Introduction
Previous studies have reported that curcumin
exhibited a wide spectrum of biological activities, including
anti-inflammatory (1,2), anti-tumor (3–6),
antioxidant (7,8), anti-microbial (9,10),
choleretic (11), neuroprotective
(12,13) and anti-mutagenic (14) activities; curcumin was also found
to be involved in numerous drug-drug interactions (15,16).
Evidence suggested that curcumin was markedly efficacious and safe
at high doses (17,18); however, due to low absorption and
rapid metabolism, the bioavailability of curcumin was not ideal
(19). Previous studies on the
pharmacokinetics of curcumin led to the identification of key
metabolites, including tetrahydrocurcumin, curcumin-O-glucuronide,
curcumin-O-sulfate, hexacurcumin, monoacetylcurcumin and
octahydrocucumin (20), of which
tetrahydrocurcumin was characterized as the primary metabolite and
has been widely investigated for its biological activities.
Tetrahydrocurcumin was reported to induce autophagic
cell death through coordinated modulation of the phosphoinositide
3-kinase/Akt-mechanistic target of rapamycin and mitogen-activated
protein kinase signaling pathways in human leukemia HL-60 cells
(21). Tetrahydrocurcumin was
reported to reduce HT1080-cell invasion and migration via
downregulation of extracellular matrix (ECM)-degrading enzymes and
inhibition of cell adhesion to ECM proteins (22). The anti-cancer and anti-angiogenic
effects of curcumin and tetrahydrocurcumin were also demonstrated
in implanted hepatocellular carcinoma in nude mice (23). Furthermore, tetrahydrocurcumin was
shown to be more effective than curcumin in preventing
azoxymethane-induced colon carcinogenesis (24).
However, previous studies on the anti-inflammatory
effect of tetrahydrocurcumin and the biological activities of other
curcumin metabolites are limited (25). One of these studies identified
metabolites of curcuminoids in the feces and urine of rats
following oral administration (26). The aim of the present study was to
investigate and compare the anti-inflammatory activities of
curcumin and its three metabolites, tetrahydrocurcumin,
hexahydrocurcumin and octahydrocurcumin in lipopolysaccharide
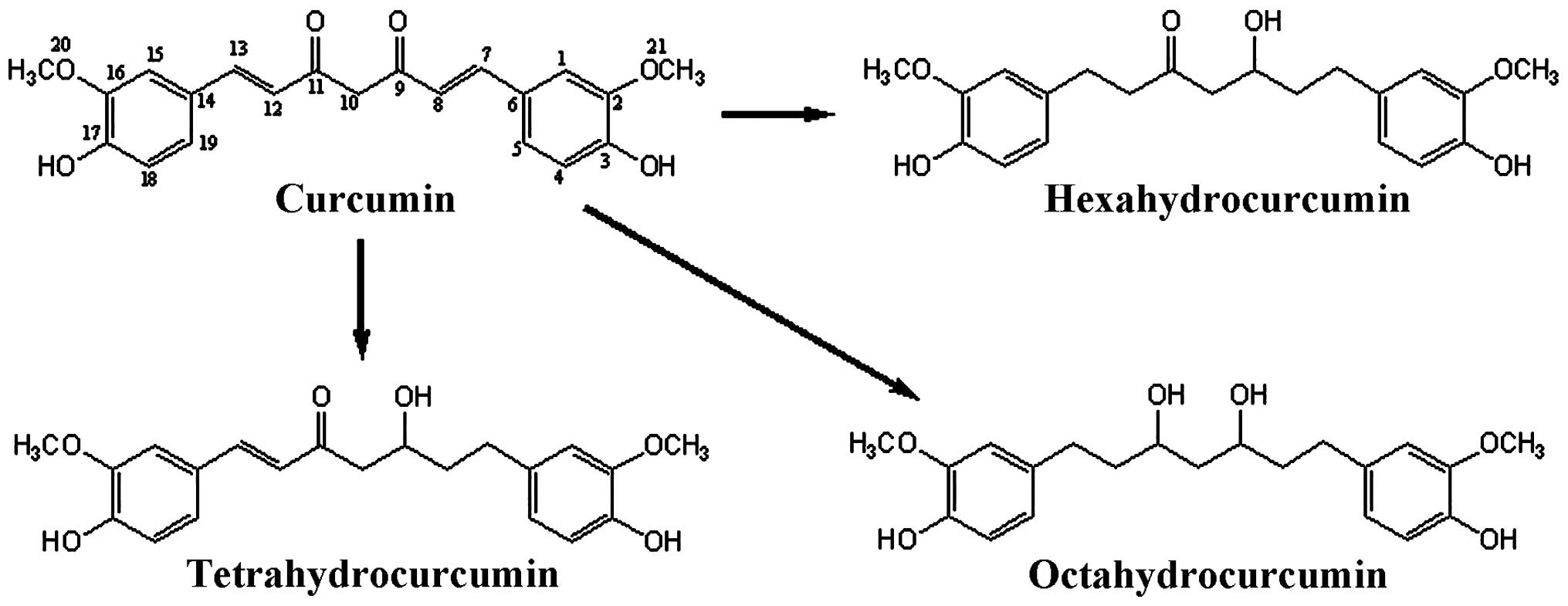
(LPS)-stimulated RAW 264.7 macrophage cells. Chemical structures of
curcumin and its three metabolites are shown in Fig. 1.
Materials and methods
Curcumin and its metabolites
A mixture of curcuminoids (curcumin, 55.2%;
demethoxycurcumin, 22.9%; and bisdemethoxycurcumin, 21.8%) was
purchased from Tianjin Jian Feng Pharmaceutical Co., Ltd (Tianjin,
China). Male Wistar-derived rats (200–250 g body weight, 8–10 weeks
old, provided by the Laboratory Animal Center of the Shenyang
Pharmaceutical University, Shenyang, China) were housed under
controlled temperature (22±2°C), humidity (55±10%) and light (8:00
a.m. to 8:00 p.m.) conditions in a breeding room. Normal food and
water were available ad libitum but were withdrawn 24 h
prior to administration. Curcuminoids were administered per
os as a 30% aqueous 1,2-propylene glycol solution. Urine and
feces were collected for 48 h from the animals housed in stainless
steel metabolism cages equipped with a urine and feces separator
(B6–10, Suhang Technology Equipment Co., Ltd., Suzhou, China).
