Introduction
The survival of osteoblasts is one of the
determinants of the development of osteoporosis. The drugs
currently used in the treatment of osteoporosis are bone resorption
inhibitors, including bisphosphonates, calcitonin and estrogen
(1,2). However, the positive effect of these
drugs on the recovery of bone mass is moderate (1). Therefore, satisfactory anabolic
agents are urgently required. Through increasing the proliferation
of an osteoblastic lineage, or inducing differentiation and
mineralization, these agents stimulate an increase in bone tissue
and the prevention of bone destruction (3,4).
As mediators in the cell signaling pathways
associated with bone formation, bone morphogenetic proteins (BMPs)
have an important role in the differentiation of osteoblasts
(5–7). The Smad and mitogen-activated protein
kinase (MAPK) pathways are essential components of BMP signaling
during osteoblast differentiation (8–10).
Among the members of the BMP subfamily, BMP-2 is able to induce
bone formation and differentiation in vivo and in
vitro (11,12). BMP-2 activates ERK1/2, p38, c-Jun
kinases and Smad1/5/8 proteins (13–16),
and induces the expression of core binding factor
(Cbfa)1/Runt-related transcription factor 2 (Runx2) (17,18),
an important transcription factor in osteoblastic differentiation
(19).
A previous study by our group demonstrated that RTS
effectively inhibited osteoporosis in ovariectomized rats.
Additionally, RTS has been shown to enhance MC3T3-E1 cell
differentiation, potentially due to its role in increasing the
expression levels of BMP-2 (20).
However, the detailed molecular mechanisms of the osteogenic
effects of RTS remain to be determined. In the present study, the
effects of RTS on the osteogenic activities of MC3T3-E1 cells were
investigated. In addition, in order to establish the potential
mechanisms involved in the osteoprotective effects of RTS, the
levels of BMP-2, Smad1/5/8, MAPKs and Runx2 were assayed, proteins
which are associated with the osteogenesis signaling pathways.
Materials and methods
Cells and reagents
MC3T3-E1 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and maintained in α-minimum
essential medium (α-MEM; HyClone Laboratories, Inc., Logan, UT,
USA) containing 10% fetal bovine serum (FBS; Hyclone Laboratories,
Inc.) and 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO,
USA) in a humidified atmosphere of 5% CO2 and 95% air at
37°C. Radix Dipsaci was purchased in a local Chinese Medicine store
and was identified morphologically, histologically and chemically
using standard Chinese Pharmacopoeia procedures (Chinese
Pharmacopeia Commission, 2010). In brief, RTS was isolated and
purified by refluxing in 60% ethanol and D101 macroporous resin,
repectively. RTS was analyzed by colorimetric determination using
asperosaponin VI (Push Bio-Technology Co. Ltd, Chengdu, China) as
the standard and the content of RTS was 76.5% (23).
Alkaline phosphatase (ALP) activity
assay
Osteoblasts were seeded at a density of
2×104 cells/well and cultured in 24-well plates with
α-MEM containing 10% FBS, L-ascorbic acid (50 μg/ml) and
β-glycerophosphate (10 mM) in the presence or absence of RTS (30,
100 or 300 μg/ml). The cells were washed twice with
phosphate-buffered saline (PBS; Wuhan Boster Biological Technology,
Inc., Wuhan, China), lysed with 0.2% Triton X-100 (Tianjin Damao
Chemical Reagent Factory, Tianjin, China) and the lysate was
centrifuged at 14,000 × g for 5 min. The supernatant was collected
in order to measure the ALP activity and protein concentration
using an ALP activity assay kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, Chian) and a BCA protein assay kit (Beyotime,
Shanghai, China), respectively (21).
Assessment of osteocalcin
Osteocalcin ELISA kits (Elabscience Biotechnology
Co., Ltd, Wuhan, China) were used to detect osteocalcin levels.
Cells were treated with RTS (30, 100 or 300 μg/ml) for eight
days. The samples were placed in 96-well microtiter plates coated
with mouse monoclonal detective antibodies for osteocalcin from the
kit and incubated for 2 h at room temperature according to the
manufacturer’s instructions. Following removal of the unbound
material using washing buffer, horseradish peroxidase
(HP)-conjugated streptavidin was added to bind to the antibodies.
