, Wang HS and Soong YK:
Paclitaxel-induced cell death: where the cell cycle and apoptosis
come together. Cancer. 88:2619–2628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sánchez-Pérez I, Martínez-Gomariz M,
Williams D, Keyse SM and Perona R: CL100/MKP-1 modulates JNK
activation and apoptosis in response to cisplatin. Oncogene.
19:5142–5152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Matsui T and Rosenzweig A: Convergent
signal transduction pathways controlling cardiomyocyte survival and
function: the role of PI3-kinase and Akt. J Mol Cell Cardiol.
38:63–71. 2005. View Article : Google Scholar
|
|
52
|
She QB, Solit DB, Ye Q, O’Reilly KE, Lobo
J and Rosen N: The BAD protein integrates survival signaling by
EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor
cells. Cancer Cell. 8:287–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Finucane DM, Bossy-Wetzel E, Waterhouse
NJ, Cotter TG and Green DR: Bax induced caspase activation and
apoptosis via cytochrome c release from mitochondria is inhibitable
by Bcl-xL. J Biol Chem. 274:2225–2233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Ann Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar
|
|
55
|
Desagher S and Martinou JC: Mitochondria
as the central control point of apoptosis. Trends Cell Biol.
10:369–377. 2000. View Article : Google Scholar : PubMed/NCBI
|