Animal experiments were performed in accordance with the Guide for
the Care and Use of Laboratory Animals of the Shenyang
Pharmaceutical University (approval date: 08/10/2010, no. 100810;
Liaoning, China). Tetrahydrocurcumin, hexahydrocurcumin and
octahydrocurcumin (Fig. 1) were
isolated from feces and urine of rats over 48 h following oral
administration of the curcuminoid mixture, as previously described
(26). The purity of
tetrahydrocurcumin, hexahydrocurcumin and octahydrocurcumin was
determined using high-performance liquid chromatography (HPLC;
Waters 600; Waters Corp., Milford, MA, USA) and a ultraviolet
detector (Waters 490; Waters Corp.); peak areas were normalized and
the purities of the metabolites were 98.2, 98.6 and 98.5%,
respectively.
Reagents
RPMI 1640 medium and fetal bovine serum (FBS) were
obtained from Invitrogen Life Technologies (Carlsbad, CA, USA).
Mouse tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)
ELISA kits as well as a bicinchoninic acid (BCA) protein
concentration assay kit were obtained from Yantai Science and
Biotechnology Co., Ltd (Shandong, China). Anti-NOS2 antibody
(sc-651, rabbit polyclonal IgG, dilution 1:1,000), anti-Cox-2
antibody (sc-1746, goat polyclonal IgG, dilution 1:1,000),
anti-IκB-α antibody (sc-371, rabbit polyclonal IgG, dilution
1:1,000), anti-NFκB p65 antibody (sc-372, rabbit polyclonal IgG,
dilution 1:1,000) and anti-actin antibody (sc-1616, goat polyclonal
IgG, dilution 1:1,000), as well as horseradish peroxidase
(HRP)-conjugated secondary antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Phenylmethylsulfonyl
fluoride, dithiothreitol, E. coli, LPS and all other
chemicals used in the present study were purchased from
Sigma-Aldrich (St. Louis, MO, USA). A 50 mM solution of tested
samples was prepared in 100% cell culture grade dimethyl sulfoxide,
stored as small aliquots at −20°C, and then diluted to the required
concentrations prior to use. Hydrocortisone (H4001; purity, ≥98%;
Sigma-Aldrich) was used as the positive control drug for all
experiments performed.
Cell culture
Mouse monocyte-macrophage RAW 264.7 cells (TIB-71;
American Type Culture Collection, Manassas, VA, USA) were
maintained in RPMI 1640 medium supplemented with penicillin (100
U/ml; Yantai Science and Biotechnology Co., Ltd.), streptomycin
(100 μg/ml; Yantai Science and Biotechnology Co., Ltd.) and 10%
heat-inactivated FBS at 37°C in a humidified incubator with 5%
CO2 and 95% air. Medium was routinely replaced every two
days and cells were passaged using 0.25% trypsin (Yantai Science
and Biotechnology Co., Ltd.) until they attained confluence.
Nitric oxide (NO) analysis
The amount of nitrite in the cell culture
supernatant was determined using a commercial Griess reagent kit (a
mixture of Griess reagent A and Griess reagent B, ratio 1:1, A: 1%
sulphanilamide in 5% H3PO4 and B: 0.1%
naphthylethylene diamine dihydrochloride) (Yantai Science and
Biotechnology, Co., Ltd.) as previously described (27). RAW 264.7 cells were treated with
LPS (1 μg/ml) and curcumin or its respective metabolites (12.5–100
μM) for 24 h. Cell culture supernatant (100 μl) was added to 100 μl
Griess reagent and then incubated at room temperature for 10 min.
Absorbance was measured at 540 nm (Biotek Synergy HT; BioTek
Instruments, Inc., Winooski, VT, USA) and inhibitory rates were
calculated using a standard calibration curve of different
concentrations of sodium nitrite as previously prepared (28).
Determination of cytokines, TNF-α and
IL-6 expression
RAW 264.7 cells were treated with LPS (1 μg/ml) and
curcumin or its respective metabolites (12.5–100 μM) for 6 h. Mouse
tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) ELISA kits
(Yantai Science and Biotechnology Co., Ltd.) were used according to
the manufacturer’s instructions in order to determine the protein
expression levels of TNF-α or IL-6 using 100 μl culture supernatant
as previously described (29).
Total protein, cytoplasmic and nuclear
protein extraction
Total protein was extracted by using western cell
lysis buffer (Yantai Science and Biotechnology Co., Ltd.) in order
to determine the protein expression levels of iNOS and COX-2.
Cytoplasmic and nuclear proteins were separated using a nuclear and
cytoplasmic protein extraction kit according to the manufacturer’s
instructions (Yantai Science and Biotechnology Co., Ltd.) for
further use for the western blot analysis of IκB-α and nuclear
factor kappa B (NF-κB) p65 subunit (30).
Western blot analysis
Protein concentrations were determined using a BCA
protein concentration assay kit (Yantai Science and Biotechnology
Co., Ltd.). Each respective protein (30 μg) was boiled in SDS-PAGE
loading buffer (Yantai Science and Biotechnology Co., Ltd.),
subjected to gel electrophoresis and then electrophoretically
transferred onto nitrocellulose membranes (Pall Corporation, Port
Washington, NY, USA). The membranes were blocked using 5% nonfat
dried milk in Tris-buffered saline with Tween 20 (TBST; 20 mM
Tris-HCl, 150 mM NaCl and 0.05% Tween 20) at room temperature for 1
h. Samples were then washed and incubated in their respective
primary antibody solution (anti-iNOS, anti-COX-2, anti-IκB-α,
anti-p65 or anti-β-actin, dilution 1:1,000) overnight at 4°C.