HP catalyzes the conversion of the chromogenic tetramethylbenzidine
to a colored solution, with the color intensity in proportion to
the amount of protein present in the sample. The absorbance of each
well was measured at a wavelength of 450 nm (Synergy™ HT Multi-Mode
Microplate Reader; Bio-Tek Instruments, Inc., Winooski, V T, USA).
Results are presented as the percentage change in the activity of
the treated cells compared with that of the untreated control.
Mineralized matrix assay
Mineralization was determined via staining with
Alizarin Red-S (Aldrich, Milwaukee, WI, USA). MC3T3-E1 cells were
seeded into 12-well plates at a density of 2×105
cells/well. Following two days of incubation, cells were washed
twice with PBS solution, treated with or without RTS and cultured
in α-MEM containing 10% FBS, L-ascorbic acid (50 μg/ml;
Sigma-Aldrich) and β-glycerophosphate (10 mM; Sigma-Aldrich).
During this period, the medium was changed every three days.
Following a 14-day incubation with or without drugs, the cells were
fixed with 70% ethanol for 1 h, washed three times with distilled
water and then incubated with 40 mmol/l Alizarin Red-S (pH 4.2) for
10 min at 37˚C. Once stained, the cultures were washed three times
with deionized water and then incubated with PBS for a further 15
min. Images of the mineralized matrices were captured using a
microscope (80i; Nikon, Tokyo, Japan). To quantify the matrix
mineralization, Alizarin Red-S-stained cultures were incubated in
100 mmol/l cetylpyridinium chloride (Tianjin Damao Chemical Reagent
Factory) for 1 h in order to solubilize and release calcium-bound
Alizarin Red-S into the solution. The absorbance of the released
Alizarin Red-S (Sigma-Aldrich) was measured at a wavelength of 570
nm (Synergy™ HT Multi-Mode Microplate Reader; Bio-Tek) (24).
Western blot analysis
To detect protein expression following RTS
treatment, MC3T3 -E1 cells werelysed and the cell lysates were
harvested and maintained on ice for 30 min. Once the soluble
fractions of nuclear and cytoplasmic proteins were obtained they
were used for western blotting. Equal amounts of protein were
subjected to 15% SDS-PAGE (Wuhan Boster Biological Technology,
Inc.). The proteins were transferred to nitrocellulose membranes
(Pall, Port Washington, NY, USA) using transfer buffer (50 mM Tris,
190 mM glycin and 10% methanol; Tianjin Damao Chemical Reagent
Factory) at 50 V for 2.5 h. The membranes were incubated with
blocking buffer containing 0.05% Tween-20 (Tianjin Damao Chemical
Reagent Factory) and 5% non-fat milk for 12 h at 4°C. Following
washing three times with PBS, the blot was incubated with primary
antibodies (rabbit polyclonal anti-BMP-2, 1:200; rabbit polyclonal
anti-Smad1/5/8, 1:200; rabbit polyclonal anti-phosphorylated
(P)-Smad1/5/8, 1:200; rabbit polyclonal anti-p38, 1:200; rabbit
polyclonal anti-P-p38, 1:200; rabbit polyclonal anti-ERK1/2, 1:200;
rabbit polyclonal anti-P-ERK1/2, 1:200; rabbit polyclonal anti-JNK,
1:200; rabbit polyclonal anti-P-JNK, 1:200; rabbit polyclonal
anti-Runx2, 1:200; rabbit polyclonal anti-histone, 1:500; and mouse
monoclonal anti-β-actin, 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 12 h at 4°C. Subsequently, the membranes were
washed three times for 10 min with tris-buffered saline (TBS)
buffer containing 0.05% Tween-20 and subsequently incubated with
anti-rabbit or anti-mouse immunoglobulin G secondary antibodies
(1:5,000 dilution) for 1 h at room temperature. The membranes were
washed three times for 10 min with Tris-buffered saline (Tianjin
Damao Chemical Reagent Factory) and once for 10 min with PBS.
Following reaction and coloration using enhanced chemiluminescence
(Millipore, Billerica, MA, USA), the relative values for the
absorbance of the bands and the absorbance of β-actin or histone
were compared.