Membranes were then washed with TBST and incubated with
HRP-conjugated secondary antibody solution for 1 h at room
temperature. Blots were then washed three times in TBST, detected
using enhanced chemiluminescence (Beyotime Institute of
Biotechnology, Haimen, China) and exposed to photographic films
(Kodak, Tokyo, Japan). Images were collected and the corresponding
protein bands for iNOS, COX-2, IκB-α, p65 and β-actin were
quantified by densitometric analysis using DigDoc100 software
(Alpha Innotech Corporation, San Leandro, CA, USA).). β-actin was
used as the internal control.
Statistical analysis
Data were analyzed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). Values are expressed as the mean ± standard
deviation. Statistical comparisons were conducted using a one-way
or two-way analysis of variance followed by a two-way post hoc
test. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
Curcumin and its metabolites do not
affect cell viability
A preliminary MTT assay showed that curcumin and its
metabolites (3.125–100 μM) did not affect the viability of RAW
264.7 cells within 24 h.
Curcumin and its metabolites inhibit
LPS-induced NO overproduction in RAW 264.7 cells
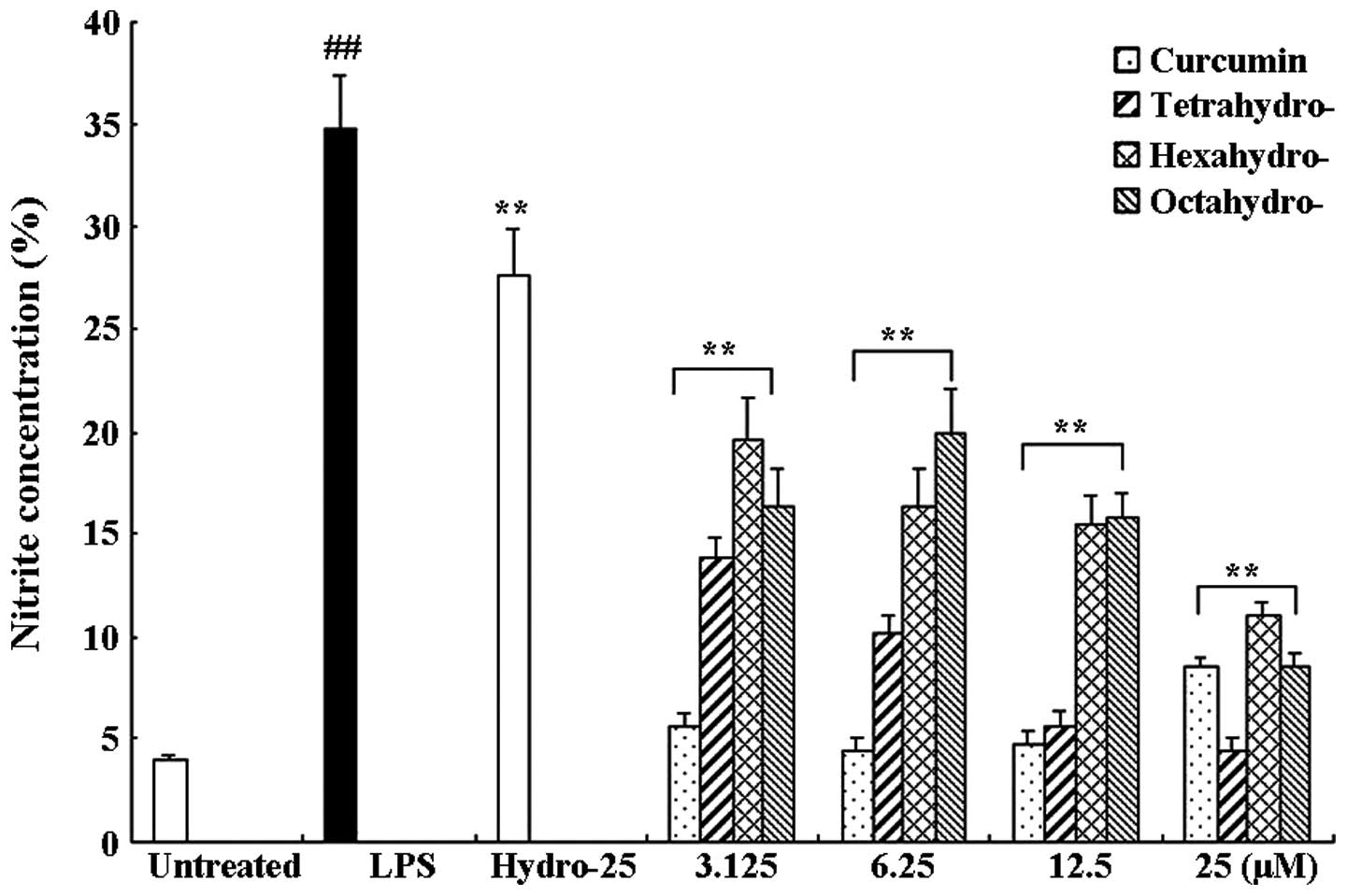
RAW 264.7 cells were treated with 1 μg/ml of LPS
only or in combination with different concentrations of curcumin
and its metabolites or hydrocortisone as the positive control. The
concentration of nitrite was determined by detecting NO production
levels 24 h following treatment (Fig.
2). LPS induced a significant increase in nitrite concentration
compared with that of the untreated group (P<0.01; 34.78±2.54
and 3.96±0.29 μM, respectively). In addition, curcumin and its
three metabolites significantly inhibited the LPS-induced NO
overproduction in a dose-dependent manner (P<0.01); however,
curcumin exhibited a more potent inhibition of nitrate
concentrations compared to that of its metabolites.
Curcumin and tetrahydrocurcumin
significantly inhibit the release of TNF-α and IL-6 in RAW 264.7
cells
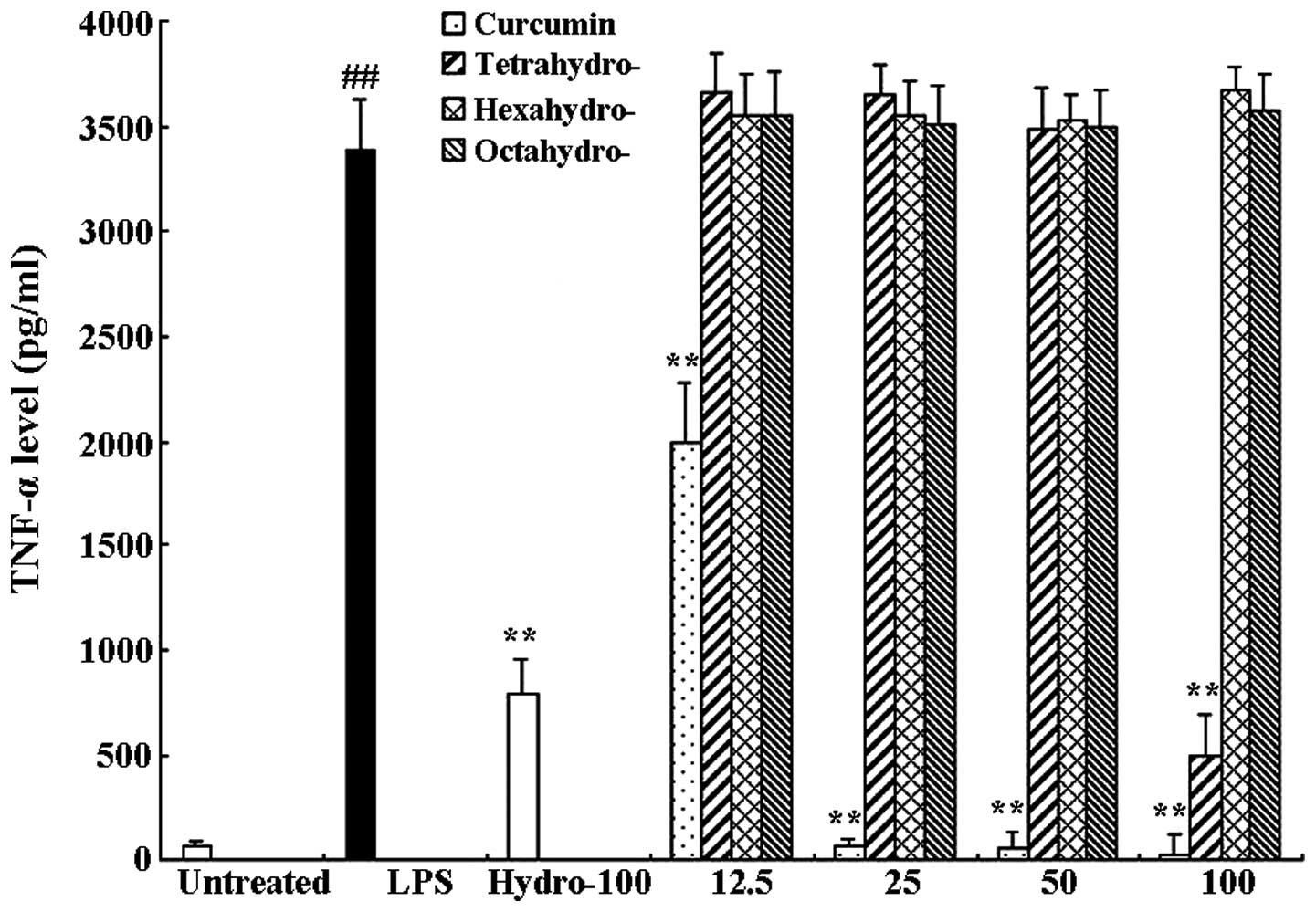
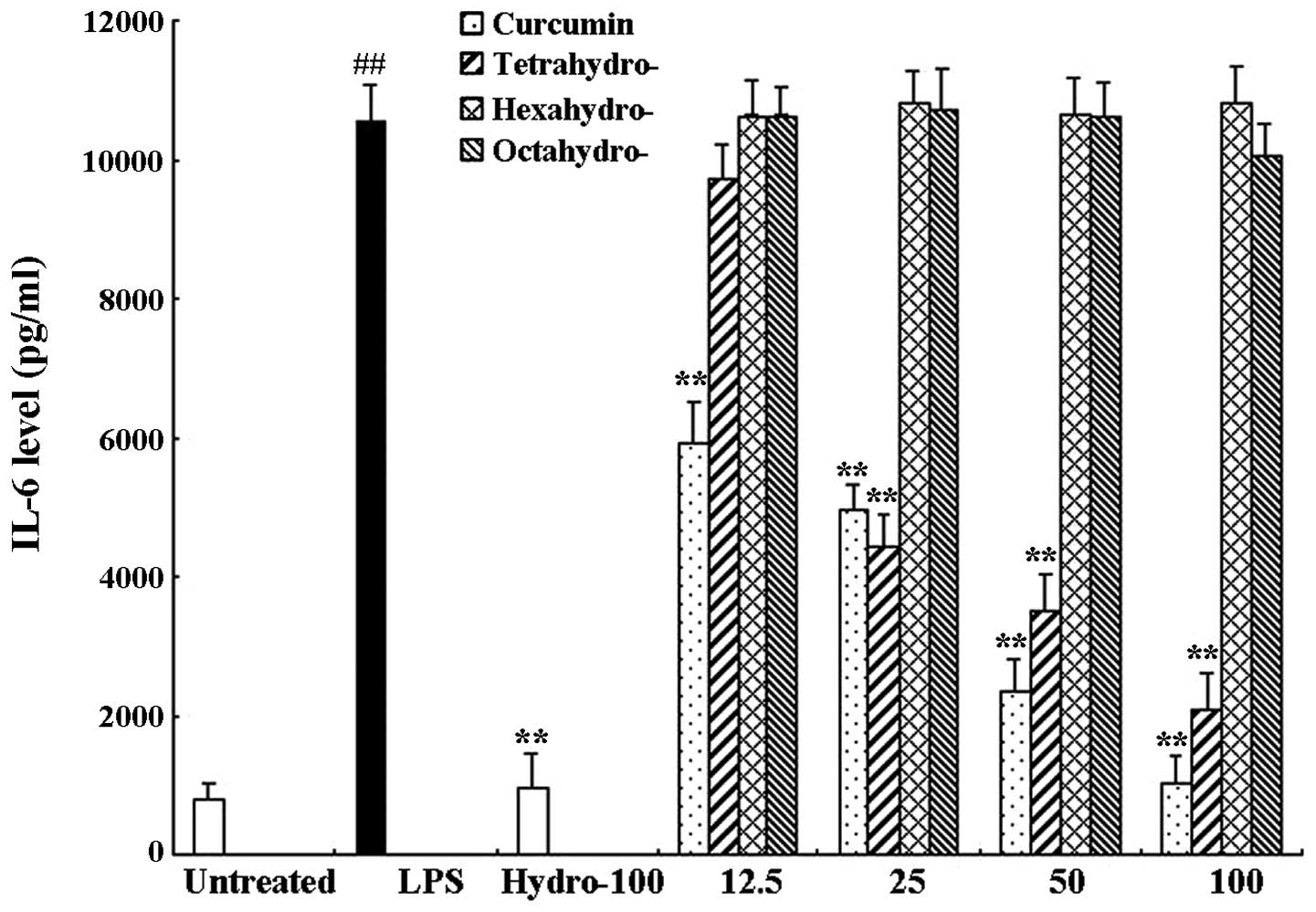
RAW 264.7 cells were treated with 1 μg/ml LPS only
or in combination with different concentrations of curcmin and its
metabolites or hydrocortisone as the positive control. Following 6
h of incubation, an ELISA assay was used to determine the levels of
TNF-α and IL-6 in the supernatants. As shown in Figs. 3 and 4, compared to the untreated control
group, LPS significantly induced the release of proinflammatory
cytokines TNF-α (63.57±6.41 and 3389.97±39.11 pg/ml, respectively)
and IL-6 (811.95±32.84 and 10536.81±833.99 pg/ml, respectively).
Curcumin exerted a significant inhibitory effect on TNF-α as well
as IL-6 release compared to that of the LPS-only group; LPS-induced
TNF-α and IL-6 release were almost completely inhibited following
100 μM curcumin treatment. In addition, the curcumin metabolite
tetrahydrocurcumin significantly inhibited the release of TNF-α and
IL-6, whereas hexahydrocurcumin and octahydrocurcumin did not