Statistical analysis
Data were analyzed using a one-way analysis of
variance test to compare the different groups. Results are
presented as the mean ± standard deviation (SD). Statistical
analyses were performed using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
RTS enhances the differentiation of
MC3T3-E1 cells
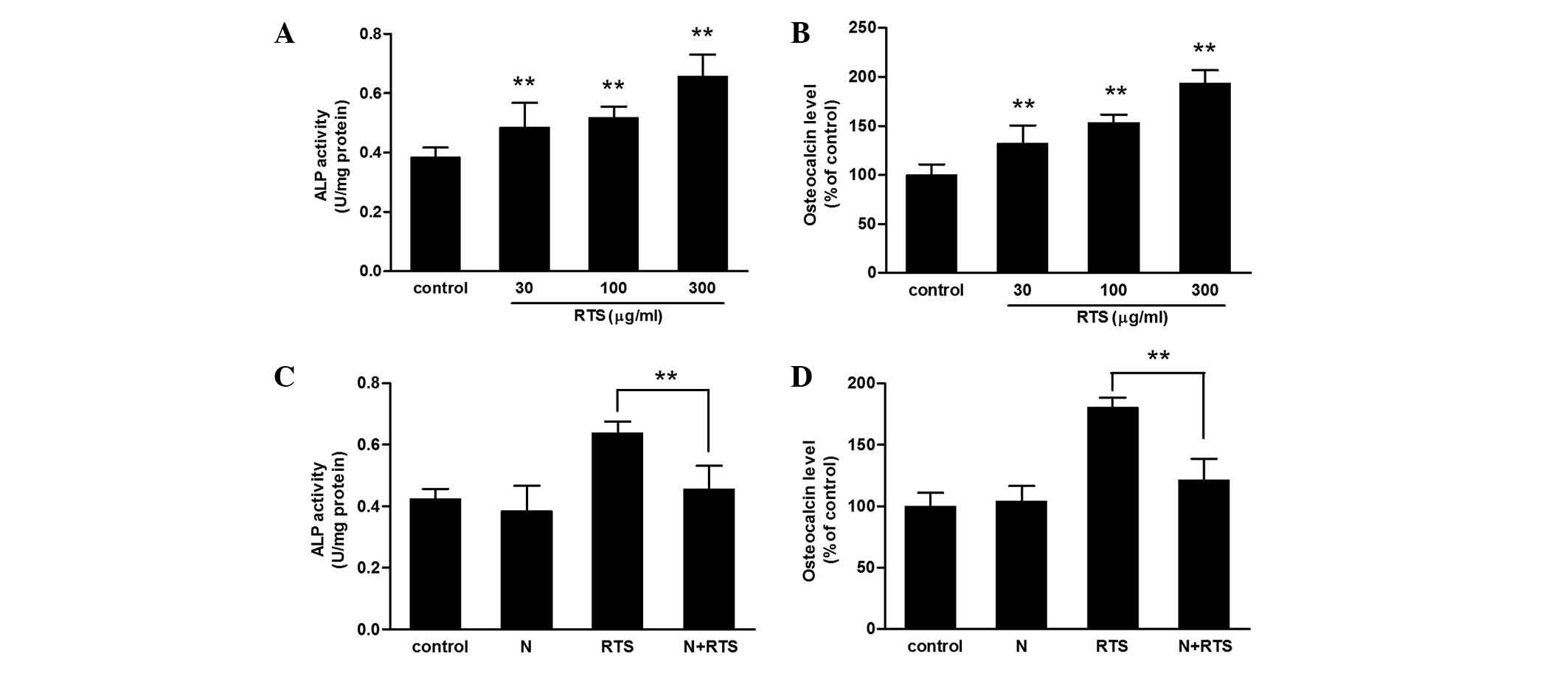
As shown in Fig.
1A, RTS treatment increased the level of ALP activity in a
concentration-dependent manner. Additionally, the expression levels
of osteocalcin protein were increased by RTS in a
concentration-dependent manner in following 7 days of treatment
(Fig. 1B); the levels in the 30,
100 and 300 μg/ml RTS-treated groups were significantly
higher than those in the controls (P<0.01).
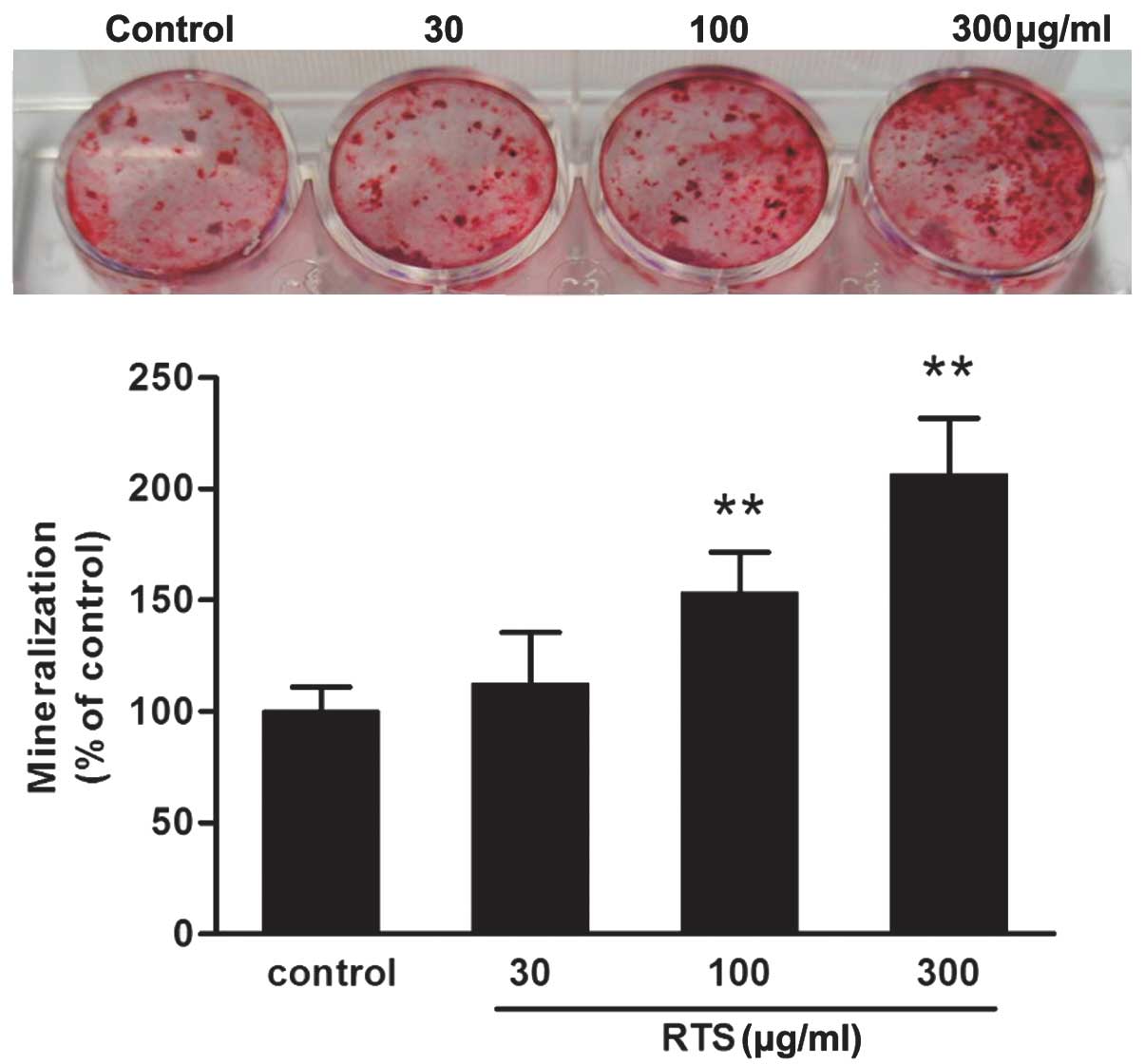
RTS promotes mineralization of MC3T3-E1
cells
The calcified nodules stained positive with Alizarin
Red-S (Fig. 2). The 100 and 300
μg/ml RTS-treated groups had the highest number of
mineralized nodules (P<0.01 compared with the control
group).