exhibit any marked inhibitory effects on the release of TNF-α and
IL-6 at concentrations of 12.5–100 μM.
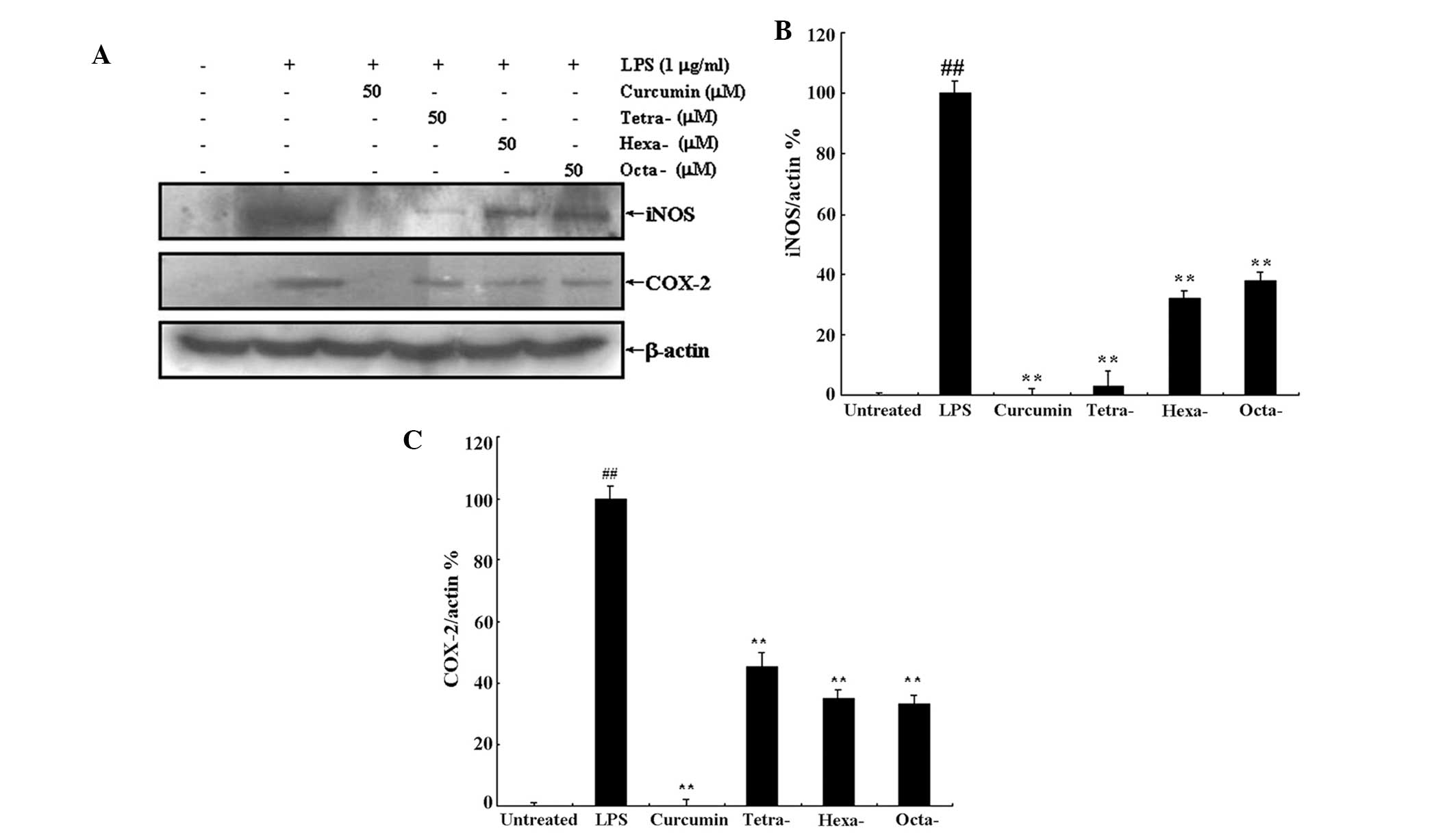
Curcumin and its metabolites inhibit
LPS-induced iNOS and COX-2 overexpression
As demonstrated above, curcumin and its metabolites
were able to inhibit LPS-induced NO overproduction (Fig. 2). NO overproduction is associated
with the upregulation of iNOS expression; therefore, in the present
study, western blot analysis was used to determine the expression
levels of iNOS and COX-2 following treatment with LPS in the
presence or absence of 50 μM curcumin or its metabolites (Fig. 5A). Densitometric analysis revealed
that 50 μM curcumin completely inhibited LPS-induced expression of
iNOS and COX-2 (Fig. 5B and C,
respectively); in addition, curcumin metabolites significantly
inhibited iNOS and COX-2 expression compared to that of the LPS
only treatment. Furthermore, the metabolite tertrahydrocurcumin
appeared to exhibit a more potent effect on iNOS expression
compared to that of the other metabolites; however, statistical