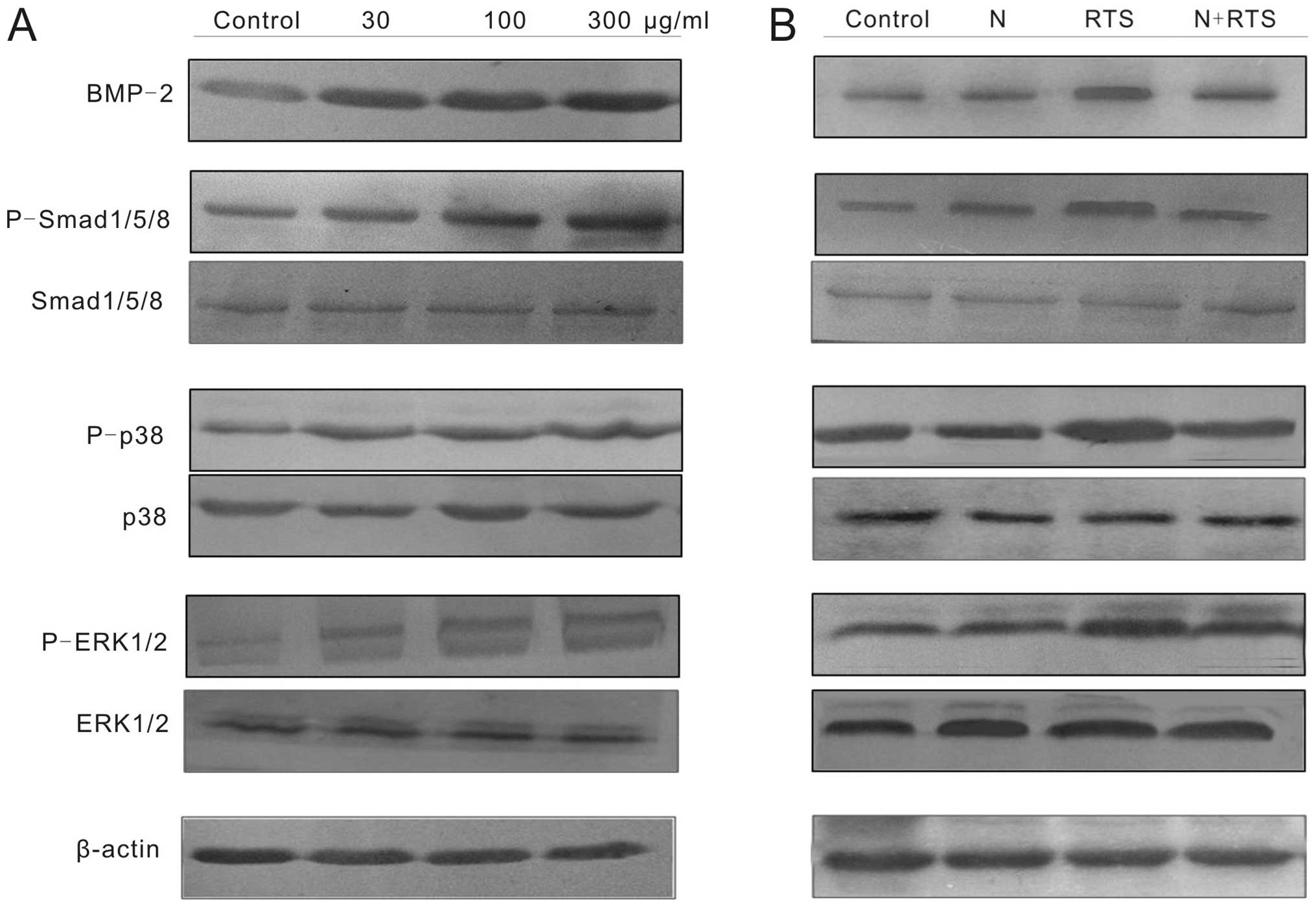
RTS induces the differentiation of
MC3T3-E1 cells via the BMP-2 pathway
As shown in Fig.
3A, BMP-2 protein levels were markedly increased by RTS
treatment in a concentration-dependent manner. MC3T3-E1 cells were
pretreated with noggin protein (100 ng/ml) for 2 h, then 300
μg/ml RTS was added for 24 h. RTS-induced BMP-2 protein
expression was diminished by the concurrent treatment with noggin
(Fig. 3B). In addition, the
RTS-induced ALP activity and osteocalcin protein levels were
significantly diminished in cells treated concurrently with noggin
compared with those in untreated cells (Fig. 1C and D). Therefore, RTS-mediated
cell differentiation may proceed via a BMP-2-dependent pathway.