analyses were not performed to confirm this observation.
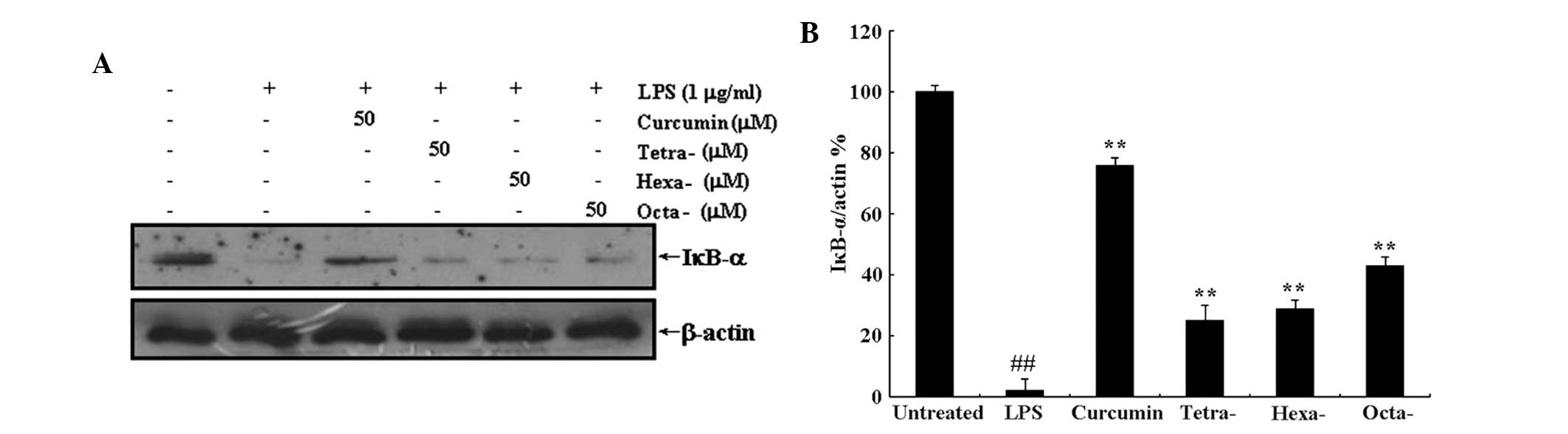
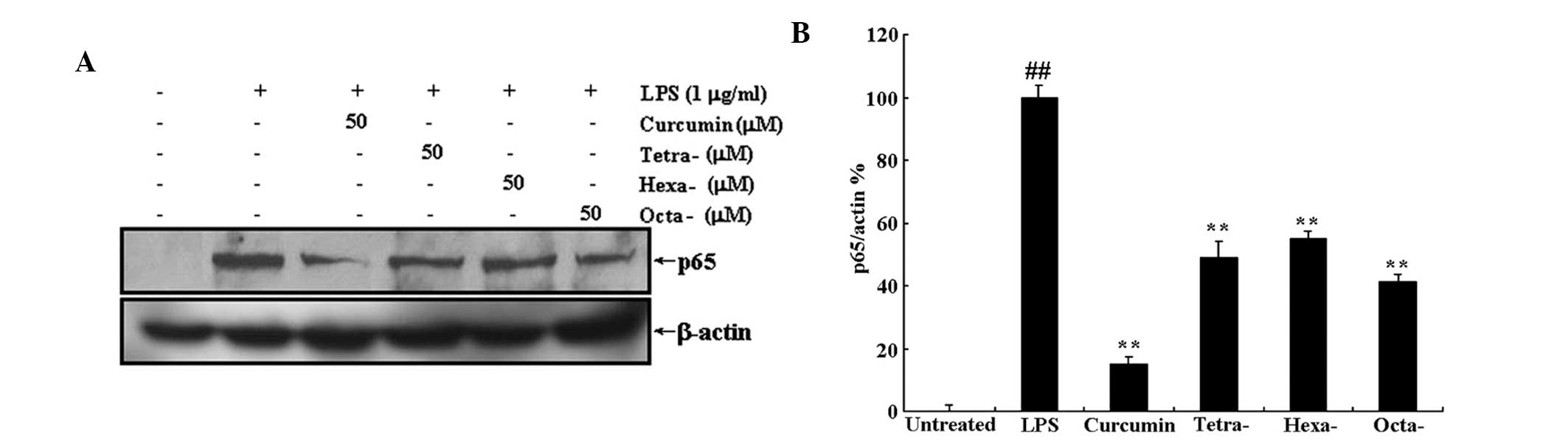
Curcumin and its metabolites prevent the
translocation of NF-κB to the nucleus via inhibition of LPS-induced
IκB-α protein degradation
Translocation of NF-κB to the nucleus is the result
of IκB-α protein degradation. Western blot analysis was used to
determine the effect of curcumin and its metabolites on LPS-induced
IκB-α degradation and translocation of NF-κB to the nucleus. As
shown in Figs. 6 and 7, treatment with 50 μM curcumin or its
metabolites significantly inhibited the LPS-induced decrease in
cytoplasmic IκB-α expression as well as the LPS-induced increase in
nuclear protein expression of the NF-κB subunit p65. This therefore
indicated that curcumin and its metabolites inhibited IκB-α
degradation and, as a result, prevented the translocation of NF-κB
to the nucleus. Futhermore, the three metabolites of curcumin
produced significant results; however, they appeared to have a less
potent effect on LPS-induced degradation of IκB-α and p65
expression than that of curcumin.