BMP-2 is involved in the activation of
Smad1/5/8, ERK and p38 in RTS-treated cells
Binding of BMP-2 to the BMP receptor activates MAPKs
or SMADs via phosphorylation (15,16).
RTS treatment significantly increased the expression of
P-Smad1/5/8, P-p38 and P-ERK1/2 (Fig.
3A). The activation of Smad1/5/8, p38 and ERK1/2 was blocked in
MC3T3-E1 cells pretreated with 100 ng/ml noggin protein for 2 h and
then co-treated with 300 μg/ml RTS for 24 h (Fig. 3B).
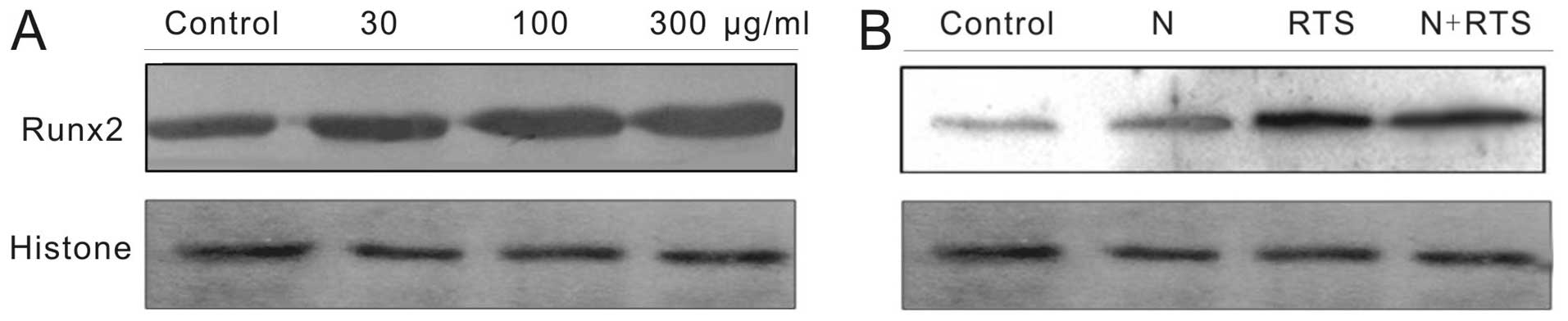
BMP-2 is required to increase expression
of Runx2 in RTS‑treated MC3T3-E1 cells
Runx2 is a vital transcription factor required for
osteoblast differentiation (19).
Following treatment with RTS for 24 h, the expression levels of
Runx2 protein in cells were markedly increased (Fig. 4A). When cells were incubated with
RTS in the presence of noggin, the stimulatory effect of RTS on the
expression of Runx2 protein were markedly reduced (Fig. 4B). These results indicated that RTS
activated osteogenic differentiation through the
BMP-2/MAPK/Smad-dependent Runx2 signaling pathway.
Discussion
The anti-osteoporosis effect of RTS in vivo
was systemically evaluated in a previous study by our group
(20). In the present study, the
effects of RTS on the osteogenic activities in MC3T3-E1 cells and
the potential mechanisms of action were evaluated. Treatment of
MC3T3-E1 cells with RTS not only raised the activity of ALP (a
marker of maturation) but also increased the levels of osteocalcin
proteins (late stage markers of differentiation). This indicated
that RTS may affect cell differentiation processes from the early
to terminal stages. In addition, RTS increased the mineralization
(a marker of bone formation) of MC3T3-E1 cells.