Discussion
Elucidation of metabolite structures is among the
most challenging tasks of drug metabolism studies. At present,
comparisons of electrospray ionization multi-stage tandem mass
spectrometry data and HPLC retention times with synthetic standards
are generally used to identify the structures of metabolites
(31). However, when metabolites
are difficult to obtain or synthesize, biological studies into the
activities of these drug metabolites may be challenging or even
impossible.
In the present study, the anti-inflammatory
activities of three major metabolites of curcumin were investigated
and compared to those of curcumin. Among these metabolites,
tetrahydrocurcumin was previously reported to be the primary
metabolite of curcumin and has been investigated for its biological
activities (21–23); however, these studies primarily
focused on the anti-tumor activities of tetrahydrocurcumin, whereas
studies into the other metabolites of curcumin and their numerous
biological activities are rare and insufficient. The results of the
present study indicated that three major metabolites of curcumin,
including tetrahydrocurcumin, hexahydrocurcumin and
octahydrocurcumin (25), inhibited
various LPS-induced responses of RAW 264.7 macrophage cells,
including excess NO production, increased iNOS and COX-2 protein
expression as well as LPS-induced degradation of IκB-α and
overexpression of nuclear p65; in addition, tetrahydrocurcumin
significantly inhibited the LPS-induced release of pro-inflammatory
cytokines TNF-α and IL-6. However, the results also determined that
curcumin exerted more potent inhibitory effects on the LPS-induced
responses of RAW 264.7 cells.
In conclusion, the results of the present study
confirmed the anti-inflammatory effects of curcumin and its
metabolites in RAW 264.7 macrophage cells and, to the best of our
knowledge, the present study was the first direct study to compare
the anti-inflammatory activities of curcumin and its main
metabolites. In addition, the results indicated that the
anti-inflammatory mechanism of curcumin and its metabolites may
proceed via the inhibition of IκB-α degradation, which in turn
prevented the translocation of NF-κB to the nucleus. These results
may therefore provide the theoretical basis for further studies
into the pharmacokinetics of curcuminoids.
Acknowledgements
The present study was supported by the Taishan
Scholar Project to Fenghua Fu (Shangdong, China) and the
Undergraduate Scientific and Technological Innovation Project of
Yantai University (grant no. 131407; Shangdong, China).
References
|
1
|
Nandal S, Dhir A, Kuhad A, Sharma S and
Chopra K: Curcumin potentiates the anti-inflammatory activity of
cyclooxygenase inhibitors in the cotton pellet granuloma pouch
model. Methods Find Exp Clin Pharmacol. 31:89–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adapala N and Chan MM: Long-term use of an
antiinflammatory, curcumin, suppressed type 1 immunity and
exacerbated visceral leishmaniasis in a chronic experimental model.
Lab Invest. 88:1329–1339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian F, Fan T, Zhang Y, Jiang Y and Zhang
X: Curcumin potentiates the antitumor effects of 5-FU in treatment
of esophageal squamous carcinoma cells through downregulating the
activation of NF-κB signaling pathway in vitro and in vivo. Acta
Biochim Biophys Sin (Shanghai). 44:847–855. 2012. View Article : Google Scholar
|
|
4
|
Wang K, Zhang T, Liu L, Wang X, Wu P, Chen
Z, Ni C, Zhang J, Hu F and Huang J: Novel micelle formulation of
curcumin for enhancing antitumor activity and inhibiting colorectal
cancer stem cells. Int J Nanomedicine. 7:4487–4497. 2012.PubMed/NCBI
|
|
5
|
Sadzuka Y, Nagamine M, Toyooka T, Ibuki Y
and Sonobe T: Beneficial effects of curcumin on antitumor activity
and adverse reactions of doxorubicin. Int J Pharm. 432:42–49. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang H, Geng QR, Wang L and Lu Y: Curcumin
potentiates antitumor activity of L-asparaginase via inhibition of
the AKT signaling pathway in acute lymphoblastic leukemia. Leuk
Lymphoma. 53:1376–1382. 2012. View Article : Google Scholar
|
|
7
|
Aftab N and Vieira A: Antioxidant
activities of curcumin and combinations of this curcuminoid with
other phytochemicals. Phytother Res. 24:500–502. 2010.
|
|
8
|
Masuda T, Hidaka K, Shinohara A, Maekawa
T, Takeda Y and Yamaguchi H: Chemical studies on antioxidant
mechanism of curcuminoid: analysis of radical reaction products
from curcumin. J Agric Food Chem. 47:71–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jordan WC and Drew CR: Curcumin - a
natural herb with anti-HIV activity. J Natl Med Assoc.