BMPs have potent osteogenic effects and control
osteoblast differentiation during osteogenesis. BMP-2, one of the
BMP subfamilies, promotes differentiation through enhanced
intracellular ALP activity as well as osteocalcin and collagen
protein synthesis (23). The
effect of BMPs is mediated by heterotetrameric serine/threonine
kinase receptors and the downstream transcription factors
Smad1/5/8. Upon phosphorylation by type I receptors, Smad1/5/8 form
complexes with Smad4, translocate to the nucleus and regulate the
transcription of target genes associated with differentiation
(13). Several natural or chemical
compounds, including daidzein, osthole and syringetin, have been
reported to induce osteoblast differentiation by induction of BMP
and/or SMAD signaling (24–6). The results of the present study
showed that the expression levels of BMP-2 were enhanced and the
phosphorylation of SMAD1/5/8 was significantly increased in
RTS-treated MC3T3-E1 cells. RTS-mediated SMAD1/5/8 activation was
blocked by the BMP antagonist noggin and cell differentiation was
attenuated in MC3T3-E1 cells. Hence, the BMP-2 signaling system has
an important role in RTS-mediated cell maturation and
differentiation in MC3T3-E1 cells.
In addition to Smad activation, BMP-2 can activate
Smad-independent pathways, for example the MAPK signaling pathway.
BMP-2 can stimulate two MAPKs: ERK and p38. The activation of p38
and ERK is essential in the BMP-2-induced upregulation of A P, type
I collagen, osteocalcin and osteopontin (27–9). The results of the
present study showed an increase in p38 and ERK activity in
RTS-treated cells. This suggested that the activation of p38 and
ERK may have an important role in increasing BMP-2 levels and the
cell differentiation in MC3T3-E1 cells stimulated by RTS. The
increase in the expression of Runx2 protein by RTS is prevented by
the BMP inhibitor noggin, which demonstrates an involvement of the
BMP-2 pathway in the stimulatory effect of RTS on Runx2.
In conclusion, the present study clearly
demonstrated that RTS stimulates osteoblast differentiation at
various stages in MC3T3-E1 cells. The effect of RTS on cell
maturation and differentiation is strongly associated with the
BMP-2/MAPK/Smad1/5/8-dependent Runx2 signaling pathway. This
suggests that RTS may be beneficial in stimulating osteoblastic
activity resulting in bone formation.
Acknowledgments
This study was financially supported by grants from
the National Natural Science Foundation of China (no. 81202457),
the China Postdoctoral Science Foundation (no. 2012T50822) and the
Fundamental Research Foundation of Northwestern Polytechnical
University (no. 3102014JKY15013).
References
|
1
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schelonka EP and Usher A: Ipriflavone and
osteoporosis. JAMA. 286:1836–837. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berg C, Neumeyer K and Kirkpatrick P:
Teriparatide. Nat Rev Drug Discov. 2:257–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lane NE and Kelman A: A review of anabolic
therapies for osteoporosis. Arthritis Res Ther. 5:214–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reddi AH: Bone and cartilage
differentiation. Curr Opin Genet Dev. 4:737–44. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakou T, Onishi T, Yamamoto T, Nagamine T,
Sampath T and Ten Dijke P: Localization of Smads, the TGF-beta
family intracellular signaling components during endochondral
ossification. J Bone Miner Res. 14:1145–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang CH, Yang RS, Huang TH, Liu SH and Fu
WM: Enhancement of fibronectin fibrillogenesis and bone formation
by basic fibroblast growth factor via protein kinase C-dependent
pathway in rat osteoblasts. Mol Pharmacol. 66:440–449.
2004.PubMed/NCBI
|
|
8
|
Gallea S, Lallemand F, Atfi A, et al:
Activation of mitogen-activated protein kinase cascades is involved
in regulation of bone morphogenetic protein-2-induced osteoblast
differentiation in pluripotent C2C12 cells. Bone. 28:491–498. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujii M, Takeda K, Imamura T, et al: Roles
of bone morphogenetic protein type I receptors and Smad proteins in
osteoblast and chondroblast differentiation. Mol Biol Cell.
10:3801–3813. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamamoto N, Akiyama S, Katagiri T, Namiki
M, Kurokawa T and Suda T: Smad1 and smad5 act downstream of
intracellular signalings of BMP-2 that inhibits myogenic
differentiation and induces osteoblast differentiation in C2C12
myoblasts. Biochem Biophys Res Commun. 238:574–580. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia TL, Wang HZ, Xie LP, Wang XY and Zhang
RQ: Daidzein enhances osteoblast growth that may be mediated by
increased bone morphogenetic protein (BMP) production. Biochem
Pharmacol. 65:709–715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takuwa Y, Ohse C, Wang EA, Wozney JM and
Yamashita K: Bone morphogenetic protein-2 stimulates alkaline
phosphatase activity and collagen synthesis in cultured
osteoblastic cells, MC3T3-E1. Biochem Biophys Res Commun.
174:96–101. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nohe A, Keating E, Knaus P and Petersen
NO: Signal transduction of bone morphogenetic protein receptors.
Cell Signal. 16:291–299. 2004. View Article : Google Scholar
|
|
14
|
Sykaras N and Opperman LA: Bone
morphogenetic proteins (BMPs): how do they function and what can
they offer the clinician? J Oral Sci. 45:57–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gallea S, Lallemand F, Atfi A, et al:
Activation of mitogen-activated protein kinase cascades is involved
in regulation of bone morphogenetic protein-2-induced osteoblast
differentiation in pluripotent C2C12 cells. Bone. 28:491–498. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guicheux J, Lemonnier J, Ghayor C, Suzuki
A, Palmer G and Caverzasio J: Activation of p38 mitogen-activated
protein kinase and c-Jun-NH2-terminal kinase by BMP-2
and their implication in the stimulation of osteoblastic cell
differentiation. J Bone Miner Res. 18:2060–2068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsieh TP, Sheu SY, Sun JS, Chen MH and Liu
MH: Icariin isolated from Epimedium pubescens regulates osteoblasts
anabolism through BMP-2, SMAD4, and Cbfa1 expression.
Phytomedicine. 17:414–423. 2010. View Article : Google Scholar
|
|
18
|
Lee KS, Hong SH and Bae SC: Both the Smad
and p38 MAPK pathways play a crucial role in Runx2 expression
following induction by transforming growth factor-beta and bone
morphogenetic protein. Oncogene. 21:7156–7163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakai S, Tamura M, Mishima H, Kojima H and
Uemura T: Bone regeneration induced by adenoviral vectors carrying
til-1/Cbfa1 genes implanted with biodegradable porous materials in
animal models of osteonecrosis of the femoral head. J Tissue Eng
Regen Med. 2:164–167. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niu YB, Li YH, Kong XH, et al: The
beneficial effect of Radix Dipsaci total saponins on bone
metabolism in vitro and in vivo and the possible mechanisms of
action. Osteoporos Int. 23:2649–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong RW, Rabie AB and Hägg EU: The effect
of crude extract from Radix Dipsaci on bone in mice. Phytother Res.
21:596–598. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang RS, Lin WL, Chen YZ, et al:
Regulation by ultrasound treatment on the integrin expression and
differentiation of osteoblasts. Bone. 36:276–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sumanasinghe RD, Bernacki SH and Loboa EG:
Osteogenic differentiation of human mesenchymal stem cells in
collagen matrices: effect of uniaxial cyclic tensile strain on bone
morphogenetic protein (BMP-2) mRNA expression. Tissue Eng.
12:3459–3465. 2006. View Article : Google Scholar
|
|
24
|
Jia TL, Wang HZ, Xie LP, Wang XY and Zhang
RQ: Daidzein enhances osteoblast growth that may be mediated by
increased bone morphogenetic protein (BMP) production. Biochem
Pharmacol. 65:709–715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuo PL, Hsu YL, Chang CH and Chang JK:
Osthole-mediated cell differentiation through bone morphogenetic
protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway
in human osteoblast cells. J Pharmacol Exp Ther. 314:1290–1299.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu YL, Liang HL, Hung CH and Kuo PL:
Syringetin, a flavonoid derivative in grape and wine, induces human
osteoblast differentiation through bone morphogenetic
protein-2/extracellular signal-regulated kinase 1/2 pathway. Mol
Nutr Food Res. 53:1452–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Attisano L and Wrana JL: Signal
transduction by the TGF-beta superfamily. Science. 296:1646–1647.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai CF and Cheng SL: Signal transductions
induced by bone morphogenetic protein-2 and transforming growth
factor-beta in normal human osteoblastic cells. J Biol Chem.
277:15514–15522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weston CR, Lambright DG and Davis RJ:
Signal transduction. MAP kinase signaling specificity. Science.
296:2345–2347. 2002. View Article : Google Scholar : PubMed/NCBI
|