88:3331996.
|
|
10
|
Kim MK, Choi GJ and Lee HS: Fungicidal
property of Curcuma longa L. rhizome-derived curcumin against
phytopathogenic fungi in a greenhouse. J Agric Food Chem.
51:1578–1581. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deters M, Siegers C, Muhl P and Hänsel W:
Choleretic effects of curcuminoids on an acute cyclosporin-induced
cholestasis in the rat. Planta Med. 65:610–613. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Li Z, Qiu D, Gu Q, Lei Q and Mao L:
The inhibitory effects of different curcuminoids on β-amyloid
protein, β-amyloid precursor protein and β-site amyloid precursor
protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci Lett.
485:83–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao J, Zhao Y, Zheng W, Lu Y, Feng G and
Yu S: Neuroprotective effect of curcumin on transient focal
cerebral ischemia in rats. Brain Res. 1229:224–232. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shukla Y, Arora A and Taneja P:
Antimutagenic potential of curcumin on chromosomal aberrations in
Wistar rats. Mutat Res. 515:197–202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Volak LP, Ghirmai S, Cashman JR and Court
MH: Curcuminoids inhibit multiple human cytochromes P450,
UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas
piperine is a relatively selective CYP3A4 inhibitor. Drug Metab
Dispos. 36:1594–1605. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Graber-Maier A, Büter KB, Aeschlimann J,
Bittel C, Kreuter M, Drewe J and Gutmann H: Effects of Curcuma
extracts and curcuminoids on expression of P-glycoprotein and
cytochrome P450 3A4 in the intestinal cell culture model LS180.
Planta Med. 76:1866–1870. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lao CD, Ruffin MT IV, Normolle D, Heath
DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL and Brenner
DE: Dose escalation of a curcuminoid formulation. BMC Complement
Altern Med. 6:102006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng AL, Hsu CH, Lin JK, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
19
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhongfa L, Chiu M, Wang J, Chen W, Yen W,
Fan-Havard P, Yee LD and Chan KK: Enhancement of curcumin oral
absorption and pharmacokinetics of curcuminoids and curcumin
metabolites in mice. Cancer Chemother Pharmacol. 69:679–689. 2012.
View Article : Google Scholar :
|
|
21
|
Wu JC, Lai CS, Badmaev V, Nagabhushanam K,
Ho CT and Pan MH: Tetrahydrocurcumin, a major metabolite of
curcumin, induced autophagic cell death through coordinative
modulation of PI3K/Akt-mTOR and MAPK signaling pathways in human
leukemia HL-60 cells. Mol Nutr Food Res. 55:1646–1654. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yodkeeree S, Garbisa S and Limtrakul P:
Tetrahydrocurcumin inhibits HT1080 cell migration and invasion via
downregulation of MMPs and uPA. Acta Pharmacol Sin. 29:853–860.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoysungnoen P, Wirachwong P, Changtam C,
Suksamrarn A and Patumraj S: Anti-cancer and anti-angiogenic
effects of curcumin and tetrahydrocurcumin on implanted
hepatocellular carcinoma in nude mice. World J Gastroenterol.
14:2003–2009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai CS, Wu JC, Yu SF, Badmaev V,
Nagabhushanam K, Ho CT and Pan MH: Tetrahydrocurcumin is more
effective than curcumin in preventing azoxymethane-induced colon
carcinogenesis. Mol Nutr Food Res. 55:1819–1828. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan MH, Lin-Shiau SY and Lin JK:
Comparative studies on the suppression of nitric oxide synthase by
curcumin and its hydrogenated metabolites through down-regulation
of IkappaB kinase and NFkappaB activation in macrophages. Biochem
Pharmacol. 60:1665–1676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Liu Y, Wei JQ, Wang K, Chen LX, Yao
XS and Qiu F: Isolation and identification of phase 1 metabolites
of curcuminoids in rats. Planta Med. 78:1351–1356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao F, Wang L and Liu K: In vitro
anti-inflammatory effects of arctigenin, a lignan from Arctium
lappa L., through inhibition on iNOS pathway. J Ethnopharmacol.
122:457–462. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishihara T, Kohno K, Ushio S, Iwaki K,
Ikeda M and Kurimoto M: Tryptanthrin inhibits nitric oxide and
prostaglandin E(2) synthesis by murine macrophages. Eur J
Pharmacol. 407:197–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao F, Gao Z, Jiao W, Chen L, Chen L and
Yao X: In vitro anti-inflammatory effects of beta-carboline
alkaloids, isolated from Picrasma quassioides, through inhibition
of the iNOS pathway. Planta Med. 78:1906–1911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao F, Chen L, Zhang M, Bi C, Li L, Zhang
Q, Shi C, Li M, Zhou S and Kong L: Inhibition of
lipopolysaccharide-induced iNOS and COX-2 expression by indole
alkaloid,
3-(hydroxymethyl)-6,7-dihydroindolo[2,3-a]quinolizin-(12H)-one, via
NF-κB inactivation in RAW 264.7 macrophages. Planta Med.
79:782–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pursley J, Shen JX, Schuster A, Dang OT,
Lehman J, Buonarati MH, Song Y, Aubry AF and Arnold ME: LC-MS/MS
determination of apixaban (BMS-562247) and its major metabolite in
human plasma: an application of polarity switching and monolithic
HPLC column. Bioanalysis. 6:2071–2082. 2014. View Article : Google Scholar : PubMed/NCBI
|